Abstract
Background
Revascularization availability at US hospitals varies and may impact care quality for acute coronary syndrome patients.
Hypothesis
The hypothesis of this study was that there would be differences in care quality at Get With The Guidelines–Coronary Artery Disease (GWTG‐CAD) hospitals based on revascularization capability.
Methods
For acute coronary syndrome patients admitted to GWTG‐CAD hospitals between 2000 and 2010, care quality at hospitals with or without revascularization capability was examined by assessing conformity with performance and quality measures.
Results
This study included 95 999 acute coronary syndrome patients admitted to 310 GWTG‐CAD hospitals. There were 89 000 patients admitted to 226 revascularization‐capable hospitals and 6999 patients admitted to 84 hospitals without revascularization capability included. Adjusted multivariate analysis demonstrated that 8 of the 19 measures were more frequently performed in the revascularization cohort: aspirin (odds ratio [OR]: 1.41, 95% confidence interval [CI]: 1.04‐1.92), clopidogrel (OR: 2.31, 95% CI: 1.78‐3.00), lipid‐lowering therapies at discharge (OR: 1.39, 95% CI: 1.04‐1.87), lipid‐lowering therapies for low‐density lipoprotein >100 mg/dL (OR: 1.85, 95% CI: 1.23‐2.77), achievement of blood pressure <140/90 mm Hg (OR: 1.20, 95% CI: 1.03‐1.40), LDL recorded (OR: 1.47, 95% CI: 1.05‐2.06), and recommendations offered for physical activity (OR: 3.82, 95% CI: 2.23‐6.55) or weight management (OR: 1.74, 95% CI: 1.12‐2.69).
Conclusions
The GWTG‐CAD revascularization hospitals were associated with better performance in some, but not all, measures assessed. Although the difference in conformity between hospital types was modest for performance measures but more variable for quality measures, room for improvement exists in key aspects of care.
Introduction
Deviation from the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for acute coronary syndrome (ACS) treatment exists across both patient populations and hospital types.1, 2, 3 Though most ACS admissions occur at nonacademic medical centers lacking revascularization capabilities, data from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines (CRUSADE) registry suggest that high‐risk patients presenting to these hospitals are paradoxically less likely to be transferred to specialized centers for percutaneous or surgical revascularization.1, 2 And although guideline adherence is also less likely at these centers, data from the Get With The Guidelines–Coronary Artery Disease (GWTG‐CAD) registry suggests that despite the availability of either revascularization strategy, ACS patients undergoing surgical revascularization were less likely to be treated with important secondary prevention strategies. This may be partially explained by separate surgical and cardiology care pathways at contemporary facilities.2, 3 However, given disparate care based on revascularization strategy, the lack of revascularization capabilities at most hospitals, and the suboptimal transfer rates of high‐risk ACS patients to revascularization centers, a comparative analysis of guideline adherence predicated on revascularization capability of admitting hospitals in the GWTG‐CAD registry may help identify areas in ACS care delivery that require improvement.
Methods
Study Population
The GTWG‐CAD national, prospective, observational database was used for this study. This program seeks to improve the care of patients with CAD by offering feedback to participating hospitals regarding guideline adherence and recommending strategies optimizing care.4, 5, 6 Through a Web‐based data collection tool (Patient Management Tool; Outcome Sciences Inc., Cambridge, MA) providing real‐time feedback on quality‐of‐care indices, the GWTG‐CAD initiative seeks to improve predefined performance and quality measures at participating hospitals.6 Collaborating with outcomes researchers and professional organizations, the GWTG‐CAD program provides instruction on evidence‐based care strategies, recommendations on implementation, and sessions sharing best practices among participants. Teleconferences are held discussing these practices and topics to improve care quality. Non‐network hospitals are recruited and participants are encouraged to enroll all eligible patients. The AHA recognizes hospitals attaining 85% adherence with performance measures over various periods. Data were analyzed on 282 585 patients diagnosed with ACS (unstable angina, non–ST‐elevation myocardial infarction [MI], or ST‐elevation MI) admitted to 432 fully participating hospitals in the GWTG‐CAD network from January 13, 2000 to March 21, 2010. Hospitals were dichotomized based on revascularization capability—(1) no revascularization capability available and (2) any revascularization capability available (ie, percutaneous and/or surgical)—and were referred to as “no revascularization” and “revascularization” cohorts, respectively. All data derived from patients lacking a diagnosis of ACS (104 096 patients), transferred in from hospitals not in the GWTG‐CAD program (41 845 patients), or transferred from GWTG‐CAD facilities (12 918 patients) were excluded. The rates of compliance with the indices or endpoints assessed in each group were not influenced by any excluded patients. Hospitals were excluded due to a lack of information regarding available on‐site capabilities (25 135 patients) or misclassification (2592 patients). The final study population consisted of 95 999 patients admitted to 310 hospitals.
