Abstract
Background
A fecal test followed by diagnostic colonoscopy for a positive result is a widely endorsed screening strategy for colorectal cancer (CRC). However, the relationship between the time delay from the positive test to the follow-up colonoscopy and CRC mortality has not been established.
Methods
From a population-based screening program, we identified CRC patients newly diagnosed from 2005 through 2015 by a positive fecal occult test followed by a colonoscopy. The primary outcome measure was CRC-specific mortality according to four categories for the time elapsed between the positive result and the subsequent colonoscopy.
Results
The 1749 patients underwent colonoscopies within 0–3 months (n = 981, 56.1%), 4–6 months (n = 307, 17.5%), 7–12 months (n = 157, 9.0%), and later than 12 months (n = 304, 17.4%). CRC-specific deaths according to exposure groups were: 13.8% (135 of 981) for 0–3 months, 10.7% (33 of 307) for 4–6 months (crude hazards ratio [HR] = 0.74, 95% confidence interval [CI] = 0.51 to 1.14), 11.5% (18 of 157) for 7–12 months (crude HR = 0.83, 95% CI = 0.51 to 1.42), and 22.7% (69 of 304) for longer than 12 months (crude HR = 1.40, 95% CI = 1.04 to 1.90). The only variable that was associated with mortality risk was the number of positive slides (P = .003). High positivity was twice the value in the 0–3 as the longer-than-12 months group: 51.9% vs 25.0% and similar for the 4–6 and 7–12 months groups (38.1% and 36.5%), respectively. The adjusted HRs for CRC mortality were 0.81 (95% CI = 0.55 to 1.19); 0.83 (95% CI = 0.50 to 1.41), and 1.53 (95% CI = 1.13 to 2.12, P = .006) for the 4–12, 7–12, and longer-than-12-months groups, respectively, compared with the shortest delay group.
Conclusions
Among screen-diagnosed CRC patients, performance of colonoscopy more than 12 months after the initial positive fecal occult blood test was associated with more advanced disease and higher mortality due to CRC.
Colorectal cancer (CRC) is a leading cause of cancer burden worldwide (1). Because its natural course is modifiable by early detection, it is a target for screening and mortality reduction interventions (2–5). A two-step approach, based on a fecal test followed by colonoscopy, is the most common worldwide-endorsed screening method in average-risk populations (6–9). However, the window of time that is needed to prevent increased risk of advanced CRC disease and disease-specific mortality has not been determined. Consequently, sound recommendations defining the timeliness of the diagnostic follow-up have not been set (6–8,10). These issues can no longer be studied directly in randomly assigned trials due to ethical reasons (8,11) and should be explored by observational studies in the setting of screening programs.
Most CRCs develop in an adenoma-carcinoma pathway (1,12). The sojourn time, during which removal of precancerous precursor lesions can modify outcomes, has not been defined (13–15). The prolonged natural history of CRC requires long-term follow-up for estimating mortality reduction (16–18). Because population-based CRC screening has been fully implemented in Israel for over one decade, we were able to monitor not only an interim endpoint of stage shift but CRC mortality as well. We explored the time within which a colonoscopy should be performed following a positive fecal test to prevent increased risk of advanced CRC and disease specific mortality.
Methods
Study Design, Population, and Setting
This retrospective cohort study is set in the organized screening program of Clalit Health Services (CHS), the largest health maintenance organization in Israel. The health-care system in Israel is founded and based on the National Health Insurance Law (1995), which ensures universal coverage of health-care needs to all Israeli citizens as a fundamental right. Four official not-for-profit health maintenance organizations provide health care to the entire population. CHS insures about 50% of the 8.7 million population. A uniform list of health services, the “Health Basket,” covers all costs of diagnosis, treatment, and preventive and palliative medicine. The health maintenance organizations are obliged to establish organized screening programs for common cancers, outreaching to target populations.
The study was approved by the Institutional Review Board at Carmel Medical Center, Haifa, Israel (approval number 0149-16-CMC).
All eligible insurees aged 50–74 years are actively invited to perform an annual fecal occult blood screening test. Patients with CRC history and with inflammatory bowel disease are not included in the target screening population and do not receive fecal occult blood test (FOBT) kits. During the study period, January 2005 to December 2015, the Hemoccult Sensa (Beckman Coulter) method was used. The kit included a testing card with six fields designed to test two samples from each of three consecutive bowel movements. A detailed instruction sheet was enclosed. Kits were delivered annually by mail to the target population. All completed tests were returned by mail to the central laboratory and processed. Reminders were issued to insurees who received a kit but failed to perform and return the test. Both clinicians and patients were notified regarding a positive result. The positive results were delivered electronically to the primary physicians and necessitated a timely colonoscopic follow-up. Colonoscopies following positive fecal test results are free of charge for patients. Reminders were issued monthly to physicians and to colonoscopy avoidants if the performance of an investigative colonoscopy was not recorded in the electronic medical records. All FOBT tests with positive results were followed and information on colonoscopies, surgical procedures, and pathologic findings was collated.
