Abstract
Background
Despite overall improvements in cardiovascular‐disease therapies and outcomes, medication nonadherence remains an important barrier to effective secondary prevention of atherothrombotic disease.
Hypothesis
Long‐term medication adherence in outpatients with stable atherothrombotic disease is impacted by demographic and clinical factors.
Methods
We examined data from the prospective international Reduction of Atherothrombosis for Continued Health (REACH) Registry. Analyses were derived from 25 737 patients with established atherothrombotic disease with complete adherence data at enrollment and at year 4. Adherence was defined as patients' self‐report of taking medications based on class I American College of Cardiology/American Heart Association guidelines for secondary prevention as defined, including antiplatelet agents, statins, and antihypertensive medications.
Results
Among patients with atherothrombotic disease, 12 500 (48.6%) were deemed adherent to guideline‐recommended medications. Adherent patients were younger, white, and had less polyvascular disease. Hispanic and East Asian patients were less likely to be adherent as compared with white patients (odds ratio [OR]: 0.72, 95% confidence interval [CI]: 0.59‐0.88; and OR: 0.67, 95% CI: 0.53‐0.83, respectively). Patients who had a nonfatal MI or underwent coronary angioplasty/stenting during follow‐up were more likely to be adherent compared with patients without these events (OR: 1.73, 95% CI: 1.25‐2.38; and OR: 2.15, 95% CI: 1.72‐2.67, respectively). On the other hand, nonfatal stroke during follow‐up was inversely associated with adherence (OR: 0.77, 95% CI: 0.61‐0.97).
Conclusions
Using a large international registry of outpatients with atherothrombotic disease, we found that age, region, race/ethnicity, and incident cardiovascular events were predictive of long‐term guideline adherence for secondary prevention, suggesting that certain patient groups may benefit from targeted interventions to improve adherence.
Introduction
Despite compelling evidence that medical therapy for atherothrombotic disease improves clinical outcomes,1, 2, 3 medication nonadherence remains an important barrier to secondary prevention.2, 4, 5, 6, 7 Medication nonadherence is associated with both higher costs and adverse clinical outcomes.1, 5, 8 Ensuring adherence to evidence‐based drug regimens remains a central public‐health priority.
Several medications have been demonstrated in large clinical trials to be efficacious in reducing cardiovascular (CV) endpoints of death and recurrent events.3, 9, 10, 11 Among these, secondary prevention through the long‐term use of antiplatelet agents, statins, and antihypertensives has been shown to improve outcomes, reduce readmissions, and decrease overall health care costs.2, 12, 13
Medication nonadherence has been linked to patient, disease, and system factors.1, 14, 15 However, studies have largely focused on in‐hospital and postdischarge adherence to CV medications. Few studies have specifically explored long‐term medication‐adherence patterns for patients with stable atherothrombotic disease at an international level, and, specifically, which factors predict adherence.
Methods
The global Reduction of Atherothrombosis for Continued Health (REACH) registry recruited 69 055 consecutive patients from 44 countries in outpatient clinical settings. The methods of the REACH Registry have been well described in prior publications.16, 17, 18 Briefly, patients age >45 years with ≥3 risk factors for atherosclerosis, and patients with established cerebrovascular disease (documented ischemic stroke or transient ischemic attack), coronary artery disease (stable angina, unstable angina, history of percutaneous coronary intervention, history of coronary artery bypass grafting [CABG] or previous myocardial infarction [MI]), and peripheral arterial disease (intermittent claudication, ankle‐brachial index <0.9 or prior intervention), were eligible for enrollment. All participants provided informed consent, and the ethics committees in each country approved the study.
Patient follow‐up was conducted at years 1, 2, 3, and 4 after enrollment. Patients were enrolled between 2003 and 2004 and followed up to 2008. Participating physicians were asked to provide data on subjects' clinical outcomes and medication use.
Study Variables
Primary Outcome
Adherence was defined as meeting class I American College of Cardiology/American Heart Association guidelines for secondary prevention in patients with stable atherothrombotic disease.19, 20 Data relating to medication use were patient self‐reported at the time of study visit and collected centrally via use of a standardized international case‐report form. In patients with a previous history of a cerebrovascular disease or peripheral arterial disease, complete adherence included the use of ≥1 antiplatelet agent (defined as aspirin or other antiplatelet agent), a statin and/or other lipid‐lowering agent, and ≥1 antihypertensive medication if blood pressure was documented as >140/90 mm Hg at the initial visit or >130/80 mm Hg in a patient with diabetes mellitus or chronic kidney disease. For eligible patients, antihypertensive‐agent adherence was defined as use of either angiotensin‐converting enzyme inhibitors, angiotensin II receptor antagonists, β‐blockers, calcium channel blockers, diuretics, nitrates, or other antihypertensive agents. For patients with coronary artery disease, adherence was defined as above in addition to the concomitant use of a β‐blocker. Medication use was assessed at time of enrollment and at each year of follow‐up. Nonadherence was defined as failure to adhere to any of the recommended medications.
