Abstract
Background
Diabetic patients have a worse prognosis than nondiabetic patients after myocardial infarction. Although exercise improves risk factors, exercise capacity, and mortality, it is still unclear if these benefits are the same as in nondiabetics. Furthermore, although exercise tolerance is predicted by systolic and diastolic dysfunction in nondiabetics, its role as a predictor of exercise capacity in diabetics remains unclear.
Hypothesis
Diabetics and nondiabetics see a similar improvement in their cardiac risk factors and exercise parameters from exercise‐based cardiac rehabilitation (CR).
Methods
A series of 370 diabetics and 942 nondiabetics entered a 36‐session outpatient CR program after interventions for coronary heart disease or after bypass or cardiac valve surgery. The program consisted of physical exercise, lifestyle modification, and pharmacotherapy.
Results
Quality of life, weight, blood pressure, and lipid profiles improved significantly in both groups during the 12‐week program. Baseline metabolic equivalents (METs) were lower in diabetics vs nondiabetics at the start of CR (2.4 vs 2.7, P < 0.001). Although both groups increased their exercise capacity, diabetics had less improvement (change in METs 1.7 vs 2.6, P < 0.001). Significant predictors for improvement after CR included age, sex, and weight, as well as both systolic and diastolic function. After adjustment for these, diabetes remained a significant predictor of reduced improvement in exercise capacity.
Conclusions
Diabetics saw a significant benefit in quality of life, weight, exercise tolerance, and cardiac risk factors, but to a lesser extent when compared with nondiabetics. The mechanisms for poorer improvement in diabetics following CR also include noncardiac factors and require further study.
Introduction
Diabetes mellitus (DM) is increasing in prevalence in the United States1 and is now more common in patients entering cardiac rehabilitation (CR).2 The association between DM and cardiovascular disease is well established,3, 4, 5, 6 and diabetic patients who have suffered a myocardial infarction (MI) have worse outcomes than nondiabetics.4, 5 Despite improvements in cardiovascular disease treatments, diabetic outcomes after a MI continue to lag.4
Exercise‐based CR improves prognosis in patients with coronary artery disease.7, 8, 9 Although improving cardiopulmonary fitness is also beneficial in diabetics, in the United States, only 25% of older cardiac patients with DM meet recommendations for physical activity based on the American Diabetes Association guidelines.10 The number of diabetics in CR has increased in the last 10 years,11 but it is still unclear if they gain the same benefit from a CR program as do nondiabetics.3, 12, 13, 14, 15
The aim of this study was to compare the effects of exercise‐based CR on cardiac risk factors and exercise parameters in a series of diabetic and nondiabetic individuals.
Methods
Patient Population
Between 2004 and 2012, 1312 patients were enrolled into the outpatient CR program at Wake Forest Baptist Medical Center after interventions for coronary artery disease, including coronary artery bypass graft surgery and/or valvular disease. These disorders were defined by clinical criteria and/or cardiac catheterization or echocardiography. Of these patients, 370 had DM, diagnosed using standard criteria.16 Clinical data were taken from patient records, and the study was approved by our institutional review board.
Program
Our CR program consists of 3 sessions per week for 12 weeks (total of 36 sessions) and includes both exercise and health and nutrition education sessions. An individualized exercise plan was created by an exercise physiologist based upon history, comorbidities, physical fitness, and clinical status according to the American College of Sports Medicine Guidelines for Exercise Testing and Prescription.17 Exercise sessions lasted 30 to 40 minutes and consisted of 5 minutes of warm‐up and cool‐down activity; upper‐ and lower‐body training, including walking on a track, cycle ergometry, treadmills, and stair‐climbers; and light resistance exercises using hand weights and tubing. Level of activity was increased by 0.5 to 1.0 metabolic equivalents (METs) as tolerated, to a rating of perceived exertion of 11 to 14 on a Borg scale of 6 to 20.17 Heart rates were obtained by telemetry at the highest level of exercise during each modality.
