Abstract
Background
Tamoxifen decreases mammographic density. Whether compliance affects this relationship is unclear as is the relationship between other types of adjuvant treatment and changes in mammographic density.
Methods
This prospective cohort study included 2490 women diagnosed with breast cancer during 2001–2015 in Sweden. Mammographic density was assessed within 3 months of diagnosis and 6–36 months post diagnosis. Logistic regression was performed to study the association between each respective adjuvant treatment and mammographic density reduction (annual dense area decrease >15%).
Results
Intention-to-treat analyses using treatment information from the regional cancer registries showed that tamoxifen-treated patients more frequently experienced mammographic density reductions compared with nontreated patients (odds ratio [OR] = 1.58, 95% confidence interval [CI] = 1.25 to 1.99), as did chemotherapy-treated patients (OR = 1.28, 95% CI = 1.06 to 1.54). For chemotherapy, the association was mainly seen in premenopausal women. Neither aromatase inhibitors nor radiotherapy was associated with density change. Tamoxifen use based on prescription and dispensation data from the Swedish Prescribed Drug Register showed that users were more likely to have density reductions compared with nonusers (adjusted OR = 2.24, 95% CI = 1.40 to 3.59). Moreover, among tamoxifen users, tamoxifen continuers were more likely than discontinuers to experience density reductions (adjusted OR = 1.50, 95% CI = 1.04 to 2.17).
Conclusions
Our results indicate that adherence influences the association between tamoxifen and mammographic density reduction. We further found that chemotherapy was associated with density reductions and propose that this is largely secondary to chemotherapy-induced ovarian failure.
Breast cancer is a heterogeneous group of diseases. Attempts at tailoring treatment for each individual are made based on molecular subtype, stage, age, and menopausal status. Even so, currently used therapy predictors and prognosticators are still somewhat blunt, and over- and undertreatment is a clinical problem. Furthermore, because surgery is the primary treatment for breast cancer, adjuvant therapy (ie, postoperative, oncologic treatment) is given without the possibility of assessing therapy response other than retrospectively.
Mammographic density (MD) is a well-established risk factor for breast cancer and a dynamic and hormonally responsive trait (1). MD has been shown to decrease during treatment with the selective estrogen receptor modulator tamoxifen (2), and women whose density decreases during tamoxifen treatment also seem to have a reduction in breast cancer risk (2). Furthermore, a decrease in MD in the unaffected breast during adjuvant tamoxifen treatment has been found to be associated with improved survival (3,4). Thus, it has been proposed that MD could be a potential biomarker of tamoxifen response. However, whether changes in density during adjuvant tamoxifen treatment are a reflection of treatment response, compliance, or a combination of the two is unknown.
Although MD is a hormonally responsive trait, nonhormonal mechanisms could also theoretically affect MD. Based on histological studies, increased MD in healthy breast tissue has been shown to be associated with an increased number of epithelial and stromal cells (5,6), increased amount of collagen (6), stromal fibrosis (7), and increased aromatase expression (6,8,9). Furthermore, regions with high MD may reflect a proinflammatory environment because these regions have been found to have increased COX-2 expression (10), reduced amounts of alternatively activated macrophages, and increased amounts of vimentin+ immune cells (6).
Only three previous studies, to our knowledge, have explored the association between nonendocrine breast cancer treatments and changes in MD (11–13), of which two of the studies exclusively investigated the association of chemotherapy and short-term changes in MD (during chemotherapy treatment) using magnetic resonance imaging (12,13). Despite the discrepancies in modality and timing of follow-up, all three studies found that chemotherapy was associated with a reduction in density. Only the study by Knight et al. (11) has investigated the association between radiotherapy and density reduction and found no association. Although chemotherapy could affect MD of the unaffected breast by causing primary ovarian failure in premenopausal women, radiotherapy of the breast and locoregional lymph nodes has no known hormonal effects but may have systemic effects through its impact on the immune system (14). We thus conducted this study to more thoroughly investigate the relationship between different types of adjuvant treatment—tamoxifen (specifically studying adherence), aromatase inhibitors (AIs), chemotherapy, and radiotherapy—and changes in MD.
