Abstract
Background
Atrial fibrillation (AF) and heart failure (HF) often coexist; the consequences of such coexistence are unclear.
Hypothesis
HF in patients with AF is associated with poor outcomes.
Methods
This post hoc analysis of RealiseAF, a survey of AF patients, compared symptoms, hospitalizations, management, and AF control in patients with vs without HF. A total of 10,523 AF patients were analyzed according to presence/absence of HF.
Results
History of HF was present in 45.8%, and in more patients with permanent vs persistent, paroxysmal, or first‐episode AF (55.6%, 44.3%, 32.9%, and 29.8%, respectively; P < 0.0001). Patients with vs those without history of HF, and patients with HF and reduced ejection fraction (HF‐REF) vs those with HF and a preserved ejection fraction (HF‐PEF), had more frequent cardiovascular (CV) risk factors and more severe symptoms. Presence vs absence of HF, and HF‐REF vs HF‐PEF, were associated with lower rates of AF control (54.6% vs 62.8% and 49.3% vs 60.3%, respectively; both P < 0.0001). The rate‐control strategy was used more frequently in HF patients, particularly those with HF‐REF, than the rhythm‐control strategy. CV hospitalizations occurred more frequently in patients with HF than those without (41.8% vs 17.5%; P < 0.001) and more frequently in patients with HF‐REF than in those with HF‐PEF (51.6% vs 35.6%; P < 0.0001).
Conclusions
AF patients with HF, particularly HF‐REF, experience heavy symptom and hospitalization burdens, and have relatively low rates of AF control. Further studies are needed to identify ways to improve the management and treatment outcomes of this very high‐risk patient population.
Introduction
The prevalence of atrial fibrillation (AF) and heart failure (HF) are increasing steadily due to an aging population and improved management of cardiovascular (CV) disease.1, 2 These conditions coexist in >1% of the general population.3 AF can cause or worsen pre‐existing HF, and the prevalence of AF in patients with HF is high, ranging from ∼15% in patients with New York Heart Association (NYHA) class II or III to approximately half of patients with NYHA class IV.4 This dual epidemic has major consequences for public health and expenditure because of the symptom burden and frequent hospital admissions.1, 4 Overall, patients with AF are at increased risk of mortality and morbidity from stroke and thromboembolism.5
The association of HF with AF control, symptoms, and hospital admissions is unclear. Using data from the RealiseAF6 survey, we examined the association of the presence/absence of HF and of HF type (with or without reduced left ventricular ejection fraction [LVEF]) with AF management and control, symptoms, and hospitalizations.
Methods
Design, Patients, and Data Collection
RealiseAF was an international, cross‐sectional, observational survey of >10 000 patients with all types of AF, involving >800 sites. It was designed to provide reliable contemporary information on patient characteristics, CV risk, AF types, symptoms, medical history, impact on quality of life (QoL), and AF management worldwide. The survey has been described in detail previously.6 Briefly, RealiseAF includes data from men and women with documented current AF or a history of ≥1 AF episode documented in the previous 12 months by standard electrocardiogram (ECG) or by ECG Holter monitoring.
Definitions
HF was defined as a history of HF at any time based on physician judgment; the physician recorded when HF was first diagnosed (before/after AF/unable to state), NYHA class, and etiology (ischemic, valvular, or hypertensive). AF control was defined as being either in sinus rhythm (SR) or in AF with a heart rate (HR) ≤ 80 beats per minute (bpm)7 at the time of the visit on resting ECG. HF with reduced ejection fraction (HF‐REF) was defined as HF and LVEF ≤ 50%, and HF with preserved ejection fraction (HF‐PEF) was defined as HF and LVEF > 50%.8 LVEF was assessed within 12 months (unless the patient had a myocardial infarction), depending on what techniques were available to the physician at the time, by ECG, multiple‐gated acquisition scan, cardiac catheterization, magnetic resonance imaging, or a computed tomographic scan. HF symptoms were classified according to the NYHA.9
The reasons for hospitalization relating to a CV event in the prior 12 months were collected without additional information, and based purely on physician judgment.
Goals
The primary aims of RealiseAF were to determine the frequency of AF control and describe the CV risk profile for AF patients.6 The present post hoc analysis assessed the association between a history of HF and AF control, symptoms, management, and hospitalizations in the year before the visit.
Statistical Analysis
Population characteristics were summarized into mean, standard deviation for continuous variables, and count and percentages for qualitative variables, unless otherwise indicated. Percentages reported were based only on those patients with data available for each given variable. Descriptive analyses were conducted according to history of HF and within HF patients according to LVEF. Comparisons between subgroups were made using χ2 tests, trend tests (for ordinal variables), or Student t test. Analyses were performed using SAS statistical software, version 9.2 (SAS Institute, Cary, NC).
