ABSTRACT
Background
Dobutamine stress echocardiography (DSE) is commonly used to risk stratify patients in the cardiac evaluation prior to orthotopic liver transplantation (OLT). Data remain limited regarding the accuracy to predict obstructive coronary artery disease (CAD) using this approach.
Hypothesis
We hypothesize that DSE may have limitations in the investigation of underlying CAD in patients with end‐stage liver disease.
Methods
A retrospective chart review of all patients who underwent OLT at Mayo Clinic in Florida between 1998 and 2010 was performed. Sixty‐six underwent invasive coronary angiography (ICA) within 1 year of DSE and were included in our study. Based on DSE results, patients were stratified into 1 of 3 groups: nonischemic, ischemic, and indeterminate. The relationship between DSE, ICA, and death from all cause and cardiac‐related cause with a minimum 3‐year follow‐up period were analyzed.
Results
A total of 66 patients were included in our cohort. There was no difference in age, gender, severity of liver disease, and echocardiographic findings among the groups. Forty‐three percent of patients (n = 12) with an abnormal result on DSE were found to have moderate or severe obstructive CAD on cardiac catheterization, whereas 49% of patients (n = 17) with a normal finding on DSE had moderate or severe CAD. Of 5 patients who died from a documented cardiac etiology, 3 had normal stress test results, 1 had abnormal findings, and 1 had an indeterminate DSE result. When compared with ICA, our study demonstrated that DSE has a sensitivity of 41.4% (95% confidence interval [CI]: 0.24‐0.61), specificity of 47.1% (95% CI: 0.30‐0.65), positive predictive value of 40.0% (95% CI: 0.23‐0.59), and negative predictive value of 48.0% (95% CI: 0.31‐0.66) in identification of underlying CAD.
Conclusions
Although widely used, DSE may not always accurately reflect the severity of obstructive CAD in patients undergoing OLT.
Introduction
Patients undergoing orthotopic liver transplantation (OLT) generally undergo a very thorough preoperative cardiac evaluation. Dobutamine stress echocardiography (DSE) is an integral part of this evaluation for risk stratification in this population.1 The American College of Cardiology/American Heart Association (ACC/AHA) has no specific guidelines for patients undergoing OLT but recommends against more invasive testing, whereas the American Association for the Study of Liver Disease (AASLD) recommends that DSE should be utilized in this patient population.1, 2
Although widely used, studies have been somewhat inconclusive as to how accurate DSE is in predicting underlying obstructive coronary artery disease (CAD) in patients undergoing OLT. Patients with end‐stage liver disease are at greater risk than the general population for CAD. Up to 26% of patients undergoing OLT are thought to have undiagnosed CAD, and studies have shown that the cardiac‐related mortality rate in such patients can exceed 40% at 1 year.3, 4 Therefore, accurate pretransplantation CAD identification is important for transplant evaluation and perioperative management.
Our study aimed to evaluate the efficacy of DSE in predicting underlying CAD in patients undergoing OLT, considering that cardiac‐related morbidity and mortality remain high despite thorough pretransplantation evaluations. Although widely used, we hypothesize that DSE may have limitations in the investigation of underlying CAD in patients with end‐stage liver disease.
Methods
A retrospective chart review of 2010 patients who underwent OLT at Mayo Clinic in Florida was performed. Of those patients, 66 underwent invasive coronary angiography (ICA) within 1 year of DSE and were included in our study. Patients were stratified into 1 of 3 groups based on DSE results: (1) ischemic, (2) nonischemic, or (3) indeterminate. Patients in the ischemic group were noted to have either electrocardiogram changes indicative of ischemia or new wall motion abnormalities. Patients in the nonischemic group reached a minimum rate of 85% of the goal heart rate and did not have ischemic changes. Patients in the indeterminate group did not achieve a goal heart rate of 85% of maximum predicted or had nondiagnostic electrocardiographic changes.