Covariates
We explored the association between patient‐level factors and adherence at baseline and at 4 years of follow‐up. Predictors included patient age as a continuous variable and sex. Attained educational level was defined at baseline based on the number of years of formal education completed, defined by 4 categories: 0 to 8 years, 9 to 12 years (or high school), trade or technical school, or university or college. Race/ethnicity was defined by self‐report. Participants were categorized as white, Hispanic, East Asian, South Asian, black, or other. Employment status was characterized as full‐time, part‐time, unemployed, retired, incapacitated, or other according to self‐report at time of enrollment.
Additional covariates included region (North America, Latin America, Western Europe, Eastern Europe, Middle East, and Asia, including Japan). Clinical variables were smoking status (former, current, or never smoker), diabetes mellitus, body mass index, and polyvascular disease vs single vascular disease.
We also explored the association between incident CV events, including myocardial infarction (MI), coronary artery bypass grafting, coronary angioplasty or stenting, and stroke with adherence to evidence‐based medical therapies for secondary prevention.
Statistical Analysis
Analyses were restricted to patients who were eligible for the 4‐year follow‐up study, defined as patients who completed ≥1 postbaseline follow‐up visit and who were enrolled at centers that agreed to participate in the 4‐year study. Patients with missing data for the 4‐year follow‐up study (defined as patient attending visit or death recorded by investigator) and patients without established atherothrombotic disease were excluded from the final analyses. Continuous variables are expressed as means ± SD; categorical variables are expressed as frequencies and percentages.
In a secondary analysis, the study sample was restricted to the US population eligible for follow‐up at 4 years (n = 7210).
To compare categorical variables, χ2 tests of independence were used; 2‐sided t tests were used for continuous variables. Multivariate logistic regression models were conducted to explore the relationship between covariates and adherence at 4 years.
We used SAS version 9.2 (SAS Institute Inc., Cary, NC) for all analyses. All reported P values are 2‐tailed, and values <0.05 were considered statistically significant.
Results
Analyses were derived from 25 737 patients with established atherothrombotic disease who had adherence data at enrollment and at year 4 (Figure 1). At baseline, 12 500 (48.6%) received all classes of medications and were thus deemed complete guideline‐adherent. The baseline characteristics of the study sample are shown in Table 1. Fully adherent patients were likely to be younger, have less polyvascular disease, be of white race, and report full‐time employment as compared with nonadherent patients. Greater adherence was observed in North America, Europe, and the Middle East, whereas participants from Latin America and Asia were less likely to be complete guideline‐adherent.
Figure 1.
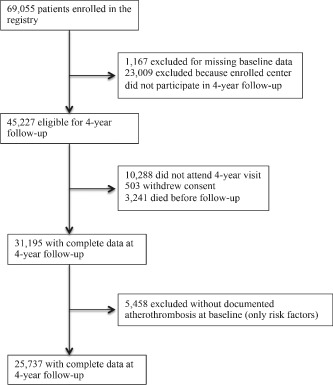
Flow of diagram of study participants.
Table 1.