Results
Study Population
From October 2009 to May 2010, 831 sites included 10 523 patients (Supplementary Figure 1) from 26 participating countries. Mean patient age was 66.6 ± 12.2 years, and 56.4% were male. AF was paroxysmal in 2606 (24.8%), persistent in 2341 (22.3%), and permanent in 4869 (46.4%) patients. In the remaining 675 patients (6.4%), AF was classified as first episode.
Prevalence and Clinical Characteristics of HF
Information regarding HF was missing in 57 (0.5%) patients. A history of HF was present in 45.8% of the remaining 10 466, of whom the majority were classified as having NYHA class I/II HF. AF patients with a history of HF were slightly older, had AF for longer, and were more frequently female than AF patients with no HF history. In addition, AF patients with a history of HF were more likely to have a high symptom burden (European Heart Rhythm Association [EHRA] class II–IV), known CV risk factors, comorbidities, or a CHADS2 score of ≥2 than those with no history of HF (Table 1). AF patients with HF had a higher HR than patients without HF, but blood pressure was similar between the groups. Permanent AF was more frequently present in patients with HF (56.4%) than in patients without HF (38.0%; Table 1).
Table 1.
Clinical Characteristics of Patients With AF According to Presence or Absence of HF and of LVEF a
| Characteristic | All HF, n = 4790 | No HF, n = 5676 | P, HF vs No HF | HF‐REF, n = 1861b | HF‐PEF, n = 1905b | P, HF‐REF vs HF‐PEF |
|---|---|---|---|---|---|---|
| Age, y, mean (SD) | 67.6 (11.7) | 65.7 (12.5) | <0.0001 | 66.9 (11.4) | 67.0 (11.9) | 0.72 |
| Males, % | 54.7 | 57.9 | 0.0009 | 65.4 | 46.2 | <0.0001 |
| BMI, kg/m2, mean (SD) | 28.6 (5.4) | 28.1 (5.0) | <0.0001 | 28.7 (5.4) | 28.6 (5.4) | 0.72 |
| HR, bpm, mean (SD) | 84.4 (22.7) | 81.3 (23.4) | <0.0001 | 87.4 (24.3) | 81.4 (21.1) | <0.0001 |
| SBP, mmHg, mean (SD) | 132.6 (20.2) | 133.1 (18.8) | 0.17 | 132.5 (21.6) | 132.8 (19.0) | 0.66 |
| DBP, mmHg, mean (SD) | 79.9 (11.9) | 79.8 (10.9) | 0.61 | 80.6 (12.6) | 79.7 (11.3) | 0.02 |
| CV risk factors, % | ||||||
| Physical inactivity | 64.6 | 58.1 | <0.0001 | 67.1 | 60.0 | <0.0001 |
| Hypertension | 76.8 | 68.3 | <0.0001 | 76.7 | 76.8 | 0.93 |
| Diabetes mellitus | 24.3 | 18.8 | <0.0001 | 27.0 | 20.6 | <0.0001 |
| Dyslipidemia | 53.1 | 40.7 | <0.0001 | 56.5 | 50.1 | 0.0001 |
| Comorbidities, % | ||||||
| CAD | 48.4 | 19.2 | <0.0001 | 56.1 | 39.8 | <0.0001 |
| CVD | 18.6 | 10.3 | <0.0001 | 18.0 | 17.1 | 0.50 |
| Valvular heart disease | 37.4 | 17.7 | <0.0001 | 37.9 | 42.0 | 0.01 |
| CPD | 15.4 | 7.2 | <0.0001 | 15.0 | 14.6 | 0.69 |
| Chronic advanced renal failurec | 6.0 | 2.1 | <0.0001 | 8.4 | 4.2 | <0.0001 |
| Liver disease | 6.2 | 3.0 | <0.0001 | 7.2 | 5.4 | 0.02 |
| Malignancies | 4.7 | 4.4 | 0.56 | 4.5 | 4.0 | 0.51 |
| CHADS2 score ≥ 2, % | 85.6 | 38.2 | <0.0001 | 85.9 | 84.2 | 0.15 |
| Time since AF diagnosis, mo, mean (SD) | 61.0 (75.8) | 46.5 (65.2) | <0.0001 | 56.3 (73.2) | 61.5 (76.2) | 0.03 |
| Type of AF, % | <0.0001 | <0.0001 | ||||
| Paroxysmal | 17.9 | 30.8 | 14.7 | 22.0 | ||
| Persistent | 21.5 | 22.9 | 24.9 | 21.9 | ||
| Permanent | 56.4 | 38.0 | 56.4 | 52.8 | ||
| First episode | 4.2 | 8.3 | 4.0 | 3.3 | ||
| EHRA classes, % | <0.0001 | <0.0001 | ||||
| I | 11.2 | 38.8 | 9.2 | 13.5 | ||
| II | 53.0 | 50.8 | 46.4 | 57.4 | ||
| III | 32.4 | 9.7 | 39.5 | 27.0 | ||
| IV | 3.4 | 0.7 | 4.9 | 2.0 | ||
| EHRA class II, III, and IV, % | 88.8 | 61.2 | <0.0001 | 90.8 | 86.5 | <0.0001 |
| HF characteristics | ||||||