Clinical, demographic, and echocardiographic data were collected in addition to results from both the DSE and ICA. Preoperative resting echocardiographic imaging within 1 year prior to OLT was included. Patients in the study were defined as having a positive tobacco history, with either a report of greater than 5 pack‐years or active tobacco use at the time of transplantation. Patients were classified as having renal dysfunction if the calculated glomerular filtration rate was below 60 mL/min/1.73 m2. All laboratory data used in our study were collected as routine, nonemergent blood draws within a 1‐week period of DSE. Resting echocardiograms were performed within a 1‐week period of DSE as well. If a patient had a subsequent transplantation, only data from the first transplantation were used. Patients were excluded with <36 months of follow‐up, unless deceased.
Patients included in our study had a goal target heart rate of at least 85% of the maximum predicted during DSE. DSE was performed according to institutional protocol, with intravenous dobutamine infusion at an incremental rate of 5 to 10 µg/kg/min until target heart rate or study end points were achieved, including new wall motion abnormalities. Board certified cardiologists with experience reading these imaging modalities reported the results. ICA was performed according to the standardized Judkins technique with multiple views. Catheterization results were classified as mild, moderate, and severe, based on definitions from previously published studies (Figure 1).5 Mild CAD was defined as any abnormality <50% stenosis of a major coronary artery, moderate as 50% to 70% stenosis, and severe as >70% stenosis.
Figure 1.
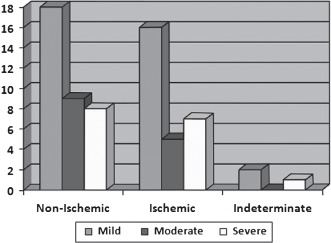
Percentage based on catheterization results that are found to have nonischemic, ischemic, or indeterminate finding on stress echocardiography (P = 0.533).
All‐cause and cardiac‐related mortality events were obtained via chart review. Time of follow‐up was measured by review of the electronic medical record. Institutional review board approval was obtained prior to the initiation of the study.
Analysis of variance and t test and were used to analyze our results. P values of <0.05 were considered to be significant.
Results
Sixty‐six patients underwent ICA within 1 year of DSE and were included in our study. No difference in baseline demographics was noted among the groups (Table 1). The overall age at stress test was 60 years in the nonischemic group, 59 years in the ischemic group, and 51 years in the indeterminate group. There was no statistical difference in follow‐up status among the groups, yet a higher percentage of patients were deceased in the indeterminate group. Patients in the indeterminate group were found to have either nondiagnostic electrocardiograms for ischemia or an inability to reach a heart rate of 85% of the maximum.
Table 1.
Baseline Patient Characteristics Prior to Transplantation
| Nonischemic, n = 35 | Ischemic, n = 28 | Indeterminate, n = 3 | P Value | |
|---|---|---|---|---|
| Age at stress testing, y | 60.0 | 59.0 | 51.0 | 0.244 |
| Age at transplant, y | 60.3 | 59.1 | 51 | 0.209 |
| Sex, n (%) | 0.838 | |||
| Male | 25 (71.4%) | 18 (64.3%) | 2 (66.7%) | |
| Female | 10 (28.6%) | 10 (35.7%) | 1 (33.3%) | |
| Follow‐up, n (%) | 0.545 | |||
