Abstract
Background
The recently introduced pocket‐sized portable transthoracic echocardiography (pTTE) is accurate for measurement of cardiac chamber size and function as well as for assessment of valvular regurgitation. This study aimed to compare the diagnostic accuracy of the pocket‐sized pTTE with the standard TTE (sTTE) and assess its cost‐effectiveness.
Hypothesis
The use of pocket‐sized pTTE, as an initial screening tool, may be feasible, accurate and cost‐effective in the diagnostic strategy of cardiac abnormalities.
Methods
The study subjects were 200 patients scheduled for sTTE and an electrocardiogram (ECG). Each patient underwent pTTE examination with the Vscan (GE Medical Systems, Milwaukee, WI) immediately after sTTE. The findings of pTTE and the ECG were compared with the results of sTTE. Cost‐effectiveness was calculated.
Results
There was a strong agreement in the detection of abnormal findings between pTTE and sTTE (agreement = 90%), whereas the agreement between the ECG and sTTE was 65%. When pTTE or the ECG was used as an initial screening tool prior to sTTE, similar cost reduction was obtained (approximately 30%) by reducing the number of referrals for sTTE. However, the negative predictive value of a diagnostic strategy with pTTE (92%) was superior to that with an ECG (67%).
Conclusions
This study demonstrates that the pocket‐sized pTTE provides accurate detection of cardiac structural and functional abnormalities beyond the ECG. In addition, the use of pTTE as an initial screening tool prior to sTTE is cost‐effective, suggesting that the pocket‐sized pTTE is poised to alter the current diagnostic strategy in clinical practice.
Introduction
Advances in electronics have resulted in the advent of portable transthoracic echocardiography (pTTE) imaging devices. Visualization of the heart by pTTE strengthens the diagnosis of various cardiac diseases and assists in clinical decision making.1, 2, 3, 4, 5 The use of pTTE as an initial screening tool reduces the number of referrals for standard TTE (sTTE) examination, resulting in cost saving in the diagnosis of cardiac abnormalities.6, 7, 8, 9, 10 However, conventional pTTE imaging devices are still too heavy (approximately 2.5 kg) for physicians to carry around like a stethoscope.
A pocket‐sized pTTE imaging device was recently introduced in clinical practice. We and others recently reported the usefulness of a pocket‐sized device for the measurement of cardiac chamber size and function11, 12, 13, 14, 15 and assessment of valvular heart diseases.13, 14, 16 Considering the portability of pocket‐sized imaging devices, similar to a stethoscope, the use of a pocket‐sized pTTE as an initial screening tool has the potential to significantly alter current diagnostic testing patterns in clinical practice. However, the cost‐effectiveness of the pocket‐sized pTTE in the diagnostic strategy of cardiac diseases has not been investigated. The present multicenter study was designed to test the feasibility and diagnostic accuracy of the pocket‐sized pTTE compared with the sTTE and to investigate its cost‐effectiveness as an initial screening tool.
Methods
Study Population
This study consisted of 200 consecutive patients who were referred for sTTE and an electrocardiogram (ECG) to evaluate 1 or more of the following questions: left ventricular (LV) function in 127 (64%) patients, coronary artery disease in 71 (36%) patients, arrhythmia in 33 (17%) patients, hypertension in 29 (15%) patients, valvular heart diseases in 29 (15%) patients, pulmonary hypertension in 4 (2%) patients, infective endocarditis in 3 (1.5%) patients, and pericardial disease in 2 (1%) patients. Patients were gathered at 4 collaborating institutions: Asakayama Hospital (n = 58), Osaka Ekisaikai Hospital (n = 79), Osaka City General Hospital (n = 12), and Okayama University Graduate School of Medicine (n = 51). An sTTE was performed by an expert sonographer and supervised by an experienced physician in each institution. This was immediately followed by pTTE examination performed by another expert physician, who was blinded to all clinical information and the results of sTTE and the ECG.
Patients were divided into low‐ and high‐risk groups according to the presence of at least 1 of the following factors: hypertension (blood pressure ≥140/90 mm Hg on repeated measurements or being treated with an antihypertensive medication), the history of coronary artery disease, diabetes (fasting plasma glucose level >126 mg/dL, taking glucose‐lowering agents or insulin, or a combination of the 2), history of cerebrovascular disease, and peripheral arterial disease. This study was approved by the ethics committee of each participating institution.
