Abstract
Background
One year of adjuvant trastuzumab in combination with chemotherapy is the standard of care in early-stage human epidermal growth factor receptor 2 (HER2)-positive breast cancer. Existing data on shortening trastuzumab treatment show conflicting results.
Methods
A search of PubMed and abstracts from key conferences identified randomized trials that compared abbreviated trastuzumab therapy to 1 year of treatment in early-stage HER2-positive breast cancer. Hazard ratios (HRs) and 95% confidence intervals (CIs) were extracted for disease-free survival (DFS) and overall survival (OS). Subgroup analyses evaluated the effect of nodal involvement, estrogen receptor expression, and the duration of abbreviated trastuzumab (9–12 weeks vs 6 months). Odds ratios (ORs) and 95% confidence intervals were computed for prespecified cardiotoxicity events including cardiac dysfunction and congestive heart failure. P values were two-sided.
Results
Analysis included six trials comprising 11 603 patients. Shorter trastuzumab treatment was associated with worse DFS (HR = 1.14, 95% CI = 1.05 to 1.25, P = .002) and OS (HR = 1.15, 95% CI = 1.02 to 1.29. P = .02). The effect on DFS was not influenced by estrogen receptor status (P for the subgroup difference = .23), nodal involvement (P = .44), or the different duration of trastuzumab in the experimental arm (P = .09). Shorter trastuzumab treatment was associated with lower odds of cardiac dysfunction (OR = 0.67, 95% CI = 0.55 to 0.81, P < .001) and congestive heart failure (OR = 0.66, 95% CI = 0.50 to 0.86, P = .003).
Conclusions
Compared with 1 year, shorter duration of adjuvant trastuzumab is associated with statistically significantly worse DFS and OS despite favorable cardiotoxicity profile. One year of targeted HER2 treatment should remain the standard adjuvant treatment in early-stage HER2-positive disease with appropriate cardiac monitoring.
Overexpression of human epidermal growth factor receptor 2 (HER2) occurs in 15%–20% of early-stage breast cancers (1). Randomized control trials (RCTs) exploring the addition of trastuzumab to standard adjuvant chemotherapy have shown a statistically significant improvement in outcome (2–4). The majority of studies explored 1 year of trastuzumab therapy, which subsequently became the standard of care in early-stage HER2- positive breast cancer (5).
There is uncertainty regarding the optimal duration of adjuvant trastuzumab. The decision to treat for 1 year was somewhat arbitrary, because there were no clinical or preclinical data to guide the optimal duration of treatment (6). Although most women tolerate treatment with trastuzumab well, this drug can cause cardiac dysfunction, and longer duration of treatment is associated with higher odds for cardiotoxicity (4,7–9). Additionally, trastuzumab requires periodic heart function monitoring and regular 3-weekly treatment administration and has financial implications (10).
The FinHER study showed that the addition of 9 weeks of trastuzumab to standard chemotherapy improved disease-free survival (DFS) and overall survival (OS) (11,12) compared with placebo, with a magnitude of effect similar to this seen in RCTs evaluating 1 year of trastuzumab (2–4). Consequently, several RCTs compared a shorter duration of adjuvant trastuzumab with the standard 1-year treatment with conflicting results (13–18). Although the PERSEPHONE trial, the largest study evaluating abbreviated duration of adjuvant trastuzumab, did meet its objective of observing noninferiority (17), other large-scale studies, including the SOLD study and the recently updated PHARE study, failed to show noninferiority (16,18).
The magnitude of benefit from adjuvant trastuzumab varies and depends typically on stage and hormone receptor expression, with lower absolute benefit in patients with less-advanced stage and hormone receptor–positive disease (19–21). The influence of these classical prognostic factors on the difference in benefit between 1 year of trastuzumab and abbreviated therapy is unknown.
Here, we report on a meta-analysis evaluating the efficacy and toxicity of shorter duration of adjuvant trastuzumab compared with 1 year of treatment in women with HER2- positive early-stage breast cancer. We also aimed to identify whether specific subgroups, defined by nodal status and estrogen receptor (ER) expression, have a differential relative effect from shorter trastuzumab treatment. We hypothesized that a shorter duration of trastuzumab would be associated with worse outcome despite favorable cardiac toxicity.