Methods
Data Sources and Study Population
This study was performed within the LIBRO1 (15,16) and KARMA (17) cohorts. Details regarding the inclusion and exclusion criteria, recruitment procedures, participants’ characteristics, and follow-up of LIBRO1 and KARMA can be found elsewhere (15–17). In short, LIBRO1 is a case-only cohort comprised of 5175 women diagnosed with breast cancer between January 1, 2001 and December 31, 2008 in the healthcare region of Stockholm-Gotland in Sweden who were identified through the Regional Breast Cancer Register of Stockholm-Gotland. KARMA is a prospective cohort study initiated in 2011 and comprises 70 877 women attending mammography screening or clinical mammography at four hospitals in Sweden (17). Using the unique personal identity number (18) assigned to all Swedish residents, breast cancer patients were identified by linkage to the regional breast cancer registers, with follow-up until December 31, 2015. The Swedish regional breast cancer registers include information on diagnosis, surgery, postoperative treatment, tumor characteristics, and follow-up and have a completeness of 98% (19). Women in both studies answered questionnaires, donated blood at enrollment, and consented to the retrieval of their mammograms and the linkage of their medical records from various Swedish health registers by using the personal identity number. Questionnaires and study materials were largely similar for both studies because LIBRO1 was the pilot study of KARMA (16).
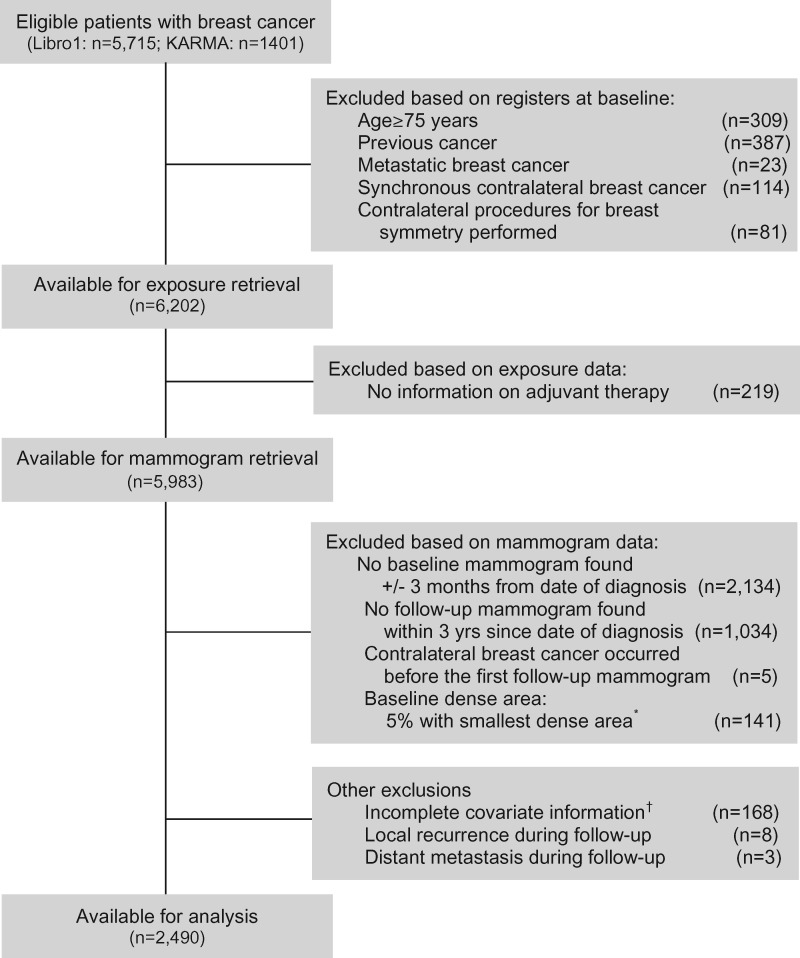
Details on study population selection can be found in Figure 1. Both LIBRO1 and KARMA were approved by the Regional Ethical Review Board in Stockholm, Sweden. All women gave written informed consent.
