Abstract
The recent surge in incorporation of metallic and metal oxide nanomaterials into consumer products and their corresponding use in occupational settings have raised concerns over the potential for metals to induce size-specific adverse toxicological effects. Although nano-metals have been shown to induce greater lung injury and inflammation than their larger metal counterparts, their size-related effects on the immune system and allergic disease remain largely unknown. This knowledge gap is particularly concerning since metals are historically recognized as common inducers of allergic contact dermatitis, occupational asthma, and allergic adjuvancy. The investigation into the potential for adverse immune effects following exposure to metal nanomaterials is becoming an area of scientific interest since these characteristically lightweight materials are easily aerosolized and inhaled, and their small size may allow for penetration of the skin, which may promote unique size-specific immune effects with implications for allergic disease. Additionally, alterations in physicochemical properties of metals in the nano-scale greatly influence their interactions with components of biological systems, potentially leading to implications for inducing or exacerbating allergic disease. Although some research has been directed toward addressing these concerns, many aspects of metal nanomaterial-induced immune effects remain unclear. Overall, more scientific knowledge exists in regards to the potential for metal nanomaterials to exacerbate allergic disease than to their potential to induce allergic disease. Furthermore, effects of metal nanomaterial exposure on respiratory allergy have been more thoroughly-characterized than their potential influence on dermal allergy. Current knowledge regarding metal nanomaterials and their potential to induce/ exacerbate dermal and respiratory allergy are summarized in this review. In addition, an examination of several remaining knowledge gaps and considerations for future studies is provided.
Keywords: Nanotoxicology, allergy, metal nanoparticles, lung function, immune response
Introduction
Over the past several decades, an extensive amount of scientific attention has been invested in the field of nanotechnology. Significant advances have been made in understanding the unique behaviors of matter with nano-scale dimensions. This progress has facilitated the capacity for manipulation of material properties to optimize their functional utility. Subsequently, nanomaterials have proven useful in diverse applications ranging from pharmaceutics and energetics to transportation and electronics. The exponential growth of the nanotechnology field has left few sectors unaffected by its momentum, as the global nanotechnology market has been valued at over $20 billion US (Nanomaterials Future Markets 2015). Although the resounding impact of these technological advancements has generated comparisons to the impact of the industrial revolution, the expansion of nanotechnology has also generated several notable concerns. In addition to the environmental, legal, ethical, and regulatory challenges imposed by the expanding presence of nanotechnology, the potential risk for adverse health effects following exposure to nanomaterials has also become a major concern.
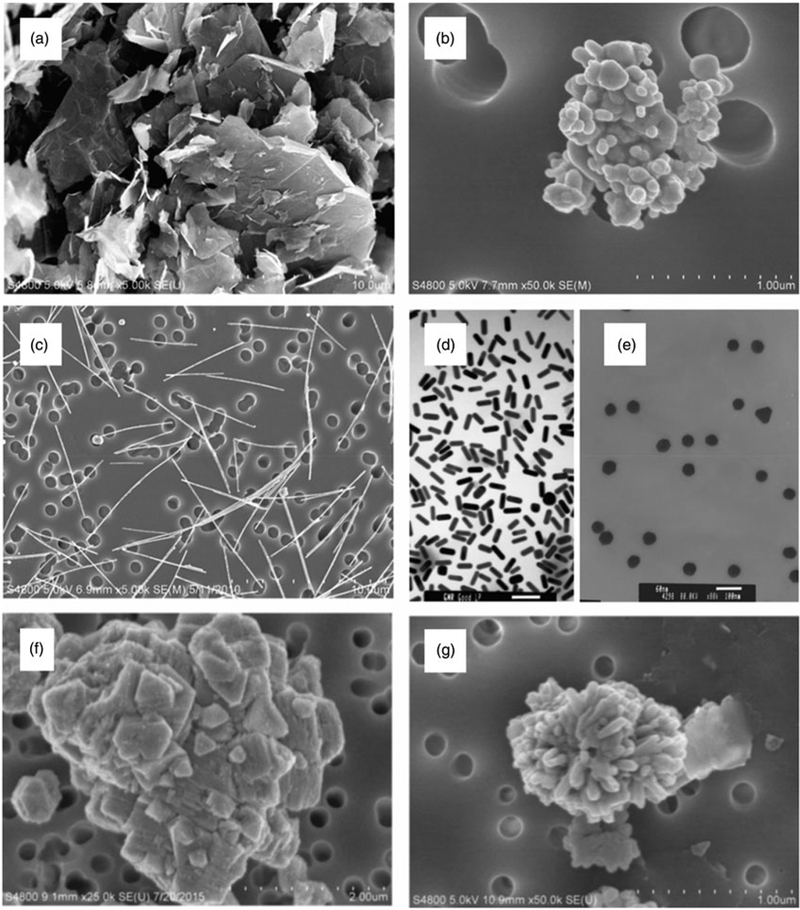
As a result, a unique discipline of toxicology has emerged to evaluate the potential health effects of nanomaterials. Nanotoxicology studies have consistently demonstrated that the unique properties of nanomaterials that render their industrial functionality also implicate unique interactions with biological systems. A general correlation between decreasing size and increased toxic potential has been observed for many nanomaterials (Shang et al. 2014). However, additional physical and chemical properties of nanomaterials have been implicated in their biological activity. Nanomaterials exist in various morphologies (Figure 1) with diverse surface textures, and can assume differing degrees of agglomeration. These physical properties contribute to variations in their chemical properties, which include surface charge, dissolution kinetics, and surface reactivity (Oberdorster et al. 2005; Castranova 2011; Gatoo et al. 2014).
Figure 1.
Different morphologies of nanomaterials are shown: (a) graphene sheets, (b) silver nanoparticles, (c) silver nanowires, (d) gold nanorods, (e) gold nanoparticles, (f) nickel oxide nanoparticles, and (g) copper oxide nanoparticles.
One of the greatest challenges presented to nano-toxicologists arises from the discord between the rapid emergence of vast quantities of new nanomaterials and the significant amount of time and resources required to evaluate the safety of each material individually. A novel risk assessment approach proposed to mitigate this issue involves delineation of relationships between specific physicochemical properties and toxicological modes of action (Kuempel et al. 2012; Braakhuis et al. 2016). Subsequently, emerging materials can be categorized by this scheme, providing preliminary safety information and prioritization of resources for in vivo studies (Schulte et al. 2014). Significant advancements have been made using this approach with respect to toxic effects on the lungs, but the correlation of nanomaterial physicochemical properties with adverse effects on other systems, such as the immune system, are less clear.
In addition to protecting the host from both endogenous and exogenous threats, the immune system is a critical regulator in hundreds of other disorders, as inflammation is a critical component in the pathophysiology of nearly all chronic diseases states (Pawelec et al. 2014). Accordingly, deviations in optimal immune functioning can have resounding effects on host health, whether polarized towards being either stimulatory or suppressive in nature. One of the immunological disorders presenting a significant and continually expanding global public health burden is allergy. The term “allergic disease” refers to a collective assortment of disorders involving diverse inciting agents, underlying immunological mechanisms, and clinical manifestations. However, all hypersensitivity disorders are characterized by commonality in hyperactivation of adaptive immune responses directed at otherwise innocuous exogenous antigens (Pawankar 2014).
Rates of allergic disease have been on the rise for decades, and the American Academy of Allergy, Asthma, and Immunology reports that worldwide, sensitization rates to one or more common allergens are approaching 40–50% in school-aged children (AAAAI 2015). In the United States, allergic diseases are the sixth leading cause of chronic illness with an annual cost exceeding $18 billion US (Centers for Disease Control and Prevention 2017). Although the development of allergy is dependent on a multitude of genetic, behavioral, and environmental factors, exposures to immunotoxic agents are a major underlying contributor to allergic diseases (Boverhof et al. 2008). Immunotoxic agents with the capacity to impact allergic disorders generally exert one of two effects. First, the agent can act as an allergen or sensitizer. Following exposure to these agents, the resultant adaptive immune response is specific to the agent and subsequent encounters trigger allergic reactions. Contrarily, agents can augment immunological processes involved in allergic disorders specific to differing agent. These agents are often referred to as “adjuvants” or “immuno-modulators” and their effects can range from increasing host susceptibility to sensitization, decreasing the allergen dose required to induce sensitization, decreasing the dose required to elicit allergic responses, or exacerbating the severity of allergic responses (Zunft 1996).
As the nanotechnology market continues to expand and the global prevalence of allergic disease continues to increase, the knowledge gap regarding the immunotoxic potential of nanomaterials is becoming increasingly relevant. Specifically, the capacity for nanomaterials to cause or exacerbate allergic disease remains largely unknown, which is particularly concerning with respect to a specific class of nanomaterials. Metal-based nanomaterials (e.g. metallic, oxidic, alloy, and salt forms) are one of the classes of nanomaterials being produced in the largest quantities. Noteworthy metal nanomaterials, their applications, and corresponding rates of production are shown in Table 1. These emerging materials present a specific concern with respect to allergy, as many of the metal-based nanomaterials being manufactured in large volumes are comprised of metals known to cause allergic contact dermatitis (ACD), asthma, and allergy adjuvancy (Warshaw et al. 2013; Schmidt and Goebeler 2015).
Table 1.
Metal nanomaterial production rates and corresponding applications.
| Nanomaterial | Global production volume (tons) | Applications |
|---|---|---|
| Silicon dioxide | 185,000–1,400,000 | Nanocomposite filler, cement additive, drug delivery, cosmetics |
| Titanium dioxide | 60,000–150,000 | Ceramics, sunscreens, construction, energy, cosmetics |
| Zinc oxide | 32,000–36,000 | Sunscreen, LED and LCD displays, antimicrobial, water filtration |
| Aluminum oxide | 5000–10,100 | Drilling equipment, energetic fuels, and additives, filtration |
| Cerium oxide | 880–1400 | Catalysts, slurry polishing, UV absorption, anti-corrosion additive |
| Copper oxide | 290–570 | Heat transfer, batteries, antimicrobials, sensors, semiconduction |
| Silver | 135–420 | Biomedicine, antimicrobial textiles, cosmetics, conductive inks |
| Antimony tin oxide | 120–225 | Electronics, composites, coatings, research |
| Zirconium oxide | 80–300 | Heat-resistant coatings, ceramics, catalysts, dental composites |
| Iron oxide | 9–45 | Ground and wastewater cleanup, color imaging, drug delivery |
| Nickel | 5–20 | Ceramic additive, catalyst, energy absorption, electronics |
| Cobalt oxide | 5–10 | Electronics, catalysts, drug delivery, solar energy absorption |
| Quantum dots | 4.5–9 | Photovoltaic devices, photodetecting devices, electronics |
| Manganese oxide | 2–3.5 | Bleaching agent, biomedical diagnostics, plastics additive |
| Gold | 1–3 | Data storage, gene therapy, biosensors, fuel cell additive |
| Palladium | – | Hydrogen sensor, antimicrobial activity, catalysts |
Metal nanomaterials, various applications, and reported global production volume for 2014, reported in tons. Adapted from Metal and Metal Oxide Nanomaterials Future Markets Report, Table 1 (Nanomaterials Future Markets 2015).
Metal nanomaterials are being increasingly incorporated into nano-enabled products and consumer goods, increasing the potential for exposures in the general public (Vance et al. 2015). Although the unknown immune effects of many metal nanomaterials present a risk for consumers, workers involved in the manufacture, handling, and transportation of metal nanomaterials present a population particularly susceptible to adverse allergic effects. The National Science Foundation estimates that by 2020, at least 2 million workers in the United States alone will be employed by nanotechnology-related fields (Roco 2011). New nanomaterials are continually being developed, and workers are often the first individuals in society to encounter emerging materials, and often in much greater quantities than consumers. Moreover, occupational settings are known to contribute to the development and progression of allergic diseases (Arruda et al. 2005). Of the 24 million Americans affected by allergic asthma, 10–25% of adult cases are related to workplace conditions (Petsonk 2002). Skin allergies are even more common, affecting an estimated 15–20% of the general population, wherein an estimated 25–60% of ACD cases are related to occupational settings (Diepgen and Coenraads 1999; Peiser et al. 2012).
Several specific concerns have emerged with respect to metal nanomaterials and their potential effects on allergic disease. First, the characteristic size profile of metal nanomaterials may confer enhanced potential for skin penetration and more efficient deposition in the lungs, circumventing one of the major barriers associated with limiting adverse immune effects induced by larger-sized metals. Secondly, the exposure threshold required for dermal and respiratory sensitization by metal nanomaterials may be lowered as a result of their unique chemistry. Lastly, the induction of inflammation and tissue injury caused by many metal nanomaterials may serve as an adjuvant, promoting the development of allergic disease to environmental allergens or exacerbating the severity of established allergic conditions. This review aims to summarize current scientific knowledge regarding these concepts. In addition to dermal and respiratory studies that examine specific metal nanomaterial effects, studies designed to delineate the role of physical and chemical properties in these effects are emphasized. Finally, considerations and knowledge gaps in the field are highlighted as potential directions for future research.
Metals and allergic disease
Metals are a class of agents associated with expansively diverse immune effects including irritancy, autoimmunity, sensitization, and adjuvancy (Lawrence and McCabe 2002). The potential for such diverse biological effects is reflective of the expansive potential speciation of metals, which can include elemental forms, ions, salts, and organified compounds (Templeton 2015). Moreover, as exemplified by the transition metal series, many metals exist in and transition between different oxidation states that have distinctive immunological activity (Artik et al. 1999; Crichton 2017). The chemical behavior unique to these potential states dictates the molecular and cellular interactions responsible for metal immunogenicity. Since many of these properties are known to be altered on the nano-scale, metal-induced immune effects with relevance to allergy are detailed, with emphasis on specific processes subject to impact by metal nanomaterial physicochemical properties.
Metals and dermal allergy
The most common metal-induced allergic disorder of the skin is ACD, a T-cell-mediated delayed-type hypersensitivity response. Dermal sensitization and the subsequent induction of ACD requires several key molecular and cellular events (Figure 2), which have been outlined in an adverse outcome pathway (AOP) by the Organization for Economic Co-operation and Development (OECD) (OECD 2014).
Figure 2.
Steps of the Adverse Outcome Pathway (AOP) for dermal sensitization adapted from the Organization for Economic Cooperation and Development (OECD).
The preliminary requirement for skin sensitization is bioavailability of the sensitizing agent. Since a primary function of the skin is to serve as an effective barrier between the host and environment, the sensitizing potential of many antigens is limited by their capacity to evade this barrier (Jaitley and Saraswathi 2012). Passage through the uppermost layers of the epidermis is heavily dependent on antigen physical and chemical properties. Likewise, most dermal sensitizers tend to be low molecular weight (LMW, < 500 Daltons) chemicals with adequate lipophilicity (logP ~2) (Chilcott and Price 2008; Karlberg et al. 2008). Metals associated with skin sensitization present the greatest concern when formulated as soluble salts that release ions capable of penetrating the physical barrier presented by the epidermis (Di Gioacchino et al. 2007; Kubo et al. 2013).
The next steps in the skin sensitization AOP involve the molecular initiating event of skin sensitization-antigen formation. The small size required for antigen passage through the stratum corneum is not conducive with cellular recognition (Anderson et al. 2011). As a result, most skin sensitizers are referred to as haptens, which must acquire or possess inherent chemical reactivity that facilitates binding to carrier molecules (Büdinger et al. 2000; Chipinda et al. 2011). This process generates adequate size for recognition by an antigen-presenting cell (APC). The APC most frequently implicated in dermal sensitization is the resident dendritic cell (DC) of the epidermis, the Langerhans cell (LC) (Thyssen and Menne 2010).
In addition to uptake of the hapten/carrier complex, activation of LC requires an additional antigen-nonspecific signal indicative of an elevated threat level. Many mediators capable of fulfilling this signal are released by non-immune cells including keratinocytes in response to injury (Dearman and Kimber 2003). Presence of both antigen-specific and nonspecific signals induce LC maturation, upregulation of co-stimulatory molecules, antigen processing, and migration to the lymph nodes (Tončić et al. 2011).
Once the LC reaches the lymph nodes, the processed hapten is presented via major histocompatibility Class I (MHC I) molecules to naïve CD8+ T-lymphocytes until recognition occurs by antigen-specific T-cell receptor (TCR). Given adequate costimulatory signals from the LC, T-lymphocytes undergo proliferation producing a pool of clonal antigen-specific effector T-cells. The T-cells enter the circulation, and following resolution of inflammation, a subset of these effector cells will survive and become memory T-cells, completing the process of sensitization.
Upon future exposures to the allergen, memory and effector T-cells are recruited to the site of exposure where CD8+ T-cells exhibit immediate cytotoxic effector functions. CD4+ T-helper (TH) of the TH1 phenotype have regulatory roles in ACD and produce high levels of the cytokines interleukin (IL)-2 and interferon (IFN)-γ, contributing to inflammatory cell recruitment (Sasseville 2008; Tončić et al. 2011). Within 48 h, the inflammatory process originally orchestrated to destroy the antigen results in the clinical manifestations of ACD, including localized skin redness, swelling, and itching at the site of allergen contact.
Metals are among the most common inducers of ACD in the general population. Patch test studies have generated data from thousands of subjects and reveal that the most common inducers of metal ACD are nickel, gold, cobalt, and chromium (Belloni Fortina et al. 2015). Interestingly, studies using subjects exclusive various geographical locations have demonstrated that these four metals are consistently problematic with respect to ACD worldwide (Kanerva et al. 2000; Mattila et al. 2001; Goon and Goh 2005; Cheng et al. 2008; Davis et al. 2011; Nonaka et al. 2011; Khatami et al. 2013; Mahler et al. 2014; Kim et al. 2015; Malinauskiene et al. 2015; Linauskiene et al. 2017). Though less frequently associated with ACD, copper, aluminum, and platinum group metals are also known to cause skin allergy in some individuals (Hostynek et al. 1993; Bergfors et al. 2005; Faurschou et al. 2011; Fage et al. 2014).
Metals and respiratory allergy
Metals are also associated with respiratory allergy and IgE-mediated asthma. An AOP specific to the events of respiratory sensitization has not been adopted by the OECD, but many of the same steps of the dermal sensitization AOP are involved in the development of asthma. Accordingly, bioavailability of the sensitizing agent is also a preliminary limiting factor in respiratory sensitization potential. Since the primary function of the respiratory tract is to facilitate gas exchange between the host and environment, it is particularly vulnerable to adverse effects from a diverse assortment of agents in the inhalable (< 20 μm) and respirable size range (< 10 μm) (Elder and Oberdorster 2006). Likewise, bioavailable metals capable of sensitizing the respiratory tract are not limited to ions, like in the skin (Linde et al. 2017). Respirable metals may be encountered as particulate matter, vapors, or fumes and can be constituents of compounds including oxides, sulfides, and salts, or as complexes with ammonia, carbon monoxide, and organic nitrogen (Malo et al. 2013).
Since both low and high molecular weight (HMW) agents are capable of inducing asthma, the molecular initiating events of respiratory sensitization may differ accordingly. Similar to the skin, pulmonary immune responses following inhalation of metals can be nonspecific and self-limiting, or can result in the recruitment of the adaptive immune system. Lung-resident DC take up antigen, and given adequate second signals, the antigen is processed and DC migrate to the lung-draining lymph nodes. Here, the peptide is presented to naïve CD4+ T-lymphocytes along with costimulatory molecules, resulting in the preferential expansion of the TH−2 phenotype lymphocytes. These cells produce high levels of IL-4, IL-5, and IL-13, and stimulate isotype switching and allergen-specific IgE-production by B-cells. The Fc portion of secreted IgE is bound to FceRI receptors present on tissue-resident mast cell surfaces and circulating basophils, exposing the antigen-recognizing motif, completing the sensitization process (Verstraelen et al. 2008).
Upon subsequent encounters, the allergen is bound by allergen-specific IgE on the surface of mast cells and basophils. Binding induces crosslinking of receptors and the subsequent release of preformed mediators such as histamine, beginning the anaphylactic cascade responsible for the early asthmatic reaction experienced minutes after antigen encounter. Acute clinical manifestations of allergic asthma range from rhinitis and bronchoconstriction to anaphylactic shock. The late phase asthmatic response occurs 4–6 h later as a result of mast cell mediators and recruitment of inflammatory cells (Possa et al. 2013). Clinical presentations of the late phase asthmatic response tend to be more severe than early phase responses, and include excessive mucus production, increased vascular permeability, and airway constriction. Chronic cycles of allergic inflammation and subsequent repair are associated with structural alterations in the airways that can have physiological implications, such as a decline in lung function (Erle and Sheppard 2014).
Compared to metal-induced ACD, metal-induced asthma occurs far less frequently. Cases tend to be isolated to individuals working in occupations involving metalwork where metal fumes, dust, or vapors are generated and inhaled (Wyman and Hines 2018). Nickel, chromium, cobalt, vanadium, zinc, platinum and aluminum have all been associated with cases of occupational asthma (Musk and Tees 1982; Hong et al. 1986; Malo et al. 2013). However, metal-specific IgE has only been implicated in cases caused by nickel, platinum, chromium, and cobalt (Malo et al. 1982; Murdoch et al. 1986; Shirakawa et al. 1988, 1990, 1992; Kusaka et al. 1996). Metal-specific IgG molecules have also been implicated in cases of cobalt and platinum-induced asthma (Pepys et al. 1979; Cirla 1994). The presence of metal-specific IgE has also been confirmed in the absence of asthmatic symptoms, emphasizing the potential for numerous immunological mechanisms in metal-specific asthma (El-Zein et al. 2005; Tončić et al. 2013).
Metals and allergy adjuvancy
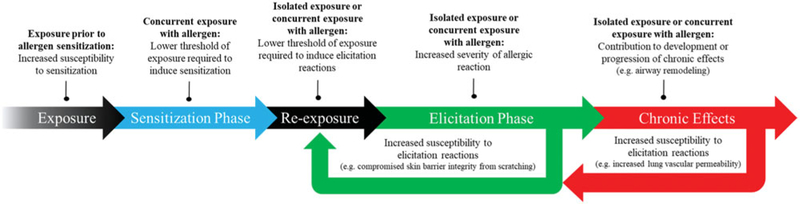
In addition to their potential to induce sensitization, metals are also associated with the capacity to modulate allergic responses to nonmetal allergens. Contrasted with the consistent, sequential series of cellular processes involved in allergic sensitization and elicitation, adjuvant effects can emerge as a result of various mechanisms in various phases of allergic disorders (Figure 3).
Figure 3.
Potential adverse outcomes with respect to the sensitization and elicitation phases of allergy following exposure to immunotoxic agents. Adjuvant effects resulting from exposure prior to allergen sensitization can manifest as increased susceptibility to sensitization. Exposure concurrent to sensitization may lower the threshold of allergen exposure required to induce sensitization. Following sensitization to allergen, exposure to an immunotoxic agent either in the absence or presence of allergen may result in a lower threshold of exposure required to induce elicitation reactions or increased severity of elicitation symptoms. These effects may further increase susceptibility to elicitation reactions as result of physiological alterations such as compromised skin barrier integrity. Furthermore, isolated exposure to immunotoxic agents or concurrent to allergens in established allergic disease conditions may also contribute to the progression of chronic effects, such as airway remodeling, which can also further contribute to elicitation reactions.
An example of metal adjuvant effects on the development of adaptive immune responses is best demonstrated by aluminum hydroxide, which is one of the most frequently used vaccine adjuvants. When administered with poorly-immunogenic antigens, aluminum hydroxide induces adequate stimulation of the innate immune system to generate antigen-specific immunological memory. Mechanisms of immunopotentiation associated with aluminum hydroxide include triggering release of alarmins, activation of inflammasomes (intracellular multi-protein complexes involved in innate immune responses), and DC activation; however, numerous other mechanisms including immune cell recruitment and activation, modulated cytokine production, and altered antigen delivery kinetics, can also enhance sensitization (Naim et al. 1997; Aimanianda et al. 2009).
Similarly, metals are also associated with adjuvant effects on established allergic conditions, as demonstrated by metal-rich ambient air pollution, which is known to exacerbate the severity of asthmatic responses to environmental allergens (Gavett et al. 2003; Schaumann et al. 2004). Adjuvant effects on allergic elicitation can involve mechanisms including induction of pulmonary oxidative stress, enhanced degranulation of mast cells, and recruitment of inflammatory cells (Walczak-Drzewiecka et al. 2003; Ghio 2008).
Unique properties associated with metal immune effects
Metals are known to induce many unique immune effects implicated in allergic disease. With respect to sensitization, the modulation of innate immune reactivity by some metals has been associated with their immuno-genicity. For example, some metal ions are known to produce functional mimicry of pathogen-associated molecular patterns (PAMP) (Schmidt and Goebeler 2015). Gold ions have the capacity to bind and activate Toll-like receptor (TLR)-3, while nickel, cobalt, and palladium ions have the capacity to bind and activate human TLR-4 (Schmidt et al. 2010; Rachmawati et al. 2013, 2015). The subsequent induction of pro-inflammatory signaling generates the antigen nonspecific signals required for DC activation, promoting sensitization.
Metals are also known to modulate mechanisms of communication between innate and adaptive immune cells. Accordingly, antigen presentation is another step in allergic sensitization that is subject to interference by metals. Beryllium and noble metals have been shown to induce structural alterations in MHC molecules, impacting subsequent interactions with TCR (de Wall et al. 2006; Falta et al. 2013). Similarly, peptide-independent linking of MHC and TCR by nickel has been demonstrated (Gamerdinger et al. 2003; Thierse et al. 2005). Adaptive immune responses can also be affected by metals, as demonstrated by CD4+ nickel-specific T-cell clones, which were shown to cross-react when presented with other transition metals including copper and palladium (Moulon et al. 1995; Pistoor et al. 1995).
Many of the unique immune effects associated with metals emerge as a result of their capacity to alter molecular and cellular interactions on a biochemical level. Accordingly, their modulation of processes involved in allergic disease is critically dependent on physicochemical properties including special geometry, oxidation state, and solubility (Schuhmann et al. 1990; Kinbara et al. 2011). Many of these properties are altered in nanoparticulate form, suggesting that metal nanomaterials may exhibit novel mechanisms of immune interaction with implications for allergic disease.
Metal nanomaterials and dermal hypersensitivity
The potential for adverse immune effects following dermal exposure to metal nanomaterials is a growing concern due to their increasingly frequent incorporation into consumer goods intended to have prolonged contact with the skin (Yang and Westerhoff 2014). The unique optical properties of titanium dioxide nanoparticles (TiO2 NP) and zinc oxide nanoparticles (ZnO NP) have led to their incorporation in sunscreens and cosmetics for their protective effects against ultraviolet radiation (UVR) (Smijs and Pavel 2011; Yoshioka et al. 2017). Silver nano-particles (AgNP) are being incorporated into clothes, medical textiles, toys, and cleaning products due to their antimicrobial properties, and silica-based nanoparticles (SiNP) have been frequently used in cosmetics and as a coating material to alter the properties of other materials (Contado 2015; Tulve et al. 2015). Likewise, the dermal effects of TiO2 NP, ZnO NP, AgNP, and SiNP are a particular concern with respect to the general public (Weir et al. 2012). These nanomaterials are also a concern for workers, but other metal nanomaterials with high rates of production (listed in Table 1) are also associated with dermal exposures in the workplace.
The potential for metal nanomaterials to penetrate the skin, induce dermal sensitization, and modulate skin allergy development/responses are the three main areas discussed in this section with respect to size and other physicochemical properties. In correspondence with the review of the literature, Table 2 summarizes studies characterizing effects of individual metal nanomaterials on skin allergy. Table 3 summarizes studies designed to examine effects of physicochemical properties of metal nanomaterials on dermal allergy. Table 4 highlights key events involved in dermal sensitization and elicitation that have been shown to be subject to modulation by metal nanomaterials and their corresponding physicochemical properties.
Table 2.
Summary of major findings from studies characterizing the effect of metal nanomaterials on dermal allergy grouped by metal
| Metal | Author/year | Material | Size | Animal or cell type | Model | Exposure route | Dose | Findings |
|---|---|---|---|---|---|---|---|---|
| Aluminum | Varlamova et al. 2015 | AI2O3 | – | M/F BALB/c, CBA/CaLac, out-bred mice and guinea pig | LLNA | Subcutaneous, intramuscular, intravenous, intra-dermal injection | No intensification of anaphylaxis systemic reaction, no inflammatory reaction to ConA, no delayed allergic reaction, no redness or edema at site of application | |
| Brown et al. 2008 | AI2O3 | 50–120 nm | HaCaT human keratinocytes | In vitro | 10–10,000 μg/mL | 24 h exposure resulted in IL-8 expression, IL-1α release, indicating potential for irritation or sensitization | ||
| Cobalt | Choet al. 2012 | Co3O4 | 18.4±5.0nm | F C57BL/6 mouse | OVA | Subcutaneous injection | 25 μg | Balanced Th1/Th2 response when used as adjuvant, causing higher specific lgG2c and IgGI and less IgE |
| Gold | Ishii et al. 2008 | Au | 5.2±1.3nm | F Japan White Rabbit | Intradermal injection | 1 mg | Azobenzene dye hapten conjugation to AuNP led to high yield of IgG specific to the agent indicating the capacity for AuNP to act as both a carrier and adjuvant | |
| Iron | Shen et al. 2012 | Fe3O4 | 58.7 nm | M BALB/c mouse | OVA | Intravenously | 0.2–10 mg/kg | Decreased footpad swelling, infiltration of macro-phages and T-cells, and IFN-y, IL-6, TNF-α levels |
| Hsiao et al. 2018 | Fe3O4 | 58.7 nm | M BALB/c mouse | OVA | Intravenously | 1–100 μg | Attenuation in TH17 responses | |
| Silica | Choi et al. 2011 | SiO2 | 7nm | CBA/N mouse | HSEM LLNA | Dermal | 10–1,000 μg | SiO2NP did not induce phototoxicity or skin sensitization |
| Hirai et al. 2015 | SiO2 | 30 nm | F NC/Nga slc mouse | HDM | Dermal | 20 μl @ 12.5mg/mL | Concurrent exposure to allergen and particles resulted in low-level production of allergen-specific IgG subtypes and increased sensitivity to anaphylaxis | |
| Ostrowski et al. 2014 | SiO2 | 55±6nm | M SKH1 mouse | Oxazolone | Dermal | – | Functionalized nanoparticles had no impact on allergic response to oxazolone in an ACD model | |
| Smulders et al. 2015 | SiO2 | 19nm | M BALB/c mouse | DNCB | Dermal | 0.4, 4.0, or 40mg/mL×3d | SiO2NP exposure prior to sensitization with DNCB did not alter the stimulation index | |
| Silver | Kim et al. 2013 | Ag | 10nm | M SPF guinea pig | GPMT | Intradermal injection | 0.1 mL @ 1:1 (v/v) | No eye or skin irritation or corrosion. 1/20 guinea pigs developed erythema following subcutaneous injection, leading to its classification as a weak skin sensitizer |
| M New Zealand White rabbit | – | Occular application | 100 mg | No eye irritation effects 1–72 hours after exposure | ||||
| Bhol et al. 2005 | Ag | <50nm | F BALB/c mouse | DNFB | Dermal | 100 mg 1% nanocrystalline | Reductions in ear swelling, erythema, and inflammation were seen after 4 days of treatment with nanopartide-containing cream | |
| Smulders et al. 2015 | Ag | 25–85 nm | M BALB/c mouse | DNCB | Dermal | 0.4, 4.0, or 40mg/mLx3d | Exposure prior to sensitization with DNCB did not alter the stimulation index | |
| Zelga et al. 2016 | Ag | - | Guinea pig | GPMT | Dermal | 2% | AgNP-containing dressings for chronic wounds were tested via GPMT, wherein 2/10 animals developed slight erythema that resolved after 72 hours, leading to classification as a mild sensitizer | |
| Korani et al. 2011 | Ag | <100nm | M Harley Albino guinea pig | - | Dermal | 100–10,000 μg/ml | Dose-dependent increase in number of Langerhans cells recruited to skin | |
| Titanium | Parket al. 2011 | TiO2 | <25nm | F CBA/N mouse | LLNA | Dermal | 10–1000 μg/mL | TiO2 did not induce skin sensitization |
| F Hartley Albino guinea pig | - | Dermal | 50 μg | TiO2 did not induce phototoxicity or acute cutaneous irritation | ||||
| Hussain et al. 2012 | TiO2 | 12±2nm | M BALB/c mouse | DNCB | Subcutaneous Injection | 0.004–0.4 mg/mL | TH2 adjuvancy, increased DNCB dermal sensitizer potency | |
| Auttachoat et al. 2014 | TiO2 | <25nm | F BCC3F1 mouse | - | Dermal, Subcutaneous Injection | 1.25–250 mg/kg | Dermal exposure did not induce auricular lymph node expansion, despite irritancy response at 5% and 10%, no ear swelling, but lymph node cell proliferation resulted following subcutaneous injection | |
| Smulders et al. 2015 | TiO2 | 15nm | M BALB/c mouse | DNCB | Dermal | 0.4, 4.0, or 40mg/mL×3d | Exposure to 4.0 mg/mL of TiO2 prior to sensitiza-tion with DNCB resulted in increased stimulation index | |
| Piasecka-Zelga et al. 2015 | TiO2 | New Zealand albino rabbit | Acute Dermal Irritation | Dermal | 0.5 g | 5 UV-absorbers containing nano-sized particles were assessed for irritation and sensitization potential. Anatase Ti02-containing agent did not induce irritation, but caused mild sensitization | ||
| Dunkin-Hartley guinea pig | GPMT | Dermal | 0.1 mL/site | |||||
| Zinc | Piasecka-Zelga et al. 2015 | ZnO | APS 396 nm, containing nanoparticles | New Zealand albino rabbit | Acute Dermal Irritation | Dermal | 0.5 g | 5 UV-absorbers containing nano-sized particles were assessed for irritation and sensitization potential. Z11 modifier caused minor dermal irritation and mild sensitization |
| Dunkin-Hartley guinea pig | GPMT | Dermal | 0.1 mL/site | |||||
| Kim et al. 2016 | ZnO | 20–50 nm, 13.1 m2/g | M Sprague-Dawley Rat, M New Zealand White rabbit, M guinea pig | GPMT | Dermal | 50% | ZnONP did not induce dermal sensitization, acute dermal toxicity, irritation, or corrosion |
Summary of studies investigating metal nanomaterial immune effects in the skin and select in vitro studies using dermal cells, grouped by metal. APS: average particle size, DNCB: dinitrochlorobenzene; GPMT: guinea pig maximization test; HDM: house dust mite; HSEM: Human Skin Equivalent Model; LLNA: Local Lymph Node Assay; OVA: ovalbumin; UV: ultraviolet.
Table 3.