Methods
Literature Review and Study Identification
A literature search using MEDLINE (Host: PubMed) identified RCTs published between January 2008 and December 31, 2018 that compared 1 year of adjuvant trastuzumab with a shorter duration of therapy in early-stage HER2-positive breast cancer. The terms “adjuvant,” “breast cancer,” and “trastuzumab” and similar terms were cross-searched by using the following search algorithm: (adjuvant OR neoadjuvant) AND (breast neoplasm MeSH OR [(breast OR mammary) AND (carcinoma OR malignan* OR neoplasm OR tumor)]) AND (Herceptin OR trastuzumab OR anti HER2 OR HER2 monoclonal antibody). To improve the sensitivity, we also searched databases from the Annual Meetings of the American Society of Clinical Oncology (2015–2018) and the San Antonio Breast Cancer Symposium (2015–2018) and reviewed citation lists. The search was restricted to the English language only.
Data Extraction
Data were collected independently by two reviewers (H. Goldvaser and Y. Korzets). Discrepancies were resolved by a third reviewer (E. Amir). Collected data included year of publication, number of patients, median age, proportion of premenopausal patients, and median duration of follow-up. We also collected trial-level tumor characteristics including median tumor size and the proportion of patients with nodal involvement, high-grade tumors, and hormone receptor–positive disease. For the efficacy analyses, data on the hazard ratio (HR) and 95% confidence intervals (CIs) for DFS and OS were collected. Data on the number of DFS and distant relapse events were also collected as were the number of patients at risk in each group. When available, data on DFS for subgroups based on ER status (positive and negative) and nodal status (positive and negative) were extracted. We then collected data on cardiac toxicity including the number of congestive heart failure (CHF) and cardiac dysfunction events as defined by the Cardiac Review and Evaluation Committee (22) or definitions closely related to these criteria (referred to as cardiac dysfunction henceforth).
Data Synthesis and Statistical Analysis
The primary analysis compared DFS and OS between patients who were randomly assigned to short trastuzumab treatment with those randomly assigned to 1 year of treatment. HR and 95% CI for DFS and OS were pooled in a meta-analysis using RevMan 5.3 (The Cochrane Collaboration, Copenhagen, Denmark) using generic inverse variance. Subgroup analyses were performed to explore the effect of abbreviated trastuzumab treatment based on ER expression and nodal status. Additionally, the difference between the shorter-duration treatments of the included studies (9–12 weeks vs 6 months) was also analyzed in a subgroup analysis. Differences between the subgroups were assessed using methods describes by Deeks et al. (23). For the number of DFS, distant relapse and cardiotoxicity events, pooled estimates of odds ratio (OR) were computed using the Mantel-Haenszel odds ratio method (24) unless the absolute event rates in the experimental and control groups were less than 1% in at least one study, in which case the Peto one-step odds ratio (25) was used. Absolute difference in outcomes and the number needed to treat (NNT) with shorter trastuzumab therapy to avoid one event were computed. Statistical heterogeneity was reported using Cochran Q and I2 statistics. Statistically significant heterogeneity was defined as Cochran Q P less than .10 or I2 greater than 50%. Fixed-effects modeling was used in the absence of statistically significant heterogeneity; otherwise, the random-effects model was used. To address the potential influence of clinical heterogeneity between studies, a sensitivity analysis using random-effects modeling was conducted for analyses performed initially using fixed effects. Additional sensitivity analyses included the exclusion of studies in which trastuzumab was not always given concurrently with chemotherapy, the exclusion of studies in which there was contamination reported in more than 10% of the study population, and the exclusion of studies in which anthracycline dosage of the experimental and the control arm was not identical. Meta-regression analyses explored the influence of duration of follow-up, median age, and the proportion of premenopausal women and those with larger (>2 cm) tumor size and high-grade tumors. The analysis was performed first for DFS and then repeated for OS. Meta-regression was performed using SPSS version 25 (IBM Corp, Armonk, NY) using the weighted least squares (mixed-effects) function (26). Statistical significance was defined as P less than .05. No corrections were made for multiple testing.
