Abstract
The prevalence of resistant hypertension (ReHy) is not well established. Furthermore, diuretics, angiotensin‐converting enzyme inhibitors or angiotensin‐receptor blockers, and calcium channel blockers are largely used as the first 3‐drug combinations for treating ReHy. However, the fourth drug to be added to the triple regimen is still controversial and guided by empirical choices. We sought (1) to determine the prevalence of ReHy in patients with stage II hypertension; (2) to compare the effects of spironolactone vs clonidine, when added to the triple regimen; and (3) to evaluate the role of measuring sympathetic and renin‐angiotensin‐aldosterone activities in predicting blood pressure response to spironolactone or clonidine. The Resistant Hypertension Optimal Treatment (ReHOT) study (ClinicalTrials.gov NCT01643434) is a prospective, multicenter, randomized trial comprising 26 sites in Brazil. In step 1, 2000 patients will be treated according to hypertension guidelines for 12 weeks, to detect the prevalence of ReHy. Medical therapy adherence will be checked by pill count monitoring. In step 2, patients with confirmed ReHy will be randomized to an open label 3‐month treatment with spironolactone (titrating dose, 12.5–50 mg once daily) or clonidine (titrating dose, 0.1–0.3 mg twice daily). The primary endpoint is the effective control of blood pressure after a 12‐week randomized period of treatment. The ReHOT study will disseminate results about the prevalence of ReHy in stage II hypertension and the comparison of spironolactone vs clonidine for blood pressure control in patients with ReHy under 3‐drug standard regimen.
Introduction
Resistant hypertension (ReHy) is an emerging clinical and public health problem that tends to increase because of increasing life expectancy,1, 2 and there is a growing global epidemic of obesity, diabetes, and sleep apnea.3, 4, 5, 6 It is also tempting to speculate that the excessive dietary salt ingestion reported in many countries7 can contribute substantially to the risk of ReHy development. The prevalence of ReHy is not well established.8 Until recently, the prevalence of ReHy was mainly derived from post hoc analyses of clinical trials.9 For instance, results from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack trial (ALLHAT) suggested that approximately 50% of patients enrolled used 3 or more drugs in an attempt to control blood pressure (BP).10 Egan et al, analyzing data from the National Health and Nutrition Examination Survey (NHANES), found that the estimated prevalence of ReHy has been increasing progressively over the past several decades, reaching 11.8% between 2005 and 2008.11 Using NHANES data collected from 2003 through 2008, Persell estimated that the prevalence of resistant hypertension was 12.8% of all adults being treated for hypertension in the USA.12 In a large cohort (68 045 patients) of treated hypertensive patients from the Spanish Ambulatory Blood Pressure Monitoring Registry, 8295 (12.2% of the database) had resistant hypertension (office BP ≥ 140 and/or 90 mm Hg while being treated with ≥3 antihypertensive drugs, one of them being a diuretic). After ambulatory blood pressure monitoring (ABPM), 62.5% of patients were classified as being true resistant hypertensive, the remaining 37.5% having white‐coat resistance.13 A recent report found an incidence rate for ReHy of just 1.9% after 4 years of follow‐up for patients who were started on antihypertensive therapy for newly diagnosed hypertension.14 However, this study did not report the prevalence of ReHy in relation to all treated hypertensive patients. Such an assessment would have strengthened the current estimates of prevalence, because it would have allowed for exclusion of patients who did not adhere to their prescribed medications. Lack of this correction remains an important limitation of current determinations of prevalence.9
ReHy is associated with target‐organ damage, higher risk of cardiovascular events, and poor prognosis.8, 14 Hence, it is important to investigate and define the efficacy of different antihypertensive combinations to control this condition.15 Few data are available about the standardization of the first 3‐drug combinations. However, a simplified treatment algorithm has demonstrated that a combination of a diuretic plus an angiotensin‐converting enzyme inhibitor (ACEi) or an angiotensin‐receptor blocker (ARB), adding a calcium channel blocker when necessary, controlled 64% of hypertensive patients and, in addition, was even more efficient than the available guideline‐based management.16 By contrast, the fourth drug to be added to the triple regimen is still controversial and guided by empirical choices or personal preferences. Recent studies suggest the emerging role of spironolactone as the first‐line fourth drug for treating ReHy.17, 18, 19 Conversely, because of the pathophysiological rationale, others have proposed the use of β‐blockers8 or even centrally acting agents for managing sympathetic hyperactivity.8, 20, 21 This has presented concerns about the limited BP‐reducing effect of β‐blockers, especially in elderly people.22, 23, 24, 25 Therefore, centrally acting agents are attractive candidates as the fourth drug for treating ReHy.