Performance and Quality Measures
The goal of this study was to examine the quality of care administered to treat ACS patients at GWTG‐CAD hospitals with or without revascularization capability by conducting a hospital‐level analysis of compliance with recognized GWTG‐CAD performance and quality measures. In aggregate, hospital compliance with both types of measures was used to gauge overall quality of ACS care. The performance measures, initially utilized by the Centers for Medicare and Medicaid services to assess hospital performance (ie, pay for performance) in acute MI (AMI) care and co‐opted by the GWTG‐CAD program, included angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) treatment in patients with documented left ventricular systolic dysfunction (LVSD); β‐blockers at discharge; lipid‐lowering therapies for low‐density lipoprotein cholesterol (LDL) levels >100 mg/dL; aspirin (ASA) within 24 hours of admission and at discharge, as well as documentation of smoking‐cessation counseling offered; and a composite performance measure documenting 100% compliance 100% compliance (i.e. defect‐free care).5 The GWTG‐CAD quality measures, based on ACC/AHA guidelines for ACS care, included ACEI or ARB at discharge for AMI; achieving systolic blood pressure (SBP) and diastolic blood pressure (DBP) <140 and 90 mm Hg, respectively; clopidogrel at discharge for eligible patients; β‐blocker administration within 24 hours of admission; LDL level recorded; documentation of physical‐rehabilitation and weight‐management recommendations offered; documentation of diabetes mellitus (DM) teaching and treatment (available from 2008); lipid‐lowering therapies at discharge; and door‐to‐thrombolytic time ≤30 minutes.4 Rates of in‐hospital mortality and length of stay >4 days based on revascularization capability were also analyzed. The rates of compliance with the indicated measures were included in the overall analysis even in those patients experiencing in‐hospital death.
Statistical Analyses
Classification of hospital capabilities was the primary independent variable of interest. Percentages were reported for categorical variables; medians with 25th and 75th percentiles were reported for continuous variables. Baseline characteristics were compared using Pearson χ2 tests for all categorical variables and χ2 rank‐based group means score statistics for all continuous variables. Multivariable logistic regression models using generalized estimating equations to account for intrahospital correlations were used to determine if hospital type independently influences adherence to performance and quality measures.7 Adjustment for the potential confounders of age, sex, race (white vs nonwhite), chronic obstructive pulmonary disease (COPD) or asthma, stroke or transient ischemic attack (TIA), diabetes mellitus (DM; insulin dependent and non–insulin dependent), heart failure, hyperlipidemia, hypertension, prior MI, peripheral vascular disease (PVD), renal insufficiency, smoking, geographic region, teaching hospital, number of hospital beds, and rural/urban designation. All variables had missing rates <7%. Missing values were imputed to the most frequent category or median, with the exception of sex and hospital characteristics, which were not imputed. Odds ratios (ORs) with their corresponding 95% confidence intervals (CIs) were reported for each measure.
All tests were 2‐sided, and P values <0.05 were considered statistically significant. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
Results
The study included 95 999 patients admitted to 310 hospitals in the GWTG‐CAD registry from January 13, 2000 to March 21, 2010. The characteristics of the analyzed hospitals, patient demographics, and hospital‐presentation data are shown in Tables 1 and 2. The majority of centers were revascularization‐capable, larger, academic, and urban (Table 1). Revascularization‐incapable sites were smaller and rural. Most patients were admitted to revascularization hospitals (Table 2) and were younger, male, and white. A higher prevalence of older, female, and minority patients were admitted to no‐revascularization centers and associated with more prior CAD, MI, stroke, DM, hypertension, PVD, coronary artery bypass grafting (CABG), heart failure, pulmonary disease, and renal insufficiency. Patients admitted to revascularization hospitals were more associated with prior PCI, smoking, hyperlipidemia, and BMI ≥30 kg/m2. Patients at revascularization centers more likely lacked insurance, Medicare, or Medicaid.
