Abstract
Background
The association between lymphedema of the arm and impaired health-related QoL (HR-QoL) has led to changes in clinical practice. However, data on lymphedema of the breast (ie, breast edema) are lacking. We prospectively evaluated patient-reported prevalence and determinants of breast edema and its effect on patient-reported HR-QoL and breast pain.
Methods
We prospectively included 836 patients undergoing breast-conserving surgery followed by radiotherapy between October 2013 and October 2016 (UMBRELLA cohort). Patient-reported breast edema, HR-QoL, and breast pain were assessed by means of European Organisation for Research and Treatment of Cancer-C30/BR23 before starting radiotherapy and at 3, 6, 12, and 18 months thereafter. We assessed which patient, tumor, and treatment characteristics were associated with breast edema. With mixed-effects models, we assessed the impact of breast edema on patient-reported HR-QoL domains and breast pain over time, adjusting for confounders.
Results
Within a median follow-up of 28 months (interquartile range [IQR] = 15), 207 (24.8%) patients reported breast edema at some point in time. Prevalence of breast edema was highest at 6 months (12.4%, 95% confidence interval [CI] = 10.0 to 14.7). Larger tumor size, oncoplastic surgery, axillary lymph node dissection, locoregional radiotherapy, radiotherapy boost on the tumor bed, and adjuvant chemotherapy were associated with breast edema. Breast edema was independently associated with more breast pain and with poorer QoL, physical functioning, and body image.
Conclusions
Breast edema occurs frequently within the first year after breast-conserving surgery and radiotherapy and is independently associated with impaired HR-QoL and more breast pain. This information is important for use in clinical practice and should be discussed with patients during shared decision making.
Because of earlier detection of breast cancer and more effective treatment, breast cancer prognosis has improved substantially over the past decades (1–3). As such, long-term side effects of treatment and health-related quality of life (HR-QoL) are becoming increasingly relevant (4,5).
Breast-conserving therapy (BCT), which consists of breast-conserving surgery (BCS) followed by whole-breast irradiation, has become the standard of care for early-stage breast cancer, because the oncologic outcome is similar to that of mastectomy (1–3). With the advent of neoadjuvant systemic therapy and oncoplastic surgical techniques, BCT can nowadays also be offered to women with larger tumors (6,7). With the increasing proportion of patients undergoing BCT, physicians more often report isolated lymphedema of the breast (ie, breast edema), the reasons for which are still not well understood (8).
Although the association between lymphedema of the arm and impaired HR-QoL is widely acknowledged (9–11), less is known about the impact of breast edema on HR-QoL (12). Compared with arm edema, fewer studies are available, most of which have been performed retrospectively or cross-sectionally and without the use of patient-reported outcomes (PROs) (13). Breast edema is most often described as part of physician-reported toxicity scores in studies that evaluate experimental interventions. Studies primarily aimed at assessing breast edema in routine care are rare (8,12). Also, the effects of modern treatment options, such as oncoplastic breast-conserving surgery and neoadjuvant chemotherapy, on the risk of breast edema have not been evaluated (13).
Evidence-based treatments for breast edema are not yet available, but a substantial amount of women with breast edema are treated with long-term interventions, such as manual lymphatic drainage, taping of the breast, and compression therapy (14). Understanding prevalence and risk factors for breast edema is important to guide clinical decision making, to adequately inform patients about its impact on HR-QoL, and to serve as a starting point for developing targeted evidence-based interventions.
The aim of this study was to evaluate the prevalence and determinants of breast edema, and the association between breast edema and patient-reported HR-QoL and breast pain in a large prospective cohort of women undergoing BCT.
Methods
Participants
This study was conducted within the prospective Utrecht cohort for Multiple Breast Cancer Intervention Studies and Long-term Evaluation (UMBRELLA). The UMBRELLA study includes women with (in situ) breast cancer referred for radiation treatment at the University Medical Center Utrecht in the Netherlands (15). All participants gave written informed consent for longitudinal data collection at regular intervals during and after treatment (see Data Collection). The UMBRELLA study was approved by the institutional review board, adheres to the Declaration of Helsinki, and is registered on clinicaltrials.gov (16).
We prospectively included all women, age 18 years and older, who underwent BCS followed by whole-breast irradiation (with or without additional regional radiotherapy) between October 2013 and October 2016. All patients with at least 12 months' follow-up, who had completed surgery, radiotherapy, and, if applicable, adjuvant chemotherapy and who returned at least one questionnaire assessing PROs, were included in the analyses.
Data Collection
Patient, tumor, and treatment characteristics were prospectively collected and obtained from electronic patient files and quarterly provided data from the Netherlands Cancer Registry.
Presence of patient-reported breast edema was assessed prior to the start of radiotherapy and at 3, 6, 12, and 18 months thereafter. Breast edema was evaluated by means of the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-BR23 question 51 (ie, “During the past week; Was the area of your affected breast swollen?”) on a four-point Likert scale (ie, “not at all,” “a little,” “quite a bit,” or “very much”) (17).
