Abstract
Background
To achieve sufficient myocardial perfusion in ST‐segment elevation myocardial infarction (STEMI) patients receiving primary percutaneous coronary intervention (PPCI), many adjunctive therapies have been proposed. Previous trials have reported variances in myocardial perfusion improvement for statin pretreatment, which made it inconvincible to confirm the beneficial effects of statins. Therefore, we performed a systematic review and meta‐analysis to determine whether statin pretreatment was effective in improving myocardial perfusion.
Hypothesis
Statin pretreatment could improve myocardial perfusion in STEMI patients undergoing PPCI.
Methods
We searched the PubMed, Web of Knowledge, and the Cochrane Library databases for studies evaluating the impact of statin pretreatment on myocardial perfusion in STEMI patients receiving PPCI.
Results
Twelve trials were finally included in our meta‐analysis. There were no significant differences in patients' baseline characteristics between the statin pretreatment and control groups. Overall pooled analysis showed that patients in the statin pretreatment groups had significantly better epicardial coronary blood flow (measured by Thrombosis in Myocardial Infarction [TIMI] grade, odds ratio [OR]: 0.49, 95% confidence interval [CI]: 0.28 to 0.84; measured by corrected TIMI frame count, mean difference: −5.63; 95% CI: −9.66 to −1.6). A trend toward myocardial tissue level perfusion improvement was seen in the statin pretreatment arm rather than the control arm (measured by myocardial blush grade, OR: 0.74; 95% CI: 0.50 to 1.09).
Conclusions
This present meta‐analysis suggests that statin pretreatment might be effective in improving myocardial perfusion in STEMI patients.
Introduction
Primary percutaneous coronary intervention (PPCI) is currently the most effective method to treat ST‐segment elevated myocardial infarction (STEMI).1 The therapeutic goal is to restore epicardial blood flow and myocardial tissue level perfusion rapidly and successfully to ensure myocardial salvage.2, 3, 4 Epicardial blood flow is usually measured by Thrombolysis in Myocardial Infarction (TIMI) grade or corrected TIMI frame count (cTFC) during coronary angioplasty.5, 6 Myocardial tissue level perfusion is commonly assessed by TIMI myocardial perfusion grade or the myocardial blush grade (MBG).2, 4 Although PPCI could reopen most of the infarct‐related arteries in STEMI patients, complete and sustained restoration of blood flow in the epicardial coronary artery and/or sufficient reperfusion of the infracted myocardium are sometimes difficult to achieve. The no‐reflow phenomenon refers to an open epicardial artery without flow into the myocardium, which predicts complications and adverse left ventricular remodeling.7, 8 Thus, effective adjunctive therapies are necessary to improve epicardial blood flow and myocardial tissue level perfusion in STEMI patients receiving PPCI.
Previous evidence has suggested that statins have a number of protective effects involving the improvement of endothelial function, stabilization of atherosclerotic plaque, decrease of oxidative stress and inflammation, and inhibition of thrombogenic response, which were not directly related to their impact on lipid metabolism.9 These pleiotropic effects could contribute to the preservation of microvascular function during ischemia and reperfusion after STEMI.10, 11
In the last few years, there have been several studies evaluating the effects of prior statin use on epicardial blood flow and myocardial tissue level perfusion PPCI in STEMI patients receiving PPCI. However, the results contained discrepancies, which made it difficult to reach definitive conclusions based on the results of individual studies. Thus, we performed a meta‐analysis of trials that evaluated the impact of statin pretreatment on epicardial coronary blood flow and myocardial tissue level perfusion in STEMI patients treated with PPCI.
Methods
Search Strategy
We searched the PubMed, Web of Knowledge, the Cochrane Library databases (all from their inception to March 2013). For “statin,” the search terms used were as follows: hydroxymethylglutaryl‐coenzyme A reductase inhibitors, statin, atorvastatin, pravastatin, lovastatin, simvastatin, cerivastatin, fluvastatin. For “myocardial perfusion,” we included the terms as follows: TIMI flow, TIMI frame count, TIMI myocardial perfusion grades, myocardial blush grades, TIMI myocardial perfusion frame count. For “ST‐segment elevation myocardial infarction,” we included the terms as follows: acute myocardial infarction, ST‐segment elevation myocardial infarction. We used the Boolean operator “AND” to combine the 3 comprehensive search themes. No language or journal type restriction was used.
