Abstract
Background
In the Women’s Health Initiative Dietary Modification trial, a low-fat dietary pattern reduced deaths after breast cancer. Mortality from other cancer sites has not been reported.
Methods
A low-fat dietary pattern influence on deaths from and after site-specific cancers was examined during 8.5 years (median) of dietary intervention and cumulatively during 17.7 years (median) of follow-up. A total 48 835 postmenopausal women, ages 50–79 years, were randomly assigned from 1993 to 1998 at 40 US clinical centers to dietary intervention (40%, n = 19 541 or a usual diet comparison group (60%, n = 29 294). Dietary intervention influence on mortality from protocol-specified cancers (breast, colon and rectum, endometrium and ovary), individually and as a composite, represented the primary analyses.
Results
During the dietary intervention period, a reduction in deaths after breast cancer (HR = 0.65 95% CI = 0.45 to 0.94, P = .02) was the only statistically significant cancer mortality finding. During intervention, the HRs for deaths after the protocol-specified cancer composite were 0.90 (95% CI = 0.73 to 1.10) and 0.95 (95% CI = 0.85 to 1.06) for deaths after all cancers. During 17.7 years of follow-up with 3867 deaths after all cancers, reduction in deaths after breast cancer continued in the dietary intervention group (HR = 0.85, 95% CI = 0.74 to 0.99, P = .03). However, no dietary intervention influence on deaths from or after any other cancer or cancer composite was seen.
Conclusions
A low-fat dietary pattern reduced deaths after breast cancer. No reduction in mortality from or after any other cancer or cancer composite was seen.
In the Women’s Health Initiative (WHI) Dietary Modification (DM) trial, 48 835 postmenopausal women were randomly assigned to a dietary modification group (40%, n = 19 541) or usual diet comparison group (60%, n = 29 294) to assess low-fat dietary pattern effects on breast cancer and colorectal cancer as co-primary endpoints. The dietary modification program reduced percent caloric intake from fat (mean [SD] to 24.3 [7.5]% from 35.1 [6.9]% at year 1), increased intake of fruit, vegetables, and grains (about 1 serving per day), and was associated with modest weight loss (2.9% loss after 1 year; −2.2 kg; P < .001) with differences between randomization groups maintained throughout the 8.5-year (median) dietary intervention period (1).
At the protocol specified end of dietary intervention, although the breast cancer incidence was somewhat lower in the dietary intervention group, the difference was not statistically significant (HR = 0.91, 95% CI = 0.83 to 1.01, P = .07) and no effect on colorectal cancer incidence was seen (1,2). Subsequent analyses through 16.1 years of cumulative follow-up identified a statistically significant reduction in deaths after breast cancer measured from random assignment (HR = 0.82, 95% CI = 0.70 to 0.96, P = 0.01) (3) or measured from cancer diagnosis (HR = 0.78, 95% CI = 0.65 to 0.94, P = .01) (4). Dietary differences between randomization groups attenuated during the post-intervention period but remainde statistically significant (5).
The breast cancer findings prompted interest in examining the cumulative influence of the WHI dietary intervention on mortality from other cancers. Although information on the incidence of several other cancers has been reported (5–8), mortality information on individual cancers, including deaths from cancer and after cancer, have not been previously described. Therfore, we updated information on dietary intervention influence on breast cancer mortality and provide, for the first time, to our knowledge, similar information for select other cancers and cancer composite groups during 8.5 years (median) of dietary intervention and cumulatively throughout 17.7 years (median) of follow-up.
Methods
Participants
Details of the WHI DM trial, conducted at 40 US clinical centers with enrollment from 1993 through 1998, have been provided elsewhere (9). Eligible were postmenopausal women, ages 50–79 years, with no previous breast or colorectal cancer and with no other cancer in the 10 years prior to randomization, dietary fat intake greater than 32% of total energy by food frequency questionnaire (FFQ), a mammogram not suspicious for breast cancer, and anticipated survival of at least 3 years. The trial was approved by institutional review boards at the clinical centers and participants provided written informed consent.
Random Assignment
Participants were randomly assigned to a low-fat dietary pattern intervention group or a usual diet comparison group in a 40:60 ratio at a specified level of power, using a randomized permuted block algorithm, stratified by clinical center and age. The algorithm was developed and implemented electronically by the WHI Clinical Coordinating Center (Seattle, WA) (1).
Procedures
Baseline characteristics were collected by interview for medication use and by questionnaires for lifestyle and behavioral variables. Body weight, height, and waist circumference were measured, with body mass index (BMI (kg/m2) calculated at baseline and annually during the dietary intervention. Mammography screening was biannually or annually for the 16% also participating in WHI hormone therapy trials. Colorectal cancer screening was not protocol mandated but screening information was collected. Physicians outside the WHI directed cancer therapy.
The low-fat dietary program was designed to reduce fat intake to 20% of total energy and increase vegetable, fruit, and grain intake (10). Calorie restriction or weight loss were not intervention targets. Dietary group participants received 18 group sessions led by centrally trained, registered dietitians/nutritionists in year one and quarterly maintenance sessions throughout the dietary intervention period. Comparison group participants received only written diet-related education materials. Participants provided a 4-day food record and a FFQ at baseline. Additional FFQs were obtained after 1 year and thereafter in a rotating subgroup sample yearly. Post-intervention findings are based on a subsample of single 24-hour dietary recalls for 1311 participants who reconsented four assessments between 2005 and 2010.