The main objectives of the Resistant Hypertension Optimal Treatment (ReHOT) trial are to assess prospectively: (1) the prevalence of ReHy in a cohort of outpatients with stage II hypertension; (2) the effect of spironolactone on BP, in comparison to clonidine, when added to a multidrug combination consisting of chlorthalidone plus ACEi (or ARB) plus amlodipine, all 3 up‐titrated to the highest dose; and (3) the role of measuring sympathetic nervous system activity and renin‐angiotensin‐aldosterone activity in predicting the response of BP to spironolactone and clonidine.26, 27
Methods and Analysis
The inclusion and exclusion criteria are depicted on Table 1. This multicenter prospective study will be divided into 2 steps (Figure 1).
Table 1.
Inclusion and Exclusion Criteria in the ReHOT Trial
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age between 18 and 75 years | Systolic blood pressure ≥ 220 mm Hg |
| Systolic blood pressure ≥ 160 mm Hg and/or diastolic blood pressure ≥ 100 mm Hg | Patients evaluated in an acute episode of hypertensive urgency or emergency |
| Chronic kidney disease stages IV and V (MDRD formula estimated GFR ≤ 30 mL/min) | |
| Hyperkalemia (>5.0 mEq/L) | |
| Renovascular disease and hyperaldosteronism | |
| Major cardiovascular event (stroke, myocardial infarction, myocardial revascularization, etc) occurring in the previous 6 months | |
| Retinopathy grade III or IV | |
| Heart failure stages III and IV | |
| Malignant disease with life expectancy < 2 years | |
| Alcoholism | |
| Psychiatric disorders | |
| Women in the fertility stage not using an efficient contraceptive method | |
| Pregnancy | |
| Complex arrhythmias, second‐ and third‐degree AV block | |
| Severe liver disease | |
| Patients with a history of hypersensitivity to study drugs | |
| Patients with compelling indication for β‐blocker use (heart failure, coronary disease) |
Abbreviations: AV, atrioventricular; GFR, Glomerular Filtration Rate; MDRD, Modification of Diet in Renal Disease; ReHOT, Resistant Hypertension Optimal Treatment.
Figure 1.
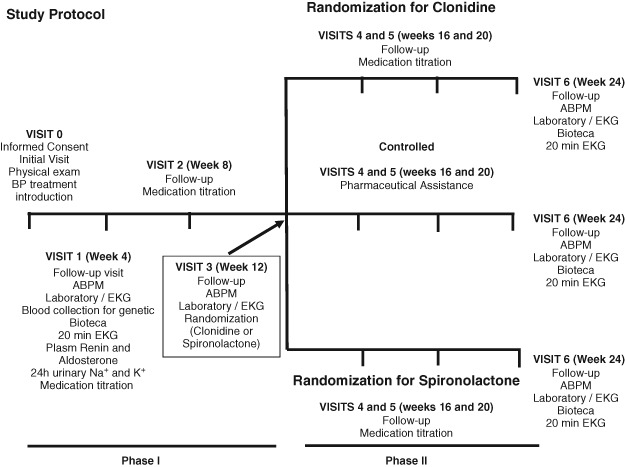
Schematic view of the protocol. Laboratory includes hemogram, plasma Na and K, fasting glucose, urea, creatinine, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, triglycerides, uric acid, summary of urine. Abbreviations: ABPM, ambulatory blood pressure monitoring; BP, blood pressure; EKG, electrocardiogram.