Study Sample Baseline Characteristics (N = 25 737)
| Fully Adherent, n = 12 500 | Nonadherent, n = 13 237 | P Valuea | |
|---|---|---|---|
| Age, y, mean (SD) | 66.3 (9.7) | 68.7 (9.8) | <0.0001 |
| Male sex, n (%) | 8612 (48.90) | 8999 (51.10) | 0.1146 |
| DM, n (%) | 4407 (48.93) | 4600 (51.07) | 0.4312 |
| PVD, n (%) | 1975 (41.30) | 2807 (58.70) | <0.0001 |
| BMI, kg/m2, mean (SD) | 28.1 (5.0) | 27.1 (5.1) | <0.0001 |
| Region, n (%) | <0.0001 | ||
| North America | 3861 (53.55) | 3349 (46.45) | <0.0001 |
| Latin America | 371 (45.47) | 445 (54.53) | 0.0096 |
| Western Europe | 4456 (52.56) | 4022 (47.44) | <0.0001 |
| Eastern Europe | 1923 (51.51) | 1810 (48.49) | 0.0644 |
| Middle East | 206 (64.98) | 111 (35.02) | <0.0001 |
| Asia | 1683 (32.47) | 3500 (67.53) | <0.0001 |
| Smoking status, n (%) | 0.0009 | ||
| Former | 5549 (50.11) | 5524 (49.89) | 0.8122 |
| Current | 1730 (47.45) | 1916 (52.55) | 0.0021 |
| Never | 4925 (47.86) | 5365 (52.14) | <0.0001 |
| Race/ethnicity, n (%) | <0.0001 | ||
| White | 8077 (52.86) | 7204 (47.14) | <0.0001 |
| Hispanic | 402 (44.08) | 510 (55.92) | 0.0003 |
| East Asian | 1236 (29.58) | 2942 (70.42) | <0.0001 |
| South Asian | 119 (53.13) | 105 (46.88) | 0.3496 |
| Other Asian | 629 (47.98) | 682 (52.02) | 0.1433 |
| Black | 278 (52.06) | 256 (47.94) | 0.3411 |
| Other | 512 (55.96) | 403 (44.04) | 0.0003 |
| Educational attainment, n (%) | <0.0001 | ||
| 0–8 years | 3902 (48.18) | 4196 (51.82) | 0.0011 |
| 9–12 years | 3576 (47.44) | 3962 (52.56) | <0.0001 |
| Trade/technical | 2147 (51.30) | 2038 (48.70) | 0.0920 |
| University | 2319 (50.78) | 2248 (49.22) | 0.2934 |
| Employment status, n (%) | <0.0001 | ||
| Full time | 2450 (53.96) | 2090 (46.04) | <0.0001 |
| Part time | 799 (50.54) | 782 (49.46) | 0.6690 |
| Unemployed | 660 (38.35) | 1061 (61.65) | <0.0001 |
| Retired | 7474 (48.23) | 8024 (51.77) | <0.0001 |
| Incapacitated | 680 (48.99) | 708 (51.01) | 0.4523 |
| Other | 342 (44.71) | 423 (55.29) | 0.0034 |
| Antiplatelet agents, n (%) | |||
| ASA | 10 725 (57.60) | 7894 (42.40) | <0.0001 |
| Other | 4231 (58.22) | 3036 (41.78) | <0.0001 |
| Lipid‐lowering agents, n (%) | |||
| Statins | 11 774 (66.30) | 5984 (33.70) | <0.0001 |
| Other | 1653 (62.40) | 996 (37.60) | <0.0001 |
| CV agents, n (%) | |||
| CCBs | 3682 (41.89) | 5107 (58.11) | <0.0001 |
| β‐Blockers | 10 046 (74.29) | 3477 (25.71) | <0.0001 |
| Nitrates/other antianginal agents | 3593 (48.50) | 3815 (51.50) | 0.8794 |
| Diuretics | 4809 (50.11) | 4788 (49.89) | 0.0001 |
| ACEIs | 6386 (53.84) | 5476 (46.16) | <0.0001 |
| ARBs | 2511 (47.92) | 2729 (52.08) | 0.3239 |
| Other antihypertensives | 907 (44.75) | 1120 (55.25) | 0.0004 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid (aspirin); BMI, body mass index; CABG, coronary artery bypass surgery; CCB, calcium channel blocker; CV, cardiovascular; DM, diabetes mellitus; PVD, polyvascular disease; SD, standard deviation.
P values were derived using the independent sample t test for continuous variables and the χ2 test for categorical values.
Table 2 describes adherence by medication category at each year of follow‐up. Patients reported the highest adherence rates for antihypertensive agents followed by antiplatelet drugs, with similar rates of adherence during each year of follow‐up.
Table 2.
Overall Adherence Rates by Medication Class and Year of Follow‐up
| Overall Adherence, % | Antiplatelet Agents, %a | Lipid‐Lowering Agents, %a | Antihypertensives, %a | β‐Blockers, %b | |
|---|---|---|---|---|---|
| Baseline | 48.57 | 85.80 | 73.83 | 99.11 | 64.13 |
| Year 1 | 49.31 | 85.86 | 74.28 | 97.92 | 65.25 |
| Year 2 | 48.73 | 85.35 | 74.92 | 97.64 | 65.23 |
| Year 3 | 46.70 | 84.32 | 75.63 | 97.16 | 65.02 |
| Year 4 | 47.45 | 84.20 | 76.69 | 96.97 | 65.32 |
Defined as being on ≥1 agent in each class at enrollment and each year of follow‐up.
β‐Blocker adherence rates are based on patients with coronary artery disease.