Outcome ascertainment was six monthly throughout the dietary intervention period. Dietary intervention ended after 8.5 years at the protocol-specified trial completion date of March 31, 2005. Subsequent outcome ascertainment required reconsent, obtained from 84.4% versus 81.1% of comparison and dietary group surviving participants, respectively, for follow-up through 2010 and 86.2% of surviving participants for subsequent, open-ended follow-up. National Death Index queries complete through September 2014 provided additional survival information regardless of reconsent status.
All cancers were confirmed after medical record review by clinical center physician adjudicators. Final adjudication and coding was performed at the WHI Clinical Coordinating Center. Cause of death was determined centrally by medical record or death certificate review and, in some cases, by participant relative report. All adjudicators were blind to randomization assignment.
Women with incident cancers continued to participate in subsequent dietary group meetings and activities (3). Thus, dietary group participants diagnosed with cancer shortly after randomization would have most nutritionist contacts after cancer diagnosis. In contrast, women diagnosed with cancer later in the dietary intervention period would have most nutritionist contacts before cancer diagnosis.
The protocol co-primary endpoints were incident invasive breast and colorectal cancer. The current analyses were not protocol mandated and represent secondary analyses. Ovarian and endometrial cancer also were identified as dietary targets in the original protocol (1), and the mortality outcomes for these four cancers individually and as a composite are the primary study outcomes in the current analyses. Secondary outcomes include mortality from a composite of “other cancers” (cancers not included in the primary analysis) and a composite of “total cancers.” Exploratory analyses examined mortality in lung cancer and pancreatic cancer (where the number of deaths was sufficient to support individual analyses). In addition, a composite of 13 cancer sites where the strength of evidence was judged sufficient to support an association between obesity and cancer risk by the World Cancer Research Fund/ International Agency for Research on Cancer (WCRF/IARC) including cancers of the esophagus (adenocarcinoma), gastric cardia, colon, rectum, liver, gallbladder, pancreas, breast, endometrial, ovaries, kidneys (renal-cell), meninges, thyroid, and multiple myeloma was also examined (11).
Statistical Analysis
The primary analysis endpoints of annualized rates of deaths from a specific cancer or cancer composite group and annualized rates of deaths after a specific cancer or cancer composite group are assessed by randomization group, during the dietary intervention period and cumulatively throughout all follow-up, by dividing the event number by the corresponding person-time in each period. Hazard ratios, 95% confidence intervals, and P values were computed from Cox regression models stratified by age at random assignment, randomization status in the WHI hormone trials, and study period (time dependent). Definitions include deaths from cancer (cancer incidence followed by death attributed to the cancer) and deaths after cancer (cancer incidence followed by death from any cause). Analyses of deaths from and after specific cancers and the cancer composites include all 48 835 study participants measured from randomization. A CONSORT diagram outlining the flow of participants in the study through 16.5 years (median) follow-up has been recently published (3).
Because a previous analysis identified fewer deaths after breast cancer in dietary intervention group women with waist circumference greater than 88 cm (3), analyses in subgroups defined by BMI and waist circumference were investigated. Less than one statistically significant (P ≤ .05) interaction would be expected by chance alone. All statistical tests were two-sided. Analyses used SAS 9.4 (SAS Institute, Cary, NC).
Results
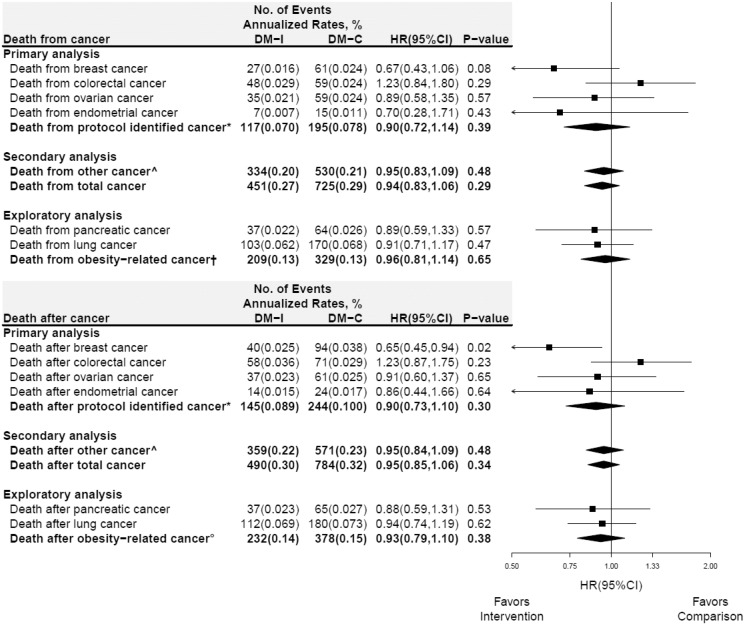
Cancer risk factors including age, BMI, smoking status, alcohol consumption, menopausal hormone therapy use, weekly total energy expenditure, and 5-year breast cancer risk were not different between randomization groups (Table 1). Deaths from specific cancers and cancer composite groups during dietary intervention are presented by randomization group in Figure 1 (upper panel). As previously reported (3), fewer deaths from breast cancer were seen in the dietary intervention group during the intervention period (HR = 0.67, 95% CI = 0.43 to 1.06, P = .08), but the difference was not statistically significant. Although there were fewer deaths from endometrial cancer in the dietary intervention group, the number of deaths was limited (n = 22) and the finding was not statistically significant (HR = 0.70, 95% CI = 0.28 to 1.71, P = .43). For no other specific cancer or cancer composite group was a low-fat dietary pattern effect on death from cancer seen. Deaths after specific cancers and cancer composite groups during the dietary intervention period are presented in Figure 1 (lower panel). During intervention, the hazard ratios for deaths after the protocol-specified cancer composite were 0.90 (95% CI = 0.73 to 1.10) and 0.95 (95% CI = 0.85 to 1.06) for deaths after all cancers. As previously reported, the risk of death after breast cancer was lower in the dietary intervention group (annualized rates, 0.025% vs 0.038%, respectively, HR = 0.65, 95% CI = 0.45 to 0.94, P = .02). However, for no other specific cancer, cancer composite, or total cancer group was a low-fat dietary pattern effect on death after cancer seen. Of note, there was strong agreement between deaths from cancer and after cancer (upper vs lower panels) for HRs across all cancer sites.
