Abstract
Frequent detection of serous tubal intraepithelial carcinoma (STIC) among BRCA1/2 mutation carriers undergoing risk-reducing surgery prompted the hypothesis that many adnexal high-grade serous carcinomas (HGSCs) arise from the fallopian tube, rather than the ovary, as supposed. The changing paradigm has important implications for HGSC prevention. Most data related to the frequency of STIC are derived from case series and estimates vary widely. Therefore, we analyzed population-based data from 10 523 surgeries including salpingectomy (Jan 2014–Dec 2016) that were examined using the “Sectioning and Extensively Examining the Fimbria” protocol, which optimizes STIC detection. Overall, STIC was detected in 40 (0.38%) specimens, including 32 diagnosed with concurrent gynecologic cancer. STIC was detected in 8 (<0.01%) of 9392 cases with benign diagnoses. We conclude that the relative rarity of STIC diagnoses in routine pathology practice has critical implications for research aiming to elucidate the pathogenesis of HGSC and developing prevention strategies.
Frequent detection of occult serous tubal intraepithelial carcinoma (STIC) among pathogenic BRCA1 or BRCA2 mutation carriers at risk-reducing surgery prompted the hypothesis that many adnexal high-grade serous carcinomas (HGSCs) arise in the fallopian tube, rather the ovary, as previously supposed (1–3). STIC may consist of only tiny microscopic (<1 mm) foci, which are easily missed unless the entire fallopian tube is subjected to meticulous microscopic examination to increase the probability of STIC being identified if present, as is achieved with the “Sectioning and Extensively Examining the Fimbria” (SEE-Fim) protocol (4).
Defining the frequency of STIC is central to understanding its relationship to the pathogenesis of HGSC, but published data are derived largely from case series and vary widely. Among women undergoing risk-reducing surgery, STIC has been reported in up to 70% of specimens when a concurrent invasive cancer is present (5,6) and 2–8% when invasive cancer is absent (7,8). In the general population, STIC has been identified in 18–71% of surgical pathology specimens removed for HGSC (9–11) and incidentally in less than 1% of women undergoing benign surgery (12,13). The frequency of STIC in different clinical contexts remains uncertain for several reasons (9,12,14–18): 1) metastases to the tube may mimic STIC, including serous carcinomas of uterine, tubal, or unknown origin (19,20); 2) poor diagnostic reproducibility (2,15,21); 3) dependence on the extent of fallopian tube processing for microscopic examination; (22) and 4) STIC may not be recognizable when fallopian tube microanatomy is effaced by masses of carcinoma. A recent survey found that SEE-Fim is not routinely performed for benign gynecologic pathology specimens or frank cancers in most US pathology laboratories, likely because STIC is rare in the former instance and may not alter management in the latter (23). Herein, we report population-based data from Calgary Laboratory Services (CLS) in Alberta, Canada, which performs total or modified SEE-Fim processing on all fallopian tubes, including histologic examination of all tubal segments and the entire fimbria, where most STIC arises. CLS data provide an estimate of the frequency of STIC in a population-based practice serving 1.3 million residents in which SEE-Fim processing is routine. Hematoxylin and eosin-stained glass slides of formalin-fixed paraffin-embedded tissue sections are reviewed for morphologic changes indicative of STIC, and these are usually confirmed by p53 immunohistochemical staining. Diagnostically challenging cases are reviewed by the laboratory’s gynecological pathologists.
Under a Human Subjects Research Protection waiver, we extracted de-identified pathological and clinical data, including synoptic reporting from the CLS database for 10 523 consecutive surgeries including salpingectomy, processed between January 2014 and December 2016. Surgical indications were classified into nonmutually exclusive categories: 1) cancer-related (including cancer rule-out), 2) post-cancer, 3) risk-reducing, and 4) benign/nonmalignant. The median age of the women at surgery was 45.9 (13.0) years. Data were extracted by free text searches; 112 pathology reports mentioning “STIC” and 166 reporting p53 immunohistochemical staining (which is used to assess possible STIC diagnoses) were reviewed. We generated statistics for the frequency of STICs by age, surgical indication, and pathology diagnosis and evaluated associations with unconditional logistic regression.
