Abstract
Background
Although most patients with acute pulmonary embolism (PE) remain hospitalized during initial therapy, some may be suitable for partial or complete outpatient management, which may have a significant impact on healthcare costs.
Hypothesis
This article reviews the state‐of‐the‐art data regarding recognition of very‐low‐risk PE patients who are potentially eligible for outpatient treatment, along with the safety, management, and cost‐effectiveness of this strategy. We propose an algorithm based on collected data that may be useful/practical for identifying patients truly eligible for early discharge.
Methods
Comprehensive review of scientific data collected from the MEDLINE and Cochrane databases. Studies selected based on potential scientific interest. Qualitative information extracted regarding feasibility, safety, and cost‐effectiveness of outpatient treatment, postdischarge management, and selection of truly low‐risk patients.
Results
Early discharge of low‐risk patients seems feasible, safe, and particularly cost‐effective. Several risk scores have been developed and/or tested as prediction tools for the recognition of low‐risk individuals: the Pulmonary Embolism Severity Index (PESI), simplified PESI, Hestia criteria, Geneva score, the Low‐Risk Pulmonary Embolism Decision rule, and the Global Registry of Acute Cardiac Events, among others. PESI is the most well‐validated model, offering the safest approach at the current time, especially when combined with additional parameters such as troponin I, N‐terminal prohormone of brain natriuretic peptide, and echocardiographic markers of right‐ventricular dysfunction.
Conclusions
Recognition of truly low‐risk patients entitled to early hospital discharge and outpatient treatment is possible with current risk‐stratification schemes along with selected prognostic parameters, and it may have a colossal impact on healthcare costs.
Introduction
Acute pulmonary embolism (PE) is a common and potentially lethal condition. Despite diagnostic advances in the last 2 decades, delays in PE diagnosis are not infrequent and may associate with increased morbidity and mortality. Massive PE is one of the most prevalent causes of sudden death, and this form of presentation is often the first manifestation of this condition.1 Nevertheless, nonmassive PE is the most common presentation, bearing a much lower mortality rate when appropriate treatment is started early (<5% in the first 3–6 months).2
Some PE patients who were initially considered low risk based on physical and echocardiographic findings and overall comorbidity may experience progressive clinical deterioration, exposing traditional risk‐stratification weaknesses. However, although most patients with acute PE remain hospitalized during initial therapy, some may be suitable for partial or complete outpatient management, which may have a significant positive impact on healthcare costs. As these patients may still be at potential risk for hemorrhagic or recurrent thromboembolic complications during the subsequent months, reliable risk stratification is warranted and, as such, may be considered the cornerstone of PE management.
This article aims to review the state‐of‐the‐art data regarding recognition of very‐low‐risk PE patients who are potentially eligible for outpatient treatment. Furthermore, we propose a prognostic algorithm based on recent clinical research that may be useful and practical for identifying patients truly eligible for early hospital discharge.
Safety and Feasibility of Early Discharge
Several investigators have tested the hypothesis that selected low‐risk patients with acute PE can safely be treated entirely as outpatients or after early hospital discharge. Despite the controversy and complexity of this topic, a systematic review by Janjua et al,3 published in 2008, suggested that carefully selected, compliant low‐risk patients with small or medium‐sized PEs could safely be treated in an outpatient context, as long as they could have easy access to inpatient care, if deemed necessary. For the selection of articles, early discharge was defined as an average hospital stay ≤3 days, including 6 studies where patients were treated entirely as outpatients and 2 investigations where patients were discharged early in the course of the disease. In the former group of studies, recurrent PE occurred in 0% to 6.2% of patients, major bleeding occurred in 0% to 2.8%, and only 1 death was reported (as a result of an intracerebral bleed). In the latter group of studies, there were no episodes of recurrent PE, whereas major bleeding occurred in 0% to 3.7% of patients (causing 1 death).
A more recent meta‐analysis performed by Zondag and colleagues4 compared outpatient treatment with inpatient treatment in low‐risk patients with acute PE. Outcomes were 3‐month recurrent venous thromboembolism (VTE), major bleeding, and all‐cause mortality. The pooled incidence of recurrent VTE was 1.7% in outpatients (1657 patients discharged in <24 hours), 1.1% in patients discharged early (256 individuals discharged within 72 hours), and 1.2% in inpatients (383 patients). Major bleeding occurred in 0.97% of outpatients, in 0.78% of those discharged early, and in 1.0% of inpatients, whereas the pooled incidence of mortality was 1.9% in outpatients, 2.3% in early discharge patients, and 0.74% in inpatients. The authors reported that incidences of recurrent VTE, major bleeding, and, after correction for malignancies, mortality were statistically comparable between outpatients, patients discharged early, and inpatients, concluding that home treatment or early discharge of selected low‐risk patients with PE is as safe as inpatient treatment.
These meta‐analyses suggest that carefully selected low‐risk patients may be discharged early and treated as outpatients, as long as easy and fast access to healthcare is possible. However, these findings should still be accepted with caution, as most studies have not considered some rarer complications of acute PE, such as chronic pulmonary hypertension, post‐thrombotic syndrome, atrial and ventricular arrhythmias, subsequent and unexpected need for vasopressors or thrombolysis, pleural effusion, paradoxical embolism, and side effects other than bleeding associated with anticoagulant therapy, which, although less likely to occur in low‐risk patients, would still be better prevented or treated in an inpatient regimen.
Over the past decade, subcutaneous low‐molecular‐weight‐heparins (LMWH) have replaced much intravenous unfractionated heparin (UFH) therapy and have facilitated outpatient deep‐vein thrombosis (DVT) therapy. However, recently, the new oral anticoagulants (NOAC) dabigatran5 and rivaroxaban6 have emerged as new ambulatory therapeutics for patients with VTE. The EINSTEIN project and the RE‐COVER study were of pivotal importance, supporting the use of new single oral agents with no need for laboratory monitoring and dose adjustment in patients with VTE. Apixaban is currently still being tested in a randomized double‐blind study for the prevention of VTE recurrence or death in patients with DVT or PE (Efficacy and Safety Study of Apixaban for the Treatment of Deep Vein Thrombosis or Pulmonary Embolism). It is hoped that future studies may investigate the feasibility of early discharge in patients treated with one of the NOACs and whether they associate with safer outpatient treatment.
