Abstract
Novel oral anticoagulants, including dabigatran, rivaroxaban, and apixaban, represent new options for preventing stroke in patients with atrial fibrillation, as shown by the results from large, randomized phase III trials. Because of their greater specificity, rapid onset of action, and predictable pharmacokinetics, the novel oral anticoagulants (dabigatran, rivaroxaban, and apixaban) address several limitations of warfarin or other vitamin K antagonists in day‐to‐day clinical practice. However, a range of practical questions relating to the novel oral anticoagulants has emerged, including topics such as patient selection, treatment of patients with renal impairment, risk of myocardial infarction, drug interactions, switching between anticoagulants, and management of bleeding, in addition to use of these agents in patients requiring antiplatelet drug treatment or undergoing cardioversion or percutaneous interventions (eg, ablation). In this review, practical aspects of the use of novel oral anticoagulants in patients with atrial fibrillation are discussed, with reference to available data and guidance from prescribing information.
Introduction
Atrial fibrillation (AF) is a major cause of morbidity and mortality. In particular, because of the potential for thrombus formation within the left atrial appendage and resulting emboli that can occlude cerebral vessels, AF increases the risk of stroke 5‐fold and is associated with 15% to 20% of all strokes.1, 2 Strokes in patients with AF are likely to be more severe than in patients without AF, as shown by higher death rates, a lower likelihood of being discharged to home, and a greater risk of functional or neurologic deficits.3, 4 Because AF is predominantly a disease of elderly patients (prevalence rates: 10%–20% in those age ≥85 years vs 0.4%–1.0% in those age 55–60 years), the overall prevalence of AF is predicted to more than double by 2050, in line with the aging population.5, 6, 7
To reduce the risk of stroke or systemic embolism, oral anticoagulant (OAC) therapy is recommended in all patients with AF who have additional risk factors.8, 9 For more than 50 years, vitamin K antagonists (VKAs) such as warfarin were the only OACs available. Meta‐analysis data have shown that warfarin reduces the occurrence of stroke in patients with AF by 64% compared with controls.10 However, VKAs have a range of well‐known limitations, including a slow onset of action, need for dose adjustments and regular monitoring to ensure that patients remain within a narrow therapeutic range, dietary restrictions, and multiple interactions with other drugs. These limitations prompted the development of novel OACs that are now entering clinical practice for patients with AF. Although these agents have clear advantages compared with VKAs, several questions need to be considered regarding the use of novel OACs in clinical practice and what type of differences exist between agents.
The aims of this review were to introduce briefly the novel OACs and their clinical characteristics and to discuss practical considerations relating to day‐to‐day use.
Clinical Trial Findings and Characteristics of Novel Oral Anticoagulants for Patients With Atrial Fibrillation
Novel OACs that are available for the prevention of stroke in patients with AF include dabigatran, rivaroxaban, and apixaban. Compared with warfarin, the novel OACs have greater specificity (single vs multiple targets within the clotting cascade), a more rapid onset of action (time to peak concentration: 2–4 hours vs 72–96 hours), shorter half‐lives (5–17 hours vs 40 hours), considerably fewer interactions with other drugs, and no food interactions or dietary restrictions (Table 1).11, 12, 13 Because they have more predictable pharmacokinetic characteristics than warfarin, novel OACs can be administered using fixed doses and do not require routine coagulation monitoring. Unlike warfarin, each of the novel OACs is eliminated renally to differing degrees,13 which has important implications that will be discussed.
Table 1.
| Warfarin | Dabigatran | Rivaroxaban | Apixaban | |
|---|---|---|---|---|
| Target | Synthesis of vitamin K‐dependent clotting factors (factors II, VII, IX, and X) | Thrombin | Factor Xa | Factor Xa |
| Bioavailability | >95% | ∼6% | >80% | >50% |
| Time to peak activity | 72–96 hours | 2 hours | 2.5–4 hours | 3 hours |
| Half‐life | 40 hours | 14–17 hours | 5–9 hours (young healthy patients),11–13 hours (elderly patients) | 8–15 hours |
| Dosing frequency in patients with AF | Once daily | Twice daily | Once daily | Twice daily |
| Interactions | Numerous drugs including substrates of CYP2C9, CYP3A4, and CYP1A2; various foods | Strong P‐gp inhibitors and inducers | Strong CYP3A4 inducers, strong inhibitors of both CYP3A4 and P‐gp | Strong inhibitors/inducers of both CYP3A4 and P‐gp |
| Renal elimination (absorbed active drug) | <1% | ∼80% | ∼33%a | ∼27% |
Abbreviations: AF, atrial fibrillation; CYP, cytochrome P450; P‐gp, P‐glycoprotein.
An additional 33% of the absorbed rivaroxaban dose inactivated in the liver is also eliminated renally.
Four phase III trials of dabigatran, rivaroxaban, or apixaban in patients with nonvalvular AF have been completed: RE‐LY (Randomized Evaluation of Long‐term anticoagulation therapY), ROCKET AF (Rivaroxaban Once daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation), ARISTOTLE (Apixaban for Reduction In STroke and Other ThromboemboLic Events in atrial fibrillation), and AVERROES (Apixaban VErsus acetylsalicylic acid to Reduce the Risk Of Embolic Stroke).14, 15, 16, 17 In RE‐LY, ROCKET AF, and ARISTOTLE, a novel OAC (dabigatran, rivaroxaban, and apixaban, respectively) was compared with warfarin. In AVERROES, apixaban was compared with acetylsalicylic acid (ASA). ROCKET AF, ARISTOTLE, and AVERROES were all double‐blind, double‐dummy trials, whereas RE‐LY was open label between the dabigatran and warfarin arms but double blind between the 2 dabigatran arms (110 mg and 150 mg twice daily [bid]).18, 19, 20, 21
The warfarin‐based trials of novel OACs had important differences that preclude direct comparisons.18, 19, 21 In particular, patients with enrolled in ROCKET AF were, on average, at higher risk of thromboembolic events than patients enrolled in the phase III trials of dabigatran or apixaban. The ROCKET‐AF trial enrolled patients who had had a prior stroke, transient ischemic attack (TIA), or systemic embolism, or otherwise had at least 2 other risk factors used to calculate their CHADS2 score (ie, congestive heart failure and/or left ventricular ejection fraction of ≤35%, hypertension, age ≥75 years, or diabetes mellitus).8, 18 Furthermore, the proportion of patients in ROCKET AF with only 2 risk factors not including prior stroke/TIA/systemic embolism was limited to 10% (per protocol); remaining patients were required to either have had a prior stroke/TIA/systemic embolism or have 3 or more risk factors.18 In contrast, in the RE‐LY and ARISTOTLE trials, patients at least 1 risk factor were enrolled.19, 21 As a result, the mean CHADS2 score was higher in ROCKET AF (3.5) compared with the mean scores in RE‐LY and ARISTOTLE (both 2.1).14, 15, 16 Among patients in the warfarin arm of each trial, the mean time spent in the therapeutic range (TTR) for the international normalized ratio (INR) (2.0–3.0) was lower in ROCKET AF (55%) than in RE‐LY (64%) and ARISTOTLE (62%), although subgroup analyses from these studies found that rates of primary efficacy events did not differ across quartiles defined by mean TTR of each study center.15, 16, 22 In an analysis of secondary outcomes in the RE‐LY study, such as vascular events, nonhemorrhagic events, and mortality, the advantages of dabigatran 150 mg bid were greater at sites with a low mean TTR and reduced at sites with a high mean TTR.23
Broadly similar efficacy and safety trends were reported for each of the novel OACs compared with warfarin (Table 2). In the intention‐to‐treat populations, an overall risk reduction in the primary end point of stroke or systemic embolism was reported for each novel OAC compared with warfarin, which was noninferior for rivaroxaban and dabigatran 110 mg and superior for apixaban and dabigatran 150 mg.14, 15, 16, 22 For rivaroxaban, the annual rate of stroke or systemic embolism in the intention‐to‐treat analysis was significantly lower than for warfarin during treatment (hazard ratio [HR]: 0.79; 95% confidence interval [CI]: 0.66‐0.96), but was noninferior when events occurring both on and off study treatment were included (HR: 0.88; 95% CI: 0.75‐1.03).15 In safety analyses, rates of major bleeding events were similar (rivaroxaban and dabigatran 150 mg) or lower (apixaban and dabigatran 110 mg) compared with warfarin. However, all novel OACs significantly reduced the rate of intracranial hemorrhage (ICH) compared with warfarin. Each novel OAC showed a trend for a reduced risk of all‐cause mortality compared with warfarin of approximately 10% to 15%, which reached statistical significance for apixaban.15, 16, 22 Subgroup analyses showed that the favorable efficacy and safety of novel OACs was sustained in patients with previous stroke or TIA.24, 25, 26 However, the effect of novel OACs in patients with a recent stroke has not been established because the studies excluded patients who had had an acute stroke within 2 weeks (RE‐LY, ROCKET AF) or 1 week (ARISTOTLE) of randomization, or severe disabling stroke within 3 to 6 months (RE‐LY, ROCKET AF) of randomization.15, 18, 19, 21
Table 2.