Results
The search identified 1674 studies. After exclusions, nine publications (13–18,27–29) reporting on outcomes from six studies were included in the analysis (cardiotoxicity outcomes from two studies were reported separately from efficacy data [27,28]). Details of the study selection schema are shown in Figure 1. Included studies comprised 11 603 patients. Individual study characteristics are shown in Table 1. In one study, reassessment of HER2 status found that 17.6% of the patients were HER2 negative (29). This study was therefore subjected to a sensitivity analysis. The duration of adjuvant trastuzumab in the control group was 1 year in all studies, and the duration of trastuzumab in the experimental group was either 9–12 weeks (15,16,29) or 6 months (13,14,17,18). In four studies, the adjuvant chemotherapy composed of an anthracycline and taxane regimen and treatment with trastuzumab was started concurrently with chemotherapy (13,15,16,29). Of note, in one study, compared with the control arm, the dose of anthracycline given in the abbreviated arm was lower (15); therefore, this study was also subject to sensitivity analysis. In two studies, different combinations were allowed, but most patients were treated with a combination of anthracyclines and taxanes, and only a minority of the patients (approximately 10%) were treated with taxanes only (14,18) or taxane-based chemotherapy (17). In these two studies, trastuzumab was given both in sequence and concurrently with chemotherapy (14,17,18).
Figure 1.
Study selection schema.
Table 1.
Characteristics of included studies
| Trial; median follow-up | Chemotherapy* | Duration of short trastuzumab | Sample size | Age (median or mean), y | Premenopausal No., % | Tumor size | Nodal status† | Hormone- receptor–positive, % | Grade 3, % |
|---|---|---|---|---|---|---|---|---|---|
| Investigators' choice of chemotherapy (at least 4 cycles)‡ followed by trastuzumab | 6 months | 3380 | 55 | NR |
|
|
58.3 | 55.8 | |
|
P(175)+H q 21*4→ AC (60,600) q21*4 | 12 weeks | 227 | 49 | NR | NR |
|
61.7 | NR |
|
FEC (700,75,700) q14*4→ D (75)+H q14*4 | 6 months | 481 | 54–56 | 38.1 | NR |
|
66.7 | 52.4 |
|
D (80/100)+H q 21*3→ FEC (600,75,600) q21*3 | 9 weeks | 2174 | 56 | 33 |
|
|
66 | 66.5 |
|
9 weeks | 1253 | 55 | 36 |
|
|
68.2 | NR | |
|
Different regimens‡ | 6 months | 4088 | 56 | 39 |
|
|
69 | 67 |
In parentheses the dose of chemotherapy is in mg/m2, respectively. A = adriamycin; C = cyclophosphamide; D = docetaxel; DFS = disease-free survival; E = epirubicin; F = fluorouracil; H = trastuzumab (Herceptin); NR = not reported; P = paclitaxel; T = tumor size.
Nodal status: N0: no lymph node metastases, N1: 1–3 lymph nodes, N2: 4–9 lymph nodes, N3: 10 or more.
Chemotherapy regimens used in the PHARE study: anthracycline based: 15.7%, anthracycline and taxanes: 73.3%, taxanes: 10.9%, other: 0.1%. Trastuzumab was given concurrently with chemotherapy in 56.4% of the patients. Chemotherapy used in the PERSEPHONE study: anthracycline based: 42%, anthracycline and taxanes: 48%, taxane based: 10%, other: less than 1%. Trastuzumab was given concurrently with chemotherapy in 47% of the patients.
Patients age 65 years and older received 80 mg/m2 docetaxel. In the control group 11% received 175 mg/m2 paclitaxel.
Data for tumor size were not available; detailed data on stage.