Cost‐Effectiveness of Early Discharge
The overall economic burden of PE in the United States is estimated to be > US$1.5 billion a year in healthcare costs. The Office for Healthcare Economics estimated that the current annual cost in the United Kingdom of treating patients who developed postsurgical DVT and PE is estimated at approximately £640 million.7
Some investigators have studied the cost‐effectiveness of diagnostic and treatment strategies in patients with VTE, although most studies have focused on DVT. Van den Belt et al performed an economic evaluation to assess the cost consequences of an outpatient management strategy with LMWH, concluding that this option would reduce resource utilization directly related to the treatment of DVT and associated costs by 64% (95% confidence interval [CI]: 56%‐72%).8 Rodger and colleagues corroborated these findings in their cost‐effectiveness analysis of treatment strategies for DVT. The authors found that the cost to treat 1 inpatient was $2993 for LMWH and $3048 for UFH, but even more would be saved if LMWH were delivered on an outpatient basis (cost of $1641 per patient). Their cost‐effectiveness analysis showed that LMWH was more cost‐effective than UFH in any treatment setting.9 Gould et al demonstrated that LMWH treatment of acute DVT became cost‐saving when its pharmacy cost could be reduced by ≥31%, when it reduced the yearly incidence of late complications by ≥7%, when ≥8% of patients were treated entirely as outpatients, or when ≥13% were eligible for early discharge.10 Other investigators stated that the LMWH strategy would result in lower costs compared with the UFH strategy when the proportion of patients treated at home was >14%.11 A different study conducted by Canadian investigators revealed cost savings of $3045 per outpatient using LMWH, despite the absence of any major difference in quality of life between the 2 groups (LMWH vs UFH).12 A total cost savings of $1 108 587 with outpatient treatment of acute DVT during a 2‐year program evaluation was reported in a study including 391 patients.13 Likewise, several more recent investigations and a subsequent systematic review have clearly suggested that total direct costs are significantly lower for the outpatient treatment strategy for DVT compared with the inpatient treatment strategy, without any significant negative health impact.14, 15, 16, 17
Conversely, Guanella and colleagues suggested that concomitant PE, unprovoked DVT, development of post‐thrombotic syndrome during follow‐up, and management of DVT in the inpatient setting were independent predictors of increased economic burden in patients with DVT, and, therefore, favoring outpatient care of low‐risk patients with DVT would have the potential to diminish costs.18 Early detection and appropriate treatment of patients at highest risk for recurrent DVT, PE, or post‐thrombotic syndrome would also have the potential for both clinical and economic benefits. Although the economic benefit of treating low‐risk PE individuals as outpatients has not been extensively evaluated (when compared with DVT), Aujesky et al found LMWH treatment of PE to be cost‐saving if ≥8% of patients were eligible for early discharge, or if ≥5% of patients could be treated in an outpatient regimen.19
The NOACs may facilitate an outpatient treatment strategy for low‐risk patients. However, cost analyses are still missing. Although these medications cost the patient considerably more than warfarin, economic analyses will be essential to determine the true cost of anticoagulation with warfarin vs rivaroxaban or dabigatran (for example) when taking into consideration laboratory monitoring, person‐time to adjust doses, medical costs of unintended supra‐ and subanticoagulation, and travel costs. It is noteworthy that some studies have suggested that rivaroxaban may be cost‐saving in the prevention of VTE in patients undergoing total hip or knee replacement, when compared with LMWH.20, 21, 22
Considering the growing evidence that early discharge or outpatient management is safe and feasible in a selected subgroup of low‐risk patients with nonmassive PE, the British Thoracic Society recommended consideration of outpatient treatment for clinically stable patients with PE,23 a measure that could lead to a substantial reduction in healthcare costs.
A reduction in the costs associated with the treatment of truly low‐risk PE patients would allow a diversion of funds for the treatment of those at highest risk for a poorer outcome or for the development of more‐accurate risk‐stratification schemes or novel lifesaving treatments. Furthermore, outpatient management of low‐risk individuals with PE is likely to improve quality and efficiency of care by reducing resource utilization and increasing patient satisfaction.
Exclusion Criteria for Early Discharge
It is widely accepted that patients with clinical or echocardiographic evidence of right ventricular (RV) dysfunction or pressure overload are not candidates for outpatient therapy, irrespective of symptomatology or hemodynamic stability. Moreover, high levels of traditional prognostic biomarkers such as troponin (Tn) and N‐terminal prohormone brain natriuretic peptide (NT‐proBNP) identify a high‐risk cohort that must be followed more closely and, in selected cases, treated more aggressively.
Studies aiming at the identification of truly low‐risk patients should first exclude symptomatic individuals, those who are hemodynamically unstable and/or with clinical or echocardiographic signs of acute RV pressure overload. Contrary to other published studies with similar goals,24, 25, 26 the Low‐Risk Pulmonary Embolism Decision (LR‐PED) rule research group excluded these subgroups of patients, focusing on apparently very‐low‐risk patients.27 Paradoxically, mortality rates reported in the LR‐PED rule derivation cohort were unexpectedly high, which was probably a result of the higher average age and prevalence of most comorbid illnesses when compared with the Pulmonary Embolism Severity Index (PESI) and Geneva scores derivation samples.
Therefore, when evaluating the feasibility of early discharge, attending physicians should look not only at symptomatic status or hemodynamic stability, but also at the degree of comorbidity.
Identifying Truly Low‐Risk Patients
The identification of truly low‐risk patients eligible for outpatient treatment is probably as relevant as identifying high‐risk patients eligible for fibrinolytic therapy. In fact, although the latter quest is definitely a more urgent one and often lifesaving, the huge economic impact of early discharge of low‐risk patients may enable a more profitable distribution of resources to cardiovascular prevention and the management of high‐risk cohorts.
