Abstract
Background
Stereotactic body radiotherapy (SBRT) is a treatment option for stage I non–small cell lung cancer (NSCLC), providing a potentially curative therapy for patients who are nonsurgical candidates. This study describes the adoption of SBRT vs other treatment options across the United States, as well as commonly used dose-fractionation regimens.
Methods
We analyzed patients in the National Cancer Data Base. A total of 107 233 stage IA NSCLC patients diagnosed from 2008 to 2013 were included. We described the proportions of patients who received different surgical and radiation treatment options by year. A multivariable model was constructed to assess factors associated with patients receiving SBRT. In patients who received SBRT, we described the proportion of patients who received common dose/fractionation regimens.
Results
Use of SBRT increased from 6.7% to 16.3% from 2008 to 2013, with a corresponding decrease in lobectomy/pneumonectomy (49.5% to 43.7%). The rates of wedge resection, conventional radiotherapy, and no treatment remained relatively constant. Adoption of SBRT was lowest in small community centers (8.6% of patients by 2013). On multivariable analysis, older age and treatment at larger centers were associated with higher SBRT receipt, and black race and higher comorbidity were associated with lower SBRT receipt. There was statistically significant geographic variation. Common SBRT schemes were 10 Gy × 5 (19%), 18–20 Gy × 3 (31%), and 12 Gy × 4 (16%).
Conclusions
SBRT adoption has been modest over time and has not substantially replaced less curative treatments. Lack of access to this technology in smaller cancer centers may have partly contributed to the slow adoption.
In 2016, an estimated 224 390 patients were diagnosed with non–small cell lung cancer (NSCLC) in the United States, with 16% of these patients diagnosed with localized disease (1). With increased use of low-dose computed tomography (CT) screening for lung cancer, the incidence of stage I disease is expected to rise (2).
Management of stage I lung cancer has evolved over the past decade. Lobectomy has been considered the standard treatment, with five-year local control rates of greater than 90% and overall survival greater than 70% (3,4). However, because the majority of lung cancers are smoking related with a median age at diagnosis of 70 years, many patients may not be ideal candidates for aggressive surgery (5). Less aggressive treatment options include wedge resections or conventional external beam radiotherapy, but published outcomes are comparably worse than lobectomy. In a randomized trial conducted by the Lung Cancer Study Group that compared lobectomy vs sublobar resection, the local failure rate was statistically significantly higher with sublobar resection (0.06 per person/year vs 0.02 for lobectomy, P = .008) (6). Long-term outcomes after conventional external beam radiotherapy have been similarly disappointing, with local control rates of 32% to 76% (7).
Stereotactic body radiotherapy (SBRT) is a technology that has been developed more recently that delivers highly precise radiation treatment. Used for stage I lung cancer, SBRT delivers a high dose of treatment each day for a total of one to five treatments, which contrasts with conventional external beam radiotherapy, which delivers a protracted course of treatment, usually over six to seven weeks. Prospective and retrospective studies have consistently reported that SBRT for stage I NSCLC results in long-term local control rates greater than 90% (8–10), providing a promising alternative for patients who are not candidates for lobectomy or who wish to avoid surgery. However, SBRT adoption requires that each facility acquire the necessary equipment and expertise, and its uptake across the United States is unknown. In addition, many dose and fractionation regimens for SBRT have been studied in the published literature, and it is unknown what regimens are actually used in routine practice.
Therefore, the purpose of this study is to describe patterns of care across the United States in the treatment of stage IA NSCLC in a contemporary nationwide cohort of patients and to examine factors associated with SBRT use. We chose to study stage IA patients specifically because the tumor size (≤3 cm) may be most suitable for SBRT. We also describe common dose and fractionation regimens used for SBRT in these patients.
Methods
The National Cancer Data Base (NCDB) is the largest cancer registry in the United States and contains approximately 70% of cancer patients nationwide. The database is jointly maintained by the American College of Surgeons and the American Cancer Society. The NCDB contains patient-level demographic information including age, sex, race, and insurance status as well as census-level household income. Additional data include Charlson-Deyo comorbidity score, TNM stage, and details of first course of treatment. Radiotherapy total dose and number of fractions are included. Treatment facility is classified as academic, comprehensive community (standalone center, >500 cancer cases per year), community (standalone center, 100–500 cancer cases per year), and integrated network (integrated multifacility cancer network). Use of NCDB data for this study was granted through an institutional review board waiver.