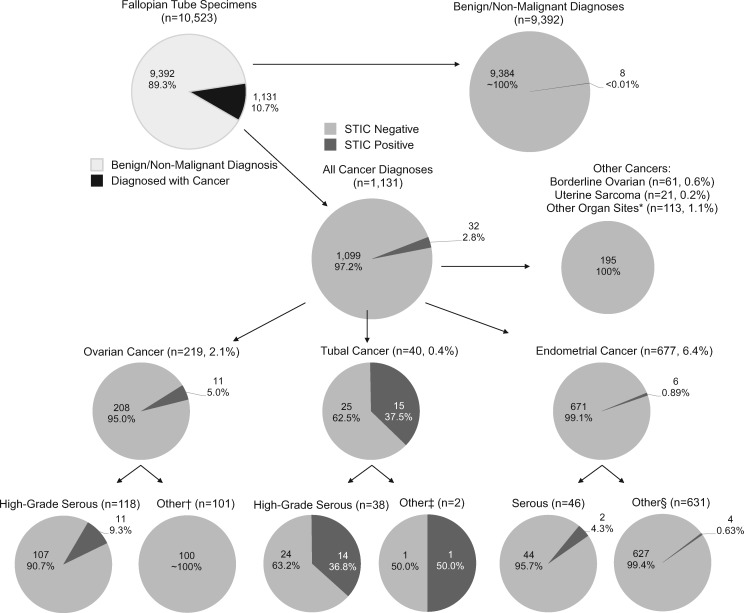
Table 1 describes the clinical characteristics of the 10 523 surgeries involving salpingectomy included in this analysis. Cancer was diagnosed in 1131 (10.7%) specimens; of 6023 surgeries sent to pathology with a mention of cancer (often cancer rule-out), 18% (n = 1085) actually contained cancer whereas among the 4500 surgeries submitted without a mention of cancer, only 1% (n = 46) were diagnosed with cancer. Endometrial, ovarian, or tubal carcinoma were the most frequently diagnosed cancers (Figure 1) . Risk-reducing surgeries accounted for 354 (3.4%) specimens.
Table 1.
Descriptive characteristics of 10 523 surgeries involving salpingectomy in the Calgary Laboratory Services database (2014–2016) overall and by STIC status
| Total population (n = 10 523) |
STIC positive (n = 40, 0.4%) |
STIC negative (n = 10 483, 99.6%) |
||||
|---|---|---|---|---|---|---|
| Descriptive characteristics | No. (%) | No. (%) | No. (%) | |||
| Age, mean (SD), y | 45.9 (13.0) | 60.8 (10.7) | 45.9 (13.0) | |||
| <40 | 3963 (37.7) | 1 (2.5) | 3962 (37.8) | |||
| 40–49 | 3112 (29.6) | 5 (12.5) | 3107 (29.6) | |||
| 50–59 | 1771 (16.8) | 16 (40.0) | 1755 (16.7) | |||
| 60+ | 1677 (15.9) | 18 (45.0) | 1659 (15.8) | |||
| Collection year | ||||||
| 2014 | 3316 (31.5) | 11 (27.5) | 3305 (31.5) | |||
| 2015 | 3538 (33.6) | 10 (25.0) | 3528 (33.7) | |||
| 2016 | 3669 (34.9) | 19 (47.5) | 3650 (34.8) | |||
| Surgical indication* | ||||||
| Cancer† | ||||||
| Yes | 6023 (57.2) | 35 (87.5) | 5988 (57.1) | |||
| No | 4500 (42.8) | 5 (12.5) | 4495 (42.9) | |||
| Post-cancer treatment‡ | ||||||
| Yes | 321 (3.1) | 10 (25.0) | 311 (3.0) | |||
| No | 10 202 (96.9) | 30 (75.0) | 10 172 (97.0) | |||
| Risk reducing§ | ||||||
| Yes | 354 (3.4) | 5 (12.5) | 349 (3.3) | |||
| No | 10 169 (96.6) | 35 (87.5) | 10 134 (96.7) | |||
| Benign/nonmalignant‖ | ||||||
| Yes | 7737 (73.5) | 11 (27.5) | 7726 (73.7) | |||
| No | 2786 (26.5) | 29 (72.5) | 2757 (26.3) | |||
Categories are not mutually exclusive; cases could be classified as having more than one reason for surgery. STIC = serous tubal intraepithelial carcinoma.