Patients
This study comprised individuals diagnosed with CRC, following a positive FOBT test performed during the study period, according to the CHS screening program. Values of high and low test positivity were defined as 4–6 and 1–3 positive fields, respectively. Patients who are actually symptomatic or anemic may not be recognized as such during the average-risk population screening process. Consequently, misclassification of symptom-detected cancers as screen-detected cancers may bias the true association of screening with outcomes. We were able to identify and ascertain patients with anemia before the FOBT test and to exclude them from the study cohort main analysis, thus reducing misclassification bias. Mortality outcomes of anemic patients were compared in a separate analysis with those of the study population to demonstrate their different course. Patients with CRC were identified using CHS databases. Demographic characteristics, body mass index, socioeconomic status according to residence, smoking status, and comorbidities were extracted. Diagnoses were verified and tumor stage and location sites identified through linkage with the Israel National Cancer Register records. FOBT test history, the date of laboratory analysis, test results, the number of positive FOBT fields, and colonoscopic follow-up history were extracted from the screening program databases. Date of death and ethnicity were derived from the Central Bureau of Statistics, and the causes of death were extracted manually using medical reports from the databases.
Exposure and Cancer Outcomes
The exposure was defined as the time elapsed between a positive screening FOBT result and the subsequent colonoscopy according to 4 categories: 0–3 months (0–90 days), 4–6 months (91–180 days), 7–12 months (181–365 days), and more than 12 months (≥366 days). Disease stage was defined by the National Cancer Registry according to the Surveillance, Epidemiology and End Results (SEER) Program Coding and Staging Manual. Advanced-stage cancers were classified as code 3 (disease in the regional lymph nodes), code 4 (regional disease with direct extension and spread to the regional lymph node), or code 7 (distant metastasis). The medical records of the 304 patients who had a colonoscopy after over 1 year were reviewed manually to understand the reasons for the delayed follow-up. The primary outcome was the cumulative incidence of CRC-specific death according to exposure for each group. The secondary outcome was disease stage at diagnosis.
Statistical Analysis
IBM statistics (SPSS) version 24 was used. Continuous and ordinal variables were presented as means and standard deviations. Categorical variables were presented as percentages. Baseline clinical and sociodemographic characteristics were compared among the four time-interval categories using the χ2 test for the categorical variables and one-way analysis of variance for the continuous variables. Time was included in the model as a continuous variable as well. The time delay between the positive FOBT result and the colonoscopy was divided into 1-month intervals.
Crude incidence rates per 1000-person years with 95% confidence intervals (CIs) of CRC death were estimated using the Poisson distribution. Death from CRC was evaluated by univariate and multivariable cause-specific hazard models using Cox regression. Hazard ratios (HRs) and 95% confidence intervals are presented. The distribution of time to CRC death event was estimated by the Cumulative Incidence Function using SAS9.4 version 12.3. Mortality due to other reasons was considered a competing event. P less than .05 was considered statistically significant. All tests were two-sided. Sensitivity analyses included redefining time to mortality from the diagnostic colonoscopy date, redefining the comparison groups using each study group as reference, and restricting the whole cohort and each of the study comparison groups to include only patients who survived at least 12 months following the positive FOBT.
Results
Cohort Characteristics
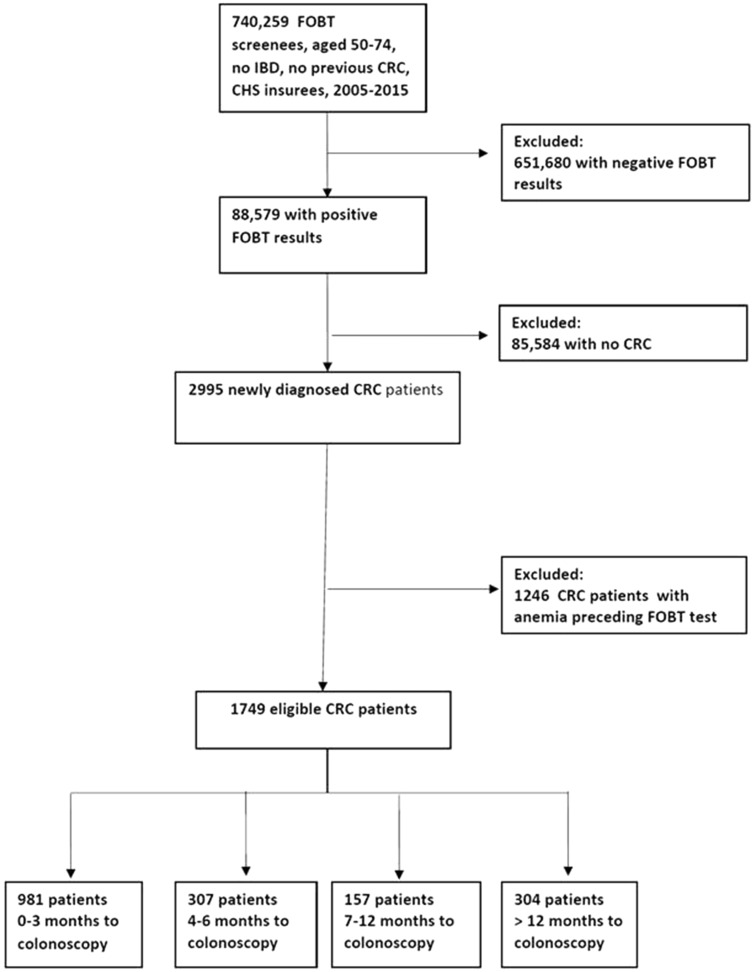
During 2005–2015, 1 901 131 FOBT tests were performed by 740 259 individuals. Of them, 88 579 patients had a positive result. A total of 2995 of the positive-result screenees had CRC. After excluding 1246 CRC patients due to anemia before the FOBT test, 1749 patients were eligible for analysis (Figure 1).