Study Selection, Data Extraction, and Quality Assessment
Inclusion criteria for retrieved studies were: (1) randomized controlled trials (RCTs) or non‐RCTs comparing statin pretreatment effects on STEMI patients receiving PPCI, and (2) angiographic assessment data on epicardial coronary blood reflow and/or myocardial tissue level reperfusion. Data abstraction was independently performed by 2 unblinded reviewers. Discrepancies were resolved by consensus. We assessed the quality of cohort studies by reporting the key components of study designs instead of providing aggregate scores, including characteristics of study populations, type of statin, statin duration, and statistical control for potential confounding factors.12 Methodological quality for RCTs was evaluated using the Jadad score, which assigns 0 or 1 point to each of the following 5 items: (1) with or without randomization, (2) with or without a double‐blind design, (3) the appropriateness of the randomization methods if used, (4) the appropriateness of a double‐blind design if used, and (5) the analysis and reasons for withdrawals or dropouts.13 Thus, the Jadad scores can range from 0 to 5. The quality of studies to be included was assessed independently by 2 reviewers. Studies would be deemed of excellent quality if they received a Jadad score of 5 out of a possible 5 points, good quality if the score was 3 or 4, and poor quality if the score was ≤2.
Data Synthesis and Data Analysis
We combined study results by using the actual counts of every outcome in all of the trials. Data were pooled and calculated by random effect model for odds ratio (OR) or weighted mean difference, respectively. Statistical heterogeneity was measured by the I 2 statistic (I 2 > 50% was considered representative of significant statistical heterogeneity). We used RevMan version 5.2.0 provided at the Cochrane Collaboration Web site (http://ims.cochrane.org/revman) for statistical analysis.
Results
The selection of studies flowchart for inclusion in the meta‐analysis is shown in Figure 1. Database searches retrieved an initial 169 articles. These included editorials, reviews, and letters. Through abstracts screening, 15 articles were deemed relevant. We completed full‐text screening of the 15 articles, which resulted in 14 full‐text articles of the studies and one abstract from American College of Cardiology meeting.14 Of the 15 studies, two studies were excluded for having no data on myocardial perfusion status,15, 16 and 1 study was excluded for myocardial perfusion status assessed by intracoronary myocardial contrast echocardiography.17 Finally, twelve studies meeting inclusion/exclusion criteria were retained in the analysis, of which 9 were cohort studies, and 3 were RCTs.14, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28
Figure 1.
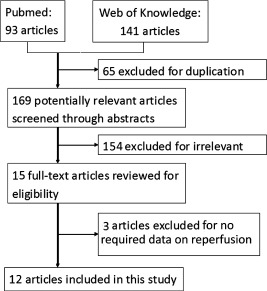
Study selection process.
The overall characteristics of the included studies are listed in Table 1. Patients in most studies received chronic statin treatment before undergoing PPCI. Eight studies had data on TIMI grade, 5 studies had data on cTFC, and 5 studies had data on MBG. In the study by Dong et al, only patients who achieved post‐percutaneous coronary intervention (PCI) TIMI grade ≥2 were included.18 The study by Kim et al was an RCT that compared high‐dose statin pretreatment effects on myocardial perfusion with a low‐dose statin.23 Demographic and clinical properties of included patients are shown in Table 2; relevant data of 2 studies were not available. The demographic and clinical data of the statin pretreatment group in the study by Dong et al were not available because the statin pretreatment group was divided into 3 subgroups according to their statin pretreatment duration. No obvious differences on the baseline characteristics comparison were observed between these subgroups and the control group.18 Although relevant data of the included patients in the study by Oduncu et al were not available, lipid levels including low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, total cholesterol, and triglyceride of the patients on admission were mentioned, which revealed no obvious differences between the 2 groups.25 There were no significant differences with respect to most of the demographic and clinical parameters between the statin pretreatment group and the control group. Two studies showed statistical differences on hypercholesterolemia history between the statin pretreatment group and the control group.21, 22 Although the patients in the study by Lev et al in the statin group had a substantially higher risk profile than the control group, the results of myocardial perfusion comparison still came out in favor of the statin group.24 The Jadad scores of the 3 RCTs were all 5, which revealed good quality of the studies.
Table 1.