Pocket‐Sized Transthoracic Echocardiography
Pocket‐sized pTTE was performed using the Vscan (GE Medical Systems, Milwaukee, WI). Standard parasternal long‐ and short‐axis views and the apical 2‐, 3‐, and 4‐chamber views were obtained. These views were then repeated with the color Doppler method. The following parameters were visually assessed: (1) LV hypertrophy, (2) LV dilatation, (3) LV systolic dysfunction, (4) LV regional wall motion abnormalities, (5) left atrial (LA) enlargement, (6) dilated ascending aorta, (7) pericardial effusion, and (8) more than moderate valvular heart disease. Mitral and tricuspid regurgitation were qualitatively estimated by color Doppler flow mapping of spatial distribution of the regurgitant jet in the left and right atrium, respectively.17 Aortic regurgitation was assessed by the spread of regurgitant jet within the LV outflow tract on the parasternal long view.17 For valvular stenosis, 2‐dimensional and color Doppler aspects of the valve, such as leaflet movement/calcification and accelerated abnormal flow, were estimated due to the lack of pulse‐ and continuous‐wave Doppler capabilities.
Standard Transthoracic Echocardiography
Commercially available echocardiographic systems were used for sTTE examination, including iE‐33 (Philips Medical Systems, Andover, MA), Vivid7 and Vivid E9 (GE Medical Systems), Artida and Xario (Toshiba Medical Systems, Tokyo, Japan), and Sequoia 512 (Siemens Medical Solutions, Mountainview, CA). Standard TTE was performed in the standard manner.18 The dimensions of the cardiac chambers were measured, and LV ejection fraction was obtained by using the Simpson method. Clinically significant abnormal findings were defined as follows: LV wall thickness >12 mm, LV end‐diastolic dimension >55 mm, LV ejection fraction <50%, LA dimension >40 mm, and ascending aortic diameter >35 mm. Quantitative assessment was added for valvular heart disease if more than moderate was visually suspected. Pulsed‐wave Doppler examination of mitral inflow was performed to measure peak velocity and deceleration time of the early diastolic flow. Early diastolic mitral annular velocity was also measured from tissue Doppler imaging in the septal wall. LV diastolic function was then graded according to the recommendation of the American Society of Echocardiography.19 Abnormal age‐adjusted diastolic filling was considered to reflect LV diastolic dysfunction.20 Right ventricular (RV) systolic pressure was estimated by the simplified Bernoulli equation,21 adding the right atrial pressure estimated from the diameter of the inferior vena cava during respiration.22 Pulmonary hypertension was defined as RV systolic pressure >30 mm Hg. TTE result was classified as normal or abnormal, according to the presence of at least 1 abnormality.
Electrocardiogram
A standard 12‐lead ECG was performed, and ECG abnormalities included sinus tachycardia, atrial fibrillation, ventricular ectopies, any ST–T wave abnormalities, pathological Q waves, and LV hypertrophy (Sokolow‐Lyon criteria).23
Statistical Analysis
Categorical variables are presented as frequencies and continuous variables as mean ± standard deviation. Differences between proportions were assessed by χ2 analysis. Variables were compared by the Student t test for continuous variables. Differences were considered significant at P < 0.05.
Results
Standard TTE Examination
Of the 200 patients, 70 (35%) patients were classified into the low‐risk group, and the remaining 130 (65%) patients were considered the high‐risk group. The clinical characteristics of patients of each group are summarized in Table 1. At least 1 echocardiographic abnormality was observed in 118 (59%) of the 200 patients. These sTTE abnormalities were significantly less common in patients of the low‐risk (33/70 [47%]) than the high‐risk group (85/130 [65%]) (P = 0.01). The findings of sTTE are summarized in Table 1. LV diastolic dysfunction always occurred concurrently with other structural and/or functional abnormalities, including LV hypertrophy, LV systolic dysfunction, and/or LA dilatation. Left ventricular regional wall motion abnormalities were observed in 1 or more of the following areas: anterior wall in 13 patients, lateral wall in 12 patients, and inferior wall in 14 patients.
Table 1.