Figure 1.
Selection of the study population. The source population comprises all 50- to 74-year-old individuals who were screened by fecal occult blood test (FOBT) during the study period. The study population comprises newly diagnosed screen-detected colorectal cancer (CRC) cases without anemia before the screening process. The four exposure groups denote the time delay from the positive FOBT result to diagnostic colonoscopy in months. CHS = Clalit Health Services; IBD = inflammatory bowl disease.
Of the 1749 patients included in the analysis, 981 (56.1%) underwent colonoscopies within 3 months, 307 (17.5%) within 4–6 months, 157 (9.0%) within 7–12 months, and 304 (17.4%) after more than 1 year (Figure 1). Baseline characteristics of the entire cohort and across time-to-colonoscopy exposure groups are presented in Table 1.
Table 1.
Baseline characteristics according to the time delay between fecal positivity and colonoscopy
| Time to colonoscopy, mo |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Total 1749 | 0–3 | n = 981 | 4–6 | n = 307 | 7–12 | n = 157 | >12 | n = 304 | P |
| Age, y | 63.4 (6.6) | 63.8 (6.5) | 64.5 (6.7) | 64.6 (6.8) | 64.6 (6.3) | .143 | ||||
| Age quartiles, y | .485 | |||||||||
| ≤60 | 480 (27.4) | 285 (29.1) | 77 (25.1) | 40 (25.5) | 78 (25.7) | |||||
| 60–65 | 421 (24.1) | 240 (24.5) | 74 (24.1) | 37 (23.6) | 70 (23.0) | |||||
| 65–70 | 439 (25.1) | 247 (25.2) | 73 (23.8) | 36 (22.9) | 83 (27.3) | |||||
| >70 | 409 (23.4) | 209 (21.3) | 83 (27.0) | 44 (28.0) | 73 (24.0) | |||||
| Sex, % men | 737 (42.1) | 407 (41.5) | 145 (47.2) | 64 (40.8) | 121 (39.8) | .237 | ||||
| SES | .002 | |||||||||
| Low | 630 (36.0) | 317 (32.3) | 109 (35.5) | 68 (43.3) | 136 (44.7) | |||||
| Medium | 687 (39.3) | 406 (41.4) | 119 (38.8) | 49 (31.2) | 113 (37.2) | |||||
| High | 314 (19.3) | 188 (19.2) | 59 (19.2) | 32 (20.4) | 35 (11.5) | |||||
| Missing | 118 (6.7) | 70 (7.1) | 20 (6.5) | 8 (5.1) | 20 (6.6) | |||||
| Ethnicity, % Jews | 1503 (85.9) | 858 (87.5) | 271 (88.3) | 134 (85.4) | 240 (78.9) | .001 | ||||
| Diabetes | 380 (21.7) | 187 (19.1) | 60 (19.5) | 56 (35.7) | 77 (25.3) | <.0001 | ||||
| Ischemic heart disease | 218 (12.5) | 106 (10.8) | 41 (13.4) | 31 (19.7) | 40 (13.2) | .015 | ||||
| Smoking | 671 (38.4) | 364 (37.1) | 117 (38.1) | 69 (43.9) | 121 (39.8) | .391 | ||||
| BMI, kg/m2 | .007 | |||||||||
| 18–25 | 292 (16.7) | 189 (19.3) | 41 (13.4) | 26 (16.6) | 36 (11.8) | |||||
| 25–30 | 613 (35.0) | 359 (36.6) | 108 (35.2) | 52 (33.1) | 94 (30.9) | |||||
| >30 | 648 (37.0) | 335 (34.1) | 121 (39.4) | 62 (39.5) | 130 (42.8) | |||||
| Missing | 196 (11.2) | 98 (10.0) | 37 (12.1) | 17 (10.8) | 44 (14.5) | |||||
| FOBT previous year | 298 (17.0) | 167 (17.0) | 54 (17.6) | 31 (19.7) | 46 (15.1) | .645 | ||||
| Any previous FOBT | 831 (47.5) | 475 (48.4) | 140 (45.6) | 83 (52.9) | 133 (43.8) | .231 | ||||
| No. of fields 4–6 | 764 (43.7) | 509 (51.9) | 117 (38.1) | 62 (36.5) | 76 (25.0) | <.0001 | ||||
| Tumor location | ||||||||||
| Proximal | 379 (21.7) | 209 (21.3) | 66 (21.5) | 32 (20.4) | 72 (23.7) | .221† | ||||
| NOS | 14 (0.8) | 6 (0.6) | 2 (0.7) | 2 (1.3) | 4 (1.3) | |||||
| Distal | 609 (34.8) | 337 (34.4) | 121 (39.4) | 56 (35.7) | 95 (31.3) | |||||
| Rectum | 575 (32.9) | 352 (35.9) | 89 (29.0) | 45 (28.7) | 89 (29.3) | |||||
| Missing | 172 (9.8) | 77 (7.8) | 29 (9.4) | 22 (14.0) | 44 (14.5) | |||||
| Disease stage | .001 | |||||||||
| 0–1 | 624 (35.7) | 363 (37.0) | 130 (42.3) | 59 (37.6) | 72 (23.7) | |||||
| 2 | 398 (22.8) | 220 (22.4) | 63 (20.0) | 34 (21.7) | 81 (26.6) | |||||
| 3–4 | 397 (22.7) | 230 (23.4) | 60 (19.5) | 30 (19.1) | 77 (25.3) | |||||
| 7 | 113 (6.5) | 63 (6.4) | 18 (5.9) | 7 (4.5) | 25 (8.2) | |||||
| Missing | 217 (12.4) | 105 (10.7) | 36 (11.7) | 27 (17.2) | 49 (16.1) | |||||
Data are presented as No. (%), except age, which is mean (SD). BMI = body mass index; FOBT = fecal occult blood test; NOS = not otherwise specified; SES = socioeconomic status.