The primary objective of this study was to describe patterns of care for stage IA NSCLC. Patients diagnosed with clinical stage IA NSCLC (cT1N0M0) between 2008 and 2013 formed the basis for the patient cohort. The year 2008 was chosen based on the publication of several large US phase II studies reporting patient outcomes after SBRT (11–13). Inclusion of only stage IA tumors was chosen because stage IB can include tumors up to 7 cm in size, which may be too large for optimal treatment with SBRT. Patients were excluded if they had missing information on clinical stage or treatment.
Patients were classified as receiving SBRT if they received radiation as first course of treatment, the radiation was targeted at the chest or lungs, and the radiation modality was coded as stereotactic radiation; further, patients who met these criteria but for whom fractionation data indicated they received 10 treatments or more were classified as having received conventional external beam radiotherapy. We performed sensitivity analysis whereby only up to five fractions were allowed for definition of SBRT, and this changed the patterns of care results minimally. All patients who were treated with external beam radiation as the first course of treatment who did not meet the above criteria were coded as receiving conventional external beam radiation. Patients who had surgery as their first course of treatment were further categorized as having received ablation, surgery less than a lobectomy, or lobectomy/pneumonectomy (combined because of the small number of pneumonectomies).
Statistical Analysis
Descriptive statistics were used to report the proportions of patients who received each treatment type by year. We also examined the uptake of SBRT over time by different types of cancer facilities ranging from community centers to academic programs. A multivariable log-binomial model was created to examine factors associated with receipt of SBRT. Finally, we describe the most commonly used radiation dose and fractionation regimens for SBRT. All statistics were performed using Stata/IC 13.1 (StataCorp LP, College Station, TX), and a two-sided P value of less than .05 was used for statistical significance.
Results
The median age of the patient cohort was 71 years, 56.1% were women, and 89.1% were white (Table 1). More than half of patients were treated at community or comprehensive community cancer centers.
Table 1.
Patient characteristics
| No. (%) | |
|---|---|
| Age, median (range) | 71 (18–90) |
| Year | |
| 2008 | 15 066 (13.6) |
| 2009 | 17 260 (15.6) |
| 2010 | 18 442 (16.6) |
| 2011 | 19 189 (17.3) |
