Abstract
Caseous calcification of the mitral annulus (CCMA) is a rare variant of mitral annular calcification (MAC). Since most cardiologists are unfamiliar with CCMA, it is commonly misdiagnosed as an abscess, tumor or infective vegetation on the mitral valve. In most cases, conservative management for this lesion is sufficient. In this review, we will discuss the various aspects of this condition and illustrate the gross and histologic pathology as well as various imaging modalities (Ultrasound, Computed tomography, Cardiac Magnetic resonance) to assess this unusual cardiac mass.
Clinical Vignette
The following is an illustration of a case that was referred for evaluation of a vegetation and possible surgery. A 41‐year‐old female with end‐stage renal disease and type 1 diabetes mellitus who underwent a kidney and pancreas transplant 8 years back presented with worsening shortness of breath over the course of 2 weeks. Electrocardiogram showed accelerated idioventricular rhythm. A transthoracic echocardiogram showed a large mass on the posterior mitral annulus resulting in moderate mitral stenosis. The patient was started on intravenous antibiotics for concerns of infective vegetation causing conduction system abnormalities and was transferred to our facility for surgical evaluation.
Upon arrival to our facility, physical examination was significant for a mid‐diastolic rumbling murmur on auscultation. The electrocardiogram showed a normal sinus rhythm. A repeat transthoracic echocardiogram revealed a 2.6 × 2.2‐cm mass located over the posterior mitral annulus, associated with moderate mitral stenosis, with a mean transmitral diastolic pressure gradient of 10 mm Hg and valve area of 1.32 cm2. Caseous calcification of the mitral annulus was diagnosed by cardiac ultrasonography.
A left heart catheterization showed a 70% to 75% mid left anterior descending coronary artery stenosis with a fractional flow reserve value of 0.69. The mitral valve area was calculated to be 1.1 to 1.3 cm2, with an estimated diastolic pressure gradient 10 to 12 mm Hg, suggesting moderate mitral stenosis.
Cardiac surgery revealed a large 2 × 2‐cm mass with a calcific envelope on the posterior mitral annulus, which was resected, the mitral valve was replaced, and a left internal thoracic artery was anastomosed to the distal part of the left anterior descending coronary artery. An incidental finding of a patent foramen ovale was surgically closed during the procedure.
Introduction
Mitral annular calcification (MAC) is defined as chronic degeneration of the mitral valve fibrous ring involving mainly the posterior annulus. This disorder is common in the elderly, particularly in women.1 MAC may occur in younger patients with advanced renal disease or other metabolic disorders that result in abnormal calcium metabolism. Caseous calcification of the mitral annulus (CCMA) is a lesser known and rarely described variant of MAC.2, 3
In 1970, Pomerance described cases of caseation of the calcified mitral rings in 7 of 258 cases in his autopsy series.1 Later on in 1983, Kronzon et al described 5 cases of sterile, caseous mitral annular abscess in a series of 9000 echocardiograms, and the authors concluded that these masses had a similar appearance to the caseous abscesses reported by Pomerance, and thought that these masses may represent a similar process.4 Since then, CCMA has been considered an odd variant of the more common MAC.
The terminology of CCMA is peculiar, as the term caseous usually refers to a type of necrosis, which is often encountered in tuberculosis.
CCMA appears as a round, sometimes semilunar, large, echo‐dense, soft mass with central echolucencies seen on both transthoracic and, in particular, transesophageal echocardiography, resembling a periannular mass. It is located at the posterior annular region of the mitral valve, unlike MAC, which usually involves the midbase of the posterior leaflet but may also involve other segments of the mitral annulus.5
Because CCMA is a rare condition, cardiac imagers may not be familiar with this incidental calcific mass; therefore, it is easily confused with a calcific periannular tumor or abscess. Even unnecessary surgeries for such lesions have been reported in the past when they were misdiagnosed as abscesses or cardiac tumors.4, 5
A PubMed search of the literature was performed using the terms “caseous calcification of the mitral annulus” and “liquefaction necrosis of the mitral annulus.” The search was limited to the title and abstract of full‐length articles written in English and published until the present. The last update of the search was performed in March 2013. To ensure that no potentially important studies were missed, the reference lists from these articles were also checked.