| Current NYHA class, % | NA | <0.0001 | ||||
| I | 12.1 | — | 15.3 | 8.3 | ||
| II | 55.0 | — | 60.0 | 47.6 | ||
| III | 29.3 | — | 22.2 | 38.5 | ||
| IV | 3.7 | — | 2.4 | 5.6 | ||
| LVEF, mean (SD) | 50.2 (12.9)d | 59.5 (9.0)e | <0.0001 | 39.7 (8.7)f | 60.5 (6.3)g | <0.0001 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; bpm, beats per minute; CAD, coronary artery disease; CPD, chronic pulmonary disease; CV, cardiovascular; CVD, CV disease; DBP, diastolic blood pressure; EHRA, European Heart Rhythm Association; HF, heart failure; HF‐PEF, HF with preserved ejection fraction; HF‐REF, HF with reduced ejection fraction; HR, heart rate; LVEF, left ventricular ejection fraction; NA, not applicable; NYHA, New York Heart Association; SBP, systolic blood pressure; SD, standard deviation.
Data are not complete for all patients: the reported percentage is for the number of patients with data available for each given variable.
Records with missing values were excluded from statistical analysis.
Creatinine clearance <30 mL/min, as reported by the physician.
n = 3766.
n = 3988.
n = 1861.
n = 1905.
HF With Preserved LVEF
Among patients with HF, information about LVEF within the past 12 months was available in 3766 (78.6%); 1905 (50.6%) were diagnosed as having HF‐PEF (ie, LVEF > 50%). Patients with AF and HF‐PEF were similar in age and body mass index compared to those with AF and HF‐REF (Table 1). However, HF‐PEF patients had longer time since AF diagnosis, were more frequently female, and were less likely to have CV risk factors or a high symptom burden (EHRA class II–IV). HF‐PEF was associated with a lower prevalence of coronary artery disease, but a similar prevalence of cerebrovascular disease, and a higher prevalence of valvular heart disease than HF‐REF. In addition, HF‐PEF patients were more likely to have paroxysmal AF than those with HF‐REF (Table 1). HF‐PEF patients were less symptomatic (based on the NYHA class) than those with HF‐REF.
Etiology of HF and NYHA Functional Class According to Type of AF
Patients with permanent AF had a history of more severe HF symptoms than other forms of AF, as reflected by a higher prevalence of patients in NYHA classes III or IV (Table 2). Additional results relating to etiology are shown in Table 2.
Table 2.
Etiology of HF and NYHA Functional Class According to the Type of AF (%)a
| HF/NYHA Class | First Episode of AF, n = 675 | Paroxysmal AF, n = 2606 | Persistent AF, n = 2341 | Permanent AF, n = 4869 | P |
|---|---|---|---|---|---|
| HF | 29.8 | 32.9 | 44.3 | 55.6 | <0.0001 |
| Main cause of HF | <0.0001 | ||||
| Ischemic | 38.2 | 48.5 | 44.0 | 40.0 | |
| Valvular | 31.2 | 12.7 | 18.2 | 31.9 | |
| Hypertensive | 30.6 | 38.9 | 37.8 | 28.0 | |
| HF NYHA classes | <0.0001 | ||||
| I | 11.8 | 16.2 | 13.4 | 10.2 | |
| II | 49.7 | 61.4 | 55.3 | 53.3 | |
| III or IV | 38.5 | 22.4 | 31.3 | 36.5 |
Abbreviations: AF, atrial fibrillation; HF, heart failure; NYHA, New York Heart Association.
Data are not complete for all patients; the reported percentage is for the number of patients with data available for each given variable.
AF Control and Treatment
On the day of the visit, AF was controlled in ∼60% of patients, with better control rates seen in patients with no HF vs HF, and in patients with HF‐PEF vs those with HF‐REF (Table 3). In HF patients, control of AF was mainly due to being in AF with an HR ≤ 80 bpm, whereas in patients without HF, AF control was almost equally divided between being in SR or in AF with a HR ≤ 80 bpm.
Table 3.