Summary of Key Patient Characteristics and Findings From the Phase III Trials of Novel Oral Anticoagulants in Patients With Nonvalvular Atrial Fibrillation14, 15, 16, 17, 22
| RE‐LY | ROCKET AF | ARISTOTLE | AVERROES | ||
|---|---|---|---|---|---|
| Novel OAC examined | Dabigatran | Rivaroxaban | Apixaban | Apixaban | |
| Comparator drug | Warfarin | Warfarin | Warfarin | Acetylsalicylic acid | |
| Patients | 18 113 | 14 264 | 18 201 | 5599 | |
| Mean or median age, y | Mean: 71 | Median: 73 | Median: 70 | Mean: 70 | |
| Mean CHADS2 score | 2.1 | 3.5 | 2.1 | 2.0–2.1 | |
| Prior vitamin K antagonist treatment, % | 50 | 62 | 57 | 15 | |
| Prior stroke or transient ischemic attack, % | 20a | 55 | 19a | 14 | |
| Mean TTR, warfarin arm; % | 64 | 55 | 62 | N/A | |
| Novel OAC dosing arm | 110 mg bid | 150 mg bid | 20 mg odb | 5 mg bidc | 5 mg bidc |
| Relative risk (95% CI) for novel OAC versus comparator | |||||
| Stroke or systemic embolism (ITT population) | 0.90 (0.74‐1.10) | 0.65 (0.52‐0.81) | 0.88 (0.75‐1.03) | 0.79 (0.66‐0.96) | 0.45 (0.32‐0.62) |
| Major bleeding | 0.80 (0.70‐0.93) | 0.93 (0.81‐1.07) | 1.04 (0.90‐1.20) | 0.69 (0.60‐0.80) | 1.13 (0.74‐1.75) |
| Intracranial hemorrhage | 0.30 (0.19‐0.45) | 0.41 (0.28‐0.60) | 0.67 (0.47‐0.93) | 0.42 (0.30‐0.58) | 0.85 (0.38‐1.90) |
| Myocardial infarction | 1.29 (0.96‐1.75) | 1.27 (0.94‐1.71) | 0.81 (0.63‐1.06) | 0.88 (0.66‐1.17) | 0.86 (0.50‐1.48) |
| Death | 0.91 (0.80‐1.03) | 0.88 (0.77‐1.00) | 0.85 (0.70‐1.02) | 0.89 (0.80‐0.99) | 0.79 (0.62‐1.02) |
Abbreviations: ARISTOTLE, Apixaban for Reduction In STroke and Other ThromboemboLic Events in atrial fibrillation; AVERROES, Apixaban VErsus acetylsalicylic acid to Reduce the Risk Of Embolic Stroke; bid, twice daily; CHADS2, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke or transient ischemic attack (2 points); CI, confidence interval; ITT, intention to treat; N/A, not available; OAC, oral anticoagulant; od, once daily; RE‐LY, Randomized Evaluation of Long‐term anticoagulation therapy; ROCKET AF, Rivaroxaban Once daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation; TTR, time in therapeutic range for international normalized ratio.
Prior stroke, transient ischemic attack, or systemic embolism.b15 mg od in patients with creatinine clearance 30 to 49 mL/min. c2.5 mg bid in patients with 2 or more of the following criteria: age ≥80 years, body weight ≤60 kg, or serum creatinine ≥1.5 mg/dL (≥133 µmol/L).
The AVERROES trial was terminated early because of a treatment benefit in favor of apixaban compared with ASA. For the primary end point, the rate of stroke or systemic embolism was reduced by more than 50% with apixaban compared with ASA (Table 2). Rates of major bleeding events or ICH showed no significant difference.17
Overall, phase III trials confirm that dabigatran, rivaroxaban, and apixaban are viable alternatives to previous therapies for the prevention of stroke or systemic embolism in patients with nonvalvular AF and that they may also have potential benefits. Dabigatran and rivaroxaban have been approved for the treatment of patients with nonvalvular AF by various regulatory authorities, including those in Europe and the United States.27, 28, 29, 30 Apixaban was also recently approved in Europe and in the United States for prevention of stroke and systemic embolism in patients with nonvalvular AF.31, 32
Practical Considerations for the Use of Novel Oral Anticoagulants in Stroke Prevention
Several practical questions have emerged that are relevant to the day‐to‐day use of novel OACs. The remainder of this review will discuss areas of debate with reference to available data and European/United States prescribing information for dabigatran, rivaroxaban, and apixaban.
Guidelines
In 2012, there were significant updates to anticoagulation guidelines, particularly with respect to use of the newer oral antithrombotic agents to prevent stroke in patients with AF. A recent scientific advisory update from the American Heart Association/American Stroke Association now recommends rivaroxaban and apixaban in addition to already recommended dabigatran and warfarin to prevent a first or recurrent stroke in patients with AF.33 More specific guidance is provided regarding dosing adjustments for the novel OACs based on renal function and individual patient stroke risk factors. Warfarin remains the preferred antithrombotic in patients at moderate to high risk of stroke who can safely receive a VKA. In contrast, based on improved benefit–risk profiles and convenience, the 2012 European Society of Cardiology (ESC) guideline updates recommend that any of the 3 novel OACs be considered instead of VKAs in most patients with nonvalvular AF who should receive oral anticoagulation (Figure 1).34 Dose‐adjustment recommendations for rivaroxaban and dabigatran based on bleeding risk and renal function also feature in the update. The 2012 Canadian Cardiovascular Society guidelines also recommend dabigatran, rivaroxaban, and apixaban in preference to warfarin for patients in whom oral anticoagulation is indicated.35 The ninth edition of the American College of Chest Physicians clinical practice guidelines36 and the 2011 American College of Cardiology Foundation/American Heart Association/Heart Rhythm Society Task Force focused update37 currently only recommend dabigatran 150 mg bid as an alternative to warfarin in patients with nonvalvular AF. However, at the time these guidelines were written, this was the only novel OAC and dose that was available.
Figure 1.
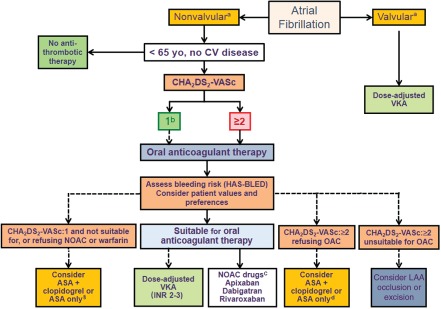
Selection of stroke prevention therapies in patients with nonvalvular atrial fibrillation (AF) and risk factors based on the CHA2DS2‐VASc score (modified from the 2012 focused update of the European Society of Cardiology Guidelines for the management of atrial fibrillation). aValvular AF is defined as AF related to the presence of rheumatic disease (predominantly mitral stenosis) or a prosthetic valve. bFemale patients with lone AF age ≤65 years with no other stroke risk factors are considered “truly low risk,” and no antithrombotic therapy should be considered. cBecause of its low antithrombotic efficacy, acetylsalicylic acid (ASA) alone should be considered only in patients unable to tolerate ASA + clopidogrel. dNovel oral anticoagulants (OACs) are listed in alphabetical order; when using novel OACs, renal function monitoring (at least yearly) should be performed. Dashed lines: less preferable or less well‐validated. Abbreviations: CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥75 years (2 points), diabetes mellitus, stroke or transient ischemic attack (2 points), vascular disease, age 65 to 74 years, sex category (female); CV, cardiovascular; HAS‐BLED, hypertension, abnormal renal/liver function (1 point each), stroke, bleeding history or predisposition, labile INR, elderly (≥65 years old), drugs/alcohol concomitantly (1 point each); INR, international normalized ratio; LAA, left atrial appendage; NOAC, novel oral anticoagulant; VKA, vitamin K antagonist.
Who Should Receive Novel Oral Anticoagulants?
Dabigatran, rivaroxaban, and apixaban are treatment options for the prevention of stroke and systemic embolism in adult patients with nonvalvular AF who have at least 1 additional risk factor. Depending on overall patient presentation, novel OACs may be most appropriate for patients who are unable or unwilling to take a VKA or who have an unstable INR during VKA therapy (Table 3). However, contraindications and regulatory recommendations for novel OACs should be considered (Table 4). For example, dabigatran, rivaroxaban, and apixaban should not be prescribed to patients with active pathological bleeding or severely impaired renal function (creatinine clearance [CrCl] <15 mL/min); in Europe, dabigatran is also contraindicated in patients with CrCl <30 mL/min. Dabigatran should not be prescribed to patients with liver enzyme levels elevated 2‐fold above the upper limit of normal.27, 28 Rivaroxaban should not be prescribed to patients with severe hypersensitivity reaction to the drug or hepatic disease associated with coagulopathy and clinically relevant bleeding risk (including Child‐Pugh B or C).29, 30 Apixaban is contraindicated in patients with severe hypersensitivity reaction to the drug. In addition, the European Summary of Product Characteristics lists hepatic disease associated with coagulopathy and clinically relevant bleeding risk, lesion or condition at significant risk of major bleeding, or concomitant treatment with any other anticoagulant agent (except under specific circumstances such as switching) as contraindications.38, 39
Table 3.
Suitability of Different Groups of Patients With Atrial Fibrillation for Treatment With Novel Oral Anticoagulants
| Patient Group | Suitability for Novel OAC | Comments |
|---|---|---|
| Eligible for oral anticoagulation, unwilling or unable to take a VKA | Novel OAC should be considered. | May include patients with allergy or increased sensitivity to VKAs, or those not receiving VKA because of fear of bleeding (particularly ICH). |
| Eligible for oral anticoagulation, naïve to VKA (newly diagnosed) | All available options, including VKAs and novel OACs, should be considered. | Factors influencing therapeutic choice should include contraindications for novel OACs (eg, creatinine clearance <15 mL/min; see Table 4) and costs (direct and indirect). |
| Receiving VKA with unstable INR | Novel OAC should be considered depending on reason for INR instability. | If INR is unstable because of nonadherence, novel OAC therapy may not be an improvement over VKA therapy. If INR is unstable because of drug or food/alcohol interactions, novel OAC therapy may be beneficial. If INR is frequently higher than the therapeutic range, novel OAC therapy may be beneficial to reduce the risk of ICH. |
| Receiving VKA with stable INR | Limited justification for novel OAC (in the absence of other factors). |
Benefits of VKAs are greater in patients with good INR control. Transition between anticoagulants requires careful management. Dabigatran is associated with gastrointestinal tolerability issues. |
Abbreviations: ICH, intracranial hemorrhage; INR, international normalized ratio; OAC, oral anticoagulant; VKA, vitamin K antagonist.