Summary of major findings from studies comparing the effects of various physicochemical properties of metal nanomaterials on dermal allergy grouped by property of interest.
| Property investigated | Author/year | Metal | Study design | Property variations | Findings | ||
|---|---|---|---|---|---|---|---|
| Size | Kang H et al. 2017 | Ag | RBL-2H3 mast cells F NC/Nga mouse, HDM AD: 40 ng | 5, 100 nm AgNP | 5 nm AgNP induced increases in ROS levels, intracellular calcium, and granule release in mast cells in vitro and earlier and more severe lesions in an AD model in vivo | ||
| Nabeshi et al. 2010 | Si | XS52 mouse epidermal Langerhans cell, 0.1–1000 ug/mL | 70, 300, 1000 nm SiNP | Cellular uptake and cytotoxicity increased with reductions in particle size | |||
| Yoshida et al. 2010 | Si | XS52 mouse epidermal Langerhans cell, 0–100 μg/mL | 70, 300, 1000 nm SiNP | ROS generation by LC was higher following exposure to the smaller amorphous SiNP | |||
| Hirai et al. 2012b | Si | M NC/Nga mouse HDM ACD Intradermal injection: 250 μg | 1136nm SiO2NP: 33.2 mV, 264 nm SiO2NP: 25.8 mV, 106 nm SiO2NP: 24.3 mV, 76 nm SiO2NP: 19.5 mV, 39 nm SiO2NP: 14.0mV | Reduction in size of SiO2NPs caused enhanced IL-18 and TSLP production, leading to an enhanced systemic Th2 response and aggravation of skin lesions following challenge with house dust mite | |||
| Kang S et al. 2017b | Au | Mouse footpad injection OVA | 7, 14, 28 nm AuNP | Size-dependent increase in cellular uptake by DC, T-cell cross-priming, and activation.after injection into footpad, higher delivery efficiency to lymph nodes was associated larger NPs | |||
| Yanagisawa et al. 2009 | Ti | M NC/Nga mouse HDM Intradermal injection: 20 μg | 15 nm TiO2NP-110 m2/g 50 nm TiO2NP-20–25 m2/g 100 nm TiO2NP-10–15 m2/g | TiO2NP aggravated AD skin lesions, caused increased IL-4 production, IgE levels, and histamine levels, but decreased IFN-γ expression. Effects were not dependent on size | |||
| lives et al. 2014 | Zn | F BALB/c mice, OVA ACD Dermal application: 16.67 mg/mL | 20, 240 nm ZnONP | Smaller sized ZnONPs were able to penetrate the skin, whereas larger particles were not. Both particles diminished local skin inflammation, but ZnONPs exhibited higher suppressive effects and increased IgE production | |||
| CRG | |||||||
| Jang et al. 2012 | Zn | CBA/N Mouse LLNA: 25, 50, or 100 μg/mL ZnONP HSEM EpiDerm skin irritation Draize skin irritation: 50 μg/mL | 20 nm, 29 mV or 40 mV ZnONP 100 nm, 24 mV or 29 mV ZnONP | ZnO are not dermal sensitizers and do not induce skin irritation irrespective of size and zeta potential, but may induce phototoxicity | |||
| Jatana et al 2017b | Si | M/F hairless C57BL6 mouse DNFB ACD Dermal application | 32.7 nm SiO2 nanosphere: 25.4 mV 66.5 nm SiO2 nanosphere: 45.7 mV 69.3 nm SiO2 nanosphere: 17.7mV 184.9 SiO2 nanosphere: 33.5 mV 440.0 nm SiO2 nanosphere: 66.0 mV | Small negative and neutral-charged nanoparticles exhibited an immunosuppressive effect, whereas positively-charged particles did not. Positively-charged nanoparticles penetrated skin to a lesser extent. Studies also included lOOnm TiO2NP, 20 nm AgNP, and 20 nm AuNP | |||
| Schaeublin et al. 2010 | Au | HaCaT human keratinocyte cells 10 μg/mL–25 μg/mL | 1.5 nm AuNP Positive, neutral, or negatively-charged | Cell morphology was disrupted by AuNPs of all 3 charges in a dose-dependent manner. Charged AuNPs caused dose-dependent cytotoxicity and mitochondrial stress | |||
| CRY | SA | Lee et al. 2011 | Si | J774A.1 mouse macrophages: 0–1,000 ug/mL, 1 or 3 d LLNA: F BALB/c, 1 mg/ ear × 3 tx | 100 nm spherical: mesoporous SiO2 1150 m2/g colloidal SiO2-40 m2/g | Higher surface area caused decreased cytotoxic and poptotic cell death. Similarly, higher surface area induced lower expression of pro-inflammatory cytokines. Lower surface area Si particles acted as an immunogenic sensitizer in the LLNA | |
| Size | Maquieira et al. 2012 | Al | Mice and rabbits Intradermal injection | 40, 3000 nm amorphous AI2O3, 300 nm crystalline AI2O3 | AINP served as both carrier and adjuvant leading to hapten-specific antibody production dependent on size and crystallinity | ||
| Braydich-Stolle et al. 2009 | Ti | HEL-30 mouse keratinocytes 0–150 μg/mL 24 h exposure | 100% anatase TiO2: 6.3, 10, 40, 50, 100 nm 61% anatase, 39% rutile TiO2: 39 nm 40% anatase, 60% rutile TiO2: 39 nm 75% anatase, 25% rutile TiO2: 26 nm Amorphous TiO2: 40 nm 100% rutile TiO2: 51 nm | Both size and crystal structure contributed to toxicity in vitro. Smaller size and less agglomeration increased cytotoxicity. 100% anatase TiO2 particles, regardless of size, induced cell necrosis, whereas the rutile TiO2 nanoparticles initiated apop-tosis through formation of ROS | |||
| MOD | Orlowski et al. 2013 | Ag | 291.03C mouse keratinocyte 1–10 μg/mL 24 h exposure | Tannic acid-modified AgNP: 13, 33, and 46 nm Unmodified AgNP: 10 −65 nm | Unmodified, but not modified, AgNP increased production of MCP-1 by keratinocytes and upregulation of TNF-α, attributable to increased ROS production | ||
| Li et al. 2016 | Si | F C57BL/6 mouse 5mg injection | Unmodified mesoporous SiNP PEG, PEG-RGD, PEG-RDG- modified SiNP | PEG modification significantly enhanced DC activation in vitro and innate immune cell infiltration in vivo. PEG-modification resulted in less recruitment of DC to area of injection |
Summary of study design and major findings from studies comparing the effects of various physicochemical properties of metal nanomaterials on dermal allergy grouped by study property of interest. Properties of interest: size, CRY (crystallinity), CRG (surface charge), SA (surface area), and MOD (surface modification). Reported particle size (nm), specific surface area (m2/g), zeta potential (mV), pore volume (cm3/g), in vitro dose concentration (mg/mL). DC: dendritic cell; DNFB: dinitrofluorobenzene; HDM: house dust mite; LC: Langerhans cell; LLNA: Local Lymph Node Assay; HSEM: Human Skin Equivalent Model; OVA: ovalbumin; PEG: poly(ethyleneglycol) modification, PEG-RDG/RGD; ROS: reactive oxygen species; TSLP: thymic stromal lymphopoietin.
Table 4.
Metal nanomaterials and corresponding physicochemical properties shown to influence immunological processes involved in the development and augmentation of ACD.
| AOP Step | Metal nanomaterial effect | Metal | Properties implicated | Source |
|---|---|---|---|---|
| Sensitization Bioavailability | Increased potential for nanomaterial penetration of intact skin | Au | size, crg | Labouta et al. 2011 |
| Increased potential for nanomaterial penetration of damaged skin | Ti | size, cry | Monteiro-Riviere et al. 2011 | |
| Increased release of ions from parent nanomaterials | Pd | size | Filon et al. 2016 | |
| Accumulation of nanomaterial in follicles and skin folds | Zn | mod | Leite-Silva et al. 2013 | |
| Molecular initating event | Increased potential for metal antigen formation | Ti | size | Vamanu et al. 2008 |
| Adsorbed protein conformational changes | Au | size | Bastus et al. 2009 | |
| Cellular response | Selective uptake by LC | Si | size | Nabeshi et al. 2010 |
| Direct activation of DC | Ti | size, cry, mor | Schanen et al. 2009 | |
| Release of DAMPs from skin epithelial cells > activation of DC | Fe | size | Murray et al. 2013 | |
| Release of DAMPs from dermal immune cells > activation of DC | Si | size, SA | Lee et al. 2011 | |
| Altered immunogenicity from adsorption of LPS to surface | Au | size, mod, hyd | Li et al. 2017 | |
| Organ response | Depot formation > altered delivery kinetics | Si | agg | Hirai et al. 2015 |
| Nanomaterial antigen vehicle > altered delivery kinetics | Ti | – | Smulders et al. 2015 | |
| Accumulation in DC endocytic compartments > interference with antigen processing | Si | mod, crg | Shahbazi et al. 2014 | |
| Enhanced capacity for cross-presentation to CD8+ T-cells | Fe | crg | Mou et al. 2017 | |
| Increased TH1 signaling | Co | Cho et al. 2012 | ||
| Elicitation Organism response | Increased permeability of endothelial cells | Al | – | Oesterling et al. 2008 |
| Increased effector T-cell recruitment | many | – | Lozano-Fernandez et al. 2014 | |
| Increased number of DC for T-cell activation | Si | mod | Li et al. 2016 | |
| Increased number of skin macrophages | Fe | – | Yun et al. 2015 | |
| Increased neutrophil influx to the skin | Ti | – | Goncalves, 2011 | |
| Increased number of skin mast cells | Ag | size | Kang H et al. 2017 | |
| Altered T-cell response to mitogens/allergens | Pd | size, sol | Reale et al. 2011 | |
| Increased IgE-independent mast cell degranulation | many | size, SA, crg | Johnson et al. 2017 | |
| Chronic effects | Compromised skin repair mechanisms | Ag | – | Vieira et al. 2017 |
| Aggravation of allergic lesion severity | Si | size | Hirai et al. 2012b | |
| Compromised barrier integrity > increased penetration of allergens | Ag | size, sol | Koohi et al. 2011 |
Adverse Outcome Pathway (AOP) steps in the sensitization and elicitation phases of allergic contact dermatitis, metal nanomaterials shown to impact individual steps and cells involved, and physicochemical properties associated with effects are shown. Physicochemical properties of interest include size, agglomeration (agg), surface modification (mod), surface area (SA), solubility (sol), surface charge (crg), morphology (mor), crystallinity (cry), hydrophobicity (hyd), surface chemistry (SC). ND (not determined) notation in metal column indicates a study demonstrating a critical role for a specific nanomaterial physicochemical property on the cellular event, but was demonstrated using nonmetal nanomaterials. Findings may be applicable to metals, but have not been demonstrated with individual metal nanomaterials.
Skin penetration and translocation studies
Adverse immune effects following dermal exposure to an agent are heavily dependent on the degree to which the skin protects from its entry into the body. Likewise, one mechanism by which dermal exposure to metal nanomaterials may lead to increased potential for adverse immune effects compared to larger-sized metals is by size-mediated evasion of skin barrier function. Although it seems logical that the small size of nanomaterials would inherently provide increased opportunity for absorption via the skin, there is currently no general consensus on the skin-penetrating capabilities of nanomaterials as a collective class of agents (Vogt et al. 2006; Baroli 2010; Try et al. 2016).
Numerous studies have demonstrated that metal nanomaterials (< 100 nm) can penetrate skin in various in vivo and in vitro models. Iron-based nanoparticles (FeNP), gold nanoparticles (AuNP), palladium nanoparticles (PdNP), nickel nanoparticles (NiNP), AgNP, SiNP, and metal-based quantum dots (QD) have all been associated with penetration of the skin (Baroli et al. 2007; Chu et al. 2007; Filon et al. 2011, 2016; Labouta et al. 2011; Hirai et al. 2012a; Rancan et al. 2012; George et al. 2014; Crosera et al. 2016; Kraeling et al. 2018). Moreover, many of these studies have established a relationship between decreased particle size and increased potential for skin permeation (Ryman-Rasmussen et al. 2006; Sonavane et al. 2008; Matsuo et al. 2016; Raju et al. 2018). Hydrophobicity, surface charge, and morphology are additional properties that have been shown to be influential in the capacity for these nanomaterials to pass through the stratum corneum (Rancan et al. 2012; Lee et al. 2013; Iannuccelli et al. 2014; Fernandes et al. 2015; Tak et al. 2015; Mahmoud et al. 2018).
By comparison, the majority of studies investigating the skin-penetrating potential of metal nanomaterials have been conducted with ZnO NP and TiO2 NP and have not generated equally consistent findings. Numerous studies have demonstrated that the stratum corneum effectively restricts passage of TiO2 NP, irrespective of size shown to facilitate penetration of the skin by other metal nanomaterials. Repeated application of different forms of TiO2 NP did not lead to skin penetration in hairless rats, elevated levels of titanium in lymph nodes of minipigs, or penetration of human skin transplanted onto immunodeficient mice (Kiss et al. 2008; Sadrieh et al. 2010; Adachi et al. 2013). Although TiO2 NP were shown to accumulate in and around furrows of the skin, microscopic analysis was used to confirm that 20–100 nm TiO2 NP remained restricted to the uppermost 3–5 layers of corneocytes of the stratum corneum (Lademann et al. 1999; Pflücker et al. 1999; Gontier et al. 2008; Senzui et al. 2010). Contrarily, a few studies using TiO2 NP-containing sunscreens have reported penetration of particles into the viable epidermis of human skin (Tan et al. 1996; Coelho et al. 2016; Naess et al. 2016).
Similar observations have been reported for ZnO NP. Despite associations with hair follicles, ZnO NP were not capable of penetrating the stratum corneum in multiple models, irrespective of alterations in size, morphology, and surface characteristics (Schulz et al. 2002; Zvyagin et al. 2008; Leite-Silva et al. 2013). However, ion release from ZnO NP and ZnO NP-containing sunscreens has been observed, highlighting a potential risk associated with soluble metal nanomaterials (Holmes et al. 2016).
Adverse effects following dermal exposure to nanomaterials may result from penetration of the particulate material or ions released from the parent material. Likewise, physicochemical properties of interest may be differentially implicated in effects associated with soluble and insoluble metal nanomaterials. With respect to soluble materials, properties associated with accelerated ion release may indicate increased potential for skin penetration (Lansdown 1995; Hostynek 2003; Thyssen and Menne 2010). The rate of ion release is proportional to specific surface area (SSA; surface area per mass unit), which is exponentially increased on the nano-scale (Laudańska et al. 2002; Zhang et al. 2011; Larese Filon et al. 2015). This concept explains the observation that application of sunscreens containing ZnO NP caused greater increases in blood, urine, and organ Zn ion levels than sunscreens containing larger-sized ZnO particles (Gulson et al. 2010, 2012; Osmond-McLeod et al. 2014). Moreover, the manipulation of properties with implications for dissolution potential, such as particle coating, vehicle, and suspension pH have been shown to promote Zn ion release from ZnO NP following dermal exposure (Leite-Silva et al. 2013; Holmes et al. 2016).
Although penetration through corneocytes of the stratum corneum is the primary pathway associated with skin penetration by materials, appendages including hair follicles, sebaceous glands, sweat glands, and skin folds can mediate an additional mechanism of skin penetration. This pathway has notable relevance to nanomaterials, as evidenced by the utility of the trans-follicular delivery route for nano-scale pharmaceutics and vaccines (Mahe et al. 2009). Compared to the thickness of the stratum corneum, which measures 10–20 mm, hair follicles can reach a tissue depth of 2000 mm (Toll et al. 2004). Since the base of hair follicles extends into the dermis and receives generous lymph and blood supply, they may promote access into the circulation (Elder et al. 2009). Moreover, hair follicles can serve as a potential reservoir, promoting accumulation of nanomaterials. Retention in hair follicles can extend the duration of exposure 10-fold, raising specific concerns for continual ion release (Lademann et al. 2006, 2009, 2015; Patzelt et al. 2011; Mahmoud et al. 2017). This pathway of skin penetration may also favor immune responses since hair follicles are surrounded by dense networks of LC and specialized keratinocyte subpopulations known to have critical roles in the early events of sensitization (Toews et al. 1980; Vogt et al. 2006; Nagao et al. 2012).
The diameter of hair follicles can vary greatly in response to anatomical location, but the smallest follicles tend to be located on the forehead and forearm and measure between 66 and 78 mm (Otberg et al. 2004). Interestingly, the optimal size for penetration of hair follicles is significantly larger than the < 100 nm size range associated with increased skin penetration of several metal nanomaterials. Particles with 600–700 nm diameter have been shown to deposit in the deepest depths of hair follicles, suggesting that agglomerates of nanomaterials in this size range are potentially more hazardous than primary particles (Patzelt et al. 2011; Lademann et al. 2015). Furthermore, preferential accumulation in follicles has been observed in hydrophobic and neutrally-charged nanomaterials (Mahmoud et al. 2017).
Although physicochemical properties of metal nanomaterials have been shown in some instances to impact skin penetration, an assortment of host factors can also impact this process. Variations in epidermal thickness, integrity, degree of hydration, and skin pH, all of which may further differ between gender, can greatly influence skin permeability (Sandby-Moller et al. 2003; Senzui et al. 2010; de Matteis et al. 2016). However, the role of disrupted skin barrier integrity is one of the most commonly-examined host factors with applicability to allergic disease since skin permeability can be increased 4–100 times in individuals with skin allergy (Larese Filon et al. 2016).
Scratching to alleviate itching associated with allergic skin lesions leads to mechanical damage to the upper layers of skin. Similar degrees of damage have been shown to increase in vivo penetration of some metal nanomaterials in humans and rodents (Zhang and Monteiro-Riviere 2008; Gopee et al. 2009; Ravichandran et al. 2011; Prow et al. 2012). In vitro simulations using a human skin model called the Franz Method have demonstrated increased capacity for passage through damaged skin by 25 nm AgNP, 6 nm PtNP, 5 nm rhodium nanoparticles (RhNP), 10 nm PdNP, 78 nm NiNP, and 80 nm CoNP (Larese et al. 2009; Larese Filon et al. 2013; Mauro et al. 2015; Crosera et al. 2016; Filon et al. 2016). Contrarily, studies have shown that penetration of various sizes of TiO2 NP and ZnO NP are not increased in skin damaged by chemical irritants, tape-strip-ping, hair removal, or mechanical force (Senzui et al. 2010; Lin et al. 2011; Miquel-Jeanjean et al. 2012; Crosera et al. 2015; Xie et al. 2015; Leite-Silva et al. 2016).
A few in vivo studies have also investigated effects of skin barrier dysfunction resulting from existing skin allergy on the penetration of metal nanomaterials (Larese Filon et al. 2016). In a mouse model of skin allergy, ZnO NP skin penetration of allergic skin was size-dependently increased, as 240 nm ZnO particles did not penetrate the skin to a similar degree as 20 nm ZnO NP (Ilves et al. 2014). Studies using nonmetal nanomaterials have also demonstrated that penetration of nanomaterials in allergic skin is size-dependent (Try et al. 2016). ZnO NP were also shown to penetrate allergic skin ex vivo using human skin samples (Szikszai et al. 2011). Contrarily, application of 35 nm ZnO NP to skin of living human subjects with atopic dermatitis did not lead to penetration into viable skin (Lin et al. 2011). Discrepancies between these studies may be reflective of varying exposure durations, as the study reporting penetration involved continuous exposure of up to 2 weeks, compared to the 4-hr exposure wherein no penetration was observed.
Comparatively, equally prolonged exposure to AgNP-containing textiles did not lead to increased skin penetration in individuals suffering from atopic dermatitis compared to control subjects. Sleeves containing silver particles (30–500 nm) were worn by human subjects for 8 h a day for 5 consecutive days, following which levels of AgNP and aggregates in the skin were quantified. Compromised skin barrier was not associated with increases in AgNP skin accumulation; moreover, no differences in urine Ag levels were observed, indicating that atopic dermatitis did not impact the absorption of ions released from the textiles either (Pluut et al. 2015; Bianco et al. 2016).
Discrepancies in findings regarding the importance of skin barrier integrity on metal nanomaterial skin penetration may be explained by several observations. The diverse degrees of epidermal barrier function disruption between studies represent a potential source of variation. Complete ablation of epidermal function is only observed in response to severe burns and lacerations; likewise, the diverse mechanisms of experimentally-induced disruptions of the stratum corneum should be compared cautiously. In addition, the pathogenesis of atopic dermatitis between experimentally-induced animal models and humans may explain some discordant findings. The severity of lesions between subjects of human studies is also subject to extreme variation, as well. Since chronic skin inflammation can result in epidermal thickening, enhanced barrier function is not uncommon in many skin disorders (Nohynek et al. 2007). Lastly, differences in exposure conditions and duration, test material formulation, and method of penetration assessment can also serve as a source of variation in conclusions between studies. A notable distinction should be made between test materials, since some studies used pristine metal nanomaterials, and others used commercially-available TiO2 NP/ZnO NP-containing sunscreens. As noted by Gulson et al. (2012), their observations regarding ZnO NP skin penetration may have been subject to modulation by excipients of the commercial sunscreens used in their study. The sunscreen contained isopropyl myristate, a chemical known to enhance the permeability of the skin, as well as EDTA, a chelating agent which may have influenced the release of ions from ZnONP.
In addition to nanomaterial properties and host factors known to influence the capacity for metal nanomaterials to penetrate the skin, environmental factors may also impact this process. One environmental factor with particular relevance to metal nanomaterials and their use in sunscreens is UVR. Although high levels of UV exposure and subsequent sunburn can significantly disrupt epidermal barrier function, low doses of UV exposure are also known to compromise the integrity of the epidermis (Wolf et al. 1993; Holleran et al. 1997; Biniek et al. 2012). Accordingly, several studies have shown that UV exposure prior to topical application of nanomaterials results in greater depth of penetration by ZnO NP, TiO2 NP, and QD (Mortensen et al. 2008; Monteiro-Riviere et al. 2011; Mortensen et al. 2013). Moreover, UVR can induce alterations in physicochemical properties of metal nanomaterials that may facilitate their passage through the stratum corneum, such as agglomerate disaggregation and ion release (Martorano et al. 2010; Bennett et al. 2012; Zhou et al. 2012; Ma et al. 2014).
Simultaneously, UVR-induced photoactivation of some metal nanomaterials can facilitate their penetration of the skin. In vitro, UVR-induced ROS production by TiO2 NP, QD, and ZnO NP has been associated with DNA damage, lipid peroxidation, and mitochondrial permeability in skin cells (Tiano et al. 2010; Wang et al. 2013; Petersen et al. 2014; Mortensen et al. 2015; Xue et al. 2015, 2016). Subsequent cytotoxicity to dermal fibroblasts, keratinocytes, and melanocytes is another mechanism by which skin barrier integrity can become compromised as a result of UVR. In vivo, UVR-induced photoactivation of TiO2 NP has been associated with increased adherence to the skin, structural rearrangement of the lipid bilayer, and facilitation of large molecule transdermal penetration (Bennett et al. 2012; Turci et al. 2013; Peira et al. 2014; Pal, Alam, Chauhan, et al. 2016; Pal, Alam, Mittal, et al. 2016). Since the degree of ROS produced in response to UVR has been associated with nanoparticle surface area and reactivity, other related properties such as size, degree of agglom-eration, and surface modification may also contribute to skin penetration following UVR exposure (Shen B et al. 2006; Jassby et al. 2012; Yin et al. 2012; Xiong et al. 2013).
Although UVR may contribute to adverse effects following dermal exposure to metal nanomaterials by facilitating skin penetration, it may also present a unique concern with respect to allergy. Many signaling pathways and pro-inflammatory mediators involved in sensitization have been associated with UVR-dependent photoactivation of metal nanomaterials (Murray et al. 2013; Rancan et al. 2014). Moreover, UVR is known to modulate the immune status of the skin by a number of mechanisms. For example, UVA and UVB are known to augment costimulatory molecule expression, compromise antigen presentation, and induce apoptosis of LC (Rattis et al. 1998; Seite et al. 2003; Schwarz 2005). Subsequent effects on the immunological fate of metal nanomaterials on the skin have been demonstrated. In a mouse model, significant depletion of LC (~80%) following UVR exposure increased skin penetration of QD, but resulted in lower levels of metal ion constituents in the lymph nodes (Mortensen et al. 2013).
Skin sensitization studies
The skin sensitizing potential of metal nanomaterials has been investigated in a few studies using traditional in vivo approaches. SiO2 NP, ZnO NP, and TiO2 NP have all been incorporated into the Local Lymph Node Assay (LLNA) (Mandervelt et al. 1997; Basketter et al. 1999). Accordingly, it was demonstrated that topical exposure to 100 nm mesoporous and colloidal SiO2 NP, 7 nm SiO2 NP, and ZnO NP were not capable of inducing the 3-fold increase in lymphocyte proliferation associated with classification as a dermal sensitizer (Choi et al. 2011; Lee et al. 2011; Kim et al. 2016). Similarly, topical exposure to 25 nm TiO2 NP did not induce dermal sensitization in multiple studies; however, subcutaneous injection of equal doses resulted in significant increases in lymphocyte proliferation, suggesting that the inability for TiO2 NP to penetrate the skin might be a limiting factor in the potential to induce dermal sensitization (Park et al. 2011; Auttachoat et al. 2014).
The guinea pig maximization test (GPMT) is another in vivo technique used to evaluate dermal sensitization potential that has been employed in the investigation of several metal-based nanomaterials. In one study, five UV-absorbing materials containing SiO2 NP, ZnO NP, and TiO2 NP were assessed. One out of 10 animals exhibited slight erythema following topical exposure to the ZnO NP and TiO2 NP-containing agents, leading to their classifications as mild skin sensitizers (Piasecka-Zelga et al. 2015). In another study, 1 of 20 animals exhibited discrete patchy erythema following intradermal injection with 10 nm AgNP, leading to its classification as a weak skin sensitizer (Kim et al. 2013). Similarly, AgNP were classified as a Grade II (mild sensitizer) after 2 of 10 guinea pigs exhibited lesions 48 h after application of AgNP-containing sterile gauze (Zelga et al. 2016). However, similar AgNP-containing dressings were actually shown to improve the healing of burn wounds in rats over an 18-d period as compared to rats with dressings lacking AgNP, but the study only examined the localized effects (Pannerselvam et al. 2017). The GPMT has also been used to demonstrate that surface-modified FeNP and hydroxyapatite nanoparticles did not induce skin sensitization (Geetha et al. 2013; Mohanan et al. 2014).
The sensitization potential of 5 and 10 nm AgNP was investigated by Hirai et al. (2016) in a mouse model. Mice were injected with AgNP or Ag ions and lipopolysaccharide (LPS) once a week for 4 weeks, and then intradermally challenged. Interestingly, mice administered Ag ions in the sensitization phase did not develop ear swelling following challenge with any form of Ag. Contrarily, AgNP exposure induced sensitization, wherein the smaller AgNP appeared to have stronger sensitizing potential, which was dependent on CD4+ T-cells and IL-17a, but not IFNγ. Moreover, ear swelling was observed in response to additional sizes of AgNP (50 and 100 nm) and Ag ions, suggesting that the immune response is not nanoparticle-specific. Further examination revealed that 3 nm NiNP was also capable of inducing sensitization in the model, whereas minimally-ionizable 10 nm AuNP and 10 nm SiNP were not (Hirai et al. 2016).
In addition to in vivo approaches to assess skin sensitization, three non-animal alterative assessment methods based on different steps of the skin sensitization AOP are currently validated by the OECD. While metal nanomaterials have not been incorporated into any of the assays, studies with similar cell lines and endpoints have indicated that many metal nano-materials can induce effects similar to those of other skin sensitizers.
The Direct Peptide Reactivity Assay (DRPA) is an in chemico assay based on the requirement for haptens to bind skin proteins to acquire immunogenicity. Accordingly, the molecular imitating event of dermal sensitization is evaluated by quantification of reactivity of an agent towards synthetic lysine and cysteine residues (Gerberick et al. 2004). While some studies have investigated metal nanomaterials and their interactions with proteins and specific amino acids, implications for their capacity to form hapten/carrier complexes are still unclear. However, cysteine has been associated with decreased stability, increased dissolution, and accelerated ion release from metal alloy nanoparticles and AgNP (Hahn et al. 2012; Ravindran et al. 2013; Siriwardana et al. 2015). Moreover, various amino acids have been associated with preferential binding affinities with respect to AuNP size and TiO2 NP surface charge, supporting a role for multiple physicochemical properties in the molecular initiating event of skin sensitization (Liu et al. 2016; Shao and Hall 2016).
The second validated in vitro assay for determination of skin sensitizing potential involves evaluation of the keratinocyte response to test agents, since they are a source of numerous mediators that facilitate LC migration, antigen presentation, and T-cell activation during sensitization (Kimber and Cumberbatch 1992). Since many of these mediators are released in response to sensitizer-induced activation of the antioxidant/electrophile sensing pathway Keap1/Nrf2/ARE, its activation is suggestive of potential for the test agent to contribute to the cellular response of the sensitization AOP (Natsch and Emter 2008; 2016; Ramirez et al. 2014). The human keratinocyte cell line associated with this assay, HaCaT, has been frequently used to investigate potential metal nanomaterial effects on the skin in vitro. Correspondingly, PdNP, AuNP, and PtNP have all been shown to activate the Nrf2 pathway in keratinocytes in vitro (Goldstein et al. 2016; Tsuji et al. 2017). Similarly, zinc-containing QD, ZnO NP, and CuO NP have all been shown to alter expression of several specific genes associated with the Nrf2 pathway, including HMOX1 (Rice et al. 2009; Romoser et al. 2011; Lee et al. 2012).
Prior to the establishment of Nrf2 pathway involvement in keratinocyte responses to skin sensitizers, cytokine release by keratinocytes in vitro was evaluated as an indicator of sensitizing potential (Jung et al. 2016; Koppes et al. 2017). Tumor necrosis factor (TNF)-α is a keratinocyte-derived cytokine involved in sensitization and is critically involved in skin sensitization by chromium and nickel (Lisby et al. 1995; Wang et al. 2007). Dose-dependent TNFα release has been noted following keratinocyte exposure to AgNP, QD, and ZnO NP, indicating high doses may promote LC maturation and dermal sensitization (Samberg et al. 2010; Romoser et al. 2011; Jeong et al. 2013). IL-18 and IL-1β (cytokines critical for LC activity) have also been shown to be increased by QD, SiO2 NP, TiO2 NP, and AgNP (Ryman-Rasmussen et al. 2007; Samberg et al. 2010; Yazdi et al. 2010; Romoser et al. 2011; Hiroike et al. 2013; Zhang and Monteiro-Riviere 2019).
Another mediator involved in skin sensitization that is differentially-released by keratino-cytes in response to irritants and sensitizers is IL-1α (Coquette et al. 2003; Koppes et al. 2017). Though it can also be actively secreted after inflammasome activation, IL-1α is an intracellular molecule that functions as an alarmin (Ansel et al. 1988). During programed cell-death, IL-1α remains associated with chromatin and its sequestration prevents effector functions. Contrarily, under necrotic conditions, it is passively released and bioactive. Accordingly, the mechanism of metal nanomaterial-induced keratinocyte cytotoxicity may significantly impact the development of ACD as a result of differential IL-1α release. Although mechanisms associated the preferential induction of necrosis or apoptosis by nanomaterials have yet to be established, some properties have been correlated to these effects (de Stefano et al. 2012; Mohammadinejad et al. 2019). For example, surface charge of 1.5 nm AuNP was demonstrated to be responsible for the mechanism of cell death in HaCaT cells in vitro. Charged AuNP led to disruptions in mitochondrial membrane potential and intracellular calcium levels causing apoptosis, whereas neutral AuNP were associated with necrotic cell death (Schaeublin et al. 2011). Preferential HaCaT apoptosis or necrosis has also been associated with AgNP surface coating and TiO2 NP crystal phase (Braydich-Stolle et al. 2009; Bastos et al. 2016). Collectively, these findings assert that surface chemistry/reactivity of metal nanomaterials may be a critical property in determining whether dermal exposure results in irritation responses or sensitization.
The last validated alternative approach to evaluate skin sensitizing potential involves assessment of the potential for an agent to induce upregulation of activation markers (CD 86 and CD 54) on human APC. However, since the recommended cell lines for these assays (THP-1 and U937) are representative of general DC and not skin-specific DC, these studies will be discussed in the in vitro section of this review, as they may also apply to respiratory sensitization and augmentation of allergy.
Very few studies have been conducted to investigate metal nanomaterial effects specific to LC. However, topical exposure to < 100 nm AgNP in guinea pigs was shown to increase the number of LC at the site of exposure in a dose- and time-dependent manner (Korani et al. 2011). This observation is relevant to skin sensitization since the concentration of LC present in the skin has been correlated with increased susceptibility to ACD development. Other in vivo studies confirmed metal nanomaterials including QD are taken up by LC and subsequently transported to lymph nodes (Jatana et al. 2017b). In vitro, associations with LC have been shown to be influ-enced by SiNP size and surface functionalization (Vogt et al. 2006; Rancan et al. 2012). Smaller SiNP size has also been correlated to increased uptake, ROS production, and cytotoxicity to LC in vitro (Nabeshi et al. 2010; Yoshida et al. 2014).
A specific observation regarding DC that has implications for ACD and dermal sensitization is that some metal nanomaterials can promote DC cross-presentation. Cross-presentation describes uptake of exogenous antigens and their subsequent processing by pathways normally associated with endogenous antigens. As a result, the exogenous antigen is presented by MHC I molecules to CD8+ T-cells, generating the cytotoxic effector cells characteristic of ACD.
Aluminum nanoparticles (AlNP), AuNP, FeNP, and SiNP have all been shown to modify DC antigen cross-presentation capacity (Blank et al. 2011; Li et al. 2011; Hirai et al. 2012; Jiménez-Periáñez et al. 2013; Kang S et al. 2017; Mou et al. 2017; Dong et al. 2018). The mecha-nism of antigen uptake by DC is known to influence the preferential association of antigens with MHC I or II molecules. Small lipophilic haptens associated with skin sensitization often enter APC via passive diffusion and bind cytoplasmic proteins, favoring their processing by endogenous pathways and presentation by MHC I molecules (Rustemeyer et al. 2006). Accordingly, passive diffusion through cell membranes similar to that demonstrated by charged 15 nm AuNP may result in promotion of cross-presentation (Arvizo et al. 2010; Lin et al. 2010; Taylor et al. 2010). Contrarily, receptor-mediated endocytosis of larger antigens has been associated with cross-presentation when uptake occurs by Fc and mannose receptors (Blum et al. 2013). In this regard, the adsorption of macromolecules, including immunoglobulins, to the surface of nanomaterials and physicochemical properties associated with the adsorption of proteins may be critically influential in determining the route of antigen processing.
Another major determinant of antigen association with MHC I or II is persistence inside DC. Antigens resistant to degradation in endosomes are more likely to be processed by MHC I pathways (Lin et al. 2008; Humeniuk et al. 2017). Likewise, metal nanomaterials with physicochemical properties capable of compromising lysosomal acidification (dissolution rate, surface reactivity) may promote cross-presentation (Accapezzato et al. 2005; Savina et al. 2006). Similarly, endosomal escape following uptake by DC can result in binding to cytosolic proteins and subsequent perception as an endogenous antigen (Lin et al. 2008). One major mechanism of endosomal antigen release leading to cross-presentation is oxidative stress and lipid peroxidation, causing antigen leakage from compromised endosome membranes (Shen H et al. 2006; Shahbazi et al. 2014; Dingjan et al. 2016). Oxidative stress induced by CuNP, FeNP, and TiO2 NP have been shown to cause lipid peroxidation, and these metal nanomaterials have also been associated with enhancing DC cross-presentation (Shukla et al. 2011; Napierska et al. 2012; Manke et al. 2013). Metal nanomaterials have also been associated with the induction of autophagy and production of exosomes by DC, both of which have also been associated with antigen cross-presentation (Moron et al. 2004; Chaput et al. 2006; Crotzer and Blum 2009; Li et al. 2011; Shen T et al. 2018).
Augmentation of existing or developing skin allergy
Since dermal exposure to metal nanomaterials nearly always occurs simultaneously to other exposures, their potential to augment skin allergy has been investigated using various allergy models. Metal nanomaterial effects on skin allergy have been studied with respect to both T-cell-mediated ACD and IgE-mediated atopic dermatitis. The effects of metal nanomaterials in ACD models have demonstrated findings suggestive of potential effects during both allergic sensitization and elicitation. In one study, subcutaneous exposure to TiO2NP 1 hr prior to skin sensitization with dinitrochlorobenzene (DNCB) increased susceptibility of mice to sensitization, as evidenced by a lower concentration of DNCB required to induce sensitization (Hussain et al. 2012; Smulders et al. 2015). The authors noted that although DNCB is known to induce a TH1-dominant response characteristic of ACD, exposure to TiO2NP resulted in a TH2-dominant response in the regional lymph nodes. In a similar study, TiO2NP were applied topically 1 day prior to sensitization with DNCB, and the same effect on sensitization as observed (Smulders et al. 2015). A diminished TH1 response was observed and TiO2NP were detectable in the lymph nodes. Contrarily, SiO2NP and AgNP did not induce alterations to DNCB sensitizer potency in the same model.