Clinical Characteristics and Results of Standard Transthoracic Echocardiography Findings in Each Group
| Overall Group | Low‐Risk Group | High‐Risk Group | P Value | |
|---|---|---|---|---|
| Age, y | 70 ± 14 | 67 ± 16 | 71 ± 12 | 0.06 |
| Males, n (%) | 104 (52) | 31 (44) | 73 (56) | 0.1 |
| Hypertension, n (%) | 111 (56) | 0 (0) | 111 (85) | |
| Diabetes, n (%) | 40 (20) | 0 (0) | 40 (31) | |
| History of coronary artery disease, n (%) | 34 (17) | 0 (0) | 34 (26) | |
| History of cerebrovascular disease, n (%) | 16 (8) | 0 (0) | 16 (12) | |
| History of peripheral arterial disease, n (%) | 9 (4.5) | 0 (0) | 9 (6.9) | |
| Echocardiographic results | ||||
| LV | ||||
| Hypertrophy, n (%) | 29 (15) | 3 (4) | 26 (20) | <0.001 |
| Dilatation, n (%) | 32 (16) | 11 (16) | 21 (16) | 0.9 |
| Systolic dysfunction, n (%) | 55 (28) | 15 (21) | 40 (31) | 0.2 |
| Diastolic dysfunction, n (%) | 18 (9) | 3 (4) | 15 (12) | 0.09 |
| Regional wall motion abnormalities, n (%) | 30 (15) | 7 (10) | 23 (18) | 0.1 |
| LA enlargement, n (%) | 80 (40) | 18 (26) | 62 (48) | 0.002 |
| Dilated ascending aorta, n (%) | 2 (1) | 1 (1) | 1 (1) | 0.7 |
| Pericardial effusion, n (%) | 11 (6) | 3 (4) | 8 (6) | 0.6 |
| More than moderate valvular heart disease | ||||
| Mitral regurgitation, n (%) | 20 (10) | 7 (10) | 13 (10) | 1 |
| Mitral stenosis, n (%) | 1 (0.5) | 0 (0) | 1 (0.8) | 0.5 |
| Aortic regurgitation, n (%) | 7 (4) | 1 (1) | 6 (5) | 0.2 |
| Aortic stenosis, n (%) | 11 (6) | 1 (1) | 10 (8) | 0.06 |
| Tricuspid regurgitation, n (%) | 19 (10) | 7 (10) | 12 (9) | 0.9 |
| Pulmonary hypertension, n (%) | 42 (21) | 14 (20) | 28 (22) | 0.8 |
Abbreviations: LA, left atrium; LV, left ventricle.
Values are mean ± standard deviation or n (%). P values shown are the comparisons between low‐risk and high‐risk groups.
Comparison Between sTTE and pTTE Findings
Echocardiographic assessment was completed for pTTE in all 200 patients (feasibility 100%). The mean time of pTTE examination per patient was shorter than that of sTTE (5.1 ± 2.3 minutes vs 28.8 ± 18.2 minutes, P < 0.001).
There was a strong agreement between the findings of pTTE and sTTE in each group for both normal and abnormal findings (Figure 1). The pocket‐sized pTTE missed the diagnosis of the presence of abnormalities detected by sTTE in 7 patients, LA dilatation in 2 patients, and pulmonary hypertension in 5 patients (estimated RV pressure ranging from 30 to 46 mm Hg). The latter 5 patients had pulmonary hypertension without other TTE abnormalities. On the other hand, pTTE overestimated the echocardiographic abnormalities in 14 patients, LV regional wall motion abnormalities in 4 patients (anterior wall in 2 patients, lateral wall in 1 patient, and inferior wall in 1 patient), LA dilatation in 2 patients, and valvular heart disease in 8 patients (aortic regurgitation in 2 patients, mitral regurgitation in 3 patients, and tricuspid regurgitation in 3 patients). Two‐thirds of these underestimated or overestimated results were observed in the low‐risk group. Therefore, the accuracy of pTTE was slightly better in the high‐risk group than in the low‐risk group, as shown in Figure 1.
Figure 1.