Therefore, reliable risk‐stratification models are a crying need for all physicians caring for patients with acute PE.
Erkens et al suggested that the PESI and simplified PESI scores could accurately identify patients with acute PE who were at low and high risk for short‐term adverse events.28 A total of 118 (48.6%) and 81 (33.3%) patients were classified as low risk using the original and simplified PESI prognostic models, respectively, and none of the low‐risk patients died within the 3‐month period of follow‐up. However, 30% to 47% of patients with a high‐risk PESI score were safely managed as outpatients as well, suggesting a suboptimal sensitivity and negative predictive value for prognostic assessment. Venetz et al studied 15 531 patients with PE, comparing the proportions of patients classified as low‐risk vs higher‐risk between the original and simplified PESI and estimated 30‐day mortality within each risk group.29 The authors concluded that, although the simplified PESI could accurately identify patients at low risk of adverse outcomes, the original PESI classified a higher proportion of patients as low‐risk and had a greater discriminatory power than the simplified PESI.
Jiménez et al compared the discriminatory prognostic power of PESI and Geneva scores and concluded that the first one quantified the prognosis of patients with PE better than Geneva and allowed the selection of patients with very low adverse event rates during the initial days of acute PE therapy.30
Some authors have demonstrated that the 48‐hour recalculation of the PESI or simplified PESI scores in patients admitted for acute PE could more accurately help identify low‐risk patients eligible for early discharge and outpatient treatment.31 Serial calculation of the PESI or simplified PESI scores and a decision on potential discharge at the 24‐ to 48‐hour mark could be a comprehensive risk‐stratification strategy with higher certainty of safety.
Although Moores and colleagues suggested TnI values do not add prognostic power to PESI in terms of low‐risk patient identification,26 Lenkeit et al did not corroborate that idea.32 In fact, the latter study concluded that high‐sensitivity TnT (hs‐TnT) assay may yield additive prognostic information beyond PESI. In their normotensive cohort with acute PE, both hs‐TnT ≥14 pg/mL and simplified PESI ≥1 point(s) emerged, besides renal failure, as independent predictors of early death and complications. Twenty‐four percent of the study sample were identified as low risk by a simplified PESI of 0 and hs‐TnT <14 pg/mL, and none of them had an adverse 30‐day outcome.
The LR‐PED rule was developed by our research group.27 This preliminary new scoring system primarily designed for the detection of patients potentially eligible for early discharge and treatment in the outpatient setting favorably compared with current gold‐standard prognostic stratification scores in PE, namely simplified PESI and Geneva, showing higher sensitivity and negative predictive value for the detection of the lowest‐risk patients, with a false‐negative rate of 0% in our cohort. The net reclassification improvement index unveiled very significant successful upward reclassification of patients who reached primary or secondary outcomes (45% of patients reaching the primary endpoint were correctly reclassified by LR‐PED into higher‐risk groups, a finding highly significant). Although the small sample size and the as‐yet lack of validation in an independent patient sample mitigates its current clinical applicability, the LR‐PED rule has shed some light into the importance of combining analytical parameters (TnI, creatinine, C‐reactive protein, and glycemia) and focusing on rhythm alongside heart rate when selecting patients for outpatient treatment. In this regard, a recent article has unveiled the independent prognostic value of atrial fibrillation in patients with PE.33
The Hestia study was a prospective cohort study of patients with proven acute PE triaged with predefined criteria for eligibility for outpatient treatment with nadroparin followed by vitamin K antagonists (VKA).34 Triaging for outpatient treatment of PE was carried out using a 11‐point questionnaire (including questions regarding symptomatic status, hemodynamic stability, bleeding risk, oxygen saturation, renal and liver function, pregnancy, history of heparin‐induced thrombocytopenia, social support, and previous anticoagulant treatment). Only patients for whom the answer was “no” to each question were allowed to be treated at home. All 297 included patients were sent home either immediately or within 24 hours after PE was objectively diagnosed. Outpatient treatment was evaluated with respect to recurrent VTE (2.0%), including PE (1.7%) or DVT (0.3%); major hemorrhage (0.6%); and total mortality (1.0%) during 3 months of follow‐up. The authors concluded that patients with PE selected for outpatient treatment with predefined criteria could be treated with anticoagulants on an outpatient basis.
Zondag et al compared the performance of the Hestia criteria with that of simplified PESI,35 concluding that both schemes selected low‐risk patients with high sensitivity and negative predictive values for 30‐day mortality. Although the 2 scores classified different patients eligible for outpatient treatment with similar accuracy, the former could potentially identify a proportion of high‐risk simplified PESI patients who could be safely treated at home. Surprisingly, the Hestia criteria have been helpful in selecting patients, including those with RV dysfunction, who have very low risk for adverse outcome and could be candidates for outpatient treatment.36 In fact, of the patients treated at home according to the Hestia criteria, 35% were normotensive but had RV dysfunction and were classified as intermediate risk, according to the European Society of Cardiology criteria. Nevertheless, no adverse events occurred in these patients treated at home.
The clinical versatility of the Global Registry of Acute Cardiac Events (GRACE) risk score has been demonstrated by Paiva et al in their retrospective observational cohort study of 206 consecutive PE patients.37 Although widely used to estimate mortality risk in patients with a myocardial infarction, the authors assessed its applicability in PE, suggesting that it could predict improve risk stratification when compared with the Geneva score, the shock index, the European Society of Cardiology score, and simplified PESI. Importantly, no adverse outcomes were observed in patients with a GRACE score ≤113, suggesting potential utility in the selection of truly low‐risk individuals entitled to outpatient treatment.
A different study has suggested that out‐of‐hospital treatment is safe in hemodynamically stable patients with PE with low (<500 pg/mL) NT‐proBNP levels, as no deaths, major bleeding complications, or recurrent VTE occurred in the first 10 days of treatment or in the follow‐up period of 3 months in 152 (out of 351) patients fulfilling these inclusion criteria.