Prevalence
The exact prevalence of CCMA is unknown. Two studies looked through the prevalence of this disease in echocardiographic laboratories and found that MAC occurs in about 10.6% of the population; among these cases CCMA was found in 0.63% to 0.64% (ie, CCMA is found in 0.06%–0.07% of the population).3, 6 The prevalence of CCMA on a necropsy series was found to be 2.7% of the cases of MAC.1
The discrepancy between the pathologic and echocardiographic prevalence is likely due to underdiagnosis, as many cardiologists are not familiar with this condition. Another potential cause for this difference is the lower sensitivity of echocardiography compared to autopsy. We expect that the number of recognized cases will continue to rise due to the widespread use and enhancements of the different cardiac imaging modalities.7, 8
CCMA tends to occur in older patients (mean age 69 years) and is associated with hypertension.3 CCMA typically involves the posterior mitral annulus,6 and it rarely involves only the anterior annulus or both annuli.2, 9
Pathology and Pathophysiology
In MAC, the calcification usually begins between the posterior atrioventricular groove and the base of the posterior mitral leaflet, although in the elderly it can involve the entire annulus. CCMA represents a rare evolution of a calcified mitral ring due to caseous transformation of the inner material.10
The precise mechanism involved in liquefaction and caseation in CCMA is not well understood.3, 7 As the prevalence of CCMA is higher in patients with end‐stage renal disease, especially those on dialysis, an altered calcium phosphate metabolism is implicated in its pathogenesis.11 It is thought that lipid‐laden macrophages may be implicated in the liquefaction necrosis and development of CCMA, because high serum cholesterol is associated with MAC.2, 3
During cardiotomy, CCMA appears as a large intramyocardial mass situated in the region of the posterior annulus. The posterior leaflet appears stretched and arched over the mass. Upon perforation of the mass, toothpaste‐like, milky, caseous material is expressed. A large, calcified envelope that is noncollapsible surrounds this caseous material (Figure 1).3, 6, 12
Figure 1.
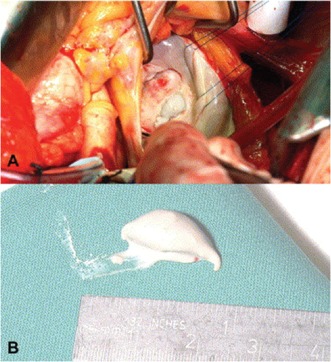
Intraoperative photograph demonstrating the caseous calcification after incision (A). Pasty white material was subsequently aspirated (B). Reprinted with permission from Elsevier.
A central region of amorphous eosinophilic acellular material surrounded by macrophages and lymphocytes characterizes the microscopic examination of the expressed caseous material. The periphery shows multiple calcifications and zones of necrosis (Figure 2).6, 12, 13
Figure 2.
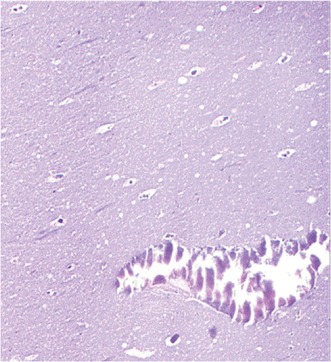
Histologic section of the aspirate shows an amorphous, basophilic substance with scattered calcifications and sparse histiocytes (magnification ×200; hematoxylin‐eosin stain). Reprinted with permission from Elsevier.