Cancer includes: cancer-related surgery, cancer rule-out, adenocarcinoma in situ, high-grade squamous intraepithelial lesion, mass, tumor.
Post-cancer treatment: clinical information text field indicated surgery was occurring post-chemotherapy and/or adjuvant therapy.
Risk-reducing includes: risk reducing, prophylactic, BRCA positive, BRCA test pending, family history of cancer (breast/ovarian/uterine), and family history of BRCA positive.
Benign/nonmalignant includes: adenomyosis, bleeding, cesarean section, cyst, dyspareunia, dysplasia, ectopic pregnancy, endometriomas, endometriosis, fibroid, GI tract related, gynecological abnormalities, hyperplasia, incontinence, IUD issue, pain, pelvic adhesion, pelvic inflammatory disease, pregnancy complications, prolapse, torsion, transgender surgery, tubal ligation (sterilization), tubal related, and sepsis.
Figure 1.
Diagnoses of the 40 serous tubal intraepithelial carcinomas identified from 10 523 cases in the Calgary Laboratory Services database from 2014 to 2016. Numbers and percentage of cases that were diagnosed with cancer, and the proportion of cases within the various histotypes of cancer that were diagnosed with STIC. *Other organ sites: vaginal (squamous), breast (ductal), cervix (squamous, adenocarcinoma, glandular), pelvic wall (leiomyosarcoma), small intestine (mixed), renal (clear cell), peritoneum (serous), liver (cholangiocarcinoma), colon (adenocarcinoma, adenoneuroendocrine, mucinous, signet-ring cell carcinoma, squamous), bladder (urothelial carcinoma), and appendix (adenocarcinoma, goblet cell carcinoid, mucinous, neuroendocrine). †Ovarian cancer (other): low-grade serous, endometrioid, clear cell, mucinous and non-epithelial cancers (carcinosarcoma, germ cell, granulosa, melanoma, mixed, sex cord, teratocarcinosarcoma). ‡Tubal cancer (other): MMMT, carcinosarcoma.§Endometrial cancer (other): endometrioid, mucinous, clear cell, MMMT, carcinosarcoma, choriocarcinoma, mixed, neuroendocrine, undifferentiated.
Overall, STICs were identified in 40 (0.38%) of 10 523 total specimens. STIC was associated with older age (OR per 5-year increase in age = 1.44, 95% CI = 1.30 to 1.60; P < .01), although this association was attenuated with adjustment for cancer diagnosis (any vs none) (OR = 1.13 = 95% CI = 0.996 to 1.29; P = .06). Although the number of STIC diagnoses was higher in 2016 compared with earlier years, calendar year was not a predictor of STIC.
Of 354 risk-reducing surgeries, STIC was identified in 5 (1.4%), including three with benign findings and two related to HGSC (one tubal and one endometrial) (Supplementary Table 1, available online). In addition, a total of five STICs were incidentally identified in gynecologic surgeries (not risk-reducing) with benign diagnoses, of which 2 were found among women younger than 50 years (Supplementary Table 1, available online). Similar to our data, Meserve et al. identified only two incidental STICs with SEE-Fim processing among 1747 specimens unrelated to cancer or risk reduction among women older than 50 years (13). The rarity of STIC among low-risk women without cancer in this and previous studies underscores the challenges posed for developing population-based screening tests with acceptable positive predictive value (few false positives) without improved risk stratification. Further, the low prevalence of STIC may have implications for the relationship of STIC to HGSC. Specifically, our results are consistent with rapid progression of STIC to clinical HGSC, unless such lesions regress, spread from microscopic foci that are missed with SEE-Fim processing, or are unrelated to the pathogenesis of HGSC in some cases. A national gynecologic bio-bank could amass sufficient numbers of STICs and early cancers to support genetic and other molecular analysis to uncover the relationship of STIC to clinical HGSC (24). Increased use of SEE-Fim processing and dramatic increases in the diagnosis of early stage tubal HGSC suggest that this may be feasible (25).
