Abstract
Background
For BRCA1 and BRCA2 mutation carriers, the association between oral contraceptive preparation (OCP) use and breast cancer (BC) risk is still unclear.
Methods
Breast camcer risk associations were estimated from OCP data on 6030 BRCA1 and 3809 BRCA2 mutation carriers using age-dependent Cox regression, stratified by study and birth cohort. Prospective, left-truncated retrospective and full-cohort retrospective analyses were performed.
Results
For BRCA1 mutation carriers, OCP use was not associated with BC risk in prospective analyses (hazard ratio [HR] = 1.08, 95% confidence interval [CI] = 0.75 to 1.56), but in the left-truncated and full-cohort retrospective analyses, risks were increased by 26% (95% CI = 6% to 51%) and 39% (95% CI = 23% to 58%), respectively. For BRCA2 mutation carriers, OCP use was associated with BC risk in prospective analyses (HR = 1.75, 95% CI = 1.03 to 2.97), but retrospective analyses were inconsistent (left-truncated: HR = 1.06, 95% CI = 0.85 to 1.33; full cohort: HR = 1.52, 95% CI = 1.28 to 1.81). There was evidence of increasing risk with duration of use, especially before the first full-term pregnancy (BRCA1: both retrospective analyses, P < .001 and P = .001, respectively; BRCA2: full retrospective analysis, P = .002).
Conclusions
Prospective analyses did not show that past use of OCP is associated with an increased BC risk for BRCA1 mutation carriers in young middle-aged women (40–50 years). For BRCA2 mutation carriers, a causal association is also not likely at those ages. Findings between retrospective and prospective analyses were inconsistent and could be due to survival bias or a true association for younger women who were underrepresented in the prospective cohort. Given the uncertain safety of long-term OCP use for BRCA1/2 mutation carriers, indications other than contraception should be avoided and nonhormonal contraceptive methods should be discussed.
Women with germline mutations in BRCA1 and BRCA2 are at substantially increased risk of breast and ovarian cancers (1). The use of oral contraceptive preparations (OCPs) has been found to be associated with reduced ovarian cancer risk for both BRCA1 and BRCA2 mutation carriers (2–7). However, a key issue in the clinical management of these mutation carriers is whether or not use of OCPs is associated with breast cancer (BC) risk. Prior findings are inconsistent and based on only a few retrospective studies with the potential for recall, survival, and testing biases. To evaluate the risk of BC associated with use of OCPs for mutation carriers, we harmonized and combined the data from three large international cohort consortia. We analyzed the association between the use of OCPs and BC risk in the first prospective analysis. For comparison with the literature with a larger sample size, we also conducted left-truncated and full cohort retrospective analyses in the same cohort at younger ages.
Methods
Study Design and Study Participants
We harmonized and pooled information from three large cohorts: the International BRCA1/2 Carrier Cohort Study (IBCCS), the Kathleen Cuningham Foundation Consortium for Research Into Familial Breast Cancer (kConFab) Follow-Up Study, and the Breast Cancer Family Registry (BCFR). The IBCCS/kConFab/BCFR collaboration combined 21 cohort studies conducted in Western countries (1,8–11). Women were eligible if they had a pathogenic mutation in either BRCA1 or BRCA2 and were between age 18 and 80 years at study enrollment. Women were excluded if they had mutations in both genes or were born before 1920, because their reproductive years preceded the availability of OCPs. The total eligible group, enrolled in the prospective, retrospective, or both analyses, consisted of 6030 BRCA1 and 3809 BRCA2 mutation carriers.
Data Collection
Baseline and consecutive follow-up questionnaires elicited detailed information on known or suspected risk factors for breast and ovarian cancer. Data collection on OCP use included ever use (no/yes), age at first or last use (years), and duration of use (years); age sat first and last use for each period of use were available for 75% of the women. Data on preventive surgeries and cancer occurrence were collected from questionnaires, from medical record validation, and from linkages to cancer and/or pathology registries. Information on vital status was obtained from municipal or death registries or from contact persons in the family.
Statistical Analysis
To estimate hazard ratios (HRs), time-dependent Cox proportional hazards regression models were used, with age as the time scale, and stratified by birth cohort and study. The proportional hazard assumption was not violated when comparing log-minus-log and hazard curves with each other. The effect of familial clustering on estimates of precision was accounted for using robust variance estimation. All tests of statistical significance were two-sided. Associations by birth cohort, attained age (≤35 years, >35 years), and study were also assessed. Hazard ratios were estimated for both prospective and retrospective (left-truncated and full-cohort) models in the same study population to enable a comparison with the literature, and to investigate potential biases and age-specific differences. For prospective analyses, OCP data from baseline and follow-up questionnaires were combined and imputed for the interval between age at last questionnaire and age at linkage with cancer registries. For retrospective analyses, only data from the baseline questionnaire were used. Characteristics of OCP use that were identified a priori for investigation included ever use of OCPs (use for more than six months), recency of use (time since last use, in years), age at first use (years), calendar year at first use (<1975, ≥1975, proxy for changes in OCP formulations; specific formulations unknown), total duration of use (years), and duration of use before first full-term pregnancy (FFTP); they were time-dependently included in the model, for each year of observation. Potential confounders were family history, defined as the number of first- or second-degree relatives diagnosed with BC (none, 1, ≥2), parity (no/yes, time-dependent), age at FFTP (time-dependent), breast feeding (never/ever and duration in years, time-dependent), and risk-reducing salpingo-oophorectomy (RRSO; no/yes and “no or <3 years since RRSO”/“yes, ≥3 years since RRSO”; time-dependent). None of these variables changed the hazard ratio for OCP exposure and BC risk by more than 10%, and therefore they were not included in the final analyses.
All statistical tests were two-sided, a P value of less than .05 was considered statistically significant. The analyses were performed using STATA-13.0 (Statcorp, College Station,TX).
Prospective Cohort Analysis
Prospective follow-up started at baseline cohort recruitment or mutation testing, whichever came last, to standardize the potential influence of being aware of the mutation on use of OCPs during follow-up. If age at mutation testing was unknown (1%) or if women were tested in a research setting and it was unknown whether they opted for a clinical test (6%), follow-up started at baseline questionnaire completion. At start of follow-up, women had no history of cancer (exclusive nonmelanoma skin cancer) and had not undergone a bilateral risk-reducing mastectomy (RRM). Person-years were censored at age of diagnosis of first primary BC (invasive or in situ), diagnosis of another cancer, RRM, death, last follow-up, or age 80 years, whichever came first. Last follow-up was defined as last questionnaire, contact, or linkage, whichever came last.
Retrospective Cohort Analysis
Two retrospective analyses were conducted. In “full-cohort” retrospective analyses, follow-up commenced at birth. The “left-truncated” retrospective analysis started follow-up five years preceding the date of the baseline questionnaire to reduce the possibility of survival bias. At the start of follow-up, carriers were included in this left-truncated analysis only if they had not been diagnosed with cancer (exclusive nonmelanoma skin cancer) or undergone RRM. In both retrospective analyses, person-years were censored at age of diagnosis of the first primary BC (invasive or in situ), diagnosis of another cancer, RRM, mutation test, or baseline questionnaire, whichever came first. By taking age at mutation testing into account, all women were unaware of carrying the mutation during retrospective person-years. To account for the oversampling of affected individuals, a weighted cohort approach was used, as described by Antoniou et al. (12).
Details on study design, study participants, data collection, and statistical analyses can be found in the Supplementary Methods (available online).
Results
BRCA1 Mutation Carriers
The prospective cohort comprised 2276 women, of whom 269 (11.8%) were diagnosed with incident BC during follow-up. The left-truncated retrospective and full-cohort retrospective analyses included 3828 women (1095, 28.6%, with BC) and 5705 women (2525, 44.3%, with BC), respectively (Table 1). The proportion of young women (<35 years) with BC was lowest in the prospective cohort (14.1% vs 27.9% and 29.7%, respectively). In total, 83% of all women in the prospective cohort and 73% and 75% of women in the retrospective cohorts had ever used OCPs by the end of follow-up (Table 2).
Table 1.