Some less‐common parameters have shown promising results as predictors of a complicated outcome in a wide range of cardiovascular diseases, including acute PE. In particular, the growth‐differentiation factor 15, a stress‐responsive member of the transforming growth factor‐β cytokine superfamily, has been shown to independently predict complicated 30‐day outcome in patients with PE, enhancing the predictive value of TnT, NT‐proBNP, and echocardiographic findings of RV dysfunction.38 Its role in the detection of low‐risk patients eligible for outpatient treatment is mostly unknown at the time. The same idea is probably valid for RV strain and strain rate assessment. Although several studies have suggested a potential role for strain imaging in the prognostication of patients with acute PE,39, 40, 41 none has tried to demonstrate its utility in the selection of low‐risk patients potentially eligible for outpatient treatment. However, as RV dysfunction is clearly associated with increased mortality, it seems wise to suggest that the detection of even subclinical dysfunction through RV strain analysis should preclude early discharge.
Moreover, although the potential role of contrast‐enhanced multidetector computed tomography as a prognosticator in PE has been the subject of several studies in the last few years (the RV/left ventricular diameter ratio, proximal superior vena cava diameter, pulmonary artery obstruction index, ventricular septal bowing, and embolic burden are among the parameters studied as potential predictors of mortality),42, 43, 44, 45, 46, 47, 48, 49 to this date no consensus has been reached regarding which radiological parameters can predict mortality with the highest discriminative performance. Most importantly, no study has clarified whether the addition of radiological parameters is able to improve PE risk stratification beyond standard risk models such as PESI or the Hestia criteria.
Unsolved Questions
Several unanswered questions reinforce the need for further studies. The safety and feasibility of early discharge in patients treated with one of the NOACs are still not clearly established. Moreover, the incidence of less‐common adverse events (such as post‐thrombotic syndrome, chronic pulmonary hypertension, pleural effusion, paradoxical embolism, minor bleeding) and subsequent complications in patients treated at home is unknown at the moment. The potential applicability of strain imaging in the selection of low‐risk patients eligible for outpatient treatment deserves some attention, as it would be useful to determine whether subclinical RV dysfunction (detectable through strain imaging) precludes safe early discharge. Furthermore, it is not determined whether the routine 24‐ to 48‐hour reapplication of risk‐stratification schemes would translate into improved clinical outcomes. The best prognostic cutoffs for TnI/TnT and NT‐proBNP and the safety of early discharge in the mid‐ to long‐term are also unsolved questions. Hopefully, future studies will address these subjects.
Algorithm for the Selection of Truly Low‐Risk Patients
The Table 1 lists currently used or proposed risk‐stratification schemes potentially applicable for the selection of low‐risk PE patients eligible for early discharge and/or outpatient treatment.
Table 1.
Currently Available Prognostic Scores for the Selection of Low‐Risk Patients With PE
| Risk Score | Advantages | Disadvantages |
|---|---|---|
| PESI | The most well‐validated risk score in this context (including consistent prospective validation)61, 62 | Exclusive use of dichotomous variables may oversimplify prognostic assessment. |
| Large derivation sample | A significant percentage of patients assigned to the high‐risk category can still be safely managed as outpatients.28 | |
| Outperforms the Geneva score in prognostic assessment30 | Requires computation of a score based on 11 variables, each with a different weight | |
| Accurate in both high‐ and low‐risk patient detection28 | ||
| Serial calculation and a decision on potential discharge at the 24‐ to 48‐hour mark may be an even more accurate risk‐stratification strategy with higher certainty of safety.31 | ||
| Simplified PESI | Easier to use than original PESI score | Although applied to a group of patients with prospectively collected data, simplified PESI has not been validated in a prospective sample. |
| Similar63 or slightly lower29 accuracy as the original PESI in prognostic assessment | Exclusive use of dichotomous variables | |
| Accurately identifies patients at low risk for adverse events63 | ||
| Easily usable bedside prediction | ||
| Hestia criteria | Prospectively derived score34 | Small size of derivation sample |
| Accurately detects patients at low‐risk of adverse events | ||
| May identify a proportion of simplified PESI high‐risk patients who can be safely treated as outpatients35 | ||
| May identify a proportion of patients with RV dysfunction who can be safely treated as outpatients36 | ||
| Easily usable bedside prediction | ||
| Geneva | Useful for assessing clinical probability of pulmonary embolism64 | Variables have different weights, which may lead to miscalculations in an acute setting. |
| Easily usable bedside prediction | Primarily developed for diagnostic purposes | |
| Prognostic value consistently outperformed by PESI30, 65 | ||
| Simplified Geneva66 | Similar diagnostic accuracy of original Geneva score but easier to use | Developed for diagnostic purposes |
| Does not require arterial blood‐gas sample to be collected | Probably outperformed by PESI (although not directly compared) | |
| LR‐PED rule27 | The only score derived from a cohort of apparently low‐risk patients | Lack of proper validation (retrospective or prospective) |
| Detects truly low‐risk patients with very high accuracy | Small size of derivation sample | |
| The first score to demonstrate the importance of rhythm alongside heart rate33 | Requires a regression equation and a calculator | |
| GRACE37 | The most comprehensive risk score available; applicable to different clinical contexts | Lack of proper validation (retrospective or prospective) in the context of an acute PE |
| Detects truly low‐risk patients (GRACE score <113) with very high accuracy | Small size of derivation sample | |
| The first score to include ECG parameters | Requires a calculator | |
| Shock index | Very easy to calculate | Extremely reductive |
| Most patients are considered low risk | ||
| Limited accuracy in the selection of low‐risk patients67 | ||
| Agterof et al68 | Very easily applicable (only 4 variables) | Lack of proper validation (retrospective or prospective) |
| Very low 10‐day adverse event rate in low‐risk patients | Small size of derivation sample | |
| Uresandi et al69 | Prospective derivation in a multicenter registry | Lack of proper validation |
| High accuracy in the selection of low‐risk patients | Exclusion of admission hemodynamic parameters (although reliably explained by the authors) | |
| Included minor bleeding (complication with significant impact in patients' well‐being) | ||
| Easily usable bedside prediction |
Abbreviations: ECG, electrocardiographic; GRACE, Global Registry of Acute Cardiac Events; LR‐PED, Low‐Risk Pulmonary Embolism Decision; PE, pulmonary embolism; PESI, Pulmonary Embolism Severity Index; RV, right ventricular.