Efficacy
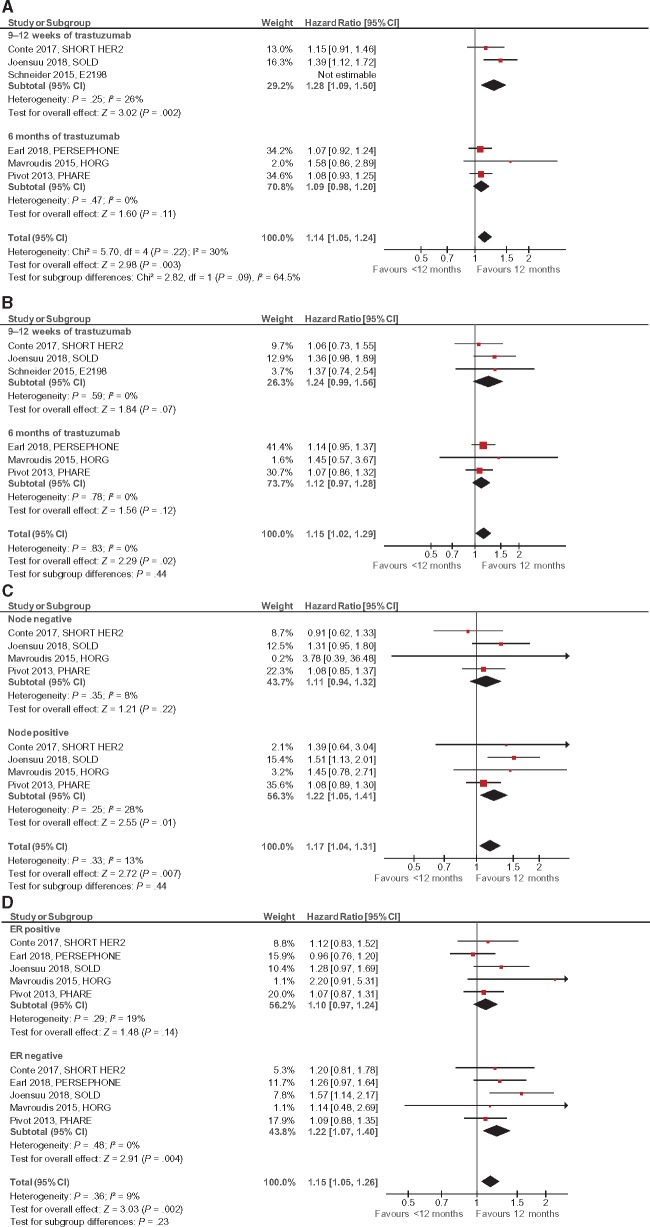
All six studies reported on DFS and OS (13–18,29) and three studies reported on distant relapse (13,14,16,18). Compared with 1 year of treatment, shorter trastuzumab treatment was associated with worse DFS (HR = 1.14, 95% CI = 1.05 to 1.24, P = .003) and OS (HR = 1.15, 95% CI = 1.02 to 1.29, P = .02) (see Figure 2, A and B). Studies using trastuzumab for 9–12 weeks showed worse DFS than trastuzumab treatment for 6 months (HR = 1.28, 95% CI = 1.09 to 1.50 vs HR = 1.09, 95% CI = 0.98 to 1.20), but this did not reach statistical significance (Psubgroup difference = .09). For OS no difference was seen between the two abbreviated trastuzumab durations (P = .44). After an estimated median follow-up of 71 months, shorter treatment with trastuzumab was associated with an absolute increase in DFS events of 2.3% (NNT 43). Similarly, after an estimated median follow-up of 76.8 months, there was a 1.5% higher absolute risk of distant relapse with abbreviated trastuzumab therapy (NNT 67).
Figure 2.
Forest plots for efficacy, hazard ratio for A) disease-free survival (DFS), B) overall survival, C) DFS by nodal involvement (negative or positive), and D) DFS by estrogen receptor status (positive or negative). Hazard ratios for each trial are represented by squares, the size of the square represents the weight of the trial in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval (CI). The diamonds represent the estimated pooled effect. All P values are two-sided.