Characteristics of BRCA1 mutation carrier cohort, by study design
| Prospective (n = 2276) |
Retrospective “left-truncated” (n = 3828) |
Retrospective “full-cohort” (n = 5705) |
||||
|---|---|---|---|---|---|---|
| BRCA1 | BC+ | BC- | BC+ | BC- | BC+ | BC- |
| No. (%) | 269 (11.8) | 2007 (88.2) | 1095 (28.6) | 2733 (71.4) | 2525 (44.3) | 3180 (55.7) |
| Mean age at start of FUP (SD), y | 40.7 (10.3) | 37.5 (11.8) | 38 (9.4) | 34.1 (11.8) | Birth | Birth |
| Mean age at end of FUP (SD), y | 44.9 (10.3) | 43.1 (12.2) | 41 (9.3) | 38.4 (11.6) | 40.1 (8.8) | 38.7 (11.6) |
| Age at end of FUP, No. (%) | ||||||
| <35 y | 38 (14.1) | 560 (27.9) | 305 (27.9) | 1111 (40.7) | 750 (29.7) | 1237 (38.9) |
| 35–64 y | 216 (80.3) | 1327 (66.2) | 770 (70.3) | 1555 (56.9) | 1746 (69.2) | 1869 (58.7) |
| 65+ y | 15 (5.6) | 120 (6.0) | 20 (1.8) | 67 (2.5) | 29 (1.2) | 74 (2.3) |
| Mean years of FUP (SD), y | 4.2 (3.3) | 5.6 (3.7) | 3.0 (1.3) | 4.3 (1.1) | 40.1 (8.8) | 38.7 (11.6) |
| Censored for, No. (%) | ||||||
| Breast cancer | 269 (100.0) | — | 1095 (100.0) | — | 2525 (100.0) | — |
| Ovarian cancer* | — | 49 (2.4) | — | 240 (8.8) | — | 413 (13.0) |
| Other cancer* | — | 45 (2.2) | — | 34 (1.2) | — | 145 (4.6) |
| Bilateral RRM*,† | — | 301 (15.0) | — | 201 (7.4) | — | 252 (7.9) |
| Death* | — | 5 (0.3) | — | 0 (0.0) | — | 0 (0.0) |
| Maximum FUP* | — | 1607 (80.1) | — | 2258 (86.6) | — | 2370 (74.5) |
| Year at end of FUP, No. (%) | ||||||
| 1959–1989 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 573 (22.7) | 114 (3.6) |
| 1990–2000 | 26 (9.7) | 32 (1.6) | 558 (51.0) | 902 (33.0) | 1327 (52.6) | 1150 (36.2) |
| 2001–2012 | 243 (90.3) | 1975 (98.4) | 537 (49.0) | 1831 (67.0) | 625 (24.8) | 1916 (60.3) |
| Birth year, No. (%) | ||||||
| 1920–1959 | 111 (41.3) | 552 (27.5) | 508 (46.4) | 886 (32.4) | 1637 (64.8) | 1181 (37.1) |
| 1960–1970 | 114 (42.4) | 640 (31.9) | 422 (38.5) | 916 (33.5) | 699 (27.7) | 1026 (32.3) |
| 1971–1992 | 44 (16.4) | 815 (40.6) | 165 (15.1) | 931 (34.1) | 189 (7.5) | 973 (30.6) |
| Study, No. (%) | ||||||
| 1. EMBRACE‡ | 41 (8.7) | 432 (91.3) | 309 (30.4) | 707 (69.6) | 740 (47.4) | 820 (52.6) |
| 2. GENEPSO‡ | 46 (9.4) | 442 (90.6) | 136 (17.3) | 649 (82.7) | 325 (32.0) | 691 (68.0) |
| 3. HEBON‡ | 40 (16.5) | 202 (83.5) | 84 (21.1) | 314 (78.9) | 330 (41.2) | 472 (58.9) |
| 4. kConFab | 55 (16.9) | 270 (83.1) | — | — | — | — |
| 5. BCFR | 50 (15.3) | 277 (84.7) | 257 (39.4) | 396 (60.6) | 456 (51.4) | 432 (48.7) |
| 6. Other‡,§ | 37 (8.8) | 384 (91.2) | 309 (31.7) | 667 (68.3) | 674 (46.8) | 765 (53.2) |
| RRSO, No. (%) | ||||||
| No | 153 (56.9) | 1284 (64.0) | 1059 (96.7) | 2601 (95.2) | 2465 (97.6) | 3041 (95.6) |
| Yes | 116 (43.1) | 723 (36.0) | 36 (3.3) | 132 (4.8) | 60 (2.4) | 139 (4.4) |
| No. of breast cancers among 1st- and 2nd-degree relatives, No. (%) | ||||||
| No BC | 54 (20.1) | 546 (27.2) | 259 (23.7) | 678 (24.8) | 593 (23.5) | 817 (25.7) |
| 1 | 91 (33.8) | 628 (31.3) | 317 (29.0) | 763 (27.9) | 679 (26.9) | 888 (27.9) |
| ≥2 | 108 (40.2) | 629 (31.3) | 288 (26.3) | 872 (31.9) | 669 (26.5) | 961 (30.2) |
| Missing | 13 (4.8) | 192 (9.6) | 190 (17.4) | 398 (14.6) | 524 (20.8) | 489 (15.4) |
| Cancer type unknown | 3 (1.1) | 12 (0.6) | 41 (3.7) | 22 (0.8) | 60 (2.4) | 25 (0.8) |
| Parity, No. (%) | ||||||
| Nulliparous | 51 (19.0) | 602 (30.0) | 250 (22.8) | 914 (33.4) | 513 (20.3) | 1020 (32.1) |
| Parous | 208 (77.3) | 1405 (70.0) | 845 (77.2) | 1819 (66.6) | 2012 (79.7) | 2160 (67.9) |
| Missing | 10 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
After exclusion of women in the preceding categories. BC = breast cancer; FUP = follow-up; RRM = risk-reducing mastectomy.
RRM occurring at the same age or within one year of BC diagnosis was ignored.
IBCCS is a collaboration of 1) EMBRACE, 2) GENEPSO, 3) HEBON, and 6) other.
“Other” included the following studies: MUV, MODSQUAD, GC-HBOC, Lund-BRCA, OUH, NIO, IHCC, HCSC, Stockholm-BRCA, INHERIT, CNIO, Milan Italy, HSP, DKFZ, Dusseldorf Germany, Belgium (order is based on number of carriers included in the analyses).
Table 2.
Breast cancer HR estimates by OCP use characteristics for BRCA1 mutation carriers, by study design
| Prospective |
Retrospective “left-truncated” |
Retrospective “full-cohort” |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BC+ | BC- | HR (95% CI)‡ | BC+ | BC- | Weighted* | BC+ | BC- | Weighted* | |
| No. (%)† | No. (%)† | No. (%)† | No. (%)† | HR (95% CI)§ | No. (%)† | No. (%)† | HR (95% CI)§ | ||
| OCP use | |||||||||
| Never (<6 mo) | 39 (14.5) | 290 (14.5) | 1.00 | 205 (18.7) | 552 (20.2) | 1.00 | 603 (23.9) | 672 (21.1) | 1.00 |
| Ever | 227 (84.4) | 1665 (83.0) | 1.08 (0.75 to 1.56) | 848 (77.4) | 2039 (74.6) | 1.26 (1.06 to 1.51)¶ | 1804 (71.5) | 2335 (73.4) | 1.39 (1.23 to 1.58)¶ |
| Ever, starting age unknown | 9 (3.4) | 69 (3.4) | 20 (1.8) | 95 (3.5) | 68 (2.7) | 110 (3.5) | |||
| Missing | 3 (1.1) | 52 (2.6) | 22 (2.0) | 47 (1.7) | 50 (2.0) | 63 (2.0) | |||
| Calendar year at start | |||||||||
| Never (<6 mo) | 39 (14.5) | 290 (14.5) | 1.00 | 205 (18.7) | 552 (20.2) | 1.00 | 603 (23.9) | 672 (21.1) | 1.00 |
| ≤1975 | 61 (22.7) | 256 (12.8) | 1.25 (0.79 to 1.96) | 246 (22.5) | 377 (13.8) | 1.32 (1.06 to 1.65) | 845 (33.5) | 512 (16.1) | 1.49 (1.29 to 1.71) |
| >1975 | 157 (58.4) | 1340 (66.8) | 0.99 (0.66 to 1.48) | 602 (55.0) | 1662 (60.8) | 1.23 (1.02 to 1.49) | 959 (38.0) | 1823 (57.3) | 1.27 (1.09 to 1.48) |
| Ever, starting year unknown | 9 (3.4) | 69 (3.4) | 20 (1.8) | 95 (3.5) | 68 (2.7) | 110 (3.5) | |||
| Missing | 3 (1.1) | 52 (2.6) | 22 (2.0) | 47 (1.7) | 50 (2.0) | 63 (2.0) | |||
| Time since last use, y | |||||||||
| Never (<6 mo) | 39 (14.5) | 290 (14.5) | 1.00 | 205 (18.7) | 552 (20.2) | 1.00 | 603 (23.9) | 672 (21.1) | 1.00 |
| ≤3 | 45 (16.7) | 392 (19.5) | 0.96 (0.60 to 1.55) | 218 (19.9) | 772 (28.3) | 1.42 (1.12 to 1.80) | 528 (20.9) | 885 (27.8) | 1.53 (1.30 to 1.80) |
| >3–9 | 48 (17.8) | 328 (16.3) | 1.14 (0.72 to 1.80) | 104 (9.5) | 277 (10.1) | 1.16 (0.88 to 1.52) | 300 (11.9) | 317 (10.0) | 1.42 (1.19 to 1.70) |
| ≥10 | 95 (35.3) | 622 (31.0) | 1.11 (0.75 to 1.65) | 283 (25.8) | 583 (21.3) | 1.31 (1.05 to 1.63) | 552 (21.9) | 681 (21.4) | 1.44 (1.22 to 1.69) |
| Ever, no period-specific data | 39 (14.5) | 323 (16.1) | 263 (24.0) | 502 (18.4) | 492 (19.5) | 562 (17.7) | |||
| Missing | 3 (1.1) | 52 (2.6) | 22 (2.0) | 47 (1.7) | 50 (2.0) | 63 (2.0) | |||
| Ptrend per year | .3 | .58 | .71 | ||||||
| Starting age, y | |||||||||
| Never (<6 mo) | 39 (14.5) | 290 (14.5) | 1.00 | 205 (18.7) | 552 (20.2) | 1.00 | 603 (23.9) | 672 (21.1) | 1.00 |
| ≤17 | 71 (26.4) | 592 (29.5) | 1.16 (0.76 to 1.79) | 275 (25.1) | 745 (27.3) | 1.43 (1.16 to 1.77) | 498 (19.7) | 846 (26.6) | 1.85 (1.55 to 2.20) |
| 18–22 | 109 (40.5) | 733 (36.5) | 1.11 (0.75 to 1.65) | 392 (35.8) | 924 (33.8) | 1.17 (0.97 to 1.42) | 850 (33.7) | 1043 (32.8) | 1.44 (1.24 to 1.67) |
| >22 | 38 (14.1) | 271 (13.5) | 0.96 (0.60 to 1.53) | 181 (16.5) | 370 (13.5) | 1.27 (1.01 to 1.59) | 456 (18.1) | 446 (14.0) | 1.17 (1.00 to 1.37) |
| Ever, starting age unknown | 9 (3.4) | 69 (3.4) | 20 (1.8) | 95 (3.5) | 68 (2.7) | 110 (3.5) | |||
| Missing | 3 (1.1) | 52 (2.6) | 22 (2.0) | 47 (1.7) | 50 (2.0) | 63 (2.0) | |||
| Ptrend per year | .57 | .8 | 6.00E to 05 | ||||||
| Total duration of use, y | |||||||||
| Never (<6 mo) | 39 (14.5) | 290 (14.5) | 1.00 | 205 (18.7) | 552 (20.2) | 1.00 | 603 (23.9) | 672 (21.1) | 1.00 |
| <5 | 67 (24.9) | 484 (24.1) | 1.13 (0.75 to 1.71) | 135 (12.3) | 209 (15.0) | 1.28 (0.99 to 1.65) | 321 (12.7) | 466 (14.7) | 1.25 (1.04 to 1.50) |
| 5–9 | 55 (20.5) | 537 (26.8) | 0.93 (0.60 to 1.43) | 186 (17.0) | 573 (21.0) | 1.27 (1.00 to 1.61) | 437 (17.3) | 663 (20.9) | 1.45 (1.23 to 1.72) |
| ≥10 | 92 (34.2) | 545 (27.2) | 1.16 (0.77 to 1.73) | 284 (25.9) | 650 (23.8) | 1.37 (1.10 to 1.70) | 622 (24.6) | 754 (23.7) | 1.67 (1.42 to 1.96) |