Table 4.
Summary of Guidance for Treatment of Patients With Nonvalvular Atrial Fibrillation With Dabigatran or Rivaroxaban From European Medicines Agency Summaries of Product Characteristics and US Food and Drug Administration Prescribing Information27, 28, 29, 30, 38, 39
| Dabigatran | Rivaroxaban | Apixaban | |
|---|---|---|---|
| Recommended dose | 150 mg bid | 20 mg od (must be taken with food) | 5 mg bid |
| Alternative doses | EMA: 110 mg bid in patients ≥80 years old or if receiving verapamil; consider 110 mg bid when thromboembolic risk is low and bleeding risk is high. | EMA: 15 mg od if CrCl is 15–49 mL/min. | EMA: 2.5 mg bid for patients with serum creatinine 1.5 mg/dL (133 micromole/L) associated with age 80 years or body weight 60 kg 2.5 mg bid for patients with creatinine clearance 15–29 mL/min |
| FDA: 75 mg bid if CrCl is 15–30 mL/min; consider 75 mg bid if CrCl is 30–50 mL/min and patient is receiving dronedarone or systemic ketoconazole. | FDA: 15 mg od if CrCl is 15–50 mL/min. | FDA: 2.5 mg bid for patients with 2 of the following: age ≥80 years, body weight ≤60 kg, or serum creatinine ≥1.5 mg/dL. | |
| Assessment of renal function | Renal function should be assessed before initiating dabigatran and periodically during treatment. | ||
| EMA: Renal function should be assessed at least once per year or more frequently as needed; in clinical situations, renal function could decline or deteriorate (eg, hypovolemia, dehydration, and with certain comedications). | |||
| FDA: Periodically assess renal function as clinically indicated (ie, more frequently in clinical situations that may be associated with a decline in renal function) and adjust therapy accordingly. | |||
| Contraindications or patient groups in which use is not recommended | EMA: CrCl <30 mL/min | CrCl <15 mL/min; clinically significant active bleeding; hepatic disease associated with coagulopathy and clinically relevant bleeding risk (including Child‐Pugh B and C); age <18 years; pregnancy or breast feeding; hypersensitivity to the active substance or excipients. | EMA: CrCl <15 mL/min; clinically significant active bleeding; hepatic disease associated with coagulopathy and clinically relevant bleeding risk; hypersensitivity to the active substance or excipients; lesion or condition at significant risk of major bleeding; concomitant treatment with any other anticoagulant agent. |
| FDA: No dosing recommendations if CrCl is <15 mL/min; clinically significant active bleeding; organic lesion at risk of bleeding; spontaneous or pharmacologic impairment of hemostasis; hepatic impairment or liver disease expected to have any impact on survival; liver enzymes elevated 2‐fold higher than the upper limit of normal; age <18 years; hypersensitivity to the active substance or excipients. | FDA: Active pathological bleeding, severe hypersensitivity. | ||
| Use with caution (close monitoring for bleeding/anemia is needed) | EMA: Patients with moderate renal impairment (CrCl 30–50 mL/min), body weight <50 kg, or age ≥75 years; patients with an increased bleeding risk (eg, congenital or acquired coagulation disorders; thrombocytopenia or functional platelet defects; active ulcerative GI disease; recent GI bleeding; recent biopsy or major trauma; recent intracerebral hemorrhage; brain, spinal, or ophthalmic surgery; bacterial endocarditis. | EMA: CrCl 15–29 mL/min, patients with an increased bleeding risk (eg, congenital or acquired bleeding disorders; uncontrolled severe arterial hypertension; active ulcerative GI disease; recent GI ulcerations; vascular retinopathy; recent intracranial or intracerebral hemorrhage; intraspinal or intracerebral vascular abnormalities; recent brain, spinal, or ophthalmic surgery; bronchiectasis or history of pulmonary bleeding. | EMA: All patients should be closely monitored for hemorrhage risk; take care with concomitant antiplatelet therapy; surgery and invasive procedures (discontinue apixaban 48 hours prior); neuraxial anesthesia; renal and hepatic impairment; elderly patients (≥80 years); drug interactions. |
| FDA: Can cause serious, potentially fatal bleeding. Prompt evaluation of signs and symptoms of blood loss required. Prosthetic heart valves are not recommended. | |||
| Special warnings | FDA: Because of an increased risk of thrombotic events, consider administering another anticoagulant if rivaroxaban needs to be discontinued for any reason other than pathological bleeding. | FDA: If anticoagulation with apixaban must be discontinued for a reason other than pathological bleeding, coverage with another anticoagulant should be strongly considered. | |
| Drugs that should not be administered concomitantly (all patients) | EMA: P‐gp inducers (eg, rifampicin, St. John's wort, carbamazepine, or phenytoin), HIV protease inhibitors (eg, ritonavir); contraindicated: systemic ketoconazole, cyclosporine, itraconazole, tacrolimus, and dronedarone. | EMA: Strong inhibitors of both P‐gp and CYP3A4 such as azole antimycotics (eg, ketoconazole, itraconazole, voriconazole, and posaconazole), HIV protease inhibitors (eg, ritonavir). |
EMA: Strong inhibitors of both CYP3A4 and P‐gp, such as azole antimycotics (eg, ketoconazole, itraconazole, voriconazole, and posaconazole) and HIV protease inhibitors (eg, ritonavir); strong inducers of both CYP3A4 and P‐gp (eg, rifampicin, phenytoin, carbamazepine, phenobarbital, or St. John's wort). |
| FDA: P‐gp inducers (eg, rifampin), P‐gp inhibitors (eg, dronedarone or systemic ketoconazole). | FDA: Combined P‐gp and strong CYP3A4 inhibitors (eg, ketoconazole, itraconazole, lopinavir/ritonavir, ritonavir, indinavir/ritonavir, and conivaptan); combined P‐gp and strong CYP3A4 inducers (eg, carbamazepine, phenytoin, rifampin, St. John's wort). | FDA: Strong dual inducers of CYP3A4 and P‐gp (eg, rifampin, carbamazepine, phenytoin, St. John's wort). | |
| Drugs that should not be administered concomitantly in patients with renal impairment | FDA: P‐gp inhibitors (eg, systemic ketoconazole, dronedarone, verapamil, amiodarone, quinidine, and clarithromycin) if CrCl is <30 mL/min. | ||
| Drugs requiring caution during concomitant treatment (all patients) | EMA: Some strong P‐gp inhibitors (may increase dabigatran concentrations) (eg, amiodarone, verapamil, quinidine, or clarithromycin); drugs that may increase risk of bleeding (eg, antiplatelet agents, heparin, fibrinolytic therapy, or NSAIDs). | EMA: Strong CYP3A4 inducers (may reduce rivaroxaban concentrations) (eg, rifampin/rifampicin, St. John's wort, carbamazepine, phenytoin, phenobarbital); drugs that may increase risk of bleeding (eg, NSAIDs, ASA, platelet aggregation inhibitors, or other antithrombotic agents). | FDA: Dosage reduction recommended (to 2.5 mg bid) when coadministered with strong dual inhibitors of CYP3A4 and P‐gp (eg, ketoconazole, itraconazole, ritonavir, or clarithromycin). |
| Drugs requiring caution during concomitant treatment in patients with renal impairment | EMA: Potent inhibitors of CYP3A4 (may increase rivaroxaban concentrations) (eg, clarithromycin, telithromycin). | EMA: Strong inhibitors of both CYP3A4 and P‐gp, such as azole antimycotics (eg, ketoconazole, itraconazole, voriconazole, and posaconazole) and HIV protease inhibitors (eg, ritonavir) are not recommended in patients with severe renal impairment. | |
| FDA: Combined P‐gp inhibitors and weak/moderate CYP3A4 inhibitors (may increase rivaroxaban concentrations) (eg, amiodarone, diltiazem, verapamil, quinidine, ranolazine, dronedarone, felodipine, erythromycin, and azithromycin); these agents should only be used in patients with renal impairment if the potential benefit justifies the potential risk. | FDA: No mention. | ||
| Guidance for switching from a VKA | Discontinue VKA and start dabigatran when INR is <2.0. | Discontinue VKA and start rivaroxaban when INR is ≤3.0 (EMA) or <3.0 (FDA). | EMA: When converting from a VKA to apixaban, discontinue warfarin or other VKA therapy and start apixaban when INR is <2.0. |
| FDA: Discontinue warfarin and start apixaban when INR is <2.0. | |||
| Guidance for switching to a VKA | VKA starting time should be based on CrCl; if CrCl is ≥50 mL/min, start VKA 3 days before discontinuing dabigatran; if CrCl is 30–<50 mL/min, start VKA 2 days before discontinuing dabigatran. | EMA: VKA and rivaroxaban should be given concurrently until the INR is ≥2.0; standard initial VKA dosing should be given for the first 2 days of the conversion period, followed by INR‐guided VKA dosing; INR should not be tested earlier than 24 hours after the previous rivaroxaban dose. | EMA: When converting patients to a VKA, continue apixaban for at least 2 days after beginning VKA therapy. After 2 days of coadministration, obtain an INR prior to the next scheduled dose of apixaban and continue coadministration until the INR is ≥2.0. |
| FDA: If CrCl is 15–30 mL/min, start VKA 1 day before discontinuing dabigatran. | FDA: Discontinue rivaroxaban and begin both a parenteral anticoagulant and a VKA at the time the next dose of rivaroxaban would have been taken. | FDA: INR measurements during coadministration with warfarin may not be useful for determining appropriate warfarin dose. If continuous anticoagulation is necessary, discontinue apixaban and then begin both a parenteral anticoagulant and warfarin at the time the next dose of apixaban would have been taken, discontinuing the parenteral anticoagulant when INR reaches an acceptable range. | |
| Missed dose | No double dose should be taken to make up for a missed dose | EMA: The dose should not be doubled within the same day to make up for a missed dose. | The dose should not be doubled to make up for a missed dose. |
| If a dose is not taken at the scheduled time, the dose should be taken as soon as possible on the same day; the missed dose should be skipped if it cannot be taken at least 6 hours before the next scheduled dose. | If a dose is missed, the patient should take the dose immediately and continue on the following day with the once daily intake as recommended. | If the drug is not taken at the scheduled time, the dose should be taken as soon as possible on the same day and twice daily administration should be resumed. | |
| FDA: If a dose is not taken at the scheduled time, administer the dose as soon as possible on the same day. For patients receiving 20 or 15 mg od: the patient should take the missed dose immediately |
Abbreviations: AF, atrial fibrillation; ASA, acetylsalicylic acid; bid, twice daily; CrCl, creatinine clearance; CYP, cytochrome P450; EMA, European Medicines Agency; FDA, US Food and Drug Administration; GI, gastrointestinal; HIV, human immunodeficiency virus; INR, international normalized ratio; NSAID, nonsteroidal anti‐inflammatory drug; od, once daily; P‐gp, P‐glycoprotein; VKA, vitamin K antagonist.