The following potential determinants were studied: age; (neo)adjuvant systemic therapy (chemotherapy, endocrine therapy, immunotherapy, alone or in combination); oncoplastic surgery; sentinel node biopsy; axillary lymph node dissection (ALND); tumor size; radiotherapy boost to the tumor bed (ie, local radiotherapy boost); and regional lymph node irradiation (ie, axillary and/or periclavicular lymph nodes). In line with Dutch guidelines, oncoplastic surgery was defined as breast-conserving oncological resection combined with immediate redistribution of local breast tissue after a large proportion of the breast (generally more than 20%) had to be resected as part of BCS (18). Simple full-thickness closure was not considered as oncoplastic surgery.
For all patients, completion dates for questionnaires were registered, and the time between dates of the start of radiotherapy, chemotherapy, and all other treatments were assessed. Treatment variables were assessed as potential determinants when the start of the concerning treatment (radiotherapy, chemotherapy, and all other treatments) preceded the date of completing the questionnaire. For example, for patients who received adjuvant chemotherapy 4 months after baseline (ie, initiation of radiotherapy), chemotherapy was not assessed as a potential determinant for breast edema at baseline and 3 months, but only at 6, 12, and 18 months.
To estimate the effects of breast edema on patient-reported QoL, physical functioning, sexual functioning, body image, and breast pain, we assessed EORTC QLQ-C30 and the breast cancer-specific module BR23 before the start of radiation treatment and 3, 6, 12, and 18 months thereafter. Breast pain was assessed by means of EORTC OLQ-BR23 question 50 (“During the past week; Have you had any pain in the area of your affected breast?” on a four-point Likert scale), whereas scores for the EORTC domains QoL, physical functioning, sexual functioning, and body image were calculated according to EORTC QLQ-C30 and BR23 guidelines (17,19).
Statistical Analysis
Frequencies, proportions, and means with standard deviations for normally distributed variables—and medians with interquartile ranges (IQRs) otherwise—were used to describe clinical characteristics of study participants and prevalence of breast edema.
To identify determinants that had a statistically significant association with breast edema, we compared differences in the percentages of breast edema between groups for each possible determinant. This was performed at each time point using a t test or Mann-Whitney U test for continuous variables and χ2 test for categorical variables using complete case analysis. For this analysis, data on patient-reported breast edema (ie, EORTC-QLQ-BR23 question 51: “During the past week; Was the area of your affected breast swollen?”) were dichotomized to “not at all or a little” vs “quite a bit or very much.” Questionnaires returned later than 4 weeks after the planned assessment interval were excluded from the analysis.
To compare QoL-related domains (ie, QoL, physical functioning, sexual functioning, and body image) and breast pain between patients with and without edema, we used linear mixed-effects models for repeated measures. For this analysis, data from patients who returned at least two PRO measures were included. An autoregressive covariance structure was included with the assumption that measurements closer together in time would be more correlated than measurements further apart. Fixed effects in the model were time (time after start of radiotherapy, categorical); group (breast edema vs no breast edema); the interaction between time and group; and potential confounders (ie, age, ALND, tumor size, local radiotherapy boost, regional lymph node irradiation, adjuvant systemic treatment). For this analysis, outcome data on patient-reported breast pain were linearly transformed into a continuous score ranging from 0 to 100, according to the EORTC manual for symptom scores (ie, higher scores indicate more symptoms). Results were presented as estimated marginal means and mean differences (MD).
All reported P values were two sided, and values less than .05 were considered statistically significant. Statistical analyses were performed with IBM Statistical Package for Social Sciences (SPSS) software, version 24 (IBM Corp, Armonk, NY).
Results
In total, we included 836 patients who were treated with BCT, with a median follow-up of 28 months (IQR = 15). This included 734 (88%) patients with invasive breast cancer and 102 (12%) patients with ductal carcinoma in situ. A total of 656 (78%) patients received whole-breast irradiation only, and 180 (22%) patients received additional regional lymph node irradiation (ie, locoregional radiotherapy) (Table 1).
Table 1.