In another study by (Jatana 2017a), a panel of metal nanomaterials with various physicochemical properties was analyzed for effects on chemical-induced ACD both during sensitization and challenge. When mice were sensitized to dinitrofluorobenzene (DNFB), co-administration of QD did not impact the severity of the challenge response to DNFB, irrespective of particle charge. However, QD administration simultaneous to DNFB challenge did impact the allergic response. Moreover, the effect was dependent on the charge of the materials. The negatively-charged particles suppressed inflammation, whereas the positively-charged materials enhanced ear swelling. The authors confirmed that sensitization to QD did not occur and suggested that variations in skin penetrating capacity of the differently-charged materials was responsible for the observed effects. The conclusions regarding a critical role for nanomaterial size and charge on modulation of ACD elicitation responses is supported by other findings, as well. Suppressive effects on allergic elicitation have also been demonstrated following applica-tion of 20 nm SiNP and <50 nm AgNP-containing cream on ACD reactions to DNFB and 2-deoxyurushiol (Jatana 2017a). Contrarily, exposure to positively-charged functionalized 56 nm SiNP did not augment the severity of oxazolone-induced elicitation responses when topically applied for five consecutive days (Ostrowski et al. 2014).
As highlighted by (Jatana 2017a), ACD responses may be subject to modulation as a result of chemical modifications induced by interactions with metal nanomaterials. In their study, the topical application of nanomaterials was subject to removal prior to application of DNFB. As a result, the particle-specific modulation of allergic skin inflammation was not reflective of blocked adduct formation. Although metal nanomaterials exhibit characteristically increased surface reactivity and catalytic potential, their capacity to impact the chemical properties of skin sensitizing chemicals has not been extensively studied. However, a few studies have demonstrated the potential for such effects to impact both ACD sensitization and elicitation. AlNP and AuNP have been shown to act as non-protein carriers of haptens capable of facilitating the generation of hapten-specific adaptive immune responses in vivo (Ishii et al. 2008; Maquieira et al. 2012). Similarly, topical application of ointment containing calcium-based nanoparticles has been shown to capture nickel ions by cation exchange, compromising bioavailability and subsequently preventing the elicitation of nickel-specific ACD (Vemula et al. 2011).
In addition to ACD, metal nanomaterial effects on IgE-mediated atopic dermatitis have also been examined. Atopic dermatitis is generally associated with protein allergens, which under normal circumstances are not capable of penetrating the skin (Smith Pease et al. 2002). However, 100 nm ZnO NP and 5 nm AuNP have been shown to enhance skin penetration by albumin and protein drugs (Huang et al. 2010; Shokri and Javar 2015). Likewise, increased permeability of the skin associated with some metal nanomaterials may represent a mechanism by which exposure may increase susceptibility to atopic dermatitis onset.
Simultaneous exposure to TiO2 NP, AgNP, and SiO2 NP during sensitization to house dust mite (HDM) in atopic dermatitis models has been associated with an amplification of TH2 responses. This effect was shown to be more pronounced with decreasing size with respect to AgNP and SiO2 NP, but not for TiO2 NP (Yanagisawa et al. 2009; Hirai et al. 2012b; Hirai et al. 2015). Exposure to 5 nm AgNP during sensitization was associated with an augmentation of mast cell activity that resulted in more severe skin lesions that appeared earlier than those induced by 100 nm AgNP (Kang H et al. 2017). Decreases in SiO2NP size were also associated with enhanced TH2 responses, as evidenced by increased thymic stromal lymphopoeitin (TSLP) and IL-18 production (Hirai et al. 2012b). Decreased particle size has been associated with increased aggravation of atopic dermatitis skin inflammation by nonmetal nanoparticles, as well (Yanagisawa et al. 2010).
Metal nanomaterial-induced modulation of allergic inflammation in the challenge phase of atopic dermatitis has also been demonstrated. Topical application of both 240 and 20 nm ZnO NP resulted in diminished localized inflammation. However, the smaller particle was associated with more pronounced suppression of local inflammation, but simultaneous increases in systemic production of IgE (Ilves et al. 2014).
An interesting observation by Hirai et al. (2015) highlights a potentially critical variation between studies that may explain discordant immune effects induced by similar nanomaterials. distinction between allergy model studies that may contribute to discordant findings. The authors demonstrated that, in their study, exacerbation of allergic sensitization was dependent on co-administration of HDM antigen and SiO2 NP. When SiO2 NP agglomerates were administered at a site distal to that of the allergen, the modulation of antibody production was no longer observed. The dependence of physical associations between nanomaterial and antigen on the subsequent adaptive immune response has been similarly demonstrated by FeNP. In multiple studies, intravenous administration of 58 nm FeNP 1 h prior to subcutaneous ovalbumin (OVA) sensitization resulted in decreased levels of IgG1 and IgG2 and suppression of TH1 and TH17 responses in mice (Shen et al. 2011, 2012; Hsiao et al. 2018). Contrarily, when FeNP and OVA were co-administered intravenously or subcutaneously, enhanced antibody responses were seen in mice (Powles et al. 2017; Shen I et al. 2018). Similar immune-stimulating effects of FeNP have been demonstrated when used as an adjuvant by various exposure routes during immunization to various other antigens in the context of vaccine studies (Pusic et al. 2013; Hoang et al. 2015; Sungsuwan et al. 2015; Neto et al. 2018; Zhao et al. 2018).
The capacity for the same metal nanomaterial to induce divergent immune effects depending on its physical association with antigen likely reflect the different mechanisms of immune modulation associated with prototypical vaccine adjuvants. Some adjuvants exert effects based on “immune modulating” mechanisms. These adjuvants often bear structural resemblance to PAMP, which emphasizes their similar mechanisms of innate immune activation that promote antigen-specific adaptive responses. Since these adjuvants alter the systemic immune environment, their effects are not dependent on physical association with the antigen (Singh and O’Hagan 2003). Contrarily, physical association with antigen is required for adjuvants whose efficacy reflects their capacity to modulate antigen delivery to the immune system. The strength and nature of the adaptive immune response are heavily dependent on the dose and duration of antigen exposure to lymphocytes, so many adjuvants enhance adaptive immune responses by augmenting this process (Johansen et al. 2008).
In accordance with these two divergent mechanisms of sensitization adjuvancy, different physicochemical properties of metal nanomaterials may be implicated in immune effects resulting from each mode of action. For example, simultaneous administration of adjuvant and antigen is often implemented with the intent of depot formation, which increases local antigen retention (Glenny et al. 1931; Demento et al. 2012). Accordingly, metal nanomaterial adjuvant effects dependent on this mechanism are subject to influence by properties involved in antigen trapping, such as size, degree of agglomeration, surface area, and surface charge (Henriksen-Lacey et al. 2010a; Henriksen-Lacey et al. 2010b; Kaur et al. 2012a, 2012b).
Knowledge gaps in metal nanomaterial effects on skin allergy
Knowledge regarding metal nanomaterial effects on skin sensitization is largely limited to TiO2 NP, SiO2 NP, and ZnO NP. Though the selective investigation of these metals is likely reflective of their significance to consumer skin exposures, titanium, silver, and zinc are not historically associated with clinically-significant rates of ACD in the general population. Accordingly, the observation that some of these metals may have increased potential to induce skin sensitization in nanoparticulate form raises additional concerns over the lack of investigations into nanomaterials comprised of metals commonly associated with ACD (nickel, gold, cobalt).
Metal-based nanomaterials and asthma
Respiratory exposure to nanomaterials from naturally-occurring and anthropogenic sources has been taking place for centuries; however, the emergence of engineered nanomaterials and their widespread incorporation into consumer goods pose a risk for inhalation exposures to higher doses of materials with diverse chemical compositions and unique properties (Buzea et al. 2007). In this regard, metal nanomaterials of concern for consumers include many of the materials mentioned above, such as ZnO NP, AgNP, TiO2 NP, and SiO2 NP, as a result of their incorporation into construction materials, sunscreen sprays, disinfectants, and cosmetic powders, which upon use can lead to their inhalation. Workers are at risk for inhalation exposure to these and other highly-produced metal-based nanomaterials (Table 1) (Nanomaterials Future Markets 2015). Effects of metal nanomaterials on pulmonary immunity and asthmatic conditions have been extensively studied and summarized in this section. In addition to studies reporting adverse immune effects in workers subject to metal nanomaterial inhalation, animal studies that have generated evidence of potential for respiratory sensitization and augmentation of asthmatic conditions are discussed. Likewise, Table 5 summarizes studies characterizing individual metal nanomaterial effects on pulmonary immunity. Table 6 summarizes studies to examine the effects of metal nanomaterial physicochemical properties on asthma. Table 7 highlights some processes involved in respiratory sensitization and elicitation demonstrated to be subject to modulation by metal nanomaterials.
Table 5.
Summary of major findings from studies characterizing the effect of metal nanomaterials on respiratory allergy grouped by metal.
| Metal | Author/year | Material | Size | Animal or cell type | Mode | Exposure route | Dose | Findings |
|---|---|---|---|---|---|---|---|---|
| Aluminum | Braydich-Stolle et al. 2010 | Al | 48.08±21.0 nm | Human A549 type ∥ pneumocyte U937 alveolar macrophage 3:1 co-culture |
In vitro | 5–500 μg/mL 24 h | Exposure impaired bacterial phagocytic function | |
| AI2O3 | 32.71 ± 28.3 nm | Exposure impaired bacterial phagocytic function, induction of NFκB pathway | ||||||
| Cerium | Park E-J et al. 2009 | CeO2 | 130 nm | M ICR mouse | - | Intratracheal Instillation | 50, 100, 200, 400 mg/kg | Differentiation of naive T-cells and TH1 cytokine production |
| Meldrum et al. 2018 | CeO2 | <25nm APS 166.5 nm agglomerates | F BALB/c mouse | HDM | Intranasal Instillation | 75 or 750 μg/kg | Repeated exposure to CeO2NP in the presence of HDM caused increased lung eosinophils, mast cells, plasma IgE, IL-4, and goblet cell metaplasia | |
| Cobalt | Cho et al. 2012 | Co3O4 | 18.4± 5.0 nm | F Wistar rat | - | Intratracheal Instillation |
150 cm2 SA | Exposure caused pulmonary alveolar proteinosis and TH1/TH17 dominant response |
| Verstaelen et al. 2014 | CoO | 7.1 nm | BEAS-2B, A549 epithelial cells | In vitro | 1–60 μg/mL | Alterations in expression of genes associated with innate immunity, T-cell activation, and leukocyte adhesion | ||
| Copper | Cho et al. 2012 | CuO | 23.1 ± 7.2 nm | F Wistar rat | - | Intratracheal Instillation |
150 cm2 SA | No immunoinflammatory reaction |
| Park et al. 2015 | CuO | <50 nm | F BALB/c mouse | OVA | Intranasal Instillation | 25, 50, 100 μg/kg | Increased AHR, IgE and mucus production | |
| Lai et al. 2018 | CuO | 46.5 nm | C57BL/6 mouse | - | Intranasal delivery | 1, 2.5, 5, 10 mg/kg | Aggravated pulmonary inflammation, collagen accumulation and expression of progressive fibrosis markers in lungs | |
| Iron | Park et al. 2015 | Fe2O3 | 1013 ± 4.2 nm | M ICR mouse | - | Intratracheal instillation | 0.5, 1, or 2 mg/kg | TH1-polarized inflammatory response, GM-CSF, MCP-1, and MIP-1 increase, and increased expression of CD80, CD86, and MHC II expression on lung APCs |
| Park et al 2010c | Fe3O4 | 5.3 + 3.6 nm | M ICR mouse | - | Intratracheal instillation | 250, 500, 1,000 μg/kg | Increases in TH1/TH2 cytokines, B cells, and IgE levels | |
| Gold | Hussain et al. 2011 | Au | 40 nm | M BALB/c mouse | TDI | Aspiration | 40 μL @ 0.8 mg/kg | 3x AHR increase |
| Baretto et al. 2015 | Au | 6.3 nm | Swiss Webster and A/J mouse | OVA | Intranasal Instillation | 6, 60 μg/kg | Inhibited allergen-induced accumulation of inflammatory cells, pro-inflammatory cytokine production. In A/J mice, AuNPs prevented mucus production and AHR | |
| Nickel | Cho et al. 2012 | NiO | 5.3 nm | F Wistar rat | - | Intratracheal Instillation | 150 cm2 SA | Exposure caused pulmonary alveolar proteinosis and TH1/TH17 dominant responses |
| Baker et al. 2016 | Ni | 20 nm | M C57BL/6 WT or T-bet−/− mouse | - | Aspiration | 4 mg/kg | Increased airway remodeling in T-bet knockout mice with susceptibility to TH2 responses | |
| Lee et al. 2016 | NiO | 5.3 ± 0.4 nm | F Wistar rat | - | Intratracheal Instillation | 50, 100, 200 cm2 | Acute neutrophilic inflammation, and eosinophils recruited at days 3 and 4 via eotaxin release | |
| Chang et al. 2017 | NiO | - | M Wistar rat | - | Intratracheal Instillation | 0.015–0.24 mg/kg | Alterations in TH1/TH2 balance were indicative of nitrative stress and NFk-B activation | |
| Platinum | Park et al. 2010b | Pt | 20.9 ± 11.4 nm | M ICR mouse | - | Intratracheal Instillation | - | Increase in serum IgE, lung TH2 cytokines, and decrease in CD4/8 ratio |
| Onizawa et al. 2009 | Pt | 2 ± 0.4 nm | DBA/2 mouse | - | Intranasal instillation | x3 d | PtNP exerted protective effects from cigarette smoke, prevented NFk-B activation, and neutrophilic inflammation | |
| Silica | Brandenberger et al. 2013 | Si | 90 nm | F BALB/c mouse | OVA | Intranasal instillation | 0, 10, 100,400 μg | Co-exposure during sensitization caused dose-dependent enhancement of OVA-specific IgE, lung eosinophils, mucus cell metaplasia, and TH2/TH17 cytokine production |
| Silver | Park H et al. 2010 | Ag | 6 ± 0.29 nm | F C57BL/6 mouse | OVA | Inhalation | 5 × 20 ppm, 40 mg/kg | Decreased AHR, TH2 cytokines, and ROS levels |
| Jang et al. 2012 | Ag | 6.0 ± 0.29 nm | F BALB/c mouse | OVA | Inhalation | 20 ppm/40 mg/kg 5× for 24 h | Suppressed mucus production via VEGF signaling alterations | |
| Su et al. 2013 | Ag | 33 nm | F BALB/c mouse | OVA | Inhalation | 3.3 ± 0.7 mg/m3 6h/7 × 7 d | Increased OVA IgE, proteins associated with immune processes were altered | |
| Chuang et al. 2013 | Ag | 33 nm | F BALB/c mouse | OVA | Inhalation | 3.3 mg/m3 6h/d × 7 d | Increased Penh, recruitment of neutrophils, lymphocytes, and eosinophils to the airways | |
| Xu et al. 2013 | Ag | 141 nm | F BALB/c mouse | OVA | IP Injection | 0.4, 2, 10 mg/kg | ncreased OVA-IgG and TH2 responses, local activation and recruitment of leukocytes | |
| Titanium | Ahn et al. 2005 | TiO2 | 0.29 μm | M Sprague-Dawley rat | - | Intratracheal Instillation | 4 mg/kg | Increased BAL IL-13 levels, IL-13-producing mast cells, and goblet cell hyperplasia |
| Park et al. 2009 | TiO2 | 20 nm | ICR mouse | - | Intratracheal Instillation | 5, 20, or 50 mg/kg | Increased BAL and serum IgE levels, altered TH1/TH2 cytokines, increased B cell distribution | |
| Larsen et al. 2009 | TiO2 | 28 nm | F BALB/c mouse | OVA | IP Injection | 2–250 μg | TH2 adjuvancy, increased IgE, IgG1, and eosinophil levels | |
| Rossi et al. 2010 | TiO2 | 10 × 40 nm | F BALC/c/Sca mouse | OVA | Inhalation | 2 h/d, 3 d/w, x4 w @ 10 mg/m3 | Allergic pulmonary inflammation suppressed by TiO2NP | |
| Gustafsson et al. 2011 | TiO2 | 21 nm | M Dark Agouti rat | - | Intratracheal instillation | 5 mg/kg | ncreased eosinophil, DC numbers in lungs, lymphocytes recruited mostly CD4+, also included CD8+ T-cells, B-cells, and CD25+ T-cells | |
| Hussain et al. 2011 | TiO2 | 15 nm | M BALB/c mouse | TDI | Aspiration | 40 μL @ 0.8 mg/kg | 2× increase AHR | |
| Scarino et al. 2012 | TiO2 | 5 nm APS, 168/171 nm agg. | M Brown Norway rat | OVA | Inhalation | 9.4 or 15.7 mg/m3 | Significantly decreased lung leukocytes and plasma/BAL IL-4, IL-6, and IFN-γ over OVA controls | |
| Jonasson et al. 2013 | TiO2 | 21 nm | F BALB/c mouse | OVA | Inhalation | 32 ± 1 μg | Aggravated allergic response dependent on dose and timing | |
| Fu et al. 2014 | TiO2 | 21 nm | M Sprague-Dawley rat | - | Intratracheal Instillation | 0.5, 4.0, 32 mg/kg 2x/w × 4 w | Deposition in lymph nodes, increased T and B-cell proliferation following mitogen stimulation, enhanced NK activity in spleen, increased B-cells in the blood | |
| Choi et al. 2014 | TiO2 | P25 | M New Zealand White Rabbit | - | Intratracheal instillation | 10, 50, 250 μg | Dose-dependent eosinophil influx and inflammation in the lung, but not neutrophil or lymphocyte influx | |
| Gustafsson et al. 2014 | TiO2 | 21 nm | M Dark Agouti rat M Brown Norway rat | OVA | Inhalation | 168–159 μg/d × 10 d | Exposure decreased eosinophilia in OVA-sensitized DA and BN rats, but neutrophils/lymphocyte increase in DA rats | |
| Mishra et al. 2016 | TiO2 | 4–8 nm | BALB/c mouse | OVA | IP Injection | 200 μg | Augmented AHR, biochemical markers of damage, and induced a mixed TH1/TH2 response | |
| Kim et al. 2017 | TiO2 | 75 nm | F BALB/c mouse | OVA | Inhalation | 50 μg/m3 × 3 d | Exposure exacerbated AHR and inflammation, increases in IL-1, IL-18 | |
| Zinc | Roy et al 2014b | ZnO | <50nm | F BALB/c mouse | OVA | IP Injection | 0.25, 0.5, 1, 3 mg | Administration with OVA caused increased OVA-IgG1, IgE, eosinophil, and mast cell numbers in lungs and spleen |
| Roy et al 2014a | ZnO | <50nm | F BALB/c mouse | OVA | IP Injection | 1, 2, 4, and 12 mg/mL | Adjuvant effect on OVA allergy by signaling through TLRs and Src kinase leading to inflammatory responses | |
| Huang et al. 2015 | ZnO | 181.5 nm low dose 360.0 nm high dose | F BALB/c mouse | OVA | Aspiration | 0.1/0.5 mg/kg | Exposure simultaneous to OVA sensitization resulted in eosinophil recruitment and TH2 adjuvancy |
Summary of findings from in vivo studies investigating immune effects of metal nanomaterials in the lung and select in vitro studies in pulmonary cells, grouped by metal. AHR: airway hyperreactivity; APC: antigen-presenting cell; APS: average particle size; DC: dendritic cell; HDM: house dust mite; IP: intraperitoneal; LPS: lipopolysaccharide; MHC: major histocompatibility complex; OVA: ovalbumin; ROS: reactive oxygen species; RSV: respiratory syncytial virus; TLR: Toll-like receptor; WT: wild-type.
Table 7.
Metal nanomaterials and corresponding physicochemical properties shown to influence immunological processes involved in the development and augmentation of asthma.
| AOP step | Metal nanomaterial effect | Metal | Properties implicated | Source |
|---|---|---|---|---|
| Sensitization Bioavailability | Increased potential for inhalation | many | dustiness | Evans et al. 2003 |
| Evasion of uptake by pulmonary macrophages | Si | size, crg, mod | Oh et al. 2010 | |
| Evasion of entrapment by pulmonary mucus | ND | size, crg | Murgia et al. 2016 | |
| Prolonged retention in airways | Al | spec, mor | Park et al. 2017 | |
| Direct translocation across lung epithelial tissue to lymphatics | Au | size, crg | Kreyling et al. 2014 | |
| Molecular initating event | Increased potential for metal antigen formation | Ti | size | Vamanu et al. 2008 |
| Increased protease activity of protein allergens | Au | – | Radauer-Preiml et al. 2016 | |
| Cellular response | Adsorption of LPS to nanomaterial surface | Au | size, hyd, mod | Li et al. 2017 |
| Increased recruitment of DC to lung | Al | mod | Li et al. 2010 | |
| Direct activation of DC | Ti | size, cry | Winter et al. 2011 | |
| Release of DAMPs from immune cells > activation of DC | Zn | size, mor, SA | Hsaio et al. 2011 | |
| Release of DAMPs from epithelial cells > activation of DC | Ag | size, mod, SA, sol | Hamilton et al. 2014 | |
| Organ response | Increased CD4þ T-cell presentation efficiency | Ti | size, mor, cry | Schanen et al. 2009 |
| Increased polarization of CD4þ T-cells to TH2 phenotype | Si | size, mod, SA | Vallhov et al. 2012 | |
| Increased number of B-cells | Ti | – | Park et al. 2009 | |
| Alteration in B-cell expansion/maturation | Au | size | Lee et al. 2014 | |
| Increased production of total IgE | Pt | – | Park et al. 2010b | |
| Increased production of allergen-specific IgE | Zn | size, sol, cry | Horie et al. 2015 | |
| Elicitation | ||||
| Organism response-early phase reaction | Increased IgE-dependent mast cell degranulation | Au | size, mod | Huang et al. 2009 |
| Increased IgE-independent mast cell degranulation | many | size, SA, crg | Johnson et al. 2017 | |
| Increased number of lung mast cells | Ce | – | Meldrum et al. 2018 | |
| Altered mast cell exocytic function and granule release | Si | SA | Maurer-Jones et al. 2010 | |
| Altered expression of Fc receptors on immune cells | Ti | size | Liu et al. 2010 | |
| Organism response- late phase reaction | Increased endothelial adhesion molecule expression | Al | – | Oesterling et al. 2008 |
| Increased neutrophil recruitment | Ag | size, sol | Arai et al. 2015 | |
| Increased eosinophil recruitment | Co | sol | Jeong et al. 2015 | |
| Increased lymphocyte recruitment | Zr | mod | Vennemann et al. 2017 | |
| Increased AHR | Ag | size, mod | Seiffert et al. 2015 | |
| Increased airway smooth muscle contractility | Co/Fe | – | Kapilevich et al. 2012 | |
| Mucus cell metaplasia/muus hyeprsecretion | Ti | – | Chen et al. 2011 | |
| Chronic effects | Epithelial cell proliferation | Zn | sol | Cho et al. 2011 |
| Increased fibroblast MMP activity extracellular matrix remodeling | Ti | size, cry, mor | Armand et al. 2012 | |
| Myofibroblast accumulation | Cu | – | Lai et al. 2018 |
Adverse Outcome Pathway (AOP) steps involved in the sensitization and elicitation phases of asthma, metal nanomaterials shown to impact individual steps, and physicochemical properties associated with effects are shown. Physicochemical properties of interest include size, metal speciation (spec), agglomeration (agg), surface modification (mod), surface area (SA), solubility (sol), surface charge (crg), morphology (mor), crystallinity (cry), and hydrophobicity (hyd). ND (not determined) notation in metal column indicates a study demonstrating a critical role for a specific nanomaterial physicochemical property on the cellular event, but was demonstrated using nonmetal nanomaterials. Findings may be applicable to metals, but have not been demonstrated with individual metal nanomaterials.
Human studies demonstrating pulmonary immune effects of metal nanomaterials
A 2014 case study best illustrates the concerns associated with the unknown allergic effects of metal nanomaterials. In the report, a chemist who accidentally inhaled NiNP in the work-place subsequently developed clinical symptoms indicative of IgE-mediated respiratory allergy including throat irritation, nasal congestion, facial flushing, and respiratory distress upon future encounters with NiNP. The chemist also developed previously-nonexistent symptoms indicative of T-cell-mediated ACD in response to non-nanoparticulate forms of nickel in her earrings and belt buckles (Journeay and Goldman 2014). In addition to reinforcing existing concerns over increased potential for allergic sensitization as a result of decreased size, the case also emphasized additional concerns reflective of the unique mechanisms of metal allergy. The case showed that sensitization via one exposure route may not limit future elicitation reactions to the same tissue; moreover, sensitization by metal ions, irrespective of original parent material size, may result in elicitation reactions following exposure to both nano- and bulk-sized metal materials.
Adverse immune effects with implications for allergy have been investigated in human subjects with risk of inhalation exposure to metal nanomaterials in their workplaces. In one study, it was shown that workers of nanomaterial-handling facilities in Taiwan exhibited increased prevalence of sneezing, dry cough, and productive cough compared to workers with no nanomaterial exposures (Liao et al. 2014). Although the workers were employed by facilities handling SiO2 NP, Fe2O3 NP, AuNP, AgNP, and TiO2NP, it is unclear whether the observed respiratory effects were mediated by adaptive immune responses specific to the metals, or nonspecific irritant mechanisms. Interestingly, increased rates of ACD were also observed in the workers of the nanomaterial-handling facilities, but the inciting agents were not determined. As such, it is unknown if exposure to the nanomaterials induced sensitization or caused increased susceptibility to ACD development in workers.
Similar studies have also demonstrated that exposure to nanomaterials in the workplace can cause elevations in various immune-related biomarkers indicative of potential effects on allergy (Andujar et al. 2014; Glass et al. 2017; Kurjane et al. 2017) For example, elevations in breath condensate leukotriene (LT) levels were observed in subjects exposed to TiO2 NP aerosol (< 100 nm) in their workplaces (Daniela et al. 2016). The exposed workers had elevated levels of LTB4, a lipid mediator associated with the recruitment, activation, and prolongation of survival of leukocytes in the lung, as well as multiple cysteinyl leukotrienes (i.e. LTC4, LTE4, LTD4), that are potent mediators of bronchoconstriction (Luster and Tager 2004; Zakharov et al. 2016). Although no alterations in lung function were observed in the exposed workers, elevations in levels of lipid mediators involved in the pathogenesis of asthma suggest the potential for exposure to TiO2NP in the workplace to influence the severity of asthmatic conditions.
Collectively, these reports emphasize the potential occupational hazards associated with metal nanomaterials. Although numerous immune markers have been shown to be modulated in workers following inhalation exposure to metal nanomaterials, specific implications for asthma remain unclear. Limitations of human studies arise from inconsistencies between exposure conditions, subject histories, and the requirement for noninvasive, measurable endpoints. Accordingly, the effects of metal nanomaterials on pulmonary immunity and underlying mechanisms have been assessed in animal models wherein controlled dosing, consistent environments, and additional endpoints have helped identify some of the potential underlying mechanisms of metal nanomaterial-induced pulmonary immune effects.
Evidence for increased potential for respiratory sensitization from animal studies
Assessment of respiratory sensitization presents numerous challenges underscored by the absence of validated in vivo, in vitro, or in silico approaches for identification of potential sensitizers. However, biomarkers with proposed utility for in vivo identification of potential respiratory sensitizers following pulmonary exposure include IgE and TH2 cytokines (de Jong et al. 2009; Chary et al. 2018). These markers have not been employed for direct evaluation of respiratory sensitization potential by metal nanomaterials; however, numerous studies have reported increased IgE levels following in vivo pulmonary exposure to TiO2 NP, PtNP, FeNP, AgNP, and ZnO NP (Park et al. 2009; Park et al. 2010; Cho et al. 2011; Huang et al. 2015; Seiffert et al. 2015). Many of the same nanomaterials have also been associated with increased TH2 cytokine levels (i.e. IL-4, IL-5, IL-13) following pulmonary exposure (Pettibone et al. 2008; Park 2010; Marzaioli et al. 2014). Although these findings are suggestive of the potential for metal nanomaterials to induce asthma, since the specificity of IgE molecules was not determined in any studies, the capacity for respiratory sensitization remains speculative.
Assessment of respiratory sensitization potential is further complicated by the absence of an AOP specific to the events associated with asthma development. Moreover, discrepancies in some key events involved in asthma inception by LMW and HMW agents indicate the potential requirement for multiple respiratory sensitization AOP. However, many steps are known to be conserved with respect to sensitization of the skin and lungs; knowledge of metal nanomaterial effects on these processes can provide potential insight regarding their potential to cause asthma.
The induction of respiratory sensitization is ultimately dependent on antigen bioavailability. Although it remains unclear whether nano-scale dimensions of metals increase the likelihood for absorption following dermal exposures, the respiratory tract presents a portal of entry known to be increasingly susceptible to smaller materials (Mercer et al. 2018). Nano-materials exhibit a characteristically increased level of “dustiness,” a property which describes the propensity for a material to become airborne following disruption (Evans et al. 2013). Accordingly, the potential for aerosolization and inhalation of metal nanoparticles increases with decreasing size, thereby overcoming one of the limiting steps of respiratory sensitization associated with larger-sized metal particles.
Sensitization of the lungs also requires interactions between the sensitizing agent and APC. The respiratory tract is equipped with an expansive repertoire of defense mechanisms that prevent such interactions, but metal nanomaterials have been shown to have increased capacity to circumvent many of these mechanisms, increasing their potential for uptake by DC. In the upper airways, a layer of mucus lining the lumen functions to trap inhaled antigens and facilitate their translocation out of the trachea by the mucociliary escalator (Moldoveanu et al. 2009). Evasion of the ~5 mm thick mucus layer has been associated with nanomaterial physicochemical properties including size, surface modification, and surface charge (Samet and Cheng 1994; Yang et al. 2008; Liu et al. 2015; Murgia et al. 2016). Generally, hydrophilic, neutrally-charged nanomaterials with smaller diameters have been shown to penetrate mucus to a greater degree than counterparts with opposing properties (Schuster et al. 2013).
In the lower airways, a similar mechanism of antigen neutralization is facilitated by pulmonary surfactant (Chroneos et al. 2010). In addition to optimizing the mechanics of respiration, surfactant contains proteins capable of binding aeroallergens, accelerating their clearance, and preventing their uptake by APC, thereby inhibiting antigen-specific responses (Malhotra et al. 1993; Wang et al. 1996; Hohlfeld 2002; Ruge et al. 2011). Two of these proteins, surfactant protein (SP)-A and SP-D, have been shown to bind to various metal nanomaterials leading to accelerated clearance by phagocytic mechanisms (Ruge et al. 2011). Accordingly, nanomaterials with properties that deter binding to surfactant proteins, such as surface charge, may exhibit increased potential for evasion of clearance by this mechanism, increasing potential for interaction with lung DC (Schulze et al. 2011).
Increased potential for antigen/nanomaterial interaction with DC can also result from evasion of pulmonary macrophage-mediated clearance. Uptake and sequestration of antigen by pulmonary macrophages results in intracellular chemical degradation or physical translocation out of the lungs, preventing their interception by DC (Elder and Oberdörster 2006). Nano-materials can evade clearance by this mechanism by numerous effects. First, since macrophages have been shown to selectively phagocytose nanomaterials according to size, charge, and surface modification, physicochemical properties may contribute to their persistence in the respiratory tract. Their clearance may also be compromised as a result of selective cytotoxic effects on pulmonary macrophages and subsequently fewer viable macrophages capable of neutralizing the nanoparticles. Pulmonary macrophage cytotoxicity has been associated with physicochemical properties including morphology, surface charge, and rate of dissolution (Oh et al. 2010; Hamilton et al. 2014; Shim et al. 2017). Metal nanomaterial-induced alterations in phagocytic activity of pulmonary macrophages, as demonstrated by TiO2 NP, ZnO NP, and AlNP, may also contribute to evasion of clearance (Wagner et al. 2007; Liu et al. 2010; Liu H et al. 2013). In addition to compromising the clearance capacity of the phagocytic system on a cellular level, nanomaterials are also associated with maximizing the clearance capacity of the collective phagocytic system. Volumetric loading of alveolar macrophages following inhalation of nanomaterials decreases the efficiency of clearance, extending biopersistence, and increasing the potential for interception by DC (Oberdorster et al. 1992; Blank et al. 2017).
Metal nanomaterial cytotoxic effects on pulmonary macrophages may also promote sensitization by additional mechanisms. Since alveolar macrophages are known to antagonize TH2 responses in the lung and downregulate APC functions, cytotoxic effects may disrupt the maintenance of an immunological tolerant state (Tang et al. 2001). Moreover, their depletion leads to significantly increased recruitment of DC and DC precursors to the lungs (Holt et al. 1993; Jakubzick et al. 2006). Numerous metal nanomaterials are also known to trigger the release of alarmins including IL-1β and −1α by alveolar macrophages; in turn, these can activate DC and facilitate sensitization (Braydich-Stolle et al. 2010; Scherbart et al. 2011; Sandberg et al. 2012; Hamilton et al. 2014; Rabolli et al. 2014; Arai et al. 2015).
Similar to their roles in the development of skin allergy, epithelial cells of the respiratory tract are integral in the development of asthma, and their disruption by inhaled materials can have profound influence on the early events of sensitization (Bergamaschi et al. 2006). A major function of airway epithelial cells is to serve as a physical barrier between inhaled agents that deposit in the airway lumen and DC in the epithelium (Hammad and Lambrecht 2015). The importance of barrier integrity in preventing the development of asthma is illustrated by the barrier-disrupting proteolytic activity shared by many aeroallergens with high rates of sensitivity in the population (Kauffman et al. 2006; Lambrecht and Hammad 2012). The frequent observation that metal nanomaterials are capable of inducing cytotoxicity to pulmonary epithelial cells suggests their potential to increase permeability and passage of antigens from the airway lumen to compartments associated with DC.
Airway epithelial cell cytotoxicity has also been associated with the release of alarmins that have potential to promote DC activation and sensitization. Similar to keratinocytes in the skin, the mechanism of cell death can critically influence the nature of the resultant immune response. For example, the necrotic cell death following pulmonary exposure to beryllium results in the release of mediators including cellular DNA, which is recognized as a DAMP by TLR-9, and promotes the unique TH1-mediated effects associated with chronic beryllium disease (McKee et al. 2015). NiNP, AgNP, and CoNP have been shown to induce similar necrotic cell death of bronchial and alveolar epithelial cells (Holt et al. 1993; von Garnier et al. 2005; de Haar et al. 2008; Capasso et al. 2014; Ortega et al. 2014). Contrarily, ZnO NP, CuO NP, TiO2 NP, and CrNP have all been associated with induction of apoptotic cell death in pulmonary epithelial cells (Park et al. 2007, 2008; Ahamed et al. 2011; Sun et al. 2012; Senapati et al. 2015). This effect may further influence the development of respiratory allergy since uptake of these cells is a property exclusive to CD103+ DC, a subset of DC associated with cross-presentation and the subsequent induction of CD8+effector responses (Desch et al. 2011).