Relating STICs to diagnosis post-surgery, 32 of 40 STICs (80%) were associated with gynecologic cancers with the remaining 8 STICs identified in cases with benign diagnoses. Of the 32 cancer-associated STICs, 11 (34.4%) were associated with ovarian HGSC, 14 (43.8%) with tubal HGSC, and 2 (6.3%) with endometrial HGSC, equating to 27 of 202 HGSC (13.4%) as associated with STIC (Figure 1). Detection of p53 accumulation by immunohistochemistry was found in 11 (91.7%) of 12 STICs tested (Supplementary Table 1, available online).
In a mutually adjusted model including age, factors associated with STIC included risk-reducing surgery (OR = 4.04, 95% CI = 1.36 to 11.96), any HGSC diagnosis (OR = 10.25, 95% CI = 6.29 to 16.72), and report of p53 positive tumor staining (OR = 3.85, 96% CI = 1.67 to 8.88).
Improved diagnosis of STIC and elucidation of its relationship with HGSC would provide a solid basis for evidence-based guidelines for prevention of these often lethal cancers and for clinical management of women with STIC. Reported outcomes for women diagnosed with STIC suggest that recurrences are rare, but data are limited by the number of reported cases and length of follow-up. Evidence suggests that STIC may metastasize (26–29), and undiagnosed STIC has been suggested as the source of primary peritoneal cancers diagnosed years after benign gynecologic surgery (30). Further, diagnosis of STIC may identify women who would benefit from genetic testing and counseling. Given that salpingectomy truncates the natural history of STIC, innovative approaches and large consortia may be needed to advance our understanding of the risks posed by STIC alone and its role in the pathogenesis of HGSC.
Note
Affiliations of authors: Division of Cancer Prevention (GS) and Division of Cancer Epidemiology and Genetics (BT, AMG), National Cancer Institute, Bethesda, MD; Department of Pathology and Laboratory Medicine, Cumming School of Medicine, University of Calgary, Calgary, Canada (MAD); Division of Epidemiology, Mayo Clinic, Jacksonville, FL (MES).
Supplementary Material
References
- 1. Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195(4):451–456. [DOI] [PubMed] [Google Scholar]
- 2. Crum CP, Drapkin R, Kindelberger D, et al. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5(1):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kurman RJ, Shih IM.. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol. 2016;186(4):733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Medeiros F, Muto MG, Lee Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30(2):230–236. [DOI] [PubMed] [Google Scholar]
- 5. Callahan MJ, Crum CP, Medeiros F, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25(25):3985–3990. [DOI] [PubMed] [Google Scholar]
- 6. Przybycin CG, Kurman RJ, Ronnett BM, et al. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34(10):1407–1416. [DOI] [PubMed] [Google Scholar]
- 7. Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100(1):58–64. [DOI] [PubMed] [Google Scholar]
- 8. Powell CB, Chen LM, McLennan J, et al. Risk-reducing salpingo-oophorectomy (RRSO) in BRCA mutation carriers: experience with a consecutive series of 111 patients using a standardized surgical-pathological protocol. Int J Gynecol Cancer. 2011;21(5):846–851. [DOI] [PubMed] [Google Scholar]
- 9. Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31(2):161–169. [DOI] [PubMed] [Google Scholar]
- 10. Carlson JW, Miron A, Jarboe EA, et al. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol. 2008;26(25):4160–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horn LC, Kafkova S, Leonhardt K, et al. Serous tubal in situ carcinoma (STIC) in primary peritoneal serous carcinomas. Int J Gynecol Pathol. 2013;32(4):339–344. [DOI] [PubMed] [Google Scholar]
- 12. Tang SG, Onuma K, Deb P, et al. Frequency of serous tubal intraepithelial carcinoma in various gynecologic malignancies: a study of 300 consecutive cases. Int J Gynecol Pathol. 2012;31(2):103–110. [DOI] [PubMed] [Google Scholar]
- 13. Meserve EEK, Mirkovic J, Conner JR, et al. Frequency of “incidental” serous tubal intraepithelial carcinoma (STIC) in women without a history of or genetic risk factor for high-grade serous carcinoma: a six-year study. Gynecol Oncol. 2017;146(1):69–73. [DOI] [PubMed] [Google Scholar]