In unadjusted analyses, predictors of complete adherence at 4 years of follow‐up included younger age, male sex, higher educational attainment, full‐time employment, geographic region, body mass index, polyvascular disease, smoking, and Hispanic, East Asian, and other Asian ethnicity (Table 3). As compared with patients in North America, patients from Latin America and Asia were less likely to be complete guideline‐adherent at 4 years. Similarly, nonfatal incident MI, coronary artery bypass grafting, and coronary angioplasty were strongly associated with full medication adherence, whereas incident stroke was inversely associated with adherence. On multivariate analyses, younger age, region, polyvascular disease, Hispanic and East Asian ethnicity, and current smoking status remained predictive of nonadherence. As compared with patients who did not experience CV events during follow‐up, incident coronary angioplasty and nonfatal MI were predictive of complete guideline adherence (odds ratio [OR]: 2.15, 95% confidence interval [CI]: 1.72‐2.67; and OR: 1.73, 95% CI: 1.25‐2.38, respectively), whereas incident nonfatal stroke was associated with a lower odds of adherence (OR: 0.77, 95% CI: 0.61‐0.97).
Table 3.
Crude and Adjusted Logistic Regression Models of Medication Adherence
| Unadjusted Models | Adjusted Models | |||
|---|---|---|---|---|
| Predictors | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Male sex | 1.11 (1.05‐1.17) | <0.0001 | 1.04 (0.98‐1.12) | 0.2312 |
| Age, ya | 0.97 (0.97‐0.98) | <0.0001 | 0.97 (0.97‐0.98) | <0.0001 |
| Region (ref = North America) | <0.0001 | <0.0001 | ||
| Latin America | 0.67 (0.57‐0.77) | <0.0001 | 0.78 (0.63‐0.96) | 0.0216 |
| Western Europe | 1.05 (0.99‐1.12) | 0.1271 | 0.92 (0.85‐1.00) | 0.0403 |
| Eastern Europe | 1.06 (0.98‐1.12) | 0.1449 | 0.95 (0.86‐1.05) | 0.2920 |
| Middle East | 1.50 (1.19‐1.89) | 0.0005 | 1.39 (1.05‐1.83) | 0.0197 |
| Asia | 0.45 (0.41‐0.48) | <0.0001 | 0.49 (0.40‐0.61) | <0.0001 |
| Race/ethnicity (ref = white) | <0.0001 | <0.0001 | ||
| Hispanic | 0.59 (0.51‐0.67) | <0.0001 | 0.72 (0.59‐0.88) | 0.0015 |
| East Asian | 0.39 (0.37‐0.42) | <0.0001 | 0.67 (0.53‐0.83) | 0.0004 |
| South Asian | 0.99 (0.76‐1.29) | 0.9649 | 1.30 (0.95‐1.78) | 0.0988 |
| Other Asian | 0.71 (0.63‐0.79) | <0.0001 | 0.99 (0.80‐1.23) | 0.9089 |
| Black | 0.85 (0.72‐1.01) | 0.0697 | 0.93 (0.76‐1.15) | 0.5125 |
| Other | 1.09 (0.95‐1.25) | 0.2031 | 1.01 (0.87‐1.18) | 0.8825 |
| Attained educational level (ref = 0–8 years) | 0.0001 | 0.9872 | ||
| 9–12 years | 0.95 (0.89‐1.01) | 0.1012 | 1.01 (0.94‐1.09) | 0.7764 |
| Trade/technical | 1.11 (1.03‐1.20) | 0.0060 | 1.00 (0.92‐1.10) | 0.9401 |
| University | 1.07 (1.00‐1.15) | 0.0651 | 1.00 (0.91‐1.09) | 0.9324 |
| Employment status (ref = full time) | <0.0001 | 0.1032 | ||
| Part time | 0.83 (0.74‐0.94) | 0.0019 | 1.04 (0.91‐1.19) | 0.5879 |
| Unemployed | 0.59 (0.53‐0.66) | <0.0001 | 1.10 (0.96‐1.27) | 0.1679 |
| Retired | 0.77 (0.72‐0.82) | <0.0001 | 1.07 (0.97‐1.17) | 0.1605 |
| Incapacitated | 0.87 (0.77‐0.98) | 0.0210 | 0.90 (0.78‐1.04) | 0.1496 |
| Other | 0.71 (0.61‐0.83) | <0.0001 | 1.17 (0.97‐1.41) | 0.0973 |
| BMI, kg/m2a | 1.03 (1.03‐1.04) | <0.0001 | 1.00 (1.00‐1.01) | 0.2228 |
| Smoking status (ref = never) | 0.0163 | 0.0870 | ||
| Former | 1.07 (1.02‐1.13) | 0.0090 | 1.00 (0.94‐1.07) | 0.9346 |
| Current | 1.08 (1.01‐1.17) | 0.0386 | 0.91 (0.83‐1.00) | 0.0486 |
| DM (ref = no) | 1.00 (0.95‐1.05) | 0.9577 | 1.06 (1.00‐1.13) | 0.0591 |
| PVD (ref = SVD) | 0.70 (0.65‐0.74) | <0.0001 | 0.69 (0.64‐0.74) | <0.0001 |
| Incident CV events | ||||
| CABG | 1.68 (1.21‐2.34) | 0.0019 | 1.20 (0.82‐1.77) | 0.3465 |