Figure 1.
Flow chart of study participants. *Women with a baseline dense area less than 4.88 cm2 (the lowest 5% of baseline dense area) were excluded because of the sensitivity to small errors and the inability to assess percentage change for these women. †Women lacking information on body mass index and age at menarche were excluded because these two variables were adjusted for as continuous variables in the multivariable analyses.
Mammographic Density
Details on the collection of mammograms and measurement of MD have been described elsewhere (17,20). In brief, full-field film (74.4%) or digital (25.6%) mammograms from the mediolateral oblique view of the unaffected breast were used to measure MD two-dimensionally using the newly developed STRATUS program (21). Only mediolateral oblique views were used because it was the only view routinely used for screening mammography in Sweden. Of the 2490 women included in the final analyses, 183 (7.3%) women who had film mammograms at baseline had digital mammograms as first follow-up mammograms. All patients who had a baseline digital mammogram were subsequently examined with digital mammograms. Using the STRATUS alignment protocol, images were first aligned prior to density measurement (illustrated in Supplementary Figure 1, available online) to minimize measurement error. STRATUS then calculated the absolute dense area (in cm2), absolute nondense area (in cm2), total breast area (in cm2), and percentage density (dense area/total breast area).
Because we lacked information on body mass index (BMI) at follow-up, we chose to analyze changes in the absolute dense area rather than changes in percentage density, because percentage density is highly, inversely correlated with BMI (through BMI’s strong association with the nondense area), whereas dense area has been shown to be only weakly associated with BMI (22), if at all (23). Our primary outcome was dense area reduction, defined as an annual decrease of dense area greater than 15%. This was calculated under the assumption of a constant yearly percentage change in dense area, that is, we compared the dense area of the first follow-up mammogram with the baseline mammogram using the formula: dense area at follow up < dense area at baseline*(100%–15%)years_since_diagnosis. The cutoff of 15% reduction was chosen based on results from our previous study (24) in which we found that a density reduction of more than 20% during a mean of 1.4 years of follow-up—which translates into an annual decrease of more than 15%—was associated with an improved survival compared with women with stabile density. All analyses were restricted to the first 3 years because we wished to investigate whether MD change could be used as a possible early biomarker of therapy response in breast cancer patients. Furthermore, this was the time scale used in our previous, aforementioned study (24), during which the change of dense area was found to be associated with breast cancer outcomes.
Adjuvant Therapy
Information on adjuvant treatment was derived from the respective regional breast cancer registers. Of the 1918 women who were prescribed endocrine treatment, information on type of endocrine treatment was missing for 259 individuals. Because AIs were first approved in October, 2003, in Sweden, women with unknown type of endocrine treatment and a diagnosis before 2003 were coded as having received tamoxifen (n = 164).
For the analysis of tamoxifen compliance and density change, more detailed information on prescription and dispensation of tamoxifen was retrieved from the Swedish Prescribed Drug Register. Based on our definition of tamoxifen adherence below and the fact that the Swedish Prescribed Drug Register was established July 1, 2005, we further restricted this analysis to women with follow-up mammograms taken after January 1, 2006. Tamoxifen use was categorized accordingly: women who were not prescribed endocrine treatment were considered “nonusers”; women prescribed tamoxifen and to whom tamoxifen was dispensed within 180 days before their follow-up mammogram were considered “continuers”; and women prescribed tamoxifen but to whom tamoxifen was not dispensed within 180 days before their follow-up mammogram were considered “discontinuers.” Because a 3-month supply is the maximum that is allowed to be dispensed at each time in Sweden, an interval of more than 180 days indicates that at least two dispensations have been missed, resulting in a shortage of the drug.