Table 1.
Hospital Characteristics
| No Revascularization, n = 84 | Revascularization, n = 226 | P Value | |
|---|---|---|---|
| Academic, % | 25.0 | 57.1 | <0.0001 |
| No. of bedsa | 104 (49, 183) | 320 (215, 489) | <0.0001 |
| Region, % | |||
| West | 15.5 | 19.5 | 0.1941 |
| South | 46.4 | 41.2 | |
| Midwest | 15.5 | 23.9 | |
| Northeast | 22.6 | 15.5 | |
| Location, % | |||
| Urban | 54.8 | 92.0 | <0.0001 |
| Rural | 45.2 | 8.0 |
Continuous variables are expressed as median (25th, 75th percentile).
Table 2.
Patient Demographic and Hospital Presentation Data
| No Revascularization, n = 6999 | Revascularization, n = 89 000 | P Value | |
|---|---|---|---|
| Patient demographics, % | |||
| Age, ya | 77 (64, 85) | 66 (55, 78) | <0.0001 |
| Male sex | 48.0 | 60.5 | <0.0001 |
| Race | |||
| White | 57.5 | 74.7 | <0.0001 |
| Black | 8.1 | 8.3 | |
| Hispanic | 9.7 | 7.5 | |
| Other | 15.1 | 2.9 | |
| CAD | 24.2 | 17.0 | <0.0001 |
| Prior MI | 24.4 | 21.2 | <0.0001 |
| Prior CABG | 2.8 | 2.4 | 0.0375 |
| Prior PCI | 1.7 | 3.7 | <0.0001 |
| DM | 37.1 | 32.0 | <0.0001 |
| Smoking | 18.6 | 29.5 | <0.0001 |
| Hyperlipidemia | 36.8 | 46.0 | <0.0001 |
| Hypertension | 70.6 | 68.0 | <0.0001 |
| PVD | 10.3 | 8.7 | <0.0001 |
| Prior stroke | 13.1 | 9.0 | <0.0001 |
| HF | 28.5 | 14.1 | <0.0001 |
| COPD or asthma | 19.2 | 13.3 | <0.0001 |
| Renal insufficiency | 16.6 | 9.0 | <0.0001 |
| BMI ≥30 kg/m2 | 20.9 | 30.3 | <0.0001 |
| Payment source, % | |||
| Medicare | 41.9 | 28.4 | <0.0001 |
| Medicaid | 13.8 | 6.2 | |
| Other | 32.2 | 42.7 | |
| Uninsured | 4.7 | 9.0 | |
| Presentation data | |||
| SBP, mm Hga | 135 (115, 156) | 138 (119, 158) | <0.0001 |
| DBP, mm Hga | 74 (62, 87) | 77 (65, 90) | <0.0001 |
| Total cholesterol, mg/dLa | 159 (130, 194) | 167 (138, 199) | <0.0001 |
| LDL, mg/dLa | 92 (68, 119) | 99 (75, 127) | <0.0001 |
| Triglycerides, mg/dLa | 109 (77, 158) | 122 (84, 182) | <0.0001 |
| HbA1c, %a | 7.1 (6.3, 8.2) | 7.3 (6.4, 8.8) | 0.0088 |
| Hospital facilities | |||
| Academic, % | 51.0 | 60.9 | <0.0001 |
| No. of bedsa | 203 (101, 279) | 367 (265, 530) | <0.0001 |
| Hospital location, % | |||
| Urban | 57.7 | 95.0 | <0.0001 |
| Rural | 42.3 | 5.0 | |
| Hospital region, % | |||
| West | 32.9 | 25.9 | <0.0001 |
| South | 28.0 | 31.2 | |
| Midwest | 5.6 | 28.1 | |
| Northeast | 33.5 | 14.8 | |
| Medications on admission, % | |||
| None | 1.3 | 2.0 | <0.0001 |
| ASA | 19.6 | 14.5 | 0.3403 |
| β‐Blocker | 9.9 | 6.2 | <0.0001 |
| ACEI | 14.0 | 9.9 | 0.0150 |
| ARB | 6.5 | 4.0 | <0.0001 |
| Other antiplateletb | 7.7 | 5.7 | 0.4665 |
| CCB | 6.6 | 4.7 | 0.2113 |
| Nitrates | 5.1 | 2.5 | <0.0001 |
| Statins | 17.2 | 13.5 | 0.0747 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, aspirin; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCB, calcium channel blocker; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; DM, diabetes mellitus; HbA1c, glycated hemoglobin; HF, heart failure; LDL, low‐density lipoprotein cholesterol; MI, myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; SBP, systolic blood pressure; TIA, transient ischemic attack.
