Abstract
Experts have expressed concerns that patients with chronic conditions are being excessively excluded from cancer randomized clinical trials (RCTs), limiting generalizability. Accordingly, we queried clinicaltrials.gov to determine the extent to which patients with chronic conditions were excluded from phase III cancer trials, using National Cancer Institute-sponsored breast cancer RCTs as a test case. Two physicians independently coded for the presence of 19 prevalent chronic conditions within eligibility criteria. They also coded for exclusions based on performance status and vague criteria that could have broadly excluded patients with chronic conditions. The search identified 58 RCTs, initiated from 1993 to 2012. Overall, 88% of trials had at least one exclusion for a chronic condition, performance status, or vague criterion. The three most commonly excluded conditions were chronic kidney disease, heart failure, and ischemic heart disease. Our study demonstrated that patients with prevalent chronic conditions were commonly excluded from National Cancer Institute-sponsored RCTs.
With advances in medical therapy and an aging population, the number of cancer patients living with multiple chronic conditions (MCCs) is increasing (1,2). Experts have expressed concerns that patients with chronic conditions are being excessively excluded from cancer randomized clinical trials (RCTs) (3), limiting generalizability of trial results to the growing number of cancer survivors with comorbidities who may respond differently to treatments (4,5). Prior reviews have shown that older patients and those from lower socioeconomic backgrounds are underrepresented in cancer RCTs, in part due to restrictive eligibility criteria (6–10). Less is known about exclusions for patients with MCCs (11). Accordingly, our goal was to determine the extent to which patients with chronic conditions were excluded from phase III cancer trials, using a contemporary sample of National Cancer Institute (NCI)-sponsored breast cancer (BC) RCTs as a test case.
Clinicaltrials.gov was searched on February 20, 2015 for all closed NCI-sponsored RCTs enrolling women with BC from database inception (1999) until December 31, 2014. Two physicians (LC, KF) manually reviewed trial records to ensure relevance to BC and then independently coded for the presence of 19 prevalent chronic conditions (13 medical, 6 psychiatric) within eligibility criteria of relevant records (12). Coders specified whether conditions were explicitly (named a specific condition) or implicitly excluded (eligibility criterion encompassed the condition without directly naming it) as well as the threshold used to define exclusions. Inclusion criteria indicative of normal states were reverse coded as exclusions (eg, an inclusion for normal renal function was coded as an exclusion for chronic kidney disease [CKD]). Coders additionally extracted exclusions relating to performance status and vague criteria that could have excluded individuals with MCCs without referencing specific conditions (eg, uncontrolled illness) (13). Differences in coding were reconciled through consensus with a third physician (IK). Data pertaining to other trial characteristics were extracted using a SAS program. Descriptive statistics were used to characterize the frequency of exclusions. Nonparametric tests were used to compare differences in the number of excluded conditions by study type.
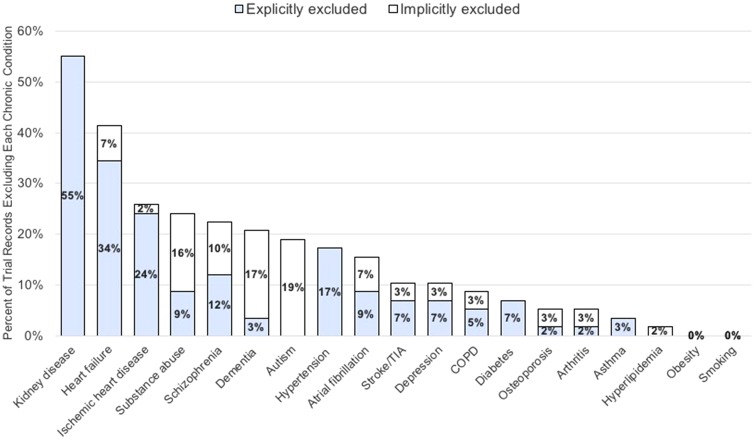
The search identified 59 phase III RCTs; one trial was excluded because it was not relevant to BC, leaving 58 RCTs for coding. Trials were initiated from June 1, 1993 to February 1, 2012 and enrolled a median of 334 participants (range = 28–19 747 participants). The median (range) number of chronic condition exclusions per trial was 2 (0–12). The five most commonly excluded conditions were CKD, heart failure, ischemic heart disease, substance abuse, and psychotic disorders (Figure 1). Overall, 66% of trials had 1 or more exclusions for a medical condition and 29% had 1 or more exclusions for a psychiatric condition.
Figure 1.
Prevalence of exclusions for 19 common chronic conditions explicitly or implicitly excluded from National Cancer Institute-funded breast cancer trials (N = 58 trials)*. COPD = chronic obstructive pulmonary disease; TIA = transient ischemic attack. *Conditions were adapted from the list of the most impactful chronic conditions compiled by a multiple chronic conditions working group at the Office of the Assistant Secretary of Health (12). Conditions were coded as explicitly excluded if exclusion criteria directly named a condition and as being implicitly excluded if the exclusion criteria used terms that encompassed the condition without directly naming it.