Covariates
Information on BMI, age at menarche, parity, family history of breast cancer, and use of hormonal contraception and hormone replacement therapy (HRT) was gathered from the questionnaires. The remaining patient, tumor, and treatment characteristics were retrieved from the respective regional breast cancer registers. For all categorical variables with missing information, a missing category was created and included in analyses.
Statistical Analysis
The Pearson χ2 test of association was used to assess differences in the distribution of descriptive characteristics between the group of women with and without a MD reduction. Only variables with a P value less than .2 were included as covariates in subsequent multivariable analyses. Furthermore, we also mutually adjusted for adjuvant therapy in all of the multivariable analyses (eg, radiotherapy and chemotherapy were included as adjustment factors when investigating the association between adjuvant hormone therapy and density reduction).
Logistic regression was performed to study the association between adjuvant therapy and MD reduction. Because the change of dense area differs greatly between pre- and postmenopausal women (25), analyses were also stratified on menopausal status. To assess the robustness of our findings, we conducted further analyses by recategorizing our study population by tamoxifen and chemotherapy usage into the following four groups: 1) no tamoxifen and no chemotherapy, 2) tamoxifen only, 3) chemotherapy only, and 4) tamoxifen and chemotherapy.
Logistic regression was also performed to study the association of tamoxifen compliance and change in MD, specifically comparing differences in MD reduction among women not prescribed endocrine therapy, tamoxifen discontinuers, and tamoxifen continuers.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) or Stata version 13.0 (Stata Corporation, College Station, TX). Statistical significance was determined at a two-sided alpha level of .05.
Results
Patient Characteristics
As compared with those defined as having no MD reduction, patients with MD reduction were more likely to be younger, be premenopausal, have a higher BMI, ever have used HRT, and have a higher baseline dense area (Table 1). Consistent results were found when we further stratified by study (KARMA and LIBRO1) (Supplementary Table 1, available online).
Table 1.
Baseline characteristics and their relation with mammographic density decline in breast cancer patients*
| Mammographic density reduction |
|||
|---|---|---|---|
| Characteristics | No (n = 1307) | Yes (n = 1183) | P† |
| Age at diagnosis, y | <.0001 | ||
| <50 | 190 (39.6) | 290 (60.4) | |
| 50–59 | 485 (52.9) | 431 (47.1) | |
| ≥60 | 632 (57.8) | 462 (42.2) | |
| Menopausal status | <.0001 | ||
| Premenopausal | 266 (43.1) | 351 (56.9) | |
| Postmenopausal | 969 (56.5) | 747 (43.5) | |
| Unknown | 72 (45.9) | 85 (54.1) | |
| Cohort | .009 | ||
| Karma | 201 (46.7) | 229 (53.3) | |
| LIBRO1 | 1106 (53.7) | 954 (46.3) | |
| Tumor size, mm | .97 | ||
| <20 | 548 (52.4) | 498 (47.6) | |
| ≥20 | 752 (52.5) | 681 (47.5) | |
| Unknown | 7 (63.6) | 4 (36.4) | |
| No. of metastatic nodes | .25 | ||
| 0 | 1214 (52.8) | 1085 (47.2) | |
| ≥1 | 88 (48.4) | 94 (51.6) | |
| Unknown | 5 (55.6) | 4 (44.4) | |
| Grade | .28 | ||
| 1 | 140 (50.9) | 135 (49.1) | |
| 2 | 401 (54.3) | 338 (45.7) | |
| 3 | 179 (49.4) | 183 (50.6) | |
| Unknown | 587 (52.7) | 527 (47.3) | |
| Estrogen receptor status | .58 | ||
| Positive | 800 (51.6) | 749 (48.4) | |
| Negative | 118 (53.6) | 102 (46.4) | |
| Unknown | 349 (52.8) | 312 (47.2) | |