The Figure suggests an easily applicable decision algorithm for the selection of truly low‐risk patients eligible for outpatient treatment, based on previous research. This algorithm requires validation in prospective multicenter studies and should supplement, but not replace, clinical judgment.
Postdischarge Management
Pulmonary embolism–associated morbidity and mortality go beyond the initial acute phase, as it may associate with long‐term complications such as recurrent PE and chronic thromboembolic pulmonary syndrome. Up to 40% of patients develop a VTE recurrence within 10 years of the primary event,50 whereas chronic thromboembolic pulmonary syndrome is diagnosed in 4% within 2 years of the index event.51
The optimal management of low‐risk patients following early discharge is unknown, as no recommendations or clinical guidelines have addressed the issue. In fact, although patients eligible for early discharge and outpatient treatment are at lower short‐ and mid‐term risk, no study to date has evaluated their long‐term risk of complications, and, therefore, they must be followed as closely as those individuals treated as inpatients.
Following the acute management of PE, secondary treatment aims to prevent thromboembolic recurrences. Anticoagulant therapy is recommended for ≥3 months in most patients.52 Further extension of anticoagulation lacks evidence‐based data, and physicians need to rely on their clinical judgment, taking into consideration the risk for recurrent VTE and the risk for anticoagulant‐induced bleeding. Overlap in risk factors for bleeding and recurrent thrombosis may add complexity to therapeutic decisions. The risk of thromboembolic recurrence is greatest during the first 6 months after the initial event and decreases thereafter, but without ever reaching nil.53 Risk varies greatly among patients and is influenced by many factors, including the presence/absence of identifiable triggers (ie, trauma, thrombophilic abnormalities), the characteristics of the index event (presence and the location of distal venous thrombosis, number of previous events), and patient demographic and clinical features (age, body mass index, and male sex).53, 54 After anticoagulant withdrawal, abnormal plasma levels of D‐dimer may be the strongest analytical predictor of VTE recurrence. To help guide risk stratification, some risk‐prediction scores have been developed, such as the Vienna Risk Prediction Model55 and the D‐dimer, age, sex, and hormonal therapy (DASH) score.56 However, they have not been prospectively validated and therefore cannot be adopted into clinical practice for the time being.
The traditional long‐term PE management options comprise VKAs and LMWH. Warfarin is the most‐used oral VKA, yet its slow onset of action, narrow therapeutic window, and multiple food and drug interactions require frequent medical monitoring and dose adjustment. Furthermore, it may associate with worrisome bleeding episodes, especially in patients with active cancer.57 Low‐molecular weight heparins (eg, enoxaparin, dalteparin) have several advantages over VKAs, as they display a rapid onset and offset of action, predictable dose–response, fewer drug‐drug interactions, and a potential net benefit in patients with active cancer, and they usually require no routine monitoring of anticoagulant effect. However, they are mainly limited by their parenteral route of administration, affecting patients' comfort and convenience.
The role of the NOACs (eg, dabigatran and rivaroxaban) for the long‐term management of VTE has been recently established.5, 6 These agents seem as effective in reducing the risk for recurrent VTE as VKAs but may associate with lower bleeding risk. Compared with warfarin, the NOACs have a more rapid onset of anticoagulant effect (1–4 vs 72–96 hours), may eliminate the need for parenteral anticoagulant bridge therapy for the treatment of VTE, have fewer drug and food interactions, have a more predictable therapeutic effect, and need no routine anticoagulation monitoring. As a result, these drugs may facilitate the earlier discharge of lower‐risk patients.
Nevertheless, as previously stated, clinicians must be aware of their patients' risk of bleeding. Although the NOACs appear to have a lower risk for major bleeding compared with traditional agents, controlled trials that support the use of NOACs have excluded subgroups of patients at increased risk for bleeding, such as those in need of concomitant use of antiplatelet therapy, with significant renal and hepatic impairment, and who use medications that interfere with homeostasis (eg, nonsteroidal anti‐inflammatory drugs), and very elderly patients with multiple comorbidities (ie, cancer, history of gastrointestinal bleeding, alcohol abuse). Thus, the true clinical net benefit of these new agents in real‐life anticoagulant users remains to be clarified. From the Computerized Registry of Patients With Venous Thromboembolism (RIETE) Registry data,58 a bleeding‐risk score was developed and validated in patients with acute VTE for predicting major bleeding within 3 months of anticoagulant therapy, using 6 variables: age >75 years, recent major bleeding, cancer, renal function, anemia, and PE. This model may help clinicians in the quantification of their patients' bleeding risk. Patients eligible for outpatient treatment have been shown to be at lower risk of PE recurrence and mortality, and we could be tempted to conclude that their bleeding risk is somewhat lower as well, due to their overall lower comorbidity burden. However, the hemorrhagic risk of this subgroup of patients has not been thoroughly addressed, and we should not forget that their outpatient treatment regimen may preclude an early detection of bleeding episodes. Preference should be given to drugs with higher safety profile in regard to bleeding.
Figure 1.
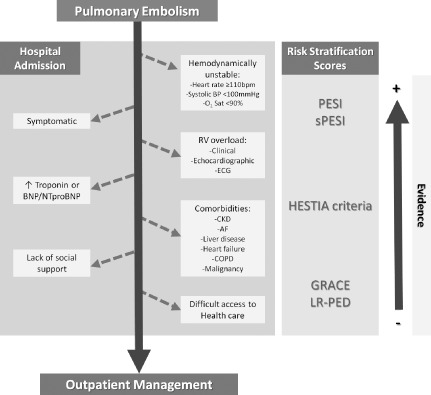
Decision algorithm for the selection of truly low‐risk patients eligible for outpatient treatment, based on previous research. COPD step should include other clinically significant respiratory conditions. Abbreviations: AF, atrial fibrillation; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease.