- 14. Schneider S, Heikaus S, Harter P, et al. Serous tubal intraepithelial carcinoma associated with extraovarian metastases. Int J Gynecol Cancer. 2017;27(3):444–451. [DOI] [PubMed] [Google Scholar]
- 15. Visvanathan K, Vang R, Shaw P, et al. Diagnosis of serous tubal intraepithelial carcinoma based on morphologic and immunohistochemical features: a reproducibility study. Am J Surg Pathol. 2011;35(12):1766–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roh MH, Kindelberger D, Crum CP.. Serous tubal intraepithelial carcinoma and the dominant ovarian mass: clues to serous tumor origin? Am J Surg Pathol. 2009;33(3):376–383. [DOI] [PubMed] [Google Scholar]
- 17. Chen F, Gaitskell K, Garcia MJ, et al. Serous tubal intraepithelial carcinomas associated with high-grade serous ovarian carcinomas: a systematic review. BJOG. 2017;124(6):872–878. [DOI] [PubMed] [Google Scholar]
- 18. Powell CB. Risk reducing salpingo-oophorectomy for BRCA mutation carriers: twenty years later. Gynecol Oncol. 2014;132(2):261–263. [DOI] [PubMed] [Google Scholar]
- 19. Eckert MA, Pan S, Hernandez KM, et al. Genomics of ovarian cancer progression reveals diverse metastatic trajectories including intraepithelial metastasis to the fallopian tube. Cancer Discov. 2016;6(12):1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDaniel AS, Stall JN, Hovelson DH, et al. Next-generation sequencing of tubal intraepithelial carcinomas. JAMA Oncol. 2015; doi:10.1001/jamaoncol.2015.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carlson JW, Jarboe EA, Kindelberger D, et al. Serous tubal intraepithelial carcinoma: diagnostic reproducibility and its implications. Int J Gynecol Pathol. 2010;29(4):310–314. [DOI] [PubMed] [Google Scholar]
- 22. Rabban JT, Krasik E, Chen LM, et al. Multistep level sections to detect occult fallopian tube carcinoma in risk-reducing salpingo-oophorectomies from women with BRCA mutations: implications for defining an optimal specimen dissection protocol. Am J Surg Pathol. 2009;33(12):1878–1885. [DOI] [PubMed] [Google Scholar]
- 23. Samimi G, Trabert B, Duggan MA, et al. Processing of fallopian tube, ovary, and endometrial surgical pathology specimens: a survey of U.S. laboratory practices. Gynecol Oncol. 2018;148(3):515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sherman ME, Drapkin RI, Horowitz NS, et al. Rationale for developing a specimen bank to study the pathogenesis of high-grade serous carcinoma: a review of the evidence. Cancer Prev Res (Phila). 2016;9(9):713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trabert B, Coburn SB, Mariani A, et al. Reported incidence and survival of fallopian tube carcinomas: a population-based analysis from the North American Association of Central Cancer Registries. J Natl Cancer Inst. 2017; doi:10.1093/jnci/djx263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chay WY, McCluggage WG, Lee CH, et al. Outcomes of incidental fallopian tube high-grade serous carcinoma and serous tubal intraepithelial carcinoma in women at low risk of hereditary breast and ovarian cancer. Int J Gynecol Cancer. 2016;26(3):431–436. [DOI] [PubMed] [Google Scholar]
- 27. Wethington SL, Park KJ, Soslow RA, et al. Clinical outcome of isolated serous tubal intraepithelial carcinomas (STIC). Int J Gynecol Cancer. 2013;23(9):1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patrono MG, Corzo C, Iniesta M, et al. Management of preinvasive lesions. Clin Obstet Gynecol. 2017;60(4):771–779. [DOI] [PubMed] [Google Scholar]
- 29. Patrono MG, Iniesta MD, Malpica A, et al. Clinical outcomes in patients with isolated serous tubal intraepithelial carcinoma (STIC): a comprehensive review. Gynecol Oncol. 2015;139(3):568–572. [DOI] [PubMed] [Google Scholar]
- 30. Seidman JD, Zhao P, Yemelyanova A.. “Primary peritoneal” high-grade serous carcinoma is very likely metastatic from serous tubal intraepithelial carcinoma: assessing the new paradigm of ovarian and pelvic serous carcinogenesis and its implications for screening for ovarian cancer. Gynecol Oncol. 2011;120(3):470–473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.