Where differences in guidance exist between documents, the source of the guidance (EMA or FDA) is noted. Where both sources provide the same guidance, no source is listed.
Other characteristics indicate that dabigatran, rivaroxaban, and apixaban should be used with caution in certain patients (Tables 3 and 4). As with any anticoagulant, caution is needed in patients who are at increased risk of bleeding, such as those who have bleeding‐associated conditions or are receiving ongoing treatment with antithrombotic agents. In such patients, close monitoring for signs of bleeding or anemia is needed.27, 29, 38 Patients with renal impairment require special consideration, which will be discussed in later sections.
In the RE‐LY trial, patient age significantly influenced the risk of bleeding. Compared with warfarin, both dabigatran doses reduced the risk of extracranial bleeding in younger patients but increased the risk in older patients. The overall risk of major bleeding was lower with both doses of dabigatran compared with warfarin in patients age <75 years (relative risk [RR] for dabigatran 110 mg and 150 mg of 0.62 [95% CI: 0.50‐0.77] and 0.70 [95% CI: 0.57‐0.86], respectively), whereas in patients age ≥75 years, the risk was similar for dabigatran 110 mg (RR: 1.01; 95% CI: 0.83‐1.23) and showed a trend toward higher risk for dabigatran 150 mg (RR: 1.18; 95% CI: 0.98‐1.42). Both dabigatran doses were associated with a lower risk of ICH, irrespective of age.40 As a result, the European Summary of Product Characteristics for dabigatran states that age ≥75 years (in addition to body weight <50 kg) is associated with an increased bleeding risk, and that patients age ≥80 years should receive the lower dabigatran dose (110 mg bid).27 For rivaroxaban, age and body weight had no significant impact on trial findings,15, 41 and the European Summary of Product Characteristics states that no dose adjustment of rivaroxaban is needed based on these characteristics.29 In the ARISTOTLE study, apixaban 5 mg was administered twice daily, with 2.5‐mg doses used in patients with 2 or more of the following criteria: ≥80 years of age, body weight ≤60 kg, or a serum creatinine level ≥1.5 mg per dL (133 µmol/L).16 Both the US Prescribing Information and the European Summary of Product Characteristics recommend this dosage schedule (Table 4).38, 39
The 2012 update of the ESC guidelines provides further suggestions for selection of dabigatran dose. When dabigatran is prescribed, the 150 mg bid dose should be considered in most patients in preference to the 110 mg bid dose.34 The 110 mg bid dose is recommended in patients age ≥80 years; patients concomitantly receiving interacting drugs (eg, verapamil); patients with a higher risk of bleeding (ie, HAS‐BLED score [hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly {>65 years}, drugs/alcohol] of ≥3); or patients with moderate renal impairment (CrCl 30–49 mL/min).34 In countries where dabigatran 110 mg is not approved, such as the United States,42 dabigatran 150 mg would remain an option in all of the above scenarios. The same guidelines also recommend the 20‐mg once‐daily rivaroxaban dose, except in patients with a HAS‐BLED score of ≥3 or CrCl 30 to 49 mL/min.34
Based on exclusion criteria used in phase III trials, novel OACs should be considered untested in specific subpopulations of patients with AF. In addition to those with valvular disease or reversible AF, RE‐LY, ROCKET AF, ARISTOTLE, and AVERROES also excluded patients with any history of, or significant risk factors for, bleeding (including ICH), liver disease/dysfunction, severe renal impairment, planned AF ablation, or conditions other than AF that required chronic anticoagulation. In ROCKET AF and ARISTOTLE, but not in RE‐LY, patients treated recently with ASA plus a thienopyridine (clopidogrel or ticlopidine) were excluded; in all 3 trials, patients requiring ASA at high doses (>100 mg/day in RE‐LY and ROCKET AF, and >165 mg/day in ARISTOTLE) were ineligible. In AVERROES, patients taking a thienopyridine were ineligible. In the warfarin‐based trials, patients with a recent stroke (within 7–14 days) were excluded; ROCKET AF also excluded patients who had experienced a TIA within 3 days. In RE‐LY and ARISTOTLE, patients with uncontrolled hypertension were excluded.14, 16 In ROCKET AF, patients with a CHADS2 score of <2 were also excluded, although 3 patients, 1 in the rivaroxaban arm and 2 in the warfarin arm, had a score of 1 at baseline.15
Patients With Renal Impairment
During treatment with dabigatran, rivaroxaban, or apixaban, the proportion of active bioavailable drug eliminated renally is approximately 80%, 33%, and 27%, respectively (Table 1). Compared with subjects with normal renal function, patients with renal dysfunction receiving the same dose of a novel OAC will have increased drug exposure.13
Each of the phase III trials of novel OACs in patients with AF included specific rules relating to renal function. Patients with severe renal impairment (CrCl <25 mL/min [ROCKET AF, ARISTOTLE, AVERROES] or ≤30 mL/min [RE‐LY]) were ineligible.18, 19, 20, 21 In RE‐LY, 19% of participants had CrCl <50 mL/min, but dosing was not adjusted based on renal function.14 In ROCKET AF, rivaroxaban 15 mg once daily (od) was given to patients with CrCl of 30 to 49 mL/min, comprising 21% of patients.18, 43 In ARISTOTLE and AVERROES, apixaban 2.5 mg (instead of 5.0 mg) bid was given to patients expected to have higher apixaban drug exposure, defined as meeting any 2 of the following criteria: age ≥80 years, body weight <60 kg, or serum creatinine level ≥1.5 mg/dL (133 µmol/L); 5% to 6% of apixaban‐treated patients received 2.5 mg bid.16, 17, 19, 20 In all studies, irrespective of treatment assignment, patients with renal dysfunction had numerically higher rates of stroke or major bleeding events than patients with normal renal function.14, 15, 16, 17, 40, 43
In RE‐LY, ROCKET AF, and AVERROES, efficacy and safety findings for the novel OAC vs control were consistent in subgroups defined by renal function.14, 17, 40, 43 In ARISTOTLE, patients with moderate or severe renal impairment had a greater reduction in major bleeding with apixaban vs warfarin than patients with no or mild impairment.16
Because of the potential for reduced elimination and increased plasma drug levels in patients with renal impairment, dabigatran, rivaroxaban, and apixaban should be used with caution.27, 28, 29, 30, 38, 39 For dabigatran, prescribing information specifies that renal function should be assessed in all patients prior to therapy and periodically during therapy.27, 28 A lower rivaroxaban dose (15 mg od instead of 20 mg od) is recommended for patients with AF who have CrCl of 15 to 49 mL/min.29, 44 As mentioned previously, for apixaban, a 2.5‐mg dose is recommended for patients with 2 or more of the following criteria: ≥80 years old, body weight ≤60 kg, or a serum creatinine level ≥1.5 mg per dL (133 µmol/L).38, 39 For dabigatran, no dose adjustment based on renal impairment for patients with AF has been recommended by European authorities (CrCl <30 mL/min is a contraindication for dabigatran), whereas based on pharmacokinetic and pharmacodynamic modeling, US Prescribing Information states that dabigatran 75 mg bid (instead of 150 mg bid) should be used in patients with CrCl of 15 to 30 mL/min.27, 28, 42, 45, 46 However, caution should be exercised in patients with moderate renal impairment (CrCl 30–50 mL/min) receiving dabigatran, particularly those at high risk of bleeding (HAS‐BLED ≥3), and dabigatran 110 mg bid should be preferred to 150 mg bid in these individuals.34 During dabigatran therapy, renal function should be reassessed if there is a risk of declining renal function, and in patients over 75 years old, renal function should be evaluated at least once a year.27 Renal function is an additional consideration when switching patients from dabigatran to a VKA and for some comedications (Table 4; see later sections).