Overall Characteristics of Included Studies
| Author | Year | Design | Total Patients | Type of Statin | Statin Dosage (mg/d) | Statin Duration | Control | Measure Method |
|---|---|---|---|---|---|---|---|---|
| Dong | 2006 | Retrospective | 87 | Atorvastatin | 10 | >6 months (n = 21), 3–6 months (n = 25); <3 months (n = 18) | No statin | TIMI |
| Celik | 2005 | Retrospective | 200 | Atorvastatin | 40 | ≥6 months | No statin | cTFC |
| Hahn | 2011 | RCT | 173 | Atorvastatin | 80 | Once | 10 mg/d | MBG |
| Hoffmann | 2008 | Retrospective | 253 | Atorvastatin (46.5%), pravastatin (23.3%), cerivastatin (15.1%), simvastatin (14.0%), lovastatin (2.3%) | Atorvastatin (12 ± 3), pravastatin ( 26 ± 6), cerivastatin (0.3 ± 0.1), simvastatin (38 ± 7), lovastatin (18 ± 4) | 1 week | No statin | TIMI, cTFC, MBG |
| Ishii | 2006 | Retrospective | 386 | Pravastatin (62.5%), fluvastatin (25.0%), atorvastatin (7.5%), simvastatin ( 5.0%) | NA | Chronically | No statin | TIMI, cTFC |
| Kadro | 2008 | Retrospective | 44 | NA | NA | NA | No statin | TIMI, cTFC |
| Kim | 2010 | RCT | 171 | Atorvastatin | 80 | Once | Placebo | TIMI, cTFC, MBG |
| Lev | 2009 | Retrospective | 950 | NA | NA | Chronically | No statin | TIMI |
| Oduncu | 2011 | Retrospective | 1617 | Atorvastatin (51%), simvastatin (15%), fluvastatin (9%), rosuvastatin (12%), pravastatin (13%) | Atorvastatin (18 ± 12), simvastatin (20 ± 10), fluvastatin (61 ± 20), rosuvastatin (15 ± 8), pravastatin (20 ± 11) | Chronically | No statin | TIMI, MBG |
| Otsuka | 2010 | Retrospective | 458 | Atorvastatin (41%), pravastatin (32%), simvastatin (16%), fluvastatin (5%), rosuvastatin (3%), pitavastatin (3%) | NA | Chronically | No statin | MBG |
| Post | 2012 | RCT | 42 | Atorvastatin | 80 | Once | 10 mg/d atorvastatin | TIMI, MBG |
| Zhao | 2009 | Retrospective | 153 | Pravastatin (50%), atorvastatin (10%), simvastatin (40%), | NA | Chronically | No statin | TIMI |
Abbreviations: cTFC, corrected TIMI frame count; MBG, myocardial blush grade; NA, not available; RCT, random controlled trial; TIMI, Thrombolysis In Myocardial Infarction.
Table 2.
Baseline Characteristics of Included Studies
| Author | Groups | n | Age, y | Male, % | Diabetes Mellitus, % | Hypercholesterolemia, % | Hypertension, % | Active Smoker, % | Previous MI, % | Door‐to‐Needle Time | TIMI Before PCI, % (TIMI ≤2) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dong | Statin (+) | 64 | NA | NA | 28.1 | NA | 46.9 | 25.0 | NA | NA | 100 |
| Statin (−) | 23 | 55.8 ± 11.2 | 52.2 | 26.1 | NA | 43.5 | 21.7 | NA | NA | 100 | |
| P | |||||||||||
| Celik | Statin (+) | 102 | 61 ± 7 | 82.4 | 35.3 | NA | 52.9 | 38.2 | 17.7 | NA | NA |
| Statin (−) | 98 | 63 ± 7 | 77.6 | 29.6 | NA | 43.9 | 29.6 | 10.2 | NA | NA | |
| P | 0.12 | 0.16 | 0.31 | 0.2 | 0.2 | 0.18 | |||||
| Hahn | Statin (+) | 89 | 55.5 ± 12.1 | 85.4 | 28.1 | 50.6 | 43.8 | 64 | 0 | 84 (63−105) | 86.5 |
| Statin (−) | 84 | 59.7 ± 12.8 | 82.1 | 21.4 | 51.2 | 46.4 | 48.8 | 0 | 75 (59–111) | 78.6 | |
| P | 0.03 | 0.56 | 0.30 | 0.93 | 0.73 | 0.04 | 0.35 | 0.21 | |||
| Hoffmann | Statin (+) | 86 | 58.7 ± 11.7 | 88.4 | 15.1 | 95.3 | NA | 59.3 | NA | 2.0 ± 2.0 | 66.3 |