| Alive | 23 (65.7%) | 18 (64.3%) | 1 (33.3%) | |
| Deceased | 12 (34.3%) | 10 (35.7%) | 2 (66.7%) | |
| Renal dysfunction, n (%) | 16 (45.7%) | 13 (46.4%) | 2 (66.7%) | 0.784 |
| Tobacco, n (%) | 13 (37.1%) | 6 (21.4%) | 1 (33.3%) | 0.412 |
| Hyperlipidemia, n (%) | 7 (20.0%) | 7 (25.0%) | 0 (0%) | 0.595 |
| Diabetes mellitus, n (%) | 22 (62.9%) | 6 (21.4%) | 2 (66.7%) | 0.003 |
| Hypertension, n (%) | 21 (60.0%) | 13 (46.4%) | 3 (100%) | 0.169 |
| Hemoglobin, g/dL | 11.4 | 11.5 | 10.4 | 0.62 |
| MELD score | 16.8 | 15.3 | 26.3 | 0.023 |
| Tbili, mg/dL | 4.24 | 2.81 | 7.63 | 0.314 |
| INR | 1.58 | 1.39 | 1.83 | 0.266 |
| Creatinine, mg/dL | 1.56 | 1.41 | 2.23 | 0.498 |
| Body mass index, kg/m2 | 30.3 | 29.7 | 29.1 | 0.919 |
| LV EF, % | 64.8 | 62.0 | 69.3 | 0.27 |
| Wall motion abnormalities, n (%) | 3 (8.6%) | 9 (32.1%) | 1 (33.3%) | 0.054 |
| LV size, n (%) | 0.133 | |||
| Normal | 34 (97.1%) | 24 (85.7%) | 2 (66.7%) | |
| Mild enlargement | 1 (2.9%) | 3 (10.7%) | 1 (33.3%) | |
| Moderate enlargement | 0 (0%) | 1 (3.6%) | 0 (0%) | |
| RV size, n (%) | 0.45 | |||
| Normal | 32 (91.4%) | 27 (96.4%) | 2 (66.7%) | |
| Mild enlargement | 3 (8.6%) | 0 (0%) | 1 (33.3%) | |
| Moderate enlargement | 0 (0%) | 1 (3.6%) | 0 (0%) | |
| RV pressure, mm Hg | 32.3 | 32.0 | 36.0 | 0.86 |
| Intrapulmonary shunt, n (%) | 6 (17.1%) | 8 (28.6%) | 1 (33.3%) | 0.52 |
Abbreviations: EF, ejection fraction; INR, international normalized ratio; LV, left ventricle; MELD, Model for End‐Stage Liver Disease; RV, right ventricle; Tbili, total bilirubin.
All groups had a higher proportion of male patients. Cardiovascular risk factors were commonly observed, with a significant difference in diabetes among the groups. Patients in the nonischemic (n = 22, 62.9%) and indeterminate (n = 2, 66.7%) groups were found to have a higher incidence of diabetes mellitus compared to those in the ischemic group (n = 6, 21.4%) (P = 0.003). There were no statistically significant differences in echocardiographic parameters, including left ventricular ejection fraction, wall motion abnormalities, left and right ventricular sizes, and right ventricular pressure, although there was a higher incidence of wall motion abnormalities in the ischemic (n = 9, 32.1%) and indeterminate (n = 1, 33%) groups compared to the nonischemic group (n = 3, 8.6%) (P = 0.054). A higher proportion of intrapulmonary shunts were seen in the ischemic (n = 8, 28.6%) and indeterminate (n = 1, 33%) groups compared to the nonischemic group (n = 6, 17.1%) (P = 0.52). No statistical difference in type of liver disease was seen, yet the majority of patients were found to have disease from either alcohol abuse or hepatitis C (Table 2).
Table 2.
Comparison of Types of Liver Disease
| Type of Liver Disease | Nonischemic, n (%) | Ischemic, n (%) | Indeterminate, n (%) |
|---|---|---|---|
| Alcohol‐induced | 6 (17.1%) | 6 (21.4%) | 1 (33.0%) |
| Hepatitis B | 2 (5.7%) | 2 (7.1%) | — |
| Hepatitis C | 9 (25.7%) | 8 (28.6%) | 2 (66.6%) |
| NASH | 5 (14.3%) | 4 (14.3%) | — |
| PBC | 1 (2.9%) | 4 (14.3%) | — |
| Cryptogenic | 7 (20.0%) | 2 (7.1%) | — |
| Autoimmune | 2 (5.7%) | 1 (3.6%) | — |
| Alpha 1 antitrypsin | 1 (2.9%) | 1 (3.6%) | — |
| PSC | 2 (5.7%) | — | — |
Abbreviations: NASH, nonalcoholic steatohepatitis, PBC, primary biliary cirrhosis, PSC, primary sclerosing cholangitis.