| Progesterone receptor status | .09 | ||
| Positive | 647 (50.8) | 627 (49.2) | |
| Negative | 251 (55.4) | 202 (44.6) | |
| Unknown | 409 (53.6) | 354 (46.4) | |
| HER2 status | .95 | ||
| Positive | 25 (47.2) | 28 (52.8) | |
| Negative | 218 (46.7) | 249 (53.3) | |
| Unknown | 1064 (54.0) | 906 (46.0) | |
| Family history of breast cancer | .21 | ||
| No | 1020 (51.8) | 949 (48.2) | |
| Yes | 250 (55.1) | 204 (44.9) | |
| Unknown | 37 (55.2) | 30 (44.8) | |
| BMI, kg/m2 | <.0001 | ||
| <25 | 769 (56.8) | 584 (43.2) | |
| 25–29 | 417 (49.0) | 434 (51.0) | |
| ≥30 | 121 (42.3) | 165 (57.7) | |
| Age at menarche, y | .10 | ||
| <12 | 135 (47.7) | 148 (52.3) | |
| 12–13 | 649 (51.9) | 601 (48.1) | |
| ≥14 | 523 (54.6) | 434 (45.4) | |
| Parity | .57 | ||
| 0 | 214 (54.9) | 176 (45.1) | |
| 1 | 218 (51.4) | 206 (48.6) | |
| ≥2 | 875 (52.2) | 801 (47.8) | |
| Ever use of hormonal contraception | .36 | ||
| No | 291 (54.3) | 245 (45.7) | |
| Yes | 1008 (52.1) | 928 (47.9) | |
| Unknown | 8 (44.4) | 10 (55.6) | |
| HRT use at diagnosis | .02 | ||
| Never user | 432 (58.6) | 305 (41.4) | |
| Ever user | 566 (53.2) | 497 (46.8) | |
| Unknown | 309 (44.8) | 381 (55.2) | |
| Mammogram type | .003 | ||
| Film | 977 (54.4) | 820 (45.6) | |
| Digital | 330 (47.6) | 363 (52.4) | |
| Baseline mammographic dense area, cm2 | <.0001 | ||
| <20 | 335 (57.2) | 251 (42.8) | |
| 20–39 | 578 (57.3) | 431 (42.7) | |
| ≥40 | 394 (44.0) | 501 (56.0) | |
| Follow-up mammographic dense area, cm2 | <.0001 | ||
| <20 | 252 (30.8) | 565 (69.2) | |
| 20–39 | 574 (55.1) | 467 (44.9) | |
| ≥40 | 481 (76.1) | 151 (23.9) | |
Mammographic density reduction was defined as an annual decrease in dense area greater than 15%. BMI= body mass index; HRT= hormone replacement therapy.
The Pearson χ2 test of association was used to assess the difference among groups.
Adjuvant Tamoxifen Therapy
Tamoxifen users were more likely to have a reduction in dense area compared with patients not treated with tamoxifen (adjusted OR = 1.58; 95% confidence interval [CI] = 1.25 to 1.99), with an adjusted OR of 1.91 (95% CI = 1.19 to 3.09) for premenopausal women and an adjusted OR of 1.32 (95% CI = 0.99 to 1.76) for postmenopausal women (Table 2).
Table 2.
Mammographic density reduction in breast cancer patients, overall and stratified by menopausal status*
| Overall |
Premenopausal |
Postmenopausal |
|||||
|---|---|---|---|---|---|---|---|
| Treatment | No. | Univariate OR (95% CI) | Multivariable† OR (95% CI) | No. | Multivariable‡ OR (95% CI) | No. | Multivariable§ OR (95% CI) |
| Endocrine treatment | |||||||
| None | 565 | 1.00 (Reference) | 1.00 (Reference) | 145 | 1.00 (Reference) | 385 | 1.00 (Reference |
| Tamoxifen | 1372 | 1.44 (1.18 to 1.75) | 1.58 (1.25 to 1.99) | 373 | 1.91 (1.19 to 3.09) | 907 | 1.32(0.99 to 1.76) |
| Aromatase inhibitors | 374 | 0.90 (0.69 to 1.17) | 1.03 (0.76 to 1.39) | — | — | 355 | 0.91(0.65 to 1.26) |
| Radiotherapy | |||||||
| No | 530 | 1.00 (Reference) | 1.00 (Reference) | 147 | 1.00 (Reference) | 353 | 1.00 (Reference) |
| Yes | 1959 | 1.08 (0.89 to 1.31) | 0.98 (0.80 to 1.20) | 469 | 1.28 (0.85 to 1.90) | 1363 | 0.94 (0.73 to 1.20) |
| Chemotherapy | |||||||
| No | 1729 | 1.00 (Reference) | 1.00 (Reference) | 362 | 1.00 (Reference) | 1261 | 1.00 (Reference) |
| Yes | 759 | 1.35 (1.14 to 1.61) | 1.28 (1.06 to 1.54) | 254 | 1.75 (1.21 to 2.51) | 454 | 1.07 (0.85 to 1.35) |
Mammographic density reduction was defined as an annual decrease in dense area greater than 15%. BMI = body mass index; CI = confidence interval; HRT = hormone replacement therapy; OR = odds ratio.