CCMA is believed to be a benign process; however, it may result in abnormal flow across the mitral valve, commonly chronic mitral regurgitation, and less likely mitral stenosis, and less likely mitral stenosis. Because the mitral annulus lies close to the atrioventricular node and the conduction system, CCMA may cause bradyarrhythmia and atrioventricular blocks.3, 6, 7, 14
Clinical Manifestations
Incidental findings of an intracardiac mass during cardiac imaging is the most common clinical presentation of CCMA; therefore, it is important for cardiologists, particularly cardiac imagers, to be familiar with this diagnosis. However, the most common symptoms encountered by patients with this condition are palpitations and dyspnea (secondary to pulmonary venous congestion); rarely, some patients may develop syncope (secondary to atrioventricular blocks).3, 6
Both CCMA and MAC are believed to be associated with a high calcium score, coronary artery disease, aortic valve disease, atrial fibrillation, and hypertension.3, 6, 12, 14, 15
CCMA may be complicated by systemic embolization (eg, cerebrovascular accidents, retinal artery occlusion, and acute coronary syndrome). The postulated mechanisms of embolization include embolization of small calcified parts, ulceration of the surface complicated by thrombus formation, and subsequent embolization or fistulization of the caseous necrosis in the lumen of the left atrium or left ventricle.9, 16, 17, 18
Imaging
CCMA has no specific clinical presentation; therefore, imaging—particularly echocardiography—is necessary for diagnosis. In fact, most cases of CCMA are diagnosed as a part of the workup for an intracardiac mass. Multimodality imaging, including 3‐dimensional (3D) transesophageal echocardiography (TEE), cardiac magnetic resonance imaging (MRI), and computed tomography (CT) can be used in suspicious cases to avoid unnecessary surgery.19, 20, 21, 22
Transthoracic echocardiography (TTE) is considered the most reliable method for diagnosis of this condition.10 CCMA appears as a large, round, echo‐dense mass with smooth borders situated in the periannular region, with no acoustic shadowing artifact and contains central areas of echolucencies resembling liquefaction. It may appear as a semilunar mass in the parasternal short‐axis view. The mass is usually located in the posterior region, at the junction between the left atrium and the left ventricle, bulging into the left atrial cavity or into the adjacent left ventricular posterior wall. The mass rarely extends along the whole mitral annulus (Figure 3).6, 23 On the contrary, MAC appears as a J‐, C‐, or U‐shaped dense echo band, which creates an acoustic shadowing and lacks the echolucent center.24, 25 On color Doppler, the central zone of CCMA lacks any flow.10
Figure 3.
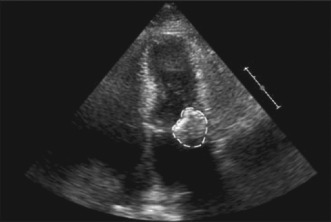
Two‐dimensional echocardiogram, in the apical 4‐chamber view, of a calcific mass attached to the mitral annulus. Copyright 2008 by the Texas Heart Institute, Houston. Reprinted with permission.
TTE is usually sufficient to confirm the diagnosis of CCMA; however, in suspicious cases, especially those with limited acoustic viewing or poor quality of echocardiography images, patients may be referred for 2‐dimensional TEE for better evaluation of this mass, as TEE gives better visualization for posteriorly located structures. CCMA appears as a mass with a central area of echolucency resembling liquefaction, with no flow in the central zone by color Doppler, surrounded by a hyperechogenic structure. It may have the appearance of a mass with “string of pearls‐like” appearance.6, 13, 25
On 3D TTE or 3D TEE, CCMA appears as a large area of liquefaction necrosis, with an irregular surface on the posterior mitral annulus, that extends to the most basal area at the region of the P1‐P2 scallops. The mass may distort the shape of the annulus.19, 25, 26
With the wide use of cardiac CT and cardiac MRI, CCMA can be accidently discovered during one of these examinations. On CT scan, CCMA appears as a well‐defined oval or crescent‐shaped hyperdense mass with peripheral calcification, usually along the posterior mitral annulus, has very high Hounsfield units, and lacks contrast enhancement. The central hyperdensity is thought to be secondary to the liquefactive material that fills the center of the mass (Figure 4).6, 12, 15, 27, 28, 29, 30, 31
Figure 4.