Characteristics of study participants treated with breast-conserving surgery and adjuvant radiotherapy between October 2013 and October 2016, with at least 12 months' follow-up (n = 836)
| Characteristic | No. of patients (%) |
|---|---|
| Age at inclusion, median (IQR), y | 58 (16) |
| Neo-adjuvant systemic treatment | |
| None | 699 (83) |
| Chemotherapy | 49 (6) |
| Chemotherapy and immunotherapy | 88 (11) |
| Oncoplastic surgery | |
| Yes | 92 (11) |
| No | 396 (47) |
| Unknown | 348 (42) |
| Sentinel node biopsy | |
| Yes | 705 (84) |
| No | 131 (16) |
| Axillary lymph node dissection | |
| Yes | 120 (14) |
| No | 716 (86) |
| Pathological tumor stage | |
| Ductal carcinoma in situ | 102 (12) |
| T1 | 594 (71) |
| T2 | 133 (16) |
| ≥T3 | 7 (1) |
| Radiotherapy treatment | |
| Local radiotherapy | 656 (78) |
| Locoregional radiotherapy* | 180 (22) |
| Local radiotherapy boost (ie, tumor bed) | |
| Yes | 286 (34) |
| No | 459 (55) |
| Unknown | 91 (11) |
| Adjuvant chemotherapy | |
| Yes | 232 (28) |
| No | 604 (72) |
| Adjuvant endocrine therapy | |
| Yes | 656 (79) |
| No | 180 (21) |
Includes radiotherapy on axillary and/or periclavicular lymph nodes. IQR = interquartile range.
Within the first 18 months after cohort enrollment, 207 (24.8%) patients had experienced breast edema at some point in time. At baseline (ie, prior to the start of radiotherapy), 12.0% (100 of 836; 95% confidence interval [CI] = 9.8% to 14.1%) of patients reported breast edema. Prevalence of breast edema was 7.1% (58 of 819; 95% CI = 5.3% to 8.8%) at 3 months, 12.4% (96 of 777; 95% CI = 10.0% to 14.7%) at 6 months, 8.2% (58 of 709; 95% CI = 6.1% to 10.2%) at 12 months, and 5.5% (33 of 601; 95% CI = 3.6% to 7.3%) at 18 months (Table 2).
Table 2.
Patient-reported presence of breast edema in patients receiving breast-conserving therapy (ie, breast-conserving surgery followed by radiotherapy)
| Breast edema* |
||
|---|---|---|
| Time intervals† | % (95% CI) | n/N |
| Baseline | 12.0 (9.8 to 14.1) | 100/836 |
| 3 months | 7.1 (5.3 to 8.8) | 58/819 |
| 6 months | 12.4 (10.0 to 14.7) | 96/777 |
| 12 months | 8.2 (6.1 to 10.2) | 58/709 |
| 18 months | 5.5 (3.6 to 7.3) | 33/601 |
Edema was defined by the European Organisation for Research and Treatment of Cancer BR23 question 50 (“no edema” consists of “not at all” and “a little”; “edema” consists of “quite a bit” and “very much”). CI = confidence interval.
Baseline is after breast-conserving surgery but before radiotherapy; 3 months measurement is after the completion of radiotherapy (and at least 2 months after the initiation of radiotherapy). Because this is an ongoing, actively recruiting cohort, the denominator decreases over time.
Several factors had a statistically significant association with breast edema (see Table 3). Oncoplastic surgery was associated with breast edema at baseline, but not at other time points. ALND was associated with breast edema at all time points from baseline up to 18 months. Locoregional radiotherapy was associated with breast edema at 3, 6, and 12 months after the initiation of radiotherapy. Local radiotherapy boost was associated with breast edema at 18 months. Adjuvant chemotherapy was associated with breast edema at 6, 12, and 18 months after the initiation of radiotherapy. Also, women with breast edema had larger tumors (17 mm, IQR = 14 mm) than women without breast edema (13 mm, IQR = 10 mm).