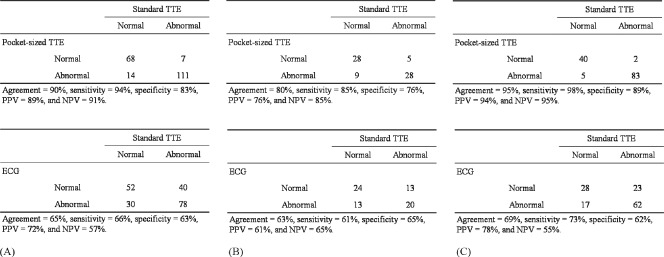
Agreement between standard transthoracic echocardiography (TTE) and portable TTE (upper row), and electrocardiogram (ECG) (lower row) in the detection of patients with abnormal echocardiography overall (A), in the low‐risk group (B), and the high‐risk group (C), respectively. Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
Results of the ECG
Of the 200 patients, 108 (54%) patients had at least 1 of the following ECG abnormalities: sinus tachycardia in 1 (0.5%) patient, atrial fibrillation in 28 (14%) patients, ventricular ectopics in 18 (9%) patients, any ST T‐wave abnormalities in 55 (28%) patients, pathological Q waves in 8 (4%) patients, and LV hypertrophy in 25 (13%) patients. These abnormalities tended to be less frequently observed in the low‐risk group (33 [47%]) than in the high‐risk group (75 [58%]), but the difference was not statistically significant (P = 0.2). The agreement between sTTE and ECG findings was lower than that between sTTE and pTTE for the entire group (Figure 1). The negative predictive value (NPV) of the ECG was approximately 60%.
Cost‐Effective Analysis
The costs of sTTE and ECG examinations were ¥8800 [US$88] and ¥1300 [US$13] in 2010 reimbursement in Japan, respectively (¥100 = US$1). In contrast, no reimbursement was received for pTTE, being considered part of the physical examination.24 Considering that the capital cost of the pTTE device was about ¥1,000,000 (US$100,000) and the 5‐year equipment depreciation was about ¥200,000 (US$2,000) per year, the cost of pTTE would be ¥200 (US$2), based on the number of patients referred for echocardiography with an estimate of 1000 per year in our institution (Osaka Ekisaikai Hospital).
Applying these data on our results, the total cost was estimated for the different diagnostic strategies, as shown in Figure 2. In strategy 1, where all patients had sTTE examination without a prior ECG or pTTE screening, the total cost of sTTE was ¥1,760,000 (US$17,600). If an ECG was used as an initial screening test for all patients (strategy 2), the sTTE cost would be ¥260,000 (US$2600). Based on the ECG results, sTTE would be required in 108 patients with ECG abnormalities (¥950,400 [US$9504]). Therefore, the total cost would be ¥1,210,400 (US$12,104), leading to a 31% reduction of medical costs compared with the results of strategy 1. However, sTTE abnormalities were missed in 40 patients, resulting in a relatively low NPV. Such calculations were performed for 7 different strategies, and the results are summarized in Table 2. The cost reduction was relatively large in strategies 2, 3, and 7, whereas NPV, which is crucial when using a screening test, was excellent in strategies 3, 4, and 6.
Figure 2.
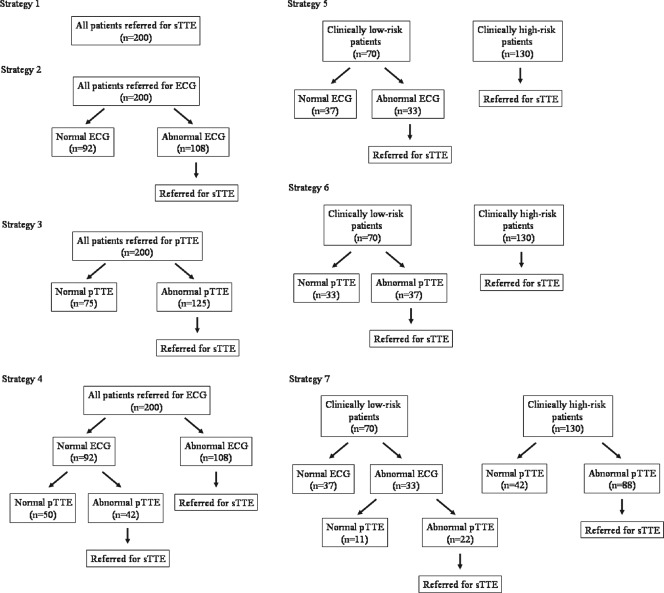
Different diagnostic strategies. Strategy 1: All patients had standard transthoracic echocardiography (sTTE) examination without prior electrocardiogram (ECG) and portable transthoracic echocardiography (pTTE) screening. Strategy 2: An ECG was obtained in all patients as an initial screening test, and sTTE was performed in patients with an abnormal ECG. Strategy 3: pTTE was used as an initial screening test instead of an ECG, and patients with abnormal pTTE were referred for sTTE. Strategy 4: Patients with an abnormal ECG had sTTE, but patients with a normal ECG had pTTE. The indication of sTTE depended on pTTE findings. Strategy 5: High‐risk patients had sTTE without any screening test. Low‐risk patients had an ECG, and patients with an abnormal ECG were referred for sTTE. Strategy 6: Similar to strategy 5, screening was performed only in low‐risk patients, but pTTE was used instead of an ECG. Strategy 7: pTTE was performed prior to sTTE in high‐risk patients. In low‐risk patients, only patients with abnormal results of an ECG and pTTE were referred for sTTE.