Four studies reported DFS by nodal status (13–16,18) and five studies reported data by ER status (13–18). The inferior outcomes with abbreviated trastuzumab treatment were of greater magnitude for node-positive compared with node-negative disease and for ER-negative compared with ER-positive disease, although neither of these differences met statistical significance (P = .44 and P = .23, respectively; Figure 2, C and D). Multiple sensitivity analyses for all efficacy endpoints did not show a statistically significant effect on the results (see Supplementary Appendix A, available online). Results of the meta-regression are shown in Table 2. None of the evaluated variables had a statistically significant impact on DFS and OS.
Table 2.
Results of meta-regression for efficacy*
| Variable | β | P |
|---|---|---|
| DFS | ||
| Median follow-up | –0.509 | .302 |
| Age | –0.068 | .898 |
| Premenopausal | –0.753 | .247 |
| T > 2 cm | –0.895 | .295 |
| Grade 3 | 0.029 | .971 |
| OS | ||
| Median follow-up | –0.596 | .212 |
| Age | 0.028 | .958 |
| Premenopausal | –0.608 | .392 |
| T > 2 cm | –0.994 | .071 |
| Grade 3 | 0.320 | .680 |
DFS = disease-free survival; ER = estrogen receptor; OS = overall survival; T = tumor size.
Cardiotoxicity
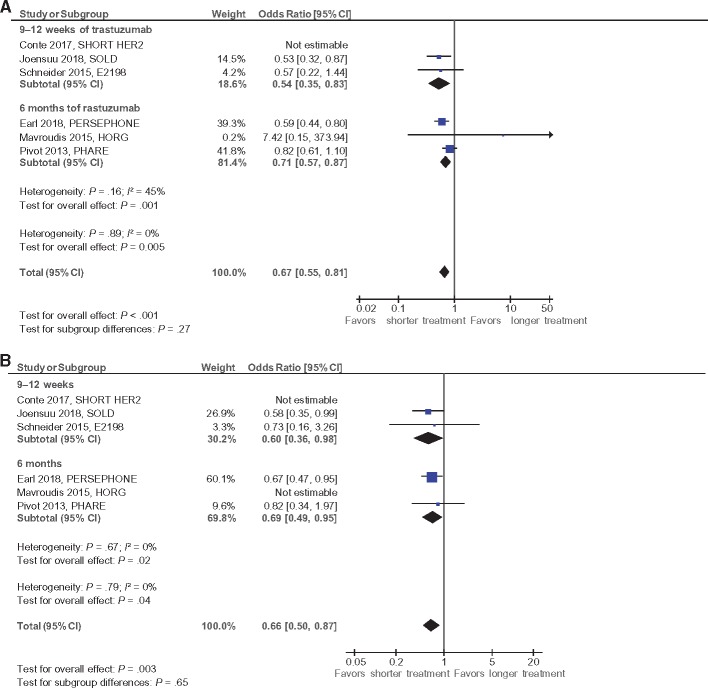
Definitions for cardiac events and frequency of cardiac monitoring in individual studies are reported in Supplementary Appendix B (available online). Data on cardiac dysfunction and CHF were available in five studies (13,16,26–28) with an estimated median follow-up of 60.7 months. Shorter duration of trastuzumab was associated with statistically significantly lower odds for cardiac dysfunction (OR = 0.67, 95% CI = 0.55 to 0.81, P < .001) and CHF (OR = 0.66, 95% CI = 0.50 to 0.86, P = .003) (see Figure 3, A and B). These differences were comparable in studies using trastuzumab for six months and those using shorter periods of trastuzumab (Psubgroup difference = .27 and P = .65 for cardiac dysfunction and CHF, respectively). The weighted absolute difference for cardiac dysfunction was 1.1% with an NNT of 89, and the weighted absolute difference for CHF was 1.0% with an NNT of 101. An additional study reported on all grade two or higher cardiac adverse events and found statistically significantly lower events with shorter trastuzumab treatment (15), but these data could not be included in the meta-analysis for cardiac dysfunction or CHF because of differences in the definition of cardiac toxicity.
Figure 3.
Forest plots for cardiotoxicity, hazard ratio for A) cardiac dysfunction and B) congestive heart failure. Odds ratios for each trial are represented by the squares, the size of the square represents the weight of the trial in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval (CI). The diamonds represent the estimated pooled effect. All P values are two-sided.