Step 1: 12 Weeks of Open Label, Forced Titration, of 3 Antihypertensive Agents (Chlorthalidone, Enalapril or Losartan, and Amlodipine), to Assess the Prevalence of ReHy
Step 1 will include 2000 patients with hypertension stage II (never treated or under antihypertensive treatment) to detect ReHy prevalence at 26 sites in Brazil (Figure 2). Table 2 describes some baseline characteristics of the first 409 patients recruited. ReHy will be defined as BP that remains above goal despite the concurrent use of 3 antihypertensive agents of different classes; one of them must be a diuretic, and all agents should be prescribed at optimal dose amounts (chlorthalidone 25 mg once daily, enalapril 20 mg twice daily [bid], losartan 50 mg bid, amlodipine 5 mg bid).8 In addition, patients whose BP is controlled but who require 4 or more medications to achieve this should also be considered resistant to treatment.8
Figure 2.
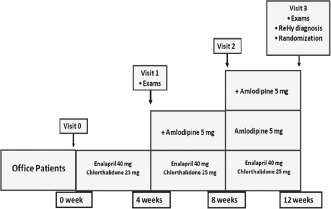
In step 1, 2000 patients with hypertension (220/130 ≥ BP ≥ 160/100 mm Hg) will be enrolled and titrated to the maximum dose of a 3‐drug combination therapy. Abbreviation: ReHy, resistant hypertension.
Table 2.
Baseline Characteristics of Participants, N = 409
| Characteristic | Value |
|---|---|
| Age, y | 57 ± 10 |
| Male, % | 38 |
| White, % | 41 |
| Body mass index, kg/m2 | 29.9 ± 5.5 |
| Diabetes, % | 20.8 |
| Smoking, % | 7.3 |
| Systolic blood pressure, mm Hg | 169 ± 15 |
| Diastolic blood pressure, mm Hg | 102 ± 11 |
| Serum creatinine, mg/dL | 0.9 ± 0.3 |
| Potassium, mEq/L | 4.1 ± 0.5 |
At each visit, the physician will measure the patient's BP in a seated position, after a 10‐minute rest period, with the validated Omron (Kyoto, Japan) HEM‐742 automatic device. At least 3 measurements will be recorded until 2 consecutive measurements differing by <4 mm Hg are obtained. The mean value of the last 2 measurements will be calculated and used as the office BP value.
Patients will be treated after visit 0 with chlorthalidone 25 mg/d and enalapril 20 mg bid. For ethical reasons, previous antihypertensive treatment other than that will be suspended without a prior washout. During all visits, medication adherence will be monitored by pill count (all medications were provided to patients, and there is a log form in the case report form of the study to control and calculate drug compliance). We will consider to be adherent those patients taking all medications correctly >80% of the time on all days. Nonadherence to treatment will be considered in the analysis. Potential reasons for poor adherence will be evaluated (side effects etc).
All patients will receive lifestyle modification counseling including dietary sodium reduction and physical activity. After 4 weeks, on visit 1, patients will undergo routine laboratory tests (including glucose, cholesterol levels, renal function, and serum K+), electrocardiography, ABPM, 24‐hour urinary Na + and K+, plasma renin and aldosterone tests, and 20‐minute electrocardiogram (ECG) for spectral analysis of RR interval. Plasma renin and aldosterone will be collected after 1 hour of supine rest, processed, and frozen (−20°C). All samples will be frozen and shipped to be centrally processed at the end of the study.