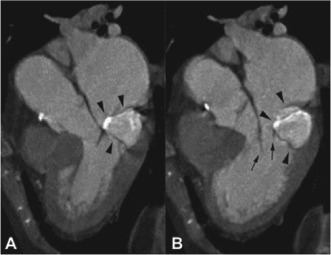
Computed tomography reconstructions parallel to the long axis of the left ventricle during midsystole (A) and mid‐diastole (B) demonstrate the centrally hyperdense, peripherally calcified mass (arrowheads) located in the posterior mitral annulus and attached to the posterior leaflet. Reprinted with permission from Elsevier.
Cardiac MRI is considered to be the technique of choice in doubtful cases. Findings of CCMA on cardiac MRI include a well‐defined mass with hyperintense center and hypointense rim, discrete from the adjacent myocardium and posterior mitral valve on T1‐weighted fast spin‐echo imaging. A similar centrally hyperintense mass with a peripheral rim of hypointensity is also visualized with T1‐weighted spoiled gradient‐echo imaging techniques (Figure 5). On T2‐weighted MRI sequences, CCMA appears as a mass devoid of a central signal but with a ring of high intensity compared with the surrounding myocardium.23, 26, 32, 33, 34, 35, 36
Figure 5.
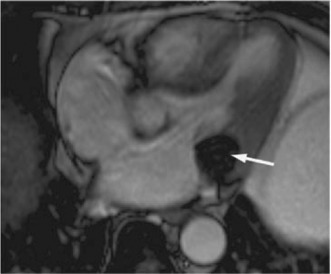
Cardiac magnetic resonance imaging scan of a hypointense mass (arrow) attached to the mitral annulus. Copyright 2008 by the Texas Heart Institute, Houston. Reprinted with permission.
Differential Diagnosis
Because CCMA is an unfamiliar condition, most of the cases are confused with other intracardiac masses such as tumors (most commonly myxoma), abscesses, and vegetations. Therefore, the clinical context is essential to establish the correct diagnosis. The key issues that can be used to differentiate CCMA from other left atrial masses are discussed below.
Tumors
Myxoma is a common intracardiac lesion. To distinguish between CCMA and a myxoma echocardiographically, it is important to note that the shape of a myxoma can be quite variable (ie, amorphous, polypoid, round, or oval). It is usually mobile, pedunculated, and attached to the myocardial wall along the interatrial septum in the region of fossa ovalis via a thin stalk. It lacks the calcifications in the borders that are seen in CCMA. Additionally, it is highly vascular compared with CCMA, in which no blood flow should be visualized by color Doppler.25
Other uncommon tumors include hemangioma of the mitral valve and malignant lesions like leiomyosarcoma. In hemangiomas, the echolucencies (representing the tumor vascularity) extend all the way to the periphery of the mass and no calcification is found. However, leiomyosarcoma rarely shows echolucencies in the areas of necrosis and calcification.25
Myocardial Abscess
A myocardial abscess appears as a mass within the myocardium or annular region, with homogenous echogenic appearance on echocardiography. It lacks calcifications and may show systolic blood flow by color Doppler. It can closely resemble CCMA. Not surprisingly, the first reported cases of CCMA were described as “sterile myocardial abscess.”4, 25
Infective Endocarditis
Infective endocarditis (IEC) is diagnosed with the use of Duke criteria. One of the major criteria is a positive echocardiographic finding. IEC appears as an oscillating mass on a support structure, an abscess (undefined structure), a partial dehiscence of a prosthetic valve, or new valve regurgitation.25
Other rare structures that may lie in the atrioventricular grooves include a dilated coronary sinus or left circumflex artery aneurysm (both may represent echolucent areas, yet on color Doppler there is blood flow), lipomatous hypertrophy, or enlarged lymph nodes.3, 25
Prognosis and Treatment