Despite the evidence supporting anticoagulant therapy in secondary PE management, there may be a potential role for antiplatelet therapy, particularly aspirin. The controlled trials Warfarin and Aspirin (WARFASA)59 and Aspirin for the Prevention of Recurrent Venous Thromboembolism (ASPIRE)60 evaluated the possibility of using low‐dose aspirin for the long‐term prevention of recurrent symptomatic VTE after initial oral anticoagulation therapy. The former trial reported a significant 42% annual reduction of VTE recurrence compared with placebo (6.6% vs 11.2% per year, P = 0.02) and no difference in the incidence of bleeding complications. Although a significant reduction of VTE recurrence was not shown in the ASPIRE study (6.5% vs 4.8% per year, P = 0.09), the combined analysis of both trials demonstrated that aspirin reduces the rate of VTE recurrence by 32% (P = 0.007), without significantly increased bleeding risk. Notwithstanding, VTE risk reduction accomplished with aspirin is about 2 × −3× lower than that achieved by anticoagulants (warfarin, as well as the NOACs).6 Considering the lower short‐ to mid‐term risk of VTE recurrence in patients eligible for outpatient treatment, aspirin might be a reasonable choice for those at higher hemorrhagic risk or who refuse any form of anticoagulant treatment.
Even low‐risk patients should be subjected to extensive etiological investigation if deemed appropriate, as PE may recur or be associated with an undiagnosed condition.
Conclusion
A fast and accurate identification of high‐risk PE patients eligible for thrombolytic therapy may be lifesaving. Conversely, the recognition of truly low‐risk patients entitled to early hospital discharge and outpatient treatment must not be underrated, as it may have a colossal impact on healthcare costs and enable a diversion of funds for the treatment of those at highest risk for a poorer outcome or for the development of more accurate risk‐stratification schemes or novel lifesaving treatments.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Lucena J, Rico A, Vázquez R, et al. Pulmonary embolism and sudden‐unexpected death: prospective study on 2477 forensic autopsies performed at the Institute of Legal Medicine in Seville. J Forensic Leg Med. 2009;16:196–201. [DOI] [PubMed] [Google Scholar]
- 2. Meyer G, Planquette B, Sanchez O. Long‐term outcome of pulmonary embolism. Curr Opin Hematol. 2008;15:499–503. [DOI] [PubMed] [Google Scholar]
- 3. Janjua M, Badshah A, Matta F, et al. Treatment of acute pulmonary embolism as outpatients or following early discharge: a systematic review. Thromb Haemost. 2008;100:756–761. [PubMed] [Google Scholar]
- 4. Zondag W, Kooiman J, Klok F, et al. Outpatient versus inpatient treatment in patients with pulmonary embolism: a meta‐analysis [published online ahead of print October 25, 2012]. Eur Respir J. doi: 10.3410/f.717962387.793468524. [DOI] [PubMed] [Google Scholar]
- 5. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–2352. [DOI] [PubMed] [Google Scholar]
- 6. Investigators EINSTEIN; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. [DOI] [PubMed] [Google Scholar]
- 7. UK House of Commons, Health Committee , Second Report. The Prevention of Venous Thromboembolism in Hospitalised Patients http://www.publications.parliament.uk/pa/cm200405/cmselect/cmhealth/99/9902.htm. Published February 23, 2005. Accessed February 2, 2013.
- 8. Van den Belt AG, Bossuyt PM, Prins MH, et al; TASMAN Study Group. Replacing inpatient care by outpatient care in the treatment of deep venous thrombosis—an economic evaluation. Thromb Haemost. 1998;79:259–263. [PubMed] [Google Scholar]
- 9. Rodger M, Bredeson C, Wells PS, et al. Cost‐effectiveness of low‐molecular‐weight heparin and unfractionated heparin in treatment of deep vein thrombosis. CMAJ. 1998;159:931–938. [PMC free article] [PubMed] [Google Scholar]
- 10. Gould MK, Dembitzer AD, Sanders GD, et al. Low‐molecular‐weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis: a cost‐effectiveness analysis. Ann Intern Med. 1999;130:789–799. [DOI] [PubMed] [Google Scholar]
- 11. Estrada CA, Mansfield CJ, Heudebert GR. Cost‐effectiveness of low‐molecular‐weight heparin in the treatment of proximal deep vein thrombosis. J Gen Intern Med. 2000;15:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Brien B, Levine M, Willan A, et al. Economic evaluation of outpatient treatment with low‐molecular‐weight heparin for proximal vein thrombosis. Arch Intern Med. 1999;159:2298–2304. [DOI] [PubMed] [Google Scholar]
- 13. Tillman DJ, Charland SL, Witt DM. Effectiveness and economic impact associated with a program for outpatient management of acute deep vein thrombosis in a group model health maintenance organization. Arch Intern Med. 2000;160:2926–2932. [DOI] [PubMed] [Google Scholar]
- 14. Spyropoulos AC, Hurley JS, Ciesla GN, et al. Management of acute proximal deep vein thrombosis: pharmacoeconomic evaluation of outpatient treatment with enoxaparin vs inpatient treatment with unfractionated heparin. Chest. 2002;122:108–114. [DOI] [PubMed] [Google Scholar]
- 15. Segal JB, Bolger DT, Jenckes MW, et al. Outpatient therapy with low molecular weight heparin for the treatment of venous thromboembolism: a review of efficacy, safety, and costs. Am J Med. 2003;115:298–308. [DOI] [PubMed] [Google Scholar]
- 16. Bäckman K, Carlsson P, Kentson M, et al. Deep venous thrombosis: a new task for primary health care. A randomised economic study of outpatient and inpatient treatment. Scand J Prim Health Care. 2004;22:44–49. [DOI] [PubMed] [Google Scholar]