| Coronary angioplasty/stenting | 2.30 (1.91‐2.76) | <0.0001 | 2.15 (1.72‐2.67) | <0.0001 |
| Nonfatal MI | 2.05 (1.58‐2.65) | <0.0001 | 1.73 (1.25‐2.38) | 0.0009 |
| Nonfatal stroke | 0.74 (0.61‐0.91) | 0.0041 | 0.77 (0.61‐0.97) | 0.0258 |
Abbreviations: BMI, body mass index; CABG, coronary artery bypass surgery; CI, confidence interval; CV, cardiovascular; DM, diabetes mellitus; MI, myocardial infarction; OR, odds ratio; ref, reference; PVD, polyvascular disease; SVD, single vascular disease.
Per 1‐unit increase.
In a secondary analysis restricted to participants in the United States eligible for 4‐year follow‐up with stable atherothrombotic disease (n = 7210), there was a total of 213 Hispanic and 115 East Asian participants. Hispanic race/ethnicity as compared with white race was associated with a lower likelihood of complete medication adherence (OR: 0.71, 95% CI: 0.52‐0.98). However, East Asian race/ethnicity was no longer associated with adherence (OR: 1.10, 95% CI: 0.73‐1.66).
Discussion
Using an international prospective registry of patients with a broad spectrum of established atherothrombotic disease, we found that <50% of eligible patients were fully adherent with cardioprotective drug regimens over a 4‐year study observation period. We identified several important independent predictors of long‐term adherence to secondary prevention therapies. Complete adherence was associated with younger age and geographic region. Hispanic and East Asian patients were less likely to be adherent at 4 years of follow‐up. We found that patients with polyvascular disease were less adherent as compared with those with single vascular disease, and that current smokers were less likely to be adherent as compared with nonsmokers. On the other hand, incident MI and coronary angioplasty were strongly associated with increased adherence. Notably, patients with incident stroke were less likely to be adherent.
Our findings are consistent with and complementary to prior studies, which have documented the importance of both clinical and demographic variables on patient adherence.1, 4, 8, 14, 15, 21, 22 Similar to other studies,23, 24, 25 age influenced complete guideline adherence, with younger patients reporting greater complete guideline adherence. Older adults are more likely to have more total medications prescribed and are less likely to live independently, both of which are important predictors of medication nonadherence.6 We also found that Hispanic and East Asian participants were less likely to be adherent at 4 years of follow‐up as compared with non‐Hispanic whites. Given the growing problem of health care disparities in cardiovascular disease,26 our finding that certain racial and ethnic groups have lower levels of adherence warrants attention. Medication nonadherence may in fact be an important contributor to the persistent gap in CV outcomes for certain racial/ethnic populations.
Similarly, it is likely that geographic region is driving some of the disparity in adherence for Hispanic and East Asian ethnic groups. The Prospective Urban Rural Epidemiological (PURE) study showed similar geographic variation in rates of self‐reported adherence to recommended therapies for secondary prevention, with the lowest adherence rates in low‐income countries.27 Statins and other medications are particularly costly in developing countries, such as in Latin America, which may limit adherence. There may also be geographic differences in prescribing patterns by providers based on differences in physician perceptions of the risks and benefits of certain drug classes, such as antiplatelet therapy and statins, particularly in East Asian countries.