A statistically significant difference in MELD (Model for End‐Stage Liver Disease) score was found among the 3 groups, with a higher score seen in the indeterminate group (MELD of 26.3), compared to patients in the ischemic (MELD of 15.3) and nonischemic (MELD of 16.8) groups (P < 0.05). However, no difference was observed between the ischemic and nonischemic groups (P = 0.3678). Total bilirubin, international normalized ratio, creatinine, and hemoglobin values were similar throughout all patients in the study.
A similar number of patients in the nonischemic group were found to have mild, moderate, and severe (n = 18, 51.4%; n = 9, 25.7%; and n = 8, 22.9%; respectively) CAD on catheterization compared to patients in the ischemic group (n = 8, 22.9%). No statistical significance was seen between echocardiographic results and catheterization results among the groups. No difference was found between follow‐up status within the mild, moderate, and severe groups (P = 0.416), yet a higher proportion (50% of patients) were deceased from all‐cause mortality in the patients with severe CAD (Table 3).
Table 3.
Stress Test Results vs Catheterization Results in All Study Patients (P = 0.533)
| Stress Test Results | |||||
|---|---|---|---|---|---|
| Nonischemic | Indeterminate | Ischemic | No. | Deceased | |
| Cardiac catheterization results | |||||
| Mild | 18 | 2 | 16 | 36 | 11 (30.6%) |
| Moderate | 9 | 0 | 5 | 14 | 5 (35.7%) |
| Severe | 8 | 1 | 7 | 16 | 8 (50.0%) |
A total of 5 patients died from a cardiac‐related cause in our cohort. The average time difference between DSE and death was 741 days. Of those, 3 patients (60%) were classified as having nonischemic finding on stress echocardiography, whereas 1 patient (20%) had ischemic findings and 1 (20%) indeterminate findings. Five of the 6 patients (83.3%) who died from cardiac‐related causes were found to have severe disease on coronary angiography. One patient (16.7%) had moderate disease on angiography. No patient with mild findings on angiography died from a cardiac‐related cause within our study period. Of those patients found to have severe disease during cardiac catheterization, 50% (n = 5) of those who did not have stents placed were deceased, and 50% (n = 3) of those who had stent placement were deceased. All patients who had died from a documented cardiac cause reportedly had an acute coronary syndrome as the cause of death. Of those who were deceased from acute cardiac syndrome, 80% did not undergo stent placement.
Of patients with normal DSE results, all‐cause mortality was 32.4%, cardiac mortality 8.8%, and 6‐month cardiac mortality of 2.9%. Patients with abnormal results had all‐cause mortality of 36.7%, cardiac mortality 10%, and 6‐month cardiac mortality of 6.7%. No statistical difference was noted between both cohorts.
A sensitivity of 0.4138 (95% confidence interval [CI]: 0.24‐0.61), specificity of 0.4706 (95% CI: 0.30‐0.65), positive predictive value of 0.400 (95% CI: 0.23‐0.59), and negative predictive value of 0.48 (95% CI: 0.31‐0.66) was seen when comparing mild and moderate/severe catheterization results with ischemic and nonischemic stress test findings (Table 4). DSE had a likelihood ratio of 0.72 to detect CAD in our cohort.
Table 4.
Stress Test Results vs Catheterization Results
| Catheterization Results | ||
|---|---|---|
| Stress Test Results | Moderate/Severe | Mild |
| Ischemic | 12 | 18 |
| Nonischemic | 17 | 16 |
Abbreviations: CI, confidence interval.
Sensitivity 0.4138 (95% CI: 0.24‐0.61), specificity 0.4706 (95% CI: 0.30‐0.65), positive predictive value 0.400 (95% CI: 0.23‐0.59), and negative predictive value 0.48 (95% CI: 0.31‐0.66).