| 2012 | 20 102 (18.1) |
| 2013 | 20 876 (18.8) |
| Race | |
| White | 98 181 (89.1) |
| Black | 9261 (8.4) |
| Other | 2688 (2.5) |
| Sex | |
| Male | 48 709 (43.9) |
| Female | 62 226 (56.1) |
| Charlson-Deyo Comorbidity Score | |
| 0 | 58 057 (52.3) |
| 1 | 36 387 (32.8) |
| 2 | 16 491 (14.9) |
| Insurance status | |
| Insured | 107 921 (98.6) |
| Uninsured/unknown | 1577 (1.4) |
| Income (census tract) | |
| Quartile 1 (0–25, lowest) | 19 777 (18.0) |
| Quartile 2 (25–50) | 26 841 (24.4) |
| Quartile 3 (50–75) | 29 547 (26.9) |
| Quartile 4 (75–100, highest) | 33 681 (30.7) |
| Region | |
| Northeast | 24 251 (21.9) |
| South | 42 125 (38.0) |
| Midwest | 30 155 (27.2) |
| West | 14 346 (12.9) |
| Treatment facility type | |
| Academic facility | 39 822 (36.1) |
| Comprehensive community cancer program | 53 919 (48.8) |
| Community cancer program | 8728 (7.9) |
| Integrated network cancer program | 7923 (7.2) |
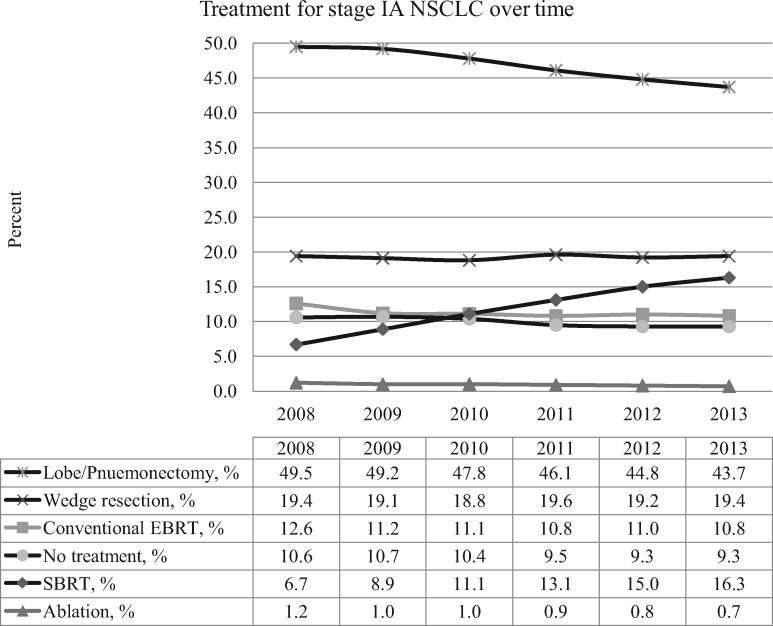
Use of SBRT steadily increased from 6.7% to 16.3% from 2008 to 2013, with a corresponding decrease in lobectomy/pneumonectomy from 49.5% to 43.7% (Figure 1). The rates of wedge resection, conventional external beam radiotherapy, ablation, and no treatment remained relatively constant over time.
Figure 1.
Treatment patterns for stage IA non–small cell lung cancer between 2008 and 2013. EBRT = external beam radiation therapy; NSCLC = non–small cell lung cancer; SBRT = stereotactic body radiotherapy.
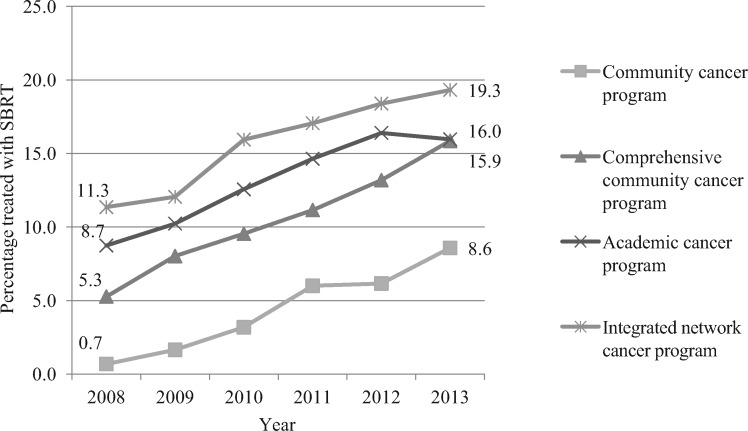
While SBRT uptake increased over time in all types of cancer centers (Figure 2), there was a dramatic difference in its use by type of center. By 2013, 19.3% of patients treated in integrated network cancer programs received SBRT, but only 8.6% of patients treated in community centers (defined as small centers with 100–500 total cancer cases per year) received SBRT.
Figure 2.
Uptake of stereotactic body radiotherapy in different facility types. SBRT = stereotactic body radiotherapy.
Multivariable analysis (Table 2) showed similar findings. Compared with smaller community programs, adoption of SBRT was higher at comprehensive community (adjusted relative risk [aRR] = 2.40, 95% confidence interval [CI] = 2.17 to 2.66), academic (aRR = 3.34, 95% CI = 3.02 to 3.70), and integrated networks (aRR = 3.44, 95% CI = 3.07 to 3.84). Older patient age was directly associated with increased use (per five years, aRR = 1.28, 95% CI = 1.27 to 1.29). There was also significant statistically variation in SBRT use by geographic region. Black race and higher comorbidity score were associated with lower SBRT receipt.
Table 2.