Thresholds to determine exclusions varied. One-third of CKD exclusions were defined as any abnormality in kidney function; the remaining were based on a wide range of cutpoints (eg, creatinine >1.5 mg/dL or creatinine >2.5 times the upper limit of normal). Similarly, thresholds to exclude heart failure varied, ranging from any heart failure to uncontrolled heart failure; various ejection fraction thresholds were also used. Diabetes and hypertension were commonly excluded, but only if uncontrolled in all but one trial. Thresholds for “uncontrolled” were infrequently provided and varied widely when provided (eg, systolic blood pressure >150 mm Hg in one trial and >180 mm Hg in another). Exclusions for psychiatric disorders were poorly specified, with many trials excluding for any psychiatric disorder.
There was an association between study type and number of chronic condition exclusions (P < .001), with treatment trials having the greatest number of exclusions (median = 3.5) compared with supportive care (median = 1) or preventive trials (median = 0; Table 1). There was also an association between intervention type and number of exclusions: studies involving behavioral interventions (median = 0) had fewer chronic condition exclusions than studies involving other types of interventions such as drugs or radiation (median range = 1.5–3; P < .001).
Table 1.
The association between clinical trial characteristic and median number of excluded chronic conditions (N = 58 trials)
| Clinical trial characteristic | Median number of excluded conditions (IQR)* | P |
|---|---|---|
| Eligible sex | .03 | |
| Women, only (41 trials) | 2 (1–6) | |
| Men and women (17 trials) | 0 (0–3) | |
| Eligible age groups | .59 | |
| Adults and seniors, 18 y or older (43 trials) | 2 (0–6) | |
| Children, adults, and seniors of all ages (10 trials) | 0.5 (0–4) | |
| Adult, only, 18–64 y (5 trials) | 3 (1–5) | |
| Study type | <.001 | |
| Treatment (30 trials) | 3.5 (2–6) | |
| Supportive care (23 trials) | 1 (0–2) | |
| Prevention (5 trials) | 0 (0–4) | |
| Overall (58 trials) | 2 (0–5) | N/A |
*Conditions were adapted from the list of the most impactful chronic conditions compiled by a multiple chronic conditions working group at the Office of the Assistant Secretary of Health (12). IQR = interquartile range.
One-half of trials had an exclusion for performance status (Karnofsky or Zubrod score). Additionally, the majority of trials (62%) included at least one vague criterion that could have excluded patients with MCCs. Examples of vague criteria included “uncontrolled illness” or “comorbid condition placing patient at high risk of complications.” Overall, 88% of trials had at least one exclusion for a prespecified condition, performance status, or vague criterion.
Our study of eligibility criteria in closed NCI-sponsored BC trials demonstrated that patients with prevalent chronic conditions, particularly CKD, heart failure, and ischemic heart disease, were commonly excluded. Of these, heart failure is among the three most common chronic conditions in Medicare-eligible cancer patients (2). Our study expands upon the literature of the applicability of cancer trials to patients with MCCs by systematically demonstrating the extent to which patients with prevalent chronic conditions are excluded from NCI-sponsored BC RCTs (14,15).
We identified substantial variability in the thresholds used to exclude comorbidities. We suspect that the thresholds used were often more stringent than necessary. For example, for CKD, experts recently recommended including cancer patients with creatinine clearance greater than 30 mL/min unless there were known risks of nephrotoxicity (16). Yet, most of the trials in our review excluded those with milder renal impairment and based exclusions on creatinine rather than creatinine clearance.
Our findings must be interpreted cautiously. We only examined eligibility criteria and did not assess actual representation of patients with MCCs in BC trials. Further, it was challenging to determine whether certain exclusion criteria indicated a transient abnormal state or a true chronic condition. Nevertheless, we employed a rigorous coding process to optimize the robustness of our findings.
Patients with many of the most prevalent chronic conditions were commonly ineligible for BC RCTs, particularly treatment trials. There are many appropriate reasons to exclude patients with comorbidities from RCTs. Foremost, it may be done to protect vulnerable patients from the hazards of experimental treatments. It may also be done to improve the ability to detect the efficacy of a new therapy or to exclude patients unable to comply with study protocols. Nevertheless, these reasons must be counterbalanced by concerns that overly restrictive criteria limit the generalizability of cancer trial findings (17). In the future, sponsors of trials should encourage research that evaluates the efficacy and safety of treatments in patients with MCCs, including promoting phase III and phase IV trials dedicated to enrolling patients with common comorbidities. Industry-independent registries could also be used to rigorously track the effects of cancer treatments being given to patients with MCCs who had been excluded from earlier trials. Investigators should provide detailed justifications for thresholds used to exclude patients with chronic conditions including psychiatric disorders. Clinical trial registries such as clinicaltrials.gov should have more rigorous standards for reporting comorbidities. These concerted steps could help ensure that future trials are applicable to the growing population of cancer survivors with MCCs.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health under contract no. 6048-S23. Additionally, Dr Hershman received support from the Komen Foundation (SAC160066). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Notes
Affiliations of authors: Department of Medicine, Columbia University Medical Center, New York, NY (IMK, KF, LC, DLH, JL); Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD (PG, JS).