Control of AF (in Sinus Rhythm or in AF with HR ≤ 80 bpm) and Management Strategy (Medication), According to Presence or Absence of HF and of LVEF (%)a
| Characteristic | All HF, n = 4790 | No HF, n = 5676 | P, HF vsNo HF | HF‐REF, n = 1861 | HF‐PEF, n = 1905 | P, HF‐REF vs HF‐PEF |
|---|---|---|---|---|---|---|
| Control of AF | 54.6 | 62.8 | <0.0001 | 49.3 | 60.3 | <0.0001 |
| In sinus rhythm | 19.8 | 32.3 | 16.1 | 26.1 | ||
| In AF with HR ≤ 80 bpm | 34.8 | 30.6 | 33.1 | 34.2 | ||
| Prior to the visit | ||||||
| Therapeutic strategy | <0.0001 | <0.0001 | ||||
| Rhythm control | 28.0 | 40.2 | 25.4 | 33.2 | ||
| Rate control | 63.3 | 45.8 | 65.9 | 58.7 | ||
| None | 8.6 | 14.0 | 8.5 | 8.1 | ||
| Both | <0.1 | <0.1 | 0.2 | 0.0 | ||
| Medication | ||||||
| At least 1 AADb | 91.8 | 84.1 | <0.0001 | 93.1 | 91.8 | 0.15 |
| Class Ia | 0.5 | 0.4 | 0.66 | 0.3 | 0.8 | 0.06 |
| Class Ic | 3.7 | 9.2 | <0.0001 | 2.5 | 5.5 | <0.0001 |
| Class II | 61.4 | 52.9 | <0.0001 | 67.6 | 59.4 | <0.0001 |
| Class II for AF reason | 44.4 | 41.3 | <0.01 | 45.7 | 45.8 | 0.95 |
| Class III | 24.9 | 23.2 | 0.04 | 27.6 | 24.2 | 0.02 |
| Amiodarone | 22.8 | 19.4 | <0.0001 | 26.6 | 20.9 | <0.0001 |
| Class IV | 15.4 | 17.2 | 0.01 | 13.3 | 16.8 | <0.01 |
| Class IV for AF reason | 6.2 | 7.7 | <0.01 | 4.7 | 7.0 | <0.01 |
| Digoxin | 40.9 | 20.1 | <0.0001 | 45.1 | 36.1 | <0.0001 |
| Antithrombotic agents | 91.5 | 82.8 | <0.0001 | 92.2 | 91.5 | 0.50 |
| Antiplatelet agents | 42.7 | 36.2 | <0.0001 | 43.8 | 39.9 | 0.014 |
| Oral anticoagulants | 56.2 | 52.2 | <0.0001 | 58.7 | 56.7 | 0.22 |
| Injectable anticoagulants | 7.5 | 4.5 | <0.0001 | 8.1 | 7.5 | 0.53 |
| At the end of the visit | ||||||
| Therapeutic strategy | <0.0001 | <0.0001 | ||||
| Rhythm control | 29.2 | 44.1 | 26.8 | 33.9 | ||
| Rate control | 67.2 | 49.5 | 70.0 | 62.2 | ||
| None | 3.5 | 6.3 | 2.9 | 3.8 | ||
| Both | 0.2 | 0.2 | 0.3 | 0.1 | ||
| Medication | ||||||
| At least 1 AADb | 85.0 | 77.5 | <0.0001 | 85.4 | 85.3 | 0.91 |
| Class Ia | 0.4 | 0.4 | 0.10 | <0.1 | 0.8 | <0.01 |
| Class Ic | 3.1 | 9.4 | <0.0001 | 2.0 | 4.5 | <0.0001 |
| Class II | 56.3 | 45.2 | <0.0001 | 61.7 | 54.8 | <0.0001 |
| Class II for AF reason | 42.3 | 37.4 | <0.0001 | 43.8 | 43.3 | 0.75 |
| Class III | 26.4 | 25.4 | 0.23 | 29.8 | 25.1 | <0.01 |
| Amiodarone | 24.1 | 20.7 | <0.0001 | 29.0 | 21.7 | <0.0001 |
| Class IV | 13.0 | 14.6 | 0.02 | 10.8 | 14.3 | <0.01 |
| Class IV for AF reason | 5.4 | 6.9 | <0.01 | 4.0 | 5.9 | <0.01 |
| Digoxin | 38.0 | 18.4 | <0.0001 | 41.5 | 33.3 | <0.0001 |
| Antithrombotic agents | 86.0 | 75.9 | <0.0001 | 86.3 | 86.6 | 0.78 |
| Antiplatelet agents | 37.1 | 32.0 | <0.0001 | 36.6 | 34.2 | 0.12 |
| Oral anticoagulants | 54.0 | 47.0 | <0.0001 | 57.0 | 55.5 | 0.36 |
| Injectable anticoagulants | 10.2 | 8.9 | 0.03 | 12.4 | 8.3 | <0.0001 |
Abbreviations: AAD, antiarrhythmic drug; AF, atrial fibrillation; bpm, beats per minute; HF, heart failure; HF‐PEF, HF with preserved ejection fraction; HF‐REF, HF with reduced ejection fraction; HR, heart rate; LVEF, left ventricular ejection fraction.