Discussion
Our study found that DSE has limitations as a screening test for underlying CAD in patients undergoing OLT. A similar number of patients with negative findings on DSE were found to have little evidence of CAD on cardiac catheterization as those with positive findings. The same can be said regarding patients with abnormal findings on DSE. Based on this study, DSE was found to have poor sensitivity, specificity, as well as positive and negative predictive values. Our results agree with the findings by Harinstein et al. In their cohort of 64 patients, DSE was shown to have a low sensitivity of 13%, high specificity of 85%, low positive predictive value of 22%, and intermediate negative predictive value of 75%. Harinstein and colleagues concluded that DSE may not be an adequate screening tool in patients with end‐stage liver disease, and our findings add additional emphasis to that argument.5 In another study by Williams et al, a total of 61 patients underwent OLT, and 8 had major perioperative events, of whom all had either normal or nondiagnostic results on DSE.6 Other authors propose that DSE is both a sufficient means of stress testing in this population and excellent in predicting underlying CAD.4, 7, 8, 9, 10, 11, 12 To the same extent, the all‐cause and 6‐month cardiac mortalities did not show any difference between those patients with normal and abnormal results, further suggesting that DSE may not be an accurate test in this population.
Interestingly, those patients with interventions performed during angiography did not have differences in mortality rates compared to those patients who did not. Based on our small cohort of patients undergoing percutaneous intervention, not much benefit was seen. This can potentially be due to an increase in in‐stent thrombosis, as the majority of the catheterizations occurred within a relatively short time period of transplantation. A larger population of patients undergoing such interventional procedures would be needed to further address this question.
Our study is the largest to date that adds to the current literature and suggests that DSE may be less accurate than previously reported. Although currently the ACC/AHA recommends against invasive testing in this population and the AASLD recommends DSE, our results suggest that other risk stratification modalities should be considered in addition to a comprehensive clinical evaluation. The addition of exercise or alternative stress testing modalities or cardiovascular computed tomography reflect other potential possibilities, although a small study assessing single‐photon emission computed tomography showed poor predictive value in the assessment of underlying CAD.13, 14
Based on our cohort, there was no significant difference in the presence of cardiac‐related risk factors within the 3 groups. It would have been expected that patients who have increased CAD would have a greater number of traditional risk factors, which would be reflected in the ischemic group, yet this was not the case. Interestingly, in comparison to the ischemic group, more patients in the nonischemic subset were found to have a history of tobacco use, diabetes mellitus, and hypertension. A much greater percentage of patients in the indeterminate group had hypertension and diabetes mellitus, yet it is difficult to form a concrete conclusion as this subset consisted of only 3 patients.
Little variation in echocardiographic findings was seen among the 3 patient groups, and no statistical significance was found in the measured parameters. Patients with both indeterminate and ischemic findings on DSE had a greater proportion of intrapulmonary shunting on echocardiography compared to those in the nonischemic group. It is unclear if the presence of intrapulmonary shunting would provide a worse cardiovascular prognosis.
Most patients in our cohort had end‐stage liver disease secondary to either alcohol‐abuse or hepatitis C, yet there was no difference in DSE results based on underlying etiology for transplantation. The MELD score did show significant difference among the 3 groups, with a higher value in the indeterminate group compared to the ischemic and nonischemic groups, yet further analysis showed that no difference was noted between the ischemic and nonischemic groups. Patients with end‐stage liver disease are known to have hemodynamic changes, including increases in sympathetic tone and cardiac output, and marked vasodilatation. This likely results in a diminished cardiac stress when compared to the general population, giving results that are difficult to interpret.15, 16 Patients in the indeterminate group were found to have a higher overall MELD score, which is indicative of more severe liver disease, and therefore are likely to have greater hemodynamic alterations, potentially confounding the DSE.