Continuous variables are expressed as median (25th, 75th percentile). b Excluding ASA.
Conformity with performance measures was generally superior in the revascularization cohort (Figure 1). Four performance measures analyzed were performed at high levels (>90% conformity) at revascularization centers, including discharge β‐blocker, smoking‐cessation counseling, and early and discharge ASA. Percent conformity with defect‐free care in the revascularization cohort was relatively lower; but, when compared with the no‐revascularization cohort, it was performed at a significantly higher rate. However, the absolute difference in conformity between cohorts for this measure and all others was small. Multivariate analysis of conformity demonstrated that 4 of these 7 indices were performed significantly more frequently in the revascularization hospitals (Table 3). Chief amongst these were important therapies such as the prescription of β‐blocker (unadjusted OR: 1.58, 95% CI: 1.19‐2.08), ASA at discharge (unadjusted OR: 2.24, 95% CI: 1.73‐2.91), and lipid‐lowering therapies for LDL >100 mg/dL (unadjusted OR: 3.09, 95% CI: 2.16‐4.40). Additionally, the rate of defect‐free care was higher (unadjusted OR: 1.34, 95% CI: 1.08‐1.66). Two measures remained significantly more likely to be performed in the revascularization cohort following adjustment: lipid‐lowering therapies for LDL >100 mg/dL (adjusted OR: 1.85, 95% CI: 1.23‐2.77) and discharge ASA (adjusted OR: 1.41, 95% CI: 1.04‐1.92).
Figure 1.
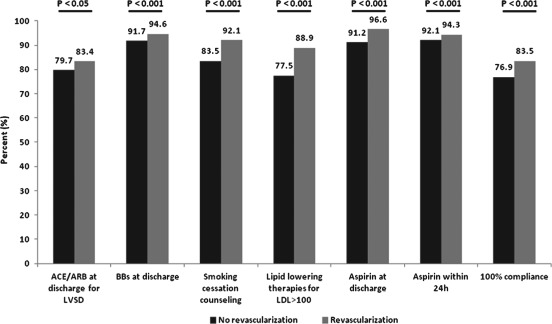
Percent conformity with performance measures for ACS patients admitted to GWTG hospitals with or without on‐site revascularization capability. Abbreviations: ACE, angiotensin‐converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; BB, β‐blocker; GWTG, Get With The Guidelines; LDL, low‐density lipoprotein cholesterol; LVSD, left ventricular systolic dysfunction.
Table 3.