In a secondary analysis, we attempted to further control for the effect of geographic region on our findings by restricting the study sample to the United States; Hispanic ethnicity remained significantly predictive of lower medication adherence, suggesting an independent association between Hispanic ethnicity and medication adherence. Another possible explanation is that certain racial/ethnic status may be linked with lower understanding of the benefits of continued medication adherence. A study by Roth and colleagues found that black and Hispanic patients had significantly higher rates of medication‐related problems and overall higher rates of nonadherence.28 Adverse cultural beliefs about the perceived benefits of medications and poor provider‐patient relationships have also been cited as reasons for reduced medication adherence among certain patient groups.5, 29, 30
Interestingly, we found a strong association between incident CV events and long‐term adherence. Evidence is mixed about adherence to therapies after a hospitalization for acute coronary syndromes.22, 25, 31 It is likely that patients with an incident event, such as an MI or angioplasty/stenting, will have additional opportunities for medication‐adherence reinforcement. Alternatively, patients who have undergone these procedures may have fewer ischemic symptoms and may therefore perceive less need of adherence to prescribed medications. After adjusting for comorbidities, we found that patients who experienced a nonfatal MI or required coronary angioplasty with or without stent placement were more likely to be fully adherent during follow‐up, whereas patients who suffered from an incident stroke were less likely to be adherent. One could speculate that stroke patients are more likely to suffer from cognitive and physical deficits that may impede complete adherence. Recent studies have suggested that posttraumatic stress disorder (PTSD) may partially explain the high rates of medication nonadherence among stroke survivors.32, 33 Stroke survivors who suffer from PTSD may be more likely to avoid medications because the drugs may serve as reminders of their triggering event. Also, PTSD has been independently linked with cognitive dysfunction, which may in turn lead to medication nonadherence.34 Alternatively, it is possible that stroke patients may have contraindications for certain therapies that we could not account for in our analyses. Because patients with a history of a stroke are at especially high risk for recurrent stroke and other CV events, these patients may benefit from additional resources, such as rehabilitation and case managers, to ensure adequate medication adherence.
To improve complete guideline adherence for secondary prevention of CVD, some have advocated the use of a polypill.35 The Use of a Multidrug Pill in Reducing Cardiovascular Events (UMPIRE) trial used a fixed‐dose combination of aspirin, a statin, and 2 blood pressure–lowering agents to improve guideline‐recommended adherence for secondary prevention of CVD.36 Preliminary results from this trial demonstrated that a polypill was associated with a 33% increase in adherence over a 15‐month period.37 Perhaps such approaches may be particularly useful in reducing the observed geographic guideline‐adherence differences between low‐income and high‐income countries, as a polypill would be associated with reduced dispensing costs of multiple medications. Government subsidies of such fixed‐dose combination pills may also improve adherence. Additionally, patients deemed at high risk for nonadherence, such as the elderly and stroke patients, may benefit from a simplified medical regimen of a single daily pill.
Several limitations warrant mention. Only a subset of the total population was eligible for the 4‐year follow‐up. We defined adherence based on physician and patient self‐report. We did not have access to direct methods, such as observed therapy or measurement of biological markers of the drugs in the blood. However, direct methods are usually not practical in the clinical setting. Self‐report methods may be biased, although we expect this finding to be nondifferential between comparison groups. Gehi and colleagues found that patient self‐report of medication nonadherence was strongly correlated with adverse CV events.38 We were not able to account for medication side‐effects or other important contributors to nonadherence. Similarly, selection bias for both patients and physicians is inherent in registry data, although this is likely to overestimate adherence rates.
This study has several important strengths. First, the study's large size and international representation increase its external validity. Our findings are applicable to a broad spectrum of outpatients with stable atherothrombotic disease followed longitudinally. Most studies on medication adherence have focused on in‐hospital and post–hospital discharge adherence and have not explored long‐term follow‐up in the outpatient setting.
Our study highlights the importance of ascertaining medication adherence at each clinic visit. Recognizing patient‐level characteristics of poor adherence is useful to help physicians and medical providers identify patients who may benefit the most from targeted interventions to improve adherence.
Conclusion
Using a large, international prospective registry of stable outpatients with atherothrombotic disease, we found that age, race/ethnicity, region, and incident CV events were strong, independent predictors of long‐term adherence with medications. These findings suggest that certain patient groups may benefit from targeted interventions to improve adherence.
The REACH Registry is sponsored by sanofi‐aventis, Bristol‐Myers Squibb, and the Waksman Foundation (Tokyo, Japan). No funding was provided for this article.