Multivariable log binomial model for receipt of stereotactic body radiotherapy*
| Unadjusted |
Adjusted |
||
|---|---|---|---|
| Patients treated with SBRT No./total (%) | RR (95% CI) | P | |
| Age (per 5 y) | 1.28 (1.27 to 1.29) | <.001 | |
| Year | |||
| 2008 | 1009/15 066 (6.7) | Ref | Ref |
| 2009 | 1536/17 260 (8.9) | 1.32 (1.22 to 1.43) | <.001 |
| 2010 | 2047/18 442 (11.1) | 1.61 (1.49 to 1.74) | <.001 |
| 2011 | 2514/19 189 (13.1) | 1.94 (1.81 to 2.09) | <.001 |
| 2012 | 3015/20 102 (15.0) | 2.19 (2.04 to 2.36) | <.001 |
| 2013 | 3403/20 876 (16.3) | 2.41 (2.25 to 2.59) | <.001 |
| Race | |||
| White | 10 898/98 181 (11.1) | Ref | Ref |
| Black | 9167/9261 (9.9) | 0.91 (0.86 to 0.97) | .006 |
| Other | 161/2688 (6.0) | 0.57 (0.49 to 0.66) | <.001 |
| Sex | |||
| Male | 5407/48 709 (11.1) | Ref | Ref |
| Female | 6720/62 226 (10.8) | 0.99 (0.96 to 1.02) | .35 |
| Charlson-Deyo Comorbidity Score | |||
| 0 | 7141/58 057 (12.3) | Ref | Ref |
| 1 | 3155/36 387 (8.7) | 0.72 (0.69 to 0.75) | <.001 |
| 2 | 1837/16 491 (11.1) | 0.87 (0.83 to 0.91) | <.001 |
| Insurance status | |||
| Insured | 11 871/107 921 (11.0) | Ref | Ref |
| Uninsured | 96/1577 (6.1) | 0.92 (0.76 to 1.11) | .36 |
| Income (census tract) | |||
| Quartile 1 (0–25, lowest) | 2211/19 777 (11.2) | Ref | Ref |
| Quartile 2 (25–50) | 3079/26 841 (11.5) | 0.97 (0.91 to 1.03) | .28 |
| Quartile 3 (50–75) | 3315/29 547 (11.2) | 0.90 (0.85 to 0.95) | <.001 |
| Quartile 4 (75–100, highest) | 3526/33 681 (10.5) | 0.81 (0.76 to 0.85) | <.001 |
| Region | |||
| Northeast | 2221/24 251 (9.2) | Ref | Ref |
| South | 4630/42 125 (11) | 1.29 (1.23 to 1.36) | <.001 |
| Midwest | 3893/30 155 (12.9) | 1.48 (1.41 to 1.55) | <.001 |
| West | 1429/14 346 (10.0) | 1.15 (1.08 to 1.22) | <.001 |
| Treatment facility type | |||
| Community cancer program | 1669/39 822 (4.2) | Ref | Ref |
| Academic facility | 7004/53 919 (13.0) | 3.34 (3.02 to 3.70) | <.001 |
| Comprehensive community cancer program | 878/8728 (10.0) | 2.40 (2.17 to 2.66) | <.001 |
| Integrated network cancer program | 1153/7923 (14.6) | 3.44 (3.07 to 3.84) | <.001 |
All adjusted estimates are adjusted for all of the other variables listed in the table. CI = confidence interval; RR = relative risk; SBRT = stereotactic body radiotherapy.
The most common SBRT dose/fractionation regimens were 10 Gy × 5 (used in 19% of SBRT patients), 18/20 Gy × 3 (31%), and 12 Gy × 4 (16%) (Table 3). Single fraction radiosurgery of 30 Gy or higher was used in 0.4% of patients.
Table 3.
Commonly used SBRT dose/fractionation schemes for stage IA non–small cell lung cancer*
| Dose and fractions | % of SBRT patients |
|---|---|
| 10 Gy × 5 | 19.1 |
| 12 Gy × 5 | 6.4 |
| 11 Gy × 5 | 2.4 |
| 12 Gy × 4 | 16.0 |
| 12.5 Gy × 4 | 5.9 |
| 18 Gy × 3 | 14.3 |
| 20 Gy × 3 | 16.2 |
| Single fraction ≥ 30 Gy | 0.4 |
| Other | 19.3 |
Gy = Gray; SBRT = stereotactic body radiotherapy.
Discussion
SBRT is a highly effective and minimally invasive treatment option for patients with stage I NSCLC. Its development has offered another attractive treatment option for patients with this disease. To our knowledge, this is the first study to examine the adoption of SBRT for stage IA NSCLC across the United States. Using the National Cancer Data Base, which includes approximately 70% of all incident cancers in the United States, we found a modest increase in the use of SBRT from 2008 to 2013 (6.7% to 16.3%). There are several findings of interest that warrant further discussion.