Adjusted for adjuvant therapy (other than the exposure of interest), age at diagnosis (continuous), age at menarche (continuous), study populations (Libro1 vs KARMA), BMI (continuous), progesterone receptor status, family history of breast cancer, mammography type (digital vs film), baseline mammographic dense area, menopausal status, and HRT.
Adjusted for adjuvant therapy (other than the exposure of interest), age at diagnosis (continuous), age at menarche (continuous), study populations (Libro1 vs KARMA), BMI (continuous), progesterone receptor status, family history of breast cancer, mammography type (digital vs film), and baseline mammographic dense area.
Adjusted for adjuvant therapy (other than the exposure of interest), age at diagnosis (continuous), age at menarche (continuous), study populations (Libro1 vs KARMA), BMI (continuous), progesterone receptor status, family history of breast cancer, mammography type (digital vs film), baseline mammographic dense area, and HRT.
Adjuvant AI Therapy
No statistically significant differences in change in dense area were observed in women treated with AIs vs those not treated with AIs. Further restricting our analysis to postmenopausal women showed consistent results (Table 2).
Adjuvant Radiotherapy
No statistically significant differences in change in dense area were observed in women who received radiotherapy vs those who did not receive radiotherapy. Stratified analyses on menopausal status showed consistent results (Table 2).
Adjuvant Chemotherapy
Patients treated with chemotherapy were more likely to have a reduction in dense area compared with patients not treated with chemotherapy, with an adjusted OR of 1.28 (95% CI = 1.06 to 1.54) (Table 2). Stratified analyses on menopausal status showed consistent results for premenopausal women, whereas no association was observed in postmenopausal women.
Adjuvant Tamoxifen + Adjuvant Chemotherapy
Compared with the group of women who received neither tamoxifen nor chemotherapy, the adjusted OR was 1.70 (95% CI = 1.28 to 2.24) for MD reduction for women treated with only tamoxifen, 1.53 (95% CI = 1.05 to 2.23) for women treated with only chemotherapy, and 2.05 (95% CI = 1.46 to 2.87) for women treated with tamoxifen plus chemotherapy (Table 3). All of the associations above were more pronounced in premenopausal women (adjusted OR = 2.30, 95% CI = 1.28 to 4.12 for tamoxifen alone, 2.70, 95% CI = 1.27 to 5.76 for chemotherapy alone, and 3.81, 95% CI = 2.01 to 7.22 for a combination of both treatments compared with women who received neither treatment). For postmenopausal women, point estimates were similarly elevated for all treatment groups compared with women who received neither tamoxifen nor chemotherapy, but the association was only statistically significant for the group of women who exclusively received tamoxifen (adjusted OR = 1.47, 95% CI = 1.06 to 2.05).
Table 3.