Author disclosures: C.P. Cannon: Research grants from Accumetrics, AstraZeneca, CSL Behring, Essentialis, GlaxoSmithKline, Merck, Regeneron, Sanofi, and Takeda; participated in consultancy or advisory board for Alnylam, Bristol‐Myers Squibb, Lipimedix, Novartis, and Pfizer; and clinical advisor, equity in Automedics Medical Systems. Ph.G. Steg: Research grant from Servier; participated in consultancy or advisory board for Eisai, Amgen, Astellas, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo–Lilly, GlaxoSmithKline, Merck, Pfizer, Roche, The Medicines Company, AstraZeneca, sanofi‐aventis, and Servier; and a stockholder in Aterovax. D.J. Kumbhani: Honoraria from the American College of Cardiology and Somahlution, Inc. S. Goto: Research grants from sanofi‐aventis, Eisai, and Boehringer Ingelheim; and participated in consultancy or advisory board for Eisai, sanofi‐aventis, and Otsuka. K.A. Eagle: Grant/research support from Bristol‐Myers Squibb, Blue Cross Blue Shield of Michigan, the National Institutes of Health (NIH), sanofi‐aventis, the Mardigian Foundation Varbedian Fund, GORE, and the Hewlett Foundation; and consultant for the NIH National Heart, Lung, and Blood Institute, sanofi‐aventis, and the Robert Wood Johnson Foundation. E.M. Ohman: Research grants from Bristol‐Myers Squibb, CV Therapeutics, Daiichi‐Sankyo, Datascope, Eli Lilly, Marquet, sanofi‐aventis, Schering‐Plough, and The Medicines Company; and consulting or other services for Abiomed, AstraZeneca, CV Therapeutics, Datascope, Gilead Sciences, Liposcience, Marquet, Northpoint Domain, Pozen, Response Biomedical, sanofi‐aventis, The Medicines Company, and WebMD (http://www.theheart.org). D.L. Bhatt: Research grants from Amarin, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Medtronic, sanofi‐aventis, and The Medicines Company; unfunded research for FlowCo, PLx Pharma, and Takeda; participated on advisory board for Elsevier PracticeUpdate Cardiology, Medscape Cardiology, and Regado Biosciences; participated on board of directors for Boston VA Research Institute and the Society of Cardiovascular Patient Care; chair of the American Heart Association Get With The Guidelines Steering Committee; honoraria from the American College of Cardiology (editor, Clinical Trials, CardioSource), Belvoir Publications (editor‐in‐chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), the Population Health Research Institute (clinical trial steering committee), Slack Publications (chief medical editor, Cardiology Today's Intervention), and WebMD (CME steering committees); other: senior associate editor of Journal of Invasive Cardiology and participated on data‐monitoring committees for Duke Clinical Research Institute, Mayo Clinic, and the Population Health Research Institute.
A complete list of investigators can be found in Bhatt DL et al.16
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. [DOI] [PubMed] [Google Scholar]
- 2. Newby LK, LaPointe NM, Chen AY, et al. Long‐term adherence to evidence‐based secondary prevention therapies in coronary artery disease. Circulation. 2006;113:203–212. [DOI] [PubMed] [Google Scholar]
- 3. Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2006;47:2130–2139. [DOI] [PubMed] [Google Scholar]
- 4. Jackevicius CA, Li P, Tu JV. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117:1028–1036. [DOI] [PubMed] [Google Scholar]
- 5. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. [DOI] [PubMed] [Google Scholar]
- 6. Kulkarni SP, Alexander KP, Lytle B, et al. Long‐term adherence with cardiovascular drug regimens. Am Heart J. 2006;151:185–191. [DOI] [PubMed] [Google Scholar]
- 7. Choudhry NK, Setoguchi S, Levin R, et al. Trends in adherence to secondary prevention medications in elderly post–myocardial infarction patients. Pharmacoepidemiol Drug Saf. 2008;17:1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155:772–779. [DOI] [PubMed] [Google Scholar]
- 9. Wijeysundera HC, Machado M, Farahati F, et al. Association of temporal trends in risk factors and treatment uptake with coronary heart disease mortality, 1994–2005. JAMA. 2010;303:1841–1847. [DOI] [PubMed] [Google Scholar]
- 10. Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980‐2000. N Engl J Med. 2007;356:2388–2398. [DOI] [PubMed] [Google Scholar]
- 11. Kumbhani DJ, Steg PG, Cannon CP, et al. Adherence to secondary prevention medications and four‐year outcomes in outpatients with atherosclerosis. Am J Med. 2013;126:693–700. [DOI] [PubMed] [Google Scholar]
- 12. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. [DOI] [PubMed] [Google Scholar]
- 13. Kolandaivelu K, Bhatt DL. Overcoming 'resistance' to antiplatelet therapy: targeting the issue of nonadherence. Nat Rev Cardiol. 2010;7:461–467. [DOI] [PubMed] [Google Scholar]