| Ever, no period-specific data | 13 (4.8) | 99 (4.9) | 263 (24.0) | 502 (18.4) | 492 (19.5) | 562 (17.7) | |||
| Missing | 3 (1.1) | 52 (2.6) | 22 (2.0) | 47 (1.7) | 50 (2.0) | 63 (2.0) | |||
| Ptrend per year | .66 | .01 | 4.9E to 10 | ||||||
| Duration by FFTP‖ | |||||||||
| Before FFTP, y | |||||||||
| Never (<6 mo) | 70 (26.0) | 487 (24.3) | 1.00 | 317 (29.0) | 775 (28.4) | 1.00 | 937 (37.1) | 946 (29.8) | 1.00 |
| <5 | 37 (13.8) | 349 (17.4) | 1.10 (0.71 to 1.72) | 144 (13.2) | 157 (16.7) | 1.05 (0.84 to 1.33) | 321 (12.7) | 514 (16.2) | 1.19 (0.99 to 1.42) |
| 5–9 | 44 (16.4) | 474 (23.6) | 0.93 (0.60 to 1.45) | 194 (17.7) | 598 (21.9) | 1.15 (0.92 to 1.44) | 401 (15.9) | 690 (21.7) | 1.39 (1.16 to 1.66) |
| ≥10 | 38 (14.1) | 278 (13.9) | 1.17 (0.73 to 1.88) | 155 (14.2) | 354 (13.0) | 1.41 (1.10 to 1.81) | 324 (12.8) | 405 (12.7) | 2.02 (1.64 to 2.50) |
| Ptrend per year | .93 | .001 | 4.5E to 11 | ||||||
| After FFTP, y | |||||||||
| Never (<6 mo) | 117 (43.5) | 1087 (54.2) | 1.00 | 514 (46.9) | 1505 (55.1) | 1.00 | 1253 (49.6) | 1752 (55.1) | 1.00 |
| <5 | 36 (13.4) | 255 (12.7) | 1.14 (0.75 to 1.73) | 136 (12.4) | 107 (11.2) | 1.15 (0.92 to 1.44) | 322 (12.8) | 365 (11.5) | 1.08 (0.91 to 1.28) |
| 5–9 | 17 (6.3) | 134 (6.7) | 0.89 (0.51 to 1.54) | 84 (7.7) | 190 (7.0) | 1.04 (0.80 to 1.35) | 214 (8.5) | 221 (7.0) | 1.12 (0.93 to 1.36) |
| ≥10 | 19 (7.1) | 112 (5.6) | 1.04 (0.60 to 1.79) | 76 (6.9) | 182 (6.7) | 1.01 (0.76 to 1.35) | 194 (7.7) | 217 (6.8) | 1.18 (0.95 to 1.45) |
| Ptrend per year | .99 | .76 | .02 | ||||||
| Ever, no period-specific data | 77 (28.6) | 367 (18.3) | 263 (24.0) | 502 (18.4) | 492 (19.5) | 562 (17.7) | |||
| OCP or FFTP data missing | 3 (1.1) | 52 (2.6) | 22 (2.0) | 47 (1.7) | 50 (2.0) | 63 (2.0) | |||
Weighted to account for the oversampling of affected individuals. BC = breast cancer; CI = confidence interval; FFTP = first full-term pregnancy; HR = hazard ratio; OCP = oral contraceptive preparation.
Distribution of variables at end of follow-up.
Intrinsically stratified on study (EMBRACE, GENEPSO, HEBON, kConFab, BCFR, other) and birth cohort (1920–1960< 1961–1992). Clustered on family membership.
Intrinsically stratified on study (EMBRACE, GENEPSO, HEBON, BCFR, other) and birth cohort (1920–1952, 1953–1964, 1965–1992). Clustered on family membership.
Duration before FFTP and after FFTP included in one multivariable model. Nulliparous women only contributed to various duration categories before FFTP and were placed in the category “never user” after FFTP. Adjusted for age at FFTP (nulliparous, ≤20, 21–25, >25 years).
Unweighted results: Left-truncated HR = 1.24, 95% CI = 1.05 to 1.45; Full cohort HR = 1.38, 95% CI = 1.25 to 1.52.
From the prospective cohort analysis, ever OCP use was not associated with BC risk (HR = 1.08, 95% confidence interval [CI] = 0.75 to 1.56). Hazard ratios did not vary according to total duration of use, age at first use, recency of use, or duration of use before FFTP (Table 2). In contrast, from the left-truncated and full-cohort retrospective analyses, we found an association between ever OCP use and BC risk (HR = 1.26, 95% CI = 1.06 to 1.51, and HR = 1.39, 95% CI = 1.23 to 1.58, respectively). Risk increased with longer lifetime duration of use (eg, left-truncated; use < 5 years: HR = 1.28; 5–9 years: HR = 1.27; ≥10 years: HR = 1.37; Ptrend = .01) and longer duration of use preceding FFTP (eg, left-truncated; <5 years: HR = 1.05; 5–9 years: HR = 1.15; ≥10 years: HR = 1.41; Ptrend = .001). From the full-cohort retrospective analysis, but not from the left-truncated analysis, there was an increased risk of BC with younger age at first OCP use (Ptrend < .001) and longer duration of use after FFTP (Ptrend = .02).
The hazard ratios for associations between ever OCP use and BC risk did not vary by birth cohort or study in either the left-truncated analysis or the full-cohort retrospective analysis. The numbers in birth cohort and study strata were too low to draw conclusions from the prospective analysis (data not shown).
Additional results were restricted to the left-truncated retrospective and prospective analyses as they are susceptible to fewer biases. When stratified by attained age (≤35, >35 years) (Table 3), the left-truncated retrospective analysis suggested that the positive trend with longer duration of use before FFTP was restricted to younger women (≤35 years; <5 years: HR = 1.19; 5–9 years: HR = 1.37; ≥10 years: HR = 1.88; >35 years; <5 years: HR = 1.10; 5–9 years: HR = 1.12; ≥10 years: HR = 1.18; Pdifference = .08). The sample size was too small to carry out a stratified analysis using the prospective data, with only two to 11 BC cases per category.
Table 3.
Breast cancer HR estimates for BRCA1 mutation carriers stratified by attained age, the left-truncated retrospective cohort
| Retrospective “left-truncated” |
||||||
|---|---|---|---|---|---|---|
| BC+ | BC- | HR (95% CI) † | BC+ | BC- | ||
| No. (%)* | No. (%)* | No. (%)* | No. (%)* | HR (95% CI)† | ||
| ≤35 y (n = 1955) | >35 y (n = 2255) | |||||
| OCP use | ||||||
| Never (<6 mo) | 40 (11.1) | 249 (15.6) | 1.00 | 165 (22.4) | 363 (23.9) | 1.00 |
| Ever | 302 (84.1) | 1283 (80.4) | 1.41 (1.01 to 1.97) | 546 (74.2) | 1062 (69.9) | 1.19 (0.99 to 1.44) |
| Ever, starting age Unknown | 6 (1.7) | 45 (2.8) | 14 (1.9) | 63 (4.2) | ||
| Missing | 11 (3.1) | 19 (1.2) | 11 (1.5) | 31 (2.0) | ||
| Duration by FFTP‡ | ||||||
| Before FFTP, y | ||||||
| Never (<6 mo) | 57 (15.9) | 312 (19.6) | 1.00 | 260 (35.3) | 553 (36.4) | 1.00 |
| <5 | 39 (10.9) | 284 (17.8) | 1.19 (0.77 to 1.84)§ | 105 (14.3) | 240 (15.8) | 1.10 (0.86 to 1.40)§ |
| 5–9.99 | 78 (21.7) | 426 (26.7) | 1.37 (0.93 to 2.02)‖ | 116 (15.8) | 249 (16.4) | 1.12 (0.87 to 1.45)‖ |
| ≥10 | 81 (22.6) | 255 (16.0) | 1.88 (1.27 to 2.79)¶ | 74 (10.1) | 157 (10.3) | 1.18 (0.87 to 1.60)¶ |
| Ptrend per year | .001# | .08 # | ||||
| After FFTP, y | ||||||
| Never (<6 mo) | 178 (49.6) | 962 (60.3) | 1.00 | 336 (45.7) | 715 (47.1) | 1.00 |
| <5 | 41 (11.4) | 176 (11.0) | 1.03 (0.70 to 1.50) | 95 (12.9) | 189 (12.4) | 1.10 (0.86 to 1.40) |
| 5–9.99 | 24 (6.7) | 101 (6.3) | 1.09 (0.68 to 1.76) | 60 (8.2) | 129 (8.5) | 1.01 (0.76 to 1.36) |
| ≥10 | 12 (3.3) | 38 (2.4) | 1.93 (0.99 to 3.75) | 64 (8.7) | 166 (10.9) | 0.89 (0.67 to 1.18) |
| Ptrend per year | .4 | .86 | ||||
| Ever, no period-specific data | 93 (25.9) | 300 (18.8) | 170 (23.1) | 289 (19.0) | ||
| OCP or FFTP data missing | 11 (3.1) | 19 (1.2) | 11 (1.5) | 31 (2.0) | ||
BC = breast cancer; CI = confidence interval; FFTP = first full-term pregnancy; HR = hazard ratio; OCP = oral contraceptive preparations.
Distribution of variables at end of follow-up..
Intrinsically stratified on study (EMBRACE, GENEPSO, HEBON, BCFR, other) and birth cohort (1920–1952, 1953–1964, 1965–1992). Clustered on family membership.
Duration before FFTP and after FFTP were included in one multivariable model. Nulliparous women only contributed to various duration categories before FFTP and were placed in the category “never user” after FFTP. Adjusted for age at FFTP (nulliparous, ≤20, 21–25, >25 years)
Test for difference: P = .406.
Test for difference: P = .757.
Test for difference: P = .055.
Test for difference: P = .077.
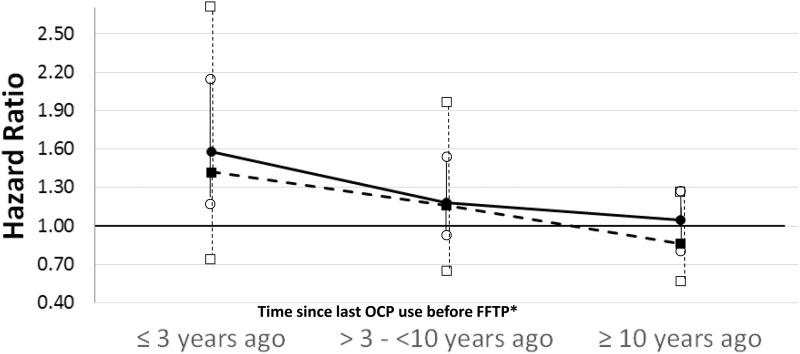
To try to explain the different findings from the prospective and left-truncated retrospective analyses, we performed exploratory analyses of time since last OCP use before FFTP. From the left-truncated retrospective analysis, BC risk decreased with a longer time since “last OCP use before FFTP” (Ptrend = .02) (Figure 1). Although the magnitude of the hazard ratios was smaller from the prospective analysis, the hazard ratios followed a similar pattern.