Table 3.
Proportions of women with breast edema after various types of treatments as part of breast-conserving therapy (n = 836)
| Variables | Breast edema |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
3 months |
6 months |
12 months |
18 months |
||||||
| % | P * | % | P * | % | P * | % | P * | % | P * | |
| Neoadjuvant chemotherapy with immunotherapy | ||||||||||
| Yes (n = 88) | 12.5 | — | 5.1 | — | 12.0 | — | 11.4 | — | 4.4 | — |
| No (n = 699) | 10.9 | .65 | 7.2 | .48 | 12.3 | .94 | 8.0 | .33 | 5.7 | .72 |
| Oncoplastic surgery | ||||||||||
| Yes (n = 92) | 20.0 | — | 10.2 | — | 12.2 | — | 4.1 | — | 5.3 | — |
| No (n = 396) | 10.2 | .04 | 7.1 | .44 | 12.2 | .99 | 6.6 | .50 | 5.5 | .99 |
| Sentinel node biopsy | ||||||||||
| Yes (n = 705) | 10.1 | 5.7 | — | 11.8 | — | 8.2 | — | 5.5 | — | |
| No (n = 131) | 11.1 | .40 | 6.3 | .45 | 15.5 | .26 | 8.0 | .95 | 5.6 | .95 |
| Axillary lymph node dissection | ||||||||||
| Yes (n = 120) | 25.8 | — | 14.4 | — | 22.0 | — | 14.7 | — | 10.7 | — |
| No (n = 716) | 9.6 | <.01 | 5.8 | <.01 | 10.8 | <.01 | 7.0 | <.01 | 4.6 | .04 |
| Radiotherapy | ||||||||||
| Local (n = 656) | N/A | — | 5.8 | — | 11.0 | — | 7.1 | — | 5.3 | — |
| Locoregional† (n = 180) | N/A | — | 10.5 | .04 | 17.1 | .04 | 12.8 | .03 | 6.8 | .65 |
| Local radiotherapy boost | ||||||||||
| Yes (n = 286) | N/A | — | 4.6 | — | 12.0 | — | 7.7 | — | 8.1 | — |
| No (n = 459) | N/A | — | 6.8 | .31 | 10.2 | .51 | 6.7 | .74 | 2.8 | .01 |
| Adjuvant chemotherapy | ||||||||||
| Yes (n = 232) | N/A | — | 5.7 | — | 22.4 | — | 14.8 | — | 11.2 | — |
| No (n = 604) | N/A | — | 7.6 | .35 | 8.4 | <.01 | 5.4 | <.01 | 2.9 | <.01 |
| Adjuvant endocrine therapy | ||||||||||
| Yes (n = 656) | N/A | — | 7.6 | — | 14.8 | — | 9.7 | — | 6.1 | — |
| No (n = 180) | N/A | — | 6.7 | .58 | 10.4 | .08 | 7.0 | .19 | 5.0 | .59 |
| Continuous variables | Median (IQR) | P ‡ | ||||||||
| Age, y | ||||||||||
| Breast edema | 58 (16) | |||||||||
| No breast edema | 58 (16) | .62 | ||||||||
| Tumor size, mm | ||||||||||
| Breast edema | 17 (14) | |||||||||
| No breast edema | 13 (10) | <.001 | ||||||||
P value based on two-sided χ2 test. IQR = interquartile range; N/A = at-baseline radiotherapy and adjuvant chemotherapy have not yet been initiated.
Includes radiotherapy on axillary and/or periclavicular lymph nodes.
P value based on Mann-Whitney U test.
Variables that were not associated with breast edema were age, neoadjuvant chemotherapy with or without immunotherapy, sentinel node biopsy, and adjuvant endocrine therapy.
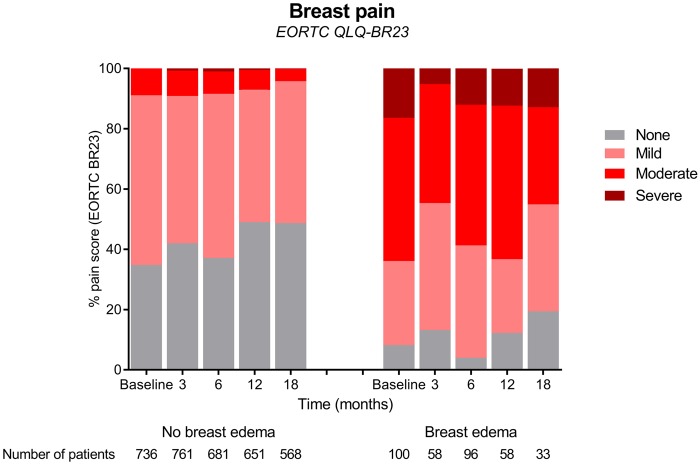
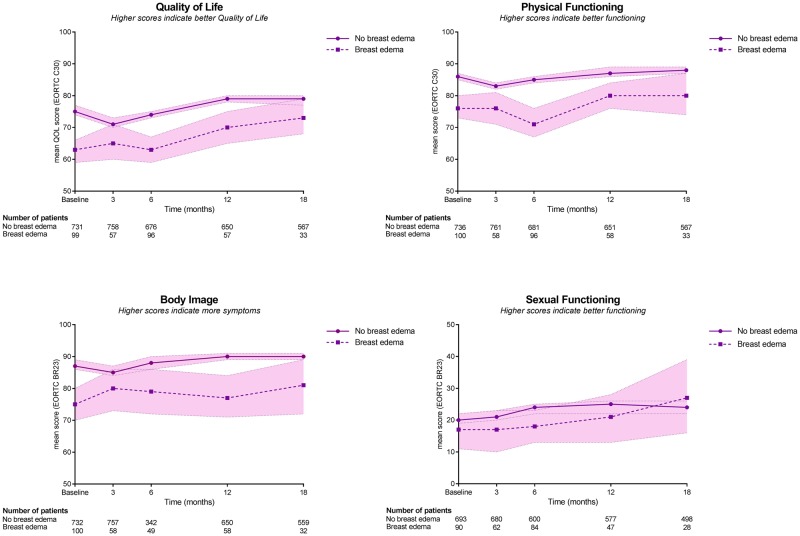
Patients with breast edema reported statistically significantly higher levels of breast pain than patients without edema at all time intervals (baseline, 3, 6, 12, and 18 months), also after adjusting the mixed model for potential confounders. Patients with breast edema reported poorer QoL, poorer physical functioning, and a poorer body image than patients without breast edema at baseline and 6 months, also after adjusting the mixed model for potential confounders (Table 4). Figures 1 and 2 demonstrate crude results.