ORs (95% CIs) for mammographic density reduction in breast cancer patients, overall and stratified by menopausal status*
| Overall |
Premenopausal |
Postmenopausal |
|||||
|---|---|---|---|---|---|---|---|
| Therapy type | No. | Univariate | Multivariable† | No. | Multivariable‡ | No. | Multivariable§ |
| No tamoxifen + | 347 | 1.00 (Reference) | 1.00 (Reference) | 79 | 1.00 (Reference) | 248 | 1.00 (Reference) |
| No chemotherapy | |||||||
| Tamoxifen only | 1044 | 1.56 (1.21 to 1.99) | 1.70 (1.28 to 2.24) | 236 | 2.30 (1.28 to 4.12) | 740 | 1.47 (1.06 to 2.05) |
| Chemotherapy only | 218 | 1.51 (1.07 to 2.13) | 1.53 (1.05 to 2.23) | 66 | 2.70 (1.27 to 5.76) | 137 | 1.32 (0.83 to 2.09) |
| Tamoxifen + | 327 | 2.20 (1.62 to 3.00) | 2.05 (1.46 to 2.87) | 136 | 3.81 (2.01 to 7.22) | 167 | 1.47 (0.96 to 2.27) |
| Chemotherapy | |||||||
Mammographic density reduction was defined as an annual decrease in dense area greater than 15%. BMI = body mass index; CI = confidence interval; HRT = hormone replacement therapy; OR = odds ratio.
Adjusted for adjuvant therapy (other than the exposure of interest), age at diagnosis (continuous), age at menarche (continuous), study populations (Libro1 vs KARMA), BMI (continuous), progesterone receptor status, family history of breast cancer, mammography type (digital vs film), baseline mammographic dense area, menopausal status, and HRT.
Adjusted for adjuvant therapy (other than the exposure of interest), age at diagnosis (continuous), age at menarche (continuous), study populations (Libro1 vs KARMA), BMI (continuous), progesterone receptor status, family history of breast cancer, mammography type (digital vs film), and baseline mammographic dense area.
Adjusted for adjuvant therapy (other than the exposure of interest), age at diagnosis (continuous), age at menarche (continuous), study populations (Libro1 vs KARMA), BMI (continuous), progesterone receptor status, family history of breast cancer, mammography type (digital vs film), and baseline mammographic dense area, and HRT.
Tamoxifen Discontinuation
Compared with women not prescribed tamoxifen, both tamoxifen discontinuers and continuers were more likely to have an MD reduction (adjusted OR = 2.24, 95% CI = 1.40 to 3.59), with an adjusted OR of 1.72 (95% CI = 1.01 to 2.92) for tamoxifen discontinuers and 2.58 (95% CI = 1.58 to 4.21) for tamoxifen continuers. Furthermore, among women prescribed tamoxifen, tamoxifen continuers were more likely to have an MD reduction than tamoxifen discontinuers, with an adjusted OR of 1.50 (95% CI = 1.04 to 2.17) (Table 4).
Table 4.
Tamoxifen usage (defined by prescribed drugs registry) and reduction in mammographic density in breast cancer patients*
| Univariate | Multivariable† | ||
|---|---|---|---|
| Tamoxifen usage | No. | OR (95% CI) | OR (95% CI) |
| Tamoxifen users | |||
| Nonusers | 271 | 1.00 (Reference) | 1.00 (Reference) |
| Users | 687 | 1.92 (1.44 to 2.55) | 2.24 (1.40 to 3.59) |
| Tamoxifen usage status | |||
| Nonusers | 271 | 1.00 (Reference) | 1.00 (Reference) |
| Tamoxifen discontinuers | 185 | 1.65 (1.13 to 2.41) | 1.72 (1.01 to 2.92) |
| Tamoxifen continuers | 502 | 2.02 (1.50 to 2.74) | 2.58 (1.58 to 4.21) |
| Tamoxifen discontinuation | |||
| Discontinuers | 185 | 1.00 (Reference) | 1.00 (Reference) |
| Continuers | 502 | 1.23 (0.88 to 1.72) | 1.50 (1.04 to 2.17) |
Mammographic density reduction was defined as an annual decrease in dense area greater than 15%. Tamoxifen users were defined as women who had at least one record of dispensed tamoxifen in the Swedish Prescribed Drug Register. Tamoxifen discontinuers were defined as patients who were prescribed tamoxifen but to whom no tamoxifen was dispensed within 180 days before the follow-up mammogram. We restricted our analyses to women with first follow-up mammograms taken after January 1, 2006, because the Swedish Prescribed Drug Register was established in July 1, 2005. BMI = body mass index; CI = confidence interval; HRT = hormone replacement therapy.