Figure 1.
Association between time since last oral contraceptive preparation use before first full-term pregnancy and breast cancer risk among BRCA1 mutation carriers. Black dot indicates retrospective “left-truncated” analysis. Black square indicates prospective analysis. Nonfilled symbols are 95% confidence intervals. *Adjusted for oral contraceptive preparation use after first full-term pregnancy. FFTP = first full-term pregnancy; OCP = oral contraceptive preparation.
BRCA2 Mutation Carriers
The prospective, left-truncated retrospective, and full retrospective cohorts comprised 1610 women (157, 9.8%, with incident BC diagnosed during follow-up), 2512 women (752, 29.9%, with BC), and 3521 women (1548, 44.0%, with BC), respectively (Table 4). As for BRCA1 mutation carriers, for BRCA2 mutation carriers, the proportion of young women (<35 years) with BC was lowest in the prospective cohort (8.2% vs 16.1% and 16.4%, respectively).
Table 4.
Characteristics of BRCA2 mutation carrier cohort, by study design
| Prospective (n = 1610) |
Retrospective “left-truncated” (n = 2512) |
Retrospective “full-cohort” (n = 3521) |
||||
|---|---|---|---|---|---|---|
| BRCA2 | BC+ | BC- | BC+ | BC- | BC+ | BC- |
| No. (%) | 157 (9.8) | 1453 (90.3) | 752 (29.9) | 1760 (70.6) | 1548 (44.0) | 1973 (56.0) |
| Mean age at start of FUP (SD), y | 45.1 (10.1) | 40 (12.6) | 40.9 (9.6) | 36.4 (12.5) | Birth | Birth |
| Mean age at end of FUP (SD), y | 49 (10.3) | 45.1 (13.0) | 44 (9.5) | 40.8 (12.3) | 43.4 (9.1) | 41 (12.4) |
| Age at end of FUP, No. (%) | ||||||
| <35 y | 13 (8.2) | 337 (23.2) | 121 (16.1) | 593 (33.7) | 254 (16.4) | 652 (33.1) |
| 35–64 y | 132 (84.1) | 993 (68.4) | 611 (81.3) | 1092 (62.0) | 1261 (81.4) | 1232 (62.5) |
| 65+ y | 12 (7.6) | 123 (8.5) | 20 (2.7) | 75 (4.3) | 33 (2.1) | 89 (4.5) |
| Mean years of FUP (SD) | 3.8 (3.1) | 5 (3.4) | 3 (1.2) | 4.5 (1.0) | 43.4 (9.1) | 41 (12.4) |
| Censored for, No. (%) | ||||||
| Breast cancer | 157 (100.0) | — | 752 (100.0) | — | 1548 (100.0) | — |
| Ovarian cancer* | — | 9 (0.6) | — | 85 (4.8) | — | 142 (7.2) |
| Other cancer* | — | 29 (2.0) | — | 36 (2.1) | — | 140 (7.1) |
| Bilateral RRM*,† | — | 182 (12.5) | — | 117 (6.7) | — | 132 (6.7) |
| Death† | — | 8 (0.6) | — | 2 (0.1) | — | 2 (0.1) |
| Maximum FUP* | — | 1225 (84.3) | — | 1520 (86.4) | — | 1557 (78.9) |
| Year at end of FUP, No. (%) | ||||||
| 1959–1989 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 267 (17.3) | 70 (3.6) |
| 1990–2000 | 15 (9.6) | 12 (0.8) | 335 (44.6) | 320 (18.2) | 767 (49.6) | 433 (22.0) |
| 2001–2012 | 142 (90.5) | 1441 (99.2) | 417 (55.5) | 1440 (81.8) | 514 (33.2) | 1470 (74.5) |
| Birth year, No. (%) | ||||||
| 1920–1959 | 86 (54.8) | 459 (31.6) | 406 (54.0) | 612 (34.8) | 1067 (68.9) | 776 (39.3) |
| 1960–1970 | 60 (38.2) | 481 (33.1) | 281 (37.4) | 598 (34.0) | 401 (25.9) | 630 (31.9) |
| 1971–1992 | 11 (7.0) | 513 (35.3) | 65 (8.6) | 550 (31.3) | 80 (5.2) | 567 (28.7) |
| Study, No. (%) | ||||||
| 1. EMBRACE‡ | 42 (8.7) | 441 (91.3) | 269 (28.9) | 662 (71.1) | 611 (45.1) | 744 (54.9) |
| 2. GENEPSO‡ | 18 (5.5) | 307 (94.5) | 71 (14.6) | 416 (85.4) | 161 (26.9) | 437 (73.1) |
| 3. HEBON‡ | 4 (5.3) | 71 (94.7) | 26 (20.3) | 102 (79.7) | 90 (38.0) | 147 (62) |
| 4. kConFab | 38 (13.2) | 250 (86.8) | — | — | — | — |
| 5. BCFR | 33 (12.9) | 222 (87.1) | 226 (43.5) | 294 (56.5) | 356 (52.6) | 321 (47.4) |
| 6. Other‡,§ | 22 (12.0) | 162 (88.0) | 160 (35.9) | 286 (64.1) | 330 (50.5) | 324 (49.5) |
| RRSO, No. (%) | ||||||
| No | 106 (67.5) | 1007 (69.3) | 725 (96.4) | 1689 (96.0) | 1494 (96.5) | 1897 (96.2) |
| Yes | 51 (32.5) | 446 (30.7) | 27 (3.6) | 71 (4.0) | 54 (3.5) | 76 (3.9) |
| No. of breast cancers among 1st- and 2nd-degree relatives, No. (%) | ||||||
| No BC | 17 (10.8) | 285 (19.6) | 131 (17.4) | 292 (16.6) | 276 (17.8) | 347 (17.6) |
| 1 | 49 (31.2) | 446 (30.7) | 231 (30.7) | 452 (25.7) | 422 (27.3) | 489 (25.2) |
| ≥2 | 78 (49.7) | 556 (38.3) | 209 (27.8) | 715 (40.6) | 446 (28.8) | 756 (38.3) |
| Missing | 13 (8.3) | 153 (10.5) | 143 (19.0) | 292 (16.6) | 350 (22.6) | 361 (18.3) |
| Cancer type unknown | 0 (0.0) | 13 (0.9) | 38 (5.1) | 9 (0.5) | 54 (3.5) | 11 (0.6) |
| Parity, No. (%) | ||||||
| Nulliparous | 22 (14.0) | 406 (27.9) | 142 (18.9) | 525 (29.8) | 274 (17.7) | 572 (29.0) |
| Parous | 127 (80.9) | 1047 (72.1) | 610 (81.1) | 1235 (70.2) | 1274 (82.3) | 1401 (71.0) |
| Missing | 8 (5.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
BC = breast cancer; FUP = follow-up; RRM = risk-reducing mastectomy.
After exclusion of women in the preceding categories.
RRM occurring at the same age or within one year of BC diagnosis was ignored.
IBCCS is a collaboration of 1) EMBRACE, 2) GENEPSO, 3) HEBON, and 6) Other.
“Other” included the following studies: GC-HBOC, MUV, MODSQUAD, INHERIT, HCSC, OUH, Lund-BRCA, CNIO, NIO, Stockholm-BRCA, Milan Italy, HSP (order is based on number of carriers included in the analyses).
In total, 83%, 75%, and 72% of all women from the prospective, left-truncated, and full retrospective cohorts, respectively, had ever used OCPs by the end of follow-up (Table 5).
Table 5.