If the patient has uncontrolled BP (systolic BP ≥ 140 mm Hg and/or diastolic BP ≥ 90 mm Hg), we will introduce amlodipine 5 mg/d, which could be titrated to 10 mg/d after 4 weeks on visit 2 if the BP goal has not been achieved. If the patient has side effects due to enalapril (such as cough), we will use losartan 50 mg bid. On visit 3, patients will undergo ABPM, and those with office BP ≥ 140/90 mm Hg and 24‐hour mean ABPM ≥ 130/80 mm Hg will be considered to be resistant hypertensive.
To access the risk of having sleep apnea, all subjects will perform the Berlin Questionnaire.28 This questionnaire classifies patients as having a low or high risk for sleep apnea. The classification is based on responses in 3 symptom categories concerning snoring, tiredness, and the presence of comorbidities. Briefly, being positive in category 1 is defined as persistent symptoms (>3–4×/wk) on 2 or more questions about snoring. In category 2, being positive is defined by the presence of persistent tiredness (>3–4×/wk). In category 3, being positive is defined by the presence of hypertension or a body mass index of at least 30 kg/m2. To be considered at high risk for sleep apnea, a patient has to be positive in at least 2 symptom categories.
Step 2: All Patients With ReHy (Defined at the End of Step 1) Will Be Randomized to Open Label 12‐Week Treatment With Spironolactone or Clonidine
As previously mentioned, the main objective of step 2 is to evaluate the BP effects of the fourth drug (spironolactone or clonidine; Table 3). True resistant hypertensive patients will be randomized (simple randomization generated by a computer) to treatment with clonidine 0.100 mg bid or with spironolactone 12.5 mg once per day. On visits 4 and 5 (4‐week intervals), dosage could be increased for clonidine to 0.200 mg bid and 0.300 mg bid and for spironolactone to 25 mg/d and 50 mg/d. Patients with controlled BP on visit 3 will receive pharmaceutical assistance every 4 weeks (visits 4 and 5) and will be evaluated after 12 weeks (visit 6). On visit 6, all patients will undergo a medical examination, routine laboratory tests, ECG, and ABPM. After 24 weeks, we will have hypertensive patients controlled with 2, 3, or 4 drugs and patients not controlled.
Table 3.
In Step 2, Patients With Resistant Hypertension Diagnosed in Step 1 Will Be Randomized to an Open Label Phase of Spironolactone vs Clonidine as Shown
| Visit 3 | Clonidine 0.1 mg 2× | Spironolactone 12.5 mg 1× |
|---|---|---|
| Visit 4 | Clonidine 0.2 mg 2× | Spironolactone 25 mg 1× |
| Visit 5 | Clonidine 0.3 mg 2× | Spironolactone 50 mg 1× |
| Visit 6 | Examinations, outcomes | Examinations, outcomes |
In addition to exploring the BP effects of spironolactone or clonidine, the ReHOT trial will also evaluate whether the BP effect is influenced by cardiovascular biomarkers (plasma renin and aldosterone dosage for renin‐angiotensin‐aldosterone system activity and low frequency [LF]/high frequency [HF] ratio from spectral analysis of RR variability for estimation of sympathetic cardiovascular modulation). Plasma renin and aldosterone dosage will be collected using standard techniques. The sympathetic and parasympathetic activity will be noninvasively estimated using spectral analysis of heart rate variability. For this purpose, we will record heart rate by using an ECG signal with the subject lying down and resting for about 20 minutes. Registration will be done on a WinCardio: MICROMED Biotecnologia Ltda, Brasília, Brazil, apparatus, and processing will be performed through Internet‐available software developed by the Biomedical Signal Analysis Group at the University of Eastern Finland (Kuopio, Finland). The processing will be performed in both the time domain (RR intervals of time) and the frequency domain (power spectrum). In the time domain, we are going to measure SDNN and pNN50. In the frequency domain, we will be able to obtain spectral components of variability associated with the HF component (∼0.25 Hz), associated with vagal tone, and the LF component (∼0.10 Hz), associated with the more sympathetic tone. The LF/HF ratio has been considered in several clinical and experimental studies as an indicator of the degree of predominance of sympathetic control over heart rate.29
Outcomes
The primary endpoint is the effective BP control from both office BP (defined as a systolic BP < 140 mm Hg and diastolic BP < 90 mm Hg) and ABPM (defined as a 24‐hour mean BP < 130/80 mm Hg) after the 12‐week randomized period of treatment with clonidine or spironolactone.