Incorporation of metal nanomaterials into asthma models
The impact of metal nano-material exposure prior to sensitization has only been addressed by a few studies using asthma models. Aspiration exposure to ZnO NP, TiO2 NP, NiO NP, CuO NP, or SiO2 NP 1 d before inhalation sensitization to OVA was followed by inhalation challenge and subsequent assessment of asthmatic severity. Soluble metal nanomaterials (NiO NP, ZnO NP, and CuO NP) were associated with elevations in OVA-specific IgE, whereas insoluble SiO2 NP and TiO2 NP were not. Subsequent investigations confirmed the importance of metal ion release in the adjuvant effects on sensitization. The increase in OVA-specific IgE production associated with soluble NiO NP was not conserved in response to insoluble NiO microparticles in the same model (Horie et al. 2015). However, ZnCl2 also did not exert the same increase in OVA-specific IgE caused by ZnO NP. As a result, it was concluded that continuous ion release from nanoparticles was required for the induction of the observed effects (Horie et al. 2016). Exposure to residual oil fly ash particles prior to allergen sensitization has also been associated with adjuvant effects attributable to soluble metal constituents (Lambert et al. 2000).
Concurrent exposure to metal nanomaterials during allergen sensitization has been explored extensively in order to evaluate the potential adjuvant effects of metal nanomaterials on asthma development. This concept has been explored with respect to both systemic and respiratory sensitization routes, as well as independent and dependent of allergen challenge. In the absence of allergen challenge, evaluation of sensitization achieved by intraperitoneal injection is limited to assessment by systemic markers, such as antigen-specific IgE and cytokine levels. Accordingly, co-administration of AgNP and ZnO NP with antigen has been associated with elevated levels of allergen-specific IgE, as well as increased levels of TH2 cytokines (Matsumura et al. 2010; Xu et al. 2013). As demonstrated with SiO2 NP, enhanced antibody production has been associated with both increasing dose and decreasing particle size (Toda and Yoshino 2016).
Though few studies have correlated metal nanomaterial properties to adjuvant effects on intraperitoneal sensitization independent of allergen challenge, existing findings are conducive with studies using larger metal particles and nonmetal nanoparticles (Naim et al. 1997; Granum 2001b). The impact of the most extensive number of physicochemical properties with respect to adjuvant effects on OVA sensitization use polystyrene nanoparticles (PSP). Nygaard et al. (2004) used PSP ranging from 58 nm to 11.4 μm to evaluate the influence of particle size, mass, surface area, and particle number. Similarly, Granum et al. (2000) used six sizes of spherical PSP to administer doses with constant mass (12.25 μg), size (0.1 μm), particle number (8 × 1010), or surface area (1300 cm2). Both studies showed that serum OVA-specific IgE levels best correlated with particle number and surface area (Granum et al. 2001a; Nygaard et al. 2004).
Similar adjuvant effects have been observed following respiratory sensitization and co-exposure to TiO2 NP, SiO2 NP, and ZnO NP (de Haar et al. 2006; Huang et al. 2015). Increases in OVA-specific IgE and TH2 cytokine levels were similarly associated with decreasing size of SiO2 NP (Yoshida et al. 2011). Moreover, SiO2NP surface properties were shown to impact sensitization independent of allergen challenge. Intranasal exposure to three variations of SiO2 NP (spherical, mesoporous, and PEGylated) simultaneous to OVA sensitization exacerbated pathological changes, inflammatory cell influx, and TH2 cytokine responses. These effects were specific to the unique surface chemistry of each type of SiO2 NP, but the most severe responses were associated with the nanoparticle with the highest surface area (Han et al. 2016).
The absence of allergen challenge in these studies helps elucidate the direct effects of metal nanomaterials on sensitization processes. However, another approach to evaluate the same effect involves evaluation of allergic parameters collected in response to allergen challenge. Studies utilizing this approach have similarly demonstrated enhanced asthmatic responses in OVA-challenged mice when intraperitoneal sensitization occurred simultaneous to TiO2 NP and ZnO NP (Larsen et al. 2010; Roy et al. 2014a; Roy et al. 2014b; Mishra et al. 2016).
Although the observed effects may reflect residual impacts of metal nanomaterial respiratory exposure during sensitization, similar adjuvant effects on elicitation responses have been observed following respiratory sensitization and simultaneous metal nanomaterial exposure. Simultaneous administration of SiO2NP, CeO2NP, QD, and TiO2NP with allergen during sensitization led to enhanced asthmatic response severity, as measured by antigen-specific antibody levels, inflammatory cell influx, and TH2 cytokine levels after challenge (Brandenberger et al. 2013; Meldrum et al. 2018; Vandebriel et al. 2018; Scoville et al. 2019). Studies using similar sensitization procedures and endpoints have also implicated TiO2 NP crystal structure in adjuvant effects on sensitization (de Haar et al. 2006; Vandebriel et al. 2018).
Metal nanomaterial exposure has also been incorporated into the challenge phase of asthma to evaluate potential modulation of asthmatic responses in established asthmatic conditions. Although some metals, including CuO NP, have been exclusively shown to induce significant aggravating effects on elicitation responses, others, including AuNP, appear to exert protective effects on asthmatic responses (Barreto et al. 2015; Park et al. 2016; Omlor et al. 2017). Contrarily, other metal nanomaterials, including TiO2 NP, have been associated with divergent effects on allergen challenge that appear increasingly susceptible to variation during this phase of asthma. Effects have been reported to be differentially induced according to dose, duration of exposure, and endpoints of assessment (Rossi et al. 2010; Hussain et al. 2011; Jonasson et al. 2013; Kim et al. 2017).
Similarly, after OVA sensitization via intraperitoneal injection, AgNP exposure during allergen challenge has been reported to induce various aggravating and attenuating effects on allergic inflammation. Inhalation exposure to 6 nm AgNP was shown in multiple studies to suppress inflammatory cell influx, airway hyper-reactivity (AHR), mucus hypersecretion, and other measures of asthmatic responses (Park, Kim, Jang, et al. 2010; Jang et al. 2012). Contrarily, in another study with very similar exposure conditions, 33 nm AgNP caused increased airway response, inflammatory cell influx, and OVA-IgE levels over control animals (Chuang et al. 2013; Su et al. 2013). The discrepancies between these studies may be attributable to AgNP size difference, as well as potential variations in particle coating, both of which have been associated with differ-ential effects on asthmatic responses (Alessandrini et al. 2017). Additionally, the first two studies used the TH1-dominant C57BL/6 mouse strain, whereas the second used a TH2-biased BALB/c strain (Jones et al. 2013). Strain-specific immune responses following respiratory exposure to metal nanomaterials during allergen challenge have been demonstrated in other studies, as well (Gustafsson et al. 2014).
Studies using SiNP and ZrO NP with variations in surface properties demonstrate that when administered during allergen challenge, surface properties of nanomaterials can differen-tially aggravate allergic inflammation (Marzaioli et al. 2014; Park, Sohn, et al. 2015; Vennemann et al. 2017). It has been suggested that particles with higher oxidant potential amplify asthmatic inflammation to a greater degree, which would implicate physicochemical properties such as surface modification in these effects (Li et al. 2009).
Potential mechanisms of asthma augmentation by metal nanomaterials
Although asthma models have characterized the potential effects of metal nanomaterial exposure on asthmatic processes, many of the underlying mechanisms of these observed effects remain unclear. However, findings from other studies suggest several mechanisms may be associated with the observed effects of metal nanomaterials on the augmentation of asthma.
Respiratory exposure to metal nanomaterials may increase susceptibility to sensitization by aeroallergens by similar mechanisms previously proposed to contribute to their respiratory sensitization potential. Release of alarmins by airway epithelial cells and resident immune cells, disruption of the TH1/TH2 balance in the lung, and amplification of oxidative stress by metal nanomaterials may also generate adjuvant effects on sensitization. Similarly, FeNP, TiO2 NP, and SiNP have all been shown induce the release of TH2 cytokines including IL-33, TSLP, GM-CSF, and IL-25 by airway epithelial cells, which are known to promote DC maturation (Hussain et al. 2009; Val et al. 2009; Mano et al. 2013; Park, Sohn, et al. 2015).
Evidence also suggests metal nanomaterial exposure can modulate inflammatory pheno-types of existing asthmatic conditions. Two major heterogeneous asthma phenotypes differ based on the presence of neutrophil (TH1/TH17)- or eosinophil (TH2)-dominated inflammation (Fahy 2009; Yu and Chen 2018). Particulate and soluble metals are known to differentially impact the nature of existing allergic airway inflammation by skewing this balance (Schneider et al. 2012). Dissolution kinetics also appear influential in this regard, as CoNP, NiNP, ZnO NP, and CuO NP and their corresponding ions have been shown to differentially recruit eosinophils and neutrophils to the lungs of rats after exposure (Cho et al. 2011; Jeong et al. 2015).
Modulation of elicitation response severity by metal nanomaterials may emerge as a result of modulation of mast cell activity. As a major effector cell in IgE-mediated allergic responses, mast cells have been shown to be potential targets of metal nanomaterial-induced adverse effects in vitro (Feltis et al. 2015; Johnson et al. 2017; Alsaleh and Brown 2018). AgNP, CuO NP, SiO2 NP, and TiO2 NP have all been shown to induce IgE-independent mast cell degranulation depending on physicochemical properties including size, surface area, charge, shape, and the presence of adsorbed surface proteins (Marquis et al. 2011; Aldossari et al. 2015; Alsaleh et al. 2016; Johnson et al. 2017). Modulation of IgE-dependent mast cell degranulation has also been demonstrated by some of the same nanomaterials. TiO2 NP, AuNP, CeO2 NP, ZnO NP, and FeNP have been shown to modulate interactions between allergen and surface-bound IgE molecules, interfering with dimerization and subsequent degranulation (Huang et al. 2009; Ortega et al. 2015). Similarly, mast cell uptake of metal nanomaterials has been associated with modulation of intracellular calcium signaling involved in mast cell degranulation (Amin 2012; Chen et al. 2012). Accordingly, differential ion release by bulk ZnO particles, ZnO NP, and soluble ZnSO4 has been correlated with the propensity for OVA-sensitized rat mast cells to degranulate when co-exposed with OVA (Yamaki and Yoshino 2009; Feltis et al. 2015).
Furthermore, TiO2 NP and AuNP have been shown to alter the exocytic kinetics of granule secretion by mast cells (Marquis et al. 2009). The qualitative and quantitative profile of granule contents has also been shown to be subject to modulation by some metal nanomaterials. The number of molecules per granule has been shown to be impacted by SiO2 NP as a function of porosity and surface area (Maurer-Jones et al. 2010). Variations in vesicle mediator content has been shown to be augmented by AuNP (Marquis et al. 2009). Since the contents of mast cell granules contribute to vascular permeability and inflammatory cell recruitment, these alterations can greatly impact the severity of allergic elicitation (Dudeck et al. 2011; Weber et al. 2015).
Aggravation of existing asthmatic conditions may also involve non-immunological mechanisms, such as metal nanomaterial-induced alterations to normal physiological processes. For example, increased mucus production by epithelial cells is a hallmark symptom of the early and late phase asthmatic response (Erle and Sheppard 2014). The observation that TiO2NP and CuONP both increased mucin secretion in human epithelial cells suggests potential to exacerbate asthmatic conditions by contributing to obstruction of airways (Chen et al. 2011; Park et al. 2016). Similarly, TiO2 NP, AuNP, and AgNP have been shown interfere with optimal pulmonary surfactant functioning, which can cause AHR and increased resistance to airflow (Hohlfeld et al. 1999; Hohlfeld 2002; Bakshi et al. 2008; Schleh et al. 2009; Zhang et al. 2018). AHR may also be modulated by metal nanomaterials as a result of alteration of airway smooth muscle (ASM) contractility. ZnO NP, CuO NP, and TiO2 NP have all been shown to alter human ASM mechanical function in vitro (Berntsen et al. 2010). Similarly, CoFe2O4 NP were shown to potentiate both histaminergic and cholinergic ASM contractility in vivo, which has the capacity to exacerbate symptoms of asthma associated with bronchoconstriction (Kapilevich et al. 2012).
Metal nanomaterial exposure may also exacerbate established asthmatic conditions by accelerating the progression of pathological alterations associated with chronic asthmatic conditions. The repetitive induction and resolution of inflammation induced by asthmatic elicitation leads to anatomical alterations referred to as airway remodeling (Fehrenbach et al. 2017). Histological indicators of these alterations have been reported to be exacerbated by various metal nanomaterials including SiNP (Han et al. 2011). Similarly, cellular indicators of accelerated airway remodeling have been implicated in response to many metal nanomaterials. For example, fibroblast accumulation and increased extracellular matrix deposition is a common contributor to airway remodeling and has been observed in response to NiNP, SiO2 NP, and CeO2 NP exposure (Warner and Knight 2008; Han et al. 2011; Ma et al. 2012; Armand et al. 2013; Glista-Baker et al. 2014).
Knowledge gaps in metal nanomaterial effects on asthma
Despite the known capacity for many metals to induce IgE-mediated asthma following inhalation, the potential for metal nanomaterials to induce sensitization of the respiratory tract remains completely unknown. Several other interesting aspects of pulmonary immunity have not been widely addressed with respect to metal nanomaterials, and may have relevance to current observations regarding their effects on asthma. The microbiome is known to significantly impact numerous aspects of allergic disorders, and while some metal nanomaterials associated with antimicrobial activity have been shown to alter the pulmonary microbiome, the implications for asthma remain unknown (Alessandrini et al. 2017; Poh et al. 2018). Similarly, the effects of metal nanomaterials on innate lymphoid cells also remain largely unstudied, but should not continue to be neglected, given the importance of this cell type in allergic disorders. Finally, the capacity for metal nanomaterials to disrupt or prevent the development of immunological tolerance has not been explored, and may be influential in both phases of asthmatic conditions.
Effects of metal nanomaterials on immune cells and allergic processes in vitro
In vivo studies have demonstrated the capacity for metal nanomaterials to augment numerous immunological processes that result in functional implications for allergic disease. However, in vitro investigations have helped elucidate some of the underlying mechanisms responsible for in vivo observations. In this section, major findings regarding the role of metal nanomaterial physicochemical properties on molecular and cellular processes with implications for both ACD and asthma are summarized.
Effects on antigen immunogenicity
Many physicochemical properties associated with the molecular and cellular processes that confer antigen immunogenicity are subject to alteration following interactions with constituents of their environment. In this regard, the immunogenicity of metal nanomaterials may be significantly altered as a result of biocorona formation. Following entry into biological media, macromolecules present in the media interact with and adsorb to the surface of nanomaterials within minutes, forming a layer that defines the bio-identity of the nanomaterial (Corbo et al. 2016). The qualitative profile of adsorbed constituents and quantitative strength of association have been shown to be influenced by nanomaterial properties including size, charge, morphology, surface modification, and hydrophilicity, among other properties (Lundqvist et al. 2008; Dobrovolskaia et al. 2014).
Independent of adsorbed constituents’ identities, macromolecule associations with metal nanomaterial surfaces may induce alterations in physicochemical properties associated with their bioactivity (Dobrovolskai et al. 2009b; Yin et al. 2015). For example, protein adsorption to 25 nm FeNP was associated with a 5-fold increase in hydrodynamic size, which can impact a number of biological effects, such as propensity for cellular uptake (Calatayud et al. 2014). Similarly, adsorption of proteins can mask reactive surfaces of metal nanomaterials, attenuating ROS generation, and subsequently inhibiting a major biochemical mechanism involved in the release of alarmins (Ilinskaya and Dobrovolskaia 2016).
Contrarily, surface adsorption of macromolecules may alter the biological activity of metal nanomaterials in a manner that is dependent on the adsorbed constituent profile. Endo-genous proteins, including immunoglobulins, cytokines, and complement proteins are all constituents of the serum and lung lining fluid known to bind metal nanomaterial surfaces (Neagu et al. 2017). The binding of complement protein C3b and IgG to nanomaterial surfaces has been shown to confer recognition by complement and Fc receptors, accelerating clearance by phagocytes, and limiting the potential of the nanomaterial to induce other immune effects (Beduneau et al. 2009; Moghimi et al. 2012; Frohlich 2015). Adsorption of exogenous agents can also lead to alterations in nanomaterial immunogenicity. One of the most notable examples is endotoxin (LPS), a frequent microbial contaminant of nanomaterial surfaces that is capable of activating innate immune cells, and inducing pro-inflammatory signaling via the TLR-4 pathway. Adsorption of LPS to nanomaterial surfaces has been shown to enhance inflammatory responses to many metal nanomaterials by lung epithelial cells, and various immune cells (Shi et al. 2010; Liu et al. 2012; Bianchi et al. 2017; Li et al. 2017; Ko et al. 2018). The chemical structure of LPS favors its adsorption to hydrophobic, positively-charged metal nanomaterial surfaces, indicating a role for physicochemical properties such as surface modification in the propensity for associations with immunogenic exogenous molecules such as LPS (Gorbet and Sefton 2005; Li et al. 2017).
Although biocorona formation can augment the immunological fate of a nanomaterial, the interactions may also facilitate alterations in immunogenicity of the adsorbed constituents. Deng et al. (2010) showed that functionalized AuNP were capable of binding fibrinogen independent of nanoparticle size, but certain sizes of AuNP induced conformational changes in the protein. Subsequent alterations in protein structure conferred its recognition by the Mac-1 receptor, subsequently activating NF-κB signaling in innate immune cells. Similarly, Bastus et al. (2009) demonstrated that while macrophages did not recognize AuNP or two biomedically-relevant peptides individually, their conjugation facilitated recognition by TLR-4 and the subsequent induction of pro-inflammatory cytokine production. Since these signaling pathways play critical roles in many of the adjuvant effects mentioned in previous sections, interactions between metal nanomaterials and host proteins can generate novel sources of immunogenicity that may promote allergic processes.
The generation of metal antigens in vitro has been shown to be impacted by the unique physicochemical properties of metal nanomaterials. The size-specific increase in surface energy of TiO2 NP was shown to promote associations between the metal and human serum albumin, resulting in increased bioavailability (Vamanu et al. 2008). This altered propensity for inter-actions with host proteins contributed to the observation that titanium antigens generated from ionic and nanoparticulate forms of TiO2 induced differential proliferation of CD4+ and CD8+ T-lymphocytes in vitro (Hol et al. 2018).
Effects on processes involved in sensitization
As the primary APC involved in allergic sensitization, the capacity for metal nanomaterials to modulate DC activity represents a prominent mechanism by which allergic sensitization can be impacted (Jia et al. 2018). Since many sensitizing agents are known to induce both antigen-specific and nonspecific signals to DC following exposure, DC activation is a step of the dermal sensitization AOP used for in vitro identification of potential sensitizers. Accordingly, the h-CLAT method has been validated for use by the OECD to determine the skin sensitizing potential of agents. In this assay, undiffer-entiated THP-1 human monocytic leukemia cells are exposed to an agent for 24 h and their activation status is subsequently assessed by quantification of CD86 and CD54 activation marker expression (Ashikaga et al. 2002, 2006; Nukada et al. 2012).
The h-CLAT assay has not been employed to evaluate the sensitizing potential of any metal nanomaterials; however, several studies have investigated metal nanomaterial effects on undifferentiated THP-1 cells following a 24-h exposure, and reported activation marker expression. Accordingly, up-regulation of CD86 expression was observed following exposure to surface-modified FeNP, SiO2 NP, and mixed-metal alloy nanoparticles (Liu, Y et al. 2013). de Marzi et al. (2017) exposed THP-1 cells to a wide range of SiO2 particle sizes (10–1430 nm); while all particles promoted activation marker expression, the 240 nm SiO2 particles induced the greatest degree of CD80 expression. Similar findings were reported by an investigation that assessed the potential for metal debris released from orthopedic implants to trigger immune activation. Both ~2 mm cobalt-chromium-molybdenum alloy particles and soluble metal ions induced elevations in THP-1 co-stimulatory molecule expression, suggesting that a wide range of metal particle sizes have the capacity to induce immune effects involved in allergic sensitization (Caicedo et al. 2010; de Marzi et al. 2017). Contrarily, no elevations in THP-1 expression of CD86 or CD54 were observed in response to 100 nm AgNP exposure (Galbiati et al. 2018).
Though CD86 and CD54 are the validated biomarkers indicative of sensitizing potential in the THP-1 line, limited reports have evaluated these specific markers following metal nanomaterial exposure. However, other markers indicative of DC activation, such as MHC II, CD11b, CD14, CCR2, and CCR5 have been reported to be up-regulated in response to exposures to ZnO NP and FeNP (Prach et al. 2013; Matuszak et al. 2015). Similarly, modulation in expression of 60 genes – several of which were correlated to monocyte differentiation and maturation – were observed in response to PtNP exposure (Gatto et al. 2018).
The THP-1 cell line has also been used to identify potential skin sensitizers in vitro based on a unifying property of rapid ROS production following exposure to skin sensitizing chemicals (Miyazawa and Takashima 2012). A similar response has been demonstrated in the cell line following exposure to < 100 nm silver-copper alloy nanoparticles, AgNP, CoO NP, PdNP, and NiNP (Monprasit et al. 2018). The degree of ROS production by THP-1 cells has been correlated to properties including particle size and corona presence, as well as exposure dose and duration (Foldbjerg et al. 2009; Casals et al. 2011; Neubauer et al. 2015). Subsequent activation of the p38 MAPK signaling pathway, alterations in expression of HMOX1 and other oxidative stress genes have also been used as in vitro biomarkers suggestive of sensitizing potential. Numerous metal nanomaterials have been associated with these effects on THP-1 cells, which suggests their potential to activate DC and promote sensitization (Mohamed et al. 2011; Khatri et al. 2013; McConnachie et al. 2013; Boonrungsiman et al. 2017).
The potential for metal nanomaterials to induce DC activation has been more extensively examined using primary DC than the cell lines used in the validated assays (Kang and Lim 2012). Although the expression of activation markers in murine bone marrow-derived DC (BMDC) or human monocyte-derived DC (MDDC) has not been validated by OECD for use in determining sensitization potential in vitro, several studies have reported their capacity to accurately predict sensitizers (Tuschl et al. 2000; Pepin et al. 2007). Accordingly, TiO2 NP, ZnO NP, and SiO2 NP have been associated with increased expression of CD80 and CD86 by murine BMDC with respect to size, surface chemistry, and crystallinity (Palom€aki et al. 2010; Heng et al. 2011; Winter et al. 2011; Zhu et al. 2014; Winkler et al. 2017; Vandebriel et al. 2018).
Nanomaterial-induced modulated DC activity can also influence sensitization indepen-dent of their capacity to induce phenotypical maturation. For example, uptake of AuNP and AgNP resulted in an enhanced capacity for DC maturation in response to other immune stimuli (Orlowski et al. 2018). This finding suggests uptake of antigens normally incapable of activating DC may trigger their maturation in the presence of nanomaterials. In addition, accumulation of metal nanomaterials has been proposed to interfere with antigen processing and presentation by DC (Thiele et al. 2003; Humeniuk et al. 2017).
Polarization of DC and the subsequent preferential generation of TH1/TH2 effector T-cells is another step in the development of allergy that has been shown to be susceptible to modulation by nanomaterials. Dermal and respiratory sensitizers are associated with divergent oxidative stress responses that induce selective alterations in three major signaling pathways responsible for DC polarization (Mizuashi et al. 2005; Antonios et al. 2009). Polarization of DC towards TH1-promoting activity has been associated with the propensity for skin sensitizers to react with cytoplasmic glutathione following which, rapid depletion leads to ROS accumulation. The rapid induction of oxidative stress induced by contact sensitizers is responsible for the selective activation of the p38 MAPK and JNK signaling pathways within minutes of encounter (Nakahara et al. 2006). Contrarily, polarization of DC towards TH2-dominant responses has been associated with delayed induction of oxidative stress resulting from the preferential association of respiratory sensitizers with intracellular amine groups (Ferreira et al. 2018). Subsequently, selective activation of the NF-κB and ERK pathways occurs.
Knowledge of these pathways and their differential activation explain the observation that metal nanomaterials with opposing catalytic properties induce different polarization profiles in DC in vitro. The oxidant capacity of TiO2 NP resulted in potentiation of DC maturation leading to a TH1-biased responses, whereas treatment with the anti-oxidant surface activity of CeO2 NP resulted in secretion of anti-inflammatory IL-10 and a TH2-dominant T-cell profile (Schanen et al. 2013). FeNP, AuNP, and GdNP have also been associated with modulation of DC polarization in vitro with respect to size and surface chemistry (Yang et al. 2010; Vallhov et al. 2012; Tomi c et al. 2014; Hoang et al. 2015).
Effects on processes involved in elicitation of allergy
Metal nanomaterials have been shown to have potential to influence elicitation reactions specific to both metals and environmental proteins in vitro. With respect to metal allergy, PBMC isolated from women with established allergic sensitivity to palladium were challenged with either 5–10 nm PdNP or palladium salts in vitro (Reale et al. 2011). Variations in TNFα and IL-10 release were noted between exposures, indicating a potential role for metal solubility on metal-specific allergy elicitation. With respect to environmental allergens, basophils isolated from patients with established sensitivity to common environmental allergens including birch pollen, timothy grass pollen, and house dust mite were exposed to AuNP-conjugated with corresponding allergenic proteins. Stable coronas were formed by all three allergens, but binding of allergen to AuNPs caused enhanced activation of basophils in response to house dust mite challenge, as well as birch pollen in some individuals (Radauer-Preiml et al. 2016).
Although lymphocyte cytotoxicity is an immunotoxic effect most often associated with immunosuppression, as major effector cells of both IgE and T-cell-mediated allergic responses, this effect has potential to impact allergic disorders, as well. Accordingly, many metal nano-materials have been shown to be cytotoxic and genotoxic to human and murine lymphocytes in vitro. Interestingly, T- and B-lymphocytes have been shown to be more resistant to adverse effects of ZnO NP compared to other immune cell types (Hanley et al. 2009). Although ion release from ZnO NP and PdNP was correlated to cytotoxicity and alteration in gene expression, DNA damage induced by CoNP was shown to be more severe than that induced by Co ions (Jiang et al. 2012; Tuomela et al. 2013; Petrarca et al. 2014; Simon-Vazquez et al. 2016). Susceptibility to cytotoxicity was shown to be reflective of cell cycle status, explaining the finding that memory T-cells were more sensitive to metal nanomaterial effects compared to naïve T-cells (Hanley et al. 2009; Shahbazi et al. 2013).
Modulation of T-lymphocyte activity by metal nanomaterials also has the potential to significantly influence allergic processes. PdNP, AuNP, CoNP, and GdNP have all been shown to induce differential TH1/TH2-biased cytokine production by lymphocytes in vitro dependent on size, solubility, and hydrophobicity (Petrarca et al. 2006; Liu et al. 2009; Boscolo et al. 2010; Moyano et al. 2012). FeNP suppressed the activity of Kv1.3 channels, which suggests a potential mechanism of lymphocyte cell signaling modulation (Yan et al. 2015). Additionally, delays in proliferation, altered mitogen responses, and morphological changes have also been observed by lymphocytes following exposure to various metal nanomaterials (Shin et al. 2007; Beer et al. 2008; Liptrott et al. 2014; Devanabanda et al. 2016).
Knowledge regarding effects of metal nanomaterials specifically on B-cells in vitro is limited to AuNP, which have been shown to be size-dependently taken up by B-cells, causing alterations in NF-κB and blimp1/pax5 signaling pathways, and altered secretion of immune-globulins in a size-dependent manner via (Sharma et al. 2013; Lee et al. 2014). The potential for metal nanomaterials to directly alter B-cell processes, such as antigen-specific interactions with T-cells, isotype switching, and affinity maturation, events critical to their effector functions in IgE-mediated allergic disorder such as asthma, remains largely unstudied (Luo et al. 2015).
Knowledge gaps in metal nanomaterial effects on immune cells and processes in vitro
Formation of the nanomaterial biocorona has been almost exclusively investigated with respect to the adsorption of proteins. However, nanomaterials are also subject to interactions with other macromolecules present in biological fluids, such as nucleic acids and lipids. Adsorption of these molecules may have notable impacts on the immune effects of nanomaterials since different nucleic acids are alarmins recognized by PRR and lipid mediators play critical roles in many aspects of allergic disorders (Schauberger et al. 2016; Muller et al. 2018). Accordingly, more research should be directed towards investigating the biological implications of surface-adsorbed macromolecules other than proteins. Similarly, it has been suggested that metal nanomaterials may act as soluble or particulate antigens, but the dynamics of metal antigen generation remains largely unstudied with respect to nanoparticles.
Important considerations for future metal-based nanomaterial allergy studies
The potential for metal-based nanomaterials to induce immune effects with implications for allergic disease following exposure by routes other than dermal contact or inhalation is a significant knowledge gap. Nanomaterials are being increasingly incorporated into foods, beverages, supplements, and packaging, rendering ingestion exposures an increasing concern (Chaudhry et al. 2008). Ingestion of metal nanomaterials has been associated with altered B-cell distribution, increased levels of IgE and IgG, and splenic toxicity, but the implications of these effects on allergy are unknown (Park et al. 2010a; Kim et al. 2014; Sheng et al. 2014). Similar immune effects have been observed following systemic administration of various metal nanomaterials. Although most current uses for metal nanomaterials are not likely to result in systemic exposures, some nanomaterials with expanding biomedical applications present a concern. The significance of this knowledge gap is demonstrated by the numerous adverse effects in patients administered Feraheme (ferumoxytol), an intravenously-administered iron replacement product containing 17–31 nm colloidal Fe3O4NP (Lu et al. 2010). In the 5 years following approval for use by the Food and Drug Administration (FDA) in 2009, 79 anaphylactic reactions were reported, of which 19 were fatal.
As more studies are conducted to advance our understanding of the effects of metal nanomaterials on allergic disease, it should be recognized that accurate assessment is dependent on the evaluation of sample contamination with endotoxin (Dobrovolskaia et al. 2009a). As a result of production and handling in non-sterile conditions, engineered nanomaterials are often carriers of impurities including LPS, a potent immunoadjuvant (Smulders et al. 2012). Accordingly, the presence of endotoxin on surfaces of metal nanomaterials could generate a subsidiary but sufficient amount of immunostimulation required to induce allergic sensitization to the metal itself, or to other allergens. Consequently, exposure to immunologically inert metal nanomaterials contaminated with LPS may lead to misidentification of such agents as sensitizers or adjuvants. Many of the studies published prior to this development do not report the presence or absence of endotoxin in samples, and results should be interpreted with caution.
Another consideration for future studies is that the successful correlation of metal nanomaterial physicochemical properties with mechanisms of toxicity is limited by the accurate assessment and reporting of test material characterization. Although thorough material character-ization has become recognized as an indispensable step in nanotoxicity studies, discrepancies in property terminology, evaluation methods, property reporting, and the biological relevance of measured properties complicate comparisons between studies. A notable example of inconsistent property terminology is the tendency for non-discriminate reporting of aggregates and agglomerates of primary particles. The irreversible bonds of aggregates and reversible bonds of agglomerates can impact the effective dose surface area, degree of primary particle dissociation, and other properties that dictate in vivo biological effects (Keene and Tyner 2011; Gualtieri et al. 2012; Sharma et al. 2014). Another property subject to inconsistent reporting is nanomaterial surface area. Differences in the material state and method of assessment can generate results representative of different parameters, including volume-specific, geometric, or specific surface area SSA (van Doren et al. 2011; Wohlleben et al. 2017). Although these metrics are often similarly reported by studies, they have been correlated to notable variations in toxic potential (Sager et al. 2016). The use of multiple assessment methods and disclosure of potential sources of measurement variation between studies will help accurately compare the impact of properties on toxic potential in future studies.
Accurate evaluation of nanomaterial physicochemical properties and their correlation to toxic effects has become increasingly relevant as emerging nanomaterials challenge the efficacy of traditional occupational exposure limits (OEL). The majority of respiratory occupational exposure limits do not discriminate for material size, so as new metal-based nanomaterials emerge, they are subject to the same mass-derived values enforced for other materials of the same elemental composition. This issue is proving problematic as nanotoxicity studies continue to demonstrate that mass may not be the best dose metric for prediction of pulmonary toxicity (Schmid and Stoeger 2016). Moreover, as demonstrated by the studies summarized here, metal nanomaterial properties other than mass have been correlated to immune effects following respiratory exposure. Accordingly, size nonspecific OELs may be ineffective in protecting workers from both nanomaterial-induced pulmonary effects, as well as subsequent immune effects (Schulte et al. 2010). This concern has become increasingly recognized, as NIOSH has recommended size-specific exposure limits for TiO2. Despite the recommended time-weighted average exposure limits of 2.4 mg/m3 for fine TiO2 and 0.3 mg/m3 for ultrafine and nanoscale TiO2, the agency responsible for regulating compliance with its own established limits(i.e. OSHA) has not yet adopted size-specific OEL values for TiO2 (NIOSH 2011).
Conclusions
Although there is a growing amount of toxicological data demonstrating the vast potential for adverse immune effects following exposure to metal nanomaterials, advancements in understanding their interactions with biological systems have allowed for their unique characteristics to be harnessed for beneficial applications, as well. Numerous studies have demonstrated the potential utility of metal nanomaterials for novel vaccine adjuvants, drug delivery vehicles, diagnostic approaches, and immunotherapies. However, to optimize the use of metal-based nanomaterials for these and other advantageous purposes, a more complete understanding of their systemic immune effects, mechanisms of immunomodulation, and capacity to induce allergic sensitization is needed.
Table 6.