- 17. Othieno R, Abu Affan M, Okpo E. Home versus in‐patient treatment for deep vein thrombosis. Cochrane Database Syst Rev. 2007;3:CD003076. [DOI] [PubMed] [Google Scholar]
- 18. Guanella R, Ducruet T, Johri M, et al. Economic burden and cost determinants of deep vein thrombosis during 2 years following diagnosis: a prospective evaluation. J Thromb Haemost. 2011;9:2397–2405. [DOI] [PubMed] [Google Scholar]
- 19. Aujesky D, Smith KJ, Cornuz J, et al. Cost‐effectiveness of low‐molecular‐weight heparin for treatment of pulmonary embolism. Chest. 2005;128:1601–1610. [DOI] [PubMed] [Google Scholar]
- 20. Ryttberg L, Diamantopoulos A, Forster F, et al. Cost‐effectiveness of rivaroxaban versus heparins for prevention of venous thromboembolism after total hip or knee surgery in Sweden. Expert Rev Pharmacoecon Outcomes Res. 2011;11:601–615. [DOI] [PubMed] [Google Scholar]
- 21. Duran A, Sengupta N, Diamantopoulos A, et al. Cost effectiveness of rivaroxaban versus enoxaparin for prevention of post‐surgical venous thromboembolism from a U.S. payer's perspective. Pharmacoeconomics. 2012;30:87–101. [DOI] [PubMed] [Google Scholar]
- 22. McCullagh L, Tilson L, Walsh C, et al. A cost‐effectiveness model comparing rivaroxaban and dabigatran etexilate with enoxaparin sodium as thromboprophylaxis after total hip and total knee replacement in the Irish healthcare setting. Pharmacoeconomics. 2009;27:829–846. [DOI] [PubMed] [Google Scholar]
- 23. British Thoracic Society Standards of Care Committee Pulmonary Embolism Guideline Development Group . British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax. 2003;58:470–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wicki J, Perrier A, Perneger TV, et al. Predicting adverse outcome in patients with acute pulmonary embolism: a risk score. Thromb Haemost. 2000;84:548–552. [PubMed] [Google Scholar]
- 25. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moores L, Aujesky D, Jiménez D, et al. Pulmonary Embolism Severity Index and troponin testing for the selection of low‐risk patients with acute symptomatic pulmonary embolism. J Thromb Haemost. 2010;8:517–522. [DOI] [PubMed] [Google Scholar]
- 27. Barra S, Paiva L, Providência R, et al. LR‐PED Rule: Low‐Risk Pulmonary Embolism Decision Rule—a new decision score for low‐risk pulmonary embolism. Thromb Res. 2012;130:327–333. [DOI] [PubMed] [Google Scholar]
- 28. Erkens PM, Gandara E, Wells PS, et al. Does the Pulmonary Embolism Severity Index accurately identify low risk patients eligible for outpatient treatment? Thromb Res. 2012;129:710–714. [DOI] [PubMed] [Google Scholar]
- 29. Venetz C, Jiménez D, Mean M, et al. A comparison of the original and simplified Pulmonary Embolism Severity Index. Thromb Haemost. 2011;106:423–428. [DOI] [PubMed] [Google Scholar]
- 30. Jiménez D, Yusen RD, Otero R, et al. Prognostic models for selecting patients with acute pulmonary embolism for initial outpatient therapy. Chest. 2007;132:24–30. [DOI] [PubMed] [Google Scholar]
- 31. Moores L, Zamarro C, Gómez V, et al; for the IRYCIS Pulmonary Embolism Study Group. Changes in PESI score predict mortality in intermediate‐risk patients with acute pulmonary embolism. Eur Respir J. 2013;41:354–359. [DOI] [PubMed] [Google Scholar]
- 32. Lankeit M, Jiménez D, Kostrubiec M, et al. Predictive value of the high‐sensitivity troponin T assay and the simplified Pulmonary Embolism Severity Index in hemodynamically stable patients with acute pulmonary embolism: a prospective validation study. Circulation. 2011;124:2716–2724. [DOI] [PubMed] [Google Scholar]
- 33. Barra SN, Paiva LV, Providência R, et al. Atrial fibrillation in acute pulmonary embolism: prognostic considerations [published online ahead of print January 24, 2013]. Emerg Med J. doi:10.1136/emermed‐2012‐202089. [DOI] [PubMed]
- 34. Zondag W, Mos IC, Creemers‐Schild D, et al; Hestia Study Investigators. Outpatient treatment in patients with acute pulmonary embolism: the Hestia Study. J Thromb Haemost. 2011;9:1500–1507. [DOI] [PubMed] [Google Scholar]
- 35. Zondag W, den Exter PL, Crobach MJ, et al; for the Hestia Study Investigators. Comparison of two methods for selection of out of hospital treatment in patients with acute pulmonary embolism. Thromb Haemost. 2013;109:47–52. [DOI] [PubMed] [Google Scholar]
- 36. Zondag W, Vingerhoets LM, Durian MF, et al; Hestia Study Investigators. Hestia criteria can safely select patients with pulmonary embolism for outpatient treatment irrespective of right ventricular function. J Thromb Haemost. 2013;11:686–692. [DOI] [PubMed] [Google Scholar]
- 37. Paiva LV, Providência RC, Barra SN, et al. Cardiovascular risk assessment of pulmonary embolism with the GRACE risk score. Am J Cardiol. 2013;111:425–431. [DOI] [PubMed] [Google Scholar]
- 38. Lankeit M, Kempf T, Dellas C, et al. Growth differentiation factor‐15 for prognostic assessment of patients with acute pulmonary embolism. Am J Respir Crit Care Med. 2008;177:1018–1025. [DOI] [PubMed] [Google Scholar]
- 39. Stergiopoulos K, Bahrainy S, Strachan P, et al. Right ventricular strain rate predicts clinical outcomes in patients with acute pulmonary embolism. Acute Card Care. 2011;13:181–188. [DOI] [PubMed] [Google Scholar]
- 40. Platz E, Hassanein AH, Shah A, et al. Regional right ventricular strain pattern in patients with acute pulmonary embolism. Echocardiography. 2012;29:464–470. [DOI] [PubMed] [Google Scholar]