Table 2.
Cost‐effectiveness and Diagnostic Accuracy Using Different Diagnostic Strategies
| Strategy 1 | Strategy 2 | Strategy 3 | Strategy 4 | Strategy 5 | Strategy 6 | Strategy 7 | |
|---|---|---|---|---|---|---|---|
| Cost analysis | |||||||
| Number of sTTE examinations | 200 | 108 | 125 | 150 | 163 | 167 | 110 |
| Number of ECG examinations | 200 | 200 | 70 | 70 | |||
| Number of pTTE examinations | 200 | 92 | 70 | 163 | |||
| Total cost, yen (US$) | 1,760,000 ($17,600) | 1,210,400 ($12,104) | 1,140,000 ($11,400) | 1,598,400 ($15,984) | 1,525,400 ($15,254) | 1,483,600 ($14,836) | 1,091,600 ($10,916) |
| Cost reduction | — | 31% | 35% | 9% | 13% | 16% | 38% |
| Accuracy | |||||||
| Sensitivity | — | 66% | 94% | 96% | 89% | 96% | 86% |
| Specificity | — | 100% | 100% | 100% | 100% | 100% | 100% |
| PPV | — | 100% | 100% | 100% | 100% | 100% | 100% |
| NPV | — | 67% | 92% | 94% | 86% | 94% | 84% |
Abbreviations: ECG, electrocardiogram; NPV, negative predictive value; PPV, positive predictive value; pTTE, portable transthoracic echocardiography; sTTE, standard transthoracic echocardiography.
Figures in parentheses are the US$ equivalent values (¥100 = US$1).
The results of this study were applied to other reimbursement schedules and to countries other than Japan (Table 3). A similar degree of cost reduction was estimated at these institutions in different countries.
Table 3.
Cost‐Effectiveness at Different Institutions in Different Countries
| Institution, Country | |||||
|---|---|---|---|---|---|
| Osaka Ekisaikai Hospital, Japan | Osaka City General Hospital, Japan | Cedars‐Sinai Medical Center, United States | University of Barcelona, Spain | Asan Medical Center, South Korea | |
| Cost, per sTTE examination | ¥8800 ($88) | ¥8800 ($88) | US$2000 | €170 (US$131) | W290,000 (US$257) |
| Cost, per ECG examination | ¥1300 (US$13) | ¥1300 (US$13) | US$200 | €9 (US$14.6) | W5902 (US$5.2) |
| Retail price of portable device, Vscan | ¥1,000,000 (US$10,000) | ¥1,000,000 (US$10,000) | US$7900 | €6000 (US$4615) | W9,900,000 (US$8761) |
| Number of sTTE examinations, per machine per year | 1000 | 1500 | 2000 | 2500 | 2600 |
| Estimated cost of pTTE | ¥ 200 (US$2) | ¥ 133 (US$1.33) | US$0.79 | €0.48 (US$0.37) | W762 (US$0.67) |
| Cost reduction | |||||
| Strategy 2 | 31% | 31% | 36% | 35% | 44% |
| Strategy 3 | 35% | 36% | 37% | 37% | 37% |
| Strategy 4 | 9% | 10% | 15% | 14% | 23% |
| Strategy 5 | 13% | 13% | 15% | 15% | 18% |
| Strategy 6 | 16% | 16% | 16% | 16% | 16% |
| Strategy 7 | 38% | 39% | 41% | 41% | 44% |
Abbreviations: ECG, electrocardiogram; pTTE, portable transthoracic echocardiography; sTTE, standard transthoracic echocardiography.