Secondary endpoints to be analyzed will be the absolute and relative BP reduction in each study arm.
Ethics and Dissemination
The study has been approved by the institutional review board (number 0758/09). It will be conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. All participants will provide written informed consent. The ReHOT investigators including the Writing Committee and Steering Committee are listed in the Appendix.
No statistical rule for early trial termination is defined, and this study will not be stopped early based on efficacy results. An independent data safety monitoring board (DSMB) will review the safety data, including death, myocardial infarction, stroke, or other serious adverse events. The DSMB will be empowered to recommend suspension of enrollment or termination of the study based on safety concerns. The Executive Committee will make the final decision for early study termination based on DSMB recommendations.
Regarding the dissemination plan, beyond the two main studies derived from this protocol, we have potential substudies to be explored.
Biomarkers in ReHy
There are several ongoing ancillary studies that will take advantage of RE‐HOT's unique resources. Biomarkers that could increase the prediction of ReHy will be explored in several different studies. Using standard and new biochemical molecules such as high‐sensitivity troponin I, asymmetric dimethylarginine, and inflammatory markers, we will test the association with both ReHy status and with different variables obtained at both baseline and 3‐month follow‐up. In addition, DNA markers will be investigated in relation to their association with stage II hypertension, ReHy, and response to BP‐lowering drugs in our sample, and will be incorporated into clinical prediction models calibrated for the Brazilian hypertensive population.
Sleep Apnea and ReHy
Recent evidence indicated that the frequency of sleep apnea is extremely high and that it is by far the most common secondary form of hypertension associated with ReHy.4 The ReHOT trial will not perform formal sleep studies, but the Berlin Questionnaire will be applied to all participants. Previous evidence showed that the Berlin Questionnaire is useful for screening sleep apnea in the general population28 and in patients with hypertension.30 The main objectives of this ReHOT substudy are 2‐fold: (1) to evaluate whether high risk of sleep apnea is associated with higher prevalence of true ReHy and (2) to evaluate potential differences in the BP effects of spironolactone and clonidine in patients with low vs high risk for sleep apnea.
Statistical Considerations
We estimate the prevalence of ReHy to be 20%. The main objective of step 1 is to identify prevalence and predictor variables for ReHy. In this scenario, at the end of step 1, we estimate we will classify 400 patients as resistant hypertensive and 1600 as controlled hypertensive. For the randomization phase, we calculate that it will be necessary to enroll 189 patients in each treatment arm. Because of the lack of evidence comparing the effects of spironolactone vs clonidine in the same trial, we made an empirical estimation of a difference of 5 mm Hg in BP between treatment groups and a standard deviation of 15 mm Hg in each group, for a 2‐sided test. We anticipate a statistical power of 90%.
Calculations and statistics will be performed using the statistical package SPSS version 15.0 (IBM SPSS Statistics, Armonk, NY). Variables that approximate a normal distribution will be summarized as mean ± standard deviation, and groups will be compared using t tests. Non‐normal variables will be summarized as median and first and third quartiles. The statistical significance of differences between study groups will be analyzed using the Mann‐Whitney U test for continuous variables and the Fisher exact test for categorical variables.