Drug Interactions
Because many patients with AF are elderly and have multiple comorbidities, potential drug interactions are an important consideration. Although drug interactions are substantially reduced for novel OACs compared with VKAs, clinically relevant interactions are known based on dabigatran, rivaroxaban, and apixaban being substrates of P‐glycoprotein (P‐gp), which is an efflux transporter, and rivaroxaban and apixaban being metabolized by cytochrome P450 3A4 (CYP3A4), which is a key liver metabolic enzyme.27, 28, 29, 30, 38
Several agents commonly administered in patients with AF or other cardiovascular diseases have relevant activity against P‐gp and/or CYP3A4. Table 4 lists drugs that should not be coadministered with novel OACs or drugs that require caution because of potential interactions.27, 28, 29, 30, 38, 39 For example, European Summary of Product Characteristics states that caution is needed if dabigatran is coadministered with amiodarone, quinidine, clarithromycin, or verapamil (strong P‐gp inhibitors).27 In the US Prescribing Information, a lower dabigatran dose (75 mg bid) is suggested for patients with CrCl of 30 to 50 mL/min who are receiving dronedarone (strong P‐gp inhibitor), whereas in patients with CrCl <30 mL/min, it is stated that coadministration with any P‐gp inhibitor should be avoided.28 Concomitant use of dabigatran with P‐gp inducers (eg, rifampicin) is also generally not recommended.27 European prescribing information for dabigatran contraindicates coadministration with dronedarone owing to increased dabigatran plasma concentrations. For rivaroxaban, the European Summary of Product Characteristics suggests that coadministration with dronedarone should be avoided because of limited clinical data.27, 29 For rivaroxaban, the US Prescribing Information states that in patients with renal impairment receiving combined P‐gp inhibitors and weak/moderate CYP3A4 inhibitors (eg, amiodarone, diltiazem, verapamil, quinidine, ranolazine, dronedarone, felodipine, erythromycin, and azithromycin), rivaroxaban should be coadministered only if the potential benefit justifies the potential risk.30 For apixaban, the European Summary of Product Characteristics contraindicates strong inhibitors of both CYP3A4 and P‐gp, such as azole antimycotics (eg, ketoconazole, itraconazole, voriconazole, and posaconazole) and human immunodeficiency virus protease inhibitors (eg, ritonavir); however, the US Prescribing Information does not contraindicate their use with apixaban, instead recommending a dose reduction of apixaban to 2.5 mg.38, 39
Concurrent Cardioversion
Cardioversion is associated with an increased risk of thromboembolic events, which is reduced by anticoagulation.8, 47 The RE‐LY study protocol recommended that patients undergoing cardioversion should remain on study drug. Of 18113 patients randomized, 1983 cardioversions were performed in 1270 patients, with numbers similar between study arms. Most cardioversions (82%–86%) were electrical. Of patients who underwent cardioversion in the dabigatran 110 mg, dabigatran 150 mg, and warfarin arms, rates of stroke or systemic embolism (0.77%, 0.30%, and 0.60%, respectively) and major bleeding events (1.7%, 0.6%, and 0.6%, respectively) within 30 days were low and comparable. Transesophageal echocardiography was performed before cardioversion in a higher proportion of patients assigned to dabigatran than to warfarin (24%–26% vs 13%). In patients treated with dabigatran 110 mg, dabigatran 150 mg, or warfarin, left atrial thrombi were detected in 1.8%, 1.2%, and 1.1%, respectively, but the proportion of patients receiving continuous treatment with study drug for ≥3 weeks before cardioversion was shorter with dabigatran than with warfarin (76%–79% vs 85.5%). Rates of stroke/systemic embolism were similar between arms with or without prior transesophageal echocardiography. These findings suggest that dabigatran is a reasonable alternative to warfarin in patients requiring cardioversion.48
In ARISTOTLE, 553 patients (272 in the apixaban group and 281 in the warfarin group) underwent cardioversion during the trial. No stroke or systemic embolism events had occurred in either group at 90 days, suggesting that apixaban may also be a safe alternative to warfarin for stroke prevention pericardioversion (ESC 2012 oral presentation).49
A recent subanalysis of ROCKET AF compared rivaroxaban with warfarin for the prevention of cardiovascular events in patients with nonvalvular AF scheduled for electrical or pharmacologic cardioversion or AF ablation. Event counts were found to be similar in patients treated with rivaroxaban or warfarin after cardioversion or ablation, including stroke or systemic embolism or death from any cause.50 Rivaroxaban will be compared with dose‐adjusted warfarin for cardioversion in the ongoing prospective, randomized, open‐label trial; a similar study with apixaban is planned.
Concurrent Antiplatelet Treatment
Because of the increased risk of bleeding in patients receiving antithrombotic agents, such as ASA, other platelet inhibitors, or nonsteroidal anti‐inflammatory drugs, coadministration of a novel OAC requires caution and close monitoring for bleeding.27, 28, 29, 30, 38, 39, 51 In the dabigatran and warfarin arms of the RE‐LY trial, concomitant use of ASA or clopidogrel increased the risk of major bleeding events, including gastrointestinal bleeding events,27, 40 and concomitant use of ASA was the most important modifiable independent risk factor for ICH.52 In ROCKET AF, concomitant ASA use (almost exclusively ≤100 mg/d) was also an independent risk factor for major bleeding events.30 In the ARISTOTLE trial, major bleeding was also more common in patients receiving concomitant ASA than those who were not, although no significant interaction between ASA use and treatment effect was reported.53
Recently, positive findings were reported from the ATLAS ACS 2 TIMI 51 (Anti‐Xa Therapy to Lower cardiovascular events in Addition to Standard therapy in subjects with ACS 2 Thrombolysis In Myocardial Infarction 51) phase III trial of rivaroxaban vs placebo in patients with a recent acute coronary syndrome (ACS) event receiving standard antiplatelet therapy with ASA alone or ASA plus a thienopyridine (eg, clopidogrel). Patients treated with rivaroxaban 2.5 mg bid had a significantly lower rate of primary end point events (cardiovascular death, myocardial infarction [MI], or stroke), cardiovascular death, and all‐cause death compared with placebo. Although rivaroxaban plus antiplatelet therapy was associated with increased rates of major and intracranial bleeding events vs antiplatelet therapy alone, fatal bleeding events did not increase.54 Importantly, rivaroxaban doses tested in ATLAS ACS 2 TIMI 51 were lower than therapeutic doses for prevention of AF‐related stroke (2.5/5.0 mg bid vs 15–20 mg od), and patients with AF were excluded.54 Thus, findings from ATLAS ACS 2 TIMI 51 have limited relevance to the prevention of stroke in patients with AF, and there is no evidence as to whether low‐dose rivaroxaban would provide effective thromboprophylaxis in patients with AF. In other trials in the ACS setting, the addition of dabigatran or apixaban to antiplatelet therapy in patients with ACS significantly increased the risk of major bleeding events without significantly reducing recurrent ischemic events. In the phase III APPRAISE‐2 (APixaban for PRevention of Acute ISchemic Events 2) trial of apixaban, AF‐equivalent doses were examined, and it is unknown whether lower APixaban doses would result in different outcomes.55
For dabigatran, both AF‐equivalent doses (110 mg and 150 mg bid) and lower doses (50 mg and 75 mg bid) were examined in the RE‐DEEM (Randomised Dabigatran Etexilate Dose Finding Study in Patients with Acute Coronary Syndromes Post Index Event with Additional Risk Factors for Cardiovascular Complications also Receiving Aspirin and Clopidogrel) study.56 Guidelines have been published for antithrombotic therapy in patients with AF presenting with ACS and/or undergoing coronary stenting.36, 57 In patients with AF who require both an anticoagulant and dual antiplatelet therapy, VKA therapy would be preferred to a novel OAC because of the greater experience with VKAs in this setting.
Risk of Myocardial Infarction
In the original RE‐LY analysis, dabigatran was associated with a significantly higher MI rate than warfarin (RR: 1.38 for dabigatran 150 mg, P = 0.048; RR: 1.35 for dabigatran 110 mg, P = 0.07).14 In a re‐analysis, which included cases of silent MI not identified in the original analysis, the difference was not statistically significant (RR of MI compared with warfarin was 1.29 for dabigatran 110 mg [P = 0.09] and 1.27 for dabigatran 150 mg [P = 0.12]).22 Irrespective of therapy, the highest rates of MI were reported in patients who: were older; had diabetes, hypertension, prior MI, prior heart failure, or moderate renal impairment; or were taking ASA and/or clopidogrel.58 Subsequently, a meta‐analysis of 7 trials of dabigatran compared with various controls (warfarin, enoxaparin, or placebo) across different indications (AF, acute venous thromboembolism, ACS, or short‐term prophylaxis of deep vein thrombosis) reported a 33% increased odds ratio with dabigatran for MI or ACS (P = 0.03).59 However, a post hoc analysis from RE‐LY analyzing composite rates of myocardial ischemic events (MI, unstable angina, cardiac arrest, or cardiac death) suggested a reduced risk with dabigatran vs warfarin (HRs vs warfarin: dabigatran 110 mg, 0.93, P = 0.24; dabigatran 150 mg, 0.88, P = 0.0252), and efficacy and safety findings were broadly consistent in patients with or without a prior MI or coronary artery disease.58 In the trials of rivaroxaban or apixaban in patients with AF, no significant difference in rates of MI vs warfarin was reported (Table 2).15, 16
Ensuring Adherence to Therapy
Poor adherence to therapy is a common problem, with typical adherence rates for prescribed medications estimated to be approximately 50%.60 Because novel OACs have much shorter half‐lives than warfarin (Table 1), close adherence to dosing regimens is needed to ensure that drug concentrations remain therapeutic. In other conditions requiring chronic daily treatment, patients generally adhere better to od compared with bid regimens,61, 62 which might suggest a potential advantage for rivaroxaban compared with dabigatran or apixaban. Because of a requirement for moisture‐free storage conditions,27, 28 dabigatran tablets cannot be transferred from their original packaging into weekly tablet organizers, which may affect adherence in elderly patients requiring multiple medications.