Table 1.
Baseline characteristics by randomization group (n = 48 835)
| Intervention (n = 19 541) |
Comparison (n = 29 294) |
||
|---|---|---|---|
| Characteristic | No. (%) | No. (%) | P |
| Age group at screening, y | .99 | ||
| 50–59 | 7206 (36.9) | 10 792 (36.8) | |
| 60–69 | 9083 (46.5) | 13 632 (46.5) | |
| 70–79 | 3252 (16.6) | 4870 (16.6) | |
| Race/ethnicity | .74 | ||
| White | 15 871 (81.2) | 23 891 (81.6) | |
| Black | 2135 (10.9) | 3127 (10.7) | |
| Hispanic | 751 (3.8) | 1094 (3.7) | |
| American Indian | 88 (0.5) | 114 (0.4) | |
| Asian/Pacific Islander | 431 (2.2) | 674 (2.3) | |
| Unknown | 265 (1.4) | 394 (1.3) | |
| Education | .65 | ||
| ≤High school/GED | 4267 (22.0) | 6468 (22.2) | |
| School after high school | 7712 (39.7) | 11 597 (39.8) | |
| College degree or higher | 7446 (38.3) | 11 044 (37.9) | |
| Marital status | .48 | ||
| Never married | 807 (4.1) | 1163 (4.0) | |
| Divorced/separated | 3128 (16.1) | 4577 (15.7) | |
| Widowed | 3029 (15.6) | 4618 (15.8) | |
| Presently married/living as married | 12 489 (64.2) | 18 806 (64.5) | |
| Self-reported health | .35 | ||
| Excellent | 3115 (16.0) | 4499 (15.4) | |
| Very good | 7947 (40.9) | 12 022 (41.2) | |
| Good | 6798 (35.0) | 10 282 (35.3) | |
| Fair/poor | 1564 (8.1) | 2351 (8.1) | |
| Time since menopause, y | .49 | ||
| <10 | 6303 (34.8) | 9362 (34.4) | |
| 10 – <20 | 6737 (37.2) | 10 081 (37.1) | |
| ≥20 | 5065 (28.0) | 7740 (28.5) | |
| Body mass index kg/m2 | .69 | ||
| <25 | 5072 (26.1) | 7587 (26.0) | |
| 25 – <30 | 6944 (35.7) | 10 452 (35.8) | |
| 30 – <35 | 4451 (22.9) | 6748 (23.1) | |
| ≥35 | 2991 (15.4) | 4377 (15.0) | |
| Smoking status | .23 | ||
| Never | 9918 (51.4) | 15 029 (51.9) | |
| Past | 8121 (42.1) | 11 979 (41.3) | |
| Current | 1273 (6.6) | 1977 (6.8) | |
| Alcohol consumption | .74 | ||
| Nondrinker | 5441 (28.1) | 8222 (28.3) | |
| ≤1 drink/d | 12 052 (62.2) | 18 105 (62.2) | |
| >1 drink/d | 1881 (9.7) | 2767 (9.5) | |
| Treated diabetes (pills or shots) | 866 (4.4) | 1337 (4.6) | .49 |
| Age at menarche, y | .93 | ||
| <12 | 4313 (22.1) | 6465 (22.1) | |
| 12 – <14 | 10 815 (55.5) | 16 166 (55.4) | |
| ≥14 | 4355 (22.4) | 6567 (22.5) | |
| Number of term pregnancies | .58 | ||
| Never/no term pregnancy | 2123 (10.9) | 3227 (11.1) | |
| 1 | 1682 (8.7) | 2463 (8.4) | |
| 2 | 4766 (24.5) | 7002 (24.0) | |
| 3 | 4714 (24.2) | 7183 (24.6) | |
| ≥4 | 6159 (31.7) | 9294 (31.9) | |
| Number first-degree female relatives breast cancer | .30 | ||
| 0 | 15 657 (85.6) | 23 542 (85.9) | |
| ≥1 | 2641 (14.4) | 3860 (14.1) | |
| Bilateral oophorectomy | 3884 (20.3) | 5997 (20.9) | .12 |
| Unopposed estrogen use status | .72 | ||
| Never used | 12 277 (62.9) | 18 477 (63.1) | |
| Past user | 2295 (11.8) | 3372 (11.5) | |
| Current user | 4954 (25.4) | 7419 (25.3) | |
| Estrogen + progesterone use status | .75 | ||
| Never used | 14 193 (72.7) | 21 298 (72.7) | |
| Past user | 1656 (8.5) | 2429 (8.3) | |
| Current user | 3685 (18.9) | 5560 (19.0) | |
| Baseline characteristics, mean (SD) | |||
| Age at screening, y | 62.3 (6.9) | 62.3 (6.9) | .99 |
| Physical functioning, RAND36 | 81.1 (19.3) | 80.9 (19.5) | .27 |
| Total energy expenditure/wk from phys act, MET-hours | 10.0 (11.7) | 10.1 (12.0) | .44 |
| Body mass index, kg/m2, baseline | 29.1 (5.9) | 29.1 (5.9) | .53 |
| Gail 5-year risk | 1.7 (0.9) | 1.7 (1.0) | >.99 |
Figure 1.