Summary of major findings from studies comparing the effects of various physicochemical properties of metal nanomaterials on respiratory allergy grouped by property of interest.
| Property investigated | Author/year | Metal | Study design | Property variations | Findings | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Size | de Haar et al. 2006 | Ti | F BALB/cANNCrl mouse | Fine TiO2: 250 nm, 6.6 m2/g | Exposure to equal mass doses of fine and ultrafine TiO2 resulted in | |||||
| Ovalbumin, 200 μg intranasal | Ultrafine TiO2: 29.0 nm, 49.8 m2/g | increased TH2 cytokines and serum OVA-specific IgE and IgGI only | ||||||||
| in animals exposed to ultrafine TiO2 | ||||||||||
| Yoshida et al. 2011 | Si | F BALB/c mouse, Ovalbumin | Amorphous silica | Smaller particles induced higher levels of OVA-specific IgE, IgG, and | ||||||
| Intranasal: 10, 50, or 250 μg/ mouse x3 | 30, 70, 300, or 1000 nm | IgG1. Splenocytes from mice exposed to the smallest particle pro- | ||||||||
| duced higher levels of TH2 cytokines than other groups. | ||||||||||
| Liu et al. 2010 | Ti | Rats, Intratracheal instillation | 5 or 200 nm TiO2 | Decreased chemotactic ability, expression of Fc receptors/MHC II by | ||||||
| 0.5, 5, or 50 μg/mL | alveolar macrophages. Phagocytic function was increased at low | |||||||||
| doses and decreased at high doses | ||||||||||
| Chang et al. 2014 | Ti | M Sprague Dawley rat | 21 nm TiO2NP: 80% anatase, 20% rutile, | Increased macrophage accumulation and alteration of TH1/TH2 status | ||||||
| Intratracheal instillation: x2, x4 w | 1–2 μm TiO2: anatase | |||||||||
| 0.5, 4, 32 mg/kg | ||||||||||
| Ban et al. 2013 | Fe | F BALB/c mouse, Ovalbumin | Submicron Fe2O3: 147±48nm, 6 m2/g | High and medium doses of both Fe particles caused decreases in | ||||||
| Intratracheal instillation: | Fe2O3NP: 35±14nm, 39 m2/g | eosinophil influx and OVA-specific IgE levels. However, at the low | ||||||||
| 4x (100, 250, or 500 μg/mouse) | dose, submicron particles had no effect on allergy, whereas nano- | |||||||||
| particles had an adjuvant effect on the TH2 response to OVA | ||||||||||
| SA | Rossi et al. 2010 | Ti | F BALB/c/Sca mouse, Ovalbumin | Rutile TiO2NP: <5 μm, 2 m2/g | Allergic pulmonary inflammation was dramatically suppressed in asth- | |||||
| Inhalation: 10±2mg/m3 × 12 | Rutile TiO2NP: 10 × 40 nm, 132 m2/g | matic mice exposed to either size TiO2. Leukocyte number, cyto- | ||||||||
| kines, chemokines, and antibodies were significantly decreased. | ||||||||||
| Parketal. 2015 | Si | F BALB/c mouse, Ovalbumin | Spherical SiNP: 12.7 m2/g | Acute SiNP exposure induced significant airway inflammation and | ||||||
| Intranasal inoculation | Mesoporous SiNP: 70.6 m2/g | AHR. Spherical SiNPs induced the greatest degree of exacerbation | ||||||||
| PEGylated SiNP: 12.7 m2/g | of allergic effects in the OVA model | |||||||||
| Han et al. 2016 | Si | F BALB/c mouse | Spherical SiNP: 12.7 m2/g, 119.6nm | Sensitized mice exposed to S-SiNP and M-SiNP exhibited elevated | ||||||
| Ovalbumin | Mesoporous SiNP: 70.6 m2/g, 100.5 nm | AHR over controls. M-SiNPs induced the greatest degree adjuvan- | ||||||||
| Intranasal inoculation: 10 mg/kg 6x | PEGylated SiNP: 12.7 m2/g, 439.1 nm | ticity, whereas PEG-SiNP caused the least toxic effects | ||||||||
| MOD | CRG Seydoux et al. 2016 | Au | F BALB/c mouse | 90 nm AuNP: | APCs preferentially took up cationic AuNPs, causing upregulation of | |||||
| AuNP intranasal instillation: 10 μg | NH2-PVA, 7.2 mV | co-stimulatory molecules. Positive AuNPs enhanced OVA-specific | ||||||||
| COOH-PVA 8.2 mV | CD4+ T-cell stimulation in the lung draining lymph nodes | |||||||||
| Marzaioli et al. 2014 | Si | F BALB/c mouse, Ovalbumin | Amorphous SiO2NP 15 nm: | Uncoated SiO2NPs induced proinflammatory and immunomodulatory | ||||||
| Intratracheal instillation: 50 μg | Uncoated 38 mV, PEGylated 26 mV, | effects with increases in lung inflammatory cells, TH2 cytokines. | ||||||||
| Phosphate-coated 43 mV, Amino-coated 0 mV | Amino and phosphate surface modifications mitigated these | |||||||||
| effects, whereas PEG coating did not. | ||||||||||
| Omlor et al. 2017 | Au | F BALB/c mouse, Ovalbumin | 5 nm AuNP, PEGylated or citrated | Asthmatic condition increased nanoparticle uptake. Systemic uptake is | ||||||
| Intranasal instillation | higher for PEGylated AuNP compared to citrated AuNPs, but both | |||||||||
| inhibited inflammatory infiltrates and AHR, wherein inhibition was | ||||||||||
| more significant following exposure to citrated AuNPS | ||||||||||
| Vennemann | Zr | F Wistar rat | APTS, TODS, PGA, or acrylic acid coated | Surface coating had minimal effects on inflammation in the lungs of | ||||||
| et al. 2017 | Intratracheal instillation | 9–1 Onm ZrO2NPs | rats, but had significant effects on allergic response. | |||||||
| Size MOD | Alessandrini | Ag | F BALB/c mice, Ovalbumin | PVP-coated AgNP: 97 nm, 6.2 m2/g, 7mV | Ag50-PVP significantly reduced OVA-induced inflammatory infiltrate in | |||||
| et al. 2017 | Intratracheal instillation: 1–50 μg | PVP-coated AgNP: 134nm, 4.5 m2/g, 7mV | sensitized mice. Lung microbiome was altered dependent | |||||||
| Citrate-AgNP: 20 nm, 30 m2/g, 45 mV | on coating. | |||||||||
| Seiffert et al. 2015 | Ag | Brown Norway and Sprague-Dawley rats | PVP-coated AgNP: 20 or iiOnm | Smaller AgNPs increased AHR on d 1, which persisted to d 7 for the | ||||||
| Intratracheal instillation: 0.1 mg/kg | Citrate-capped AgNP: 20 or iiOnm | citrate AgNPs only. 20 nm AgNP was more pro-inflammatory but | ||||||||
| little difference between different surface coatings | ||||||||||
| CRY Horie et al. 2015 | Zn | F C57BL/6N mouse, Ovalbumin | Rutile TiO2, AI(OH)3 surf: 30–50 nm 37.1 m2/g | Serum total and OVA IgE, IgG1 increased in mice treated with the | ||||||
| Ti | Pharyngeal aspiration: 50 μg | ZnO: 21 nm, 49.6 m2/g | uncoated ZnO particle. However, ZnCI2 did not produce similar | |||||||
| Si | ZnO, SiO2 Surface: 25 nm, unknown SA, ZnCI2 | exacerbations. TiO2 and SiO2 did not affect OVA-lgE or IgG levels. | ||||||||
| Amorphous SiO2: 7nm 300 m2/g, 34 nm 80 m2/g | ||||||||||
| SA | Sandberg | Si | LPS-primed RAW264.7 mouse macrophages, pri- | 64 nm Si, 650cm2/mg | Non-crystalline SiO2 particles in both nano and micron size ranges | |||||
| et al. 2012 | mary rat lung macrophages | 369 nm Si, 90 cm2/mg | induced IL-1β release from LPS-primed macrophages following | |||||||
| 0, 50, 100, 250, 500 μg/mL, 6h | ~20nm Fumed Si (aerosol), 1880cm2/mg 500nm-10 μm Fused Si (suprasil), 23cm2/mg | uptake, phagosomal leakage, and activation of the NALP3 inflam- | ||||||||
| masome. Particle surface area, reactivity, and uptake all influenced | ||||||||||
| the degree of mediator release by cells | ||||||||||
| Vandebriel | Ti | F BALB/c mouse, Ovalbumin | Uncoated TiO2NP: | Rutile TiO2NP caused the greatest increase in OVA-specific serum IgE | ||||||
| et al. 2018 | Intranasal exposure: 120 μg TiO2 | 10–30 nm rutile or 10–25 nm anatase | and IgGI. Neutrophils recruited by rutile, but not anatase | |||||||
| SOL Jeong et al. 2015 | Co | F Rat, Intratracheal Instillation | CoO: 65.4± 2.8 nm, 92.65% solubility | Soluble CoNP induced eosinophilic inflammation, whereas insoluble | ||||||
| 80, 200, 800 μg/mL @ 0.5 mL | Co3O4: 20.2 ± 0.4 nm, 11.46% solubility | CoONP induced neutrophilic inflammation | ||||||||
| Cho et al. 2011 | Zn | F Wistar rat | 10.7±0.7nm ZnONP 50 or 150cm2/rat | ZnONP induced eosinophilia, proliferation of airway epithelial cells, | ||||||
| Intratracheal instillation | Zn2+ ions 92.5 μg/rat | goblet cell hyperplasia, and increased IgE levels, and decreased | ||||||||
| IgA- findings which were also seen following instillation of Zn ions | ||||||||||
Summary of study design and major findings from studies comparing the effects of various physicochemical properties of metal nanomaterials on respiratory allergy grouped by study property of interest. Properties of interest: size, CRY (crystallinity), MOR (morphology), MOD (surface modification), CRG (surface charge), and SA (surface area), and SOL (solubility). Reported particle size (nm), specific surface area (m2/g), zeta potential (mV), pore volume (cm3/g), in vitro dose concentration (mg/mL). AHR: airway hyperreactivity; APC: antigen-presenting cell; APTS: aminopropylsilane modification; DC-SIGN: dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin; MDDC: monocyte-derived dendritic cell; MHC: major histocompatibility complex; OVA: ovalbumin; PDI: polydispersity index; PEG: poly(ethylene glycol) modification; PGA: poly(lactic-coglycolicacid) modification; PVA: PVP polyvinylpyrrolidone modification; ROS: reactive oxygen species; TODS: tetraoxidecanoic acid modification
Footnotes
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
References
- Accapezzato D, Visco V, Francavilla V, Molette C, Donato T, Paroli M, Mondelli M, Doria M, Torrisi M, Barnaba V. 2005. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J Exp Med. 202:817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi K, Yamada N, Yoshida Y, Yamamoto O. 2013. Subchronic exposure of titanium dioxide nanoparticles to hairless rat skin. Exp Dermatol. 22: 278–283. [DOI] [PubMed] [Google Scholar]
- Ahamed M, Akhtar M, Raja M, Ahmad I, Siddiqui M, AlSalhi M, Alrokayan S. 2011. ZnO nanorod-induced apoptosis in human alveolar adenocarcinoma cells via p53, survivin, and bax/bcl-2 pathways: Role of oxidative stress. Nanomed Nanotechnol Biol Med. 7:904–913. [DOI] [PubMed] [Google Scholar]
- Aimanianda V, Haensler J, Lacroix-Desmazes S, Kaveri S, Bayry J. 2009. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol Sci. 30:287–295. [DOI] [PubMed] [Google Scholar]
- Aldossari A, Shannahan J, Podila R, Brown J. 2015. Influence of physicochemical properties of silver nanoparticles on mast cell activation and degranulation. Toxicol In Vitro. 29:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandrini F, Vennemann A, Gschwendtner S, Neumann A, Rothballer M, Seher T, Wimmer M, Kublik S, Traidl-Hoffmann C, Schloter M. 2017. Pro-Inflammatory versus immuno-modulatory effects of silver nanoparticles in the lung: Critical role of dose, size, and surface modification. Nanomaterials. 7:300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaleh N, Persaud I, Brown J. 2016. Silver nanoparticle-directed mast cell degranulation is mediated through calcium and PI3K signaling independent of the high affinity IgE receptor. PloS One. 11:e0167366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaleh N, Brown J. 2018. Exposure to silver nanoparticles primes mast cells for enhanced IgE/FcER1-mediated activation. J Immunol. 200:105.113–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [AAAAI] American Academy of Allergy Asthma & Immunology. 2015. Allergy Statistics. https://www.aaaai.org/about-aaaai/newsroom/allergy-statistics. [Google Scholar]
- Amin K 2012. The role of mast cells in allergic inflammation. Respir Med.106:9–14. [DOI] [PubMed] [Google Scholar]
- Anderson S, Siegel P, Meade B. 2011. The LLNA: A brief review of recent advances and limitations. J Allergy. 2011:424203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andujar P, Simon-Deckers A, Galateau-Salle F, Fayard B, Beaune G, Clin B, Billon-Galland M, Durupthy O, Pairon J, Doucet J, et al. 2014. Role of metal oxide nanoparticles in histopathological changes observed in the lung of welders. Particle Fibre Toxicol. 11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel J, Luger T, Lowry D, Perry P, Roop D, Mountz J. 1988. The expression and modulation of IL-1 alpha in murine keratinocytes. J Immunol. 140: 2274–2278. [PubMed] [Google Scholar]
- Antonios D, Ade N, Kerdine-Römer S, Assaf-Vandecasteele H, Larangé A, Azouri H, Pallardy M. 2009. Metallic haptens induce differential pheno-type of human dendritic cells through activation of mitogen-activated protein kinase and NF-κB pathways. Toxicol In Vitro. 23:227–234. [DOI] [PubMed] [Google Scholar]
- Arai Y, Miyayama T, Hirano S. 2015. Difference in the toxicity mechanism between ion and nanoparticle forms of silver in the mouse lung and in macrophages. Toxicology. 328:84–92. [DOI] [PubMed] [Google Scholar]
- Armand L, Dagouassat M, Belade E, Simon-Deckers A, le Gouvello S, Tharabat C, Duprez C, Andujar P, Pairon J, Boczkowski J, et al. 2013. Titanium dioxide nanoparticles induce matrix metalloprotease 1 in human pulmonary fibroblasts partly via an IL-1β-dependent mechanism. Am J Respir Cell Mol Biol. 48:354–363. [DOI] [PubMed] [Google Scholar]
- Arruda L, Sole D, Baena-Cagnani C, Naspitz C. 2005. Risk factors for asthma and atopy. Curr Opin Allergy Clin Immunol. 5:153–159. [DOI] [PubMed] [Google Scholar]
- Artik S, von Vultée C, Gleichmann E, Schwarz T, Griem P. 1999. Nickel allergy in mice: Enhanced sensitization capacity of nickel at higher oxidation states. J Immunol. 163:1143–1152. [PubMed] [Google Scholar]
- Arvizo R, Miranda O, Thompson M, Pabelick C, Bhattacharya R, Robertson J, Rotello V, Prakash Y, Mukherjee P. 2010. Effect of nanoparticle surface charge at the plasma membrane and beyond. Nano Lett. 10:2543–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikaga T, Hoya M, Itagaki H, Katsumura Y, Aiba S. 2002. Evaluation of CD86 expression and MHC Class II molecule internalization in THP-1 human monocyte cells as predictive endpoints for contact sensitizers. Toxicol In Vitro. 16:711–716. [DOI] [PubMed] [Google Scholar]
- Ashikaga T, Yoshida Y, Hirota M, Yoneyama K, Itagaki H, Sakaguchi H, Miyazawa M, Ito Y, Suzuki H, Toyoda H. 2006. Development of an in vitro skin sensitization test using human cell lines: The human Cell Line Activation Test (h-CLAT): I. Optimization of the h-CLAT protocol. Toxicol In Vitro. 20:767–773. [DOI] [PubMed] [Google Scholar]
- Auttachoat W, McLoughlin C, White K, Smith M. 2014. Route-dependent systemic and local immune effects following exposure to solutions prepared from titanium dioxide nano-particles. J Immunotoxicol. 11:273–282. [DOI] [PubMed] [Google Scholar]
- Bakshi M, Zhao L, Smith R, Possmayer F, Petersen N. 2008. Metal nanoparticle pollutants interfere with pulmonary surfactant function in vitro. Biophys J. 94:855–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroli B 2010. Penetration of nanoparticles and nanomaterials in the skin:Fiction or reality? J Pharm Sci. 99:21–50. [DOI] [PubMed] [Google Scholar]
- Baroli B, Ennas M, Loffredo F, Isola M, Pinna R, Lopez-Quintela M. 2007. Penetration of metallic nanoparticles in human full-thickness skin. J Invest Dermatol. 127:1701–1712. [DOI] [PubMed] [Google Scholar]
- Barreto E, Serra M, dos Santos R, dos Santos C, Hickmann J, Cotias A, Pao C, Trindade S, Schimidt V, Giacomelli C, et al. 2015. Local Administration of gold nanoparticles prevents pivotal pathological changes in murine models of atopic asthma. J Biomed Nanotechnol. 11:1038–1050. [DOI] [PubMed] [Google Scholar]
- Basketter D, Lea L, Cooper K, Ryan C, Gerberick G, Dearman R, Kimber I. 1999. Identification of metal allergens in the local lymph node assay. Am J Contact Dermatitis. 10:207–212. [DOI] [PubMed] [Google Scholar]
- Bastos V, de Oliveira J, Brown D, Jonhston H, Malheiro E, Daniel-da-Silva A, Duarte I, Santos C, Oliveira H. 2016. The influence of citrate or PEG coating on silver nanoparticle toxicity to a human keratinocyte cell line. Toxicol Lett. 249:29–41. [DOI] [PubMed] [Google Scholar]
- Beduneau A, Ma Z, Grotepas C, Kabanov A, Rabinow B, Gong N, Mosley R, Dou H, Boska M, Gendelman H. 2009. Facilitated monocyte-macrophage uptake and tissue distribution of super-parmagnetic iron-oxide nanoparticles. PloS One. 4:e4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer A, Holzapfel K, Neudorfer J, Piontek G, Settles M, Kronig H, Peschel C, Schlegel J, Rummeny EJ, Bernhard H. 2008. Visualization of antigen-specific human cytotoxic T-lymphocytes labeled with super-paramagnetic iron-oxide particles. Eur Radiol. 18:1087–1095. [DOI] [PubMed] [Google Scholar]
- Belloni Fortina A, Cooper S, Spiewak R, Fontana E, Schnuch A, Uter W. 2015. Patch test results in children and adolescents across Europe. Analysis of the ESSCA Network 2002–2010. Pediat Allergy Immunol. 26: 446–455. [DOI] [PubMed] [Google Scholar]
- Bennett S, Zhou D, Mielke R, Keller A. 2012. Photoinduced disaggregation of TiO2 nanoparticles enables transdermal penetration. PloS One. 7:e48719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi E, Bussolati O, Magrini A, Bottini M, Migliore L, Bellucci S, Iavicoli I, Berga-maschi A. 2006. Nanomaterials and lung toxicity: Interactions with airways cells and relevance for occupational health risk assessment. Int J Immunopathol Pharmacol. 19:3–10. [PubMed] [Google Scholar]
- Bergfors E, Bjorkelund C, Trollfors B. 2005. Nineteen cases of persistent pruritic nodules and contact allergy to aluminium after injection of commonly used aluminium-adsorbed vaccines. Eur J Pediatr. 164:691–697. [DOI] [PubMed] [Google Scholar]
- Berntsen P, Park C, Rothen-Rutishauser B, Tsuda A, Sager T, Molina R, Donaghey T, Alencar A, Kasahara D, Ericsson T, et al. 2010. Biomechanical effects of environmental and engineered particles on human airway smooth muscle cells. J Royal Soc Interface. 7:S331–S340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M, Allegri M, Chiu M, Costa AL, Blosi M, Ortelli S, Bussolati O, Bergamaschi E. 2017. Lipopolysaccharide adsorbed to the bio-corona of TiO2 nanoparticles powerfully activates selected pro-inflammatory transduction pathways. Front Immunol. 8:866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C, Visser MJ, Pluut OA, Svetličić V, Pletikapić G, Jakasa I, Riethmuller C, Adami G, Larese Filon F, Schwegler-Berry D, et al. 2016. Characterization of silver particles in the stratum corneum of healthy subjects and atopic dermatitis patients dermally exposed to a silver-containing garment. Nanotoxicology. 10:1480–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biniek K, Levi K, Dauskardt R. 2012. Solar UV radiation reduces the barrier function of human skin. Proc Natl Acad Sci USA 109:17111–17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank F, Gerber P, Rothen-Rutishauser B, Sakulkhu U, Salaklang J, de Peyer K, Gehr P, Nicod L, Hofmann H, Geiser T, et al. 2011. Biomedical nanoparticles modulate specific CD4+ T cell stimulation by inhibition of antigen processing in dendritic cells. Nanotoxicology. 5:606–621. [DOI] [PubMed] [Google Scholar]
- Blank F, Fytianos K, Seydoux E, Rodriguez-Lorenzo L, Petri-Fink A, von Garnier C, Rothen-Rutishauser B. 2017. Interaction of biomedical nanoparticles with the pulmonary immune system. J Nanobiotechnol. 15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J, Wearsch P, Cresswell P. 2013. Pathways of antigen processing. Annu Rev Immunol. 31:443–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonrungsiman S, Suchaoin W, Chetprayoon P, Viriya-empikul N, Aueviriyavit S, Maniratanachote R. 2017. Shape and surface properties of titanate nanomaterials influence differential cellular uptake behavior and biological responses in THP-1 cells. Biochem Biophys Rep. 9:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo P, Bellante V, Leopold K, Maier M, Di Giampaolo L, Antonucci A, Iavicoli I, Tobia L, Paoletti A, Montalti M, et al. 2010. Effects of palladium nanoparticles on the cytokine release from peripheral blood mononuclear cells of non-atopic women. J Biol Regul Homeost Agents. 24:207–214. [PubMed] [Google Scholar]
- Boverhof D, Billington R, Gollapudi B, Hotchkiss J, Krieger S, Poole A, Wiescinski C, Woolhiser M. 2008. Respiratory sensitization and allergy: Current research approaches and needs. Toxicol Appl Pharmacol. 226: 1–13. [DOI] [PubMed] [Google Scholar]
- Braakhuis H, Oomen A, Cassee F. 2016. Grouping nanomaterials to predict their potential to induce pulmonary inflammation. Toxicol Appl Pharmacol. 299:3–7. [DOI] [PubMed] [Google Scholar]
- Brandenberger C, Rowley N, Jackson-Humbles D, Zhang Q, Bramble L, Lewandowski R, Wagner J, Chen W, Kaplan B, Kaminski N, et al. 2013. Engineered silica nanoparticles act as adjuvants to enhance allergic airway disease in mice. Part Fibre Toxicol. 10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braydich-Stolle L, Schaeublin N, Murdock R, Jiang J, Biswas P, Schlager J, Hussain S. 2009. Crystal structure mediates mode of cell death in TiO2 nanotoxicity. J Nanopart Res. 11:1361–1374. [Google Scholar]
- Braydich-Stolle L, Speshock J, Castle A, Smith M, Murdock R, Hussain S. 2010. Nanosized aluminum altered immune function. ACS Nano. 4: 3661–3670. [DOI] [PubMed] [Google Scholar]
- Büdinger L, Hertl M, Büdinger L. 2000. Immunologic mechanisms in hyper-sensitivity reactions to metal ions: An overview. Allergy. 55:108–115. [DOI] [PubMed] [Google Scholar]
- Buzea C, Pacheco I, Robbie K. 2007. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases. 2:MR17–MR71. [DOI] [PubMed] [Google Scholar]
- Caicedo M, Pennekamp P, McAllister K, Jacobs J, Hallab N. 2010. Soluble ions more than particulate cobalt-alloy implant debris induce monocyte co-stimulatory molecule expression and release of pro-inflammatory cytokines critical to metal-induced lymphocyte reactivity. J Biomed Mater Res. A 93:1312–1321. [DOI] [PubMed] [Google Scholar]
- Calatayud M, Sanz B, Raffa V, Riggio C, Ibarra M, Goya G. 2014. The effect of surface charge of functionalized Fe3O4 nanoparticles on protein adsorption and cell uptake. Biomaterials. 35:6389–6399. [DOI] [PubMed] [Google Scholar]
- Capasso L, Camatini M, Gualtieri M. 2014. Nickel oxide nanoparticles induce inflammation and genotoxic effect in lung epithelial cells. Toxicol Lett. 226:28–34. [DOI] [PubMed] [Google Scholar]
- Casals E, Pfaller T, Duschl A, Oostingh G, Puntes V. 2011. Hardening of the nanoparticle-protein corona in metal (Au, Ag) and oxide (Fe3O4, CoO, and CeO2) nanoparticles. Small. 7:3479–3486. [DOI] [PubMed] [Google Scholar]
- Castranova V 2011. Overview of current toxicological knowledge of engineered nanoparticles. J Occup Environ Med. 53:S14–S17. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2017. Allergies. Atlanta, GA:CDC. [Google Scholar]
- Chaput N, Flament C, Viaud S, Taieb J, Roux S, Spatz A, Andre F, LePecq J, Boussac M, Garin J, et al. 2006. Dendritic cell derived-exosomes: Biology and clinical implementations. J Leukoc Biol. 80:471–478. [DOI] [PubMed] [Google Scholar]
- Chary A, Hennen J, Klein S, Serchi T, Gutleb A, Blömeke B. 2018. Respiratory sensitization: Toxicological point of view on the available assays. Arch Toxicol. 92:803–822. [DOI] [PubMed] [Google Scholar]
- Chaudhry Q, Scotter M, Blackburn J, Ross B, Boxall A, Castle L, Aitken R, Watkins R. 2008. Applications and implications of nanotechnologies for the food sector. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 25:241–258. [DOI] [PubMed] [Google Scholar]
- Chen E, Garnica M, Wang Y, Chen C, Chin W. 2011. Mucin secretion induced by titanium dioxide nanoparticles. PloS One. 6:e16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Garnica M, Wang Y, Mintz A, Chen C, Chin W. 2012. A mixture of anatase and rutile TiO2 nanoparticles induces histamine secretion in mast cells. Particle Fibre Toxicol. 9:2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Tseng Y, Sun C, Chu C. 2008. Contact sensitization to metals in Taiwan. Contact Derm. 59:353–360. [DOI] [PubMed] [Google Scholar]
- Chilcott R, and Price S, editors. 2008. Principles and Practice of Skin Toxicology. Chichester: John WIley and Sons Ltd. [Google Scholar]
- Chipinda I, Hettick J, Siegel P. 2011. Haptenation: Chemical reactivity and protein binding. J Allergy. 2011:839682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W, Duffin R, Howie S, Scotton C, Wallace W, Macnee W, Bradley M, Megson I, Donaldson K. 2011. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part Fibre Toxicol 8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Park Y, Lee E, Jeong S, Kim S, Kim M, Son S. 2011. A safety assessment of phototoxicity and sensitization of SiO2 nanoparticles. Mol Cell Toxicol. 7:171–176. [Google Scholar]
- Chroneos Z, Sever-Chroneos Z, Shepherd V. 2010. Pulmonary surfactant: An immunological perspective. Cell Physiol Biochem. 25:13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M, Wu Q, Wang J, Hou S, Miao Y, Peng J, Sun Y. 2007. In vitro and in vivo transdermal delivery capacity of quantum dots through mouse skin. J Nanotechnol. 18:455103. [Google Scholar]
- Chuang H, Hsiao T, Wu C, Chang H, Lee C, Chang C, Cheng T. 2013. Allergenicity and toxicology of inhaled silver nanoparticles in allergen-provocation mice models. Int J Nanomed. 8:4495–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirla A 1994. Cobalt-related asthma: Clinical and immunological aspects. Sci Total Environ. 150:85–94. [DOI] [PubMed] [Google Scholar]
- Coelho S, Patri A, Wokovich A, McNeil S, Howard P, Miller S. 2016. Repetitive application of sunscreen containing titanium dioxide nanoparticles on human skin. JAMA Dermatol. 152:470–472. [DOI] [PubMed] [Google Scholar]
- Contado C 2015. Nanomaterials in consumer products: A challenging analytical problem. Front Chem. 3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquette A, Berna N, Vandenbosch A, Rosdy M, de Wever B, Poumay Y. 2003. Analysis of interleukin-1alpha (IL-1alpha) and interleukin-8 (IL-8) expression and release in in vitro reconstructed human epidermis for the prediction of in vivo skin irritation and/or sensitization. Toxicol In Vitro. 17:311–321. [DOI] [PubMed] [Google Scholar]
- Corbo C, Molinaro R, Parodi A, Toledano Furman N, Salvatore F, Tasciotti E. 2016. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine. 11:81–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton RR. 2017. Metal Toxicity–An Introduction In: Crichton RR, Ward RJ, Hider RC, editors. Metal Chelation in Medicine. pp. 1–23. DOI: 10.1039/9781782623892. [DOI] [Google Scholar]
- Crosera M, Adami G, Mauro M, Bovenzi M, Baracchini E, Filon F. 2016. In vitro dermal penetration of nickel nanoparticles. Chemosphere. 145: 301–306. [DOI] [PubMed] [Google Scholar]
- Crosera M, Prodi A, Mauro M, Pelin M, Florio C, Bellomo F, Adami G, Apostoli P, De Palma G, Bovenzi M, et al. 2015. Titanium dioxide nano-particle penetration into the skin and effects on HaCaT cells. Int J Environ Res Pub Health. 12:9282–9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotzer V, Blum J. 2009. Autophagy and its role in MHC-mediated antigen presentation. J Immunol. 182:3335–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniela P, Vladimir Z, Petr K, Zdenka F, Stepanka V, Martin K, Tomas N, Jaroslav S, Nadezda Z, Otakar M, et al. 2016. Leukotrienes in exhaled breath condensate and fractional exhaled nitric oxide in workers exposed to TiO2 nanoparticles. J Breath Res. 10:036004. [DOI] [PubMed] [Google Scholar]
- Davis M, Wang M, Yiannias J, Keeling J, Connolly S, Richardson D, Farmer S. 2011. Patch testing with a large series of metal allergens: Findings from more than 1,000 patients in one decade at Mayo Clinic. Dermatitis. 22: 256–271. [DOI] [PubMed] [Google Scholar]
- de Haar C, Hassing I, Bol M, Bleumink R, Pieters R. 2006. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin Exp Allergy. 36: 1469–1479. [DOI] [PubMed] [Google Scholar]
- de Haar C, Kool M, Hassing I, Bol M, Lambrecht B, Pieters R. 2008. Lung dendritic cells are stimulated by ultrafine particles and play a key role in particle adjuvant activity. J Allergy Clin Immunol. 121:1246–1254. [DOI] [PubMed] [Google Scholar]
- de Jong W, Arts J, De Klerk A, Schijf M, Ezendam J, Kuper C, van Loveren H. 2009. Contact and respiratory sensitizers can be identified by cytokine profiles following inhalation exposure. Toxicology. 261:103–111. [DOI] [PubMed] [Google Scholar]
- de Marzi M, Saraceno M, Mitarotonda R, Todone M, Fernandez M, Malchiodi E, Desimone M. 2017. Evidence of size-dependent effect of silica micro- and nano-particles on basal and specialized monocyte functions. Ther Deliv. 8:1035–1049. [DOI] [PubMed] [Google Scholar]
- de Matteis V, Cascione M, Brunetti V, Toma C, Rinaldi R. 2016. Toxicity assessment of anatase and rutile titanium dioxide nanoparticles: The role of degradation in different pH conditions and light exposure. Toxicol In Vitro. 37:201–210. [DOI] [PubMed] [Google Scholar]
- de Stefano D, Carnuccio R, Maiuri M. 2012. Nanomaterials toxicity and cell death modalities. J Drug Deliv. 2012:167896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wall S, Painter C, Stone J, Bandaranayake R, Wiley D, Mitchison T, Stern L, de Decker B. 2006. Noble metals strip peptides from Class II MHC proteins. Nat Chem Biol. 2:197–201. [DOI] [PubMed] [Google Scholar]
- Dearman R, Kimber I. 2003. Factors influencing the induction phase of skin sensitization. Am J Contact Derm. 14:188–194. [PubMed] [Google Scholar]
- Demento S, Cui W, Criscione J, Stern E, Tulipan J, Kaech S, Fahmy T. 2012. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 33:4957–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desch A, Randolph G, Murphy K, Gautier E, Kedl R, Lahoud M, Caminschi I, Shortman K, Henson P, Jakubzick C. 2011. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J Exp Med. 208:1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanabanda M, Latheef S, Madduri R. 2016. Immunotoxic effects of gold and silver nanoparticles: Inhibition of mitogen-induced proliferative responses and viability of human and murine lymphocytes in vitro. J Immunotoxicol. 13:897–902. [DOI] [PubMed] [Google Scholar]
- Di Gioacchino M, Verna N, Di Giampaolo L, di Claudio F, Turi M, Perrone A, Petrarca C, Mariani-Costantini R, Sabbioni E, Boscolo P. 2007. Immunotoxicity and sensitizing capacity of metal compounds depend on speciation. Int J Immunopathol Pharmacol. 20:15–22. [DOI] [PubMed] [Google Scholar]
- Diepgen T, Coenraads P. 1999. The epidemiology of occupational contact dermatitis. Int Arch Occup Environ Health. 72:496–506. [DOI] [PubMed] [Google Scholar]
- Dingjan I, Verboogen D, Paardekooper L, Revelo N, Sittig S, Visser L, von Mollard G, Henriet S, Figdor C, Ter Beest M. 2016. Lipid peroxidation causes endosomal antigen release for cross-presentation. Sci Rep. 6:22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia M, Germolec D, Weaver J. 2009a. Evaluation of nanoparticle immunotoxicity. Nat Nanotechnol. 4:411–414. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia M, Neun B, Man S, Ye X, Hansen M, Patri A, Crist R, McNeil S. 2014. Protein corona composition does not accurately predict hematocompatibility of colloidal gold nanoparticles. Nanomed Nanotechnol Biol Med. 10:1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia M, Patri A, Zheng J, Clogston J, Ayub N, Aggarwal P, Neun B, Hall J, McNeil S. 2009b. Interaction of colloidal gold nanoparticles with human blood: Effects on particle size and analysis of plasma protein binding profiles. Nanomedicine. 5:106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Wen Z, Chen L, Zhou N, Liu H, Dong S, Hu H, Mou Y. 2018. Polyethyleneimine modification of aluminum hydroxide nanoparticle enhances antigen transportation and cross-presentation of dendritic cells. Intl J Nanomed. 13:3353–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Kohler A, Peschke K, Vohringer D, Waskow C, Krieg T, et al. 2011. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 34:973–984. [DOI] [PubMed] [Google Scholar]
- Elder A, Oberd€orster A. 2006. Translocation and effects of ultrafine particles outside of the lung. Clin Occup Environ Med. 5:785–796. [DOI] [PubMed] [Google Scholar]
- Elder A, Vidyasagar S, DeLouise L. 2009. Physicochemical factors that affect metal and metal oxide nanoparticle passage across epithelial barriers. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 1:434–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Zein M, Infante RC, Malo J, Gautrin D. 2005. Is metal fume fever a determinant of welding related respiratory symptoms and/or increased bronchial responsiveness? A longitudinal study. Occup Environ Med. 62: 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erle D, Sheppard D. 2014. The cell biology of asthma. J Cell Biol. 205:621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Turkevich LA, Roettgers CT, Deye GJ, Baron PA. 2013. Dustiness of fine and nanoscale powders. Ann Occup Hyg. 57:261–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fage S, Faurschou A, Thyssen J. 2014. Copper hypersensitivity. Contact Derm. 71:191–201. [DOI] [PubMed] [Google Scholar]
- Fahy J 2009. Eosinophilic and neutrophilic inflammation in asthma: Insights from clinical studies. Proc Am Thorac Soc. 6:256–259. [DOI] [PubMed] [Google Scholar]
- Falta MT, Pinilla C, Mack DG, Tinega AN, Crawford F, Giulianotti M, Santos R, Clayton GM, Wang Y, Zhang X, et al. 2013. Identification of beryllium-dependent peptides recognized by CD4+ T-cells in chronic beryllium disease. J Exp Med. 210:1403–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faurschou A, Menne T, Johansen J, Thyssen J. 2011. Metal allergen of the 21st century-a review on exposure, epidemiology and clinical manifestations of palladium allergy. Contact Derm. 64:185–195. [DOI] [PubMed] [Google Scholar]
- Fehrenbach H, Wagner C, Wegmann M. 2017. Airway remodeling in asthma:What really matters. Cell Tissue Res. 367:551–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltis B, Elbaz A, Wright P, Mackay G, Turney T, Lopata A. 2015. Characterizing the inhibitory action of zinc oxide nanoparticles on allergic-type mast cell activation. Mol Immunol. 66:139–146. [DOI] [PubMed] [Google Scholar]