Measurements
At each visit, baseline vital signs were recorded for all patients. We also measured height, weight, systolic and diastolic blood pressures, resting heart rate, and MET level. Steady‐state MET levels were recorded at each session and were obtained either automatically from exercise devices or using a standardized MET formula. The MET levels represent the highest level of exertion for the patient for that specific session and were documented by the exercise physiologist or nurse. Baseline MET level was recorded during the first session and at each subsequent visit in the same manner.
Blood pressure was measured by a registered nurse using a standardized cuff after a 5‐minute rest period. Hypertension was diagnosed based on guidelines from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7),18 with normal pressure defined as a systolic pressure (SBP) <120 mm Hg and diastolic pressure (DBP) <80 mm Hg. We defined baseline pressure as the first, and post pressure as the last, measurement for each patient.
Adult Treatment Panel III (ATP III) guidelines were used to define lipid goals. Goals were low‐density lipoprotein (LDL) concentrations < 100 mg/dL, high‐density lipoprotein (HDL) concentrations > 40 mg/dL, and triglyceride concentrations < 150 mg/dL.19 Pre‐ and postlipid profiles were checked, respectively, within 1 month of starting and finishing CR.
Quality of life (QOL) score was calculated at the beginning and end of the program using the Ferrans and Powers Quality of Life Index Cardiac version IV questionnaire.20 Additional initial blood measurements included hemoglobin A1c (glycated hemoglobin). Left ventricular ejection fraction (LVEF) was measured by standard techniques.21 Approximately half of the patients (54% of diabetics and 56% of nondiabetics) underwent transthoracic echocardiograms within a month of participating in CR. Chronic obstructive pulmonary disease was documented by chart review and had been diagnosed by standard criteria.22
Statistical Analysis
Descriptive statistics (frequency and percent for categorical factors, mean and SD for continuous factors) were calculated and compared for statistical significance using χ2 or Fisher exact tests for categorical variables and Student's t test for continuous variables. Baseline and post‐CR measures were compared using paired t tests and changes calculated as mean (95% confidence interval) of post‐CR minus baseline levels. Multivariable linear regression models were constructed with metabolic equivalents as the dependent variable, and independent predictors were selected for inclusion using a backward selection methodology. All potentially significant univariate predictors of exercise capacity (P < 0.10) were included in the model, and least significant covariates were removed individually until all remaining covariates were statistically significant predictors of exercise capacity. Two‐ and 3‐way interaction terms between independent predictors in the final models were further tested for statistical significance. P < 0.05 was considered statistically significant. The SAS version 9.3 statistical software package (SAS Institute, Inc., Cary, NC) was used for all statistical analyses.
Results
Baseline Characteristics
Demographic and clinical characteristics upon entry into the program are shown in Table 1. Ninety percent of the diabetic patients had been referred for rehabilitation for coronary artery disease, and most had undergone revascularization procedures. Hypertension, obesity, lung disease, and dyslipidemia were more common in diabetic vs nondiabetic participants (P < 0.001 for all). Diabetic patients had increased resting heart rate, SBP, triglyceride concentrations, waist circumference, and weight, but decreased HDL and LDL concentrations compared with nondiabetics at the start of CR (P < 0.05 for all). Initial exercise capacity was lower in diabetics vs nondiabetics (2.4 vs 2.7 METs, P < 0.001). The proportion of patients with valvular surgery was significantly higher among the nondiabetics (P < 0.001). Mean time to CR initiation in all the patients was 32 ± 33 days (34 ± 34 for diabetics vs 32 ± 32 for nondiabetics, P = 0.38).
Table 1.