Breast cancer HR estimates by OCP use characteristics for BRCA2 mutation carriers, by study design
| Prospective |
Retrospective “left-truncated” |
Retrospective “full-cohort” |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BC+ | BC- | BC+ | BC- | Weighted* | BC+ | BC- | Weighted* | ||
| No. (%)† | No. (%)† | HR (95% CI)‡ | No. (%)† | No. (%)† | HR (95% CI)§ | No. (%)† | No. (%)† | HR (95% CI)§ | |
| OCP use | |||||||||
| Never (<6 mo) | 17 (10.8) | 214 (14.7) | 1.00 | 161 (21.4) | 337 (19.2) | 1.00 | 376 (24.3) | 408 (20.7) | 1.00 |
| Ever | 137 (87.3) | 1206 (83.0) | 1.75 (1.03 to 2.97) | 563 (74.9) | 1333 (75.7) | 1.06 (0.85 to 1.33)¶ | 1096 (70.8) | 1456 (73.8) | 1.52 (1.28 to 1.81)¶ |
| Ever, starting age unknown | 3 (1.9) | 42 (2.9) | 16 (2.1) | 63 (3.6) | 46 (3.0) | 73 (3.7) | |||
| Missing | 3 (1.9) | 33 (2.3) | 12 (1.6) | 27 (1.5) | 30 (1.9) | 36 (1.8) | |||
| Calendar year at start | |||||||||
| Never (<6 mo) | 17 (10.8) | 214 (14.7) | 1.00 | 161 (21.4) | 337 (19.2) | 1.00 | 376 (24.3) | 408 (20.7) | 1.00 |
| ≤1975 | 51 (32.5) | 226 (15.6) | 1.77 (0.98 to 3.21) | 197 (26.2) | 279 (15.9) | 1.06 (0.80 to 1.41) | 554 (35.8) | 352 (17.8) | 1.47 (1.22 to 1.78) |
| >1975 | 83 (52.9) | 938 (64.6) | 1.77 (0.96 to 3.25) | 366 (48.7) | 1054 (59.9) | 1.06 (0.83 to 1.37) | 542 (35.0) | 1104 (56.0) | 1.63 (1.30 to 2.05) |
| Ever, starting year unknown | 3 (1.9) | 42 (2.9) | 16 (2.1) | 63 (3.6) | 46 (3.0) | 73 (3.7) | |||
| Missing | 3 (1.9) | 33 (2.3) | 12 (1.6) | 27 (1.5) | 30 (1.9) | 36 (1.8) | |||
| Time since last use, y | |||||||||
| Never (<6 mo) | 17 (10.8) | 214 (14.7) | 1.00 | 161 (21.4) | 337 (19.2) | 1.00 | 376 (24.3) | 408 (20.7) | 1.00 |
| ≤3 | 22 (14.0) | 290 (20.0) | 1.56 (0.77 to 3.17) | 96 (12.8) | 431 (24.5) | 1.12 (0.80 to 1.57) | 211 (13.6) | 471 (23.9) | 1.54 (1.21 to 1.97) |
| >3–<10 | 17 (10.8) | 201 (13.8) | 1.60 (0.78 to 3.30) | 68 (9.0) | 179 (10.2) | 1.27 (0.88 to 1.83) | 173 (11.2) | 201 (10.2) | 1.85 (1.46 to 2.36) |
| ≥10 | 70 (44.6) | 507 (34.9) | 1.77 (1.02 to 3.06) | 209 (27.8) | 432 (24.6) | 1.09 (0.82 to 1.45) | 415 (26.8) | 467 (23.7) | 1.57 (1.27 to 1.94) |
| Ever, no period-specific data | 28 (17.8) | 208 (14.3) | 206 (27.4) | 354 (20.1) | 343 (22.2) | 390 (19.8) | |||
| Missing | 3 (1.9) | 33 (2.3) | 12 (1.6) | 27 (1.5) | 30 (1.9) | 36 (1.8) | |||
| Ptrend per year | .75 | .87 | .69 | ||||||
| Starting age, y | |||||||||
| Never (<6 mo) | 17 (10.8) | 214 (14.7) | 1.00 | 161 (21.4) | 337 (19.2) | 1.00 | 376 (24.3) | 408 (20.7) | 1.00 |
| ≤17 | 31 (19.8) | 373 (25.7) | 1.58 (0.82 to 3.04) | 127 (16.9) | 425 (24.2) | 0.90 (0.67 to 1.21) | 211 (13.6) | 458 (23.2) | 1.53 (1.17 to 1.99) |
| 18–22 | 69 (44.0) | 574 (39.5) | 1.88 (1.06 to 3.33) | 287 (38.2) | 648 (36.8) | 1.11 (0.86 to 1.42) | 554 (35.8) | 702 (35.6) | 1.66 (1.14 to 1.73) |
| >22 | 34 (21.7) | 217 (14.9) | 1.72 (0.94 to 3.14) | 149 (19.8) | 260 (14.8) | 1.10 (0.83 to 1.46) | 331 (21.4) | 296 (15.0) | 1.40 (1.14 to 1.73) |
| Ever, starting age unknown | 3 (1.9) | 42 (2.9) | 16 (2.1) | 63 (3.6) | 46 (3.0) | 73 (3.7) | |||
| Missing | 3 (1.9) | 33 (2.3) | 12 (1.6) | 27 (1.5) | 30 (1.9) | 36 (1.8) | |||
| Ptrend per year | .83 | .20 | .02 | ||||||
| Total duration of use | |||||||||
| Never (<6 mo) | 17 (10.8) | 214 (14.7) | 1.00 | 161 (21.4) | 337 (19.2) | 1.00 | 376 (24.3) | 408 (20.7) | 1.00 |
| <5 | 50 (31.9) | 393 (27.1) | 1.83 (1.04 to 3.25) | 101 (13.4) | 247 (14.0) | 1.12 (0.80 to 1.57) | 219 (14.2) | 276 (14.0) | 1.46 (1.15 to 1.86) |
| 5–9.99 | 30 (19.1) | 363 (25.0) | 1.40 (0.75 to 2.61) | 129 (17.2) | 359 (20.4) | 1.26 (0.93 to 1.71) | 253 (16.3) | 388 (19.7) | 1.68 (1.32 to 2.12) |
| ≥10 | 49 (31.2) | 393 (27.1) | 1.75 (0.98 to 3.16) | 143 (19.0) | 436 (24.8) | 1.01 (0.74 to 1.37) | 327 (21.1) | 475 (24.1) | 1.70 (1.35 to 2.13) |
| Ever, no period-specific data | 8 (5.1) | 57 (3.9) | 206 (27.4) | 354 (20.1) | 343 (22.2) | 390 (19.8) | |||
| Missing | 3 (1.9) | 33 (2.3) | 12 (1.6) | 27 (1.5) | 30 (1.9) | 36 (1.8) | |||
| Ptrend per year | .68 | .94 | 2.2E to 04 | ||||||
| Duration of use by FFTP‖ | |||||||||
| Before FFTP | |||||||||
| Never (<6 mo) | 43 (27.4) | 390 (26.8) | 1.00 | 237 (31.5) | 495 (28.1) | 1.00 | 603 (39.0) | 603 (30.6) | 1.00 |
| <5 | 20 (12.7) | 237 (16.3) | 1.31 (0.71 to 2.39) | 106 (14.1) | 262 (14.9) | 1.15 (0.85 to 1.56) | 197 (12.7) | 281 (14.2) | 1.30 (1.02 to 1.67) |
| 5–9.99 | 28 (17.8) | 308 (21.2) | 1.39 (0.78 to 2.48) | 117 (15.6) | 391 (22.2) | 1.00 (0.74 to 1.35) | 225 (14.5) | 413 (20.9) | 1.38 (1.07 to 1.79) |
| ≥10 | 11 (7.0) | 199 (13.7) | 0.85 (0.39 to 1.88) | 74 (9.8) | 231 (13.1) | 0.97 (0.68 to 1.38) | 150 (9.7) | 250 (12.7) | 1.64 (1.21 to 2.21) |
| Ptrend per year | .69 | .76 | .002 | ||||||
| After FFTP, y | |||||||||
| Never (<6 mo) | 54 (34.4) | 769 (52.9) | 1.00 | 353 (46.9) | 921 (52.3) | 1.00 | 749 (48.4) | 1031 (52.3) | 1.00 |
| <5 | 25 (15.9) | 202 (13.9) | 1.55 (0.91 to 2.65) | 80 (10.6) | 214 (12.2) | 0.90 (0.65 to 1.23) | 172 (11.1) | 239 (12.1) | 1.06 (0.84 to 1.35) |
| 5–9.99 | 10 (6.4) | 80 (5.5) | 1.72 (0.83 to 3.58) | 55 (7.3) | 131 (7.4) | 1.16 (0.81 to 1.65) | 132 (8.5) | 150 (7.6) | 1.34 (1.03 to 1.74) |
| ≥10 | 13 (8.3) | 83 (5.7) | 1.55 (0.78 to 3.09) | 46 (6.1) | 113 (6.4) | 0.96 (0.63 to 1.46) | 122 (7.9) | 127 (6.4) | 1.29 (0.96 to 1.72) |
| Ptrend per year | .68 | .98 | .04 | ||||||
| Ever, no period-specific data | 52 (33.1) | 286 (19.7) | 206 (27.4) | 354 (20.1) | 343 (22.2) | 390 (19.8) | |||
| OCP or FFTP data missing | 3 (1.9) | 33 (2.3) | 12 (1.6) | 27 (1.5) | 30 (1.9) | 36 (1.8) | |||
Weighted to account for the oversampling of affected individuals. BC = breast cancer; CI = confidence interval; FFTP = first full-term pregnancy; HR = hazard ratio; OCP = oral contraceptive preparations.
Distribution of variables at end of follow-up.
Intrinsically stratified on study (EMBRACE, GENEPSO, HEBON, kConFab, BCFR, other) and birth cohort (1920–1960, 1961–1992). Clustered on family membership.
Intrinsically stratified on study (EMBRACE, GENEPSO, HEBON, BCFR, other) and birth cohort (1920–1952, 1953–1964, 1965–1992). Clustered on family membership.
Duration before FFTP and after FFTP included in one multivariable model. Nulliparous women only contributed to various duration categories before FFTP and are placed in the category “never user” after FFTP. Adjusted for age at FFTP (nulliparous, ≤20, 21–25, >25 years).
Unweighted results: Left-truncated HR = 1.03, 95% CI = 0.85 to 1.24; Full-cohort HR = 1.48, 95% CI = 1.31 to 1.68.
We found a positive association between ever OCP use and BC risk from both the prospective and full-cohort retrospective analyses (HR = 1.75, 95% CI = 1.03 to 2.97, and HR = 1.52, 95% CI = 1.28 to 1.81, respectively) but not from the left-truncated analysis (HR = 1.06, 95% CI = 0.85 to 1.33). From the full-cohort retrospective analysis, BC risk was higher for women with younger age at first use (≤17 years: HR = 1.53; 18–22 years: HR = 1.66; ≥23 years: HR = 1.40; Ptrend = .02) and longer duration of use (<5 years: HR = 1.46; 5–9 years: HR = 1.68; ≥10 years: HR = 1.70; Ptrend < .001), most clearly for use before FFTP (<5 years: HR = 1.30; 5–9 years: HR = 1.38; ≥10 years: HR = 1.64; Ptrend = .002). There was no evidence of variation in the hazard ratios by any of these characteristics from the prospective or left-truncated analyses.
The association between ever OCP use and BC risk did not vary by birth cohort or study from the retrospective analyses (data not shown; the numbers were too low in the prospective cohort to perform these analyses). The results from the stratified analyses by age were consistent with the unstratified retrospective analyses (left-truncated; all ages: HR = 1.06; ≤35 years: HR = 1.01; >35 years: HR = 1.03). For BRCA2 mutation carriers, we repeated the exploratory analysis by time since last OCP use before FFTP that we conducted for BRCA1 mutation carriers, but no evidence for an association was found (data not shown).
Discussion
For BRCA1 mutation carriers, no association between ever OCP use and BC risk was found from the prospective analysis. From the left-truncated retrospective analysis, however, we found a positive association (HR = 1.26), which was stronger without left-truncation (full cohort: HR = 1.39). Longer duration of use, especially before FFTP, was associated with an increasing risk of BC from both retrospective analyses, but not from the prospective analysis. The increasing risk associated with a younger age at first use found from the full-cohort retrospective analysis was not observed in the left-truncated retrospective or prospective analyses. For BRCA2 mutation carriers, we found positive associations between ever OCP use and BC risk from both the prospective and full-cohort retrospective analyses (HR = 1.75 and HR = 1.52, respectively). However, there was no increased risk after left-truncation (HR = 1.06). From full-cohort retrospective analyses, increasing risks with younger age at first use and longer durations of use, especially before FFTP, were found, but these trends again did not persist in left-truncated retrospective or prospective analyses. It is important to note, however, that for both BRCA1 and BRCA2 mutation carriers, the confidence intervals of the estimates from the retrospective and prospective analyses overlapped.