Discussion
The discovery of trastuzumab has changed the natural history of HER2-positive breast cancer, and its addition to chemotherapy in early-stage disease is a gold standard (5). One year of adjuvant treatment has been the standard duration based on several large-scale adjuvant studies comparing 1 year of trastuzumab with placebo (2–4). Because trastuzumab is associated with cardiotoxicity and substantial direct and indirect costs, investigating the impact of a shorter treatment duration is of interest.
In this meta-analysis, we found that a shorter duration of treatment resulted in statistically significantly worse DFS compared with 1 year of adjuvant trastuzumab. Over the course of almost 6 years of follow-up, this translated to a 2.3% absolute increase in DFS events, the majority of which were distant relapses. Unsurprisingly, therefore, shorter trastuzumab treatment was associated with worse OS. The statistically significant reduction in cardiotoxicity with shorter treatment did not translate into a statistically significant impact on OS, and the overall benefit of longer trastuzumab treatment on breast cancer outcome outweighed the increased risk of cardiac disease.
The studies included in this analysis were all designed as noninferiority trials. However, because of difficulties in combining noninferiority studies into a meta-analysis (30), we used data to infer inferiority of abbreviated trastuzumab therapy.
Tumor size and nodal involvement affect the absolute benefit from adjuvant treatment in early-stage HER2-positive breast cancer. Patients with small tumors and without nodal involvement have relatively low risk of recurrence. A singlearm study has shown that in patients with small tumors without nodal involvement, deescalating chemotherapy to only 12 weeks of weekly paclitaxel in combination with 1 year of trastuzumab results in excellent outcome (31). Exploratory analysis from two of the included studies in this meta-analysis did recognize a lower-risk population with comparable DFS between both arms (20,32). These data support the importance of finding a population with a lower risk that will not be harmed by deescalating adjuvant treatment. In the current analysis, many patients had high risk of recurrence with frequent nodal involvement or large tumors. Additionally, the vast majority were treated with polychemotherapy in addition to trastuzumab. Although compared with patients with node-positive disease, patients with node-negative disease had a lower degree of inferiority from abbreviated treatment; this difference was not statistically significant. Therefore, current data do not support shortening trastuzumab treatment when using the deescalated chemotherapy regimen of 12 weeks of single-agent paclitaxel even in low-risk patients.
Deescalating trastuzumab treatment could have several advantages. It is associated with reduced risk of cardiotoxicity (7,8,10,21) and lower cost compared with 1 year of treatment. Therefore, further research in selected low-risk populations is warranted to better tailor treatment in early-stage HER2-positive disease.
Although prior meta-analyses have reported similar results (9,33), neither of these studies included data from the E2198 study (29). Additionally, the current analysis was able to include updated results from the PHARE study, with much longer follow-up, in contrast to prior meta-analyses (18). Finally, in the current analysis, we performed meta-regression to evaluate the impact of several important characteristics, including tumor size, menopausal status, age, and grade on results. To our knowledge, these variables have not been tested in meta-regression analyses in prior analyses. The combination of more- inclusive eligibility criteria, data based on longer follow-up, and more extensive meta-regression likely result in the current analysis being a more robust evaluation of deescalated tratsuzumab in early breast cancer than those reported previously.
This study has limitations. First, because this is a literature-based rather than an individual patient meta-analysis, data on comorbidities and concurrent medications were not available and could not be adjusted for. Second, there was limited information about reversibility of cardiac toxicity, and because of differences between studies in the duration of follow-up, we were unable to report actuarial rates of cardiotoxicity. Additionally, to better assess the potential benefit of reduced cardiotoxicity on mortality, a longer duration of follow-up is required. Third, there was some variability in the definitions of cardiac events among the included studies. Furthermore, we were unable to exclude ascertainment bias for cardiac toxicity because these studies were open label rather than placebo controlled. Additionally, in two studies there was a lack of consistency in the frequency of cardiac monitoring, with more assessments performed in the control group (13,29). This may have led to increased diagnosis of cardiac dysfunction in the control group. Fourth, although most included patients were treated with a combination of anthracyclines and taxanes, there was variability between included studies in the chemotherapy regimens administered, and in one study the dosage of anthracyclines was reduced in the abbreviated arm (15), which might also reduce the treatment efficacy in the experimental arm. Additionally, the duration of shorter trastuzumab treatment was not consistent among the included studies, and in two studies administration of trastuzumab in sequence with chemotherapy was allowed (14,17,18). This heterogeneity could have influenced our results.