CCMA is considered to be a dynamic and resolving process. It can resolve spontaneously and transform back to MAC. However, it may recur even after surgical excision.3, 24, 37, 38
Currently, there is no consensus on the optimal management for CCMA. The current data suggest conservative medical management for CCMA when the diagnosis is certain and there is no obstruction to left atrial emptying.25
The current indications for surgical intervention include mitral valvular dysfunction (either stenosis or regurgitation), embolic manifestations, or when it is impossible to rule out the possibility of a tumor. Mitral valve replacement is preferred to mitral valve repair.16, 25, 39 It should be noted that aggressive debridement may increase the risk of perforation of the left ventricle, and simple unroofing of the cavity would continue to expose necrotic debris to the systemic blood flow. Therefore, a technique of non‐everting annular sutures is recommended.39
Anticoagulation should be considered in patients with CCMA who present with embolic manifestations.17
Conclusion
CCMA is a cardiac mass that can be confused with other intracardiac masses (eg, tumors, vegetations, and abscesses). However, close attention to the clinical presentation and multimodality imaging can clearly differentiate CCMA from other lesions. Most cardiologists (even imagers) are unfamiliar with this condition. CCMA is thought to be a rare condition; however, the actual number may be higher due to the advances in the imaging modalities. CCMA is typically located on the posterior mitral annulus. The exact pathogenesis for this condition remains unclear. CCMA is usually diagnosed incidentally and may have a benign course, but it may be complicated with mitral valvular dysfunction, systemic embolization, or conduction abnormalities. CCMA may have a dynamic course; it can resolve spontaneously or even recur after surgical excision. TTE is the most reliable method for diagnosis. On TTE, CCMA appears as a large, echo‐dense, soft mass with central echolucencies and lacks any acoustic shadowing. Cardiac MRI is considered to be the technique of choice in suspicious cases. Multimodality imaging, including TEE, cardiac CT, and cardiac MRI, can be used to confirm the diagnosis and avoid unnecessary surgery. Uncomplicated cases of CCMA can be managed conservatively; surgical intervention (preferable mitral valve replacement) is reserved only for the complicated cases.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Pomerance A. Pathological and clinical study of calcification of the mitral valve ring. J Clin Pathol. 1970;23:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Correale M, Deluca G, Ieva R, et al. Spontaneous resolution of a caseous calcification of the mitral annulus. Clinics (Sao Paulo). 2009;64:1130–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deluca G, Correale M, Ieva R, et al. The incidence and clinical course of caseous calcification of the mitral annulus: a prospective echocardiographic study. J Am Soc Echocardiogr. 2008;21:828–833. [DOI] [PubMed] [Google Scholar]
- 4. Kronzon I, Winer HE, Cohen ML. Sterile, caseous mitral anular abscess. J Am Coll Cardiol. 1983;2:186–190. [DOI] [PubMed] [Google Scholar]
- 5. Teja K, Gibson RS, Nolan SP. Atrial extension of mitral annular calcification mimicking intracardiac tumor. Clin Cardiol 1987;10:546–548. [DOI] [PubMed] [Google Scholar]