| Statin (−) | 167 | 60.8 ± 11.6 | 77.2 | 18.6 | 53.9 | NA | 53.9 | NA | 1.9 ± 2.0 | 73.1 | |
| P | 0.262 | 0.049 | 0.612 | 0.001 | 0.493 | 0.863 | 0.333 | ||||
| Ishii | Statin (+) | 40 | 65 ± 10 | 72.5 | 37.5 | 100 | 35.0 | 30.0 | 0 | NA | 97.5 |
| Statin (−) | 346 | 63 ± 10 | 81.5 | 32.1 | 24.0 | 30.1 | 39.3 | 0 | NA | 96.5 | |
| P | 0.25 | 0.17 | 0.49 | <0.001 | 0.52 | 0.25 | 1 | 0.42 | |||
| Kadro | Statin (+) | 21 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Statin (−) | 23 | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| P | |||||||||||
| Kim | Statin (+) | 86 | 61 ± 11 | 76.7 | 24.5 | 39.5 | 52.3 | 40.7 | 2.3 | NA | 48.2 (TIMI = 0) |
| Statin (−) | 85 | 59 ± 11 | 77.6 | 18.9 | 38.1 | 46.4 | 50.6 | 2.4 | NA | 52.9 (TIMI = 0) | |
| P | 0.99 | 0.89 | 0.47 | 0.88 | 0.45 | 0.38 | 1 | 0.66 | |||
| Lev | Statin (+) | 327 | 62 ± 12 | 78 | 34.3 | 100 | 58.1 | 39.8 | 18 | 0.5 ± 1.4 | 64.8 (TIMI <2) |
| Statin (−) | 623 | 60 ± 13 | 83.9 | 20.1 | 44.6 | 38 | 48 | 8.2 | 1.4 ± 1.4 | 62 (TIMI <2) | |
| P | 0.006 | 0.03 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.5 | 0.8 | ||
| Oduncu | Statin (+) | 306 | NA | NA | NA | NA | NA | NA | NA | 76± 44 | NA |
| Statin (−) | 1311 | NA | NA | NA | NA | NA | NA | NA | 77 ± 39 | NA | |
| P | 0.4 | ||||||||||
| Otsuka | Statin (+) | 68 | 64.4 ± 11.6 | 75.0 | 44.1 | NA | 70.6 | 49.3 | 25.0 | NA | 42.6 (TIMI <2) |
| Statin (−) | 390 | 61.9 ± 11.0 | 84.1 | 24.6 | NA | 56.6 | 63.8 | 3.1 | NA | 41.5 (TIMI <2) | |
| P | 0.097 | 0.097 | 0.002 | 0.042 | 0.033 | <0.001 | 0.97 | ||||
| Post | Statin (+) | 22 | 57.5 ± 7.7 | 65 | 5 | 30 | 55 | 85 | NA | 161.9 ± 80.9 | NA |
| Statin (−) | 20 | 64.6 ± 10.3 | 86 | 23 | 19 | 23 | 68 | NA | 164.1 ± 85.2 | NA | |
| P | 0.02 | 0.15 | 0.19 | 0.48 | 0.06 | 0.28 | 0.845 | ||||
| Zhao | Statin (+) | 40 | 60 ± 11 | 0.7 | 57.5 | 75 | 62.5 | 67.5 | NA | NA | 78.1 (TIMI = 0) |
| Statin (−) | 114 | 60 ± 14 | 71.9 | 57.9 | 46.5 | 64 | 65.8 | NA | NA | 79.8 (TIMI = 0) | |
| P | NS | NS | NS | NS | <0.01 | NS | NS |
Abbreviations: MI, myocardial infarction; NA, not available; NS, not significant; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
We compared the percentage of patients who did not achieve post‐PCI TIMI grade 3 in 2 groups. The overall pooled results with random effects analysis showed that statin pretreatment was significantly superior to standard medical therapy in TIMI grade with all of the studies included (OR: 0.49; 95% CI: 0.28 to 0.84), and significant heterogeneity was observed (P = 0.03, I 2 = 52%) (Figure 2A). The TIMI grade data of the study by Kadro et al did not show in the forest plots because patients in both groups all achieved TIMI grade 3 after undergoing PPCI, which generated no statistical significance in the meta‐analysis.14 The subgroup analysis, with retrospective studies included, also showed that statin pretreatment could improve post‐PCI TIMI grade compared with the control (OR: 0.43; 95% CI: 0.23 to 0.81), with P = 0.03 and I 2 = 59% (Figure 2B). However, the pooled OR for 3 RCT studies showed no favorable effect on post‐PCI TIMI grade improvement (OR: 0.76; 95% CI: 0.22 to 2.6), and there was no significant heterogeneity (P = 0.17, I 2 = 43%) (Figure 2C).
Figure 2.