Data are not complete for all patients; the reported percentage is for the number of patients with data available for each given variable.
Class Ia, Ic, II, III, and IV; includes digoxin.
Prior to the visit, antiarrhythmic drugs (AADs), including amiodarone and digoxin, antithrombotic agents (Table 3), and other medications such as aldosterone antagonists, angiotensin‐converting enzyme (ACE) inhibitors, β‐blockers, and diuretics, were prescribed more frequently to patients with HF than without (all P < 0.0001); however, angiotensin II receptor blockers (ARBs) were prescribed more frequently to patients without HF than with HF (P = 0.04).
Prior to the visit in patients with HF‐REF and HF‐PEF, the prescription frequency of antithrombotic agents, AADs (Table 3), and ARBs was similar. Digoxin, amiodarone (Table 3), ACE inhibitors, β‐blockers, and diuretics were prescribed less frequently to patients with HF‐PEF than with HF‐REF (all P < 0.0001). Of the antithrombotic agents prescribed, the prescription frequency of oral and injectable anticoagulants was similar; however, antiplatelets were prescribed less frequently to patients with HF‐PEF than with HF‐REF (P = 0.014; Table 3).
At the end of the visit, fewer patients were on medication than prior to the visit in all categories; however, as described above the prescription pattern remained the same between HF and no HF, and between HF‐REF and HF‐PEF. The only exception was in the prescription of injectable anticoagulants, which increased at the end of the visit, and were prescribed less frequently to patients with HF‐PEF than with HF‐REF (P < 0.0001). In addition, at the end of the visit, prescription of antiplatelets was similar between HF‐REF and HF‐PEF (Table 3).
Rhythm‐Control vs Rate‐Control Strategies
Overall, rate control was preferred over rhythm control. Rhythm control was used more frequently in patients without HF or HF‐PEF than with HF or HF‐REF, both prior to and at the end of the visit (Table 3).
As the presence and severity of HF increased, rhythm control was less frequently used, ranging from 43.5% in patients with no HF or with NYHA class I HF, to 31.1% in patients with NYHA class II HF, and to 22.8% in patients with NYHA classes III or IV HF (P < 0.001; Figure 1).
Figure 1.
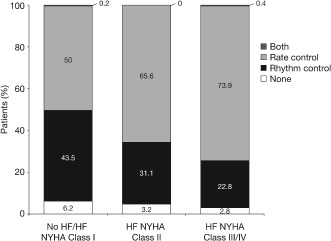
Choice of rhythm‐control or rate‐control strategy at the end of the visit according to existence of heart failure (HF) and New York Heart Association (NYHA) functional class.
Burden of AF and CV Interventions
Overall, 28.7% of all patients underwent ≥1 hospital admission relating to a CV event in the prior 12 months. The rate of CV hospitalizations was higher in patients with HF than in those without (41.8% vs 17.5%; P < 0.001), across all types of AF (paroxysmal AF: 42.5% vs 20.1%; persistent AF: 46.5% vs 16.9%; permanent AF: 39.6% vs 16.2%). Hospitalization rates were the same, regardless of whether AF preceded or followed the occurrence of HF (both 41.4%). Among patients with HF, hospital admissions in the prior 12 months were more frequent in patients with HF‐REF than with HF‐PEF (51.6% vs 35.6%; P < 0.001). AF patients with ischemic HF had more frequent CV hospitalizations than those with valvular or hypertensive HF (48.4% vs 40.8% vs 34.1%, respectively; P < 0.001). Reasons for hospitalization in patients according to presence or absence of HF and LVEF are described in Figure 2A. Acute decompensated HF was the main reason for hospitalization in patients with HF (around a quarter of all patients). Specifically, over one‐third of patients with HF‐REF and around one‐fifth of patients with HF‐PEF were hospitalized for this reason. Arrhythmic or proarrhythmic events were the main reasons for hospitalization in patients with no HF (6.4% of patients). In addition, 9.6% of patients with HF were hospitalized due to an arrhythmic or proarrhythmic event. In patients with HF‐REF and patients with HF‐PEF, supraventricular tachycardia and clinically significant bradycardia/atrioventricular block were the most frequently recorded events (ranging from 3.2% to 4.5% of patients; Figure 2B).
Figure 2.