- 41. Descotes‐Genon V, Chopard R, Morel M, et al. Comparison of right ventricular systolic function in patients with low risk and intermediate‐to‐high risk pulmonary embolism: a two‐dimensional strain imaging study. Echocardiography. 2013;30:301–308. [DOI] [PubMed] [Google Scholar]
- 42. Araoz PA, Gotway MB, Harrington JR, et al. Pulmonary embolism: prognostic CT findings. Radiology. 2007;242:889–897. [DOI] [PubMed] [Google Scholar]
- 43. Qanadli SD, El Hajjam M, Vieillard‐Baron A, et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001;176:1415–1420. [DOI] [PubMed] [Google Scholar]
- 44. Van der Meer RW, Pattynama PM, van Strijen MJ, et al. Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3‐month follow‐up in patients with acute pulmonary embolism. Radiology. 2005;235:798–803. [DOI] [PubMed] [Google Scholar]
- 45. Ghuysen A, Ghaye B, Willems V, et al. Computed tomographic pulmonary angiography and prognostic significance in patients with acute pulmonary embolism. Thorax. 2005;60:956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schoepf UJ, Kucher N, Kipfmueller F, et al. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110:3276–3280. [DOI] [PubMed] [Google Scholar]
- 47. Rodrigues B, Correia H, Figueiredo A, et al. Clot burden score in the evaluation of right ventricular dysfunction in acute pulmonary embolism: quantifying the cause and clarifying the consequences [article in Portuguese]. Rev Port Cardiol. 2012;31:687–695. [DOI] [PubMed] [Google Scholar]
- 48. Ghanima W, Abdelnoor M, Holmen LO, et al. The association between the proximal extension of the clot and the severity of pulmonary embolism (PE): a proposal for a new radiological score for PE. J Intern Med. 2007;261:74–81. [DOI] [PubMed] [Google Scholar]
- 49. Wu AS, Pezzullo JA, Cronan JJ, et al. CT pulmonary angiography: quantification of pulmonary embolus as a predictor of patient outcome‐initial experience. Radiology. 2004;230:831–835. [DOI] [PubMed] [Google Scholar]
- 50. Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism: a prospective cohort study in 1626 patients. Haematologica. 2007;92:199–205. [DOI] [PubMed] [Google Scholar]
- 51. Pengo V, Lensing AWA, Prins MH, et al; for the Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–2264. [DOI] [PubMed] [Google Scholar]
- 52. Kearon C, Akl EA, Comerota AJ, et al; Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis. 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines [published correction appears in Chest . 2012;142:1698–1704]. Chest. 2012;141(2 suppl):e419S–e494S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Agnelli G, Becattini C. Treatment of DVT: how long is enough and how do you predict recurrence. J Thromb Thrombolysis. 2008;25:37–44. [DOI] [PubMed] [Google Scholar]
- 54. Heit JA. Predicting the risk of venous thromboembolism recurrence. Am J Hematol. 2012;87:S63–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eichinger S, Heinze G, Jandeck LM, et al. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. 2010;121:1630–1636. [DOI] [PubMed] [Google Scholar]
- 56. Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost. 2012;10:1019–1025. [DOI] [PubMed] [Google Scholar]
- 57. Palareti G, Legnani C, Lee A, et al. A comparison of the safety and efficacy of oral anticoagulation for the treatment of venous thromboembolic disease in patients with or without malignancy. Thromb Haemost. 2000;84:805–810. [PubMed] [Google Scholar]
- 58. Ruíz‐Giménez N, Suárez C, González R, et al; RIETE Investigators. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100:26–31. [DOI] [PubMed] [Google Scholar]
- 59. Becattini C, Agnelli G, Schenone A, et al; for the WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959–1967. [DOI] [PubMed] [Google Scholar]
- 60. Brighton TA, Eikelboom JW, Mann K, et al; for the ASPIRE Investigators. Low‐dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367:1979–1987. [DOI] [PubMed] [Google Scholar]
- 61. Donzé J, Le Gal G, Fine MJ, et al. Prospective validation of the Pulmonary Embolism Severity Index: a clinical prognostic model for pulmonary embolism. Thromb Haemost. 2008;100:943–948. [DOI] [PubMed] [Google Scholar]
- 62. Aujesky D, Perrier A, Roy PM, et al. Validation of a clinical prognostic model to identify low‐risk patients with pulmonary embolism. J Intern Med. 2007;261:597–604. [DOI] [PubMed] [Google Scholar]
- 63. Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170:1383–1389. [DOI] [PubMed] [Google Scholar]
- 64. Chunilal SD, Eikelboom JW, Attia J, et al. Does this patient have pulmonary embolism? JAMA. 290:2849–2858. [DOI] [PubMed] [Google Scholar]
- 65. Zwierzina D, Limacher A, Méan M, et al. Prospective comparison of clinical prognostic scores in elderly patients with pulmonary embolism. J Thromb Haemost. 2012. doi: 10.1111/j.1538‐7836.2012.04929.x. [DOI] [PubMed] [Google Scholar]
- 66. Klok FA, Mos IC, Nijkeuter M, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008;168:2131–2136. [DOI] [PubMed] [Google Scholar]
- 67. Sam A, Sánchez D, Gómez V, et al. The shock index and the simplified PESI for identification of low‐risk patients with acute pulmonary embolism. Eur Respir J. 2011;37:762–766. [DOI] [PubMed] [Google Scholar]
- 68. Agterof MJ, Schutgens RE, et al. A prognostic model for short term adverse events in normotensive patients with pulmonary embolism. Am J Hematol. 2011;86:646–649. [DOI] [PubMed] [Google Scholar]
- 69. Uresandi F, Otero R, Cayuela A, et al. [‐A clinical prediction rule for identifying short‐term risk of adverse events in patients with pulmonary thromboembolism]. Arch Bronconeumol. 2007;43:617–622. [DOI] [PubMed] [Google Scholar]