In conclusion, compared with 1 year of treatment, abbreviated durations of trastuzumab in early-stage HER2-positive disease are associated with worse DFS and OS, but with reduced cardiotoxicity. One year of anti–HER2-targeted treatment along with cardiac monitoring should remain the standard of care in this population. Patients with low-risk disease, mainly small, ER-positive, node-negative disease, may be an appropriate group for further prospective study to evaluate shorter trastuzumab treatment together with deescalated chemotherapy regimens such as 12 weeks of paclitaxel.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Notes
Affiliations of authors: Division of Medical Oncology, University of Toronto and Princess Margaret Cancer Centre, Toronto, Canada (HG, DS, DR, EA); Davidoff Cancer Center, Beilinson Hospital, Rabin Medical Center, Petach Tikva, Israel (HG, YK, DS, RY, MS); Sackler Faculty of Medicine, Tel Aviv University, Israel (HG, YK, RY, MS); Department of Radiation Oncology, University of Toronto and Princess Margaret Cancer Centre, Toronto, Canada (YK); Ted Rogers Program in Cardiotoxicity Prevention, Toronto General Hospital, Peter Munk Cardiac Center, University of Toronto, Toronto, Canada (PT).
Dr Goldvaser declared an honorarium payment from Roche as an invited speaker. Dr Yerushalmi declared a contracted grant and personal fees from Roche for expert consulting. Dr Sarfaty declared an honorarium payment from Roche and Novartis as an invited speaker. Dr Amir declared personal fees from Genentech/Roche for expert testimony. The other authors have no conflicts of interest to declare.
Supplementary Material
References
- 1. Loibl S, Gianni L.. HER2-positive breast cancer. Lancet. 2017;389(10087):2415–2429. [DOI] [PubMed] [Google Scholar]
- 2. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curigliano G, Burstein HJ, P Winer E, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28(8):1700–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pinto AC, Ades F, de Azambuja E, Piccart-Gebhart M.. Trastuzumab for patients with HER2 positive breast cancer: delivery, duration and combination therapies. Breast. 2013;22(2):S152–S155. [DOI] [PubMed] [Google Scholar]
- 7. Long HD, Lin YE, Zhang JJ, Zhong WZ, Zheng RN.. Risk of congestive heart failure in early breast cancer patients undergoing adjuvant treatment with trastuzumab: a meta-analysis. Oncologist. 2016;21(5):547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Azambuja E, Procter MJ, van Veldhuisen DJ, et al. Trastuzumab associated cardiac events at 8 years of median follow-up in the Herceptin adjuvant trial (BIG 1-01). J Clin Oncol. 2014;32(20):2159–2165. [DOI] [PubMed] [Google Scholar]
- 9. Gyawali B, Niraula S.. Duration of adjuvant trastuzumab in HER2 positive breast cancer: overall and disease free survival results from meta-analyses of randomized controled trials. Cancer Treat Rev. 2017;60:18–23. [DOI] [PubMed] [Google Scholar]
- 10. Kurian AW, Thompson RN, Gaw AF, Arai S, Ortiz R, Garber AM. A cost-effectiveness analysis of adjuvant trastuzumab regimens in early HER2/neu-positive breast cancer. J Clin Oncol. 2007;25(6):634–641. [DOI] [PubMed] [Google Scholar]
- 11. Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354(8):809–820. [DOI] [PubMed] [Google Scholar]
- 12. Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27(34):5685–5692. [DOI] [PubMed] [Google Scholar]
- 13. Mavroudis D, Saloustros E, Malamos N, et al. ; Breast Cancer Investigators of Hellenic Oncology Research Group (HORG), Athens, Greece. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol. 2015;26(7):1333–1340. [DOI] [PubMed] [Google Scholar]
- 14. Pivot X, Romieu G, Debled M, et al. ; PHARE trial investigators. Six months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14(8):741–748. [DOI] [PubMed] [Google Scholar]