- Fernandes R, Smyth N, Muskens OL, Nitti S, Heuer-Jungemann A, Ardern-Jones M, Kanaras A. 2015. Interactions of skin with gold nanoparticles of different surface charge, shape, and functionality. Small. 11:713–721. [DOI] [PubMed] [Google Scholar]
- Ferreira I, Silva A, Martins J, Neves B, Cruz M. 2018. Nature and kinetics of redox imbalance triggered by respiratory and skin chemical sensitizers on the human monocytic cell line THP-1. Redox Biol. 16:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filon F, Crosera M, Adami G, Bovenzi M, Rossi F, Maina G. 2011. Human skin penetration of gold nanoparticles through intact and damaged skin. Nanotoxicology. 5:493–501. [DOI] [PubMed] [Google Scholar]
- Filon F, Crosera M, Mauro M, Baracchini E, Bovenzi M, Montini T, Fornasiero P, Adami G. 2016. Palladium nanoparticles exposure: Evaluation of permeation through damaged and intact human skin. Environ Pollut. 214:497–503. [DOI] [PubMed] [Google Scholar]
- Foldbjerg R, Olesen P, Hougaard M, Dang D, Hoffmann H, Autrup H. 2009. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol Lett. 190: 156–162. [DOI] [PubMed] [Google Scholar]
- Frohlich E 2015. Value of phagocyte function screening for immunotoxicity of nanoparticles in vivo. Intl J Nanomed. 10:3761–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati V, Cornaghi L, Gianazza E, Potenza M, Donetti E, Marinovich M, Corsini E. 2018. In vitro assessment of silver nanoparticles immunotoxicity. Food Chem Toxicol. 112:363–374. [DOI] [PubMed] [Google Scholar]
- Gamerdinger K, Moulon C, Karp D, van Bergen J, Koning F, Wild D, Pflugfelder U, Weltzien H. 2003. A new type of metal recognition by human T-cells: Contact residues for peptide-independent bridging of T-cell receptor and Major Histocompatibility Complex by nickel. J Exp Med. 197:1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatoo M, Naseem S, Arfat M, Mahmood Dar A, Qasim K, Zubair S. 2014. Physicochemical properties of nanomaterials: Implication in associated toxic manifestations. BioMed Res Int. 2014:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto F, Moglianetti M, Pompa P, Bardi G. 2018. Platinum nanoparticles decrease reactive oxygen species and modulate gene expression without alteration of immune responses in THP-1 monocytes. Nanomaterials. 8:pii E392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavett S, Haykal-Coates N, Copeland L, Heinrich J, Gilmour M. 2003. Metal composition of ambient PM2.5 influences severity of allergic airways disease in mice. Environ Health Perspect. 111:1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha C, Remya N, Leji K, Syama S, Reshma S, Sreekanth P, Varma H, Mohanan P. 2013. Cells-nano interactions and molecular toxicity after delayed hypersensitivity, in guinea pigs on exposure to hydroxyapatite nanoparticles. Colloids Surf B Biointerf. 112:204–212. [DOI] [PubMed] [Google Scholar]
- George R, Merten S, Wang T, Kennedy P, Maitz P. 2014. In vivo analysis of dermal and systemic absorption of silver nanoparticles through healthy human skin. Aus J Dermatol. 55:185–190. [DOI] [PubMed] [Google Scholar]
- Gerberick G, Vassallo J, Bailey R, Chaney J, Morrall S, Lepoittevin J. 2004. Development of a peptide reactivity assay for screening contact allergens. Toxicol Sci. 81:332–343. [DOI] [PubMed] [Google Scholar]
- Ghio A 2008. Mechanism of asthmatic exacerbation by ambient air pollution particles. Expert Rev Respir Med. 2:109–118. [DOI] [PubMed] [Google Scholar]
- Glass D, Mazhar M, Xiang S, Dean P, Simpson P, Priestly B, Plebanski M, Abramson M, Sim M, Dennekamp M. 2017. Immunological effects among workers who handle engineered nanoparticles. Occup Environ Med. 74: 868–876. [DOI] [PubMed] [Google Scholar]
- Glenny A, Buttle G, Stevens M. 1931. Rate of disappearance of diphtheria toxoid injected into rabbits and guinea-pigs: Toxoid precipitated with alum. J Pathol Bacteriol. 34:267–275. [Google Scholar]
- Glista-Baker E, Taylor A, Sayers B, Thompson E, Bonner J. 2014. Nickel nanoparticles cause exaggerated lung and airway remodeling in mice lacking the T-box transcription factor, TBX21 (T-bet). Particle Fibre Toxicol. 11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A, Soroka Y, Frušić-Zlotkin M, Lewis A, Kohen R. 2016. The bright side of plasmonic gold nanoparticles; activation of Nrf2, the cellular protective pathway. Nanoscale. 8:11748–11759. [DOI] [PubMed] [Google Scholar]
- Gontier E, Ynsa M-D, Bíró T, Hunyadi J, Kiss B, Gáspár K, Pinheiro T, Silva J-N, Filipe P, Stachura J, et al. 2008. Is there penetration of titania nano-particles in sunscreens through skin? A comparative electron and ion microscopy study. Nanotoxicology. 2:218–231. [Google Scholar]
- Goon A, Goh C. 2005. Metal allergy in Singapore. Contact Dermatitis. 52:130–132. [DOI] [PubMed] [Google Scholar]
- Gopee NV, Roberts DW, Webb P, Cozart CR, Siitonen PH, Latendresse JR, Warbitton AR, Yu WW, Colvin VL, Walker NJ, et al. 2009. Quantitative determination of skin penetration of PEG-coated CdSe quantum dots in dermabraded but not intact SKH-1 hairless mouse skin. Toxicol Sci. 111: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbet M, Sefton M. 2005. Endotoxin: The uninvited guest. Biomaterials. 26:6811–6817. [DOI] [PubMed] [Google Scholar]
- Granum B, Gaarder PI, Groeng E, Leikvold R, Namork E, Lovik M. 2001a. Fine particles of widely-different composition have an adjuvant effect on the production of allergen-specific antibodies. Toxicol Lett. 118:171–181. [DOI] [PubMed] [Google Scholar]
- Granum B, Gaarder P, Løvik M. 2001b. IgE adjuvant effect caused by particles - immediate and delayed effects. J Toxicol. 156:149–159. [DOI] [PubMed] [Google Scholar]
- Gualtieri M, Skuland T, Iversen T, Lag M, Schwarze P, Bilanicova D, Pojana G, Refsnes M. 2012. Importance of agglomeration state and exposure conditions for uptake and pro-inflammatory responses to amorphous silica nanoparticles in bronchial epithelial cells. Nanotoxicology. 6:700–712. [DOI] [PubMed] [Google Scholar]
- Gulson B, McCall M, Korsch M, Gomez L, Casey P, Oytam Y, Taylor A, McCulloch M, Trotter J, Kinsley L, et al. 2010. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol Sci. 118:140–149. [DOI] [PubMed] [Google Scholar]
- Gulson B, Wong H, Korsch M, Gomez L, Casey P, McCall M, McCulloch M, Trotter J, Stauber J, Greenoak G. 2012. Comparison of dermal absorption of zinc from different sunscreen formulations and differing UV exposure based on stable isotope tracing. Sci Total Environ. 420:313–318. [DOI] [PubMed] [Google Scholar]
- Gustafsson A, Jonasson S, Sandstrom T, Lorentzen J, Bucht A. 2014. Genetic variation influences immune responses in sensitive rats following exposure to TiO2 nanoparticles. Toxicology. 326:74–85. [DOI] [PubMed] [Google Scholar]
- Hahn A, Fuhlrott J, Loos A, Barcikowski S. 2012. Cytotoxicity and ion release of alloy nanoparticles. J Nanopart Res. 14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R, Buckingham S, Holian A. 2014. The effect of size on Ag nano-sphere toxicity in macrophage cell models and lung epithelial cell lines is dependent on particle dissolution. Int J Mol Sci. 15:6815–6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, Lambrecht B. 2015. Barrier epithelial cells and the control of Type 2 immunity. J Immunity. 43:29–40. [DOI] [PubMed] [Google Scholar]
- Han B, Guo J, Abrahaley T, Qin L, Wang L, Zheng Y, Li B, Liu D, Yao H, Yang J, et al. 2011. Adverse effect of nano-silicon dioxide on lung function of rats with or without ovalbumin immunization. PloS One. 6: e17236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Park Y, Park H, Lee K, Um K, Park J, Lee J. 2016. Toxic and adjuvant effects of silica nanoparticles on ovalbumin-induced allergic airway inflammation in mice. Respir Res. 17:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley C, Thurber A, Hanna C, Punnoose A, Zhang J, Wingett D. 2009. The influences of cell type and ZnO nanoparticle size on immune cell cytotoxicity and cytokine induction. Nanoscale Res Lett. 4:1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng B, Zhao X, Tan E, Khamis N, Assodani A, Xiong S, Ruedl C, Ng K, Loo J. 2011. Evaluation of the cytotoxic and inflammatory potential of differentially shaped zinc oxide nanoparticles. Arch Toxicol. 85:1517–1528. [DOI] [PubMed] [Google Scholar]
- Henriksen-Lacey M, Bramwell V, Christensen D, Agger E, Andersen P, Perrie Y. 2010a. Lipo-somes based on dimethyldioctadecylammonium promote a depot effect and enhance immu-nogenicity of soluble antigen. J Control Rel. 142:180–186. [DOI] [PubMed] [Google Scholar]
- Henriksen-Lacey M, Christensen D, Bramwell V, Lindenstrøm T, Agger E, Andersen P, Perrie Y. 2010b. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J Control Rel. 145:102–108. [DOI] [PubMed] [Google Scholar]
- Hirai T, Yoshikawa T, Nabeshi H, Yoshida T, Akase T, Yoshioka Y, Itoh N, Tsutsumi Y. 2012a. Dermal absorption of amorphous nanosilica particles after topical exposure for three days. Pharmazie. 67:742–743. [PubMed] [Google Scholar]
- Hirai T, Yoshikawa T, Nabeshi H, Yoshida T, Tochigi S, Ichihashi K, Uji M, Akase T, Nagano K, Abe Y, et al. 2012b. Amorphous silica nanoparticles size-dependently aggravate atopic dermatitis-like skin lesions following an intradermal injection. Particle Fibre Toxicol. 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Yoshioka Y, Izumi N, Ichihashi K, Handa T, Nishijima N, Uemura E, Sagami K, Takahashi H, Yamaguchi M, et al. 2016. Metal nanoparticles in the presence of lipopolysaccharides trigger the onset of metal allergy in mice. Nat Nanotechnol. 11:808–816. [DOI] [PubMed] [Google Scholar]
- Hirai T, Yoshioka Y, Takahashi H, Ichihashi K, Udaka A, Mori T, Nishijima N, Yoshida T, Nagano K, Kamada H, et al. 2015. Cutaneous exposure to agglomerates of silica nanoparticles and allergen results in IgE-biased immune response and increased sensitivity to anaphylaxis in mice. Particle Fibre Toxicol. 12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Yoshioka Y, Takahashi H, Ichihashi K-i, Yoshida T, Tochigi S, Nagano K, Abe Y, Kamada H, Tsunoda S-i, et al. 2012. Amorphous silica nanoparticles enhance cross-presentation in murine dendritic cells. Biochem Biophys Res Commun. 427:553–556. [DOI] [PubMed] [Google Scholar]
- Hiroike M, Sakabe J, Kobayashi M, Hirakawa S, Inoh A, Tokura Y. 2013. Acicular, but not globular, titanium dioxide nanoparticles stimulate keratinocytes to produce cytokines independently of inflammasome. J Dermatol Sci. 69:e81. [DOI] [PubMed] [Google Scholar]
- Hoang M, Lee H, Lee H, Jung S, Choi N, Vo M, Nguyen-Pham T, Kim H, Park I, Lee J. 2015. Branched polyethylenimine-superparamagnetic iron oxide nanoparticles (bPEI-SPIONs) improve immunogenicity of tumor antigens and enhance TH1 polarization of dendritic cells. J Immunol Res. 2015:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld J 2002. The role of surfactant in asthma. Respir Res. 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld J, Ahlf K, Enhorning G, Balke K, Erpenbeck V, Petschallies J, Hoymann H, Fabel H, Krug N. 1999. Dysfunction of pulmonary surfactant in asthmatics after segmental allergen challenge. Am J Respir Crit Care Med. 159:1803–1809. [DOI] [PubMed] [Google Scholar]
- Hol P, Kristoffersen E, Gjerdet N, Pellowe A. 2018. Novel nanoparticulate and ionic titanium antigens for hypersensitivity testing. Int J Mol Sci. 19: pii:E1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran W, Uchida Y, Halkier-Sorensen L, Haratake A, Hara M, Epstein J, Elias P. 1997. Structural and biochemical basis for the UVB-induced alterations in epidermal barrier function. Photodermatol Photoimmunol Photomed. 13:117–128. [DOI] [PubMed] [Google Scholar]
- Holmes A, Song Z, Moghimi H, Roberts M. 2016. Relative penetration of zinc oxide and zinc ions into human skin after application of different zinc oxide formulations. ACS Nano. 10:1810–1819. [DOI] [PubMed] [Google Scholar]
- Holt P, Oliver J, Bilyk N, McMenamin C, McMenamin P, Kraal G, Thepen T. 1993. Down-regulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 177:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Oh S, Lee H, Huh K, Lee S. 1986. Occupational asthma caused by nickel and zinc. Korean J Intern Med. 1:259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M, Stowe M, Tabei M, Kuroda E. 2015. Pharyngeal aspiration of metal oxide nanoparticles showed potential of allergy aggravation effect to inhaled ovalbumin. Inhal Toxicol. 27:181–190. [DOI] [PubMed] [Google Scholar]
- Horie M, Stowe M, Tabei M, Kuroda E. 2016. Metal ion release of manufactured metal oxide nanoparticles is involved in the allergic response to inhaled ovalbumin in mice. Occup Dis Environ Med. 04:17. [Google Scholar]
- Hostynek J 2003. Factors determining percutaneous metal absorption. Food Chem Toxicol. 41:327–345. [DOI] [PubMed] [Google Scholar]
- Hostynek J, Hinz R, Lorence C, Price M, Guy R. 1993. Metals and the skin.Crit Rev Toxicol. 23:171–235. [DOI] [PubMed] [Google Scholar]
- Hsiao Y, Shen C, Huang C, Lin Y, Jan T. 2018. Iron oxide nanoparticles attenuate T helper 17 cell responses in vitro and in vivo. Int Immunopharmacol. 58:32–39. [DOI] [PubMed] [Google Scholar]
- Huang K, Lee Y, Chen H, Liao H, Chiang B, Cheng T. 2015. Zinc oxide nanoparticles induce eosinophilic airway inflammation in mice. J Hazard Mat. 297:304–312. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu H, Xiong X, Chen Y, Tan W. 2009. Nanoparticle-mediated IgE-receptor aggregation and signaling in RBL mast cells. J Am Chem Soc. 131:17328–17334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yu F, Park Y, Wang J, Shin M, Chung H, Yang V. 2010. Co-administration of protein drugs with gold nanoparticles to enable percutaneous delivery. J Biomaterials. 31:9086–9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeniuk P, Dubiela P, Hoffmann-Sommergruber K. 2017. Dendritic cells and their role in allergy: Uptake, proteolytic processing, and presentation of allergens. Int J Mol Sci. 18:1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Boland S, Baeza-Squiban A, Hamel R, Thomassen L, Martens J, Billon-Galland M, Fleury-Feith J, Moisan F, Pairon J, et al. 2009. Oxidative stress and proinflammatory effects of carbon black and titanium dioxide nanoparticles: Role of particle surface area and internalized amount. Toxicology. 260:142–149. [DOI] [PubMed] [Google Scholar]
- Hussain S, Smulders S, de Vooght V, Ectors B, Boland S, Marano F, van Landuyt KL, Nemery B, Hoet P, Vanoirbeek J. 2012. Nano-titanium dioxide modulates the dermal sensitization potency of DNCB. Particle Fibre Toxicol. 9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Vanoirbeek J, Luyts K, de Vooght V, Verbeken E, Thomassen L, Martens J, Dinsdale D, Boland S, Marano F, et al. 2011. Lung exposure to nanoparticles modulates an asthmatic response in a mouse model. Eur Respir J. 37:299–309. [DOI] [PubMed] [Google Scholar]
- Iannuccelli V, Bertelli D, Romagnoli M, Scalia S, Maretti E, Sacchetti F, Leo E. 2014. In vivo penetration of bare and lipid-coated silica nanoparticles across the human stratum corneum. Colloids Surf B Biointerf. 122: 653–661. [DOI] [PubMed] [Google Scholar]
- Ilinskaya A, Dobrovolskaia M. 2016. Understanding the immunogenicity and antigenicity of nanomaterials: Past, present and future. Toxicol Appl Pharmacol. 299:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilves M, Palomaki J, Vippola M, Lehto M, Savolainen K, Savinko T, Alenius H. 2014. Topically-applied ZnO nanoparticles suppress allergen induced skin inflammation but induce vigorous IgE production in the atopic dermatitis mouse model. Particle Fibre Toxicol. 11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N, Fitrilawati F, Manna A, Akiyama H, Tamada Y, Tamada K. 2008. Gold nanoparticles used as a carrier enhance production of anti-hapten IgG in rabbit: A study with azobenzene-dye as a hapten presented on the entire surface of gold nanoparticles. Biosci Biotechnol Biochem. 72: 124–131. [DOI] [PubMed] [Google Scholar]
- Jaitley S, Saraswathi T. 2012. Pathophysiology of Langerhans cells. J Oral Maxillofac Pathol. 16:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick C, Tacke F, Llodra J, Rooijen N, Randolph G. 2006. Modulation of dendritic cell trafficking to and from the airways. J Immunol. 176: 3578–3584. [DOI] [PubMed] [Google Scholar]
- Jang S, Park J, Cha H, Jung S, Lee J, Jung S, Kim J, Kim S, Lee C, Park H. 2012. Silver nanoparticles modify VEGF signaling pathway and mucus hypersecretion in allergic airway inflammation. Intl J Nanomed. 7: 1329–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassby D, Farner Budarz J, Wiesner M. 2012. Impact of aggregate size and structure on the photocatalytic properties of TiO2 and ZnO nanoparticles. Environ Sci Technol. 46:6934–6941. [DOI] [PubMed] [Google Scholar]
- Jatana S, Palmer B, Phelan S, DeLouise L. 2017a. Immunomodulatory effects of nanoparticles on skin allergy. Sci Rep. 7:3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatana S, Palmer B, Phelan S, Gelein R, DeLouise L. 2017b. In vivo quantification of quantum dot systemic transport in C57BL/6 hairless mice following skin application post-ultraviolet radiation. Particle Fibre Toxicol. 14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Han Y, Poland C, Cho WS. 2015. Response-metrics for acute lung inflammation pattern by cobalt-based nanoparticles. Part Fibre Toxicol. 12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Kim H, Ryu H, Ryu W, Park Y, Bae H, Jang Y, Son S. 2013. ZnO nanoparticles induce TNF-a expression via ROS-ERK-Egr-1 pathway in human keratinocytes. J Dermatol Sci. 72:263–273. [DOI] [PubMed] [Google Scholar]
- Jia J, Zhang Y, Xin Y, Jiang C, Yan B, Zhai S. 2018. Interactions between nanoparticles and dendritic cells: From the perspective of cancer immunotherapy. Front Oncol. 8:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Liu F, Yang H, Li Y. 2012. Effects of cobalt nanoparticles on human T cells in vitro. Biol Trace Elem Res. 146:23–29. [DOI] [PubMed] [Google Scholar]
- Jiménez-Periáñez A, Abos Gracia B, Lopez Relano J, Diez-Rivero C, Reche P, Martínez-Naves E, Matveyeva E, Gomez del Moral M. 2013. Mesoporous silicon microparticles enhance MHC Class I cross-antigen presentation by human dendritic cells. Clin Develop Immunol. 2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen P, Storni T, Rettig L, Qiu Z, Der-Sarkissian A, Smith K, Manolova V, Lang K, Senti G, Mullhaupt B, et al. 2008. Antigen kinetics determines immune reactivity. Proc Nat Acad Sci USA. 105:5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Mendoza R, Raghavendra A, Podila R, Brown J. 2017. Contribution of engineered nanomaterials physicochemical properties to mast cell degranulation. Sci Rep. 7:43570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson S, Gustafsson A, Koch B, Bucht A. 2013. Inhalation exposure of nano-scaled titanium dioxide (TiO2) particles alters the inflammatory responses in asthmatic mice. Inhal Toxicol. 25:179–191. [DOI] [PubMed] [Google Scholar]
- Jones S, Roberts R, Robbins G, Perry J, Kai M, Chen K, Bo T, Napier M, Ting J, DeSimone J, et al. 2013. Nanoparticle clearance is governed by TH1/TH2 immunity and strain background. J Clin Invest. 123:3061–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journeay W, Goldman R. 2014. Occupational handling of nickel nanoparticles: A case report. Am J Ind Med. 57:1073–1076. [DOI] [PubMed] [Google Scholar]
- Jung D, Che J, Lim K, Chun Y, Heo Y, Seok S. 2016. Discrimination of skin sensitizers from non-sensitizers by interleukin-1a and interleukin-6 production on cultured human keratinocytes. J Appl Toxicol. 36:1129–1136. [DOI] [PubMed] [Google Scholar]
- Kanerva L, Jolanki R, Estlander T, Alanko K, Savela A. 2000. Incidence rates of occupational allergic contact dermatitis caused by metals. Am J Contact Derm. 11:155–160. [DOI] [PubMed] [Google Scholar]
- Kang S, Ahn S, Lee J, Kim J, Choi M, Gujrati V, Kim H, Kim J, Shin E, Jon S. 2017. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J Control Rel. 256:56–67. [DOI] [PubMed] [Google Scholar]
- Kang K, Lim J. 2012. Induction of functional changes of dendritic cells by silica nanoparticles. Immune Netw. 12:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Kim S, Lee K, Jin S, Kim S, Lee K, Jeon H, Song Y, Lee S, Seo J, et al. 2017. 5-nm Silver nanoparticles amplify clinical features of atopic dermatitis in mice by activating mast cells. Small. 13(9). DOI: 10.1002/smll.201602363. [DOI] [PubMed] [Google Scholar]
- Kapilevich L, Zaitseva T, Nosarev A, D’Iakova E, Petlina Z, Ogorodova L, Ageev B, Magaeva A, Itin V, Terekhova O. 2012. Influence of nanosize particles of cobalt ferrite on contractile responses of smooth muscle segment of airways. Ross Fiziol Zh Im Sechenova. 98:228–235. [PubMed] [Google Scholar]
- Karlberg A, Bergstrom M, Borje A, Luthman K, Nilsson J. 2008. Allergic contact dermatitis-formation, structural requirements, and reactivity of skin sensitizers. Chem Res Toxicol. 21:53–69. [DOI] [PubMed] [Google Scholar]
- Kauffman HF, Tamm M, Timmerman JAB, Borger P. 2006. House dust mite major allergens Der p 1 and Der p 5 activate human airway-derived epithelial cells by protease-dependent and protease-independent mechanisms. Clin Mol Allergy. 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R, Bramwell V, Kirby D, Perrie Y. 2012a. Manipulation of surface pegylation in combina-tion with reduced vesicle size of cationic liposomal adjuvants modifies their clearance kinetics from the injection site, and rate and type of T-cell response. J Control Rel. 164:331–337. [DOI] [PubMed] [Google Scholar]
- Kaur R, Bramwell V, Kirby D, Perrie Y. 2012b. Pegylation of DDA: TDB liposomal adjuvants reduces the vaccine depot effect and alters the TH1/TH2 immune responses. J Control Rel. 158:72–77. [DOI] [PubMed] [Google Scholar]
- Keene A, Tyner K. 2011. Analytical characterization of gold nanoparticle primary particles, aggregates, agglomerates, and agglomerated aggregates. J Nanopart Res. 13:3465–3481. [Google Scholar]
- Khatami A, Nassiri-Kashani M, Gorouhi F, Babakoohi S, Kazerouni-Timsar A, Davari P, Sarraf-Yazdy M, Dowlati Y, Firooz A. 2013. Allergic contact dermatitis to metal allergens in Iran. Int J Dermatol. 52:1513–1518. [DOI] [PubMed] [Google Scholar]
- Khatri M, Bello D, Pal A, Cohen J, Woskie S, Gassert T, Lan J, Gu A, Demokritou P, Gaines P. 2013. Evaluation of cytotoxic, genotoxic and inflammatory responses of nanoparticles from photocopiers in three human cell lines. Part Fibre Toxicol. 10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Heo Y, Choi S, Kim Y, Kim M, Kim H, Jo E, Song C, Lee K. 2016. Safety evaluation of zinc oxide nanoparticles in terms of acute dermal toxicity, dermal irritation and corrosion, and skin sensitization. Mol Cell Toxicol. 12:93–99. [Google Scholar]
- Kim T, Kim W, Mun J, Song M, Kim H, Kim B, Kim M, Ko H. 2015. Patch testing with dental screening series in oral disease. Ann Dermatol. 27: 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Lee P, Lee S, Park M, Jang A. 2017. Effect of TiO2 nanoparticles on inflammasome-mediated airway inflammation and responsiveness. Allergy Asthma Immunol Res. 9:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Nguyen H, Ignacio R, Kim J, Cho H, Maeng E, Kim Y, Kim M, Park B, Kim S. 2014. Immunotoxicity of zinc oxide nanoparticles with different size and electrostatic charge. Int J Nanomed. 9:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Song K, Sung J, Ryu H, Choi B, Cho H, Lee J, Yu I. 2013. Genotoxicity, acute oral and dermal toxicity, eye and dermal irritation and corrosion and skin sensitisation evaluation of silver nanoparticles. Nanotoxicology. 7:953–960. [DOI] [PubMed] [Google Scholar]
- Kimber I, Cumberbatch M. 1992. Dendritic cells and cutaneous immune responses to chemical allergens. Toxicol Appl Pharmacol. 117:137–146. [DOI] [PubMed] [Google Scholar]
- Kinbara M, Nagai Y, Takano-Yamamoto T, Sugawara S, Endo Y. 2011. Cross-reactivity among some metals in a murine metal allergy model. Br J Dermatol. 165:1022–1029. [DOI] [PubMed] [Google Scholar]
- Kiss B, Bíró T, Czifra G, Tóth B, Kertész Z, Szikszai Z, Kiss Á, Juhász I, Zouboulis C, Hunyadi J. 2008. Investigation of micronized titanium dioxide penetration in human skin xenografts and its effect on cellular functions of human skin-derived cells. Exp Dermatol. 17:659–667. [DOI] [PubMed] [Google Scholar]
- Ko J, Lee H, Shin N, Seo Y, Kim S, Shin I, Kim J. 2018. Silicon dioxide nanoparticles enhance endotoxin-induced lung injury in mice. Molecules. 23:2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes SA, Engebretsen KA, Agner T, Angelova-Fischer I, Berents T, Brandner J, Brans R, Clausen M-L, Hummler E, Jakasa I, et al. 2017. Current knowledge on biomarkers for contact sensitization and allergic contact dermatitis. Contact Dermatitis. 77:1–16. [DOI] [PubMed] [Google Scholar]
- Korani M, Rezayat S, Gilani K, Bidgoli S, Adeli S. 2011. Acute and sub-chronic dermal toxicity of nanosilver in guinea pig. Int J Nanomedicine. 6:855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeling M, Topping V, Keltner Z, Belgrave K, Bailey K, Gao X, Yourick J. 2018. In vitro percutaneous penetration of silver nanoparticles in pig and human skin. Regul Toxicol Pharmacol. 95:314–322. [DOI] [PubMed] [Google Scholar]
- Kubo A, Ishizaki I, Kubo A, Kawasaki H, Nagao K, Ohashi Y, Amagai M. 2013. The stratum corneum comprises three layers with distinct metal-ion barrier properties. Sci Rep. 3:1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuempel E, Castranova V, Geraci C, Schulte P. 2012. Development of risk-based nanomaterial groups for occupational exposure control. J Nanopart Res. 14:1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjane N, Zvagule T, Reste J, Martinsone Z, Pavlovska I, Martinsone I, Vanadzins I. 2017. The effect of different workplace nanoparticles on the immune systems of employees. J Nanopart Res. 19:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka Y, Iki M, Kumagai S, Goto S. 1996. Epidemiological study of hard metal asthma. Occup Environ Med. 53:188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouta H, el-Khordagui L, Kraus T, Schneider M. 2011. Mechanism and determinants of nanoparticle penetration through human skin. Nanoscale. 3:4989–4999. [DOI] [PubMed] [Google Scholar]
- Lademann J, Knorr F, Richter H, Jung S, Meinke M, Rühl E, Alexiev U, Calderon M, Patzelt A. 2015. Hair follicles as target structure for nanoparticles. J Innov Optical Health Sci. 08:1530004. [Google Scholar]
- Lademann J, Patzelt A, Richter H, Antoniou C, Sterry W, Knorr F. 2009. Determination of the cuticula thickness of human and porcine hairs and their potential influence on the penetration of nanoparticles into the hair follicles. J Biomed Opt. 14:021014. [DOI] [PubMed] [Google Scholar]
- Lademann J, Richter H, Schaefer UF, Blume-Peytavi U, Teichmann A, Otberg N, Sterry W. 2006. Hair follicles - a long-term reservoir for drug delivery. Skin Pharmacol Physiol. 19:232–236. [DOI] [PubMed] [Google Scholar]
- Lademann J, Weigmann H, Rickmeyer C, Barthelmes H, Schaefer H, Mueller G, Sterry W. 1999. Penetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orifice. Skin Pharmacol Physiol. 12:247–256. [DOI] [PubMed] [Google Scholar]
- Lambert AL, Dong W, Selgrade M, Gilmour M. 2000. Enhanced allergic sensitization by residual oil fly ash particles is mediated by soluble metal constituents. Toxicol Appl Pharmacol. 165:84–93. [DOI] [PubMed] [Google Scholar]
- Lambrecht B, Hammad H. 2012. The airway epithelium in asthma. Nat Med.18:684. [DOI] [PubMed] [Google Scholar]
- Lansdown A 1995. Physiological and toxicological changes in the skin resulting from the action and interaction of metal ions. Crit Rev Toxicol. 25: 397–462. [DOI] [PubMed] [Google Scholar]
- Larese FF, D’Agostin F, Crosera M, Adami G, Renzi N, Bovenzi M, Maina G. 2009. Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology. 255:33–37. [DOI] [PubMed] [Google Scholar]
- Larese Filon F, Bello D, Cherrie J, Sleeuwenhoek A, Spaan S, Brouwer D. 2016. Occupational dermal exposure to nanoparticles and nano-enabled products: Part I-Factors affecting skin absorption. Int J Hyg Environ Health. 219:536–544. [DOI] [PubMed] [Google Scholar]
- Larese Filon F, Crosera M, Timeus E, Adami G, Bovenzi M, Ponti J, Maina G. 2013. Human skin penetration of cobalt nanoparticles through intact and damaged skin. Toxicol In Vitro. 27:121–127. [DOI] [PubMed] [Google Scholar]
- Larese Filon F, Mauro M, Adami G, Bovenzi M, Crosera M. 2015. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul Toxicol Pharmacol. 72:310–322. [DOI] [PubMed] [Google Scholar]
- Larsen S, Roursgaard M, Jensen K, Nielsen G. 2010. Nano titanium dioxide particles promote allergic sensitization and lung inflammation in mice. Basic Clin Pharmacol Toxicol. 106:114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudańska H, Lemancewicz A, Kretowska M, Reduta T, Laudański T. 2002. Permeability of human skin to selected anions and cations-in vitro studies. Res Commun Mol Pathol Pharmacol. 112:16–26. [PubMed] [Google Scholar]
- Lawrence D, McCabe M. 2002. Immunomodulation by metals. Int Immunopharmacol. 2:293–302. [DOI] [PubMed] [Google Scholar]
- Lee O, Jeong S, Shin W, Lee G, Oh C, Son S. 2013. Influence of surface charge of gold nanorods on skin penetration. Skin Res Technol. 19: e390–e396. [DOI] [PubMed] [Google Scholar]
- Lee S, Pie J, Kim Y, Lee H, Son S, Kim M. 2012. Effects of zinc oxide nano-particles on gene expression profile in human keratinocytes. Mol Cell Toxicol. 8:113–118. [Google Scholar]
- Lee C, Syu S, Chen Y, Hussain S, Aleksandrovich Onischuk A, Chen W, Steven Huang G. 2014. Gold nanoparticles regulate the blimp1/pax5 pathway and enhance antibody secretion in B-cells. Nanotechnology. 25: 125103. [DOI] [PubMed] [Google Scholar]
- Lee S, Yun H, Kim S. 2011. The comparative effects of mesoporous silica nanoparticles and colloidal silica on inflammation and apoptosis. Biomaterials. 32:9434–9443. [DOI] [PubMed] [Google Scholar]
- Lee S, Hwang S, Jeong J, Han Y, Kim S, Lee D, Lee H, Chung S, Jeong J, Roh C. 2016. Nickel oxide nanoparticles can recruit eosinophils in the lungs of rats by the direct release of intracellular eotaxin. Particle Fibre Toxicol. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite-Silva VR, Le Lamer M, Sanchez WY, Liu DC, Sanchez WH, Morrow I, Martin D, Silva HDT, Prow TW, Grice JE, et al. 2013. The effect of formulation on the penetration of coated and uncoated zinc oxide nanoparticles into the viable epidermis of human skin in vivo. Eur J Pharm Biopharm. 84:297–308. [DOI] [PubMed] [Google Scholar]
- Leite-Silva VR, Sanchez WY, Studier H, Liu DC, Mohammed YH, Holmes AM, Ryan EM, Haridass IN, Chandrasekaran NC, Becker W, et al. 2016. Human skin penetration and local effects of topical nano zinc oxide after occlusion and barrier impairment. Eur J Pharm Biopharm. 104:140–147. [DOI] [PubMed] [Google Scholar]
- Li H, Li Y, Jiao J, Hu H. 2011. α-Alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent anti-tumor response. Nat Nanotechnol. 6:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wang M, Bramble L, Schmitz D, Schauer J, Sioutas C, Harkema J, Nel A. 2009. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ Health Perspect. 117:1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shi Z, Radauer-Preiml I, Andosch A, Casals E, Luetz-Meindl U, Cobaleda M, Lin Z, Jaberi-Douraki M, Italiani P, et al. 2017. Bacterial endotoxin (lipopolysaccharide) binds to the surface of gold nanoparticles, interferes with biocorona formation and induces human monocyte inflammatory activation. Nanotoxicology. 11:1157–1175. [DOI] [PubMed] [Google Scholar]
- Liao H, Chung Y, Lai C, Lin M, Liou S. 2014. Sneezing and allergic dermatitis were increased in engineered nanomaterial-handling workers. Ind Health. 52:199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Grice J, Butler M, Zvyagin A, Becker W, Robertson T, Soyer H, Roberts M, Prow T. 2011. Time-correlated single photon counting for simultaneous monitoring of ZnO nanoparticles and NAD(P)H in intact and barrier-disrupted volunteer skin. Pharm Res. 28:2920–2930. [DOI] [PubMed] [Google Scholar]
- Lin M, Zhan Y, Villadangos J, Lew A. 2008. The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol Cell Biol. 86: 353–362. [DOI] [PubMed] [Google Scholar]
- Lin J, Zhang H, Chen Z, Zheng Y. 2010. Penetration of lipid membranes by gold nanoparticles: Insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano. 4:5421–5429. [DOI] [PubMed] [Google Scholar]
- Linauskiene K, Malinauskiene L, Blažiene A. 2017. Metals are important contact sensitizers: An experience from Lithuania. BioMed Res Intl. 2017: 3964045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde S, Franken A, du Plessis J. 2017. Occupational respiratory exposure to platinum group metals: A review and recommendations. Chem Res Toxicol. 30:1778–1790. [DOI] [PubMed] [Google Scholar]
- Liptrott N, Kendall E, Nieves D, Farrell J, Rannard S, Fernig D, Owen A. 2014. Partial mitigation of gold nanoparticle interactions with human lymphocytes by surface functionalization with a ‘mixed matrix’. Nanomedicine. 9:2467–2479. [DOI] [PubMed] [Google Scholar]