Death from and after select cancers in the Women’s Health Initiative dietary modification trial during the 8.5-year (median) dietary intervention period. Forest plot and summary statistics of the dietary intervention’s influence on deaths from (directly attributed to) cancer (upper panel) and deaths (from any cause) after cancer (lower panel). The P value corresponds to a two-sided score (log-rank) test. Percentages are annualized. *Protocol identified cancer includes: breast, colorectal, ovarian, and endometrial cancer. ^Includes cancers that were not identified in the protocol. †Includes deaths from cancers of the esophagus, stomach, colon, rectum, liver, gallbladder, pancreas, breast, endometrium, ovaries, kidneys, meninges, thyroid, or multiple myeloma. °Includes deaths from any cause after cancers of the esophagus (adenocarcinoma), gastric cardia, colon, rectum, liver, gallbladder, pancreas, breast, endometrium, ovaries, kidneys (renal-cell), meninges, thyroid and multiple myeloma. List of incident cancers based on (12). HR = hazard ratio; CI = confidence interval.
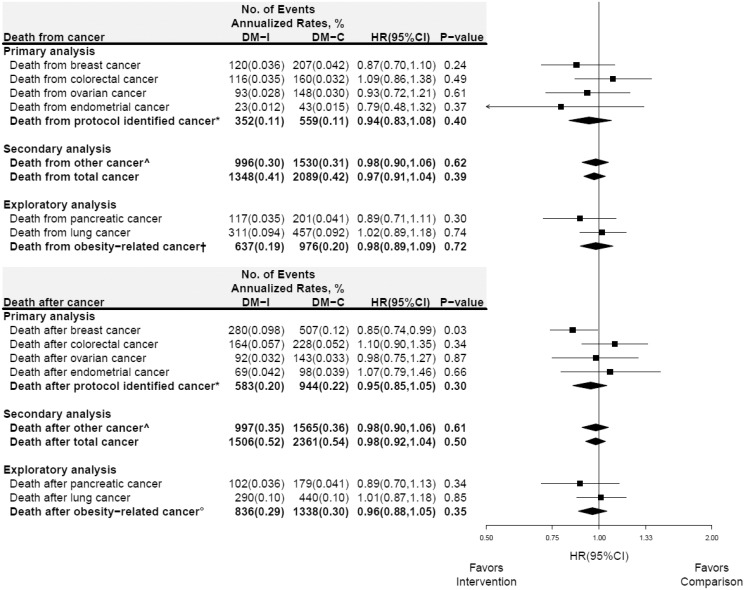
Deaths from specific cancers and cancer composite groups throughout cumulative 17.7 years follow-up (N = 3437 deaths) by randomization group are presented in Figure 2 (upper panel). There were somewhat fewer deaths from breast and endometrial cancer in the intervention group, but the findings were not statistically significant. Deaths after specific cancers and cancer composite groups throughout cumulative follow-up (n = 3867) are presented in Figure 2 (lower panel). The risk of death after breast cancer continued to be lower in the intervention group (annualized rates, 0.098% vs 0.12%, HR = 0.85, 95% CI = 0.74 to 0.99, P = .03). However, for no other specific cancer or cancer composite group was a low-fat dietary pattern effect on death from or after cancer seen.
Figure 2.
Death from and after select cancers in the Women’s Health Initiative dietary modification trial during 17.7 years (median) of cumulative follow-up (intervention + postintervention periods; randomization through 30SEP2014). Forest plot and summary statistics of the dietary intervention’s influence on deaths from (directly attributed to) cancer (upper panel) and deaths (from any cause) after cancer (lower panel). Ascertainment of incident cancer information after the intervention period ended required consent for extended follow-up. Consequently, the follow-up of nonconsenting women who did experience a cancer is censored in the analysis of death after cancer (lower panel). The P value corresponds to a two-sided score (log-rank) test. Percentages are annualized. See above for definition of: *protocol identified cancer; ^other cancer; †deaths from obesity-related cancer; and °deaths from any cause after incident obesity-related cancer. HR = hazard ratio; CI = confidence interval.
Due to the National Death Index search, death from cancer is more than 98% complete (13), whereas ascertainment of incident cancers after 2005 required consent for extended follow-up. Consequently, for some cancer sites, deaths from cancer exceeds deaths after specific cancers (eg, pancreatic cancer; Figure 2). However, a sensitivity analysis, moving the nonconsenting women into time-dependent strata wherein the outcome would be death from cancer, did not have an appreciable influence on results (Supplementary Figure 1, available online).
Cause of death is available for 3833 of 3867 cancer cases. Among the six specific cancer sites considered, lung cancer was the most common cause of death (16.9%, 648 deaths), followed by breast (7.5%, 289 deaths), pancreatic (7.3%, 280 deaths), colorectal (6.3%, 243 deaths), and ovarian cancers (5.7%, 218 deaths), with a smaller number from endometrial cancer (1.6%, 62 deaths). Deaths from cardiovascular disease were relatively common (9.4%, 360 deaths), and death from other cancers accounted for 32.6% of deaths.