Figures in parentheses are the US$ equivalent values (¥100 = US$1, €0.77 = US$1, W1130 = US$1).
Discussion
This study demonstrated that the pocket‐sized pTTE accurately detected cardiac structural and functional abnormalities, far better than the ECG, regardless of the patient background with regard to the risk of cardiac disease. This is the first study to investigate the cost‐effectiveness of the pocket‐sized pTTE using various clinical diagnostic strategies and 5 different reimbursement systems.
Diagnostic Accuracy
Several studies have already reported comparable results obtained by the pocket‐sized pTTE and sTTE with regard to measurement of cardiac chamber sizes and function, and assessment of valvular disease in various clinical settings.11, 12, 13, 14, 15, 16 It is also reported that adding pocket‐sized pTTE to physical examination improved the diagnosis of cardiac abnormalities,25, 26 and clinical decision making satisfactorily dealt with the pocket‐sized pTTE in approximately two‐thirds of the patients.27
However, pTTE analysis did not include the assessment of right ventricular function, pericardial diseases, and congenital heart disease in this study protocol, because the question regarding such complex conditions/diseases may not be appropriate to approach with a pocket‐sized pTTE. On the other hand, 8 (17%) of 46 valvular regurgitation cases were overestimated by pTTE distinguishing from less than mild regurgitation to greater than moderate regurgitation. However, pTTE did not underestimated the severity of valvular regurgitation. Our previous report was focused on the ability of pTTE in the assessment of the severity of valvular regurgitation, showing the good concordant between sTTE and pTTE results.16 Also, valvular stenosis was observed in 7 patients, aortic stenosis in 6 patients, and mitral stenosis in 1 patient. Valvular stenosis was detected by pTTE in all patients. Thus, pTTE is useful to assess significant valvular regurgitation/stenosis as an initial screening tool.
In the present study, due to the high NPV of pTTE, the use of pTTE for screening prior to sTTE, instead of an ECG, strengthened the diagnosis of cardiac diseases. Interestingly, the NPV of pTTE was slightly higher in the high‐risk group than in the low‐risk group, although screening a high‐risk population is generally thought to be more effective than screening a general‐ or low‐risk population. This may be explained by the more severe cardiac disease in the high‐risk patients, which was easy to define as an abnormal echocardiogram. In contrast, mild pulmonary hypertension was missed by pTTE due to the lack of other concomitant cardiac abnormalities. Accurate and immediate decision making by a pocket‐sized imaging device has the potential to exert a significant impact on current diagnostic strategies of cardiac diseases and in turn impacts on physical examination skills.
Cost‐Effectiveness
Previous results of cost‐effectiveness analysis reported that the use of conventional pTTE devices in patients with suspected cardiovascular disease was associated with a 20% to 30% cost reduction in health care costs.6, 7, 8, 9, 10 Using similar analysis models, this is the first study showing the cost‐effectiveness of a pocket‐sized device in clinical practice. The use of the pocket‐sized pTTE as the initial screening tool instead of the ECG allowed accurate diagnosis and was cost‐effective. The result of the cost‐effectiveness analysis was similar in 5 institutions in 4 different countries, indicating that the results of the present study are applicable internationally.
However, other additional factors may also affect the cost‐effectiveness in clinical practice. First, immediate decision making based on the findings of the pocket‐sized pTTE may make the patient available earlier for other diagnostic or therapeutic procedures, thus leading to further cost savings through improved outcome. Furthermore, the reduced demand for sTTE may result in a shorter waiting list for sTTE and reduced workloads in echocardiographic laboratories, thus improving their productivity. The results of this study could promote the widespread use of the pocket‐sized pTTE as the first‐line screening tool, in addition to detailed patient interviews and reliable identification of physical examinations.