Although INR monitoring during VKA therapy is a requirement and not an adherence tool, it does provide an indirect measure of adherence. By contrast, although INR measurements are affected by the novel OACs, INR assessment is not an informative or valid method of monitoring coagulation with these newer agents.63, 64, 65, 66 As a result, alternative measures of coagulation are needed for the novel OACs. Potential methods for assessing recent adherence to novel OACs include activated partial thromboplastin time, calibrated diluted thrombin time, or ecarin clotting time for dabigatran; or prothrombin time, HepTest, or anti‐Factor Xa chromogenic assays for rivaroxaban. These may be informative for patients who have been prescribed a novel OAC but who present with an ischemic or hemorrhagic stroke. However, limitations of alternative tests include sensitivity, variability, availability, or lack of validation.27, 29, 67, 68, 69 Furthermore, because these tests reveal only recent effects of OACs, alternative strategies for encouraging adherence between clinic visits should also be considered, such as effective patient education and counseling, and telephone follow‐up.60
Interrupting Therapy Before Invasive or Surgical Procedures
In general, novel OACs should be interrupted before invasive or surgical procedures and restarted promptly when hemostasis has been restored.27, 29 The time taken to clear dabigatran is highly dependent on renal function, therefore prescribing information recommends that dabigatran should be discontinued for 24 hours if CrCl is ≥80 mL/min, for 1 to 2 days if CrCl is 50 to <80 mL/min, and for 2 to 3 days if CrCl is ≥30 to 50 mL/min; an additional 1 to 2 days without dabigatran is recommended if there is a high risk of bleeding or if major surgery is needed.27
For rivaroxaban, prescribing information states that dosing should be stopped at least 24 hours before the intervention.29 For both dabigatran and rivaroxaban, if surgery cannot be delayed, the increased risk of bleeding should be weighed against the urgency of the intervention. OAC therapy should be resumed based on clinical judgment after hemostasis has been achieved.27, 29
For apixaban, it is recommended that the drug is discontinued at least 48 hours prior to elective surgery or invasive procedures with a moderate or high risk of unacceptable or clinically significant bleeding and at least 24 hours prior to elective surgery or invasive procedures with a low risk of bleeding.38, 39, 70
For patients who require dental extraction, most sources suggest that ongoing warfarin treatment should be continued in patients with a well‐controlled INR.70, 71 Data are not yet available for novel OACs, but there is no reason to suggest that novel OAC therapy would need to be interrupted for routine dental extraction.
A RE‐LY subanalysis has examined periprocedural bleeding with dabigatran and warfarin. Across all arms, 25% to 26% of patients underwent an invasive procedure, including pacemaker/defibrillator insertion (10%), dental procedure (10%), diagnostic procedure (10%), cataract removal (9%), colonoscopy (9%), or joint replacement (6%). The last dose of dabigatran or warfarin was administered a median of 49 or 114 hours, respectively, before the procedure. Rates of periprocedural major bleeding events were similar with dabigatran and warfarin (4%–5%), including in patients who underwent urgent surgery (18%–22%).72
There is accumulating, albeit still limited evidence for the use of dabigatran and, to a lesser extent, rivaroxaban in patients undergoing left atrial ablation. The majority of reports came from the observational cohort studies which used peri‐procedural anticoagulation with either interrupted or uninterrupted warfarin as a comparator to interrupted dabigatran. Dabigatran was held the morning of the procedure to 24–30 hours prior to ablation and resumed after hemostasis had been achieved (usually 3–6 hours). A recent meta‐analysis which included 10 studies in 3648 patients has found that the incidence of thromboembolic events (OR, 2.38; 95% CI, 0.62–6.85; P = 0.11), major bleeding (OR, 1.05, 95% CI, 0.62–1.8; P = 0.85), and minor bleeding events (OR, 0.95; 95% CI, 0.67–1.35).73 The number of thromboembolic events was low leading to wide confidence intervals. Another meta‐analysis of 9 studies in 3036 patients has yielded similar results.74 However, these meta‐analyses had not enough power to firmly establish the efficacy and safety of peri‐procedural anticoagulation with dabigatran and did not eliminate the need for the randomized controlled study or a well‐designed registry.
Switching Between Oral Anticoagulants
When transitioning patients from a VKA to a novel OAC (or vice versa), strategies are needed to ensure that adequate anticoagulation is maintained. This point is highlighted by a specific warnings in US Prescribing Information for rivaroxaban and apixaban, which states that the discontinuation of these 2 drugs places patients at an increased risk of thrombotic events, and that if they must be discontinued for a reason other than pathological bleeding, consideration should be given to the administration of another anticoagulant.30, 39 When switching between anticoagulants, it is very important to ensure adequate anticoagulation while simultaneously minimizing the bleeding risk.75 A warning is also included for dabigatran that highlights the increased risk of stroke if therapy is temporarily discontinued.28
Guidance on switching between OACs is provided in prescribing information. When switching from a VKA to a novel OAC, warfarin should be stopped and the novel OAC started when the INR is <2.0 (dabigatran and apixaban) or ≤3.0 (rivaroxaban) (Table 4).27, 28, 29, 30, 38, 39 The difference in INR cutoff points may reflect an increased degree of caution for rivaroxaban relating to poststudy events in ROCKET AF that occurred when patients were transitioned from blinded rivaroxaban to open‐label VKA therapy. The increased number of poststudy events in the rivaroxaban arm vs the warfarin arm was attributed to the longer time taken to reach therapeutic INR after study drug discontinuation (13 days vs 3 days, respectively).15, 76 In the RE‐LY and ROCKET AF studies, previous exposure to VKA therapy did not influence the effects of the novel OAC relative to warfarin,77, 78 meaning that patients starting a novel OAC without prior VKA exposure or switching from a VKA will experience similar benefits.79
To switch between novel OACs, the alternative agent should be taken instead of the original agent when the next dose is due.27, 28, 29, 30, 38, 39 To switch from a novel OAC to a VKA, a period of concurrent treatment is needed to ensure ongoing anticoagulation is adequate (Table 4). For dabigatran, the duration of concurrent treatment is based on renal function, with the VKA started 3 days, 2 days, or 1 day before discontinuing dabigatran in patients with CrCl ≥50 mL/min, 30 to <50 mL/min, or 15 to <30 mL/min, respectively.27, 28 For rivaroxaban, the VKA should be given concurrently until the INR is 2.0 or higher, with INR‐guided dosing of the VKA given from day 3 of the transition period. Because rivaroxaban can cause elevated INR levels, INR should be tested no earlier than 24 hours after the previous dose but before the next dose of rivaroxaban.29 Alternatively, rivaroxaban can be discontinued and both the VKA and a parenteral anticoagulant started when the next rivaroxaban dose would have been taken.30 In the case of apixaban, when switching to warfarin or another VKA in Europe, apixaban should be continued for at least 2 days after beginning VKA therapy. After 2 days, an INR should be obtained prior to the next scheduled dose of apixaban and coadministration continued until the INR is ≥2.0.38 In the United States, because of the effect of apixaban on INR measurements, it is recommended to discontinue apixaban and then begin both a parenteral anticoagulant and warfarin at the time the next dose of apixaban would have been taken, discontinuing the parenteral anticoagulant when INR reaches an acceptable range.39
Management of Bleeding
Monitoring patients for bleeding is essential during therapy with any anticoagulant. Before treatment with novel OACs, standard risk stratification (eg, HAS‐BLED score ≥3) should be used to identify patients at higher risk of bleeding who require closer monitoring.8 Prescribing information for novel OACs also highlights the risk of bleeding in specific patient types, such as patients with disorders associated with bleeding (eg, congenital or acquired coagulation disorders or ulcerative gastrointestinal disease) or patients receiving drugs associated with a bleeding risk (eg, other antithrombotic agents) (Table 3). In high‐risk patients, laboratory testing of hemoglobin/hematocrit to detect occult bleeding should be considered in addition to clinical surveillance. Any unexplained fall in hemoglobin or blood pressure should lead to a search for a bleeding site.27, 29
The potential issue of bleeding with dabigatran has been highlighted in an analysis of data from the US Food and Drug Administration MedWatch program during the first 3 months of 2011. During this period, more bleeding events occurred with dabigatran (n = 505) than with warfarin (n = 176). The median age of patients receiving dabigatran who experienced bleeding events was 80 years, compared with 56 years for patients with bleeding events on other drugs. The authors suggested that optimal dosing of dabigatran for elderly patients or those with moderate renal impairment needs to be reevaluated.80 Other reports have highlighted bleeding risk with dabigatran in frail elderly patients81 and poor outcomes and difficulties of managing bleeding in patients who experienced trauma while receiving dabigatran.82
Patients who develop bleeding events during novel OAC therapy should receive standard management. The OAC should be delayed or discontinued while the severity and source of bleeding are investigated. Supportive treatment such as mechanical compression, surgical hemostasis, blood volume replacement, and blood products should be used as appropriate.83 Coagulation tests might also be useful. Because dabigatran is mostly excreted renally, adequate diuresis must be maintained.27 Agents to reverse the anticoagulation of novel agents would be useful. To date, no antidotes for novel OACs have been clinically validated in patients with bleeding events. However, a clinical study in healthy subjects found that anticoagulation by rivaroxaban, but not by dabigatran, was reversed by nonactivated prothrombin complex concentrate.84 In an ex vivo study, low‐dose activated prothrombin complex concentrate (FEIBA inhibitor bypassing activity) reversed the anticoagulant activity of both rivaroxaban and dabigatran.85 Because of the low protein binding (∼35%) of dabigatran, the drug can be dialyzed in patients with an overdose,27, 28 although the feasibility of this in the emergency setting has been questioned.82 With dabigatran, rivaroxaban, and apixaban, oral charcoal may be useful shortly after an overdose.29, 30, 39, 83, 86 In vitro data suggest that dabigatran levels may be reduced by hemoperfusion over an activated charcoal filter.86 For apixaban, the US Prescribing Information and European Summary of Product Characteristics report that, in healthy subjects, daily doses of up to 50 mg for 3 to 7 days “had no clinically relevant adverse effects,” but this should not be simply extrapolated to patients with cardiovascular disease.38, 39
Which Novel Oral Anticoagulant?