- 6. Harpaz D, Auerbach I, Vered Z, et al. Caseous calcification of the mitral annulus: a neglected, unrecognized diagnosis. J Am Soc Echocardiogr. 2001;14:825–831. [DOI] [PubMed] [Google Scholar]
- 7. Pozsonyi Z, Toth A, Vago H, et al. Severe mitral regurgitation and heart failure due to caseous calcification of the mitral annulus. Cardiology. 2011;118:79–82. [DOI] [PubMed] [Google Scholar]
- 8. Novaro GM, Griffin BP, Hammer DF. Caseous calcification of the mitral annulus: an underappreciated variant. Heart. 2004;90:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stamou SC, Braverman AC, Kouchoukos NT. Caseous calcification of the anterior mitral valve annulus presenting as intracardiac mass. J Thorac Cardiovasc Surg. 2010;140:e9–e10. [DOI] [PubMed] [Google Scholar]
- 10. Marci M, Lo Jacono F. Mitral regurgitation due to caseous calcification of the mitral annulus: two case reports. Cases J. 2009;2:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akram M, Hasanin A. Caseous mitral annular calcification: is it a benign condition? J Saudi Heart Assoc. 2012;24:205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alkadhi H, Leschka S, Pretre R, et al. Caseous calcification of the mitral annulus. J Thorac Cardiovasc Surg. 2005;129:1438–1440. [DOI] [PubMed] [Google Scholar]
- 13. Di Bella G, Carerj S, Ando G, et al. Cardiac imaging in the evaluation of mitral annulus caseous calcification. Int J Cardiol. 2006;113:E30–E31. [DOI] [PubMed] [Google Scholar]
- 14. Fernandes RM, Branco LM, Galrinho A, et al. Caseous calcification of the mitral annulus. A review of six cases. Rev Port Cardiol. 2007;26:1059–1070. [PubMed] [Google Scholar]
- 15. Chahal M, Temesy‐Armos P, Stewart WJ. Big MAC: caseous calcification of the mitral annulus referred for possible cardiac tumor. Echocardiography. 2011;28:E76–E78. [DOI] [PubMed] [Google Scholar]
- 16. Chevalier B, Reant P, Laffite S, et al. Spontaneous fistulization of a caseous calcification of the mitral annulus: an exceptional cause of stroke. Eur J Cardiothorac Surg. 2011;39:e184–e185. [DOI] [PubMed] [Google Scholar]
- 17. Higashi H, Ohara T, Nakatani S, et al. A case of caseous calcification of the mitral annulus: a potential source of embolic stroke. J Cardiol Cases. 2010;2:e141–e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Durao D, Pitta Mda L, Alves M, et al. Myocardial infarction as the first probable manifestation of caseous calcification of the mitral annulus. Rev Port Cardiol. 2009;28:1271–1275. [PubMed] [Google Scholar]
- 19. Ribeiro S, Salgado A, Salome N, et al. Caseous calcification of the mitral annulus: A multi‐modality imaging perspective. Rev Port Cardiol. 2012;31:313–316. [DOI] [PubMed] [Google Scholar]
- 20. Fujiwara T, Fujita T, Toda K, et al. Multimodality imaging of caseous calcification of mitral annulus. Eur J Cardiothorac Surg. 2012;41:451. [DOI] [PubMed] [Google Scholar]
- 21. Pugliatti P, Piccione MC, Ascenti G, et al. Images in cardiovascular medicine: caseous calcification of the mitral annulus. Echocardiography. 2013;30:E30–E32. [DOI] [PubMed] [Google Scholar]
- 22. Lee C, Yoon AJ, Klipfel NE, et al. Caseous mitral annular calcification along the anterolateral annulus causing mild mitral regurgitation: multi‐modality imaging and diagnosis. Circ J. 2012;76:2898–2900. [DOI] [PubMed] [Google Scholar]
- 23. Arora H, Madan P, Simpson L, et al. Caseous calcification of the mitral annulus. Tex Heart Inst J. 2008;35:211–213. [PMC free article] [PubMed] [Google Scholar]