Study Limitations
First, patients were enrolled in echocardiographic laboratories, and therefore applying our results to other medical situations may be limited because cost‐effective screening depends on the selection of a target population for the disease in question. It was unclear which specific physician would be eligible to provide pTTE service (eg, the patient's primary care physician, cardiologist, or other referring physicians). Visual estimation strongly relies on the experience in echocardiography. Also, pTTE was examined by the expert physician, and image quality of pTTE was adequate in all 200 patients. However, the image quality of the pocket‐sized pTTE may not be enough for less‐experienced physician/technicians, especially in patients with chronic obstructive pulmonary disease or obesity.
Second, only 5 representative ECG abnormalities were evaluated for the detection of cardiac structural and/or functional abnormalities, as described in previous studies.8, 28 Because an ECG provides additional information on electrical conductivity, an ECG and pocket‐sized TTE may not be mutually exclusive.
Third, pocket‐sized pTTE missed the diagnosis of the presence of LA dilatation in 2 (1%) patients and pulmonary hypertension in 5 (2.5%) patients. The potential cost associated with missing these cardiac conditions should be addressed in a future study. Also, the mean time of pTTE examination per patient was approximately 5 minutes. The personal who performed pTTE would have his/her involvement in other clinical services hindered, although this extra time spent in enhancing care would be valuable.
Finally, additional information from the results of biochemical tests, such as serum B‐type natriuretic peptide, troponin, and C‐reactive protein is commonly used in clinical practice for overall assessment of cardiac abnormalities.29, 30 For example, evaluation of LV diastolic function is complex and may be difficult by sTTE alone.31
Conclusion
The present study demonstrated that the pocket‐sized pTTE is feasible, accurate, and cost‐effective for the initial screening of various cardiac diseases. This information has the potential to significantly alter the diagnostic strategy of cardiac disease in clinical practice.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Bruce CJ, Spittell PC, Montgomery SC, et al. Personal ultrasound imager: abdominal aortic aneurysm screening. J Am Soc Echocardiogr. 2000;13:674–679. [DOI] [PubMed] [Google Scholar]
- 2. Vourvouri EC, Poldermans D, Schinkel AF, et al. Left ventricular hypertrophy screening using a hand‐held ultrasound device. Eur Heart J. 2002;23:1516–1521. [DOI] [PubMed] [Google Scholar]
- 3. Spencer KT, Anderson AS, Bhargava A, et al. Physician‐performed point‐of‐care echocardiography using a laptop platform compared with physical examination in the cardiovascular patient. J Am Coll Cardiol. 2001;37:2013–2018. [DOI] [PubMed] [Google Scholar]
- 4. Tsutsui JM, Maciel RR, Costa JM, et al. Hand‐carried ultrasound performed at bedside in cardiology inpatient setting—a comparative study with comprehensive echocardiography. Cardiovasc Ultrasound. 2004;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coletta C, De Marchis E, Lenoli M, et al. Reliability of cardiac dimensions and valvular regurgitation assessment by sonographers using hand‐carried ultrasound devices. Eur J Echocardiogr. 2006;7:275–283. [DOI] [PubMed] [Google Scholar]
- 6. Vourvouri EC, Koroleva LY, Ten Cate FJ, et al. Clinical utility and cost effectiveness of a personal ultrasound imager for cardiac evaluation during consultation rounds in patients with suspected cardiac disease. Heart. 2003;89:727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greaves K, Jeetley P, Hickman M, et al. The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis. J Am Soc Echocardiogr. 2005;18:620–625. [DOI] [PubMed] [Google Scholar]
- 8. Galasko GI, Barnes SC, Collinson P, et al. What is the most cost‐effective strategy to screen for left ventricular systolic dysfunction: natriuretic peptides, the electrocardiogram, hand‐held echocardiography, traditional echocardiography, or their combination? Eur Heart J. 2006;27:193–200. [DOI] [PubMed] [Google Scholar]