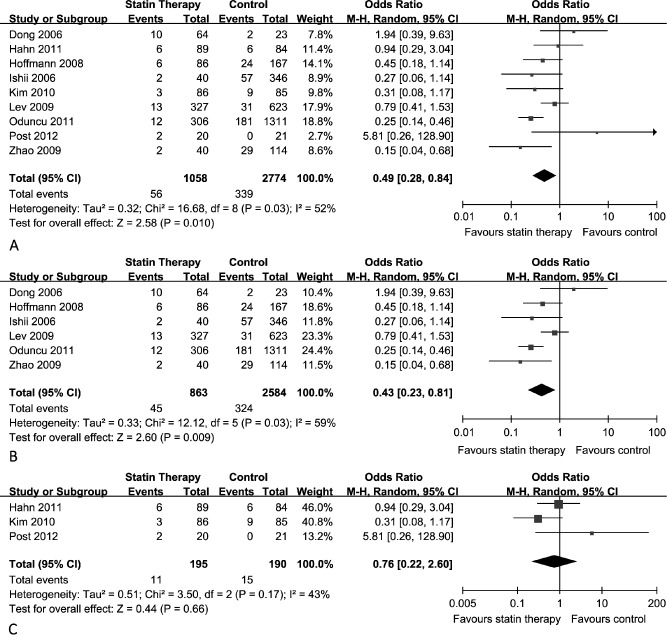
Thrombosis in Myocardial Infarction (TIMI) grade comparison between statin pretreatment group and control group. (A) Overall effect of statins on TIMI grade for all of the studies included. (B) Overall effect of statins on TIMI grade for the retrospective studies included. (C) Overall effect of statins on TIMI grade for the randomized controlled trial studies included. Abbreviations: CI, confidence interval; M‐H, Mantel‐Haensel.
For the quantitative assessment method of epicardial coronary perfusion, the overall pooled results with a random effects analysis of all of the studies included demonstrated that statin pretreatment had a favorable effect on post‐PCI cTFC compared with standard medical therapy (mean difference: −5.63; 95% CI: −9.66 to −1.6), with significant heterogeneity across all of the included studies (P < 0.00001, I 2 = 92%) (Figure 3A). The subgroup analysis with retrospective studies included showed that patients who received prior use of statins had lower cTFC compared with the controls (mean difference: −6.95; 95% CI: −11.63 to −2.27), with P < 0.00001 and I 2 = 95% (Figure 3B). However, the pooled results for 2 RCT studies showed no favorable effect on post‐PCI cTFC improvement (mean difference: −0.31; 95% CI: −15.05 to 14.43), and there was significant heterogeneity (P = 0.008, I 2 = 86%) (Figure 3C).
Figure 3.
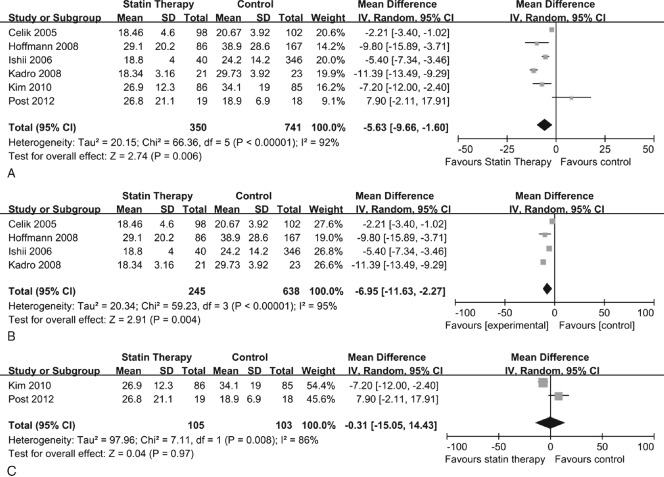
Corrected TIMI frame count (cTFC) comparison between statin pretreatment group and control group. (A) Overall effect of statins on cTFC for all the studies included. (B) Overall effect of statins on cTFC for the retrospective studies included. (C) Overall effect of statins on cTFC for the randomized controlled trial studies included. Abbreviations: CI, confidence interval; SD, standard deviation; IV, inverse variance.
Patients with MBG <3 after PPCI were considered to have insufficient myocardial tissue level perfusion. We compared the percentage of patients who did not achieve post‐PCI MBG 3 in 2 groups. The overall pooled results with random effects analysis showed a nonsignificant improvement in the statin pretreatment arm rather than the control arm on MBG (OR: 0.74; 95% CI: 0.50 to 1.09), and significant heterogeneity was observed across all of the included studies (P = 0.07, I 2 = 55%) (Figure 4A). However, subgroup analysis results, which only included the retrospective studies, showed better MBG was achieved in the statin group compared with the control (OR: 0.60; 95% CI: 0.40 to 0.90), with heterogeneity test results of P = 0.15 and I 2 = 47% (Figure 4B). MBG comparison data between the statin pretreatment group and control group in the RCT by Kim et al were given by mean value ± standard deviation (2.2 ± 0.8 vs 1.9 ± 0.8, P < 0.02),23 which made us unable to perform a quantitative analysis with the other studies. Although in the Kim et al study, a favorable impact on MBG with statin pretreatment was observed, and the pooled results with the other 2 studies showed no benefit of statin pretreatment before PPCI in STEMI patients compared with the control (OR: 1.19; 95% CI: 0.69 to 2.06) (Figure 4C).
Figure 4.