- 24. Koito H, Nakamura C, Suzuki J, et al. Reduced size of liquefaction necrosis of mitral annular calcification in chronic renal failure by using low calcium concentration hemodialysis. Jpn Circ J 1999;63:490–492. [DOI] [PubMed] [Google Scholar]
- 25. McKernan NP, Culp WC Jr, Knight WL, et al. CASE 2—2012 intraoperative diagnosis and management of caseous calcification of the mitral annulus. J Cardiothorac Vasc Anesth. 2012;26:327–332. [DOI] [PubMed] [Google Scholar]
- 26. Garcia‐Ibarrondo N, Lang RM. Caseous calcification of the mitral annulus, a rare echocardiographic finding. Rev Esp Cardiol. 2011;64:828–831. [DOI] [PubMed] [Google Scholar]
- 27. Biteker M, Duran NE, Ozkan M. Caseous calcification of the mitral annulus imaged with 64‐slice multidetector CT. Echocardiography. 2009;26:744–745. [DOI] [PubMed] [Google Scholar]
- 28. Alizade E, Acar G, Guler A, et al. Giant caseous calcification of the mitral annulus on 64‐slice multidetector computed tomography. Tex Heart Inst J. 2012;39:288–289. [PMC free article] [PubMed] [Google Scholar]
- 29. Zeina AR, Makhoul N, Nachtigal A. Mitral annulus caseous calcification imaged with 64‐slice MDCT. Acute Card Care. 2009;11:121–122. [DOI] [PubMed] [Google Scholar]
- 30. Vanovermeire OM, Duerinckx AJ, Duncan DA, et al. Caseous calcification of the mitral annulus imaged with 64‐slice multidetector CT and magnetic resonance imaging. Int J Cardiovasc Imaging. 2006;22:553–559. [DOI] [PubMed] [Google Scholar]
- 31. Lubarsky L, Jelnin V, Marino N, et al. Images in cardiovascular medicine. Caseous calcification of the mitral annulus by 64‐detector‐row computed tomographic coronary angiography: a rare intracardiac mass. Circulation. 2007;116:e114–e115. [DOI] [PubMed] [Google Scholar]
- 32. Monti L, Renifilo E, Profili M, et al. Cardiovascular magnetic resonance features of caseous calcification of the mitral annulus. J Cardiovasc Magn Reson. 2008;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fernandez‐Golfin Loban C, Jimenez Lopez‐Guarch C, Centeno J. Caseous calcification of the mitral annulus: role of cardiac magnetic resonance. Rev Esp Cardiol. 2009;62:827–830. [DOI] [PubMed] [Google Scholar]
- 34. Chen O, Dontineni N, Nahlawi G, et al. Serial cardiac magnetic resonance imaging of a rapidly progressing liquefaction necrosis of mitral annulus calcification associated with embolic stroke. Circulation. 2012;125:2792–2795. [DOI] [PubMed] [Google Scholar]
- 35. Di Bella G, Masci PG, Ganame J, et al. Images in cardiovascular medicine. Liquefaction necrosis of mitral annulus calcification: detection and characterization with cardiac magnetic resonance imaging. Circulation. 2008;117:e292–e294. [DOI] [PubMed] [Google Scholar]
- 36. Srivatsa SS, Taylor MD, Hor K, et al. Liquefaction necrosis of mitral annular calcification (LNMAC): review of pathology, prevalence, imaging and management: proposed diagnostic imaging criteria with detailed multi‐modality and MRI image characterization. Int J Cardiovasc Imaging. 2012;28:1161–1171. [DOI] [PubMed] [Google Scholar]
- 37. Gramenzi S, Mazzola AA, Tagliaferri B, et al. Caseous calcification of the mitral annulus: unusual case of spontaneous resolution. Echocardiography. 2005;22:510–513. [DOI] [PubMed] [Google Scholar]
- 38. Stone E, Cohn D, Deal C, et al. Calcific atrial mass in end‐stage renal failure. Nephrol Dial Transplant. 1997;12:807–810. [DOI] [PubMed] [Google Scholar]
- 39. de Vrey EA, Scholte AJ, Krauss XH, et al. Intracardiac pseudotumor caused by mitral annular calcification. Eur J Echocardiogr. 2006;7:62–66. [DOI] [PubMed] [Google Scholar]