Unadjusted and Adjusted ORs for Performance and Quality Measures for ACS Patients Admitted to GWTG Hospitals With On‐site Revascularization Capability (With the No‐Revascularization Cohort as the Reference Group)a
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Performance measures | ||
| ACEI/ARB at discharge for LVSD | 1.13 (0.85‐1.49) | 0.86 (0.63‐1.18) |
| β‐Blocker at discharge | 1.58 (1.19‐2.08) | 1.13 (0.81‐1.58) |
| Smoking‐cessation counseling | 2.13 (1.43‐3.19) | 1.26 (0.80‐1.99) |
| Lipid‐lowering therapies LDL >100 mg/dL | 3.09 (2.16‐4.40) | 1.85 (1.23‐2.77) |
| ASA at discharge | 2.24 (1.73‐2.91) | 1.41 (1.04‐1.92) |
| ASA within 24 h | 1.18 (0.83‐1.66) | 0.80 (0.55‐1.17) |
| 100% compliance | 1.34 (1.08‐1.66) | 0.98 (0.75‐1.28) |
| Quality measures | ||
| ACEI/ARB at discharge for AMI | 1.08 (0.91‐1.29) | 0.86 (0.71‐1.05) |
| ACEI at discharge for AMI | 1.26 (1.05‐1.52) | 1.00 (0.82‐1.22) |
| SBP <140 mm Hg and DBP <90 mm Hg | 1.47 (1.29‐1.69) | 1.20 (1.03‐1.40) |
| Clopidogrel at discharge | 3.33 (2.67‐4.15) | 2.31 (1.78‐3.00) |
| β‐Blocker within 24 h | 1.12 (0.86‐1.48) | 0.86 (0.61‐1.21) |
| LDL recorded | 2.32 (1.81‐2.98) | 1.47 (1.05‐2.06) |
| Rehabilitation/physical‐activity recommendations | 4.27 (2.45‐7.43) | 3.82 (2.23‐6.55) |
| Weight‐management/physical‐activity recommendations | 2.18 (1.51‐3.14) | 1.74 (1.12‐2.69) |
| Door to thrombolytics ≤30 min | 0.87 (0.58‐1.30) | 0.77 (0.49‐1.21) |
| DM treatment at discharge | 2.06 (0.82‐5.17) | 1.71 (0.65‐4.46) |
| DM teaching at discharge | 0.95 (0.51‐1.78) | 0.82 (0.42‐1.63) |
| Lipid‐lowering therapies at discharge | 2.48 (1.98‐3.10) | 1.39 (1.04‐1.87) |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ACS, acute coronary syndrome; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; ASA, aspirin; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; DM, diabetes mellitus; GWTG, Get With The Guidelines; HF, heart failure; LDL, low‐density lipoprotein cholesterol; LVSD, left ventricular systolic dysfunction; MI, myocardial infarction; OR, odds ratio; PVD, peripheral vascular disease; SBP, systolic blood pressure; TIA, transient ischemic attack.
The variables included in the linear regression modeling included age, sex, race (white vs nonwhite), history of COPD or asthma, stroke or TIA, DM (combined insulin‐dependent and non–insulin‐dependent), HF, hyperlipidemia, hypertension, prior MI, PVD, renal insufficiency, smoking, geographic region, teaching hospital, no. of hospital beds, and setting (urban vs rural).
Besides DM teaching and door‐to‐thrombolytic time of ≤30 minutes, percent conformity with the quality measures was generally higher in the revascularization cohort (Figure 2). However, aside from DM treatment, no quality measure was performed at >90% in either group. Although the early administration of a β‐blocker was high in both groups, conformity with other important therapies was lower. In fact, neither cohort exceeded 85% conformity for treatment to a BP <140/90 mm Hg or prescribing discharge ACEI/ARB for AMI, clopidogrel, or lipid‐lowering therapies. These were, however, all performed at significantly higher rates at the revascularization centers. Unlike the performance measures, the difference between hospital classes was more variable. A larger difference was seen for discharge clopidogrel and lipid‐lowering therapies. Negligible differences were seen for discharge ACEI or ACEI/ARB for AMI, β‐blocker within 24 hours, and treatment to BP <140/90 mm Hg. Regression analysis demonstrated that 7 quality measures were more likely performed in revascularization centers: discharge clopidogrel (unadjusted OR: 3.33, 95% CI: 2.67‐4.15), lipid‐lowering therapies (unadjusted OR: 2.48, 95% CI: 1.98‐3.10), ACEI for AMI (unadjusted OR: 1.26, 95% CI: 1.05‐1.52), and BP <140/90 mm Hg (unadjusted OR: 1.47, 95% CI: 1.29‐1.69; Table 3). Following adjustment, only discharge ACEI for AMI (adjusted OR: 1.00, 95% CI: 0.82‐1.22) did not remain more likely in the revascularization group.
Figure 2.