- Lisby S, Müller K, Jongeneel C, Saurat J, Hauser C. 1995. Nickel and skin irritants up-regulate TNFα mRNA in keratinocytes by different but potentially synergistic mechanisms. Int Immunol. 7:343–352. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang J, Wang D, Wang J. 2013. Identification of DMSA-coated Fe3O4 nanoparticles induced-apoptosis response genes in human monocytes by cDNA microarrays. Adv Mat Res. 749:377–383. [Google Scholar]
- Liu Z, Li W, Wang F, Sun C, Wang L, Wang J, Sun F. 2012. Enhancement of lipopolysaccharide-induced nitric oxide and IL-6 production by PEGylated gold nanoparticles in RAW264.7 cells. Nanoscale. 4:7135–7142. [DOI] [PubMed] [Google Scholar]
- Liu H, Yang D, Yang H, Zhang H, Zhang W, Fang Y, Lin Z, Tian L, Lin B, Yan J. 2013. Comparative study of respiratory tract immune toxicity induced by three sterilization nanoparticles: Silver, zinc oxide and titanium dioxide. J Hazard Mater. 248:478–486. [DOI] [PubMed] [Google Scholar]
- Liu R, Yin L, Pu Y, Li Y, Zhang X, Liang G, Li X, Zhang J, Li Y, Zhang X. 2010. The immune toxicity of titanium dioxide on primary pulmonary alveolar macrophages relies on their surface area and crystal structure. J Nanosci Nanotechnol. 10:8491–8499. [DOI] [PubMed] [Google Scholar]
- Liu M, Zhang J, Shan W, Huang Y. 2015. Developments of mucus penetrating nanoparticles. Asian J Pharm Sci. 10:275–282. [Google Scholar]
- Liu Y, Jiao F, Qiu Y, Li W, Lao F, Zhou G, Sun B, Xing G, Dong J, Zhao Y, et al. 2009. The effect of Gd@C82(OH)22 nanoparticles on the release of TH1/TH2 cytokines and induction of TNFα-mediated cellular immunity. Biomaterials. 30:3934–3945. [DOI] [PubMed] [Google Scholar]
- Liu S, Meng X, Perez-Aguilar J, Zhou R. 2016. An in silico study of TiO2 nanoparticles interaction with 20 standard amino acids in aqueous solution. Sci Rep. 6:37761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Suh K, Lee H, Cohen M, Rieves D, Pazdur R. 2010. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol. 85:315–319. [DOI] [PubMed] [Google Scholar]
- Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson K. 2008. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci USA 105:14265–14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Chang L, Lin P. 2015. Metal-based nanoparticles and the immune system: Activation, inflammation, and potential applications. BioMed Res Int. 2015:143720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster A, Tager A. 2004. T-Cell trafficking in asthma: Lipid mediators grease the way. Nat Rev Immunol. 4:711–724. [DOI] [PubMed] [Google Scholar]
- Malo J, Chan-Yeung M, Bernstein D, editors. 2013. Asthma in the Workplace. Boca Raton, FL: CRC Press. [Google Scholar]
- Ma J, Mercer R, Barger M, Schwegler-Berry D, Scabilloni J, Ma J, Castranova V. 2012. Induc-tion of pulmonary fibrosis by CeO nanoparticles. Toxicol Appl Pharmacol. 262:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Wallis L, Diamond S, Li S, Canas-Carrell J, Parra A. 2014. Impact of solar UV radiation on toxicity of ZnO nanoparticles through photocatalytic reactive oxygen species (ROS) generation and photo-induced dissolution. Environ Pollut. 193:165–172. [DOI] [PubMed] [Google Scholar]
- Mahe B, Vogt A, Liard C, Duffy D, Abadie V, Bonduelle O, Boissonnas A, Sterry W, Verrier B, Blume-Peytavi U, et al. 2009. Nanoparticle-based targeting of vaccine compounds to skin antigen-presenting cells by hair follicles and their transport in mice. J Invest Dermatol. 129:1156–1164. [DOI] [PubMed] [Google Scholar]
- Mahler V, Geier J, Schnuch A. 2014. Current trends in patch testing - new data from the German Contact Dermatitis Research Group (DKG) and the Information Network of Departments of Dermatology (IVDK). J Dtsch Dermatol Ges. 12:583–592. [DOI] [PubMed] [Google Scholar]
- Mahmoud N, Alkilany AM, Dietrich D, Karst U, Al-Bakri A, Khalil E. 2017. Preferential accumulation of gold nanorods into human skin hair follicles: Effect of nanoparticle surface chemistry. J Coll Interf Sci. 503:95–102. [DOI] [PubMed] [Google Scholar]
- Mahmoud N, Harfouche M, Alkilany A, Al-Bakri A, El-Qirem R, Shraim S, Khalil EA. 2018. Synchrotron-based X-ray fluorescence study of gold nanorods and skin elements distribution into excised human skin layers. Colloids Surf B Biointerf. 165:118–126. [DOI] [PubMed] [Google Scholar]
- Malhotra R, Haurum J, Thiel S, Jensenius J, Sim R. 1993. Pollen grains bind to lung alveolar Type II cells (A549) via lung surfactant protein A (SP-A). Biosci Rep. 13:79–90. [DOI] [PubMed] [Google Scholar]
- Malinauskiene L, Isaksson M, Bruze M. 2015. Patch testing with the Swedish Baseline Series in two countries. J Clin Exp Dermatol Res. 6:5. [Google Scholar]
- Malo J, Cartier A, Doepner M, Nieboer E, Evans S, Dolovich J. 1982. Occupational asthma caused by nickel sulfate. J Allergy Clin Immunol. 69: 55–59. [DOI] [PubMed] [Google Scholar]
- Mandervelt C, Clottens F, Demedts M, Nemery B. 1997. Assessment of the sensitization potential of five metal salts in the murine local lymph node assay. Toxicology. 120:65–73. [DOI] [PubMed] [Google Scholar]
- Manke A, Wang L, Rojanasakul Y. 2013. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res Int. 2013:942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano S, Kanehira K, Taniguchi A. 2013. Comparison of cellular uptake and inflammatory response via toll-like receptor 4 to lipopolysaccharide and titanium dioxide. Nanoparticles Int J Mol Sci. 14:13154–13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquieira Á, Brun EM, Garcés-García M, Puchades R. 2012. Aluminum oxide nanoparticles as carriers and adjuvants for eliciting antibodies from non-immunogenic haptens. Anal Chem. 84:9340–9348. [DOI] [PubMed] [Google Scholar]
- Marquis B, Liu Z, Braun K, Haynes C. 2011. Investigation of noble metal nanoparticle ζ-potential effects on single-cell exocytosis function in vitro with carbon-fiber microelectrode amperometry. Analyst. 136:3478–3486. [DOI] [PubMed] [Google Scholar]
- Marquis B, Maurer-Jones M, Braun K, Haynes C. 2009. Amperometric assessment of functional changes in nanoparticle-exposed immune cells: Varying Au nanoparticle exposure time and concentration. Analyst. 134: 2293–2300. [DOI] [PubMed] [Google Scholar]
- Martorano L, Stork C, Li Y. 2010. UV irradiation-induced zinc dissociation from commercial zinc oxide sunscreen and its action in human epidermal keratinocytes. J Cosmetic Dermatol. 9:276–286. [DOI] [PubMed] [Google Scholar]
- Marzaioli V, Aguilar-Pimentel J, Weichenmeier I, Luxenhofer G, Wiemann M, Landsiedel R, Wohlleben W, Eiden S, Mempel M, Behrendt H. 2014. Surface modifications of silica nanoparticles are crucial for their inert versus pro-inflammatory and immunomodulatory properties. Int J Nanomed. 9:2815–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M, Nagata M, Nakamura K, Kawai M, Baba T, Yamaki K, Yoshino S. 2010. Adjuvant effect of zinc oxide on TH2 but not TH1 immune responses in mice. Immunopharmacol Immunotoxicol. 32:56–62. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Hirobe S, Okada N, Nakagawa S. 2016. Analysis of skin permeability and toxicological properties of amorphous silica particles. Biol Pharm Bull. 39:1201–1205. [DOI] [PubMed] [Google Scholar]
- Mattila L, Kilpelainen M, Terho E, Koskenvuo M, Helenius H, Kalimo K. 2001. Prevalence of nickel allergy among Finnish university students in 1995. Contact Derm. 44:218–223. [DOI] [PubMed] [Google Scholar]
- Matuszak J, Dörfler P, Zaloga J, Unterweger H, Lyer S, Dietel B, Alexiou C, Cicha I. 2015. Shell matters: Magnetic targeting of SPIONs and in vitro effects on endothelial and monocytic cell function. Clin Hemorheol Microcirc. 61:259–277. [DOI] [PubMed] [Google Scholar]
- Maurer-Jones M, Lin Y, Haynes C. 2010. Functional assessment of metal oxide nanoparticle toxicity in immune cells. ACS Nano. 4:3363–3373. [DOI] [PubMed] [Google Scholar]
- Mauro M, Crosera M, Bianco C, Adami G, Montini T, Fornasiero P, Jaganjac M, Bovenzi M, Filon F. 2015. Permeation of platinum and rhodium nanoparticles through intact and damaged human skin. J Nanopart Res 17:253. [Google Scholar]
- McConnachie L, White C, Botta D, Zadworny M, Cox D, Beyer R, Hu X, Eaton D, Gao X, Kavanagh T. 2013. Heme oxygenase expression as a bio-marker of exposure to amphiphilic polymer-coated CdSe/ZnS quantum dots. Nanotoxicology. 7:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A, Mack D, Crawford F, Fontenot A. 2015. MyD88 dependence of beryllium-induced dendritic cell trafficking and CD4+ T-cell priming. Mucosal Immunol. 8:1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum K, Robertson S, Römer I, Marczylo T, Dean L, Rogers A, Gant T, Smith R, Tetley T, Leonard M. 2018. Cerium dioxide nanoparticles exacerbate house dust mite-induced Type II airway inflammation. Particle Fibre Toxicol. 15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Wang L, Battelli LA, Antonini JM, Roberts JR, Qian Y, Sisler JD, Castranova V, Porter DW, et al. 2018. The fate of inhaled nanoparticles: Detection and measurement by enhanced dark-field microscopy. Toxicol Pathol. 46:28–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel-Jeanjean C, Crépel F, Raufast V, Payre B, Datas L, Bessou-Touya S, Duplan H. 2012. Penetration study of formulated nanosized titanium dioxide in models of damaged and sun-irradiated skins. Photochem Photobiol. 88:1513–1521. [DOI] [PubMed] [Google Scholar]
- Mishra V, Baranwal V, Mishra R, Sharma S, Paul B, Pandey A. 2016. Titanium dioxide nanoparticles augment allergic airway inflammation and Socs3 expression via NF-κB pathway in murine model of asthma. Biomaterials. 92:90–102. [DOI] [PubMed] [Google Scholar]
- Miyazawa M, Takashima A. 2012. Development and validation of a new in vitro assay designed to measure contact allergen-triggered oxidative stress in dendritic cells. J Dermatol Sci. 68:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuashi M, Ohtani T, Nakagawa S, Aiba S. 2005. Redox imbalance induced by contact sensitizers triggers the maturation of dendritic cells. J Invest Dermatol. 124:579–586. [DOI] [PubMed] [Google Scholar]
- Moghimi S, Parhamifar L, Ahmadvand D, Wibroe P, Andresen T, Farhangrazi Z, Hunter A. 2012. Particulate systems for targeting of macrophages: Basic and therapeutic concepts. J Innate Immun. 4:509–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed B, Verma N, Prina-Mello A, Williams Y, Davies AM, Bakos G, Tormey L, Edwards C, Hanrahan J, Salvati A, et al. 2011. Activation of stress-related signalling pathway in human cells upon SiO2 nanoparticles exposure as early indicator of cytotoxicity. J Nanobiotechnol. 9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadinejad R, Moosavi M, Tavakol S, Vardar D, Hosseini A, Rahmati M, Dini L, Hussain S, Mandegary A, Klionsky D. 2019. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy. 15: 4–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanan P, Geetha C, Syama S, Varma H. 2014. Interfacing of dextran coated ferrite nano-materials with cellular system and delayed hypersensitivity on guinea pigs. Coll Surf B 116:633–642. [DOI] [PubMed] [Google Scholar]
- Moldoveanu B, Otmishi P, Jani P, Walker J, Sarmiento X, Guardiola J, Saad M, Yu J. 2009. Inflammatory mechanisms in the lung. J Inflamm Res. 2: 1–11. [PMC free article] [PubMed] [Google Scholar]
- Monprasit P, Lawanprasert S, Maniratanachote R. 2018. Effects of silver, silver-copper and silver-tin nanoparticles in THP-1 cells. Thai J Pharm Sci. 42:59–63. [Google Scholar]
- Monteiro-Riviere N, Wiench K, Landsiedel R, Schulte S, Inman A, Riviere J. 2011. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanopar-ticles in UVB-sunburned skin: An in vitro and in vivo study. Toxicol Sci. 123:264–280. [DOI] [PubMed] [Google Scholar]
- Moron G, Dadaglio G, Leclerc C. 2004. New tools for antigen delivery to theMHC Class I pathway. Trends Immunol. 25:92–97. [DOI] [PubMed] [Google Scholar]
- Mortensen L, Faulknor R, Ravichandran S, Zheng H, DeLouise L. 2015. UVB dependence of quantum dot reactive oxygen species generation in common skin cell models. J Biomed Nanotechnol. 11:1644–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen L, Jatana S, Gelein R, de Benedetto A, de Mesy Bentley K, Beck L, Elder A, DeLouise L. 2013. Quantification of quantum dot murine skin penetration with UVR barrier impairment. Nanotoxicology. 7:1386–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen LJ, Oberdörster G, Pentland AP, Delouise LA. 2008. In vivo skin penetration of quantum dot nanoparticles in the murine model: The effect of UVR. Nano Lett. 8:2779–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou Y, Xing Y, Ren H, Cui Z, Zhang Y, Yu G, Urba WJ, Hu Q, Hu H. 2017. Effect of super-paramagnetic iron oxide nanoparticle surface charge on antigen cross-presentation. Nanoscale Res Lett. 12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulon C, Vollmer J, Weltzien H. 1995. Characterization of processing requirements and metal cross-reactivities in T cell clones from patients with allergic contact dermatitis to nickel. Eur J Immunol. 25:3308–3315. [DOI] [PubMed] [Google Scholar]
- Moyano D, Goldsmith M, Solfiell D, Landesman-Milo D, Miranda O, Peer D, Rotello V. 2012. Nanoparticle hydrophobicity dictates immune response. J Am Chem Soc. 134:3965–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Prozeller D, Ghazaryan A, Kokkinopoulou M, Mailander V, Morsbach S, Landfester K. 2018. Beyond the protein corona - lipids matter for biological response of nanocarriers. Acta Biomater. 71:420–431. [DOI] [PubMed] [Google Scholar]
- Murdoch RD, Pepys J, Hughes E. 1986. IgE antibody responses to platinum group metals: A large scale refinery survey. Br J Ind Med. 43:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia X, Pawelzyk P, Schaefer U, Wagner C, Willenbacher N, Lehr C. 2016. Size-limited penetration of nanoparticles into porcine respiratory mucus after aerosol deposition. Bio-macromolecules. 17:1536–1542. [DOI] [PubMed] [Google Scholar]
- Murray AR, Kisin E, Inman A, Young S-H, Muhammed M, Burks T, Uheida A, Tkach A, Waltz M, Castranova V, et al. 2013. Oxidative stress and dermal toxicity of iron oxide nanoparticles in vitro. Cell Biochem Biophys. 67:461–476. [DOI] [PubMed] [Google Scholar]
- Musk A, Tees J. 1982. Asthma caused by occupational exposure to vanadium compounds. Med J Aust. 1:183–184. [DOI] [PubMed] [Google Scholar]
- Nabeshi H, Yoshikawa T, Matsuyama K, Nakazato Y, Arimori A, Isobe M, Tochigi S, Kondoh S, Hirai T, Akase T, et al. 2010. Size-dependent cytotoxic effects of amorphous silica nanoparticles on Langerhans cells. Pharmazie. 65:199–201. [PubMed] [Google Scholar]
- Naess EM, Hofgaard A, Skaug V, Gulbrandsen M, Danielsen TE, Grahnstedt S, Skogstad A, Holm J–ø. 2016. Titanium dioxide nanoparticles in sun-screen penetrate the skin into viable layers of the epidermis: A clinical approach. Photodermatol Photoimmunol Photomed. 32:48–51. [DOI] [PubMed] [Google Scholar]
- Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY, Ueha S, Horiuchi K, Tanizaki H, Kabashima K, et al. 2012. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol. 13:744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim J, van Oss C, Wu W, Giese R, Nickerson P. 1997. Mechanisms of adjuvancy: I-Metal oxides as adjuvants. Vaccine 15:1183–1193. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Moroi Y, Uchi H, Furue M. 2006. Differential role of MAPK signaling in human dendritic cell maturation and TH1/TH2 engagement. J Dermatol Sci. 42:1–11. [DOI] [PubMed] [Google Scholar]
- Nanomaterials Future Markets. 2015. Metal & Metal Oxide: Forecast from 2010 to 2025. Future Markets, Inc. [Google Scholar]
- Napierska D, Rabolli V, Thomassen LCJ, Dinsdale D, Princen C, Gonzalez L, Poels KLC, Kirsch-Volders M, Lison D, Martens JA, et al. 2012. Oxidative stress induced by pure and iron-doped amorphous silica nanoparticles in subtoxic conditions. Chem Res Toxicol. 25:828–837. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH). 2011. Occupational Exposure to Titanium Dioxide. Atlanta, GA: NIOSH. [Google Scholar]
- Natsch A, Emter R. 2008. Skin sensitizers induce antioxidant response element dependent genes: Application to the in vitro testing of the sensitization potential of chemicals. Toxicol Sci. 102:110–119. [DOI] [PubMed] [Google Scholar]
- Natsch A, Emter R. 2016. Nrf2 activation as a key event triggered by skin sensitisers: The development of the stable KeratinoSens reporter gene assay. Altern Lab Anim. 44:443–451. [DOI] [PubMed] [Google Scholar]
- Neagu M, Piperigkou Z, Karamanou K, Engin A, Docea A, Constantin C, Negrei C, Nikitovic D, Tsatsakis A. 2017. Protein bio-corona: Critical issue in immune nanotoxicology. Arch Toxicol. 91:1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto L, Zufelato N, de Sousa-Junior A, Trentini M, da Costa A, Bakuzis A, Kipnis A, Junqueira-Kipnis A. 2018. Specific T-cell induction using iron oxide based nanoparticles as subunit vaccine adjuvant. Human Vaccin Immunother. 18:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer N, Palomaeki J, Karisola P, Alenius H, Kasper G. 2015. Size-dependent ROS produc-tion by palladium and nickel nanoparticles in cellular and acellular environments. An indication for the catalytic nature of their interactions. Nanotoxicology. 9:1059–1066. [DOI] [PubMed] [Google Scholar]
- Nohynek GJ, Lademann J, Ribaud C, Roberts MS. 2007. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol. 37:251–277. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Nakada T, Iijima M, Maibach H. 2011. Metal patch test results from 1990–2009. J Dermatol. 38:267–271. [DOI] [PubMed] [Google Scholar]
- Nukada Y, Ashikaga T, Miyazawa M, Hirota M, Sakaguchi H, Sasa H, Nishiyama N. 2012. Prediction of skin sensitization potency of chemicals by human Cell Line Activation Test (h-CLAT) and an attempt at classifying skin sensitization potency. Toxicol In Vitro. 26:1150–1160. [DOI] [PubMed] [Google Scholar]
- Nygaard U, Samuelsen M, Aase A, Løvik M. 2004. The capacity of particles to increase allergic sensitization is predicted by particle number and surface area, not by particle mass. Toxicol Sci. 82:515–524. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Ferin J, Morrow P. 1992. Volumetric loading of alveolar macrophages (AM): A possible basis for diminished AM-mediated particle clearance. Exp Lung Res. 18:87–104. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J. 2005. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 113:823–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [OECD] (Organization for Economic Cooperation and Development). 2014Adverse Outcome Pathway for Skin Sensitisation Initiated by Covalent Binding to Proteins. Part 1: Scientific evidence. OECD Environment, Health and Safety Publications Series on Testing and Assessment 168 (2012):1–59. [Google Scholar]
- Oh W, Kim S, Choi M, Kim C, Jeong Y, Cho B, Hahn J, Jang J. 2010. Cellular uptake, cyto-toxicity, and innate immune response of silica-titania hollow nanoparticles based on size and surface functionality. ACS Nano. 4:5301–5313. [DOI] [PubMed] [Google Scholar]
- Omlor AJ, Le DD, Schlicker J, Hannig M, Ewen R, Heck S, Herr C, Kraegeloh A, Hein C, Kautenburger R, et al. 2017. Local effects on airway inflammation and systemic uptake of 5-nm PEGylated and citrated gold nanoparticles in asthmatic mice. Small. 13:1603070. [DOI] [PubMed] [Google Scholar]
- Orlowski P, Tomaszewska E, Ranoszek-Soliwoda K, Gniadek M, Labedz O, Malewski T, Nowakowska J, Chodaczek G, Celichowski G, Grobelny J, et al. 2018. Tannic acid-modified silver and gold nanoparticles as novel stimulators of dendritic cells activation. Front Immunol. 9:1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega VA, Ede JD, Boyle D, Stafford JL, Goss GG. 2015. Polymer-coated metal-oxide nanoparticles inhibit IgE receptor binding, cellular signaling, and degranulation in a mast cell-like cell line. Advanced Sci 2:1500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega R, Bresson C, Darolles C, Gautier C, Roudeau S, Perrin L, Janin M, Floriani M, Aloin V, Carmona A, et al. 2014. Low-solubility particles and a Trojanhorse-type mechanism of toxicity: The case of cobalt oxide on human lung cells. Particle Fibre Toxicol. 11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond-McLeod M, Oytam Y, Kirby J, Gomez-Fernandez L, Baxter B, McCall M. 2014. Dermal absorption and short-term biological impact in hairless mice from sunscreens containing zinc oxide nano- or larger particles. Nanotoxicology. 8:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski A, Nordmeyer D, Mundhenk L, Fluhr J, Lademann J, Graf C,Rühl E, Gruber A. 2014. AHAPS-functionalized silica nanoparticles do not modulate allergic contact dermatitis in mice. Nanoscale Res Lett. 9:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otberg N, Richter H, Schaefer H, Blume-Peytavi U, Sterry W, Lademann J. 2004. Variations of hair follicle size and distribution in different body sites. J Invest Dermatol. 122:14–19. [DOI] [PubMed] [Google Scholar]
- Pal A, Alam S, Chauhan L, Saxena P, Kumar M, Ansari G, Singh D, Ansari K. 2016a. UVB exposure enhanced the dermal penetration of zinc oxide nanoparticles and induced inflammatory responses through oxidative stress mediated by MAPKs and NF-κB signaling in SKH-1 hairless mouse skin. Toxicol Res. 5:1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Alam S, Mittal S, Arjaria N, Shankar J, Kumar M, Singh D, Pandey A, Ansari K. 2016b. UVB irradiation-enhanced zinc oxide nanoparticles-induced DNA damage and cell death in mouse skin. Mutat Res. 807: 15–24. [DOI] [PubMed] [Google Scholar]
- Palomäki J, Karisola P, Pylkkänen L, Savolainen K, Alenius H. 2010. Engineered nanomaterials cause cytotoxicity and activation on mouse antigen-presenting cells. Toxicology. 267:125–131. [DOI] [PubMed] [Google Scholar]
- Pannerselvam B, Dharmalingam Jothinathan M, Rajenderan M, Perumal P, Pudupalayam Thangavelu K, Kim H, Singh V, Rangarajulu S. 2017. An in vitro study on the burn wound healing activity of cotton fabrics incorporated with phytosynthesized silver nanoparticles in male Wistar albino rats. Eur J Pharm Sci. 100:187–196. [DOI] [PubMed] [Google Scholar]
- Park E, Bae E, Yi J, Kim Y, Choi K, Lee S, Yoon J, Lee B, Park K. 2010a. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ Toxicol Pharmacol. 30: 162–168. [DOI] [PubMed] [Google Scholar]
- Park Y, Jeong S, Yi S, Choi B, Kim Y, Kim I, Kim M, Son S. 2011. Analysis for the potential of polystyrene and TiO2 nanoparticles to induce skin irritation, phototoxicity, and sensitization. Toxicol In Vitro. 25:1863–1869. [DOI] [PubMed] [Google Scholar]
- Park E, Kim H, Kim Y, Park K. 2010b. Intratracheal instillation of platinum nanoparticles may induce inflammatory responses in mice. Arch Pharm Res. 33:727–735. [DOI] [PubMed] [Google Scholar]
- Park E, Kim H, Kim Y, Yi J, Choi K, Park K. 2010c. Inflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in mice. Toxicology. 275:65–71. [DOI] [PubMed] [Google Scholar]
- Park S, Lee Y, Jung M, Kim K, Chung N, Ahn E, Lim Y, Lee K. 2007. Cellular toxicity of various inhalable metal nanoparticles on human alveolar epithelial cells. Inhal Toxicol. 19:59–65. [DOI] [PubMed] [Google Scholar]
- Park J, Lee I, Shin N, Jeon C, Kwon O, Ko J, Kim J, Oh S, Shin I, Ahn K. 2016. Copper oxide nanoparticles aggravate airway inflammation and mucus production in asthmatic mice via MAPK signaling. Nanotoxicology. 10:445–452. [DOI] [PubMed] [Google Scholar]
- Park E, Yi J, Chung K, Ryu D, Choi J, Park K. 2008. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol Lett. 180:222–229. [DOI] [PubMed] [Google Scholar]
- Park E-J, Park Y-K, Park K-S. 2009. Acute toxicity and tissue distribution of cerium oxide nanoparticles by a single oral administration in rats. Toxicological Research. 25(2):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Yoon J, Choi K, Yi J, Park K. 2009. Induction of chronic inflammation in mice treated with titanium dioxide nanoparticles by intratracheal instillation. Toxicology. 260:37–46. [DOI] [PubMed] [Google Scholar]
- Park H, Kim K, Jang S, Park J, Cha H, Lee J, Kim J, Kim S, Lee C, Kim J. 2010. Attenuation of allergic airway inflammation and hyper-responsiveness in a murine model of asthma by silver nanoparticles. Int J Nanomed. 5:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Sohn J-H, Kim Y-J, Park YH, Han H, Park KH, Lee K, Choi H, Um K, Choi I-H, et al. 2015. Acute exposure to silica nanoparticles aggravate airway inflammation: Different effects according to surface characteristics. Exp Mol Med 47:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzelt A, Richter H, Knorr F, Schäfer U, Lehr C, Dähne L, Sterry W, Lademann J. 2011. Selective follicular targeting by modification of the particle sizes. J Control Rel. 150:45–48. [DOI] [PubMed] [Google Scholar]
- Pawankar R 2014. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organiz J. 7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Goldeck D, Derhovanessian E. 2014. Inflammation, ageing and chronic disease. Curr Opin Immunol. 29:23–28. [DOI] [PubMed] [Google Scholar]
- Peira E, Turci F, Corazzari I, Chirio D, Battaglia L, Fubini B, Gallarate M. 2014. The influence of surface charge and photo-reactivity on skin-permeation enhancer property of nano-TiO2 in ex vivo pig skin model under indoor light. Int J Pharm. 467:90–99. [DOI] [PubMed] [Google Scholar]
- Peiser M, Tralau T, Heidler J, Api A, Arts J, Basketter D, English J, Diepgen T, Fuhlbrigge R, Gaspari A, et al. 2012. Allergic contact dermatitis: Epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Current knowledge assembled at an international workshop at BfR, Germany. Cell Mol Life Sci. 69:763–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin E, Goutet M, Ban M. 2007. Murine bone marrow-derived dendritic cells as a potential in vitro model for predictive identification of chemical sensitizers. Toxicol Lett. 175:89–101. [DOI] [PubMed] [Google Scholar]
- Pepys J, Parish W, Cromwell O, Hughes E. 1979. Specific IgE and IgG antibodies to platinum salts in sensitized workers. Monogr Allergy. 14:142. [PubMed] [Google Scholar]
- Petersen E, Reipa V, Watson S, Stanley D, Rabb S, Nelson B. 2014. DNA damaging potential of photoactivated P25 titanium dioxide nanoparticles. Chem Res Toxicol. 27:1877–1884. [DOI] [PubMed] [Google Scholar]
- Petrarca C, Clemente E, di Giampaolo L, Mariani-Costantini R, Leopold K, Schindl R, Lotti L, Mangifesta R, Sabbioni E, Niu Q, et al. 2014. Palladium nanoparticles induce disturbances in cell cycle entry and progression of peripheral blood mononuclear cells: Paramount role of ions. J Immunol Res. 2014:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrarca C, Perrone A, Verna N, Verginelli F, Ponti J, Sabbioni E, di Giampaolo L, Dadorante V, Schiavone C, Boscolo P, et al. 2006. Cobalt nanoparticles modulate cytokine in vitro release by human mononuclear cells mimicking autoimmune disease. Int J Immunopathol Pharmacol. 19: 11–14. [PubMed] [Google Scholar]
- Petsonk E 2002. Work-related asthma and implications for the general public. Environ Health Perspect. 110:569–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettibone J, Adamcakova-Dodd A, Thorne P, O’Shaughnessy P, Weydert J, Grassian V. 2008. Inflammatory response of mice following inhalation exposure to iron and copper nanoparticles. Nanotoxicology. 2:189–204. [Google Scholar]
- Pflücker F, Hohenberg H, Hölzle E, Will T, Pfeiffer S, Wepf R, Diembeck W, Wenck H, Gers-Barlag H. 1999. The outermost stratum corneum layer is an effective barrier against dermal uptake of topically applied micronized titanium dioxide. Int J Cosmetic Sci. 21:399–411. [DOI] [PubMed] [Google Scholar]
- Piasecka-Zelga J, Zelga P, Górnicz M, Strzelczyk P, Sójka-Ledakowicz J. 2015. Acute dermal toxicity and sensitization studies of novel nano-enhanced UV absorbers. J Occup Health. 57:275–284. [DOI] [PubMed] [Google Scholar]
- Pistoor F, Kapsenberg M, Bos J, Meinardi M, von Blomberg M, Scheper R. 1995. Cross-reactivity of human nickel-reactive T-lymphocyte clones with copper and palladium. J Invest Dermatol. 105:92–95. [DOI] [PubMed] [Google Scholar]
- Pluut O, Bianco C, Jakasa I, Visser M, Krystek P, Larese-Filon F, Rustemeyer T, Kezic S. 2015. Percutaneous penetration of silver from a silver containing garment in healthy volunteers and patients with atopic dermatitis. Toxicol Lett. 235:116–122. [DOI] [PubMed] [Google Scholar]
- Poh T, Ali N, Mac Aogáin M, Kathawala M, Setyawati M, Ng K, Chotirmall S. 2018. Inhaled nanomaterials and the respiratory microbiome: Clinical, immunological and toxicological perspectives. Part Fibre Toxicol. 15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possa S, Leick E, Prado C, Martins M, Tibério I. 2013. Eosinophilic inflammation in allergic asthma. Front Pharmacol. 4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles LM, Wilson K, Xiang SD, Selomulya C, Plebanski M. 2017. New strategies in vaccine design for induction of CD8 T-cell responses using biodegradable iron oxide nanoparticles. J Immunol. 198:79.23. [Google Scholar]
- Prach M, Stone V, Proudfoot L. 2013. Zinc oxide nanoparticles and monocytes: Impact of size, charge and solubility on activation status. Toxicol Appl Pharmacol. 266:19–26. [DOI] [PubMed] [Google Scholar]
- Prow TW, Monteiro-Riviere NA, Inman AO, Grice JE, Chen X, Zhao X, Sanchez WH, Gierden A, Kendall MAF, Zvyagin AV, et al. 2012. Quantum dot penetration into viable human skin. Nanotoxicology. 6: 173–185. [DOI] [PubMed] [Google Scholar]
- Pusic K, Aguilar Z, McLoughlin J, Kobuch S, Xu H, Tsang M, Wang A, Hui G. 2013. Iron oxide nanoparticles as a clinically acceptable delivery platform for a recombinant blood-stage human malaria vaccine. Faseb J. 27: 1153–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabolli V, Badissi AA, Devosse R, Uwambayinema F, Yakoub Y, Palmai-Pallag M, Lebrun A, De Gussem V, Couillin I, Ryffel B, et al. 2014. The alarmin IL-1α is a master cytokine in acute lung inflammation induced by silica micro- and nanoparticles. Part Fibre Toxicol. 11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmawati D, Bontkes H, Verstege M, Muris J, von Blomberg B, Scheper R, van Hoogstraten I. 2013. Transition metal sensing by toll-like receptor-4: Next to nickel, cobalt and palladium are potent human dendritic cell stimulators. Contact Derm. 68:331–338. [DOI] [PubMed] [Google Scholar]
- Rachmawati D, Buskermolen J, Scheper R, Gibbs S, von Blomberg B, van Hoogstraten I. 2015. Dental metal-induced innate reactivity in keratinocytes. Toxicol In Vitro. 30:325–330. [DOI] [PubMed] [Google Scholar]
- Radauer-Preiml I, Andosch A, Hawranek T, Luetz-Meindl U, Wiederstein M, Horejs-Hoeck J, Himly M, Boyles M, Duschl A. 2016. Nanoparticle-allergen interactions mediate human allergic responses: Protein corona characterization and cellular responses. Particle Fibre Toxicol. 13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju G, Katiyar N, Vadukumpully S, Shankarappa S. 2018. Penetration of gold nanoparticles across the stratum corneum layer of thick-Skin. J Dermatol Sci. 89:146–154. [DOI] [PubMed] [Google Scholar]
- Ramirez T, Mehling A, Kolle S, Wruck C, Teubner W, Eltze T, Aumann A, Urbisch D, van Ravenzwaay B, Landsiedel R. 2014. LuSens: A keratinocyte based ARE reporter gene assay for use in integrated testing strategies for skin sensitization hazard identification. Toxicol In Vitro. 28:1482–1497. [DOI] [PubMed] [Google Scholar]
- Rancan F, Gao Q, Graf C, Troppens S, Hadam S, Hackbarth S, Kembuan C, Blume-Peytavi U, Rühl E, Lademann J, et al. 2012. Skin penetration and cellular uptake of amorphous silica nanoparticles with variable size, surface functionalization, and colloidal stability. ACS Nano. 6:6829–6842. [DOI] [PubMed] [Google Scholar]
- Rancan F, Nazemi B, Rautenberg S, Ryll M, Hadam S, Gao Q, Hackbarth S, Haag SF, Graf C, Rühl E, et al. 2014. Ultraviolet radiation- and nanoparticle-induced intracellular free radical gene-ration measured in human keratinocytes by electron paramagnetic resonance spectroscopy. Skin Res Technol. 20:182–193. [DOI] [PubMed] [Google Scholar]
- Rattis F, Concha M, Dalbiez-Gauthier C, Schmitt D, Péguet-Navarro J, Courtellemont P. 1998. Effects of ultraviolet B radiation on human Langerhans cells: Functional alteration of CD86 upregulation and induction of apoptotic cell death. J Invest Dermatol. 111:373–379. [DOI] [PubMed] [Google Scholar]
- Ravichandran S, Mortensen L, Delouise L. 2011. Quantification of human skin barrier function and susceptibility to quantum dot skin penetration. Nanotoxicology. 5:675–686. [DOI] [PubMed] [Google Scholar]
- Ravindran A, Dhas S, Chandrasekaran N, Mukherjee A. 2013. Differential interaction of silver nanoparticles with cysteine. J Exp Nanosci. 8:589–595. [Google Scholar]
- Reale M, Vianale G, Lotti L, Mariani-Costantini R, Perconti S, Cristaudo A,Leopold K, Anto-nucci A, di Giampaolo L, Iavicoli I, et al. 2011. Effects of Palladium nanoparticles on the cytokine release from peripheral blood mononuclear cells of palladium-sensitized women. J Occup Environ Med. 53:1054–1060. [DOI] [PubMed] [Google Scholar]
- Rice R, Vidrio E, Kumfer B, Qin Q, Willits N, Kennedy I, Anastasio C. 2009. Generation of oxidant response to copper and iron nanoparticles and salts: Stimulation by ascorbate. Chem Biol Interact. 181:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roco M 2011. The long view of nanotechnology development: The National Nanotechnology Initiative at 10 years. J Nanopart Res. 13:427–445. [Google Scholar]