Discussion
The most important design feature of the ReHOT Study is to evaluate, in Brazilian patients with stage 2 hypertension, the prevalence of ReHy in those using the most used triple therapy for hypertension: thiazide, ACEi inhibitor (or ARB), and calcium channel blockers. Although almost all treatment guidelines for high BP recommend these drugs for “real life” management and control of hypertension, the efficacy of this combination in controlling hypertension has never been studied in a multicentric clinical trial. Moreover, the study design will evaluate which drug is more efficient as the fourth drug to be added to the therapeutic regimen and whether it is necessary to evaluate the aldosterone‐renin status and/or the sympathetic nervous system activity. So far, only a single randomized study has started to address the preferred fourth drug.31 In this study, 4 weeks of a standardized triple therapy regimen including 12.5 mg daily hydrochlorothiazide, 300 mg irbesartan, and 5 mg amlodipine, with the sequential addition of low doses of 1 to 3 other diuretics acting at different nephron segments (spironolactone, furosemide, and amiloride) over a 12‐week period, achieved a larger decrease in mean daytime ambulatory systolic BP and mean daytime ambulatory diastolic BP than treatment based on sequential renin‐angiotensin system blockade.31 In the Addition of Spironolactone in Patients With Resistant Arterial Hypertension (ASPIRANT) trial, patients with ReHy were randomized to spironolactone or placebo.32 This strategy precludes us evaluating whether spironolactone is the best option for additional antihypertensive treatment in patients with ReHy. Thus, there is a strong necessity for additional randomized studies to clarify this important gap in the literature.33
There are potential limitations to be addressed in the present trial. First, this is an open label study. However, except for the office BP, all measurements (including ABPM) will be performed in a blinded fashion. Second, we are going to exclude patients with a clear indication for β‐blockers in the 3‐drug regimen (such as patients with coronary artery disease). Further studies will be necessary for addressing the best drug regimen for this subset of patients. Third, OSA will be accessed by the Berlin Questionnaire, a validated tool for predicting OSA in patients with hypertension.30 Unfortunately, most of the included centers do not have a sleep laboratory available for performing standard sleep studies (overnight polysomnography).
In conclusion, the ReHOT trial has the ambitious goals of evaluating the prevalence of ReHy in a cohort of outpatients with stage II hypertension as well as the impact of 2 antihypertensive drugs (spironolactone vs clonidine) when added to a multidrug combination consisting of chlorthalidone plus ACEi (or ARB) plus amlodipine, all 3 up‐titrated to the highest dose. Results from this multicenter trial may have direct clinical implications in providing predictors of ReHy among patients with hypertension stage II and critical evidence for the best therapeutic approach in patients with ReHy.
ReHOT Investigators
The Writing Committee consisted of Eduardo M. Krieger, Luciano F. Drager, Dante Marcelo Artigas Giorgi, Jose Eduardo Krieger, Alexandre Costa Pereira, José Augusto Soares Barreto‐Filho, Armando da Rocha Nogueira, and José Geraldo Mill.
The Steering Committee consisted of Eduardo M. Krieger, Dante Marcelo Artigas Giorgi, Jose Eduardo Krieger, Alexandre Costa Pereira, Luciano F. Drager, Alessandro Betito, and Diogo Duarte Fagundes Moia.
The Statistical Analyses Committee consisted of Paulo Lotufo and Alexandre Pereira.
The Adjudication Committee consisted of Jose Eduardo Krieger, Alexandre Pereira, Dante Marcelo Artigas Giorgi, and Luciano F. Drager.