Baseline Patient Characteristics by DM Status
| Characteristic | Diabetics, N = 370 | Nondiabetics, N = 942 | P Value |
|---|---|---|---|
| Demographics | |||
| Age, y | 62 ± 10 | 63 ± 12 | 0.34 |
| Male sex | 252 (68) | 682 (72) | 0.12 |
| Caucasian race | 288 (78) | 807 (86) | <0.001 |
| BMI, kg/m2 | 31 ± 6 | 28 ± 5 | <0.001 |
| Weight, lbs | 205 ± 46 | 187 ± 40 | <0.001 |
| Waist circumference, in | 43 ± 6 | 40 ± 5 | <0.001 |
| Medical history | |||
| Quality of life | 22 ± 5 | 23 ± 4 | <0.001 |
| Baseline METs | 2.4 ± 0.6 | 2.7 ± 0.9 | <0.001 |
| Current smoker | 30 (8) | 82 (9) | 0.72 |
| Lung disease | 149 (40) | 286 (30) | <0.001 |
| Hypertension | 336 (91) | 687 (73) | <0.001 |
| SBP, mm Hg | 127 ± 20 | 120 ± 18 | <0.001 |
| DBP, mm Hg | 71 ± 11 | 70 ± 10 | 0.02 |
| Resting HR, bpm | 79 ± 15 | 76 ± 14 | <0.001 |
| Hyperlipidemia | 325 (88) | 687 (73) | <0.001 |
| LDL, mg/dL | 91 ± 38 | 101 ± 40 | <0.001 |
| HDL, mg/dL | 38 ± 12 | 42 ± 17 | <0.001 |
| Triglycerides, mg/dL | 182 ± 170 | 131 ± 84 | <0.001 |
| Glucose, mg/dL | 121 ± 48 | 99 ± 14 | <0.001 |
| LVEF, % | 49 ± 12 | 49 ± 12 | 0.30 |
| Multivessel disease | 265 (72) | 573 (61) | <0.001 |
| Medications | |||
| β‐Blocker | 326 (88) | 823 (87) | 0.71 |
| ACEI | 263 (71) | 558 (59) | <0.001 |
| Statin | 333 (90) | 802 (85) | 0.02 |
| Reason for referral | |||
| CAD | 313 (88) | 741 (80) | <0.001 |
| Presentation | |||
| MI | 109 (31) | 336 (37) | 0.06 |
| UA | 108 (31) | 174 (19) | <0.001 |
| Stable angina | 71 (20) | 157 (17) | 0.20 |
| Treatment | |||
| CABG | 108 (31) | 270 (29) | 0.66 |
| DES | 138 (39) | 333 (36) | 0.34 |
| BMS | 26 (7) | 52 (6) | 0.25 |
| Balloon angioplasty | 7 (2) | 18 (2) | 0.98 |
| Medical therapy | 31 (9) | 56 (6) | 0.09 |
| Valvular disease | 28 (8) | 173 (19) | <0.001 |
| AVR surgery | 17 (5) | 95 (10) | 0.002 |
| MVR surgery | 11 (3) | 56 (6) | 0.03 |
| Other treatment | 1 (0.3) | 25 (3) | 0.006 |
Abbreviations; ACEI, angiotensin‐converting enzyme inhibitor; AVR, atrial valve replacement; BMS, bare‐metal stent; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; DBP, diastolic blood pressure; DES, drug‐eluting stent; HDL, high‐density lipoprotein; HR, heart rate; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; METs, metabolic equivalents; MI, myocardial infarction; MVR; mitral valve replacement; SBP, systolic blood pressure; UA, unstable angina.
Data are expressed as mean ± standard deviation or n (%).
Both diabetics and nondiabetics showed significant improvement in quality of life, weight, SBP, DBP, resting heart rate, lipid profile, and exercise capacity after CR (Table 2). Diabetic patients showed marked improvement in triglyceride concentrations and glycated hemoglobin levels compared with nondiabetics (mean difference, −37.3 vs −12.3 mg/dL, P < 0.001), (−0.5 vs 0.1, P = 0.002). Diabetics also had significant improvement in exercise capacity at peak compared with baseline (1.7 METs increase, P < 0.001); however, this was less than in nondiabetic participants (Table 2 and Figure 1).
Table 2.