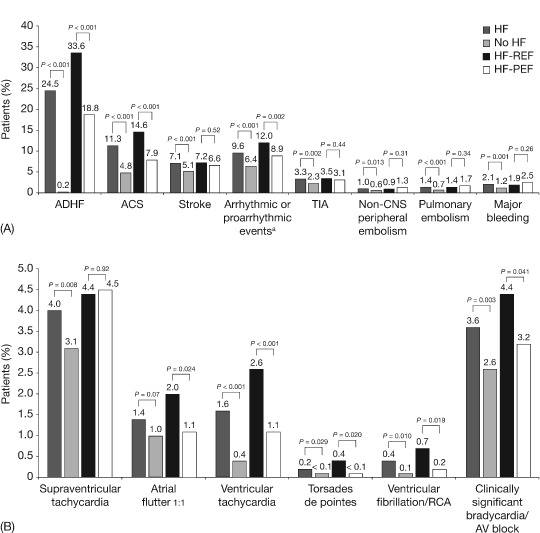
(A) Cardiovascular events leading to hospitalizations in the past 12 months according to presence or absence of heart failure (HF), HF‐REF, and HF‐PEF. (B) Arrhythmic or proarrhythmic events leading to hospitalizations in the past 12 months according to presence or absence of HF, HF‐REF, and HF‐PEF. a Supraventricular tachycardia, atrial flutter 1 to 1, ventricular tachycardia, torsades de pointes, ventricular fibrillation/RCA, clinically significant bradycardia/AV block. Abbreviations: ACS, acute coronary syndrome; ADHF, acute decompensated heart failure; AV, atrioventricular; CNS, central nervous system; HF, heart failure; HF‐PEF, HF with preserved ejection fraction; HF‐REF, HF with reduced ejection fraction; RCA, resuscitated cardiac arrest; TIA, transient ischemic attack.
A history of CV interventions in the past 12 months was more frequent in patients with HF compared with no HF (14.7% vs 10.4%; P < 0.0001) and more frequent in HF‐REF than in HF‐PEF (18.9% vs 12.8%; P < 0.0001). Intervention types according to HF and LVEF are shown in Supplementary Figure 2.
Discussion
The present post hoc analysis of the RealiseAF survey examined the association of HF with AF control, symptoms, and CV hospitalizations. The main finding was that a history of HF is extremely common in patients with AF, and was most frequently reported in patients with permanent AF. AF accompanied by HF is associated with more comorbidities and a higher CV risk profile than AF without HF; the latter is more pronounced in patients with HF‐REF than HF‐PEF. Patients with AF and concomitant HF vs patients without HF and patients with HF‐REF vs patients with HF‐PEF had more severe symptoms, less frequently controlled AF, and more CV events leading to hospitalizations. The latter was true across all types of AF and regardless of whether AF preceded HF or the reverse. Acute decompensated HF, acute coronary syndrome, arrhythmic or proarrhythmic events, and transient ischemic attack were the leading causes of hospitalization.
In the Framingham Heart Study, left ventricular systolic dysfunction increased the risk of de novo appearance of AF in men and women by 4.5 and 5.9 times, respectively.10 Similarly, in the Euro Heart Survey on AF, new HF appeared during 1 year of follow‐up in 5.0% of patients with AF, whereas deterioration of HF was registered in 24.7% patients.11 Our results are in accordance with recent studies, which indicate that, in AF patients, the coexistence of HF identifies a population at particularly high risk of CV complications, particularly HF hospitalizations.12, 13, 14
There are several possible reasons for this increased risk; HF can aggravate AF because it is associated with neurohormonal activation, ventricular and atrial interstitial fibrosis, dysregulation of myocardial intracellular calcium, and increased atrial filling pressures leading to increased atrial size.1 Conversely, AF can impair myocardial function due to the reduction of cardiac output consecutive to irregular and/or rapid HR, loss of atrial contraction, and increased mitral or tricuspid regurgitation.1
Additionally, HF is known to be closely associated with the potentially fatal effects of AADs used in AF.15, 16 AADs were more frequently prescribed to our patients with AF and HF than to those with AF and no HF.
In our study, patients in whom HF coexisted with AF were older and had more frequent CV risk factors and comorbidities than those with AF and no history of HF. These patients, and in particular those with HF‐REF, also had a higher HR, more frequent permanent AF, a higher symptom burden, and less frequently controlled AF than patients without HF or HF‐PEF. Our findings are clinically relevant because all these factors identify patients with AF and HF as a sicker population at exceedingly high CV risk.
In this survey of AF patients, the concomitance of HF increased thromboembolic risk, evidenced by the higher percentage of patients having a CHADS2 score ≥2 compared to those with AF and no HF. However, in these high‐risk patients, oral anticoagulation both prior to and at the end of the visit was relatively low, at around 55%. The underuse of oral anticoagulants in high‐risk AF patients (where undertreatment is defined as treatment of <70% of high‐risk patients) has been documented previously in a 2010 systematic review.17 Underuse of oral anticoagulation in patients at high risk of thromboembolic events could account for the increase in hospital admissions due to stroke, TIAs, and pulmonary embolism observed in our population. This is pertinent because, in some older patients with AF and HF, nontreatment with the anticoagulant warfarin has been associated with a higher mortality rate than if warfarin was prescribed.18
AF and HF share a high prevalence, an increased morbidity and mortality, and a high economic impact.8, 15 In both conditions, the main outcomes include death and hospitalizations. The prevention of these outcomes and the improvement of symptoms constitute major therapeutic goals, although the fulfillment of many of them remains elusive in the case of AF. Hospital admissions are particularly important, not only because they are the main drivers of cost but also because they are important markers for adverse outcomes.