P value refers to the comparison between proximal, distal, and rectum.
Fecal Test Positivity
Altogether, 764 (43.7%) individuals had a high number of positive fields. Distribution differed statistically significantly in the four exposure groups (P < .0001). High positivity was twice the value in the 0–3-month (51.9%) as in the longer-than-12-month group (25.0%) and similar for the 4–6-month (38.1%) and 7–12-month (36.5%) groups.
Disease Stage at Diagnosis
For the 1532 patients (87.6%) with disease stage data (Table 1; Figure 2), 1022 (66.1%) were diagnosed early: 40.1% (624 of 1532] at SEER code stage 0–I and 26.0% (398 of 1532) at stage II; 26.0% (397 of 1532) were diagnosed at stage III–IV and 7.4% (113 of 1532) were metastatic. U and inverse-U relationships were observed between disease stages and the duration of time lapse from the positive FOBT result (Figure 2).
Figure 2.
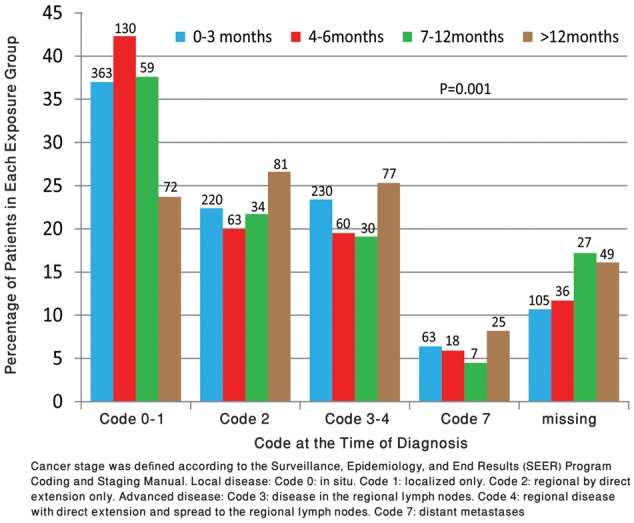
Percentage of Surveillance, Epidemiology and End Results (SEER) coding of colorectal cancer (CRC) cases in fecal occult blood test (FOBT)-positive patients according to time to diagnostic colonoscopy. Shown are the percentages of individuals diagnosed with each SEER code disease stage for each of the four exposure groups. The four exposure groups denote the time delay in months from the positive FOBT result to diagnostic colonoscopy. The numbers above the columns represent the number of patients in each subgroup. The missing stage data are represented separately. Explanation of SEER coding is included in the footnote.
Characteristics of Late Performers
Of the 304 patients (17% of the cohort) who deferred follow-up beyond 1 year, 90% did not adhere to positive fecal test follow-up guidelines (eg, some totally ignored and never repeated a positive test, others failed to act promptly yet performed a subsequent FOBT test after 1 year). Of the 304 patients, 56% developed anemia with or without symptoms or had a gradual reduction in hemoglobin, 52% had symptoms with or without anemia, and 16% had a severe comorbidity, such as another cancer, congestive heart failure, cerebrovascular accident, cirrhosis, or schizophrenia.
CRC-Specific Mortality Incidence Rate
During 11 037 person-years, 255 incident cases of CRC-specific deaths were recorded. The overall rate of CRC death was 23.1 (95% CI = 20.4 to 26.1) per 1000-person years. CRC-specific death rates were 22.6 (95% CI = 19.0 to 27.1), 16.9 (95% CI = 11.6 to 23.0), 18.8 (95% CI = 11.1 to 29.0), and 32.0 (95% CI = 24.9 to 40.5) for the four exposure groups, respectively. After adjustment for age and sex, the HR for CRC mortality among anemic patients (n = 1246) was 1.65 (95% CI = 1.39 to 1.97, P < .0001) (Supplementary Figure 1, available online).
CRC-Specific Cumulative Mortality
CRC-specific deaths according to exposure groups were 13.8% (135 of 981) for 0–3 months, 10.7% (33 of 307) for 4–6 months (crude HR = 0.74, 95% CI = 0.51 to 1.14), 11.5% (18 of 157) for 7–12 months (crude HR = 0.83, 95% CI = 0.51 to 1.42), and 22.7% (69 of 304) for longer than 12 months (crude HR = 1.40, 95% CI = 1.04 to 1.90) (Table 2). Each additional 1-month delay was associated with an increased risk of 3% for mortality (HR = 1.03, 95% CI = 1.004 to 1.06, P = .025).
Table 2.