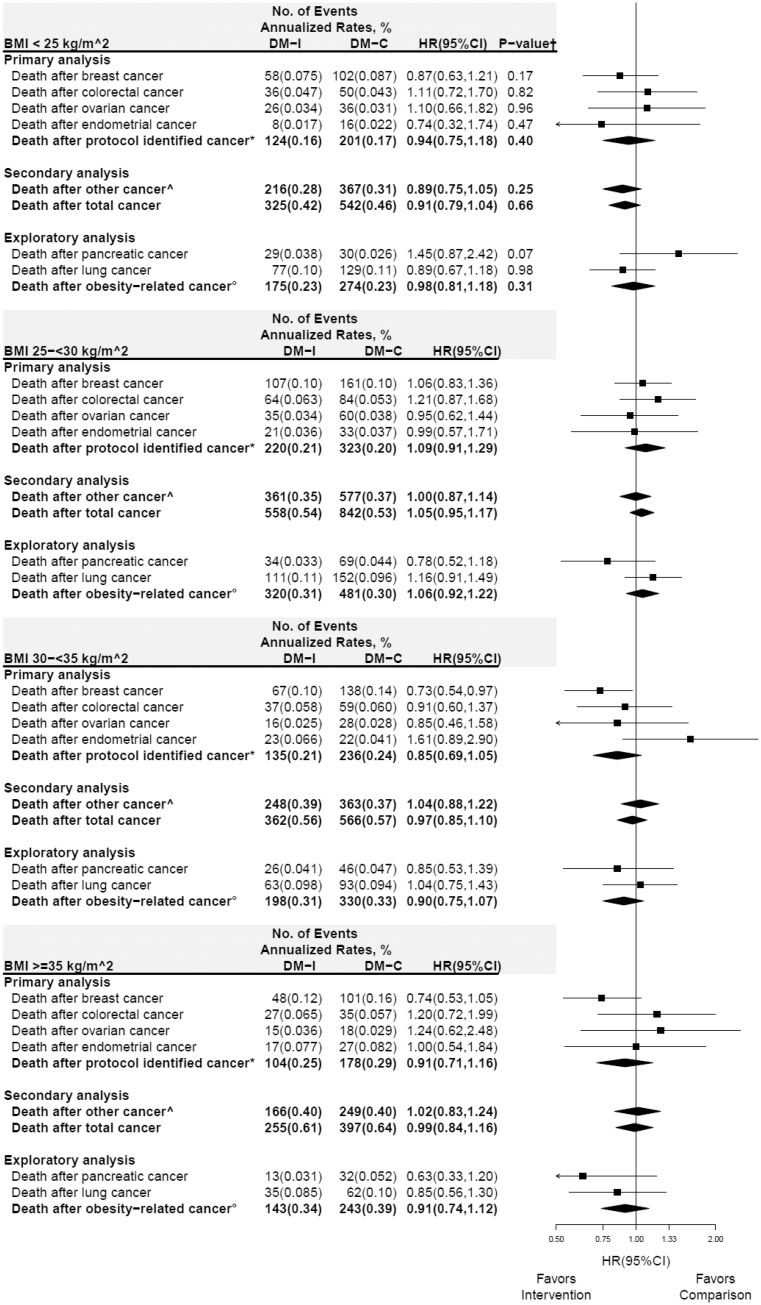
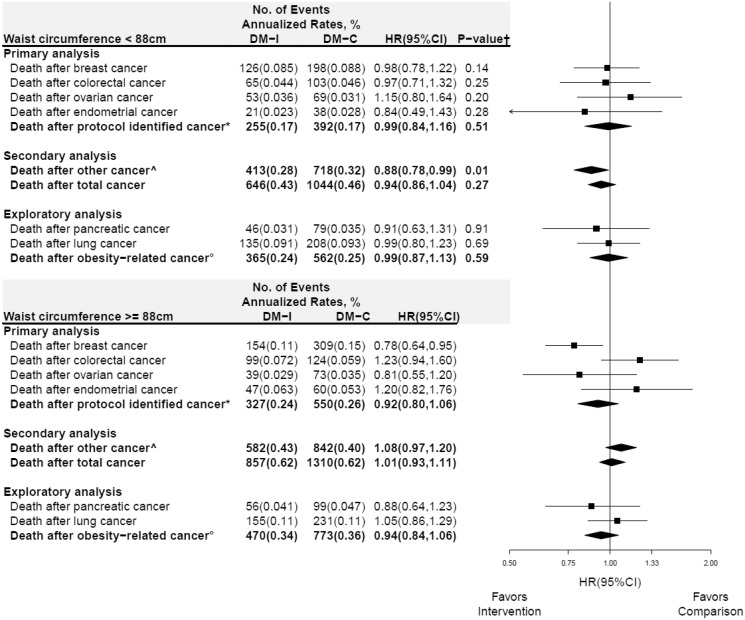
In subgroup analyses, deaths after cancer for specific cancers and cancer composite groups throughout the cumulative follow-up period for BMI and waist circumference are presented in Figures 3 and 4, respectively. Differential influence of the dietary intervention on the composite endpoint of “other cancers” was observed with HR = 0.88 (95% CI = 0.78 to 0.99) and HR = 1.08 (95% CI = 0.97 to 1.20) among those with waist circumference less than 88 and 88 cm or greater, respectively (Pinteraction= .01) (Figure 4). Additional sensitivity analyses, stratified by prior estrogen plus progestin or estrogen alone use, did not suggest that the influence of the dietary intervention could have been obfuscated by prior hormone use (Supplementary Figures 2 and 3, available online); Pinteraction across all endpoints were not statistically significant.
Figure 3.