Participating Sites
InCor, HCFMUSP, Instituto do Coração: Eduardo M. Krieger; HCFMUSP, Hospital das Clínicas da Faculdade de Medicina da USP: Décio Mion Jr; UNIFESP, AME, Maria Zélia: Carlos Alberto Machado; UNIFESP, FOR, Fundação Oswaldo Ramos: Marcelo da Costa Batista; UNIFESP, Disciplina de Cardiologia: Antônio Carlos de Camargo Carvalho; IDPC, Instituto Dante Pazzanese de Cardiologia: Celso Amodeo; USP/HCRP, Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto: Fernando Nobre; UNESP/Botucatu, Faculdade de Medicina de Botucatu: Roberto Jorge da Silva Franco; HUAP, Hospital Universitário Antônio Pedro, Universidade Federal Fluminense: Antônio Claudio Lucas da Nóbrega; UFRJ, Hospital Universitário Clementino Fraga Filho da Universidade Federal do Rio de Janeiro: Armando da Rocha Nogueira; UERJ I, Universidade do Estado do Rio de Janeiro: Maria Eliane Campos Magalhães; UERJ II, Universidade do Estado do Rio de Janeiro: Antônio Felipe Sanjuliani; UFES, Universidade Federal do Espírito Santo: José Geraldo Mill; UFMG, Hospital das Clínicas da Universidade de Minas Gerais: Antônio Luiz Pinho Ribeiro; UFOP, Universidade Federal de Ouro Preto: Raimundo Marques do Nascimento; HCPA, Hospital de Clínicas de Porto Alegre: Flávio Danni Fuchs; IC‐FUC, Fundação Universitária de Cardiologia: Iran Castro; PUC RS, Hospital São Lucas Pontifícia Universidade Católica do Rio Grande do Sul: Luís Carlos Bodanese; HAN, Hospital Ana Nery da Universidade Federal da Bahia: Armênio Costa Guimarães; HSI, Hospital Santa Izabel da Santa Casa de Misericórdia, Escola Bahiana de Medicina e Saúde Pública: Gilson Soares Feitosa; UFC, Universidade Federal do Ceará: Carlos Roberto Martins Rodrigues Sobrinho; UFPE, Universidade Federal de Pernambuco: Hilton Chaves; UFS, Universidade Federal de Sergipe: José Augusto Soares Barreto Filho; UNICSAL, Universidade Estadual de Ciências da Saúde de Alagoas: Maria do Carmo Borges Teixeira; UFPA (Universidade Federal do Pará), Hospital Universitário João de Barros Barreto: Eduardo Augusto da Silva Costa; UFG, Hospital de Clínicas da Faculdade de Medicina da Universidade Federal de Goiás: Paulo César Brandão Veiga Jardim.
This study is funded by Fundação de Amparo á Pesquisa do Estado de São Paulo (FAPESP) (2009/53282‐8), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (577030/2008‐6) and Edital MCT/CNPq/CT‐Saúde/MS/SCTIE/DECIT n° 36/2008.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Butler RN. Living longer, contributing longer. JAMA. 1997;278:1372–1373. [PubMed] [Google Scholar]
- 2. Appel LJ. Dietary patterns and longevity: expanding the blue zones. Circulation. 2008;118:214–215. [DOI] [PubMed] [Google Scholar]
- 3. Drager LF, Lopes HF, Maki‐Nunes C, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. 2010;5:e12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–817. [DOI] [PubMed] [Google Scholar]
- 5. Howard BV, Rodriguez BL, Bennett PH, et al. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group I: epidemiology. Circulation. 2002;105:e132–e137. [DOI] [PubMed] [Google Scholar]
- 6. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 7. Pimenta E, Gaddam KK, Pratt‐Ubunama MN, et al. Relation of dietary salt and aldosterone to urinary protein excretion in subjects with resistant hypertension. Hypertension. 2008;51:339–344. [DOI] [PubMed] [Google Scholar]
- 8. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. [DOI] [PubMed] [Google Scholar]
- 9. Pimenta E, Calhoun DA. Resistant hypertension: incidence, prevalence, and prognosis. Circulation. 2012;125:1594–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 11. Egan BM, Zhao Y, Axon RN, et al. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988–2008. Circulation. 2011;124:1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–1080. [DOI] [PubMed] [Google Scholar]