- 14. Maddox TM, Ho PM. Medication adherence and the patient with coronary artery disease: challenges for the practitioner. Curr Opin Cardiol. 2009;24:468–472. [DOI] [PubMed] [Google Scholar]
- 15. Kumbhani DJ, Fonarow GC, Cannon CP, et al. Predictors of adherence to performance measures in patients with acute myocardial infarction. Am J Med. 2013;126:74.e1–74.e9. [DOI] [PubMed] [Google Scholar]
- 16. Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. [DOI] [PubMed] [Google Scholar]
- 17. Ohman EM, Bhatt DL, Steg PG, et al. The Reduction of Atherothrombosis for Continued Health (REACH) Registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events—study design. Am Heart J. 2006;151:786.e1–786.e10. [DOI] [PubMed] [Google Scholar]
- 18. Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. [DOI] [PubMed] [Google Scholar]
- 19. Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–2372. [DOI] [PubMed] [Google Scholar]
- 20. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276. [DOI] [PubMed] [Google Scholar]
- 21. Baker DW, Hayes R, Fortier JP. Interpreter use and satisfaction with interpersonal aspects of care for Spanish‐speaking patients. Med Care. 1998;36:1461–1470. [DOI] [PubMed] [Google Scholar]
- 22. Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–1847. [DOI] [PubMed] [Google Scholar]
- 23. Avorn J, Monette J, Lacour A, et al. Persistence of use of lipid‐lowering medications a cross‐national study. JAMA. 1998;279:1458–1462. [DOI] [PubMed] [Google Scholar]
- 24. Balkrishnan R. Predictors of medication adherence in the elderly. Clin Ther. 1998;20:764–771. [DOI] [PubMed] [Google Scholar]
- 25. Kulik A, Shrank WH, Levin R, et al. Adherence to statin therapy in elderly patients after hospitalization for coronary revascularization. Am J Cardiol. 2011;107:1409–1414. [DOI] [PubMed] [Google Scholar]
- 26. Mensah GA, Mokdad AH, Ford ES, et al. State of disparities in cardiovascular health in the united states. Circulation. 2005;111:1233–1241. [DOI] [PubMed] [Google Scholar]
- 27. Yusuf S, Islam S, Chow CK, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high‐income, middle‐income, and low‐income countries (the PURE study) a prospective epidemiological survey. Lancet. 2011;378:1231–1243. [DOI] [PubMed] [Google Scholar]
- 28. Roth MT, Esserman DA, Ivey JL, et al. Racial disparities in quality of medication use in older adults: findings from a longitudinal study. Am J Geriatr Pharmacother. 2011;9:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shrank WH. Helping our patients to adhere to chronic medications a new arrow for the quiver. J Gen Intern Med. 2011;26:1394–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bowry AD, Shrank WH, Lee JL, et al. A systematic review of adherence to cardiovascular medications in resource‐limited settings. J Gen Intern Med. 2011;26:1479–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eagle KA, Kline‐Rogers E, Goodman SG, et al. Adherence to evidence‐based therapies after discharge for acute coronary syndromes: an ongoing prospective, observational study. Am J Med. 2004;117:73–81. [DOI] [PubMed] [Google Scholar]
- 32. Edmondson D, Horowitz CR, Goldfinger JZ, et al. Concerns about medications mediate the association of posttraumatic stress disorder with adherence to medication in stroke survivors. Br J Health Psychol. 2013; doi:10.1111/bjhp.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kronish IM, Edmondson D, Goldfinger JZ, et al. Posttraumatic stress disorder and adherence to medications in survivors of strokes and transient ischemic attacks. Stroke. 2012;43:2192–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qureshi SU, Long ME, Bradshaw MR, et al. Does PTSD impair cognition beyond the effect of trauma? J Neuropsychiatry Clin Neurosci. 2011;23:16–28. [DOI] [PubMed] [Google Scholar]
- 35. Lonn E, Bosch J, Teo KK, et al. The polypill in the prevention of cardiovascular diseases: key concepts, current status, challenges, and future directions. Circulation. 2010;122:2078–2088. [DOI] [PubMed] [Google Scholar]
- 36. Thom S, Field J, Poulter N, et al. Use of a Multidrug Pill in Reducing Cardiovascular Events (UMPIRE): rationale and design of a randomised controlled trial of a cardiovascular preventive polypill‐based strategy in India and Europe. Eur J; Prev Cardiol: 2012; doi:10.1177/2047487312463278. [DOI] [PubMed] [Google Scholar]
- 37. Thom S. Use of Multidrug Pill in Reducing Cardiovascular Events (UMPIRE): late‐breaking clinical trials. American Heart Association Scientific Sessions; November 3–7; Los Angeles, CA: doi:10.1161/CIR.0b013e318278c90d.
- 38. Gehi AK, Ali S, Na B, et al. Self‐reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the Heart and Soul Study. Arch Intern Med. 2007;167:1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]