All novel OACs have been shown to be noninferior or superior to dose‐adjusted VKA therapy. Compared with warfarin treatment, all agents reduce the risk of ICH. Overall, novel OACs reduced the primary end point of stroke or systemic embolism by ∼20% to 22%, any stroke by ∼23%, ischemic or unidentified stroke by ∼13%, hemorrhagic stroke and ICH by ∼50% to 55%, major bleeding by ∼12%, and all‐cause mortality by ∼12% compared with warfarin.87, 88, 89 The very large phase III outcome trials also allow subgroup analyses on substantial numbers of patients (eg, in patients with previous stroke).14, 15, 16, 24, 25, 26 Although such analyses may provide valuable information, they are generally underpowered and can be used only for general guidance.
Several indirect comparisons employing different statistical methods have yielded inconsistent results, but there are no significant differences between the analyses.88, 89, 90, 91 The main limitation of these analyses is that no statistical adjustment method is capable of fully accounting for the inherent dissimilarities between the studies, such as the mean CHADS2 score and the prevalence and distribution of its components and general cardiovascular risk. For example, the ROCKET AF study included patients at higher risk by means of a CHADS2 score (87% had a CHADS2 score of 3–6 compared with 30% and 33% in ARISTOTLE and RE‐LY, respectively), with a greater prevalence of previous cerebral or systemic embolic events (55% vs ∼20%), as well as a greater overall prevalence of cardiovascular risk factors such as hypertension and diabetes. Other pertinent factors such as time spent within the therapeutic INR range92 and the incidence of the primary end points in the comparator (VKA) arm also differed between the studies. Therefore, in the absence of head‐to‐head comparisons, it is difficult to provide definitive recommendations on which novel OACs should be used in which patients, although patient characteristics, drug tolerability, and cost may be considered (Figure 2).
Figure 2.
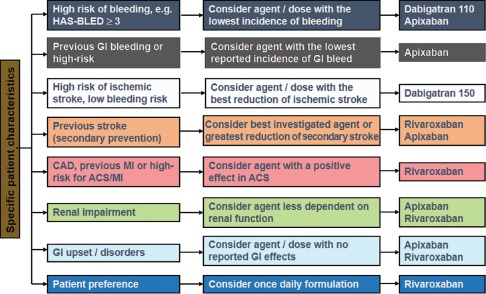
Possible considerations (authors' opinions) for selecting between novel oral anticoagulants (if preferred to a vitamin K antagonist) based on patient characteristics in the absence of head‐to‐head trials. Any clinical decision should take account of individual patient presentation and agent/dose availability based on local regulatory approval. Clinical opinions are often based on indirect comparisons, subgroup analyses, advese event profiles, and clinical trials in other patient populations. None of these data on which a choice will be made is individually valid, and together only provide a gestalt, but in the absence of other information clinicians have no other way to proceed. Abbreviations: ACS, acute coronary syndrome; AF, atrial fibrillation; CAD, coronary artery disease; GI, gastrointestinal; HAS‐BLED, hypertension, abnormal renal/liver function (1 point each), stroke, bleeding history or predisposition, labile international normalized ratio, elderly (≥65 years), drugs/alcohol concomitantly (1 point each); MI, myocardial infarction.
Conclusion
Novel OACs have heralded a new era in anticoagulation for patients with AF. To ensure that patients derive the maximum benefit from therapy, understanding the differences between novel OACs and VKAs and the practical implications for day‐to‐day practice is critical. However, clinical experience of novel OACs outside of trials remains limited, and further insights into appropriate use will undoubtedly become apparent as these agents are prescribed more widely. Prescribing information and drug characteristics also suggest that differences may exist in terms of how patients receiving different novel OACs should be managed. However, the limitations of the current evidence base are an important consideration.
Acknowledgments
The authors acknowledge Jeremy Gardner and Geraint Owens, who provided medical writing services with funding from Bayer HealthCare Pharmaceuticals and Janssen Janssen Scientific Affairs, LLC.
I. Savelieva and A. J. Camm have acted in consultant/advisor and speaker roles on behalf of Boehringer Ingelheim, Bayer HealthCare Pharmaceuticals, Bristol‐Myers Squibb, Pfizer, and Daiichi Sankyo.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147:1561–1564. [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 3. Jorgensen HS, Nakayama H, Reith J, et al. Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke. 1996;27:1765–1769. [DOI] [PubMed] [Google Scholar]
- 4. Lamassa M, Di Carlo A, Pracucci G, et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital‐based registry (The European Community Stroke Project). Stroke. 2001;32:392–398. [DOI] [PubMed] [Google Scholar]
- 5. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 6. Heeringa J, van der Kuip DAM, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 7. Naccarelli GV, Varker H, Lin J, et al. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. [DOI] [PubMed] [Google Scholar]
- 8. Camm AJ, Kirchhof P, Lip GYH, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010;12:1360–1420. [DOI] [PubMed] [Google Scholar]
- 9. Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e269–e367. [DOI] [PubMed] [Google Scholar]
- 10. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 11. Eriksson BI, Quinlan DJ, Eikelboom JW. Novel oral Factor Xa and thrombin inhibitors in the management of thromboembolism. Annu Rev Med. 2011;62:41–57. [DOI] [PubMed] [Google Scholar]
- 12. Ansell J. Warfarin versus new agents: interpreting the data. Hematology Am Soc Hematol Educ Program. 2010;2010:221–228. [DOI] [PubMed] [Google Scholar]
- 13. Harder S. Renal profiles of anticoagulants. J Clin Pharmacol. 2012;52:964–975. [DOI] [PubMed] [Google Scholar]
- 14. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE‐LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 15. Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 16. Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 17. Connolly SJ, Eikelboom J, Joyner C, et al; AVERROES Steering Committee and Investigators . Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. [DOI] [PubMed] [Google Scholar]
- 18. ROCKET AF Study Investigators . Rivaroxaban‐once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;159:340–347. [DOI] [PubMed] [Google Scholar]
- 19. Lopes RD, Alexander JH, Al‐Khatib SM, et al; ARISTOTLE Investigators . Apixaban for Reduction In Stroke and Other ThromboemboLic Events in Atrial Fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J. 2010;159:331–339. [DOI] [PubMed] [Google Scholar]
- 20. Eikelboom JW, O'Donnell M, Yusuf S, et al. Rationale and design of AVERROES: Apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. Am Heart J. 2010;159:348–353. [DOI] [PubMed] [Google Scholar]
- 21. Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE‐LY: randomized evaluation of long‐term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157:805–810. [DOI] [PubMed] [Google Scholar]
- 22. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Newly identified events in the RE‐LY trial. N Engl J Med. 2010;363:1875–1876. [DOI] [PubMed] [Google Scholar]
- 23. Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE‐LY trial. Lancet. 2010;376:975–983. [DOI] [PubMed] [Google Scholar]
- 24. Diener HC, Connolly SJ, Ezekowitz MD, et al. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE‐LY trial. Lancet Neurol. 2010;9:1157–1163. [DOI] [PubMed] [Google Scholar]
- 25. Hankey GJ, Patel MR, Stevens SR, et al; ROCKET AF Steering Committee Investigators . Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol. 2012;11:315–322. [DOI] [PubMed] [Google Scholar]
- 26. Easton JD, Lopes RD, Bahit MC, et al. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol. 2012;11:503–511. [DOI] [PubMed] [Google Scholar]
- 27. Boehringer Ingelheim International GmbH . Pradaxa (dabigatran etexilate) summary of product characteristics. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000829/WC500041059.pdf. Accessed May 17, 2013.
- 28.Boehringer Ingelheim Pharmaceuticals Inc. Pradaxa (dabigatran etexilate) prescribing information. 2012. http://bidocs.boehringer‐ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed May 17, 2013.
- 29. Bayer Pharma AG. Xarelto (rivaroxaban) summary of product characteristics. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000944/WC500057108.pdf. Accessed May 22, 2013.
- 30. Janssen Pharmaceuticals Inc . Xarelto (rivaroxaban) prescribing information. 2013. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022406s004lbl.pdf. Accessed May 22, 2013.
- 31.Bristol‐Myers Squibb Press release: ELIQUIS (apixaban) approved in Europe for prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. http://news.bms.com/press‐release/financial‐news/eliquisapixaban‐approved‐europe‐prevention‐stroke‐and‐systemic‐embolism&t=634890181706594412. Accessed January 9, 2013.
- 32.Food and Drug Administration: FDA news release. FDA approves Eliquis to reduce the risk of stroke, blood clots in patients with non‐valvular atrial fibrillation. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm333634.htm. Accessed January 9, 2013.
- 33. Furie KL, Goldstein LB, Albers GW, et al. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:3442–3453. [DOI] [PubMed] [Google Scholar]
- 34. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385–413. [DOI] [PubMed] [Google Scholar]
- 35. Skanes AC, Healey JS, Cairns JA, et al. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol. 2012;28:125–136. [DOI] [PubMed] [Google Scholar]
- 36. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e531S–e575S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:1144–1150. [DOI] [PubMed] [Google Scholar]
- 38.Bristol‐Myers Squibb, Pfizer EEIG. Eliquis (apixaban) summary of product characteristics. 2013. http://www.eliquis.com/index.aspx. Accessed May 17, 2013.
- 39. Bristol‐Myers Squibb Company, Pfizer Inc . Eliquis (apixaban) prescribing information. 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202155s000lbl.pdf. Accessed May 17, 2013.