References
- 1. Sarfati D, Koczwara B, Jackson C.. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66(4):337–350. [DOI] [PubMed] [Google Scholar]
- 2. Bluethmann SM, Mariotto AB, Rowland JH.. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beaver JA, Ison G, Pazdur R.. Reevaluating eligibility criteria—balancing patient protection and participation in oncology trials. N Engl J Med. 2017;376(16):1504–1505. [DOI] [PubMed] [Google Scholar]
- 4. Apisarnthanarax S, Swisher-McClure S, Chiu WK, et al. Applicability of randomized trials in radiation oncology to standard clinical practice. Cancer. 2013;119(16):3092–3099. [DOI] [PubMed] [Google Scholar]
- 5. Sargent D. What constitutes reasonable evidence of efficacy and effectiveness to guide oncology treatment decisions? Oncologist. 2010;15(suppl 1):19–23. [DOI] [PubMed] [Google Scholar]
- 6. Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS.. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–2067. [DOI] [PubMed] [Google Scholar]
- 7. Yee KW, Pater JL, Pho L, Zee B, Siu LL.. Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol. 2003;21(8):1618–1623. [DOI] [PubMed] [Google Scholar]
- 8. Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383–1389. [DOI] [PubMed] [Google Scholar]
- 9. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamaker ME, Stauder R, van Munster BC.. Exclusion of older patients from ongoing clinical trials for hematological malignancies: an evaluation of the National Institutes of Health Clinical Trial Registry. Oncologist. 2014;19(10):1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Srikanthan A, Vera-Badillo F, Ethier J, et al. Evolution in the eligibility criteria of randomized controlled trials for systemic cancer therapies. Cancer Treat Rev. 2016. Feb;43:67–73. [DOI] [PubMed] [Google Scholar]
- 12. Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK.. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013. Apr;10:E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jadad AR, To MJ, Emara M, Jones J.. Consideration of multiple chronic diseases in randomized controlled trials. JAMA. 2011;306(24):2670–2672. [DOI] [PubMed] [Google Scholar]
- 14. Brooks SE, Carter RL, Plaxe SC, et al. Patient and physician factors associated with participation in cervical and uterine cancer trials: an NRG/GOG247 study. Gynecol Oncol. 2015;138(1):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chao HH, Mayer T, Concato J, Rose MG, Uchio E, Kelly WK.. Prostate cancer, comorbidity, and participation in randomized controlled trials of therapy. J Investig Med. 2010;58(3):566–568. [DOI] [PubMed] [Google Scholar]
- 16. Lichtman SM, Harvey RD, Damiette Smit MA, et al. Modernizing clinical trial eligibility criteria: recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Organ Dysfunction, Prior or Concurrent Malignancy, and Comorbidities Working Group. J Clin Oncol. 2017;35(33):3753–3759. [DOI] [PubMed] [Google Scholar]
- 17. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Templeton AJ, Vera-Badillo FE, Wang L, et al. Translating clinical trials to clinical practice: outcomes of men with metastatic castration resistant prostate cancer treated with docetaxel and prednisone in and out of clinical trials. Ann Oncol. 2013;24(12):2972–2977. [DOI] [PubMed] [Google Scholar]
- 19. Unger JM, Barlow WE, Martin DP, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106(3):dju002.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mengis C, Aebi S, Tobler A, Dähler W, Fey MF.. Assessment of differences in patient populations selected for excluded from participation in clinical phase III acute myelogenous leukemia trials. J Clin Oncol. 2003;21(21):3933–3939. [DOI] [PubMed] [Google Scholar]
- 21. Adams-Campbell LL, Ahaghotu C, Gaskins M, et al. Enrollment of African Americans onto clinical treatment trials: study design barriers. J Clin Oncol. 2004;22(4):730–734. [DOI] [PubMed] [Google Scholar]
- 22. Kornblith AB, Kemeny M, Peterson BL, et al. Survey of oncologists’ perceptions of barriers to accrual of older patients with breast carcinoma to clinical trials. Cancer. 2002;95(5):989–996. [DOI] [PubMed] [Google Scholar]
- 23. Schulkes KJ, Nguyen C, van den Bos F, van Elden LJ, Hamaker ME.. Selection of patients in ongoing clinical trials on lung cancer. Lung. 2016;194(6):967–974. [DOI] [PubMed] [Google Scholar]