Table 4.
Results from mixed-model analysis. Patient-reported outcome score of patients with self-reported breast edema and patients without breast edema at different time points (n = 836)
| Patient-reported* | Group | Baseline |
3 months |
6 months |
12 months |
18 months |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Between-group difference |
Between-group difference |
Between-group difference |
Between-group difference |
Between-group difference |
|||||||
| Mean† | MD‡ (95% CI) | Mean† | MD (95% CI) | Mean† | MD (95% CI) | Mean† | MD (95% CI) | Mean† | MD (95% CI) | ||
| Breast pain | No breast edema | 25.3 | Referent | 23.0 | Referent | 25.0 | Referent | 20.4 | Referent | 19.6 | Referent |
| Breast edema | 52.2 | 26.9 (21.5 to 32.3) | 45.2 | 22.2‡ (16.0 to 28.2) | 51.9 | 26.9‡ (22.5 to 31.4) | 51.3 | 30.9‡ (25.4 to 36.3) | 42.9 | 23.2‡ (16.3 to 30.1) | |
| QoL | No breast edema | 71.7 | Referent | 69.4 | Referent | 72.4 | Referent | 76.3 | Referent | 76.1 | Referent |
| Breast edema | 63.6 | 8.1 (4.2 to 12.1) | 68.2 | 1.2(−3.2 to 5.7) | 65.1 | 7.3‡ (4.0 to 10.5) | 74.6 | 1.7 (−2.3 to 5.7) | 74.4 | 1.7 (−3.5 to 6.8) | |
| Physical functioning | No breast edema | 81.2 | Referent | 79.7 | Referent | 80.4 | Referent | 83.1 | Referent | 83.0 | Referent |
| Breast edema | 78.1 | 3.1 (0.1 to 6.0) | 81.3 | 1.6 (−4.8 to 1.7) | 76.1 | 4.3‡ (2.0 to 6.7) | 81.9 | 1.2 (−1.8 to 4.0) | 83.3 | 0.3 (−4.1 to 3.5) | |
| Body image | No breast edema | 83.4 | Referent | 83.7 | Referent | 84.6 | Referent | 87.4 | Referent | 87.0 | Referent |
| Breast edema | 73.8 | 9.6 (6.1 to 13.1) | 83.9 | 0.2 (−4.2 to 3.7) | 78.1 | 6.5‡ (3.6 to 9.4) | 85.8 | 1.6 (−2.0 to 5.0) | 83.7 | 3.3 (−1.3 to 8.0) | |
| Sexual functioning | No breast edema | 19.3 | Referent | 22.3 | Referent | 28.8 | Referent | 36.5 | Referent | 30.9 | Referent |
| Breast edema | 17.1 | 2.2 (−4.1 to 8.5) | 20.3 | 1.9 (−5.7 to 9.6) | 27.6 | 2.5 (−4.1 to 6.3) | 30.5 | 2.7 (−0.7 to 12.5) | 34.8 | 4.8 (−1.5 to 11.0) | |
Quality of life (QoL), physical functioning according to the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30; body image, sexual functioning, and breast pain according to EORTC QLQ BR23. Scores range from 0 to 100. Higher scores on QoL, physical functioning, and sexual functioning indicate better functioning. Higher scores on breast pain indicate more pain. CI = confidence interval; MD = mean difference.
Mean scores adjusted for adjusted for age, axillary lymph node dissection, tumor size, local radiotherapy boost, locoregional radiotherapy, and/or adjuvant systemic treatment, depending on the sample size.
Difference in adjusted mean scores between patients with no breast edema and breast edema at baseline, 3, 6, 12, and 18 months.
Between-group effects were assessed using mixed models including the measurements obtained at baseline and at 3, 6, 12, and 18 months.
A random intercept per patient was included in the model.
Figure 1.
Impact of breast edema on breast pain following breast-conserving surgery and radiotherapy (unadjusted scores). EORTC = European Organisation for Research and Treatment of Cancer.
Figure 2.
Impact of breast edema on health-related quality-of-life domains following breast-conserving surgery and radiotherapy (unadjusted scores) with 95% confidence bands. EORTC = European Organisation for Research and Treatment of Cancer; QOL = quality of life.
Discussion
To date, this is the largest study assessing breast edema, which was conducted prospectively and from the patients’ perspective, by using PROs to diagnose breast edema and its impact on HR-QoL and breast pain. We found that 24.8% of patients reported breast edema at some point in time within the first 18 months after BCT. The highest prevalence of breast edema was observed at 6 months (12.4%) and the lowest at 18 months (5.5%) after the start of radiotherapy. Patients who underwent oncoplastic surgery, ALND, locoregional radiotherapy, local radiotherapy boost, and adjuvant chemotherapy and patients with a larger tumor had a higher probability of developing breast edema (see Table 3 for statistically significant time points). The presence of breast edema was independently associated with more breast pain at all time intervals up to 18 months and with poorer QoL, physical functioning, and body image at baseline and 6 months.