Subgroup analysis of deaths from any cause after cancer for cumulative follow-up (intervention period + postintervention periods) according to baseline body mass index (BMI) group. Forest plot and summary statistics of the dietary modification influence on deaths among normal (BMI < 25 kg/m2; top panel), overweight (25–<30 kg/m2), obese (30–<35 kg/m2), and very obese women (≥35 kg/m2; bottom panel). P value corresponds to a 1 degree-of-freedom test for trend of the interaction between BMI group and randomization group. See above for definition of: *protocol identified cancer; ^other cancer; and °deaths from any cause after incident obesity-related cancer. HR = hazard ratio; CI = confidence interval.
Figure 4.
Subgroup analysis of deaths from any cause after cancer for cumulative follow-up (intervention period + postintervention periods) according to baseline waist circumference group. Forest plot and summary statistics of the dietary modification influence on deaths by waist circumference (<88 cm, top panel; vs ≥88 cm, bottom panel). P value corresponds to a test of the interaction between waist circumference group and randomization group. See above for definition of: *protocol identified cancer; ^other cancer; and °deaths from any cause after incident obesity-related cancer. HR = hazard ratio; CI = confidence interval.
Discussion
With additional long-term follow-up of the WHI DM trial participants, deaths after breast cancer continued to be reduced in the dietary intervention group throughout 17.7 years of cumulative follow-up. However, in no other cancers, considered individually or as prespecified composites, was a dietary intervention influence on death from or death after cancer seen.
Although obesity and dietary fat intake may be thought to have similar influence on cancer incidence, in fact, evidence regarding the association of obesity, as compared with dietary fat intake, with specific cancer risks differs substantially (11,14,15). The recent WCRF/IARC working group identified 13 cancers where the evidence was judged sufficient to support association of obesity and cancer (11). In contrast, evidence regarding dietary fat intake as a cancer risk factor is limited as outlined below.
When the WHI DM trial was planned, breast cancer and colorectal cancer incidence were identified as co-primary endpoints with endometrial cancer and ovarian cancer also identified as potential responding sites (1). Recent observational studies examining dietary fat intake and cancer incidence provide some support for ovarian cancer benefit (16), mixed results for breast cancer (17) and pancreatic cancer (18), and largely neutral results for colorectal cancer (19) and endometrial cancer (12) with ongoing controversy over validity of dietary intake methodology (20). Of note, in their seminal report, Armstrong and Doll (21) found dietary fat intake most strongly correlated with breast cancer mortality, whereas other cancers were more strongly associated with meat consumption. In any event, the findings from the WHI DM randomized trial where multi-year, sustained differences in dietary intake, including reduction in total and animal fat, were maintained in the dietary intervention group compared with the usual diet group (1,3,4,7) have substantial strengths compared with findings from observational studies commonly based on a single dietary intake assessment.
For the specific question of dietary fat intake and survival following a breast cancer diagnosis, observational studies are limited (14) and provide inconsistent results (22,23). The current randomized clinical trial results indicate adoption of a low-fat dietary pattern reduces risk of deaths after breast cancer, a finding likely influenced by favorable effects on not only breast cancer but also other causes of mortality (3,4,24,25) as well as with improvement in physical functioning, general health, vitality, and self-rated health (26).
The effect of a low-fat dietary pattern to exclusively influence breast cancer may reflect the role of progestins as drivers of short- and long-term breast cancer progression. As previously reported (1,3), there was a statistically significant reduction in poor prognosis, estrogen receptor positive but progesterone receptor negative breast cancers (27) in the dietary intervention group, a finding that explained 29% of the difference in deaths after breast cancer between randomized groups (4). The potential role of progestin and other sex hormones seems plausible because the dietary intervention changed levels of circulating hormones including estradiol and sex hormone−binding globulin (1). Of note, the benefit in breast cancer survival due to reduction of circulating estrogens has been long known for aromatase inhibitors (28).
The potential role of progestins as drivers of breast cancer progression are supported by findings from the two WHI randomized hormone therapy trials. In these trials, breast cancer risk and mortality were persistently increased with estrogen plus progestin use and persistently decreased with estrogen alone use (29). In terms of mediating mechanisms, in preclinical studies, medroxyprogesterone acetate (the progestin used in the WHI hormone therapy trials), acting as a glucocorticoid, blunts estrogen-induced apoptosis leading to mammary epithelial proliferation (30). In addition, long-term stimulation of breast cancer risk likely reflects progestin’s role in expanding stem/progenitor cell numbers (31). To our review, a similar role for progestins in other cancers has not been described.
In subgroup analyses, the previously reported (3) effect modification on death after breast cancer by waist circumference diminished and was no longer statistically significant (Figure 4). Although waist circumference was also associated with outcome of the composite “other cancers” (Figure 4), this finding was likely due to chance because the main effect of this secondary endpoint was not statistically significant.
In an exploratory analysis, no effect of the low-fat dietary pattern on a composite of 13 obesity-associated cancer sites (11) was seen. Perhaps this finding should not be surprising because the current intervention targeted dietary fat intake and diet composition rather than weight loss. In addition, adjustment for weight loss did not alter the reduction in deaths after breast cancer result (4). With respect to obesity, although the substantial, 20-kg weight loss seen in bariatric surgery populations has been associated with lower cancer risk (32), influence of lesser weight loss commonly achievable without surgery on cancer risk has not been convincingly demonstrated (11). Thus, the magnitude of the weight loss seen in the current study may have been insufficient to influence other obesity-associated cancers.