- 15. Conte PF, Bisagni G, Frassoldati A, et al. 9 weeks vs 1 year adjuvant trastuzumab in combination with chemotherapy: results of the phase III multicentric Italian study Short-HER. J Clin Oncol. 2017;35(suppl; abstr 501). [Google Scholar]
- 16. Joensuu H, Fraser J, Wildiers H, et al. Effect of adjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2–positive breast cancer the SOLD randomized clinical trial. JAMA Oncol. 2018;4(9):1199–1206. doi:10.1001/jamaoncol.2018.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Earl HM, Hiller L, Vallier AL, et al. PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results. Presented at the American Society of Clinical Oncology Annual Meeting, June 2018. J Clin Oncol. 2018;36(15 suppl):506. [Google Scholar]
- 18. Pivot X, Romieu G, Debled M, et al. PHARE randomized trial final results comparing 6 to 12 months of trastuzumab in adjuvant early breast cancer. In: 2018 San Antonio Breast Cancer Symposium; San Antonio, TX, December 4–8, 2018, GS2-07.
- 19. O'Sullivan CC, Bradbury I, Campbell C, et al. Efficacy of Adjuvant trastuzumab for patients with human epidermal growth factor receptor 2-positive early breast cancer and tumors ≤ 2 cm: a meta-analysis of the randomized trastuzumab trials. J Clin Oncol. 2015;33(24):2600–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kramar A, Bachelot T, Madrange N, et al. Trastuzumab duration effects within patient prognostic subgroups in the PHARE trial. Ann Oncol. 2014;25(8):1563–1570. [DOI] [PubMed] [Google Scholar]
- 21. Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–384. [DOI] [PubMed] [Google Scholar]
- 22. Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215–1221. [DOI] [PubMed] [Google Scholar]
- 23. Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323(7305):157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JPT, Deeks JJ, Altman DG. Meta-analysis of rare events. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0) The Cochrane Collaboration, Copenhagen, Denmark; 2011. http://www.cochrane-handbook.org.
- 25. Sweeting MJ, Sutton AJ, Lambert PC.. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351–1375. [DOI] [PubMed] [Google Scholar]
- 26. Stanley TD, Doucouliagos H.. Neither fixed nor random: weighted least squares meta-regression. Res Synth Methods. 2017;8(1):19–42. [DOI] [PubMed] [Google Scholar]
- 27. Pivot X, Suter T, Nabholtz JM, et al. Cardiac toxicity events in the PHARE trial, an adjuvant trastuzumab randomised phase III study. Eur J Cancer. 2015;51(13):1660–1666. [DOI] [PubMed] [Google Scholar]
- 28. Earl HM, Vallier AL, Dunn J, et al. Trastuzumab-associated cardiac events in the Persephone trial. Br J Cancer. 2016;115(12):1462–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schneider BP, O'Neill A, Shen F, et al. Pilot trial of paclitaxel-trastuzumab adjuvant therapy for early stage breast cancer: a trial of the ECOG-ACRIN cancer research group (E2198). Br J Cancer. 2015;113(12):1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Witte S, Victor N.. Some problems with the investigation of noninferiority in meta-analysis. Methods Inf Med. 2004;43(5):470–474. [PubMed] [Google Scholar]
- 31. Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Conte PF, Guarneri V, Bisagni G, et al. 191PD_PR 9 weeks versus 1 year adjuvant trastuzumab for HER2+ early breast cancer: subgroup analysis of the ShortHER trial allows to identify patients for whom a shorter trastuzumab administration may have a favourable risk/benefit ratio. Ann Oncol. 2018;29(suppl 8):mdy424.005. [Google Scholar]
- 33. Inno A, Barni S, Ghidini A, Zaniboni A, Petrelli F.. One year versus a shorter duration of adjuvant trastuzumab for HER2-positive early breast cancer: systemic review and meta-anlsysis. Breast Cancer Res Treat. 2019;173(2):247–254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.