All studies on OCP use and risk of BC for BRCA1/2 mutation carriers to date have performed full-cohort retrospective analyses (6,13–17). Among “independent” studies, using data not included in the present study, most found a statistical significantly increased association with BC risk for BRCA1 mutation carriers, comparable with our full-cohort retrospective analysis (13,17,18). In addition, Kotsopoulos et al. and Ursin et al. also found that the increased risk was limited to early-onset BC (<40 years) (17,18). For BRCA2 mutation carriers, results from two previous independent full-cohort analyses were very inconsistent (odds ratio = 0.94 vs HR = 2.07). However, in our study, the magnitude of the associations estimated from the full-cohort analysis became weaker (BRCA1) or even disappeared (BRCA2) when survivors of more than five years were excluded (left-truncated retrospective analyses). This might suggest the presence of survival bias, driven by a lower stage at diagnosis for OCP users compared with nonusers, as found for the general population (19). However, a true age-related effect that disappears when the cohort gets older might be another explanation. Apart from survival bias, there was evidence for testing bias, resulting from the nonrandom uptake of genetic testing. We adjusted for the oversampling of carriers with BC by using the weighted cohort approach (12). Furthermore, the presence of recall and response biases cannot be excluded in the retrospective analyses.
We consider the findings from prospective analyses to be our main results, as they are free of testing bias and hardly influenced by recall and survival bias. They showed no association between the various characteristics of OCP use and BC risk for BRCA1 mutation carriers, whereas compared with never users, ever OCP users had a higher risk of BC for BRCA2 mutation carriers. However, trends with duration, starting age, or recency of OCP use were absent. Such trends might have been expected if the association had been causal, but power was limited to detect such trends. Results of our adequately powered next-best left-truncated analyses support the absence of an association between OCP use and risk of BC for BRCA2 mutation carriers.
Although prospective results showed no associations for BRCA1 mutation carriers, weak associations between OCP use and BC risk arose in left-truncated analyses. This seemed to be driven by duration of use before FFTP. Exploratory analyses showed that duration of OCP use before FFTP was associated with BC risk diagnosed before age 35 years), consistent with findings of Pike et al. in the general population (20). The prospective cohort included a much smaller proportion of such young BC cases (≤35 years 14.1% vs 27.9%, respectively) (Table 1). Additionally, the positive association of OCP use before FFTP with risk of early-onset BC seemed to diminish with time since last use before FFTP. Thus, the prospective cohort may have been too old to detect the temporal effect of OCP use before FFTP on risk of early-onset BC.
We did not consider a combined BRCA1/2 mutation carrier analysis to increase prospective power, because of our own gene-specific findings and those of studies that reported different associations for BRCA1 and BRCA2 mutation carriers (21–23). Such differences may be caused by differences in BC subtype, being more often ER-negative in BRCA1-associated BC and ER-positive in BRCA2-associated BC in young middle-aged women (24).
This is the first prospective study to investigate the association between OCP use and BC risk for BRCA1 and BRCA2 mutation carriers. Two previous reports were based on left-truncated retrospective analyses; however, these form a subset of current retrospective analysis. Brohet et al. (IBCCS) found a hazard ratio of 1.48 (95% CI = 0.92 to 2.36) for ever OCP use in 967 BRCA1/2 mutation carriers combined (14). Haile et al. (BCFR and kConFab), restricting analyses to women younger than age 50 years, found a hazard ratio of 0.77 (95% CI = 0.53 to 1.12) for ever OCP use in 497 BRCA1 mutation carriers and a hazard ratio of 1.62 (95% CI = 0.90 to 1.92) for 307 BRCA2 mutation carriers (16). Results by Haile et al. were inconsistent with our findings in mutation carriers younger than age 50 years (BRCA1: HR = 1.32, 95% CI = 1.10 to 1.58; BRCA2: HR = 1.08, 95% CI = 0.86 to 1.36), and additionally, there was no evidence for heterogeneity between the harmonized studies in our cohort (BRCA1: P = .683; BRCA2: P = .882).
The association of OCP use with BC risk is not the only consideration in the clinical OCP management of BRCA1 and BRCA2 mutation carriers, because these mutations are also associated with high risk of ovarian cancer (1). The meta-analysis by Moorman et al. reported a consistent inverse association for OCP use and risk of ovarian cancer, proportional with duration of use (25), although potential bias was not investigated. The relative risk reduction appears to be comparable to that in the general population, so that the absolute ovarian cancer risk reduction is large. However, in many Western countries, the majority of BRCA1 and BRCA2 mutation carriers (70%–75%) now opt for RRSO between age 35 and 50 years, when childbearing is completed (26,27), whereas the uptake of RRM is lower (35%–44%) (28) and varies widely between countries. These aspects should be taken into consideration when balancing the risks and benefits of OCP use for BRCA1 and BRCA2 mutation carriers.
In conclusion, prospective analyses did not show that past use of OCP causes an increased BC risk for BRCA1 mutation carriers in young middle-aged women (40–50 years). For BRCA2 mutation carriers, a causal association is also not likely at those ages, but power was limited. For both BRCA1 and BRCA2 mutation carriers, prospective analyses do not support retrospective findings that BC risk is increased after long-term duration of use, especially before FFTP. These retrospective results were consistent between full-cohort and left-truncated analyses for BRCA1 mutation carriers, but not for BRCA2 mutation carriers. Inconsistent prospective and retrospective findings could be due to survival bias or a true association for younger women, who were under-represented in the prospective cohort. Therefore, despite this analysis of a large number of carriers with a prospective component, there remains uncertainty about the impact of OCP use on BC risk in carriers, and this uncertainty should be discussed with each individual woman to help her make a personalized decision about OCP use that takes into account her personal circumstances and values. Given the uncertain safety of long-term OCP use for BRCA1/2 mutation carriers, OCP use for indications other than contraception should be avoided and nonhormonal contraceptive methods (eg, copper intrauterine device) should be discussed.
Further extension and follow-up of these cohorts will provide more precise risk estimates and hence more clarity in the advice that can be provided to BRCA1 and BRCA2 mutation carriers.
Funding
This work was supported by Cancer Research–UK grants C12292/A20861 and C12292/A11174.
Study-specific funding: The Breast Cancer Family Registry (BCFR) was supported by grant UM1 CA164920 from the US National Cancer Institute. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the BCFR, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the BCFR.
This work was partially supported by the Spanish Ministry of Economy and Competitiveness (MINECO) SAF2014-57680-R and the Spanish Research Network on Rare diseases (CIBERER). This work was partially supported by FISPI05/2275 and the Mutua Madrileña Foundation (FMMA).
Part of this work was supported by the Canadian Institutes of Health Research (CIHR) for the “CIHR Team in Familial Risks of Breast Cancer” program (grant No. CRN-87521) and the Ministry of Economic Development, Innovation and Export Trade (grant No. PSR-SIIRI-701). The PErsonalised Risk Stratification for Prevention and Early deteCTIon of breast cancer (PERSPECTIVE) project was supported by the Government of Canada through Genome Canada and the Canadian Institutes of Health Research (GPH-129344), the Ministère de l’Économie, de la Science et de l' Innovation du Québec through Genome Québec, and The Quebec Breast Cancer Foundation.
The German Cancer Research Center (DKFZ) study was supported by the German Cancer Research Center (DKFZ).
Epidemiological Study of Familial Breast Cancer (EMBRACE) is supported by Cancer Research UK Grants C1287/A10118 and C1287/A11990. D. Gareth Evans is supported by a National Institute for Health Research (NIHR) grant to the Biomedical Research Centre, Manchester. The Investigators at The Institute of Cancer Research and The Royal Marsden National Health Service (NHS) Foundation Trust are supported by an NIHR grant to the Biomedical Research Centre at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust. Ros Eeles and Elizabeth Bancroft are supported by Cancer Research UK Grant C5047/A8385. Ros Eeles is also supported by NIHR support to the Biomedical Research Centre at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust. Antonis C. Antoniou is funded by Cancer Research–UK grants C12292/A20861 and C12292/A11174.
The German Consortium of Hereditary Breast and Ovarian Cancer (GC-HBOC) is supported by the German Cancer Aid (grant No. 110837, Rita K. Schmutzler). This work was supported by LIFE–Leipzig Research Center for Civilization Diseases, Universität Leipzig. LIFE is funded by the European Union, the European Regional Development Fund (ERDF), and the Free State of Saxony within the framework of the excellence initiative.
The national French cohort, Gene Etude Prospective Sein Ovaire (GENEPSO), had been supported by a grant from the Fondation de France and grants from the Ligue Nationale Contre le Cancer and is supported by a grant from Institute National du Cancer (INCa) as part of the European program ERA-NET on Translational Cancer Research (TRANSCAN-JTC2012, No. 2014-008).
Hospital Clinico San Carlos (HCSC) was supported by grants RD12/0036/0006 and 15/00059 from ISCIII (Spain), which is partially supported by European Regional Development FEDER funds.
The Hereditary Breast and Ovarian cancer study Netherlands (HEBON) study is supported by the Dutch Cancer Society grants NKI1998-1854, NKI2004-3088, and NKI2007-3756, the Netherlands Organisation of Scientific Research grant NWO 91109024, the Pink Ribbon grants 110005 and 2014-187.WO76, the Biobanking and BioMolecular resources Research Infrastructure (BBMRI) grant Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) 184.021.007/CP46, and the Transcan grant JTC 2012 Cancer 12-054.
The International Hereditary Cancer Centre (IHCC) was supported by grant PBZ_KBN_122/P05/2004 and The National Centre for Research and Development (NCBR) within the framework of the international ERA-NET TRANSAN JTC 2012, application No. Cancer 12-054 (Contract No. ERA-NET-TRANSCAN / 07/2014).
This work was also partially supported by grants to Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab) and the kConFab Follow-Up Study from Cancer Australia (809195), the Australian National Breast Cancer Foundation (IF 17), the National Health and Medical Research Council (454508, 288704, 145684), the US National Institutes of Health (1RO1CA159868), the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania, and South Australia, and the Cancer Foundation of Western Australia. KAP is an Australian National Breast Cancer Foundation fellow.
Modifier Study of Quantitative Effects on Disease (MODSQUAD)–Czech Republic, Brno, was supported by MH CZ - DRO (MMCI, 00209805) and by MEYS - NPS I - LO1413 to LF, MN.