- Romoser A, Chen P, Berg J, Seabury C, Ivanov I, Criscitiello M, Sayes CM. 2011. Quantum dots trigger immunomodulation of the NFjB pathway in human skin cells. Mol Immunol. 48:1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi E, Pylkkanen L, Koivisto AJ, Nykasenoja H, Wolff H, Savolainen K, Alenius H. 2010. Inhalation exposure to nanosized and fine TiO2 particles inhibits features of allergic asthma in a murine model. Particle Fibre Toxicol. 7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R, Kumar D, Sharma A, Gupta P, Chaudhari B, Tripathi A, Das M, Dwivedi P. 2014a. ZnO nanoparticles induced adjuvant effect via toll-like receptors and Src signaling in Balb/c mice. Toxicol Lett. 230:421–433. [DOI] [PubMed] [Google Scholar]
- Roy R, Kumar S, Verma AK, Sharma A, Chaudhari B, Tripathi A, Das M, Dwivedi P. 2014b. Zinc oxide nanoparticles provide an adjuvant effect to ovalbumin via a TH2 response in Balb/c mice. Int Immunol. 26:159–172. [DOI] [PubMed] [Google Scholar]
- Ruge C, Kirch J, Canadas O, Schneider M, Perez-Gil J, Schaefer U, Casals C, Lehr C. 2011. Uptake of nanoparticles by alveolar macrophages is triggered by surfactant protein A. Nanomedicine. 7:690–693. [DOI] [PubMed] [Google Scholar]
- Rustemeyer T, van Hoogstraten I, von Blomberg B, Scheper R. 2006. Mechanisms in allergic contact dermatitis In: Rustemeyer T, Elsner P, John S, Maibach H, editors. Kanerva’s Occupational Dermatology. Chapter 2. London: Springer; p. 11–43. [Google Scholar]
- Ryman-Rasmussen J, Riviere J, Monteiro-Riviere N. 2006. Penetration of intact skin by quantum dots with diverse physicochemical properties. Toxicol Sci. 91:159–165. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen J, Riviere J, Monteiro-Riviere N. 2007. Surface coatings determine cytotoxicity and irritation potential of quantum dot nanoparticles in epidermal keratinocytes. J Invest Dermatol. 127:143–153. [DOI] [PubMed] [Google Scholar]
- Sadrieh N, Wokovich AM, Gopee NV, Zheng J, Haines D, Parmiter D, Siitonen PH, Cozart CR, Patri AK, McNeil SE, et al. 2010. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol Sci. 115: 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager T, Wolfarth M, Keane M, Porter D, Castranova V, Holian A. 2016. Effects of nickel-oxide nanoparticle pre-exposure dispersion status on bio-activity in the mouse lung. Nanotoxicology. 10:151–161. [DOI] [PubMed] [Google Scholar]
- Samberg M, Oldenburg S, Monteiro-Riviere N. 2010. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ Health Perspect. 118:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet J, Cheng P. 1994. Role of airway mucus in pulmonary toxicology.Environ Health Perspect. 102:89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg W, Låg M, Holme J, Friede B, Gualtieri M, Kruszewski M, Schwarze P, Skuland T, Refsnes M. 2012. Comparison of non-crystalline silica nanoparticles in IL-1β release from macrophages. Particle Fibre Toxicol. 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandby-Moller J, Poulsen T, Wulf H. 2003. Epidermal thickness at different body sites: Relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm Venereol. 83:410–413. [DOI] [PubMed] [Google Scholar]
- Sasseville D 2008. Occupational contact dermatitis. Allergy Asthma ClinImmunol. 4:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura I, Lennon-Dumenil A, Seabra M, Raposo G, Amigorena S. 2006. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 126:205–218. [DOI] [PubMed] [Google Scholar]
- Schaeublin N, Braydich-Stolle L, Schrand A, Miller J, Hutchison J, Schlager J, Hussain S. 2011. Surface charge of gold nanoparticles mediates mechanism of toxicity. Nanoscale. 3:410–420. [DOI] [PubMed] [Google Scholar]
- Schanen B, Das S, Reilly C, Warren W, Self W, Seal S, Drake D. 2013. Immunomodulation and T helper TH1/TH2 response polarization by CeO2 and TiO2 nanoparticles. PloS One. 8:e62816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauberger E, Peinhaupt M, Cazares T, Lindsley A. 2016. Lipid mediators of allergic disease: Pathways, treatments, and emerging therapeutic targets. Curr Allergy Asthma Rep. 16:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumann F, Borm P, Herbrich A, Knoch J, Pitz M, Schins R, Luettig B, Hohlfeld J, Heinrich J, Krug N. 2004. Metal-rich ambient particles (PM2.5) cause airway inflammation in healthy subjects. Am J Respir Crit Care Med. 170:898–903. [DOI] [PubMed] [Google Scholar]
- Scherbart A, Langer J, Bushmelev A, van Berlo D, Haberzettl P, van Schooten F, Schmidt A, Rose C, Schins R, Albrecht C. 2011. Contrasting macrophage activation by fine and ultrafine titanium dioxide particles is associated with different uptake mechanisms. Part Fibre Toxicol. 8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleh C, Muhlfeld C, Pulskamp K, Schmiedl A, Nassimi M, Lauenstein H, Braun A, Krug N, Erpenbeck V, Hohlfeld J. 2009. The effect of titanium dioxide nanoparticles on pulmonary surfactant function and ultrastructure. Respir Res. 10:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid O, Stoeger T. 2016. Surface area is the biologically most effective dose metric for acute nanoparticle toxicity in the lung. J Aerosol Sci. 99: 133–143. [Google Scholar]
- Schmidt M, Goebeler M. 2015. Immunology of metal allergies. J DtschDermatol Ges. 13:653–659. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Raghavan B, Muller V, Vogl T, Fejer G, Tchaptchet S, Keck S, Kalis C, Nielsen P, Galanos C, et al. 2010. Crucial role for human toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol. 11:814–819. [DOI] [PubMed] [Google Scholar]
- Schneider B, Constant S, Patierno S, Jurjus R, Ceryak S. 2012. Exposure to particulate hexavalent chromium exacerbates allergic asthma pathology. Toxicol Appl Pharmacol. 259:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann D, Kubicka-Muranyi M, Mirtschewa J, Günther J, Kind P, Gleichmann E. 1990. Adverse immune reactions to gold. I. Chronic treatment with an Au (I) drug sensitizes mouse T-cells not to Au (I) but to Au (III) and induces autoantibody formation. J Immunol. 145:2132–2139. [PubMed] [Google Scholar]
- Schulte P, Geraci C, Murashov V, Kuempel E, Zumwalde R, Castranova V, Hoover M, Hodson L, Martinez K. 2014. Occupational safety and health criteria for responsible development of nanotechnology. J Nanopart Res. 16:2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte PA, Murashov V, Zumwalde R, Kuempel ED, Geraci CL. 2010. Occupational exposure limits for nanomaterials: State of the art. J Nanopart Res. 12:1971–1987. [Google Scholar]
- Schulz J, Hohenberg H, Pflucker F, Gartner E, Will T, Pfeiffer S, Wepf R, Wendel V, Gers-Barlag H, Wittern K. 2002. Distribution of sunscreens on skin. Adv Drug Deliv Rev. 54:S157–S163. [DOI] [PubMed] [Google Scholar]
- Schulze C, Schaefer U, Ruge C, Wohlleben W, Lehr C. 2011. Interaction of metal oxide nanoparticles with lung surfactant protein A. Eur J Pharm Biopharm. 77:376–383. [DOI] [PubMed] [Google Scholar]
- Schuster B, Suk J, Woodworth G, Hanes J. 2013. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials. 34: 3439–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz T 2005. Mechanisms of UV-induced immunosuppression. Keio JMed. 54:165–171. [DOI] [PubMed] [Google Scholar]
- Scoville D, Nolin J, Ogden H, An D, Afsharinejad Z, Johnson B, Bammler T, Gao X, Frevert C, Altemeier W, et al. 2019. Quantum dots and mouse strain influence house dust mite-induced allergic airway disease. Toxicol Appl Pharmacol. 368:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiffert J, Hussain F, Wiegman C, Li F, Bey L, Baker W, Porter A, Ryan M, Chang Y, Gow A, et al. 2015. Pulmonary toxicity of instilled silver nano-particles: Influence of size, coating and rat strain. PloS One. 10:e0119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seite S, Zucchi H, Moyal D, Tison S, Compan D, Christiaens F, Gueniche A, Fourtanier A. 2003. Alterations in human epidermal Langerhans cells by ultraviolet radiation: Quantitative and morphological study. Br J Dermatol. 148:291–299. [DOI] [PubMed] [Google Scholar]
- Senapati V, Jain A, Gupta G, Pandey A, Dhawan A. 2015. Chromium oxide nanoparticle-induced genotoxicity and p53-dependent apoptosis in human lung alveolar cells. J Appl Toxicol. 35:1179–1188. [DOI] [PubMed] [Google Scholar]
- Senzui M, Tamura T, Miura K, Ikarashi Y, Watanabe Y, Fujii M. 2010. Study on penetration of titanium dioxide (TiO(2)) nanoparticles into intact and damaged skin in vitro. J Toxicol Sci. 35:107–113. [DOI] [PubMed] [Google Scholar]
- Shahbazi M, Almeida P, Makila E, Kaasalainen M, Salonen J, Hirvonen J, Santos H. 2014. Augmented cellular trafficking and endosomal escape of porous silicon nanoparticles via zwitterionic bilayer polymer surface engineering. Biomaterials. 35:7488–7500. [DOI] [PubMed] [Google Scholar]
- Shahbazi M, Hamidi M, M€akil€a E, Zhang H, Almeida P, Kaasalainen M, Salonen J, Hirvonen J, Santos H. 2013. The mechanisms of surface chemistry effects of mesoporous silicon nanoparticles on immunotoxicity and biocompatibility. Biomaterials. 34:7776–7789. [DOI] [PubMed] [Google Scholar]
- Shang L, Nienhaus K, Nienhaus G. 2014. Engineered nanoparticles interacting with cells: Size matters. J Nanobiotechnol. 12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q, Hall C. 2016. Binding preferences of amino acids for gold nanoparticles: A molecular simulation study. Langmuir. 32:7888–7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Kodali V, Gaffrey M, Wang W, Minard K, Karin N, Teeguarden J, Thrall B. 2014. Iron oxide nanoparticle agglomeration influences dose rates and modulates oxidative stress-mediated dose-response profiles in vitro. Nanotoxicology. 8:663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Salisbury R, Maurer E, Hussain S, Sulentic C. 2013. Gold nano-particles induce transcriptional activity of NF-κB in a B-lymphocyte cell line. Nanoscale. 5:3747–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Ackerman A, Cody V, Giodini A, Hinson E, Cresswell P, Edelson R, Saltzman W, Hanlon D. 2006. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in bio-degradable nanoparticles. Immunology. 117:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Scaiano J, English AM. 2006. Zeolite encapsulation decreases TiO2- photosensitized ROS generation in cultured human skin fibroblasts. Photochem Photobiol. 82:5–12. [DOI] [PubMed] [Google Scholar]
- Shen T, Zhu W, Yang L, Liu L, Jin R, Duan J, Anderson J, Ai H. 2018. Lactosylated N-alkyl polyethylenimine-coated iron oxide nanoparticles induced autophagy in mouse dendritic cells. Regen Biomater. 5:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng L, Wang L, Sang X, Zhao X, Hong J, Cheng S, Yu X, Liu D, Xu B, Hu R, et al. 2014. Nano-sized titanium dioxide-induced splenic toxicity: A biological pathway explored using microarray technology. J Hazard Mater. 278:180–188. [DOI] [PubMed] [Google Scholar]
- Shen C, Liang H, Wang C, Liao M, Jan T. 2012. Iron oxide nanoparticles suppressed T-helper-1 cell-mediated immunity in a murine model of delayed-type hypersensitivity. Int J Nanomed. 7:2729–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Tenzer S, Storck W, Hobernik D, Raker VK, Fischer K, Decker S, Dzionek A, Krauth€auser S, Diken M, et al. 2018. Protein corona-mediated targeting of nanocarriers to B-cells allows redirection of allergic immune responses. J Allergy Clin Immunol. 142:1558–1570. [DOI] [PubMed] [Google Scholar]
- Shen C, Wang C, Liao M, Jan T. 2011. A single exposure to iron oxide nano-particles attenuates antigen-specific antibody production and T-cell reactivity in ovalbumin-sensitized BALB/c mice. Int J Nanomed. 6:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Yadav S, Wang F, Wang H. 2010. Endotoxin promotes adverse effects of amorphous silica nanoparticles on lung epithelial cells in vitro. J Toxicol Environ Health. 73:748–756. [DOI] [PubMed] [Google Scholar]
- Shim I, Choi K, Hirano S. 2017. Oxidative stress and cytotoxic effects of silver ion in mouse lung macrophages J774.1 cells. J Appl Toxicol. 37: 471–478. [DOI] [PubMed] [Google Scholar]
- Shin S, Ye M, Kim H, Kang H. 2007. The effects of nano-silver on the proliferation and cytokine expression by peripheral blood mononuclear cells. Int Immunopharmacol. 7:1813–1818. [DOI] [PubMed] [Google Scholar]
- Shirakawa T, Kusaka Y, Fujimura N, Goto S, Morimoto K. 1988. The existence of specific antibodies to cobalt in hard metal asthma. Clin Allergy. 18:451–460. [DOI] [PubMed] [Google Scholar]
- Shirakawa T, Kusaka Y, Fujimura N, Kato M, Heki S, Morimoto K. 1990. Hard metal asthma: Cross immunological and respiratory reactivity between cobalt and nickel? Thorax. 45:267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa T, Kusaka Y, Morimoto K. 1992. Specific IgE antibodies to nickel in workers with known reactivity to cobalt. Clin Exp Allergy. 22:213–218. [DOI] [PubMed] [Google Scholar]
- Shokri N, Javar H. 2015. Comparison of calcium phosphate and zinc oxide nanoparticles as dermal penetration enhancers for albumin. Indian J Pharm Sci. 77:694. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shukla R, Sharma V, Pandey A, Singh S, Sultana S, Dhawan A. 2011. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol In Vitro. 25:231–241. [DOI] [PubMed] [Google Scholar]
- Simon-Vazquez R, Lozano-Fernandez T, Davila-Grana A, Gonzalez-Fernandez A. 2016. Metal oxide nanoparticles interact with immune cells and activate different cellular responses. Int J Nanomed. 11:4657–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, O’Hagan D. 2003. Recent advances in veterinary vaccine adjuvants.Int J Parasitol. 33:469–478. [DOI] [PubMed] [Google Scholar]
- Siriwardana K, Wang A, Gadogbe M, Collier W, Fitzkee N, Zhang D. 2015. Studying the effects of cysteine residues on protein interactions with silver nanoparticles. J Phys Chem C 119:2910–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smijs T, Pavel S. 2011. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol Sci Appl. 4:95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Pease C, White I, Basketter D. 2002. Skin as a route of exposure to protein allergens. Clin Exp Dermatol. 27:296–300. [DOI] [PubMed] [Google Scholar]
- Smulders S, Golanski L, Smolders E, Vanoirbeek J, Hoet P. 2015. Nano-TiO2 modulates the dermal sensitization potency of dinitrochlorobenzene after topical exposure. Br J Dermatol. 172:392–399. [DOI] [PubMed] [Google Scholar]
- Smulders S, Kaiser J, Zuin S, van Landuyt K, Golanski L, Vanoirbeek J, Wick P, Hoet P. 2012. Contamination of nanoparticles by endotoxin: Evaluation of different test methods. Part Fibre Toxicol. 9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonavane G, Tomoda K, Sano A, Ohshima H, Terada H, Makino K. 2008. In vitro permeation of gold nanoparticles through rat skin and rat intestine: Effect of particle size. Colloids Surf B Biointerf. 65:1–10. [DOI] [PubMed] [Google Scholar]
- Su C, Chen T, Chang C, Chuang K, Wu C, Liu W, Ho K, Lee K, Ho S, Tseng H, et al. 2013. Comparative proteomics of inhaled silver nanoparticles in healthy and allergen-provoked mice. Int J Nanomed. 8: 2783–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Yan Y, Zhao Y, Guo F, Jiang C. 2012. Copper oxide nanoparticles induce autophagic cell death in A549 cells. PloS One. 7:e43442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungsuwan S, Yin Z, Huang X. 2015. Lipopeptide-coated iron oxide nanoparticles as potential glycoconjugate-based synthetic anti-cancer vaccines. ACS Appl Mater Interfaces. 7:17535–17544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szikszai Z, Kertész Z, Bodnár E, Borbíró I, Angyal A, Csedreki L, Furu E, Szoboszlai Z, Kiss Á, Hunyadi J. 2011. Nuclear microprobe investigation of the penetration of ultrafine zinc oxide into human skin affected by atopic dermatitis. Nuclear Instr Meth Physics Res Section B. 269: 2278–2280. [Google Scholar]
- Tak Y, Pal S, Naoghare P, Rangasamy S, Song J. 2015. Shape-dependent skin penetration of silver nanoparticles: Does it really matter? Sci Rep. 5:16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Commens C, Burnett L, Snitch P. 1996. A pilot study on the percutaneous absorption of microfine titanium dioxide from sunscreens. Aus J Dermatol. 37:185–187. [DOI] [PubMed] [Google Scholar]
- Tang C, Inman M, van Rooijen N, Yang P, Shen H, Matsumoto K, O’Byrne P. 2001. TH Type 1-stimulating activity of lung macrophages inhibits TH2-mediated allergic airway inflammation by an IFNc-dependent mechanism. J Immunol. 166:1471–1481. [DOI] [PubMed] [Google Scholar]
- Taylor U, Klein S, Petersen S, Kues W, Barcikowski S, Rath D. 2010. Nonendosomal cellular uptake of ligand-free, positively charged gold nanoparticles. Cytometry A. 77:439–446. [DOI] [PubMed] [Google Scholar]
- Templeton D 2015. Speciation in metal toxicity and metal-based therapeutics. Toxics. 3:170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele L, Merkle H, Walter E. 2003. Phagocytosis and phagosomal fate of surface-modified microparticles in dendritic cells and macrophages. Pharm Res. 20:221–228. [DOI] [PubMed] [Google Scholar]
- Thierse H, Gamerdinger K, Junkes C, Guerreiro N, Weltzien H. 2005. T cell receptor (TCR) interaction with haptens: Metal ions as non-classical haptens. Toxicology. 209:101–107. [DOI] [PubMed] [Google Scholar]
- Thyssen J, Menne T. 2010. Metal allergy-a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 23: 309–318. [DOI] [PubMed] [Google Scholar]
- Tiano L, Armeni T, Venditti E, Barucca G, Mincarelli L, Damiani E. 2010. Modified TiO2 particles differentially affect human skin fibroblasts exposed to UVA light. Free Rad Biol Med. 49:408–415. [DOI] [PubMed] [Google Scholar]
- Toda T, Yoshino S. 2016. Enhancement of OVA-specific TH1, TH2, and TH17 immune responses by amorphous silica nanoparticles. Int J Immunopathol Pharmacol. 29:408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews G, Bergstresser P, Streilein J. 1980. Epidermal langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 124:445–453. [PubMed] [Google Scholar]
- Toll R, Jacobi U, Richter H, Lademann J, Schaefer H, Blume-Peytavi U. 2004. Penetration profile of microspheres in follicular targeting of terminal hair follicles. J Invest Dermatol. 123:168–176. [DOI] [PubMed] [Google Scholar]
- Tomić S, Dokić J, Vasilijić S, Ogrinc N, Rudolf R, Pelicon P, Vučević D, Milosavljević P, Janković S, An zel I, et al. 2014. Size-dependent effects of gold nanoparticles uptake on maturation and antitumor functions of human dendritic cells in vitro. PLoS One. 9:e96584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tončić RJ, Lipozenčić J, Martinac I, Gregurić S. 2011. Immunology of allergic contact dermatitis. Acta Dermatovenerol Croat. 19:51–68. [PubMed] [Google Scholar]
- Try C, Moulari B, B eduneau A, Fantini O, Pin D, Pellequer Y, Lamprecht A. 2016. Size dependent skin penetration of nanoparticles in murine and porcine dermatitis models. Eur J Pharm Biopharm. 100:101–108. [DOI] [PubMed] [Google Scholar]
- Tsuji G, Hashimoto-Hachiya A, Takemura M, Kanemaru T, Ichihashi M, Furue M. 2017. Palladium and platinum nanoparticles Activate AHR and NRF2 in Human Keratinocytes-Implications in Vitiligo Therapy. J Invest Dermatol. 137:1582. [DOI] [PubMed] [Google Scholar]
- Tulve N, Stefaniak A, Vance M, Rogers K, Mwilu S, LeBouf R, Schwegler-Berry D, Willis R, Thomas T, Marr L. 2015. Characterization of silver nanoparticles in selected consumer products and its relevance for predicting children’s potential exposures. Int J Hyg Environ Health. 218:345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomela S, Autio R, Buerki-Thurnherr T, Arslan O, Kunzmann A, Andersson-Willman B, Wick P, Mathur S, Scheynius A, Krug H, et al. 2013. Gene expression profiling of immune-competent human cells exposed to engineered zinc oxide or titanium dioxide nanoparticles. PLoS One. 8:e68415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turci F, Peira E, Corazzari I, Fenoglio I, Trotta M, Fubini B. 2013. Crystalline phase modulates the potency of nanometric TiO2 to adhere to and perturb the stratum corneum of porcine skin under indoor light. Chem Res Toxicol. 26:1579–1590. [DOI] [PubMed] [Google Scholar]
- Turčić P, Marinović Kulišić S, Lipozenčić J. 2013. Patch test reactions to metal salts in patients with different types of dermatitis. Acta Dermatovenerol Croatica. 21:180–180. [PubMed] [Google Scholar]
- Tuschl H, Kovac R, Weber E. 2000. The expression of surface markers on dendritic cells as indicators for the sensitizing potential of chemicals. Toxicol In Vitro. 14:541–549. [DOI] [PubMed] [Google Scholar]
- Val S, Hussain S, Boland S, Hamel R, Baeza-Squiban A, Marano F. 2009. Carbon black and titanium dioxide nanoparticles induce pro-inflammatory responses in bronchial epithelial cells: Need for multi-parametric evaluation due to adsorption artifacts. Inhal Toxicol. 21:115–122. [DOI] [PubMed] [Google Scholar]
- Vallhov H, Kupferschmidt N, Gabrielsson S, Paulie S, Strømme M, Garcia-Bennett A, Scheynius A. 2012. Adjuvant properties of mesoporous silica particles tune the development of effector T cells. Small. 8:2116–2124. [DOI] [PubMed] [Google Scholar]
- Vamanu C, Høl P, Allouni Z, Elsayed S, Gjerdet N. 2008. Formation of potential titanium antigens based on protein binding to titanium dioxide nanoparticles. Int J Nanomed. 3:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doren E, De Temmerman P, Francisco M, Mast J. 2011. Determination of volume-specific surface area by using transmission electron tomography for characterization and definition of nanomaterials. J Nanobiotechnol. 9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance M, Kuiken T, Vejerano E, McGinnis S, Hochella M, Rejeski D, Hull M. 2015. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol. 6:1769–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebriel R, Vermeulen J, van Engelen L, de Jong B, Verhagen L, de la Fonteyne-Blankestijn L, Hoonakker M, de Jong W. 2018. The crystal structure of titanium dioxide nanoparticles influences immune activity in vitro and in vivo. Part Fibre Toxicol. 15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemula P, Anderson R, Karp J. 2011. Nanoparticles reduce nickel allergy by capturing metal ions. Nat Nanotechnol. 6:291–295. [DOI] [PubMed] [Google Scholar]
- Vennemann A, Alessandrini F, Wiemann M. 2017. Differential effects of surface-functionalized zirconium oxide nanoparticles on alveolar macrophages, rat lung, and a mouse allergy model. Nanomaterials. 7:pii E280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraelen S, Bloemen K, Nelissen I, Witters H, Schoeters G, van Den Heuvel R. 2008. Cell types involved in allergic asthma and their use in in vitro models to assess respiratory sensitization. Toxicol In Vitro. 22: 1419–1431. [DOI] [PubMed] [Google Scholar]
- Vogt A, Combadiere B, Hadam S, Stieler K, Lademann J, Schaefer H, Autran B, Sterry W, Blume-Peytavi U. 2006. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J Invest Dermatol. 126:1316–1322. [DOI] [PubMed] [Google Scholar]
- von Garnier C, Filgueira L, Wikstrom M, Smith M, Thomas J, Strickland D, Holt P, Stumbles P. 2005. Anatomical location determines the distribution and function of dendritic cells and other apcs in the respiratory tract. J Immunol. 175:1609–1618. [DOI] [PubMed] [Google Scholar]
- Wagner A, Bleckmann C, Murdock R, Schrand A, Schlager J, Hussain S. 2007. Cellular inter-action of different forms of aluminum nanoparticles in rat alveolar macrophages. J Phys Chem B. 111:7353–7359. [DOI] [PubMed] [Google Scholar]
- Walczak-Drzewiecka A, Wyczólkowska J, Dastych J. 2003. Environmentally-relevant metal and transition metal ions enhance FcεRI-mediated mast cell activation. Environ. Health Perspect. 111:708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Kishore U, Lim B, Strong P, Reid K. 1996. Interaction of human lung surfactant proteins-A and -D with mite (dermatophagoides pteronyssinus) allergen. Clin Exp Immunol. 106:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shiao J, Chen C, Lee Y, Guo Y. 2007. Tumour necrotizing factor-alpha promoter and GST-T1 genotype predict skin allergy to chromate in cement workers in Taiwan. Contact Derm. 57:309–315. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang S, Xia Q, He W, Yin J, Fu P, Li J. 2013. Phototoxicity of zinc oxide nanoparticles in HACAT keratinocytes-generation of oxidative DNA damage during UVA and visible light irradiation. J Nanosci Nanotechnol. 13:3880–3888. [DOI] [PubMed] [Google Scholar]
- Warner S, Knight D. 2008. Airway modeling and remodeling in the pathogenesis of asthma. Curr Opin Allergy Clin Immunol. 8:44–48. [DOI] [PubMed] [Google Scholar]
- Warshaw E, Belsito D, Taylor J, Sasseville D, DeKoven J, Zirwas M, Fransway A, Mathias C, Zug K, DeLeo V. 2013. North American Contact Dermatitis Group patch test results: 2009 to 2010. Dermatitis. 24:50–59. [DOI] [PubMed] [Google Scholar]
- Weber F, Nemeth T, Csepregi J, Dudeck A, Roers A, Ozsvari B, Oswald E, Puskas L, Jakob T, Mocsai A, et al. 2015. Neutrophils are required for both the sensitization and elicitation phase of contact hypersensitivity. J Exp Med. 212:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. 2012. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 46:2242–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H, Kornprobst J, Wick P, von Moos L, Trantakis I, Schraner E, Bathke B, Hochrein H, Suter M, Naegeli H. 2017. Myd88-dependent pro-IL-1β induction in dendritic cells exposed to food-grade synthetic amorphous silica. Particle Fibre Toxicol. 14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter M, Beer H, Hornung V, Kramer U, Schins R, Forster I. 2011. Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology. 5:326–340. [DOI] [PubMed] [Google Scholar]
- Wohlleben W, Mielke J, Bianchin A, Ghanem A, Freiberger H, Rauscher H, Gemeinert M, Hodoroaba V. 2017. Reliable nanomaterial classification of powders using the volume-specific surface area method. J Nanoparticle Res. 19:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf P, Donawho C, Kripke M. 1993. Analysis of the protective effect of different sunscreens on ultraviolet radiation-induced local and systemic suppression of contact hypersensitivity and inflammatory responses in mice. J Invest Dermatol. 100:254–259. [DOI] [PubMed] [Google Scholar]
- Wyman A, Hines S. 2018. Update on metal-induced occupational lung disease. Curr Opin Allergy Clin Immunol. 18:73–79. [DOI] [PubMed] [Google Scholar]
- Xie G, Lu W, Lu D. 2015. Penetration of titanium dioxide nanoparticles through slightly damaged skin in vitro and in vivo. J Appl Biomat Functional Mater. 13:356–361. [DOI] [PubMed] [Google Scholar]
- Xiong S, George S, Ji Z, Lin S, Yu H, Damoiseaux R, France B, Ng K, Loo S. 2013. Size of TiO2 nanoparticles influences their phototoxicity: An in vitro investigation. Arch Toxicol. 87:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Tang H, Liu J, Wang H, Liu Y. 2013. Evaluation of the adjuvant effect of silver nanoparticles both in vitro and in vivo. Toxicol Lett. 219:42–48. [DOI] [PubMed] [Google Scholar]
- Xue C, Li X, Liu G, Liu W. 2016. Evaluation of mitochondrial respiratory chain on the generation of reactive oxygen species and cytotoxicity in HACAT cells induced by nanosized titanium dioxide under UVA irradiation. Int J Toxicol. 35:644–653. [DOI] [PubMed] [Google Scholar]
- Xue C, Luo W, Yang X. 2015. A mechanism for nano-titanium dioxide-induced cytotoxicity in HACAT cells under UVA irradiation. Biosci Biotechnol Biochem. 79:1384–1390. [DOI] [PubMed] [Google Scholar]
- Yamaki K, Yoshino S. 2009. Comparison of inhibitory activities of zinc oxide ultrafine and fine particulates on IgE-induced mast cell activation. Biometals. 22:1031–1040. [DOI] [PubMed] [Google Scholar]
- Yan L, Liu X, Liu W, Tan X, Xiong F, Gu N, Hao W, Gao X, Cao J. 2015. Fe2O3 nanoparticles suppress Kv1.3 channels via affecting the redox activity of Kvb2 subunit in Jurkat T-cells. Nanotechnology. 26:505103. [DOI] [PubMed] [Google Scholar]
- Yanagisawa R, Takano H, Inoue K, Koike E, Kamachi T, Sadakane K, Ichinose T. 2009. Titanium dioxide nanoparticles aggravate atopic dermatitis-like skin lesions in nc/nga mice. Exp Biol Med. 234:314–322. [DOI] [PubMed] [Google Scholar]
- Yanagisawa R, Takano H, Inoue K, Koike E, Sadakane K, Ichinose T. 2010. Size effects of polystyrene nanoparticles on atopic dermatitis-like skin lesions in nc/nga mice. Int J Immunopathol Pharmacol. 23:131–141. [DOI] [PubMed] [Google Scholar]
- Yang W, Peters J, Williams R. 2008. Inhaled nanoparticles-a current review.Int J Pharm. 356:239–247. [DOI] [PubMed] [Google Scholar]
- Yang Y, Westerhoff P. 2014. Presence in, and release of, nanomaterials from consumer products In: Capco D, and Chen Y, editors. Nanomaterial: Impacts on Cell Biology and Medicine. Dordrecht, Netherlands: Springer;p. 1–17. [DOI] [PubMed] [Google Scholar]
- Yang D, Zhao Y, Guo H, Li Y, Tewary P, Xing G, Hou W, Oppenheim J, Zhang N. 2010. [Gd@C(82)(OH)22](n) nanoparticles induce dendritic cell maturation and activate TH1 immune responses. ACS Nano. 4:1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi A, Guarda G, Riteau N, Drexler S, Tardivel A, Couillin I, Tschopp J. 2010. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1α and IL-1bb. Proc Nat Acad Sci USA. 107:19449–19454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Chen R, Casey P, Ke P, Davis T, Chen C. 2015. Reducing the cytotoxicity of ZnO nanoparticles by a pre-formed protein corona in a supplemented cell culture medium. RSC Adv. 5:73963–73973. [Google Scholar]
- Yin J, Liu J, Ehrenshaft M, Roberts J, Fu P, Mason R, Zhao B. 2012. Phototoxicity of nano titanium dioxides in HACAT keratinocytesgeneration of reactive oxygen species and cell damage. Toxicol Appl Pharmacol. 263:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Kubo A, Fujita H, Yokouchi M, Ishii K, Kawasaki H, Nomura T, Shimizu H, Kouyama K, Ebihara T, et al. 2014. Distinct behavior of human langerhans cells and inflammatory dendritic epidermal cells at tight junctions in patients with atopic dermatitis. J Allergy Clin Immunol. 134:856–864. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Yoshioka Y, Fujimura M, Yamashita K, Higashisaka K, Morishita Y, Kayamuro H, Nabeshi H, Nagano K, Abe Y, et al. 2011. Promotion of allergic immune responses by intranasally-administrated nanosilica particles in mice. Nanoscale Res Lett. 6:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka Y, Kuroda E, Hirai T, Tsutsumi Y, Ishii K. 2017. Allergic responses induced by the immunomodulatory effects of nanomaterials upon skin exposure. Front Immunol. 8:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Chen Z. 2018. Establishment of different experimental asthma models in mice. Exp Ther Med. 15:2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharov S, Pelclova D, Zdimal V, Kacer P, Fenclova Z, Vlckova S, Schwarz J, Navratil T, Komarc M. 2016. Markers of inflammation among workers exposed to engineered TiO2 nanoparticles. Eur Resp J. 48:110–118. [Google Scholar]
- Zelga PJ G Górnicz MM, Głuszkiewicz JM, Piasecka-Zelga J. 2016. Outcomes of acute dermal irritation and sensitisation tests on active dressings for chronic wounds: A comparative study. J Wound Care. 25:722–729. [DOI] [PubMed] [Google Scholar]
- Zhang K, Liu L, Bai T, Guo Z. 2018. Interaction between hydrophobic Au nanoparticles and pulmonary surfactant (DPPC) monolayers. J Biomed Nanotech. 14:526–535. [DOI] [PubMed] [Google Scholar]
- Zhang L, Monteiro-Riviere N. 2008. Assessment of quantum dot penetration into intact, tape-stripped, abraded and flexed rat skin. Skin Pharmacol Physiol. 21:166–180. [DOI] [PubMed] [Google Scholar]
- Zhang L, Monteiro-Riviere N. 2019. Toxicity assessment of six TiO nanoparticles in human epidermal keratinocytes. Cutan Ocular Toxicol. 38:66–80. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yao Y, Sullivan N, Chen Y. 2011. Modeling the primary size effects of citrate-coated silver nanoparticles on their ion release kinetics. Environ Sci Technol. 45:4422–4428. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhao X, Cheng Y, Guo X, Yuan W. 2018. Iron oxide nanoparticles-based vaccine delivery for cancer treatment. Mol Pharm. 15:1791–1799. [DOI] [PubMed] [Google Scholar]
- Zhou D, Bennett S, Keller A. 2012. Increased mobility of metal oxide nano-particles due to photo and thermal induced disagglomeration. PloS One. 7:e37363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R, Zhu Y, Zhang M, Xiao Y, Du X, Liu H, Wang S. 2014. The induction of maturation on dendritic cells by TiO2 and Fe3O4@TiO2 nanoparticles via NF-κB signaling pathway. Mater Sci Eng C Mater Biol Appl. 39: 305–314. [DOI] [PubMed] [Google Scholar]
- Zunft HJ. 1996. Target Organ Toxicology Series. In: Dean JH, Luster MI, Munson AE, Kimber I, editors. Vol. 40, Issue 3, Immunotoxicology and immunopharmacology. XXII and 761 pages. Philadelphia (PA): Lippincott-Raven Publishers. [Google Scholar]
- Zvyagin A, Zhao X, Gierden A, Sanchez W, Ross J, Roberts M. 2008. Imaging of zinc oxide nanoparticle penetration in human skin in vitro and in vivo. J Biomed Optics. 13:064031. [DOI] [PubMed] [Google Scholar]