Study limitations include the relatively small number of patients, specifically within the indeterminate group. Exclusion criteria added to this limitation, as the goal was to include only those patients who had echocardiography, DSE, angiography, and laboratory testing within a narrow time period. Another limitation is the fact that our patient population included patients who underwent angiography and can be deemed to be more severely ill than the general population undergoing OLT, yet the percentage of patients deceased from cardiac‐related mortality was actually lower than the averages reported in numerous other studies. Functional studies, including fractional flow reserve and intravascular ultrasound, were not routinely reported in our cohort, as these testing modalities can likely provide a better understanding of the significance of obstructive disease.
Conclusion
Although widely used, DSE may not accurately identify underlying CAD in patients undergoing OLT. Other modalities should be investigated to help prevent cardiac‐related complications in this population.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Murray K, Carithers R Jr. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407–1432. [DOI] [PubMed] [Google Scholar]
- 2. Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines. Circulation. 2009;120:e169–e276. [DOI] [PubMed] [Google Scholar]
- 3. Mandell MS, Lindenfield J, Tsou M, et al. Cardiac evaluation of liver transplant candidates. World J Gastroenterol. 2008;14:3445–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raval Z, Harinstein M, Skaro A, et al. Cardiovascular risk assessment of the liver transplant candidate. J Am Coll Cardiol. 2011;58:223–231. [DOI] [PubMed] [Google Scholar]
- 5. Harinstein M, Flaherty J, Ansari A, et al. Predictive value of dobutamine stress echocardiography for coronary artery disease detection in liver transplant candidates. Am J Transplant. 2008;8:1523–1528. [DOI] [PubMed] [Google Scholar]
- 6. Williams K, Lewis J, Davis G, et al. Dobutamine stress echocardiography in patients undergoing liver transplantation evaluation. Transplantation. 2000;69:2354–2356. [DOI] [PubMed] [Google Scholar]
- 7. Keeffe B, Valantine H, Keeffe E. Detection and treatment of coronary artery disease in liver transplant candidates. Liver Transpl. 2001;7:755–761. [DOI] [PubMed] [Google Scholar]
- 8. Sawada SG, Segar DS, Ryan T, et al. Echocardiographic detection of coronary artery disease during dobutamine infusion. Circulation. 1991;83:1605–1614. [DOI] [PubMed] [Google Scholar]
- 9. Umphrey L, Hurst R, Eleid M, et al. Preoperative dobutamine stress echocardiographic findings and subsequent short‐term adverse cardiac events after orthotopic liver transplantation. Liver Transpl. 2008;14:886–892. [DOI] [PubMed] [Google Scholar]
- 10. Findlay JY, Keegan MT, Pellikka PP, et al. Preoperative dobutamine stress echocardiography, intraoperative events, and intraoperative myocardial injury in liver transplantation. Transplant Proc. 2005;37:2209–2213. [DOI] [PubMed] [Google Scholar]
- 11. Wolf A. Preoperative optimization of patients with liver disease. Curr Opin Anaesthesiol. 2005;18:325–331. [DOI] [PubMed] [Google Scholar]
- 12. Donovan C, Marcovitz P, Punch J, et al. Two‐dimensional and dobutamine stress echocardiography in the preoperative assessment of patients with end‐stage liver disease prior to orthotopic liver transplantation. Transplantation. 1996;61:1180–1188. [DOI] [PubMed] [Google Scholar]
- 13. Di Carli MF, Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation. 2007;115:1464–1480. [DOI] [PubMed] [Google Scholar]
- 14. Davidson C, Gheorghiade M, Flaherty J, et al. Predictive value of stress myocardial perfusion imaging in liver transplant candidates. Am J Cardiol. 100;89:359–360. [DOI] [PubMed] [Google Scholar]
- 15. Moller S, Henriksen J. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tiukinhoy‐Laing S, Rossi J, Bayram M, et al. Cardiac hemodynamic and coronary angiographic characteristics of patients being evaluated for liver transplantation. Am J Cardiol. 2006;98:178–181. [DOI] [PubMed] [Google Scholar]