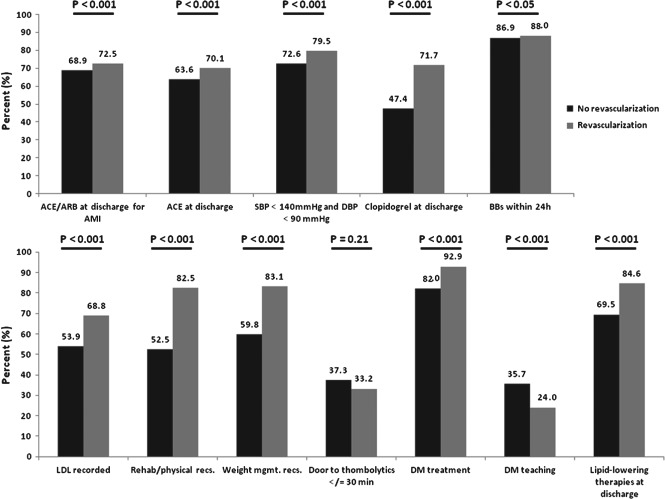
Percent conformity with quality measures for ACS patients admitted to GWTG hospitals with or without on‐site revascularization capability. Abbreviations: ACE, angiotensin‐converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; BB, β‐blocker; DBP, diastolic blood pressure; DM, diabetes mellitus; LDL, low‐density lipoprotein cholesterol; recs., recommendations; SBP, systolic blood pressure; weight mgmt., weight management.
Revascularization facilities were associated with a significantly decreased in‐hospital mortality rate (5.2% vs 10.7%; P < 0.001) and length of hospitalization (length of stay >4 days, 38.1% vs 44.6%; P < 0.001). Multivariate analysis demonstrated that the revascularization cohort was associated with lower in‐hospital mortality (unadjusted OR: 0.40, 95% CI: 0.32‐0.50) and shorter length of hospitalization (unadjusted OR: 0.84, 95% CI: 0.72‐0.98).
Discussion
This study of 95 999 ACS patients admitted to GWTG‐CAD hospitals analyzed conformity with performance and quality measures to determine whether the absence of on‐site revascularization capability was associated with significantly inferior quality of care. Though the difference in conformity between the 2 groups for performance measures was modest, more variation was noted for the quality measures. Multivariate analysis demonstrated significantly better performance at revascularization centers in some, but not all, of the analyzed indices.
Substantial variation in the quality of care administered to treat cardiovascular disease exists among hospitals. Addressing this, guidelines incorporating specific treatments supported by outcomes data have been drafted to guide practitioners. National initiatives such as AHA GWTG and the ACC Guidelines Applied in Practice (GAP) have fostered quality improvement in cardiovascular care through outcomes analysis and quality‐of‐care metrics coupled with recommendations to improve guideline adherence. Consequently, increasing guideline adherence has been associated with mortality reduction, particularly for ACS treatment.7 Given the benefits of timely revascularization but suboptimal rates of referral, the relationship between on‐site revascularization and quality of care was therefore studied.1, 2
Specifically, percent conformity with the performance measures was relatively high, consistent with previous studies, confirming that compliance has been improving.8 Compliance with quality measures was relatively lower and has also been noted.9 Multivariate analysis demonstrated that important aspects comprising acute ACS care, such as early ASA or β‐blocker treatment, were not more frequently performed in the revascularization cohort. Conversely, treatment with lipid‐lowering agents for above goal LDL levels and to achieve target blood pressure was superior at revascularization hospitals. At discharge, patients at revascularization centers were more likely to be prescribed most, but not all, crucial secondary‐prevention medications at discharge, including ASA, clopidogrel, and lipid‐lowering agents, but not β‐blockers or ACEI/ARB. As class I indications, acute or discharge ASA or β‐blocker therapy can be improved.10, 11 Substantial improvement for measures with relatively high conformity in both cohorts would prove difficult. However, with comparatively lower utilization of discharge ACEI/ARB for AMI or LVSD, respectively, which is consistent with other reports,8 improved treatment with these medications represents an area for improvement.