Several other studies assessed the occurrence of breast edema after BCT, showing a wide range from 10% to 90% (13). This is best explained by heterogeneity in methods used to measure and define breast edema in the absence of a gold standard. In clinical oncology practice, breast edema is often measured using physician-reported outcome measures, such as the Common Terminology Criteria of Adverse Events (CTCAE); however, interobserver agreement using CTCAE is known to be low (20). As a result, breast edema often remains underdiagnosed and untreated in clinical practice. Therefore, we assessed breast edema from the patient’s perspective.
Like us, two studies assessed patient-reported breast edema after BCS (13). One study included 100 patients with early-stage breast cancer who were treated with adjuvant partial breast irradiation in an experimental setting. At 5 years after treatment, nine women (9%) reported breast edema (21). A comparison with our population is not possible because the UMBRELLA cohort did not include patients who underwent partial breast irradiation, and we did not assess outcomes 5 years after treatment. The other study was performed cross-sectionally, and included 131 patients who received BCT between 2005 and 2010, of whom 75% reported breast edema at some point between 0 and 60 months following BCT (8). Differences in prevalence of breast edema are explained by the use of different PRO instruments, different breast edema definitions, and the other study's cross-sectional study design.
We identified six risk factors for breast edema following BCT: oncoplastic surgery, ALND, larger tumor size, locoregional radiotherapy, local radiotherapy boost, and adjuvant chemotherapy. Larger tumor size, local radiotherapy boost, and adjuvant chemotherapy were also identified as risk factors in other studies, whereas the association of breast edema with ALND and locoregional radiotherapy have not yet been found by previous studies (13,22,23). In contrast to some previous reports, sentinel node biopsy and adjuvant endocrine therapy did not increase the risk of breast edema in our study population (13,24,25).
To our knowledge, we were the first to assess modern-day treatment options such as oncoplastic surgery and neoadjuvant chemotherapy. Oncoplastic surgery led to an increased risk of breast edema at baseline only (ie, after surgery but before the start of radiotherapy). This may be explained by a temporary impairment of the lymphatic system after larger volumes of tissue were mobilized when applying oncoplastic breast-conserving surgery. Collecting data on oncoplastic surgery was challenging, because not all operative reports described which closure techniques were applied (although surgeons were aware that this information was part of our prospective data collection). In only 58% of the samples (488 of 836 patients), it was explicitly stated whether oncoplastic surgery was applied. Prospective studies that assess larger groups of patients who underwent oncoplastic breast-conserving surgery are required to further study its association with breast edema.
ALND was associated with breast edema at all time points. Because of the removal of all axillary lymph nodes, lymph drainage of the arm is affected. This could potentially result in more accumulation of lymph fluid in the surrounding areas, such as the breast area.
In our study, breast edema occurred more often at 18 months after a local radiotherapy boost. Breast edema also occurred more often at 3, 6, and 12 months after locoregional radiotherapy. In both cases, this may be closely linked to breast fibrosis, a process by which skin and underlying tissue gradually become less elastic, which starts to develop later in time after undergoing more extensive radiotherapy (26–28).
Results from the AMAROS trial showed that locoregional radiotherapy leads to significantly less symptomatic arm edema compared with ALND (11% vs 23% after 5 years) (29). Therefore, to reduce arm morbidity in patients with limited nodal involvement, axillary lymph node irradiation (as part of locoregional radiotherapy) is increasingly applied instead of routine ALND (30). Our study showed that both locoregional radiotherapy and ALND increase the risk of breast edema. This increased risk of breast edema may be an argument for patients with limited nodal involvement to refuse any type of additional axillary treatment. Therefore, physicians should discuss the risks of developing breast edema with patients when outweighing oncological benefits vs potential side effects of additional axillary treatment.
An important aim of our study was to assess the association between breast edema and HR-QoL, because data are lacking. This is the first study to systematically assess breast edema in relation to HR-QoL over time in a large sample of modern-day patients with breast cancer. Thus, our results provide more robust and in-depth scientific proof of the association between breast edema and impaired HR-QoL, providing physicians clinically relevant numbers to share with their patients.
Adriaenssens et al. (8) previously assessed the influence of breast edema of QoL using the same PRO instrument as we did (ie, EORTC QLQ-BR23) in 131 patients undergoing BCT. Their data were collected cross-sectionally, which hampers conclusions about the temporal occurrence relation. Nonetheless, similar to our findings, patients with breast edema reported statistically significant worse body image and no statistically significant differences in sexual functioning. Degnim et al. (12) also assessed the impact of breast edema on QoL in a group of 124 women after nonmastectomy breast procedures between 2006 and 2009. Within a median follow-up period of 11 months, they did not find statistically significant differences in QoL between patients with and without breast edema, which may best be explained by their sample size. They used a different breast cancer-specific PRO instrument (FACT-B), hampering direct comparison with our results.