Cause-specific hazard model of CRC deaths following a positive fecal test*
| Variable | HR (95% CI) | Adjusted HR (95% CI) | P † |
|---|---|---|---|
| Colonoscopy interval, mo | |||
| 0–3 | Reference | Reference | |
| 4–6 | 0.74 (0.51 to 1.14) | 0.81 (0.55 to 1.19) | .267 |
| 7–12 | 0.83 (0.51 to 1.42) | 0.83 (0.50 to 1.41) | .462 |
| >12 | 1.40 (1.04 to 1.90) | 1.53 (1.13 to 2.12) | .006 |
| Age quartiles, y | |||
| ≤60 | Reference | Reference | |
| 60–65 | 1.09 (0.76 to 1.56) | 1.10 (0.77 to 1.60) | .615 |
| 65–70 | 1.18 (0.84 to 1.70) | 1.15 (0.80 to 1.60) | .439 |
| >70 | 1.28 (0.91 to 1.81) | 1.37 (0.96 to 1.95) | .083 |
| Sex (male vs female) | 1.00 (0.78 to 1.28) | 1.10 (0.85 to 1.45) | .453 |
| SES | |||
| Missing | 0.76 (0.40 to 1.46) | 0.70 (0.36 to 1.34) | .278 |
| Low | 1.33 (0.92 to 1.92) | 1.10 (0.72 to 1.61) | .688 |
| Medium | 1.14 (0.79 to 1.64) | 1.00 (0.69 to 1.46) | .981 |
| High | Reference | Reference | |
| Ethnicity (Arabs vs Jews) | 1.33 (0.96 to 1.84) | 1.20 (0.83 to 1.74) | .328 |
| Diabetes | 1.28 (0.91 to 1.63) | 1.13 (0.82 to 1.50) | .458 |
| Ischemic heart disease | 1.14 (0.67 to 1.92) | 1.30 (0.95 to 1.92) | .109 |
| Smoking | 1.11 (0.87 to 1.41) | 1.12 (0.86 to 1.46) | .395 |
| BMI, kg/m2 | |||
| <25 | Reference | Reference | |
| 25–30 | 1.01 (0.70 to 1.45) | 0.93 (0.64 to 1.30) | .685 |
| ≥30 | 0.96 (0.67 to 1.37) | 0.82 (0.56 to 1.20) | .307 |
| Missing | 0.74 (0.46 to 1.20) | 0.65 (0.40 to 1.06) | .082 |
| FOBT in previous year | 0.71 (0.48 to 1.06) | 0.78 (0.49 to 1.20) | .274 |
| Any previous FOBT | 0.84 (0.65 to 1.09) | 0.87 (0.65 to 1.18) | .373 |
| Number of fields (4–6 vs 1–3) | 1.37 (1.07 to 1.75) | 1.50(1.14 to 1. 90) | .003 |
| Tumor location | |||
| Proximal | Reference | Reference | |
| NOS | 1.30 (0.41 to 4.30) | 1.30 (0.42 to 4.30) | .621 |
| Distal | 0.88 (0.62 to 1.24) | 0.88 (0.63 to 1.25) | .488 |
| Rectum | 1.20 (0.91 to 1.70) | 1.20 (0.89 to 1.72) | .215 |
| Missing | 0.86 (0.48 to 1.51) | 0.82 (0.45 to 1.49) | .517 |
The table is adjusted for: age quartiles, sex, socioeconomic status, ethnicity, diabetes, ischemic heart disease, smoking, FOBT previous year, any previous FOBT, BMI, number of positive fields, and tumor location. BMI = body mass index; CI = confidence interval; CRC = colorectal cancer; FOBT = fecal occult blood test; NOS = not otherwise specified; SES = socioeconomic status.
P refers to the comparison between proximal, distal, and rectum.
In the univariate model, the worst CRC mortality outcome was associated with the longest delay (>12 months), with an increased HR of 1.40% (95% CI = 1.04 to 1.90) compared with the 0–3-month group (Table 2). After adjustment in the multivariable model, patient and tumor features were not associated with increased mortality. The only variable that associated statistically significantly with mortality risk was the number of positive fields, reflecting the extent of bleeding (P = .003) (Table 2). In the multivariable model, the HR for CRC mortality for the longer-than-12-months group was 1.53 (95% CI = 1.13 to 2.1, P = .006) compared with the 0–3-month group. For the 4–6 and 7–12-month groups, HRs were 0.81 (95% CI = 0.55 to 1.19) and 0.83 (95% CI = 0.50 to 1.41), respectively, compared with the 0–3-month group (Table 2; Figure 3). In an additional analysis that compared CRC mortality for the 0–3-, 4–6-, and 7–12-month groups to the longer-than-12-months month group, HRs were 0.65 (95% CI = 0.48 to 0.88, P = .005), 0.52 (95% CI = 0.34 to 0.79, P = .002), and 0.55 (95% CI = 0.33 to 0.93, P = .027), respectively.
Figure 3.