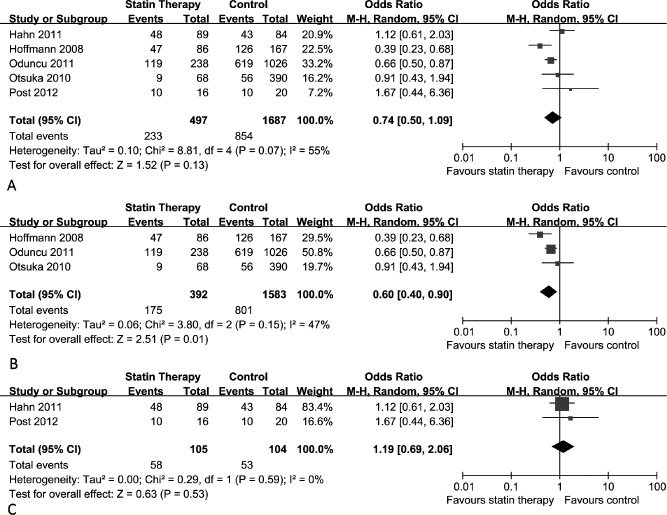
Myocardial blush grade (MBG) comparison between statin pretreatment group and control group. (A) Overall effect of statins on MBG for all the studies included. (B) Overall effect of statins on MBG for the retrospective studies included. (C) Overall effect of statins on MBG for the RCT studies included. Abbreviations: CI, confidence interval; M‐H, Mantel‐Haensel.
Discussion
To the best of our knowledge, this is the first systematic review that pooled the current evidence of the effect of statin pretreatment on improving post‐PCI epicardial coronary flow and myocardial tissue level perfusion in STEMI patients.
Animal studies had suggested that different mechanisms were involved in the occurrence of the no‐reflow phenomenon, including endothelial damage, neutrophil accumulation, reactive oxygen species, the coagulation cascade, and plaque fragments.29 It was convincible that similar mechanisms contributed to the occurrence of the no‐reflow phenomenon in STEMI patients, with supportive evidence including correlation between the no‐reflow phenomenon and endothelin‐1 level, the initial neutrophil count, C‐reactive protein value, high platelet reactivity, and thromboxane A2 level.30, 31, 32, 33, 34 Thus, given the proposed mechanisms of impaired coronary flow and myocardial tissue level perfusion, the pleiotropic effects might contribute to improvement of post‐PCI reperfusion status in STEMI patients.9 In this study, we included retrospective and prospective studies comparing the incidence of impaired myocardial perfusion incidence in a statin pretreatment group with a statin‐naïve group in STEMI patients undergoing PPCI.
We performed this meta‐analysis to evaluate the effect of statin pretreatment on both postprocedure epicardial coronary blood flow and myocardial tissue level perfusion in STEMI patients receiving PPCI. The results were that prior use of statins had beneficial effects on epicardial coronary flow measured by TIMI grade and cTFC. The subgroup analysis with retrospective studies indicated a stronger favorable effect on epicardial coronary flow improvement. However, the pooled results of 3 RCTs showed that 1 loading dose of statin before PPCI did not have a favorable effect on epicardial coronary flow improvement. On the other hand, the pooled OR with the retrospective and prospective studies involved did not show a significant effect of statin pretreatment on myocardial tissue level perfusion improvement measured by MBG in STEMI patients receiving PPCI. However, the subgroup analysis, which only included retrospective studies, showed a favorable effect on myocardial tissue level perfusion in STEMI patients receiving PPCI.
Most retrospective studies mentioned the timing of statin pretreatment before PPCI except the study by Kadro et al.14 Statin pretreatment duration in the retrospective studies was for at least 1 week. Regarding the 2 RCTs with 1 loading dose of statin pretreatment, the study by Kim et al showed atorvastatin could improve both epicardial coronary blood flow and myocardial tissue‐level perfusion, but the study by Post et al showed no favorable effect of statin pretreatment on myocardial perfusion. The time interval between first atorvastatin/placebo administration and reperfusion in the study by Post et al was 10 to 30 minutes, thus it might be too short for statins to exert an effect. Our study suggests that although 1 loading dose of statin pretreatment before PPCI suggested null impacts on myocardial perfusion, a longer time of prior use of statins showed favorable effects on myocardial perfusion. This indicated that the duration of statin therapy before PPCI in STEMI patients might influence its effect on myocardial perfusion.
Most of the included studies gave data regarding the clinical risk factors associated with impaired myocardial perfusion including advanced age, delayed reperfusion, and low TIMI flow prior to PCI. The comparison of these risk factors between the statin group and the control group turned out to be of no significant difference. However, our meta‐analysis still showed heterogeneity in the results of the trials included. Duration of statin pretreatment, use of different statins, and different dosages of statins might be contributing factors. In addition, there were several factors we should take into account. The retrospective studies were observational and nonrandomized, therefore the results might not be as convincible as RCTs. Although the 2 RCTs in our study had good quality as measured by the Jadad score, they both did not include large numbers of patients.