Study strengths include the randomized design, a population of 48 835 postmenopausal women, 3867 deaths after cancer, dietary program adherence supported by previously published (1) measured body weight and biomarker differences between randomization groups, serial screening mammography, verified cancer diagnoses, and long follow-up. Study limitations include those associated with secondary analyses and limited cancer therapy information. Because the trial was powered for dietary influence on breast cancer incidence, power is limited for cancers with lower incidence.
Although the findings of dietary intervention influence on deaths after breast cancer represent a secondary analysis in a randomized clinical trial, the absolute benefit in breast cancer overall survival seen is comparable to that with adjuvant anthracycline and taxane use (33), aromatase inhibitor benefit over tamoxifen in postmenopausal women (34), and is somewhat lower than trastuzumab addition to chemotherapy benefit for human epidermal growth factor receptor 2 positive breast cancer (35).
We have described the WHI dietary intervention as requiring a modest reduction in fat intake with minimal weight loss as achievable by many (3) where subgroup analyses suggest benefit in women with obesity or abdominal circumference greater than 88 cm (as marker of central obesity) (3,4). In this setting, referral to trained nutritionists, presented as obesity management, would likely be reimbursable by Medicare and other providers. Alternatively, centrally mediated programs for implementation of low-fat dietary regimens have been developed (36,37), which provides an even lower cost option for broad implementation.
The current breast cancer findings are generally supportive of the WCRF/AICR cancer prevention recommendations that include limiting “fast foods” and red and processed meat and and incorporating whole grains and fruits and vegetables (38). However, because only breast cancer outcome was affected by the WHI dietary intervention, other obesity-related cancers may require different intervention strategies. In summary, after long-term follow-up, women randomly assigned to a low-fat dietary pattern had a reduced risk of death after breast cancer. In no other cancer or cancer composite group was a dietary intervention effect on death from or after cancer seen.
Funding
The WHI program is supported by the National Heart, Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. This report was additionally funded by American Institute for Cancer Research Grant 30210-01 (RTC).
Notes
Affiliations of authors: Los Angeles Biomedical Research Institute, Torrance, CA (RTC); Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA (AKA, GLA, RLP); Mel and Enid Zuckerman College of Public Health, University of Arizona Cancer Center, Tucson, AZ (CAT); Harvard Medical School, Brigham and Women’s Hospital, Boston, MA (JEM); Department of Medicine, Baylor College of Medicine, Houston, TX (LJ); Department of Epidemiology, University of Washington, Seattle, WA (SAAB); Department of Epidemiology, University of Pittsburgh, Pittsburgh, PA (LHK); MedStar Health Research Institute and Georgetown/Howard Universities Center for Clinical and Translational Sciences, Washington, DC (AB); Department of Epidemiology & Population Health, Albert Einstein College of Medicine, Bronx, NY (TER); Preventive Medicine, Stony Brook University, Stony Brook, NY (DL); Methodist Healthcare, Memphis, TN (CW); School of Public Health, University of Indiana, Bloomington, IN (JL); University of Iowa, Iowa City/Davenport, IA (LS); Department of Preventive Medicine, University of Tennessee, Memphis, TN (KJ, MC); School of Public Health, University of Purdue, West Lafayette, IN (MD).
Contributors: RTC wrote the analysis proposal and initial draft of the report. Authors RTC, AKA, GLA, and RLP had full access to the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis. AKA and RLP were responsible for the statistical analysis. RTC, AKA, CAT, GLA, JEM, MSS, BVH, TER, LS, DL, WB, MV, CW, LQ, LH, FT, and RLP provided critical review of the manuscript for important intellectual content. RTC, JEM, MLS, MSS, BVH, KCJ, JW, MJO, and RLP collected the data and obtained study funding.
Additional contributions: We thank the Women’s Health Initiative investigators, staff, and the trial participants for their outstanding dedication and commitment.
Trial Registration: clinicaltrials.gov identifier NCT00000611.
Women’s Health Initiative Investigators
Program office: (National Heart, Lung, and Blood Institute, Bethesda, MD) Jacques Roscoe, Shari Ludlum, Dale Burden, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical coordinating center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kopperberg).
Investigators and academic centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thompson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; City of Hope National Medical Center, Duarte, CA) Rowan T. Chlebowski; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Additional information: A full list of all the investigators who have contributed to Women’s Health Initiative science appears at: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Inv estigator%20Long%20List.p.