It is unclear why revascularization hospitals are not associated with substantially superior quality of care, especially for critical therapies comprising optimal ACS care. Studies suggest guideline adherence is superior when care is overseen by a cardiologist.12, 13, 14, 15 This may be a factor presently, but these details are not available. However, the performance of certain measures, such as the administration of early β‐blocker or ASA, was not different between cohorts. Whereas the performance of guideline‐recommended therapies in emergency departments has been more variable in the past,16 the present study suggests that initiatives directed toward emergency department physicians have improved acute ACS care. In contrast, less uniform performance of other important measures that are less likely to be administered acutely was observed. The performance of these is more likely directed by physicians administering care after admission. Though the details of the personnel caring for these patients are not available, they are probably variable, ranging from generalists to specialists. Therefore, practice improvement efforts directed toward these providers may help increase adherence with the assessed measures. Additionally, culture effects that are different between the cohorts may also be affecting care quality, as hospital organization, strength of internal communication, and coordination of services may have a sizeable effect on outcomes.17 Though this registry cannot assess such factors, an analysis of the patterns of hospital‐associated structural and functional attributes between these classes coupled with performance‐driven measures may provide additional insights and perhaps novel approaches to further improve care quality.
There are a few study limitations that should be considered. The majority of patients were admitted to the revascularization cohort. Due to missing details regarding site capability, 26 820 patients at 94 sites were excluded. Patients hospitalized at the lesser capable facilities were older and had more comorbid illness. It is unclear then whether the severity of CAD between classes was equivalent (ie, rates of left main or multivessel disease). It is also unclear whether patients with elevated cardiovascular risk on presentation in the field (ie, hemodynamic or electrical instability, elevated Thrombolysis in Myocardial Infarction risk scores) were initially triaged and then diverted to revascularization‐capable GWTG‐CAD facilities. It is also possible that the rates of compliance with important pharmacotherapy were influenced by differing rates of comorbid conditions for the patients admitted at each hospital class (ie, decreased β‐blocker or ASA administration for patients with COPD or peptic‐ulcer disease, respectively). Related to this, although the multivariate modeling did account for a collection of clinically significant confounders, it is possible that unmeasured factors may have affected these results.
Conclusion
In summary, some but not all of the specified quality‐of‐care indices were performed significantly more frequently at revascularization‐capable GTWG‐CAD hospitals. For those measures associated with superior performance at these more capable facilities, the increment in conformity relative to the lesser capable centers was modest. In particular, strategies designed to improve the performance of measures with low rates of compliance (<90%) should be implemented. Infrequently, this applies to a single hospital class (ie, no revascularization cohort for smoking‐cessation counseling). However, for the majority of measures, substantial room for improved compliance at both types of hospitals is present and represents important targets for practice improvement that may help improve the quality of ACS care delivered at all facilities.
Dr. W. Frank Peacock discloses the following relationships ‐ Research Grants: Cardiorentis, Roche, The Medicine's Company. Consultant: Abbott, Alere, BG Medicine, Cardiorentis, Janssen, The Medicine's Company. Ownership Interest: Comprehensive Research Associates LLC, Emergencies in Medicine LLC. Dr. Deepak L. Bhatt discloses the following relationships: research grants from Amarin, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, and The Medicines Company; advisory board for Elsevier PracticeUpdate Cardiology, Medscape Cardiology, and Regado Biosciences; board of directors for Boston VA Research Institute and Society of Cardiovascular Patient Care; chair of the American Heart Association Get With The Guidelines Steering Committee; honoraria from the American College of Cardiology (editor, Clinical Trials, CardioSource), Belvoir Publications (editor‐in‐chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Population Health Research Institute (clinical trial steering committee), SLACK Incorporated (chief medical editor, Cardiology Today's Intervention), and WebMD (CME steering committees). Additional relationships for Dr. Bhatt: senior associate editor for Journal of Invasive Cardiology; data‐monitoring committees with Duke Clinical Research Institute, Mayo Clinic, and Population Health Research Institute; and unfunded research for FlowCo, PLx Pharma, and Takeda. Dr. Christopher P. Cannon discloses the following relationships: research grants from Accumetrics, AstraZeneca, CSL Behring, Essentialis, GlaxoSmithKline, Merck, Regeneron, Sanofi, and Takeda; advisory board for Alnylam, Bristol‐Myers Squibb, LipimetiX Development LLC, and Pfizer; and clinical advisor for and equity in Automedics Medical Systems.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Patel MR, Chen AY, Roe MT, et al. A comparison of acute coronary syndrome care at academic and nonacademic hospitals. Am J Med. 2007;120:40–46. [DOI] [PubMed] [Google Scholar]
- 2. Roe MT, Chen AY, Delong ER, et al. Patterns of transfer for patients with non‐ST‐segment elevation acute coronary syndrome from community to tertiary care hospitals. Am Heart J. 2008;156:185–192. [DOI] [PubMed] [Google Scholar]
- 3. Hiratzka LF, Eagle KA, Liang L, et al; Get With The Guidelines Steering Committee . Atherosclerosis secondary prevention performance measures after coronary bypass graft surgery compared with percutaneous catheter intervention and nonintervention patients in the Get With The Guidelines Database. Circulation. 2007;116(11 suppl):I207–I212. [DOI] [PubMed] [Google Scholar]
- 4. LaBresh KA, Ellrodt AG, Gliklich R, et al. Get With The Guidelines for cardiovascular secondary prevention: pilot results. Arch Intern Med. 2004;164:203–209. [DOI] [PubMed] [Google Scholar]
- 5. LaBresh KA, Fonarow GC, Smith SC Jr, et al; Get With The Guidelines Steering Committee . Improved treatment of hospitalized coronary artery disease patients with the Get With The Guidelines program. Crit Pathw Cardiol. 2007;6:98–105. [DOI] [PubMed] [Google Scholar]
- 6. LaBresh KA, Gliklich R, Liljestrand J, et al. Using “Get With The Guidelines” to improve cardiovascular secondary prevention. Jt Comm J Qual Saf. 2003;29:539–550. [DOI] [PubMed] [Google Scholar]
- 7. Amsterdam EA, Peterson ED, Ou FS, et al. Comparative trends in guidelines adherence among patients with non–ST‐segment elevation acute coronary syndromes treated with invasive versus conservative management strategies: results from the CRUSADE quality improvement initiative. Am Heart J. 2009;158:748.e1–754.e1. [DOI] [PubMed] [Google Scholar]
- 8. Kumbhani DJ, Fonarow GC, Cannon CP, et al; Get With The Guidelines Steering Committee and Investigators . Predictors of adherence to performance measures in patients with acute myocardial infarction. Am J Med. 2013;126:74.e1–74.e9. [DOI] [PubMed] [Google Scholar]
- 9. Somma KA, Bhatt DL, Fonarow GC, et al. Guideline adherence after ST‐segment elevation versus non‐ST segment elevation myocardial infarction. Circ Cardiovasc Qual Outcomes. 2012;5:654–661. [DOI] [PubMed] [Google Scholar]
- 10. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 11. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011;58:2432–2446. [DOI] [PubMed] [Google Scholar]
- 12. Nash IS, Corrato RR, Dlutowski MJ, et al. Generalist versus specialist care for acute myocardial infarction. Am J Cardiol. 1999;83:650–654. [DOI] [PubMed] [Google Scholar]
- 13. Casale PN, Jones JL, Wolf FE, et al. Patients treated by cardiologists have a lower in‐hospital mortality for acute myocardial infarction. J Am Coll Cardiol. 1998;32:885–889. [DOI] [PubMed] [Google Scholar]
- 14. Roe MT, Chen AY, Mehta RH, et al. Influence of inpatient service specialty on care processes and outcomes for patients with non–ST‐segment elevation acute coronary syndromes. Circulation. 2007;116:1153–1161. [DOI] [PubMed] [Google Scholar]
- 15. Norcini JJ, Kimball HR, Lipner RS. Certification and specialization: do they matter in the outcome of acute myocardial infarction? Acad Med. 2000;75:1193–1198. [DOI] [PubMed] [Google Scholar]
- 16. Diercks DB, Roe MT, Chen AY, et al. Prolonged emergency department stays of non–ST‐segment‐elevation myocardial infarction patients are associated with worse adherence to the American College of Cardiology/American Heart Association guidelines for management and increased adverse events. Ann Emerg Med. 2007;50:489–496. [DOI] [PubMed] [Google Scholar]
- 17. Curry LA, Spatz E, Cherlin E, et al. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? Ann Intern Med. 2011;154:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]