Breast edema was associated with more breast pain at all time points. This is a clinically relevant finding. Early recognition of breast edema as the potential underlying cause of breast pain is important to initiate targeted interventions instead of only prescribing (chronic) pain medication. However, the currently available breast edema interventions have not been properly evaluated in randomized studies. Therefore, evidence-based treatment for breast edema is lacking. Our results can help set up studies for proper evaluation of effectiveness (eg, breast pain reduction as an endpoint).
Our study has several limitations. We used EORTC-BR23 question 51 to identify patients with breast edema (“swelling of the breast”). As a result, baseline data may also include other factors that could be labeled by patients as breast swelling such as hematoma or seroma. This explains the high prevalence of breast swelling at baseline (before the start of radiotherapy), because seroma and hematoma are most often seen within days to weeks after breast-conserving surgery. Therefore, we did not assess whether edema at baseline is a determinant for persistent breast edema, because edema reported at baseline in this study may be slightly different from what has been identified as edema later in time. Unfortunately, to date no other PRO instruments exist to identify isolated patient-reported breast edema (11). Furthermore, questionnaire return rates decreased over time from 87% at baseline to 71% at 18 months. This may not be an issue when nonresponse is random, but could be problematic in case of differential nonresponse (eg, result in underestimation of breast edema prevalence when only patients with breast edema stop returning questionnaires).
In conclusion, this study identified several risk factors for breast edema that could change clinical decisions. Patients undergoing oncoplastic surgery, ALND, locoregional radiotherapy, radiotherapy boost to the tumor bed, and adjuvant chemotherapy and patients with larger tumors should be informed about their higher probability of developing breast edema. Risks and benefits of applying these treatment options should carefully be outweighed during shared decision making between patient and physician. Our study also shows that breast edema is associated with reduced HR-QoL, and especially with more breast pain. To date, there are no evidence-based interventions for breast edema, thus these findings highlight the need for systematic evaluation and development of targeted interventions for breast edema to reduce its impact on the lives of breast cancer patients and survivors.
Notes
Affiliations of authors: Imaging Division, University Medical Center, Utrecht, the Netherlands (DAYA, MLG, HMV); Department of Epidemiology, Julius Center for Health Sciences and Primary Care, University Medical Center, Utrecht, the Netherlands (DAYA, CHvG); Department of Radiation Oncology, University Medical Center, Utrecht, the Netherlands (DHvdB); Julius Center for Health Sciences and Primary Care, University Medical Center, Utrecht, the Netherlands; Department of Surgery, Diakonessenhuis, Utrecht, the Netherlands (IB); Department of Surgery, Alrijne Hospital, Leiderdorp, the Netherlands (CCvdP); Department of Surgery, University Medical Center, Utrecht, the Netherlands (AJW); Department of Medical Oncology, University Medical Center, Utrecht, the Netherlands (RMB); Department of Surgery, St. Antonius Hospital, Nieuwegein, the Netherlands (RK); Department of Surgery, Meander Medical Center, Amersfoort, the Netherlands (EJS); Alexander Monro Hospital, Bilthoven, the Netherlands (YJ); Utrecht University, Utrecht, the Netherlands (CHvG, HMV).
We would like to thank Rebecca Stellato for her statistical advice on mixed-model analysis.
The authors have no conflicts of interest to disclose.