The optimal management strategy for AF in patients with HF remains unclear due to possible causative links between AF and HF.19 Rhythm control is recommended in symptomatic AF patients in the current guidelines,15, 20 largely based on the possible QoL benefits rather than changes in hard endpoints. Rate‐control strategy is typically used for patients with HF and worsening of its functional class. The large prospective clinical trial Atrial Fibrillation and Congestive Heart Failure directly compared rate‐control and rhythm‐control strategies, and found no difference in CV mortality (the primary outcome) in patients with an LVEF ≤ 35%, symptoms of congestive HF, and a history of AF, or in secondary outcomes, including all‐cause mortality and worsening of HF.21 Other large clinical trials comparing rate and rhythm control for AF management strategies have not shown superiority of either approach in terms of major clinical outcomes7, 21, 22, 23 and have failed to confirm the apparent benefits of long‐term maintenance of SR as seen in retrospective analyses.24 This notwithstanding, retrospective data from large clinical trials suggest that long‐term maintenance of SR is associated with improved long‐term outcomes in patients with HF and paroxysmal or persistent AF.25 However, recent data show that in AF patients with signs of HF, 2 distinct clinical conditions can be recognized based on the chronologic sequence of AF and HF development. This should be considered before making a decision regarding SR restoration and maintenance.26
There are important limitations to keep in mind when interpreting our observations. Due to the cross‐sectional nature of this study, it is not possible to extract data to support or contradict the idea of restoration and maintenance of SR in patients with AF.
Nevertheless, these data call to our attention the high risk inherent in patients with AF and a history of HF. Due to the contraindications of many AADs, existing antiarrhythmic strategies are of limited value to these patients; as such, there is a need to develop targeted strategies for the monitoring and treatment of these patients to avoid symptomatic deterioration and reduce hospitalization burden.
The prevalence of severe class IV HF was low at 3.7%, presumably reflecting a low prevalence in real life among outpatients. As in all observational studies, associations between clinical characteristics and events or symptoms may be confounded. In addition, events were not adjudicated, and collection of biological variables and performance of functional tests was left to the physician's discretion; thus, the evaluation of HF and determination of LVEF were based on physician judgment and available techniques, respectively. In addition, the cross‐sectional nature of this survey prevents analysis of a direct link with outcomes and is prone to reverse causality. Conversely, the contemporary nature, the geographic scope, and the large size constitute strengths of the current data set.
In conclusion, in this large, international, observational survey, HF was highly prevalent among patients with AF. Furthermore, HF appeared strongly associated with more symptoms, less frequently controlled AF, and increased CV‐related hospitalizations. Further studies will be needed to identify the optimal management strategy for this very high‐risk patient population.
Supporting information
Figure S1 Patient flow. AF, atrial fibrillation; HF, heart failure according to New York Heart Association criteria; HF‐PEF, HF with preserved ejection fraction; HF‐REF, HF with reduced ejection fraction. a1 patient had 3 reasons for ineligibility. bRecords with missing values were excluded from statistical analysis.
Figure S2 CV interventions in the last 12 months according to presence or absence of HF, HF‐REF, and HF‐PEF. CABG, coronary arterial bypass graft surgery; HF, heart failure; HF‐PEF, HF with preserved ejection fraction; HF‐REF, HF with reduced ejection fraction; PCI, percutaneous coronary intervention.
Acknowledgments
The authors thank Leigh Prevost, BSc (PPSI, a PAREXEL company) for editing the manuscript.
The RealiseAF survey was sponsored by Sanofi, which provided assistance with data collection. The study was designed, conducted, and analyzed by an academic international steering committee, with nonvoting participation of the sponsor representatives. The sponsor had no role in submitting the article for publication. PAREXEL was funded by the sponsor.