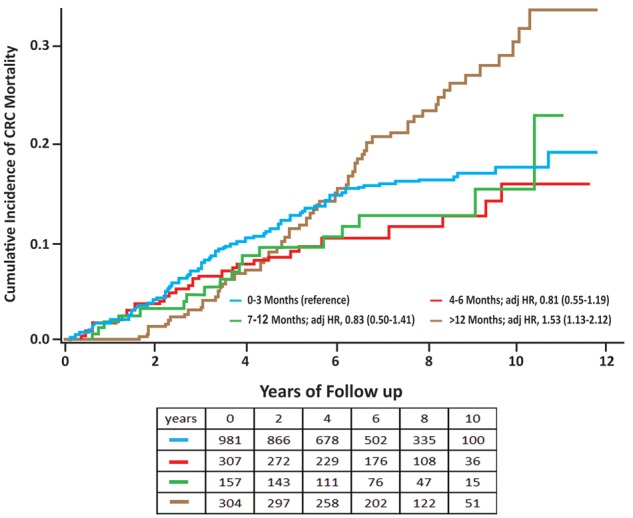
Cumulative incidence of colorectal cancer (CRC) mortality in fecal occult blood test (FOBT)-positive patients according to time to diagnostic colonoscopy. Shown are the cumulative incidence CRC mortality rates according to the time to diagnosis for each of the four exposure groups. The four exposure groups denote time delay from the positive FOBT result to diagnostic colonoscopy in months. The table below the survival curves figure shows the numbers at risk for each exposure group according to years of follow-up.
Sensitivity Analyses
In the sensitivity analyses, the pattern of increased HR estimates for CRC mortality outcomes, incidence rate, and cumulative mortality persisted with different comparison groups’ definitions and when patients who did not survive 12 months following the positive FOBT were excluded (thereby reducing the possibility of survivorship-time bias). When time to death was measured starting at the time of diagnostic colonoscopy, the mortality risk was higher mostly in the longer-than-12-months exposure group.
Discussion
In this study of a CRC fecal test-based screening program, a delay of more than 1 year in colonoscopic follow-up was associated with an increased risk of CRC mortality, evidently due to greater tumor progression.
For this group, the mortality hazard exceeded by more than 50% the 0–3-month group, 92% the 4–6-month group, and 81% the 7–12-month group. Although the 0–3-month group served as a reference, outcomes were better for the 4–6- and 7–12-month groups; the difference between the latter groups was not statistically significant. The disproportionate number of most abnormal fecal test results in the early period is presumably due to the inclusion of individuals who were actually symptomatic. Symptomatic patients have a worse prognosis, regardless of the urgent timing of the investigation (19). The recent Kaiser-Permanente study (7), with an extremely rapid follow-up, excluded the 1–7-day period to account for patients with a higher risk of worse outcome. Such an extremely rapid follow-up necessitates resources that are not available in Israel or in other regions worldwide (1,7). The first 3-month period was not excluded from our study so as to mirror an actual real-life situation in a program with a given colonoscopic capacity. The shortest delay group likely overrepresents higher-risk screenees, prioritized for follow-up due to an alarming sign of a high number of positive fields or being symptom-driven. The outcomes of this group (Figures 2 and 3) were apparently adversely affected by selection bias, favoring these patients for immediate diagnostic investigation.
Before the initiation of CRC screening, early disease detection was as low as 10–15% (5). Randomized controlled trials (RCTs) have shown that screen-detected cases are generally diagnosed at early, more manageable stages. Early detection is a prerequisite, though not a guarantee to achieve mortality reduction in later years. In our study, the distribution of disease stage is consistent with the pattern of mortality outcomes. A recent modeling study (10), designed to address the timeliness-outcome association, simulated an average-risk newly diagnosed cohort without considering the possible inclusion in the screening process of symptomatic and higher-risk patients. Thus, the reportedly steady increase in advanced disease with time directly reflected the disease progression process (10). In the current study, the U-shaped pattern of advanced disease, according to colonoscopy delay, contrasts with a curve depicted by gradual increase and reflects the real-life overrepresentation of patients with worse prognosis in the early period. The largest observational study to date, from Kaiser-Permanente, California, comprising 2191 cases of CRC (7) used endpoints of overall CRC risk and advanced disease and provided evidence of a higher risk for follow-up delay of more than 10 months. Our study, using CRC mortality as a primary endpoint, supports the idea of a safe interval from FOBT to colonoscopy that is less than 12 months.
RCTs of CRC screening performed in the 1990s did not consider higher risk of symptomatic patients. Participants were recruited based on age group and residence, usually excluding previous CRC, inflammatory bowel disease, and comorbidities. Those studies did not collect and integrate data from the participants’ personal medical records to identify individuals with higher risk conditions such as certain alarming symptoms, anemia, or family history of CRC (2–5). In a similar manner, mass screening is aimed at a certain target age range and lacks effective means to identify the actual average-risk population and to divert others to tailored assessment (20,21). Consequently, the benefits and outcomes may be biased and confounded by the misclassification of symptom-detected cancers as screen detected (22,23). Anemic patients are commonly included wrongly in a screening process, with reported proportions reaching as high as 40–60% (24). In our study, being anemic was associated with a high mortality hazard ratio of 1.65. Population programs are limited, as well, in their capacity to exclude patients who are at higher risk due to family history. Currently, authorities such as the European Union (25) include these patients in the average-risk population and have not issued specific recommendations for them (25).
Based on a long observational period, our study was able to extend previous screening-set research that used interim indicators such as shift to early diagnosis and to show an effect on mortality. Mortality for the worst prognosis (>12 months) group diverged from that of the better prognosis groups only after about 5 years. This concurs with the findings of the RCTs (2–5) in which the cumulative mortality of the screening and control subject groups began separating after 5–7 years.