- 13. de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902. [DOI] [PubMed] [Google Scholar]
- 14. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krumholz HM, Weintraub WS, Bradford WD, et al. Task force #2—the cost of prevention: can we afford it? Can we afford not to do it? 33rd Bethesda Conference. J Am Coll Cardiol. 2002;40:603–615. [DOI] [PubMed] [Google Scholar]
- 16. Feldman RD, Zou GY, Vandervoort MK, et al. A simplified approach to the treatment of uncomplicated hypertension: a cluster randomized, controlled trial. Hypertension. 2009;53:646–653. [DOI] [PubMed] [Google Scholar]
- 17. Chapman N, Dobson J, Wilson S, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839–845. [DOI] [PubMed] [Google Scholar]
- 18. Berecek KH, Farag A, Bahtiyar G, et al. Adding low‐dose spironolactone to multidrug regimens for resistant hypertension. Curr Hypertens Rep. 2004;6:211–212. [PubMed] [Google Scholar]
- 19. Carl D, Sica DA. Spironolactone use in resistant hypertension. Curr Hypertens Rep. 2007;9:297–298. [DOI] [PubMed] [Google Scholar]
- 20.Taler SJ, Textor SC, Augustine JE. Resistant hypertension: comparing hemodynamic management to specialist care. Hypertension. 2002;39:982–988. [DOI] [PubMed] [Google Scholar]
- 21. Maciel RM, Spritzer N, Spritzer TS, et al. Treatment of diuretic‐resistant arterial hypertension ‐ crossed comparative study of verapamil and clonidine [in Portuguese]. Arq Bras Cardiol. 1981;36(suppl 1):47–50. [PubMed] [Google Scholar]
- 22. Viera AJ, Hinderliter AL. Evaluation and management of the patient with difficult‐to‐control or resistant hypertension. Am Fam Physician. 2009;79:863–869. [PubMed] [Google Scholar]
- 23.Sociedade Brasileira de Cardiologia‐SBC; Sociedade Brasileira de Hipertensão‐SBH; Sociedade Brasileira de Nefrologia‐SBN. V Brazilian guidelines in arterial hypertension [in Portuguese]. Arq Bras Cardiol. 2007;89:e24–e79. [PubMed] [Google Scholar]
- 24. Che Q, Schreiber MJ Jr, Rafey MA. Beta‐blockers for hypertension: are they going out of style? Cleve Clin J Med. 2009;76:533–542. [DOI] [PubMed] [Google Scholar]
- 25. Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta‐analysis. Lancet. 2005;366:1545–1553. [DOI] [PubMed] [Google Scholar]
- 26. Laragh JH, Letcher RL, Pickering TG. Renin profiling for diagnosis and treatment of hypertension. JAMA. 1979;241:151–156. [PubMed] [Google Scholar]
- 27. Zweifler A, Esler M. Blood pressure, renin activity and heart rate changes during propranolol therapy of hypertension. Am J Cardiol. 1977;40:105–109. [DOI] [PubMed] [Google Scholar]
- 28. Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. [DOI] [PubMed] [Google Scholar]
- 29. Liao D, Barnes RW, Chambless LE, et al. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability—the ARIC Study. Am J Cardiol. 1995;76:906–912. [DOI] [PubMed] [Google Scholar]
- 30. Drager LF, Genta PR, Pedrosa RP, et al. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105:1135–1139. [DOI] [PubMed] [Google Scholar]
- 31. Bobrie G, Frank M, Azizi M, et al. Sequential nephron blockade versus sequential renin‐angiotensin system blockade in resistant hypertension: a prospective, randomized, open blinded endpoint study. J Hypertens. 2012;30:1656–1664. [DOI] [PubMed] [Google Scholar]
- 32. Václavík J, Sedlák R, Plachy M, et al. Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT): a randomized, double‐blind, placebo‐controlled trial. Hypertension. 2011;57:1069–1075. [DOI] [PubMed] [Google Scholar]
- 33. Mancia G. Additional drug treatment in resistant hypertension: need for randomized studies. J Hypertens. 2012;30:1514–1515. [DOI] [PubMed] [Google Scholar]