Limitations
The major limitation of this study was that the results were mostly based on observational studies. However, we have listed the necessary characteristics of the observational studies that were recommended by the MOOSE (Meta‐analysis Of Observational Studies in Epidemiology) group, which made the pooled results believable.12 Patients included in the studies received different statin pretreatment dosage and duration, which made it hard to conclude the exact dosage and timing of statin pretreatment that would benefit myocardial perfusion in STEMI patients receiving PPCI.
Conclusion
This meta‐analysis suggested that statin pretreatment could improve myocardial perfusion in STEMI patients receiving PPCI, and continual administration of statins had a stronger favorable impact on myocardial perfusion. Thus, relative long‐time prior use of statins may be a potential adjunctive therapy for STEMI patients receiving PPCI. However, considering most studies included are retrospective and observational, a larger prospective randomized study needs to be performed to confirm the effects of continual statin pretreatment on myocardial perfusion.
Ting Lyu, MD, and Yichao Zhao, MD, contributed equally to this work.
This work was supported by National Natural Science Foundation of China (81070176, 81270282, 30600242 and 81170192), Shanghai Shuguang Program (12SG22), Program for New Century Excellent Talents of Ministry of Education of China (NCET‐12‐0352), International Cooperation Program of Shanghai Committee of Science and Technology (12410708300), and the Shanghai Med‐X Program (YG2011MS65).
References
- 1. Steg PG, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 2. Gibson CM, Cannon CP, Murphy SA, et al. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. 2000;101:125–130. [DOI] [PubMed] [Google Scholar]
- 3. Roe MT, Ohman EM, Maas AC, et al. Shifting the open‐artery hypothesis downstream: the quest for optimal reperfusion. J Am Coll Cardiol. 2001;37:9–18. [DOI] [PubMed] [Google Scholar]
- 4. van't Hof AW, Liem A, Suryapranata H, et al. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. 1998;97:2302–2306. [DOI] [PubMed] [Google Scholar]
- 5.The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med. 1985;312:932–936. [DOI] [PubMed]
- 6. Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. [DOI] [PubMed] [Google Scholar]
- 7. Bolognese L, Carrabba N, Parodi G, et al. Impact of microvascular dysfunction on left ventricular remodeling and long‐term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004;109:1121–1126. [DOI] [PubMed] [Google Scholar]
- 8. Ito H, Maruyama A, Iwakura K, et al. Clinical implications of the “no reflow” phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223–228. [DOI] [PubMed] [Google Scholar]
- 9. Morikawa S, Takabe W, Mataki C, et al. The effect of statins on mRNA levels of genes related to inflammation, coagulation, and vascular constriction in HUVEC. Human umbilical vein endothelial cells. J Atheroscler Thromb. 2002;9:178–183. [DOI] [PubMed] [Google Scholar]
- 10. de Lorenzo F, Feher M, Martin J, et al. Statin therapy‐evidence beyond lipid lowering contributing to plaque stability. Curr Med Chem. 2006;13:3385–3393. [DOI] [PubMed] [Google Scholar]
- 11. Halcox JP, Deanfield JE. Beyond the laboratory: clinical implications for statin pleiotropy. Circulation. 2004;109:II42–II48. [DOI] [PubMed] [Google Scholar]
- 12. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 13. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 14. Kadro WY, Turkmani M, Alrahim HE, et al. Pretreatment with statins gives better frame count in the culprit artery after primary PCI for acute MI. J Am Coll Cardiol. 2008;51:B66–B66. [Google Scholar]
- 15. Chang SM, Yazbek N, Lakkis NM. Use of statins prior to percutaneous coronary intervention reduces myonecrosis and improves clinical outcome. Catheter Cardiovasc Interv. 2004;62:193–197. [DOI] [PubMed] [Google Scholar]
- 16.Fuernau GF, Eitel I, Desch S, et al. Impact of chronic statin‐pretreatment on myocardial damage as assessed by cardiac magnetic resonance findings in patients with acute ST‐elevation myocardial infarction. Circulation. 2011;124:A15418. [Google Scholar]
- 17. Iwakura K, Ito H, Kawano S, et al. Chronic pre‐treatment of statins is associated with the reduction of the no‐reflow phenomenon in the patients with reperfused acute myocardial infarction. Eur Heart J. 2006;27:534–539. [DOI] [PubMed] [Google Scholar]