The Hungarian Breast and Ovarian Cancer Study was supported by Hungarian Research grants KTIA-OTKA CK-80745 and NKFI OTKA K-112228 and the Norwegian EEA Financial Mechanism HU0115/NA/2008-3/ÖP-9.
Lund-BRCA collaborators are supported by the Swedish Cancer Society, Lund Hospital Funds, and European Research Council Advanced Grant ERC-2011-294576. Stockholm-BRCA collaborators are supported by the Swedish Cancer Society.
Notes
Authors: Lieske H. Schrijver, Håkan Olsson, Kelly-Anne Phillips, Mary Beth Terry, David E. Goldgar, Karin Kast, Christoph Engel, Thea M. Mooij, Julian Adlard, Daniel Barrowdale, Rosemarie Davidson, Ros Eeles, Steve Ellis, D. Gareth Evans, Debra Frost, Louise Izatt, Mary E. Porteous, Lucy E. Side, Lisa Walker, Pascaline Berthet, Valérie Bonadona, Dominique Leroux, Emmanuelle Mouret-Fourme, Laurence Venat-Bouvet, Saundra S. Buys, Melissa C. Southey, Esther M. John, Wendy K. Chung, Mary B. Daly, Anita Bane, Christi J. van Asperen, Encarna B. Gómez Garcia, Marian J. E. Mourits, Theo A. M. van Os, Marie-José Roos-Blom, Michael L. Friedlander, Sue-Anne McLachlan, Christian F. Singer, Yen Y. Tan, Lenka Foretova, Marie Navratilova, Rita K. Schmutzler, Carolina Ellberg, Anne-Marie Gerdes, Trinidad Caldes, Jacques Simard, Edith Olah, Anna Jakubowska, Brita Arver, Ana Osorio, Catherine Nogue‘s, Nadine Andrieu, Douglas F. Easton, Flora E. van Leeuwen, John L. Hopper, Roger L. Milne, Antonis C. Antoniou, Matti A. Rookus; on behalf of EMBRACE, GENEPSO, BCFR, HEBON, kConFab, and IBCCS.
Affiliations of the authors: Department of Epidemiology, Netherlands Cancer Institute, Amsterdam, the Netherlands (LHS, TMM, MRB, FEL, MAR); Department of Oncology, Lund University Hospital (HO) and Oncology and Pathology, Department of Clinical Sciences Lund (CE), Lund University, Lund, Sweden; Sir Peter MacCallum Department of Oncology (KAP, kConFab), Genetic Epidemiology Laboratory, Department of Pathology (MCS), and Department of Medicine, St. Vincent’s Hospital (SAM), University of Melbourne, Parkville, Victoria, Australia; Division of Cancer Medicine (KAP, SAM) and Research Department (kConFab), Peter MacCallum Cancer Centre, Victoria, Australia; Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (KAP, JLH, RLM); Department of Epidemiology (MBT, BCFR), Department of Pediatrics and Medicine (WKC), Columbia University, New York, NY; Department of Dermatology, University of Utah School of Medicine, Salt Lake City, UT (DEG); Department of Gynecology and Obstetrics, Medical Faculty and University Hospital Carl Gustav Carus, Technische Universität Dresden, Germany (KK); Institute for Medical Informatics, Statistics and Epidemiology, University of Leipzig, Germany (CE); Yorkshire Regional Genetics Service, Chapel Allerton Hospital, Leeds, UK (JA); Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Strangeways Research Laboratory, Worts Causeway, Cambridge, UK (DB, SE, EMBRACE, DF, DFE, ACA); Department of Clinical Genetics, South Glasgow University Hospitals, Glasgow, UK (RD); Oncogenetics Team, The Institute of Cancer Research and Royal Marsden NHS Foundation Trust, Sutton, UK (RE); Genomic Medicine, Manchester Academic Health Sciences Centre, Institute of Human Development, Manchester University, Central Manchester University Hospitals NHS Foundation Trust, Manchester, UK (DGE); Clinical Genetics, Guy’s and St. Thomas’ NHS Foundation Trust, London, UK (LI); South East of Scotland Regional Genetics Service, Western General Hospital, Edinburgh, UK (MEP); North East Thames Regional Genetics Service, Great Ormond Street Hospital for Children NHS Trust, London, UK (LES); Oxford Regional Genetics Service, Churchill Hospital, Oxford, UK (LW); Centre François Baclesse, Caen, France (PB); Université Claude Bernard Lyon 1, Villeurbanne, France (VB); CHU de Grenoble, Hôpital Couple-Enfant, Département de Génétique, Grenoble, France (DL); Service de Génétique Oncologique, Hôpital René Huguenin/Institut Curie, Saint-Cloud, France (EMF); Hôpital Universitaire Dupuytren, Service d’Oncologie Médicale, Limoges, France (LVB); Department of Medicine, Huntsman Cancer Institute, Salt Lake City, UT (SSB); Precision Medicine, School of Clinical Science at Monash Health, Monash University, Victoria, Australia (MCS); Department of Epidemiology, Cancer Prevention Institute of California, Fremont, CA (EMJ); Stanford Cancer Institute, Stanford University School of Medicine, Stanford, CA (EMJ); Division of Population Science, Fox Chase Cancer Center, Philadelphia, PA (MBD); Department of Pathology and Molecular Medicine, Juravinski Hospital and Cancer Centre, McMaster University, Hamilton, Ontario, Canada (AB); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJA); Department of Clinical Genetics and GROW, School for Oncology and Developmental Biology, Maastricht University Medical Center, Maastricht, the Netherlands (EBGG); Department of Gynaecologic Oncology, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands (MM); Department of Clinical Genetics, Academic Medical Center, Amsterdam, the Netherlands (TAMO); Prince of Wales Clinical School, University of New South Wales, Sydney, Australia (MLF); Department of Medical Oncology, Prince of Wales Hospital, Randwick, Australia (MLF); Department of Medical Oncology, St Vincent's Hospital, Fitzroy, Australia (SAM); Department of OB/GYN and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria (CFS, YYT); Department of Cancer Epidemiology and Genetics, Masaryk Memorial Cancer Institute, Brno, Czech Republic (LF, MN); Center for Familial Breast and Ovarian Cancer, Center for Integrated Oncology (CIO), Medical Faculty, University of Cologne and University Hospital Cologne, Germany (RKS); Department of Clincial Genetics, Rigshospitalet, København, Denmark (AMG); Molecular Oncology Laboratory, Hospital Clinico San Carlos, IdISSC, CIBERONC, Martin Lagos s/n, Madrid, Spain (TC); Genomics Center, Centre Hospitalier Universitaire de Québec Research Center and Laval University, Quebec City, Quebec, Canada (JS); Department of Molecular Genetics, National Institute of Oncology, Budapest, Hungary (EO); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ); Department of Oncology-Pathology, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden (BA); Human Genetics Group, Spanish National Cancer Centre (CNIO), Madrid, Spain (AO); Oncogénétique Clinique, Institut Paoli-Calmettes and Aix Marseille Univ, INSERM, IRD, SESSTIM, Marseille, France (CN); INSERM U900, Paris, France (NA); Institut Curie, Paris, France (NA); Ecole des Mines de Paris, ParisTech, Fontainebleau, France (NA); Cancer Epidemiology Centre, Cancer Council Victoria, Victoria, Australia (RLM).
Contributing studies (in order of size): Epidemiological Study of Familial Breast Cancer (EMBRACE); Gene Etude Prospective Sein Ovaire (GENEPSO); Breast Cancer Family Registry (BCFR); Hereditary Breast and Ovarian Cancer Study Netherlands (HEBON); Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab); International BRCA1/2 Carrier Cohort Study (IBCCS; next to EMBRACE, GENEPSO and HEBON included Medical University of Vienna (MUV), Modifier Study of Quantitative Effects on Disease (MODSQUAD), German Consortium of Hereditary Breast and Ovarian Cancer (GC-HBOC), Lund-BRCA, Odense University Hospital (OUH), HCSC, Interdisciplinary Health Research Internal Team Breast Cancer Susceptibility (INHERIT), National Institute of Oncology (NIO) Hungary, The International Hereditary Cancer Center (IHCC) Poland, Stockholm-BRCA, Centro Nacional de Investigaciones Oncológicas (CNIO), Milan Italy, Hospital de la Santa Creu i Sant Pau (HSP), The German Cancer Research Center (DKFZ), Düsseldorf Germany, Belgium.
Author contributions: LHS and MAR drafted the initial manuscript, while the complete writing group consisted of LHS, MAR, FEL, RLM, KAP, JLH, HO, and ACA. LHS and MAR performed and are responsible for the statistical analyses, while the complete analysis group additionally consists of FEL, RLM, KAP, JLH, HO, ACA, MBT, DEG, DFE, CE, KK, and NA. TMM and MRB are the database managers. MAR coordinated the collaborative study, while DEG, DFE, CN, NA, MBT, JLH, MAR, and KAP initiated and coordinated the original studies. All authors read and approved the final manuscript.
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Study-specific acknowledgements: BCFR wishes to thank members and participants in the Breast Cancer Family Registry from the Australian, New York, Northern California, Ontario, Philadelphia, and Utah sites for their contributions to the study. The Australian BCFR site wishes to acknowledge Maggie Angelakos, Judi Maskiell, Gillian Dite, and Helen Tsimiklis.
CNIO thanks Alicia Barroso, Rosario Alonso, and Guillermo Pita for their assistance.
