Abstract
Current guidance for evaluation of kidney function and drug dosing emphasize using measured or estimated glomerular filtration rate (GFR) rather than measured or estimated creatinine clearance or serum creatinine (Scr) alone. We assessed the definitions of kidney function thresholds for eligibility in cancer clinical trials. A random sample of active Phase I–III trials with cisplatin (n = 465) and studies in cancer with decreased kidney function (n = 74) were identified from clinicaltrials.gov. Among cisplatin trials, kidney function thresholds were defined by Scr alone or a composite of Scr or creatinine clearance in 46% (212/465) of studies. Only 2% (n = 11) used GFR. Among trials in participants with decreased kidney function, the proportion utilizing GFR (14%, 10/74) was modestly higher. Imprecise and logically inconsistent kidney function thresholds are in frequent use in clinical trials in cancer and may cause harm from either toxicity or impaired efficacy. We recommend the adoption and harmonization of recommended standards.
Impaired kidney function is common in cancer populations, and 50% of anticancer agents are either cleared by the kidney or impact kidney function (1–5). Methodological advances in the past 20 years have allowed for more precise estimates of kidney function (6). International clinical practice guidelines and recommendations (7,8) for evaluation of kidney function and for drug dosing now emphasize using estimated glomerular filtration rate (eGFR) rather than estimated creatinine clearance (eClcr) or serum creatinine (Scr) alone for initial evaluation. We evaluated the methods that are currently employed for defining kidney function thresholds for eligibility in clinical trials in oncology in the United States by examining trials in cancer listed on clinicaltrials.gov, the clinical trial registry and results data bank operated by the National Library of Medicine of the National Institutes of Health.
To obtain a study set of cancer-related trials in which kidney function thresholds are expected to be well defined, we focused on cisplatin trials, given that cisplatin is both dosed by level of GFR and has kidney toxicity (Clinicaltrials.gov, accessed December 30, 2017: search terms “cancer” and “cisplatin”). For contemporary data, we identified 878 of the approximately 3000 active trials using the search terms “active, not recruiting” or “recruiting.” A random sample of 500 of these trials was drawn, excluding 35 trials in which cisplatin was not used as a therapeutic agent, leaving a final sample of 465 trials. We evaluated the subset of cisplatin trials with a Phase I component (n = 292) and all active and completed trials in cancer in decreased kidney function regardless of agent (n = 74) (Supplementary Figure 1, available online).
Eligibility criteria for each trial were screened for definitions of kidney function thresholds and categorized as follows: Scr, eClcr, a composite definition using Scr or eClcr, eGFR, a composite term of eGFR and a secondary method, other uncommon definitions, absence of laboratory-based kidney function parameter (“adequate organ function”), or without any definition.
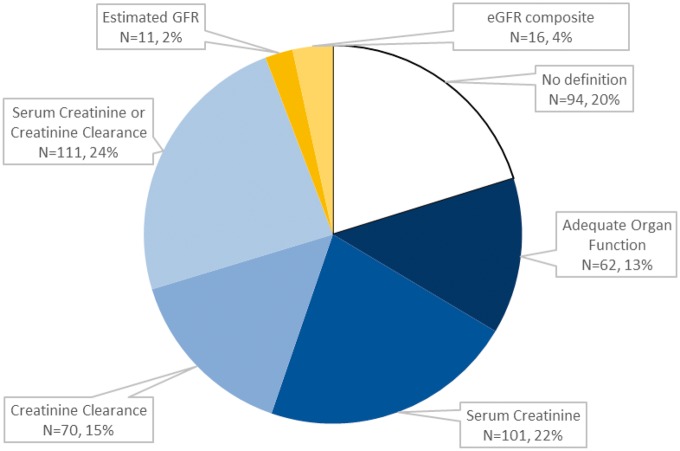
Only 1% of trials specified modern equations for GFR estimation, either the Modification of Diet in Renal Disease (MDRD) Study equation or Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (Figure 1). Among the rest, substantial heterogeneity of methods for definition of kidney function thresholds was found. The most common methods were Scr alone (22%, n = 101), a composite of Scr or eClcr (24%, n = 111), or eClcr alone (15%, n = 70). Among studies listing an eClcr-based definition, the method for eClcr was defined in just under one-half, most commonly the Cockcroft-Gault formula. Less than 5% of trials reported an eGFR alone (2%, n = 11) or an eGFR composite (4%, n = 16). Unexpectedly, one-third of trials did not articulate a specific kidney function threshold, listing “adequate kidney function” (16%, n = 62) or no specified definition (18%, n = 94).
Figure 1.
Definition of kidney function thresholds in contemporary cisplatin trials.
Among Phase I cisplatin trials (n = 292), eGFR was used in less than 5% of trials and no explicit definition was common (25%, n = 72) (Supplementary Figure 2a, available online). Among trials in the setting of decreased kidney function (n = 74), GFR methods were identified in only 14% (n = 10), with use of the modern GFR estimating equation specified in 4% (n = 3) (Supplementary Figure 2b, available online). One-quarter (24%, n = 17) did not report any kidney function criteria.
In summary, diverse methods for defining kidney function thresholds are currently in use in cancer clinical trials. These include the frequent use of imprecise measures such as Scr alone and eClcr that are no longer recommended for routine evaluation of kidney function, or composites of these measures that are logically inconsistent. Modern GFR estimating equations recommended for evaluation of kidney function by international clinical practice guidelines (7) were employed in 5% or fewer trials, even in Phase I cisplatin studies, and the proportion was only modestly higher in trials in the setting of decreased kidney function.