First, the increase in SBRT corresponded to an also modest decrease in lobectomy and pneumonectomy over time. In multivariable analysis, we found that higher patient age was associated with increased use of SBRT. These results suggest a possible trend for less aggressive treatment of stage IA NSCLC, especially for older patients. One possible explanation for our findings is a decreased use of lobectomy being replaced by the less aggressive wedge resection and a simultaneous replacement of wedge resection by SBRT—especially in borderline surgical candidates. However, lobectomy remains the most commonly used treatment by far, suggesting that many physicians continue to view surgery as a superior option to SBRT. Unfortunately, while multiple single-arm phase I and II trials have consistently demonstrated very high rates of long-term cancer control after SBRT, several international attempts at randomized trials comparing SBRT with surgical options have failed due to poor accrual (STARS trial [NCT00840749], ROSEL trial [NCT00687986], ACOSOG Z4099 trial [NCT01336894]). It is possible that some physicians may be reluctant to offer SBRT as a primary treatment option due to lack of randomized data compared with surgery, but this is the same type of physician reluctance that likely resulted in the failure of these randomized trial efforts.
We also observed relatively stable rates of conventional external beam RT and no treatment. By 2013, more than 20% of patients with stage IA NSCLC continued to receive care, which is less than optimal. According to the National Comprehensive Cancer Network guidelines, conventionally fractionated or hypofractionated are “less preferred alternatives” for institutions without SBRT availability (14). Conventionally fractionated radiotherapy results in relatively poor local control rates for early-stage lung cancer. A meta-analysis published in 2001 reported local control from 18 studies ranging from 32% to 76% (7). More recent reports of modestly hypofractionated radiotherapy have reported more promising local control rates of greater than 70%, but this remains lower than the local control reported with SBRT or surgery (15–17). As lung cancer screening becomes more widely adopted (2), more patients will be diagnosed with lung cancers at an early and most curable stage. Further efforts are needed to better understand the reasons for the relatively muted adoption of SBRT and the related observation of a persistently high proportion of patients receiving non-guideline-recommended care.
A barrier to wider adoption may be the equipment and expertise necessary for radiation oncology centers to provide SBRT. We found that SBRT adoption was slowest in smaller community centers, which is consistent with this hypothesis related to resource limitations. Prior studies in other cancers have similarly found slower adoption of SBRT in prostate cancer in smaller community cancer centers (18). However, because SBRT is a short-course treatment usually involving a total of three to five fractions, centers that do not offer SBRT may be able to refer some patients for treatments at facilities that do offer this technology.
We found that a variety of dose-fractionation regimens were used for lung SBRT. This is not surprising because published studies have used varied dosing schemes, and there is an overall lack of data comparing the efficacy across different regimens. Several ongoing or recently closed trials are comparing different treatment regimens, and results are awaited (RTOG 0813 [NCT00750269], RTOG 0915 [NCT00960999], Alberta Health 20131[NCT00351962]). In our study, the use of single-fraction SBRT was minimal (<1%), and this is appropriate given the relative immaturity of the data regarding this compared with regimens using three to five fractions (19–23).
This study has potential limitations. The NCDB does not contain information regarding tumor location; therefore, it was not possible to perform data analysis stratified by patients with central vs peripheral tumors. It is acknowledged that central tumor location is a relative contraindication to SBRT. However, we found almost no replacement of the proportions of patients receiving conventional external beam radiotherapy or no treatment with SBRT from 2008 to 2013 overall, suggesting that central tumor location is unlikely to be the only factor limiting SBRT adoption across the United States. An additional limitation is the lack of information on the frequency of treatment, so assessment of the use of daily vs nondaily regimens could not be performed. Further, Charlson-Deyo comorbidity score may not have fully captured patients’ overall health status and surgical candidacy. This is one possible explanation for our finding that patients living in more affluent areas were less likely to receive SBRT; patients may be healthier overall in these areas and therefore more likely to undergo surgery. Finally, we do not know why patients of “other race” were less likely to receive SBRT.