- 40. Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long‐term anticoagulant therapy (RE‐LY) trial. Circulation. 2011;123:2363–2372. [DOI] [PubMed] [Google Scholar]
- 41. Halperin JL, Wojdyla D, Piccini JP, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the ROCKET‐AF trial. Stroke. 2012;43:A148. [Google Scholar]
- 42. Beasley BN, Unger EF, Temple R. Anticoagulant options—why the FDA approved a higher but not a lower dose of dabigatran. N Engl J Med. 2011;364:1788–1790. [DOI] [PubMed] [Google Scholar]
- 43. Fox KAA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non‐valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32:2387–2394. [DOI] [PubMed] [Google Scholar]
- 44. Mueck W, Lensing AW, Agnelli G, et al. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep‐vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;50:675–686. [DOI] [PubMed] [Google Scholar]
- 45. Hariharan S, Madabushi R. Clinical pharmacology basis of deriving dosing recommendations for dabigatran in patients with severe renal impairment. J Clin Pharmacol. 2012;52:119S–125S. [DOI] [PubMed] [Google Scholar]
- 46. Lehr T, Haertter S, Liesenfeld KH, et al. Dabigatran etexilate in atrial fibrillation patients with severe renal impairment: dose identification using pharmacokinetic modeling and simulation. J Clin Pharmacol. 2011;52:1373–1378. [DOI] [PubMed] [Google Scholar]
- 47. Arnold AZ, Mick MJ, Mazurek RP, et al. Role of prophylactic anticoagulation for direct current cardioversion in patients with atrial fibrillation or atrial flutter. J Am Coll Cardiol. 1992;19:851–855. [DOI] [PubMed] [Google Scholar]
- 48. Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011;123:131–136. [DOI] [PubMed] [Google Scholar]
- 49. Flaker G, Lopes R, Al‐Khatib S, et al. Apixaban and warfarin are associated with a low risk of stroke following cardioversion for atrial fibrillation: results from the ARISTOTLE Trial. Eur Heart J. 2012;33:686. Abstract 4048. [Google Scholar]
- 50. Piccini JP, Stevens SR, Lokhnygina Y, et al. Outcomes following cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol. 2013;61:1998–2006. [DOI] [PubMed] [Google Scholar]
- 51. Lip GYH, Andreotti F, Fauchier L, et al. Bleeding risk assessment and management in atrial fibrillation patients: a position document from the European Heart Rhythm Association, endorsed by the European Society of Cardiology Working Group on Thrombosis. Europace. 2011;13:723–746. [DOI] [PubMed] [Google Scholar]
- 52. Hart RG, Diener HC, Yang S, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE‐LY trial. Stroke. 2012;43:1511–1517. [DOI] [PubMed] [Google Scholar]
- 53. Alexander JH, Lopes R, McMurray J, et al. Efficacy and safety of apixaban compared with warfarin for stroke prevention in atrial fibrillation in patients taking concomitant aspirin. J Am Coll Cardiol. 2012;59:E1697. [Google Scholar]
- 54. Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 55. Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699–708. [DOI] [PubMed] [Google Scholar]
- 56. Oldgren J, Budaj A, Granger CB, et al; RE‐DEEM Investigators . Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double‐blind, phase II trial. Eur Heart J. 2011;32:2781–2789. [DOI] [PubMed] [Google Scholar]
- 57. Lip GYH, Huber K, Andreotti F, et al. Antithrombotic management of atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing coronary stenting: executive summary—a Consensus Document of the European Society of Cardiology Working Group on Thrombosis, endorsed by the European Heart Rhythm Association (EHRA) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2010;31:1311–1318. [DOI] [PubMed] [Google Scholar]
- 58. Hohnloser SH, Oldgren J, Yang S, et al. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE‐LY (Randomized Evaluation of Long‐Term Anticoagulation Therapy) trial. Circulation. 2012;125:669–676. [DOI] [PubMed] [Google Scholar]
- 59. Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta‐analysis of noninferiority randomized controlled trials. Arch Intern Med. 2012;172:397–402. [DOI] [PubMed] [Google Scholar]
- 60. Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011. [DOI] [PubMed] [Google Scholar]
- 61. Saini SD, Schoenfeld P, Kaulback K, et al. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15:e22–e33. [PubMed] [Google Scholar]
- 62. Coleman CI, Roberts MS, Sobieraj DM, et al. Effect of dosing frequency on chronic cardiovascular disease medication adherence. Curr Med Res Opin. 2012;28:669–680. [DOI] [PubMed] [Google Scholar]
- 63. Barrett YC, Wang Z, Frost C, et al. Clinical laboratory measurement of direct Factor Xa inhibitors: anti‐Xa assay is preferable to prothrombin time assay. Thromb Haemost. 2010;104:1263–1271. [DOI] [PubMed] [Google Scholar]
- 64. Coleman CI, Limone B, Sobieraj DM, et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm. 2012;18:527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lindhoff‐Last E, Samama MM, Ortel TL, et al. Assays for measuring rivaroxaban: their suitability and limitations. Ther Drug Monit. 2010;32:673–679. [DOI] [PubMed] [Google Scholar]
- 66. Stangier J, Rathgen K, Stahle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Watanabe M, Siddiqui FM, Qureshi AI. Incidence and management of ischemic stroke and intracerebral hemorrhage in patients on dabigatran etexilate treatment. Neurocrit Care. 2012;16:203–209. [DOI] [PubMed] [Google Scholar]
- 68. Wilcox R, Pendleton RC, Smock KJ, et al. Hospital‐based clinical implications of the novel oral anticoagulant, dabigatran etexilate, in daily practice. Hosp Pract (1995). 2011;39:23–34. [DOI] [PubMed] [Google Scholar]
- 69. Samama MM, Martinoli JL, Le Flem L, et al. Assessment of laboratory assays to measure rivaroxaban—an oral, direct Factor Xa inhibitor. Thromb Haemost. 2010;103:815–825. [DOI] [PubMed] [Google Scholar]
- 70. Heidbuchel H, Verhamme P, Alings M, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013;15:625–51. [DOI] [PubMed] [Google Scholar]
- 71. Nematullah A, Alabousi A, Blanas N, et al. Dental surgery for patients on anticoagulant therapy with warfarin: a systematic review and meta‐analysis. J Can Dent Assoc. 2009;75:41. [PubMed] [Google Scholar]
- 72. Healey JS, Eikelboom J, Douketis J, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long‐term anticoagulation therapy (RE‐LY) randomized trial. Circulation. 2012;126:343–348. [DOI] [PubMed] [Google Scholar]
- 73. Hohnloser SH, Camm AJ. Safety and efficacy of dabigatran etexilate during catheter ablation of atrial fibrillation: a meta-analysis of the literature. Europace 2013;15:1407–11. [DOI] [PubMed] [Google Scholar]
- 74. Bin Abdulhak AA, Khan AR, Tleyjeh IM, et al. Safety and efficacy of interrupted dabigatran for peri-procedural anticoagulation in catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Europace 2013;15:1412–20. [DOI] [PubMed] [Google Scholar]
- 75.Bayer HealthCare AG: Xarelto (rivaroxaban) prescriber guide. https://www.xarelto.com/html/downloads/Xarelto‐Prescriber‐Guide.pdf. Accessed May 20, 2013.
- 76. Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF Trial (rivaroxaban once‐daily, oral, direct Factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation). J Am Coll Cardiol. 2013;61:651–658. [DOI] [PubMed] [Google Scholar]
- 77. Ezekowitz MD, Wallentin L, Connolly SJ, et al; the RE‐LY Steering Committee and Investigators . Dabigatran and warfarin in vitamin K antagonist‐naive and ‐experienced cohorts with atrial fibrillation. Circulation. 2010;122:2246–2253. [DOI] [PubMed] [Google Scholar]
- 78.Mahaffey KW, Wojdyla D, Hankey GJ, et al. Outcomes in vitamin K antagonist‐naive and ‐experienced patients: results from ROCKET AF. In: Proceedings of the European Stroke Conference; May 22–25, 2012; Lisbon, Portugal. Abstract OAID65.
- 79. Garcia DA, Alexander JA, Lopes RD, et al. Apixaban versus warfarin in Patients with Atrial Fibrillation in relation to Prior Warfarin Use: Insights from the ARISTOTLE trial. Circulation. 2012;126(suppl):A14771. [Google Scholar]
- 80.Institute for Safe Medication Practices: QuarterWatch (first quarter 2011) signals for dabigatran and metoclopramide. http://www.ismp.org/Newsletters/acutecare/showarticle.asp?ID=12. Accessed May 20, 2013.
- 81. Harper P, Young L, Merriman E. Bleeding risk with dabigatran in the frail elderly. N Engl J Med. 2012;366:864–866. [DOI] [PubMed] [Google Scholar]
- 82. Cotton BA, McCarthy JJ, Holcomb JB. Acutely injured patients on dabigatran. N Engl J Med. 2011;365:2039–2040. [DOI] [PubMed] [Google Scholar]
- 83. Peacock WF, Gearhart MM, Mills RM. Emergency management of bleeding associated with old and new oral anticoagulants. Clin Cardiol. 2012;35:730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo‐controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–1579. [DOI] [PubMed] [Google Scholar]
- 85. Marlu R, Hodaj E, Paris A, et al. Effect of non‐specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban. A randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108:217–224. [DOI] [PubMed] [Google Scholar]
- 86. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–1127. [DOI] [PubMed] [Google Scholar]
- 87. Miller CS, Grandi SM, Shimony A, et al. Meta‐analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110:453–460. [DOI] [PubMed] [Google Scholar]
- 88. Baker WL, Phung OJ. Systematic review and adjusted indirect comparison meta‐analysis of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5:711–719. [DOI] [PubMed] [Google Scholar]
- 89. Lip GYH, Larsen TB, Skjoth F, et al. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2012;60:738–746. [DOI] [PubMed] [Google Scholar]
- 90. Schneeweiss S, Gagne JJ, Patrick AR, et al. Comparative efficacy and safety of new oral anticoagulants in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5:480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rasmussen LH, Larsen TB, Graungaard T, et al. Primary and secondary prevention with new oral anticoagulant drugs for stroke prevention in atrial fibrillation: indirect comparison analysis. BMJ. 2012;345:e7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Reynolds MW, Fahrbach K, Hauch O, et al. Warfarin anticoagulation and outcomes in patients with atrial fibrillation: a systematic review and metaanalysis. Chest. 2004;126:1938–1945. [DOI] [PubMed] [Google Scholar]