We acknowledge the GENEPSO Centers: the Coordinating Center: Institut Paoli-Calmettes, Marseille, France: Catherine Noguès, Lilian Laborde, Pauline Pontois; and the Collaborating Centers: Institut Curie, Paris: Dominique Stoppa-Lyonnet, Marion Gauthier-Villars; Bruno Buecher, Institut Gustave Roussy, Villejuif: Olivier Caron; Hôpital René Huguenin/Institut Curie, Saint Cloud: Catherine Noguès, Emmanuelle Mouret-Fourme; Centre Paul Strauss, Strasbourg: Jean-Pierre Fricker; Centre Léon Bérard, Lyon: Christine Lasset, Valérie Bonadona; Centre François Baclesse, Caen: Pascaline Berthet; Hôpital d’Enfants CHU Dijon – Centre Georges François Leclerc, Dijon: Laurence Faivre; Centre Alexis Vautrin, Vandoeuvre-les-Nancy: Elisabeth Luporsi; Centre Antoine Lacassagne, Nice: Marc Frénay; Institut Claudius Regaud, Toulouse: Laurence Gladieff; Réseau Oncogénétique Poitou Charente, Niort: Paul Gesta; Institut Paoli-Calmettes, Marseille: Catherine Noguès, Hagay Sobol, François Eisinger, Jessica Moretta; Institut Bergonié, Bordeaux: Michel Longy, Centre Eugène Marquis, Rennes: Catherine Dugast; GH Pitié Salpétrière, Paris: Chrystelle Colas, Florent Soubrier; CHU Arnaud de Villeneuve, Montpellier: Isabelle Coupier, Pascal Pujol; Centres Paul Papin, and Catherine de Sienne, Angers, Nantes: Alain Lortholary; Centre Oscar Lambret, Lille: Philippe Vennin, Claude Adenis; Institut Jean Godinot, Reims: Tan Dat Nguyen; Centre René Gauducheau, Nantes: Capucine Delnatte; Centre Henri Becquerel, Rouen: Annick Rossi, Julie Tinat, Isabelle Tennevet; Hôpital Civil, Strasbourg: Jean-Marc Limacher; Christine Maugard; Hôpital Centre Jean Perrin, Clermont-Ferrand: Yves-Jean Bignon; Polyclinique Courlancy, Reims: Liliane Demange; Clinique Sainte Catherine, Avignon: Hélène Dreyfus; Hôpital Saint-Louis, Paris: Odile Cohen-Haguenauer; CHRU Dupuytren, Limoges: Brigitte Gilbert; Couple-Enfant-CHU de Grenoble: Dominique Leroux; Hôpital de la Timone, Marseille: Hélène Zattara-Cannoni.
HCSC acknowledge Alicia Tosar and Paula Diaque for their technical assistance.
The Hereditary Breast and Ovarian Cancer Research Group Netherlands (HEBON) consists of the following Collaborating Centers: Netherlands Cancer Institute (coordinating center), Amsterdam, NL: M. A. Rookus, F. B. L. Hogervorst, F. E. van Leeuwen, M. A. Adank, M. K. Schmidt, N. S. Russell, J. L. de Lange, R. Wijnands, D. J. Jenner; Erasmus Medical Center, Rotterdam, NL: J. M. Collée, A. M. W. van den Ouweland, M. J. Hooning, C. Seynaeve, C. H. M. van Deurzen, I. M. Obdeijn; Leiden University Medical Center, NL: C. J. van Asperen, J. T. Wijnen, R. A. E. M. Tollenaar, P. Devilee, T. C. T. E. F. van Cronenburg; Radboud University Nijmegen Medical Center, NL: C. M. Kets, A. R. Mensenkamp; University Medical Center Utrecht, NL: M. G. E. M. Ausems, R. B. van der Luijt, C. C. van der Pol; Amsterdam Medical Center, NL: C. M. Aalfs, H. E. J. Meijers-Heijboer, T. A. M. van Os; VU University Medical Center, Amsterdam, NL: K. van Engelen, J. J. P. Gille, Q. Waisfisz; Maastricht University Medical Center, NL: E. B. Gómez-Garcia, M. J. Blok; University of Groningen, NL: J. C. Oosterwijk, A. H. van der Hout, M. J. Mourits, G. H. de Bock; The Netherlands Comprehensive Cancer Organisation (IKNL): S. Siesling, J. Verloop; The Nationwide Network and Registry of Histo- and Cytopathology in the Netherlands (PALGA): L. I. H. Overbeek. HEBON thanks the study participants and the registration teams of IKNL and PALGA for part of the data collection.
INHERIT would like to thank Dr. Martine Dumont for sample management and skillful assistance.
We thank Heather Thorne, Eveline Niedermayr, all the kConFab research nurses and staff, the heads and staff of the Family Cancer Clinics, and the many families who contributed to kConFab for their contributions to this resource.
Czech Republic, MMCI, Brno—many thanks to Dita Hanouskova, research nurse, and Sarka Rathouska, research technician, for data collection and management.
We wish to thank the Hungarian Breast and Ovarian Cancer Study Group members (Maria Balogh, Janos Papp, Matrai Zoltan, Judit Franko Department of Molecular Genetics, National Institute of Oncology, Budapest, Hungary) and the clinicians and patients for their contributions to this study.
Swedish scientists participating as SWE-BRCA collaborators: from Lund University and University Hospital: Håkan Olsson, Carolina Ellberg; from Stockholm and Karolinska University Hospital: Brita Arver.
Supplementary Material
References
- 1. Kuchenbaecker KB, Hopper JL, Barnes DR et al. , . Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;31723:2402–2416. [DOI] [PubMed] [Google Scholar]
- 2. Cibula D, Gompel A, Mueck AO et al. , . Hormonal contraception and risk of cancer. Hum Reprod Update. 2010;166:631–650. [DOI] [PubMed] [Google Scholar]
- 3. Davidson BA, Moorman PG.. Risk-benefit assessment of the combined oral contraceptive pill in women with a family history of female cancer. Expert Opin Drug Saf. 2014;1310:1375–1382. [DOI] [PubMed] [Google Scholar]
- 4. Gadducci A, Sergiampietri C, Tana R.. Alternatives to risk-reducing surgery for ovarian cancer. Ann Oncol. 2013;24(Suppl 8):viii47–viii53. [DOI] [PubMed] [Google Scholar]
- 5. Lee E, Ma H, McKean-Cowdin R et al. , . Effect of reproductive factors and oral contraceptives on breast cancer risk in BRCA1/2 mutation carriers and noncarriers: Results from a population-based study. Cancer Epidemiol Biomarkers Prev. 2008;1711:3170–3178. [DOI] [PubMed] [Google Scholar]
- 6. Narod SA, Dube MP, Klijn J et al. , . Oral contraceptives and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2002;9423:1773–1779. [DOI] [PubMed] [Google Scholar]
- 7. Walker JL, Powell CB, Chen LM et al. , . Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;12113:2108–2120. [DOI] [PubMed] [Google Scholar]
- 8. Goldgar D, Bonnardel C, Renard H et al. , . The International BRCA1/2 Carrier Cohort Study: Purpose, rationale, and study design. Breast Cancer Res. 2000;E10. [Google Scholar]
- 9. John EM, Hopper JL, Beck JC et al. , . The Breast Cancer Family Registry: An infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res. 2004;64:R375–R389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phillips KA, Butow PN, Stewart AE et al. , . Predictors of participation in clinical and psychosocial follow-up of the kConFab breast cancer family cohort. Fam Cancer. 2005;42:105–113. [DOI] [PubMed] [Google Scholar]
- 11. Thorne H, Mitchell G, Fox S et al. , . kConFab: A familial breast cancer consortium facilitating research and translational oncology. J Natl Cancer Inst Monogr. 2011;201143:79–81. [DOI] [PubMed] [Google Scholar]
- 12. Antoniou AC, Goldgar DE, Andrieu N et al. , . A weighted cohort approach for analysing factors modifying disease risks in carriers of high-risk susceptibility genes. Genet Epidemiol. 2005;291:1–11. [DOI] [PubMed] [Google Scholar]
- 13. Bernholtz S, Laitman Y, Kaufman B et al. , . Cancer risk in Jewish BRCA1 and BRCA2 mutation carriers: Effects of oral contraceptive use and parental origin of mutation. Breast Cancer Res Treat. 2011;1292:557–563. [DOI] [PubMed] [Google Scholar]
- 14. Brohet RM, Goldgar DE, Easton DF et al. , . Oral contraceptives and breast cancer risk in the international BRCA1/2 carrier cohort study: A report from EMBRACE, GENEPSO, GEO-HEBON, and the IBCCS Collaborating Group. J Clin Oncol. 2007;2525:3831–3836. [DOI] [PubMed] [Google Scholar]
- 15. Gronwald J, Byrski T, Huzarski T et al. , . Influence of selected lifestyle factors on breast and ovarian cancer risk in BRCA1 mutation carriers from Poland. Breast Cancer Res Treat. 2006;952:105–109. [DOI] [PubMed] [Google Scholar]
- 16. Haile RW, Thomas DC, McGuire V et al. , . BRCA1 and BRCA2 mutation carriers, oral contraceptive use, and breast cancer before age 50. Cancer Epidemiol Biomarkers Prev. 2006;1510:1863–1870. [DOI] [PubMed] [Google Scholar]
- 17. Kotsopoulos J, Lubinski J, Moller P et al. , . Timing of oral contraceptive use and the risk of breast cancer in BRCA1 mutation carriers. Breast Cancer Res Treat. 2014;1433:579–586. [DOI] [PubMed] [Google Scholar]
- 18. Ursin G, Henderson BE, Haile RW et al. , . Does oral contraceptive use increase the risk of breast cancer in women with BRCA1/BRCA2 mutations more than in other women? Cancer Res. 1997;5717:3678–3681. [PubMed] [Google Scholar]
- 19. Breast cancer and hormonal contraceptives: Collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1996;3479017:1713–1727. [DOI] [PubMed] [Google Scholar]
- 20. Pike MC, Henderson BE, Casagrande JT et al. , . Oral contraceptive use and early abortion as risk factors for breast cancer in young women. Br J Cancer. 1981;431:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heemskerk-Gerritsen BA, Seynaeve C, van Asperen CJ et al. , . Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: Revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;1075:djv033. [DOI] [PubMed] [Google Scholar]
- 22. Kim J, Oktay K.. Baseline E(2) levels are higher in BRCA2 mutation carriers: A potential target for prevention? Cancer Causes Control. 2013;243:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. King MC, Wieand S, Hale K et al. , . Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA. 2001;28618:2251–2256. [DOI] [PubMed] [Google Scholar]
- 24. Mavaddat N, Barrowdale D, Andrulis IL et al. , . Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: Results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;211:134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moorman PG, Havrilesky LJ, Gierisch JM et al. , . Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: A systematic review and meta-analysis. J Clin Oncol. 2013;3133:4188–4198. [DOI] [PubMed] [Google Scholar]
- 26. Garcia C, Wendt J, Lyon L et al. , . Risk management options elected by women after testing positive for a BRCA mutation. Gynecol Oncol. 2014;1322:428–433. [DOI] [PubMed] [Google Scholar]
- 27. Mannis GN, Fehniger JE, Creasman JS et al. , . Risk-reducing salpingo-oophorectomy and ovarian cancer screening in 1077 women after BRCA testing. JAMA Intern Med. 2013;1732:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Driel CM, Eltahir Y, de Vries J et al. , . Risk-reducing mastectomy in BRCA1/2 mutation carriers: Factors influencing uptake and timing. Maturitas. 2014;772:180–184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.