References
- 1. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Litière S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol. 2012;13(4):412–419. [DOI] [PubMed] [Google Scholar]
- 3. Bosma SC, Van der Leij F, Van Werkhoven E, et al. Very low local recurrence rates after breast-conserving therapy: analysis of 8485 patients treated over a 28-year period. Breast Cancer Res Treat. 2016;156(2):391–400. [DOI] [PubMed] [Google Scholar]
- 4. Fallowfield L, Jenkins V.. Psychosocial/survivorship issues in breast cancer: are we doing better? J Natl Cancer Inst. 2015;107(1):335. [DOI] [PubMed] [Google Scholar]
- 5. Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;34(6):611–635. [DOI] [PubMed] [Google Scholar]
- 6. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Losken A, Hart AM, Chatterjee A.. Updated evidence on the oncoplastic approach to breast conservation therapy. Plast Reconstr Surg. 2017;140(5S Advances in Breast Reconstruction):14S–22S. [DOI] [PubMed] [Google Scholar]
- 8. Adriaenssens N, Verbelen H, Lievens P, Lamote J.. Lymphedema of the operated and irradiated breast in breast cancer patients following breast conserving surgery and radiotherapy. Lymphology. 2012;454:154–164. [PubMed] [Google Scholar]
- 9. Land SR, Kopec JA, Julian TB, et al. Patient-reported outcomes in sentinel node-negative adjuvant breast cancer patients receiving sentinel-node biopsy or axillary dissection: National Surgical Adjuvant Breast and Bowel Project phase III protocol B-32. J Clin Oncol. 2010;2825:3929–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;2524:3657–3663. [DOI] [PubMed] [Google Scholar]
- 11. Pusic AL, Cemal Y, Albornoz C, et al. Quality of life among breast cancer patients with lymphedema: a systematic review of patient-reported outcome instruments and outcomes. J Cancer Surviv. 2013;71:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Degnim AC, Miller J, Hoskin TL, et al. A prospective study of breast lymphedema: frequency, symptoms, and quality of life. Breast Cancer Res Treat. 2012;1343:915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verbelen H, Gebruers N, Beyers T, et al. Breast edema in breast cancer patients following breast-conserving surgery and radiotherapy: a systematic review. Breast Cancer Res Treat. 2014;1473:463–471. [DOI] [PubMed] [Google Scholar]
- 14. International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 consensus document of the International Society of Lymphology. Lymphology. 2013;461:1–11. [PubMed] [Google Scholar]
- 15. Young-Afat DA, van Gils CH, van den Bongard H, Verkooijen HM.. UMBRELLA Study Group. The Utrecht Cohort for Multiple Breast Cancer Intervention Studies and Long-term Evaluation (UMBRELLA): objectives, design, and baseline results. Breast Cancer Res Treat. 2017;164(2):445–450. doi:10.1007/s10549-017-4242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. National Library of Medicine. The UMBRELLA cohort, number NCT02839863. https://clinicaltrials.gov/. Accessed July 10, 2018.
- 17. Sprangers MAG, Groenvold M, Arraras JI, et al. The European Organisation for Research and Treatment of Cancer: breast cancer specific quality of life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14(10):2756–2768. [DOI] [PubMed] [Google Scholar]
- 18.Dutch Society for Surgery. Dutch breast cancer guidelines; 2012. https://heelkunde.nl/sites/heelkunde.nl/files/richtlijnen-definitief/Mammacarcinoom2012.pdf. Accessed July 10, 2018.
- 19. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;855:365–376. [DOI] [PubMed] [Google Scholar]
- 20. Atkinson TM, Li Y, Coffey CW, et al. Reliability of adverse symptom event reporting by clinicians. Qual Life Res. 2012;217:1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Formenti SC, Hsu H, Fenton-Kerimian M, et al. Prone accelerated partial breast irradiation after breast-conserving surgery: five-year results of 100 patients. Int J Radiat Oncol Biol Phys. 2012;843:606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toledano A, Garaud P, Serin S, et al. Concurrent administration of adjuvant chemotherapy and radiotherapy after breast-conserving surgery enhances late toxicities: long-term results of the ARCOSEIN multicenter randomized study. Int J Radiat Oncol Biol Phys. 2006;652:324–332. [DOI] [PubMed] [Google Scholar]
- 23. Kelemen G, Varga Z, Lazar G, et al. Cosmetic outcome 1–5 years after breast conservative surgery, irradiation and systemic therapy. Pathol Oncol Res. 2012;182:421–427. [DOI] [PubMed] [Google Scholar]
- 24. Chadha M, Vongtama D, Friedmann P, et al. Comparative acute toxicity from whole breast irradiation using 3-week accelerated schedule with concomitant boost and the 6.5-week conventional schedule with sequential boost for early-stage breast cancer. Clin Breast Cancer. 2012;121:57–62. doi: 10.1016/j.clbc.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 25. Barnett GC, Wilkinson JS, Moody AM, et al. The Cambridge Breast Intensity-Modulated Radiotherapy Trial: patient- and treatment-related factors that influence late toxicity. Clin Oncol. 2011;2310:662–673. [DOI] [PubMed] [Google Scholar]
- 26. Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;161:47–56. [DOI] [PubMed] [Google Scholar]
- 27. Borger JH, Kemperman H, Smitt HS, et al. Dose and volume effects on fibrosis after breast conservation therapy. Int J Radiat Oncol Biol Phys. 1994;305:1073–1081. [DOI] [PubMed] [Google Scholar]
- 28. Colette S, Collette L, Budiharto T, et al. Predictors of the risk of fibrosis at 10 years after breast conserving therapy for early breast cancer: a study based on the EORTC Trial 22881-10882 ‘boost versus no boost’. Eur J Cancer. 2008;4417:2587–2599. [DOI] [PubMed] [Google Scholar]
- 29. Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;1512:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gregorowitsch ML, Verkooijen HM, Young-Afat DA, et al. Impact of modern-day axillary treatment on patient reported arm morbidity and physical functioning in breast cancer patients. Radiother Oncol. 2018;131:221–228. [DOI] [PubMed] [Google Scholar]