Supplementary Material
References
- 1. Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):629–642. [DOI] [PubMed] [Google Scholar]
- 2. Beresford SA, Johnson KC, Ritenbaugh C, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):643–654. [DOI] [PubMed] [Google Scholar]
- 3. Chlebowski RT, Aragaki AK, Anderson GL, et al. Low-fat dietary pattern and breast cancer mortality in the Women’s Health Initiative randomized controlled trial. J Clin Oncol. 2017;35(25):2919–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chlebowski RT, Aragaki AK, Anderson GL, et al. Association of low-fat dietary pattern with breast cancer overall survival: A secondary analysis of the Women’s Health Initiative randomized clinical trial. JAMA Oncol. 2018;4(10):e181212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prentice RL, Thomson CA, Caan B, et al. Low-fat dietary pattern and cancer incidence in the Women’s Health Initiative Dietary Modification randomized controlled trial. J Natl Cancer Inst. 2007;99(20):1534–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gamba CS, Stefanick ML, Shikany JM, et al. Low-fat diet and skin cancer risk: the Women’s Health Initiative randomized controlled dietary modification trial. Cancer Epidemiol Biomarkers Prev. 2013;22(9):1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thomson CA, Horn LV, Caan BJ, et al. Cancer incidence and mortality during the intervention and post-intervention periods of the Women’s Health Initiative Dietary Modification Trial. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2924–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiao L, Chen L, White DL, et al. Low-fat dietary pattern and pancreatic cancer risk in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst. 2018;110(1):djx117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 suppl):S5–S17. [DOI] [PubMed] [Google Scholar]
- 10. Ritenbaugh C, Patterson RE, Chlebowski RT, et al. The Women’s Health Initiative Dietary Modification Trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9):S87–S97. [DOI] [PubMed] [Google Scholar]
- 11. Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer-viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang L, Hou R, Gong TT, Wu QJ.. Dietary fat intake and endometrial cancer risk: dose-response meta-analysis of epidemiological studies. Sci Rep. 2015;5:16693.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stampfer MJ, Willett WC, Speizer FE, et al. Test of the national death index. Am J Epidemiol. 1984;119(5):837–839. [DOI] [PubMed] [Google Scholar]
- 14. Chlebowski RT. Nutrition and physical activity influence on breast cancer incidence and outcome. Breast. 2013;22:S30–S37. [DOI] [PubMed] [Google Scholar]
- 15. Prentice RL, Sheppard L.. Dietary fat and cancer: consistency of the epidemiologic data, and disease prevention that may follow from a practical reduction in fat consumption. Cancer Causes Control. 1990;1(1):81–97. [DOI] [PubMed] [Google Scholar]
- 16. Qiu W, Lu H, Qi Y, Wang X.. Dietary fat intake and ovarian cancer risk: a meta-analysis of epidemiological studies. Oncotarget. 2016;7(24):37390–37406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao Y, Hou L, Wang W.. Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: a meta-analysis of prospective cohort studies. Int J Cancer. 2016;138(8):1894–1904. [DOI] [PubMed] [Google Scholar]
- 18. Shen QW, Yao QY.. Total fat consumption and pancreatic cancer risk: a meta-analysis of epidemiologic studies. Eur J Cancer Prev. 2015;24(4):278–285. [DOI] [PubMed] [Google Scholar]
- 19. Alexander DD, Miller AJ, Cushing CA, Lowe KA.. Processed meat and colorectal cancer: a quantitative review of prospective epidemiologic studies. Eur J Cancer Prev. 2010;19(5):328–341. [DOI] [PubMed] [Google Scholar]
- 20. Prentice RL, Pettinger M, Tinker LF, et al. Regression calibration in nutritional epidemiology: example of fat density and total energy in relationship to postmenopausal breast cancer. Am J Epidemiol. 2013;178(11):1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Armstrong B, Doll R.. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975;15(4):617–631. [DOI] [PubMed] [Google Scholar]
- 22. Kroenke CH, Kwan ML, Sweeney C, et al. High-and low-fat dairy intake, recurrence, and mortality after breast cancer diagnosis. J Natl Cancer Inst. 2013;105(9):616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boeke CE, Eliassen AH, Chen WY, et al. Dietary fat intake in relation to lethal breast cancer in two large prospective cohort studies. Breast Cancer Res Treat. 2014;146(2):383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prentice RL, Aragaki AK, van Horn L, et al. Low-fat dietary pattern and cardiovascular disease: results from the Women’s Health Initiative randomized controlled trial. Am J Clin Nutr. 2017;106(1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Howard BV, Aragaki AK, Tinker LF, et al. A low-fat dietary pattern and diabetes: a secondary anaylsis from the Women’s Health Initiative Dietary Modification Trial. Diabetes Care. 2018;41(4):680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Assaf AR, Beresford SA, Risica PM, et al. Low-fat dietary pattern intervention and health-related quality of life: the Women’s Health Initiative randomized controlled dietary modification trial. J Acad Nutr Diet. 2016;116(2):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cancello G, Maisonneuve P, Rotmensz N, et al. Progesterone receptor loss identifies Luminal B breast cancer subgroups at higher risk of relapse. Ann Oncol. 2013;24(3):661–668. [DOI] [PubMed] [Google Scholar]
- 28. Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor–positive breast cancer: status report 2004. J Clin Oncol. 2005;23(3):619–629. [DOI] [PubMed] [Google Scholar]
- 29. Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1(3):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sweeney E, Fan P, Jordan VC.. Molecular modulation of estrogen-induced apoptosis by synthetic progestins in hormone replacement therapy: an insight into the Women’s Health Initiative study. Cancer Res. 2014;74(23):7060–7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joshi PA, Goodwin PJ, Khokha R.. Progesterone exposure and breast cancer risk: understanding the biological roots. JAMA Oncol. 2015;1(3):283–285. [DOI] [PubMed] [Google Scholar]
- 32. Casagrande DS, Rosa DD, Umpierre D, Sarmento RA, Rodrigues CG, Schaan BD.. Incidence of cancer following bariatric surgery: systematic review and meta—analysis. Obes Surg. 2014;24(9):1499–1509. [DOI] [PubMed] [Google Scholar]
- 33. Early Breast Cancer Trialists' Collaborative Group. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Early Breast Cancer Trialists' Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. [DOI] [PubMed] [Google Scholar]
- 35. Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goodwin PJ, Segal RJ, Vallis M, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozol; the LISA trial. J Clin Oncol. 2014;32(21):2231–2239. [DOI] [PubMed] [Google Scholar]
- 37. Reeves M, Winkler E, McCarthy N, et al. The living well after breast cancer pilot trial: a weight loss intervention for women following treatment for breast cancer. Asia Pac J Clin Oncol. 2017;13(3):125–136. [DOI] [PubMed] [Google Scholar]
- 38. World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report. 2018. www.dietandcancerreport.org/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.