- 9. Trambaiolo P, Papetti F, Posteraro A, et al. A hand‐carried cardiac ultrasound device in the outpatient cardiology clinic reduces the need for standard echocardiography. Heart. 2007;93:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Badano LP, Nucifora G, Stacul S, et al. Improved workflow, sonographer productivity, and cost‐effectiveness of echocardiographic service for inpatients by using miniaturized systems. Eur J Echocardiogr. 2009;10:537–542. [DOI] [PubMed] [Google Scholar]
- 11. Fukuda S, Shimada K, Kawasaki T, et al. Pocket‐sized transthoracic echocardiography device for the measurement of cardiac chamber size and function. Circ J. 2009;73:1092–1096. [DOI] [PubMed] [Google Scholar]
- 12. Culp BC, Mock JD, Chiles CD, et al. The pocket echocardiograph: validation and feasibility. Echocardiography. 2010;27:759–764. [DOI] [PubMed] [Google Scholar]
- 13. Lafitte S, Alimazighi N, Reant P, et al. Validation of the smallest pocket echoscopic device's diagnostic capabilities in heart investigation. Ultrasound Med Biol. 2011;37:798–804. [DOI] [PubMed] [Google Scholar]
- 14. Andersen GN, Haugen BO, Graven T, et al. Feasibility and reliability of point‐of‐care pocket‐sized echocardiography. Eur J Echocardiogr. 2011;12:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prinz C, Voigt JU. Diagnostic accuracy of a hand‐held ultrasound scanner in routine patients referred for echocardiography. J Am Soc Echocardiogr. 2011;24:111–116. [DOI] [PubMed] [Google Scholar]
- 16. Kono Y, Fukuda S, Shimada K, et al. Pocket‐sized echo for evaluation of mitral and tricuspid regurgitation. JACC Cardiovasc Imaging. 2011;4:921. [DOI] [PubMed] [Google Scholar]
- 17. Zoghbi WA, Enriquez‐Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 19. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 20.How to diagnose diastolic heart failure. European Study Group on Diastolic Heart Failure. Eur Heart J. 1998;19:990–1003. [DOI] [PubMed]
- 21. Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–662. [DOI] [PubMed] [Google Scholar]
- 22. Otto CM. Echocardiographic evaluation of left and right ventricular function In: Otto MC, ed. Textbook of Clinical Echocardiography 2nd ed. Philadelphia, PA: W.B. Saunders; 2000:100–130. [Google Scholar]
- 23. Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186. [DOI] [PubMed] [Google Scholar]
- 24. Tofield A. The use of pocket size imaging devices: a position statement by the European Association of Echocardiography. Eur Heart J. 2011;32:385–386. [DOI] [PubMed] [Google Scholar]
- 25. Galderisi M, Santoro A, Versiero M, et al. Improved cardiovascular diagnostic accuracy by pocket size imaging device in non‐cardiologic outpatients: the NaUSiCa (Naples Ultrasound Stethoscope in Cardiology) study. Cardiovasc Ultrasound. 2010;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mjolstad OC, Dalen H, Graven T, et al. Routinely adding ultrasound examinations by pocket‐sized ultrasound devices improves inpatient diagnostics in a medical department. Eur J Intern Med. 2011;23:185–191. [DOI] [PubMed] [Google Scholar]
- 27. Cardim N, Fernandez Golfin C, Ferreira D, et al. Usefulness of a new miniaturized echocardiographic system in outpatient cardiology consultations as an extension of physical examination. J Am Soc Echocardiogr. 2011;24:117–124. [DOI] [PubMed] [Google Scholar]
- 28. Lim TK, Dwivedi G, Hayat S, et al. Cost effectiveness of the B type natriuretic peptide, electrocardiography, and portable echocardiography for the assessment of patients from the community with suspected heart failure. Echocardiography. 2007;24:228–236. [DOI] [PubMed] [Google Scholar]
- 29. Mueller C, Scholer A, Laule‐Kilian K, et al. Use of B‐type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350:647–654. [DOI] [PubMed] [Google Scholar]
- 30. Than M, Cullen L, Reid CM, et al. A 2‐h diagnostic protocol to assess patients with chest pain symptoms in the Asia‐Pacific region (ASPECT): a prospective observational validation study. Lancet. 2011;377:1077–1084. [DOI] [PubMed] [Google Scholar]
- 31. Unzek S, Popovic ZB, Marwick TH. Effect of recommendations on interobserver consistency of diastolic function evaluation. JACC Cardiovasc Imaging. 2011;4:460–467. [DOI] [PubMed] [Google Scholar]