Composites of Scr and eGFR or eClcr can introduce a logical inconsistency because a patient with an eligible Scr may have an ineligible eGFR or eClcr. Indeed, the composite “Scr less than 1.5 × upper limit of normal or Clcr greater than 60 mL/min” was present in one-quarter of all cisplatin trials. Older equations, such as the Cockcroft and Gault and Jeliffe equations for eClcr, were developed before standardization of creatinine assays and may no longer provide accurate estimates. In contrast, the MDRD Study and CKD-EPI equations are expressed for use with standardized creatinine assays, include a term for race (African American vs other), and are more accurate than eClcr, including in studies of cancer patients (9). The CKD-EPI equation is more accurate than the MDRD Study equation at eGFR greater than 60 mL/min/1.73 m2. GFR estimates are indexed to body surface area and can be “un-indexed” by multiplying by body surface area/1.73 m2 for patients with extremes of body size.
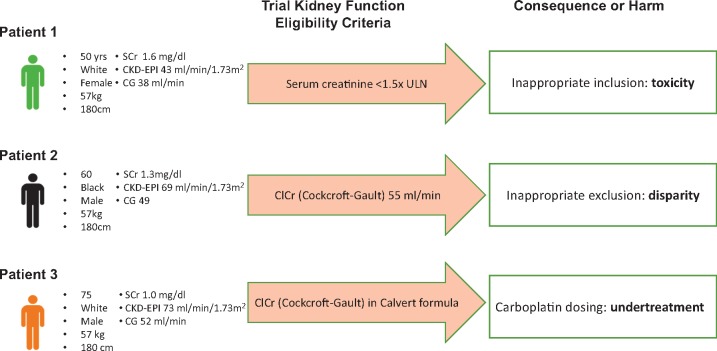
In our view, the results reflect the lack of precision in the definition of kidney function thresholds in clinical trials by sponsors, investigators, and clinicians. This imprecision has the potential to lead to harm, either by exposure to unwarranted toxicity, inappropriate exclusion from studies, or incorrect dosing leading to undertreatment and diminished efficacy (Figure 2). We call for adoption and harmonization of improved methods for assessing adequacy of kidney function for eligibility in cancer clinical trials. As recommended by the nephrology international evidence-based clinical practice guidelines (7), we support initial evaluation of kidney function as eGFR from Scr using the CKD-EPI equation. Confirmatory testing based on methods other than creatinine should be considered with measured GFR or Clcr or eGFR based on serum cystatin C if eGFR based on Scr is suspected to be inaccurate, for example due to variation in muscle mass or diet, which may affect many patients. If confirmatory tests are used, this value should be used for determination of trial inclusion, treatment regimen, drug dosage, and assessment of toxicity. Furthermore, we recommend that all clinical trials listed in clinicaltrials.gov should have adequate information on kidney function thresholds; missing data work against patients and their physicians who are in search of opportunities for participation.
Figure 2.
Examples of harm generated by imprecise and logically inconsistent definitions of kidney function thresholds in common use. eGFR = estimated glomerular filtration rate from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation; eClcr = estimated creatinine clearance using the Cockcroft and Gault equation. Body surface area for all scenarios is 1.73 m2, so values are identical whether expressed as mL/min or mL/min/1.73 m2. To convert to SI units of mL/s, multiply by 0.0167. Units of serum creatinine are mg/dL; to convert to SI units of μmol/L, multiply by 88.4.
Notes
Affiliations of authors: Tufts University School of Medicine, Boston, MA (EW); Predictive Analytics and Comparative Effectiveness Center, Institute for Clinical Research and Health Policy Studies (JKP), Pharmacy Department (DH), Division of Nephrology (LAI, ASL), and Division of Hematology-Oncology (PM), Tufts Medical Center, Boston, MA.
Supplementary Material
References
- 1. Finkel KW, Foringer JR.. Renal disease in patients with cancer. Nat Clin Pract Nephrol. 2007;3(12):669–678. [DOI] [PubMed] [Google Scholar]
- 2. Launay-Vacher V, Oudard S, Janus N, et al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer. 2007;110(6):1376–1384. [DOI] [PubMed] [Google Scholar]
- 3. Lichtman SM, Wildiers H, Launay-Vacher V, Steer C, Chatelut E, Aapro M.. International Society of Geriatric Oncology (SIOG) recommendations for the adjustment of dosing in elderly cancer patients with renal insufficiency. Eur J Cancer. 2007;43(1):14–34. [DOI] [PubMed] [Google Scholar]
- 4. Pontes Lde B, Antunes YP,, Bugano DD, Karnakis T, Giglio AD, Kaliks RA.. Prevalence of renal insufficiency in elderly cancer patients in a tertiary cancer center. Einstein. 2014;12(3):300.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang Y, Li H-y, Zhou Q, et al. Renal function and all-cause mortality risk among cancer patients. Medicine (Baltimore). 2016;95(20):e3728.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levey AS, Inker LA.. Assessment of glomerular filtration rate in health and disease: a state of the art review. Clin Pharmacol Ther. 2017;102(3):405–419. [DOI] [PubMed] [Google Scholar]
- 7. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. [Google Scholar]
- 8. US Department of Health and Human Services, F.D.A. Pharmacokinetics in Patients with Impaired Renal Function—Study Design, Data Analysis, and Impact on Dosing and Labeling Silver Spring, MD: Center for Drug Evaluation and Research (CDER); 2010.
- 9. Janowitz T, Williams EH, Marshall A, et al. New model for estimating glomerular filtration rate in patients with cancer. J Clin Oncol. 2017;35(124):2798–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.