J.S.C. has consulted and received speaker fees for Abbott, AstraZeneca Pharmaceuticals, Menarini, Merck, Merck Sharp & Dohme, Novartis, Pfizer, and Sanofi. O.J.Z. has received research grants from Sanofi. P.P. has consulted for or received speaker's fees from Abbott, Abbott Vascular, Amgen, Bayer HealthCare, Boehringer Ingelheim, MSD, Merck Serono, Novartis, Pfizer, Sanofi, Servier, and Vifor Pharma. L.N.‐B. is an employee of Sanofi S.B. is an employee of Lincoln, which is under contract with Sanofi to oversee and review statistical analyses. P.G.S. has consulted for Astellas, AstraZeneca Pharmaceuticals, Bayer HealthCare, LLC, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi, Gilead Sciences, Medtronic, Merck, Otsuka Research Institute, Pfizer, Roche Diagnostics, Sanofi, and Servier.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119:2516–2525. [DOI] [PubMed] [Google Scholar]
- 2. Kazemian P, Oudit G, Jugdutt BI. Atrial fibrillation and heart failure in the elderly. Heart Fail Rev. 2012;17:597‐613. [DOI] [PubMed] [Google Scholar]
- 3. Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. [DOI] [PubMed] [Google Scholar]
- 4. Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2D–8D. [DOI] [PubMed] [Google Scholar]
- 5. Lip GY, Huber K, Andreotti F, et al. Antithrombotic management of atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing coronary stenting: executive summary—a Consensus Document of the European Society of Cardiology Working Group on Thrombosis, endorsed by the European Heart Rhythm Association (EHRA) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2010;31:1311–1318. [DOI] [PubMed] [Google Scholar]
- 6. Steg PG, Alam S, Chiang CE, et al. Symptoms, functional status and quality of life in patients with controlled and uncontrolled atrial fibrillation: data from the RealiseAF cross‐sectional international registry. Heart. 2012;98:195–201. [DOI] [PubMed] [Google Scholar]
- 7. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 8. Dickstein K, Cohen‐Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29:2388–2442. [DOI] [PubMed] [Google Scholar]
- 9. AHA medical/scientific statement . 1994 revisions to classification of functional capacity and objective assessment of patients with diseases of the heart. Circulation. 1994;90:644–645. [PubMed] [Google Scholar]
- 10. Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 11. Nieuwlaat R, Capucci A, Camm AJ, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26:2422–2434. [DOI] [PubMed] [Google Scholar]
- 12. Connolly SJ, Camm AJ, Halperin JL, et al. Dronedarone in high‐risk permanent atrial fibrillation. N Engl J Med. 2011;365:2268–2276. [DOI] [PubMed] [Google Scholar]
- 13. Kober L, Torp‐Pedersen C, McMurray JJ, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687. [DOI] [PubMed] [Google Scholar]
- 14. Mountantonakis SE, Grau‐Sepulveda MV, Bhatt DL, et al. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of get with the guidelines‐heart failure. Circ Heart Fail. 2012;5:191–201. [DOI] [PubMed] [Google Scholar]
- 15. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 16. Digitalis Investigation Group . The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. [DOI] [PubMed] [Google Scholar]
- 17. Ogilvie IM, Newton N, Welner SA, et al. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645. [DOI] [PubMed] [Google Scholar]
- 18. Hess PL, Greiner MA, Fonarow GC, et al. Outcomes associated with warfarin use in older patients with heart failure and atrial fibrillation and a cardiovascular implantable electronic device: findings from the ADHERE registry linked to Medicare claims. Clin Cardiol. 2012;35:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liang LH, Khammy O, Byrne M, et al. Irregular rhythm adversely influences calcium handling in ventricular myocardium: implications for the interaction between heart failure and atrial fibrillation. Circ Heart Fail. 2012;5:786–793. [DOI] [PubMed] [Google Scholar]
- 20. Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation). Eur Heart J. 2006;27:1979–2030. [DOI] [PubMed] [Google Scholar]
- 21. Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. [DOI] [PubMed] [Google Scholar]
- 22. Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356:1789–1794. [DOI] [PubMed] [Google Scholar]
- 23. Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. [DOI] [PubMed] [Google Scholar]
- 24. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–1513. [DOI] [PubMed] [Google Scholar]
- 25. Hagens VE, Crijns HJ, Van Veldhuisen DJ, et al. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J. 2005;149:1106–1111. [DOI] [PubMed] [Google Scholar]
- 26. Smit MD, Moes ML, Maass AH, et al. The importance of whether atrial fibrillation or heart failure develops first. Eur J Heart Fail. 2012;14:1030–1040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Patient flow. AF, atrial fibrillation; HF, heart failure according to New York Heart Association criteria; HF‐PEF, HF with preserved ejection fraction; HF‐REF, HF with reduced ejection fraction. a1 patient had 3 reasons for ineligibility. bRecords with missing values were excluded from statistical analysis.
Figure S2 CV interventions in the last 12 months according to presence or absence of HF, HF‐REF, and HF‐PEF. CABG, coronary arterial bypass graft surgery; HF, heart failure; HF‐PEF, HF with preserved ejection fraction; HF‐REF, HF with reduced ejection fraction; PCI, percutaneous coronary intervention.