In conclusion, we found that SBRT adoption in the United States has been modest and has not significantly clinically reduced the proportions of patients who received no treatment or conventional radiotherapy for stage IA NSCLC. Barriers to adoption of this newer treatment option may relate to the resources required for SBRT and lack of randomized trial data. Efforts are needed to address these barriers so that more patients can have access to this minimally invasive and highly effective treatment modality.
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. [DOI] [PubMed] [Google Scholar]
- 3. Kelsey CR, Marks LB, Hollis D, et al. Local recurrence after surgery for early stage lung cancer: An 11-year experience with 975 patients. Cancer. 2009;115(22):5218–5227. [DOI] [PubMed] [Google Scholar]
- 4. Su S, Scott WJ, Allen MS, et al. Patterns of survival and recurrence after surgical treatment of early stage non-small cell lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. J Thorac Cardiovasc Surg. 2014;147(2):747–752, discussion 752–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howlader N, Noone AM, Krapcho M, et al. , eds. SEER Cancer Statistics Review (CSR) 1975-2012. Bethesda, MD: National Cancer Institute; 2015. [Google Scholar]
- 6. Ginsberg RJ, Rubinstein LV.. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60(3):615–622, discussion 622–613. [DOI] [PubMed] [Google Scholar]
- 7. Rowell NP, Williams CJ.. Radical radiotherapy for stage I/II non-small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable). Cochrane Database Syst Rev. 2001(2):Cd002935. [DOI] [PubMed] [Google Scholar]
- 8. Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S.. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: A retrospective analysis. Lancet Oncol. 2012;13(8):802–809. [DOI] [PubMed] [Google Scholar]
- 10. Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27(20):3290–3296. [DOI] [PubMed] [Google Scholar]
- 11. Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75(3):677–682. [DOI] [PubMed] [Google Scholar]
- 12. Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833–4839. [DOI] [PubMed] [Google Scholar]
- 13. Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7 suppl 3):S94–S100. [DOI] [PubMed] [Google Scholar]
- 14. Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 6.2015. J Natl Compr Canc Netw. 2015;13(5):515–524. [DOI] [PubMed] [Google Scholar]
- 15. Chiang A, Thibault I, Warner A, et al. A comparison between accelerated hypofractionation and stereotactic ablative radiotherapy (SABR) for early-stage non-small cell lung cancer (NSCLC): Results of a propensity score-matched analysis. Radiother Oncol. 2016;118(3):478–484. [DOI] [PubMed] [Google Scholar]
- 16. Lucas JT Jr, Kuremsky JG, Soike M, et al. Comparison of accelerated hypofractionation and stereotactic body radiotherapy for Stage 1 and node negative stage 2 non-small cell lung cancer (NSCLC). Lung Cancer. 2014;85(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bogart JA, Hodgson L, Seagren SL, et al. Phase I study of accelerated conformal radiotherapy for stage I non-small-cell lung cancer in patients with pulmonary dysfunction: CALGB 39904. J Clin Oncol. 2010;28(2):202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker BR, Basak R, Mohiuddin JJ, Chen RC.. Use of stereotactic body radiotherapy for prostate cancer in the United States from 2004 through 2012. Cancer. 2016;122(14):2234–2241. [DOI] [PubMed] [Google Scholar]
- 19. Videtic GM, Stephans KL, Woody NM, et al. 30 Gy or 34 Gy? Comparing 2 single-fraction SBRT dose schedules for stage I medically inoperable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;90(1):203–208. [DOI] [PubMed] [Google Scholar]
- 20. Hof H, Muenter M, Oetzel D, Hoess A, Debus J, Herfarth K.. Stereotactic single-dose radiotherapy (radiosurgery) of early stage nonsmall-cell lung cancer (NSCLC). Cancer. 2007;110(1):148–155. [DOI] [PubMed] [Google Scholar]
- 21. Hara R, Itami J, Kondo T, et al. Clinical outcomes of single-fraction stereotactic radiation therapy of lung tumors. Cancer. 2006;106(6):1347–1352. [DOI] [PubMed] [Google Scholar]
- 22. Fritz P, Kraus HJ, Muhlnickel W, et al. Stereotactic, single-dose irradiation of stage I non-small cell lung cancer and lung metastases. Radiat Oncol. 2006;1:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le QT, Loo BW, Ho A, et al. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J Thorac Oncol. 2006;1(8):802–809. [PubMed] [Google Scholar]