In our program, 74% of colonoscopies were performed within 6 months of FOBT and a total of 83% within 1 year. Elsewhere, patients who do not take follow-up measures within 6 months were shown to be less likely to do so at all (26). This time-point may reflect human behavior, because it is consistent across many health systems. Patients’ behavior, physicians’ attitudes, and organizational factors are considered possible barriers or risk factors for delay (27–30). Our manual review of all the medical charts of the late performers showed that these patients overlooked the alarming positive test result, despite reminders, and acted when symptoms and signs inevitably appeared or when repeated fecal tests were positive. In our health system, a patient’s refusal to undergo a colonoscopy must be recorded in the medical chart, so avoiding colonoscopy is actually an informed choice. Consequently, patients’ behavior is more likely than physician and organizational factors to serve as a barrier. A proportion of the late performers in this study had a severe comorbidity with a limited life expectancy and probably should not have been candidates for screening, as described elsewhere (26). Our findings support the possibility that utilizing windows of opportunity could contribute to the prevention of advanced disease and reduced mortality (7,10,11) and that interventions that would target the avoidant population could be beneficial (30).
After adjusting for a number of factors, we found that mortality risk was not associated with age, sex, socioeconomic status, or ethnicity. This observed equity may be consequent to full insurance by the Israeli national health-care system. Thus, all insurees, independently of personal background and features, have equal access and receive health-care from the same colonoscopic facilities (31). Surprisingly, previous screening behavior did not convey protection. The impact of tumor sidedness on prognosis is a target for extensive research; no statistically significant association was shown in our study to mortality risk. Former studies demonstrate conflicting results, such as evidence of predictive implications of sidedness in advanced but not early disease (32,33). Knowledge of the biologic process associated with tumor location is currently insufficient.
The prominent variable associated with an elevated mortality risk in our study was a higher number of positive FOBT slides with an adjusted hazard ratio of 1.50 for mortality. This increased risk is expected, because advanced lesions bleed more. The need for diagnostic prioritization according to risk was previously described in an RCT (34) as well as in recent research (35,36). In the current study, more than 50% of the patients who underwent colonoscopies within 0–3 months had a higher number of positive FOBT slides (4–6 fields), associated with a higher risk. The longer-than-12-months group proportion of a higher number of positive FOBT slides at the time of index FOBT testing was less than one-half (25%) compared with the 0–3-month group.
The observational design and possibility of residual confounding from unmeasured factors are limitations of this study. Lead time bias is a potential problem, because the longer the interval from FOBT to colonoscopy, the more advanced the disease is likely to be at diagnosis. Poor health-care behavior could explain both the longer time until colonoscopy and a higher mortality rate. Strengths are the large population-based design, setting in a large community-based health system with a stable membership, and the extensive coded and free-text clinical data linked and identified by an ID. The assumptions were evaluated through sensitivity analyses. The pattern and direction of hazard ratios for CRC mortality persisted with different group definitions.
Among screen-diagnosed CRC patients, performance of colonoscopy more than 12 months after the initial positive FOBT was associated with more advanced disease and higher mortality due to CRC. These findings support the suggestion that it is safe to delay colonoscopy for several months after a positive FOBT but not by more than 12 months.
Notes
Affiliations of authors: Department of Community Medicine and Epidemiology, Lady Davis Carmel Medical Center, Haifa, Israel (AAF, NS, IL); Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel (AAF, OS); Clalit National Cancer Control Center, Haifa, Israel (AAF, NS, IL); Department of Gastroenterology, Lady Davis Carmel Medical Center, Haifa, Israel (OS); Israel National Cancer Registry, Israel Center for Disease Control, Ministry of Health, Ramat Gan, Israel (LKB); School of Public Health, University of Haifa, Haifa, Israel (LKB).
All authors report no conflict of interest.