Pre‐ to Post–Cardiac Rehabilitation Metric Δs by DM Status
| Metric | Diabetics (N = 370) | Nondiabetics (N = 942) | ||
|---|---|---|---|---|
| n | Δ (95% CI) | n | Δ (95% CI) | |
| Peak METs | 370 | 1.7 (1.5‐1.9) | 942 | 2.5 (2.4‐2.7)a |
| Quality of life | 147 | 1.9 (1.4‐2.4) | 383 | 1.8 (1.5‐2.1) |
| Weight, lbs | 309 | −2.2 (−3.2 to −1.1) | 798 | −3.0 (−3.5 to −2.4) |
| BMI, kg/m2 | 291 | −0.4 (−0.6 to −0.2) | 762 | −0.5 (−0.6 to −0.4) |
| SBP, mm Hg | 321 | −4.9 (−6.8 to −3.0) | 822 | −3.6 (−4.8 to −2.4) |
| DBP, mm Hg | 318 | −2.2 (−3.5 to −0.9) | 821 | −1.4 (−2.2 to −0.7) |
| Resting heart rate, bpm | 315 | −4.3 (−5.8 to −2.9) | 818 | −4.3 (−5.1 to −3.5) |
| LDL, mg/dL | 234 | −13.2 (−17.6 to −8.8) | 607 | −18.3 (−21.4 to −15.3) |
| HDL, mg/dL | 250 | 2.0 (0.7‐3.4) | 629 | 1.7 (0.8‐2.6) |
| Triglycerides, mg/dL | 250 | −37.3 (−58.9 to −15.8) | 629 | −12.6 (−18.8 to −6.4)a |
| Glucose, mg/dL | 257 | 1.2 (−4.6 to 6.9) | 490 | −0.5 (−2.1 to 1.2) |
| HbA1c | 100 | −0.5 (−0.8 to −0.3) | 26 | 0.1 (−0.1 to 0.2)a |
Abbreviations: BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; HbA1c, glycated hemoglobin; LDL, low‐density lipoprotein; METs, metabolic equivalents; SBP, systolic blood pressure.
P < 0.05 vs patients with diabetes mellitus.
Figure 1.

Mean metabolic equivalents (METs) during cardiac rehabilitation in diabetic (circles) and nondiabetic patients (diamonds). † = P < 0.01 vs baseline.
We further evaluated improvement in exercise capacity by DM status stratified by sex (Figure 2). Overall, women improved less than men, with similar results in diabetic and nondiabetic women. In contrast, there was a marked improvement in diabetic men compared with nondiabetic men.
Figure 2.
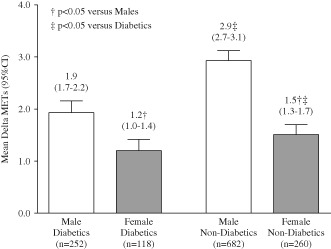
Mean change in metabolic equivalents (mean Δ METs) from baseline to peak in males (white bars) and females (gray bars), by diabetic status. Numbers above bars indicate mean value and range of values. † = P < 0.05 vs males; ‡ = P < 0.05 vs diabetic patients. Abbreviations: CI, confidence interval; METs, metabolic equivalents.
Exercise capacity in diabetics improved less than in nondiabetics regardless of diastolic function, whereas nondiabetics with normal diastolic function had significantly greater improvements in METs levels compared with those with decreased diastolic function (P < 0.05; Figure 3).
Figure 3.
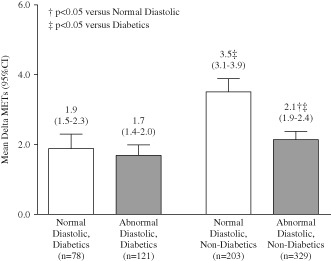
Mean change in metabolic equivalents (mean Δ METs) from baseline to peak in patients with normal (white bars) and abnormal diastolic function (gray bars), by diabetic status. Numbers above bars indicate mean value and range of values. †P < 0.05 vs normal diastolic; ‡P < 0.05 vs diabetic patients. Abbreviations: CI, confidence interval; METs, metabolic equivalents.
In multivariate analyses, DM, advanced age, female sex, increased body mass index (BMI), lung disease, and poor LVEF (<35%) were significant independent predictors of decreased improvement in peak exercise capacity after CR (Table 3). There was a statistically significant interaction between DM status, sex, and BMI, and so a 3‐way interaction term was also included in the final models. Similar results were observed in the subset of patients who had baseline diastolic function measured.