- 18. Dong BQ, Hou J, Fang YM, Effects of atorvastatin on improving myocardium reperfusion in patients with acute ST segment elevation myocardial infarction after emergency percutaneous coronary intervention. J Clin Cardiol. 2006;22:198–200. [Google Scholar]
- 19. Celik T, Kursaklioglu H, Iyisoy A, et al. The effects of prior use of atorvastatin on coronary blood flow after primary percutaneous coronary intervention in patients presenting with acute myocardial infarction. Coron Artery Dis. 2005;16:321–326. [DOI] [PubMed] [Google Scholar]
- 20. Hahn JY, Kim HJ, Choi YJ, et al. Effects of atorvastatin pretreatment on infarct size in patients with ST‐segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J. 2011;162:1026–1033. [DOI] [PubMed] [Google Scholar]
- 21. Hoffmann R, Haager P, Suliman H, et al. Effect of statin therapy before Q‐wave myocardial infarction on myocardial perfusion. Am J Cardiol. 2008;101:139–143. [DOI] [PubMed] [Google Scholar]
- 22. Ishii H, Ichimiya S, Kanashiro M, et al. Effects of receipt of chronic statin therapy before the onset of acute myocardial infarction: a retrospective study in patients undergoing primary percutaneous coronary intervention. Clin Ther. 2006;28:1812–1819. [DOI] [PubMed] [Google Scholar]
- 23. Kim JS, Kim J, Choi D, et al. Efficacy of high‐dose atorvastatin loading before primary percutaneous coronary intervention in ST‐segment elevation myocardial infarction: the STATIN STEMI trial. JACC Cardiovasc Interv. 2010;3:332–339. [DOI] [PubMed] [Google Scholar]
- 24. Lev EI, Kornowski R, Vaknin‐Assa H, et al. Effect of previous treatment with statins on outcome of patients with ST‐segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. 2009;103:165–169. [DOI] [PubMed] [Google Scholar]
- 25. Oduncu V, Tanalp AC, Erkol A, et al. Impact of chronic pre‐treatment of statins on the level of systemic inflammation and myocardial perfusion in patients undergoing primary angioplasty. Am J Cardiol. 2011;107:179–185. [DOI] [PubMed] [Google Scholar]
- 26. Otsuka F, Hibi K, Kusama I, et al. Impact of statin pretreatment on the incidence of plaque rupture in ST‐elevation acute myocardial infarction. Atherosclerosis. 2010;213:505–511. [DOI] [PubMed] [Google Scholar]
- 27. Post S, Post MC, van den Branden BJ, et al. Early statin treatment prior to primary PCI for acute myocardial infarction: REPERATOR, a randomized placebo‐controlled pilot trial. Catheter Cardiovasc Interv. 2012;80:756–765. [DOI] [PubMed] [Google Scholar]
- 28. Zhao JL, Yang YJ, Pei WD, et al. The effect of statins on the no‐reflow phenomenon: an observational study in patients with hyperglycemia before primary angioplasty. Am J Cardiovasc Drugs. 2009;9:81–89. [DOI] [PubMed] [Google Scholar]
- 29. Reffelmann T, Kloner RA. The no‐reflow phenomenon: A basic mechanism of myocardial ischemia and reperfusion. Basic Res Cardiol. 2006;101:359–372. [DOI] [PubMed] [Google Scholar]
- 30. Niccoli G, Lanza GA, Shaw S, et al. Endothelin‐1 and acute myocardial infarction: a no‐reflow mediator after successful percutaneous myocardial revascularization. Eur Heart J. 2006;27:1793–1798. [DOI] [PubMed] [Google Scholar]
- 31. Campo G, Valgimigli M, Gemmati D, et al. Value of platelet reactivity in predicting response to treatment and clinical outcome in patients undergoing primary coronary intervention: insights into the STRATEGY Study. J Am Coll Cardiol. 2006;48:2178–2185. [DOI] [PubMed] [Google Scholar]
- 32. Takahashi T, Hiasa Y, Ohara Y, et al. Relation between neutrophil counts on admission, microvascular injury, and left ventricular functional recovery in patients with an anterior wall first acute myocardial infarction treated with primary coronary angioplasty. Am J Cardiol. 2007;100:35–40. [DOI] [PubMed] [Google Scholar]
- 33. Niccoli G, Giubilato S, Russo E, et al. Plasma levels of thromboxane A2 on admission are associated with no‐reflow after primary percutaneous coronary intervention. Eur Heart J. 2008;29:1843–1850. [DOI] [PubMed] [Google Scholar]
- 34. Isik T, Kurt M, Ayhan E, et al. The impact of admission red cell distribution width on the development of poor myocardial perfusion after primary percutaneous intervention. Atherosclerosis. 2012;224:143–149. [DOI] [PubMed] [Google Scholar]