Supplementary Material
References
- 1. Lauby-Secretan B, Vilahur N, Bianchini F, et al. International Agency for Research on Cancer Handbook Working Group. The IARC perspective on colorectal cancer screening. N Engl J Med. 2018;378(18):1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;3289(19):1365–1371. [DOI] [PubMed] [Google Scholar]
- 3. Lindholm E, Brevinge H, Haglind E.. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg. 2008;95(8):1029–1036. [DOI] [PubMed] [Google Scholar]
- 4. Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–1477. [DOI] [PubMed] [Google Scholar]
- 5. Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O.. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467–1471. [DOI] [PubMed] [Google Scholar]
- 6. Doubeni CA, Gabler NB, Wheeler CM, et al. Timely follow-up of positive cancer screening results: a systematic review and recommendations from the PROSPR Consortium. CA Cancer J Clin. 2018;68(3):199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317(16):1631–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rutter CM, Inadomi JM.. Follow-up of positive fecal test results: sooner is better, but how much better? JAMA. 2017;317(16):1627–1628. [DOI] [PubMed] [Google Scholar]
- 9. Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–1649. [DOI] [PubMed] [Google Scholar]
- 10. Meester RG, Zauber AG, Doubeni CA, et al. Consequences of increasing time to colonoscopy examination after positive result from fecal colorectal cancer screening test. Clin Gastroenterol Hepatol. 2016;14(10):1445–1451.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rutter CM, Kim JJ, Meester RGS, et al. Effect of time to diagnostic testing for breast, cervical, and colorectal cancer screening abnormalities on screening efficacy: a modeling study. Cancer Epidemiol Biomarkers Prev. 2018;27(2):158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319(9):525–532. [DOI] [PubMed] [Google Scholar]
- 13. Kuntz KM, Lansdorp-Vogelaar I, Rutter CM, et al. A systematic comparison of microsimulation models of colorectal cancer: the role of assumptions about adenoma progression. Med Decis Making. 2011;31(4):530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luebeck EG, Curtius K, Jeon J, Hazelton WD.. Impact of tumor progression on cancer incidence curves. Cancer Res. 2013;73(3):1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brenner H, Altenhofen L, Katalinic A, Lansdorp-Vogelaar I, Hoffmeister M.. Sojourn time of preclinical colorectal cancer by sex and age: estimates from the German national screening colonoscopy database. Am J Epidemiol. 2011;174(10):1140–1146. [DOI] [PubMed] [Google Scholar]
- 16. Malila N, Oivanen T, Malminiemi O, Hakama M.. Test, episode, and programme sensitivities of screening for colorectal cancer as a public health policy in Finland: experimental design. BMJ. 2008;337:a2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim JJ, Tosteson AN, Zauber AG, et al. Cancer models and real-world data: better together. JNCIJ. 2016;108(2):djv316.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toes-Zoutendijk E, Kooyker AI, Elferink MA, et al. Stage distribution of screen-detected colorectal cancers in the Netherlands. Gut. 2018;67(9):1745–1746. [DOI] [PubMed] [Google Scholar]
- 19. Tørring ML, Murchie P, Hamilton W, et al. Evidence of advanced stage colorectal cancer with longer diagnostic intervals: a pooled analysis of seven primary care cohorts comprising 11 720 patients in five countries. Br J Cancer. 2017;117(6):888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G.. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med. 2000;343(3):162–168. [DOI] [PubMed] [Google Scholar]
- 21. Worthley DL, Smith A, Bampton PA, Cole SR, Young GP.. Many participants in fecal occult blood test population screening have a higher-than-average risk for colorectal cancer. Eur J Gastroenterol Hepatol. 2006;18(10):1079–1083. [DOI] [PubMed] [Google Scholar]
- 22. Singal AG, Gupta S, Lee J, et al. Importance of determining indication for colonoscopy: implications for practice and policy. Clin Gastroenterol Hepatol. 2014;12(12):1958–1963.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hubbard RA, Johnson E, Chubak J, et al. Accounting for misclassification in electronic health records-derived exposures using generalized linear finite mixture models. Health Serv Outcomes Res Method. 2017;17(2):101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hatch QM, Kniery KR, Johnson EK, et al. Screening or symptoms? How do we detect colorectal cancer in an equal access health care system? J Gastrointest Surg. 2016;20(2):431–438. [DOI] [PubMed] [Google Scholar]
- 25. von Karsa L, Patnick J, Segnan N, et al. European Colorectal Cancer Screening Guidelines Working Group. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy. 2013;45(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chubak J, Garcia MP, Burnett-Hartman AN, et al. Time to colonoscopy after positive fecal blood test in four U.S. health care systems. Cancer Epidemiol Biomarkers Prev. 2016;25(2):344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Partin MR, Gravely A, Gellad ZF, et al. Factors associated with missed and cancelled colonoscopy appointments at Veterans Health Administration facilities. Clin Gastroenterol Hepatol. 2016;14(2):259–267. [DOI] [PubMed] [Google Scholar]
- 28. Partin MR, Burgess DJ, Burgess JF Jr, et al. Organizational predictors of colonoscopy follow-up for positive fecal occult blood test results: an observational study. Cancer Epidemiol Biomarkers Prev. 2015;24(2):422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Selby K, Baumgartner C, Levin TR, et al. Interventions to improve follow-up of positive results on fecal blood tests: a systematic review. Ann Intern Med. 2017;167(8):565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gupta S, Nodora J.. Optimizing the quality of the colorectal cancer screening continuum: a call to action. J Natl Cancer Inst. 2017;109(5):djw271.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fedewa SA, Flanders WD, Ward KC, et al. Racial and ethnic disparities in interval colorectal cancer incidence: a population-based cohort study. Ann Intern Med. 2017;166(12):857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karim S, Brennan K, Nanji S, Berry SR, Booth CM.. Association between prognosis and tumor laterality in early-stage colon cancer. JAMA Oncol. 2017;3(10):1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang GJ, Gonen M.. Prognostic and predictive ability of tumor sidedness: another vexing difference between localized and advanced colon cancer. JAMA Oncol. 2017;3(10):1314–1315. [DOI] [PubMed] [Google Scholar]
- 34. Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343(22):1603–1607. [DOI] [PubMed] [Google Scholar]
- 35. Auge JM, Pellise M, Escudero JM, et al. Risk stratification for advanced colorectal neoplasia according to fecal hemoglobin concentration in a colorectal cancer screening program. Gastroenterology. 2014;147(3):628–636.e1. [DOI] [PubMed] [Google Scholar]
- 36. Grobbee EJ, Schreuders EH, Hansen BE, et al. Association between concentrations of hemoglobin determined by fecal immunochemical tests and long-term development of advanced colorectal neoplasia. Gastroenterology. 2017;153(5):1251–1259.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.