Table 3.
Multivariate Analysis of Change in Exercise Capacity From Baseline
| Model | Base Model, n = 1290a | Including Diastolic Function, n = 723a | ||||
|---|---|---|---|---|---|---|
| PE | SE | P Value | PE | SE | P Value | |
| DM | −0.86 | 0.150 | <0.001 | −0.97 | 0.209 | <0.001 |
| Age per 10 years | −0.55 | 0.051 | <0.001 | −0.63 | 0.074 | <0.001 |
| Female sex | −1.29 | 0.148 | <0.001 | −1.43 | 0.200 | <0.001 |
| BMI per kg/m2 | −0.05 | 0.011 | <0.001 | −0.06 | 0.015 | <0.001 |
| Lung disease | −0.44 | 0.123 | <0.001 | −0.33 | 0.171 | 0.05 |
| LVEF <35% vs ≥50% | −0.63 | 0.188 | <0.001 | −0.55 | 0.267 | 0.04 |
| LVEF 35%–50% vs ≥50% | −0.03 | 0.132 | 0.82 | −0.06 | 0.181 | 0.75 |
| On statin therapy | 0.23 | 0.169 | 0.18 | 0.25 | 0.220 | 0.25 |
| 3‐Way interaction term for DM, female sex, BMI | 0.02 | 0.008 | 0.006 | 0.03 | 0.011 | 0.02 |
| Abnormal diastolic function | −0.50 | 0.171 | 0.004 | |||
Abbreviations: BMI, body mass index; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; METs, metabolic equivalents; PE, parameter estimate; SE, standard error of parameter estimate.
Only patients with nonmissing values for covariates included in the model. Parameter estimates indicate the multivariable adjusted absolute mean change in METs from baseline to peak associated with each covariate.
Discussion
We found that diabetic patients entering CR had a greater prevalence of cardiac risk factors at baseline than nondiabetics, similar to other studies.3, 13, 14 Although both diabetics and nondiabetics experienced significant improvements in all risk factors after CR, particularly triglycerides, diabetic participants had significantly less improvement in exercise capacity than did nondiabetic participants.
Previous studies reported similar benefits in exercise capacity after CR in diabetic and nondiabetics, both with3, 13, 14, 23, 24, 25 and without26 coronary artery disease. Beneficial effects of such programs have been reviewed elsewhere.27 In the current study, initial exercise capacity in diabetics was lower compared with nondiabetics and overall improvement was less marked. In a 2‐month study of 59 diabetic CR participants (51 men, mean age 57 years), Verges et al15 also noted a lack of improvement relative to participants without DM. But in their study, diabetics had the same initial exercise capacity as nondiabetics,15 whereas we and others have found the opposite.3, 13, 14, 23, 24 Although the older age of our patients (62 years) may have played a role in their poorer exercise tolerance, the presence of DM itself was a stronger independent predictor of exercise capacity than age in multivariable analysis.
Diabetic participants had lower exercise capacity regardless of their systolic and diastolic left ventricular function as assessed by echocardiography. Indeed, abnormal diastolic function, a significant predictor of exercise intolerance,28 predicted a poorer improvement in exercise capacity than abnormal systolic function. Furthermore, diabetic participants with normal diastolic function still had less improvement in exercise capacity compared with nondiabetic participants. Multivariable analysis including age, sex, weight, LVEF, and diastolic function showed that DM was a stronger predictor of exercise intolerance than left ventricular function, suggesting that these patients potentially have additional noncardiac determinants of exercise capacity.
There are several possible mechanisms underlying cardiac dysfunction in DM, which have been reviewed in detail elsewhere.29 Whatever the cause, increasing exercise in diabetics is a major public‐health priority. Improving cardiopulmonary fitness in diabetic individuals improves their health outcomes,5 but only 25% of older diabetics in the United States meet recommendations for physical activity according to American Diabetes Association guidelines.10 There is thus a need for strong endorsement to enroll in exercise‐based CR programs that enhances participation.30 In addition to improved exercise capacity, the significant improvements we observed in lipid profiles and blood pressure in diabetics following CR are also noteworthy benefits.
The current study has several limitations that merit mention. This was a retrospective analysis at a single site, and the duration of participation varied. We did not modify any participant's diabetic or cardiovascular medications during this study. Furthermore, our program uses moderate‐intensity exercises without formal stress testing. However, 27 diabetic and 80 nondiabetics did undergo stress tests before and after CR (data not shown). The diabetic patients had maximal METs of 9.2 before CR and 10.8 after CR, whereas for nondiabetic patients the maximal METs were 0.5 METs at baseline and 12.5 after CR. An additional limitation of our study was that we did not perform metabolic tests (VO2 max) or evaluate peripheral muscle or vascular function, so we could not explore the possible noncardiac mechanisms of exercise intolerance in patients in detail. Future prospectively designed studies may address these shortcomings.
Conclusion
In this retrospective study of CR in diabetic patients, significant benefit was seen in quality of life, weight, exercise tolerance, and cardiac risk factors. The failure of diabetics to improve exercise levels to the same extent as in nondiabetics may be multifactorial and requires further study.
Acknowledgments
The authors thank Theresa Addison, Beverly Martin, Cynthia Hayes, Connie Paladenech, and Danylo Zorin from the cardiac rehabilitation program at Wake Forest Baptist Health for their contributions to this project. A special thanks to Karen Klein (Translational Science Institute, Wake Forest University Health Sciences) for editing the manuscript.
The authors' contributions are as follows: M.S.C. researched data, wrote manuscript, reviewed/edited manuscript; M.S. researched data, wrote manuscript, reviewed/edited manuscript; H.M. gathered data, researched data, wrote manuscript, reviewed/edited manuscript; D.J. gathered data, contributed to discussion, reviewed/edited manuscript; K.R. researched data, wrote manuscript, reviewed/edited manuscript. Dr. Killian Robinson is the guarantor for this study and takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. [DOI] [PubMed] [Google Scholar]
- 2. Audelin MC, Savage PD, Ades PA. Changing clinical profile of patients entering cardiac rehabilitation/secondary prevention programs: 1996 to 2006. J Cardiopulm Rehabil Prev. 2008;28:299–306. [DOI] [PubMed] [Google Scholar]
- 3. Banzer JA, Maguire TE, Kennedy CM, et al. Results of cardiac rehabilitation in patients with diabetes mellitus. Am J Cardiol. 2004;93:81–84. [DOI] [PubMed] [Google Scholar]
- 4. Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. [DOI] [PubMed] [Google Scholar]
- 5. Yu CM, Lau CP, Cheung BM, et al. Clinical predictors of morbidity and mortality in patients with myocardial infarction or revascularization who underwent cardiac rehabilitation, and importance of diabetes mellitus and exercise capacity. Am J Cardiol. 2000;85:344–349. [DOI] [PubMed] [Google Scholar]
- 6. Soja AM, Zwisler AD, Frederiksen M, et al. Use of intensified comprehensive cardiac rehabilitation to improve risk factor control in patients with type 2 diabetes mellitus or impaired glucose tolerance—the randomized Danish Study of impaired glucose metabolism in the settings of cardiac rehabilitation (DANSUK) study. Am Heart J. 2007;153:621–628. [DOI] [PubMed] [Google Scholar]
- 7. Taylor RS, Brown A, Ebrahim S, et al. Exercise‐based rehabilitation for patients with coronary heart disease: systematic review and meta‐analysis of randomized controlled trials. Am J Med. 2004;116:682–692. [DOI] [PubMed] [Google Scholar]
- 8. Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med. 2007;120:799–806. [DOI] [PubMed] [Google Scholar]
- 9. Suaya JA, Stason WB, Ades PA, et al. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009;54:25–33. [DOI] [PubMed] [Google Scholar]
- 10. Zhao G, Ford ES, Li C, et al. Physical activity in US older adults with diabetes mellitus: prevalence and correlates of meeting physical activity recommendations. J Am Geriatr Soc. 2011;59:132–137. [DOI] [PubMed] [Google Scholar]
- 11. Evans J, Bethell H, Turner S, et al. Characteristics of patients entering cardiac rehabilitation in the United Kingdom 1993–2006: implications for the future. J Cardiopulm Rehabil Prev. 2011;31:181–187. [DOI] [PubMed] [Google Scholar]
- 12. Suresh V, Harrison RA, Houghton P, et al. Standard cardiac rehabilitation is less effective for diabetics. Int J Clin Pract. 2001;55:445–448. [PubMed] [Google Scholar]
- 13. Hindman L, Falko JM, LaLonde M, et al. Clinical profile and outcomes of diabetic and nondiabetic patients in cardiac rehabilitation. Am Heart J. 2005;150:1046–1051. [DOI] [PubMed] [Google Scholar]
- 14. Mourot L, Boussuges A, Maunier S, et al. Cardiovascular rehabilitation in patients with diabetes. J Cardiopulm Rehabil Prev. 2010;30:157–164. [DOI] [PubMed] [Google Scholar]
- 15. Vergĕs B, Patois‐Vergĕs B, Cohen M, et al. Effects of cardiac rehabilitation on exercise capacity in Type 2 diabetic patients with coronary artery disease. Diabet Med. 2004;21:889–895. [DOI] [PubMed] [Google Scholar]
- 16. American Diabetes Association . Standards of medical care in diabetes. Diabetes Care. 2011;34(suppl 1):S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whaley MH, Brubaker PH, Otto RM, et al. ACSM's Guidelines for Exercise Testing and Prescription. 7th ed Philadelphia, PA: Lippincott, Williams & Wilkins; 2006. [Google Scholar]
- 18. Alderman MH. JNC 7: brief summary and critique. Clin Exp Hypertens. 2004;26:753–761. [DOI] [PubMed] [Google Scholar]
- 19.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed]
- 20. Ferrans CE, Powers MJ. Quality of Life Index. Chicago, IL: College of Nursing, University of Illinois at Chicago;1998–1984. [Google Scholar]
- 21. Otto CM. Textbook of Clinical Echocardiography. Philadelphia, PA: Saunders/Elsevier; 2009. [Google Scholar]
- 22. Fishman AP, ed. Fishman's Pulmonary Diseases and Disorders. New York, NY: McGraw‐Hill Medical; 2008. [Google Scholar]
- 23. Svacinová H, Nováková M, Placheta Z, et al. Benefit of combined cardiac rehabilitation on exercise capacity and cardiovascular parameters in patients with type 2 diabetes. Tohoku J Exp Med. 2008;215:103–111. [DOI] [PubMed] [Google Scholar]
- 24. Carroll S, Tsakirides C, Hobkirk J, et al. Differential improvements in lipid profiles and Framingham recurrent risk score in patients with and without diabetes mellitus undergoing long‐term cardiac rehabilitation. Arch Phys Med Rehabil. 2011;92:1382–1387. [DOI] [PubMed] [Google Scholar]
- 25. Milani RV, Lavie CJ. Behavioral differences and effects of cardiac rehabilitation in diabetic patients following cardiac events. Am J Med. 1996;100:517–523. [DOI] [PubMed] [Google Scholar]
- 26. Johannsen NM, Swift DL, Lavie CJ, et al. Categorical analysis of the impact of aerobic and resistance exercise training, alone and in combination, on cardiorespiratory fitness levels in patients with type 2 diabetes: results from the HART‐D study. Diabetes Care. 2013;36:3305–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lavie CJ, Milani RV. Cardiac rehabilitation and exercise training programs in metabolic syndrome and diabetes. J Cardiopulm Rehabil. 2005;25:59–66. [DOI] [PubMed] [Google Scholar]
- 28. Grewal J, McCully RB, Kane GC, et al. Left ventricular function and exercise capacity. JAMA. 2009;301:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. [DOI] [PubMed] [Google Scholar]
- 30. Ghisi GL, Polyzotis P, Oh P, et al. Physician factors affecting cardiac rehabilitation referral and patient enrollment: a systematic review. Clin Cardiol .2013;36:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]