Adjusted for radiotherapy, chemotherapy, age at diagnosis (continuous), age at menarche (continuous), study populations (Libro1 vs KARMA), BMI (continuous), progesterone receptor status, family history of breast cancer, mammography type (digital vs film), baseline mammographic dense area, menopausal status, and HRT.
Discussion
In this prospective cohort study, both tamoxifen and chemotherapy were associated with a reduction in MD in breast cancer patients. These associations were present in the study population as a whole and, for tamoxifen, in both pre- and postmenopausal women but were, for chemotherapy, exclusive to premenopausal women. Furthermore, for women prescribed tamoxifen, density reduction was affected by compliance. Neither AIs nor radiotherapy was associated with density change.
To our knowledge, this is the first study with adequate power to investigate the association between tamoxifen discontinuation and MD change. Only two other studies have investigated tamoxifen adherence (using prescription data) and density change (26,27) of which the second study was nested within the first. Contrary to our finding that continuers of tamoxifen treatment had a more pronounced reduction in dense area than discontinuers, neither of the studies by Nyante et al. found an association between adherence and density change. The null finding may be explained by the studies’ small sample sizes (only 15 women had >90 days without tamoxifen coverage during a mean of 12 months follow-up in the first study by Nyante et al. and in their second study, only 40 women were included) and the short follow-up period of the first study by Nyante et al.
Because continuers of tamoxifen treatment had a more pronounced reduction in dense area than discontinuers, the association between tamoxifen and density reduction is at least partially explained by compliance. This finding along with the findings of two previous studies showing that MD reduction during adjuvant tamoxifen treatment is associated with an improved survival (24,26) suggest that 1) MD reduction could be a sign of therapy response in women adhering to tamoxifen, or 2) a lack of MD reduction could be a sign of treatment discontinuation.
Further analyses of dispensed tamoxifen showed that tamoxifen users (both continuers and discontinuers) were more likely to have an MD reduction compared with women not prescribed tamoxifen. The observed OR was higher in this analysis (OR = 2.24, 95% CI = 1.40 to 3.59) than in the main analysis (OR = 1.58, 95% CI = 1.25 to 1.99) in which tamoxifen use was based on intention to treat, suggesting that most previous studies (3) that have used intention-to-treat analyses may have underestimated the association between tamoxifen use and MD reduction.
In line with the few existing studies on chemotherapy and changes in MD (11–13), we found that chemotherapy was associated with density reduction. This may reflect lobular atrophy, which, in nonneoplastic breast tissue, has been found to be associated with chemotherapy (28). To exclude the possibility that the relationship between chemotherapy and density reduction was due to residual confounding by tamoxifen, we carried out a sensitivity analysis restricted to women who had not received tamoxifen and found that the association persisted. Furthermore, congruent with the results by Chen et al. (12) who found that younger women had a more pronounced reduction in MD than older women, stratified analysis on menopausal status showed that the association between chemotherapy and density reduction was mainly seen in premenopausal women. Hence, as Chen et al. also hypothesized, we propose that the relationship between chemotherapy and density reduction is largely due to a change in the hormonal milieu (specifically, decreases in estrogen and progesterone levels) secondary to chemotherapy-induced ovarian failure. However, although not statistically significant, there was a positive association between treatment with chemotherapy and density reduction also in postmenopausal women (OR = 1.32, 95% CI = 0.83 to 2.09). We therefore cannot rule out the possibility of other nonhormonal mechanisms being at play. Further studies with larger sample sizes are needed to investigate the association between chemotherapy and dense area reduction and, if replicated, investigate whether this potential association also translates into an improved survival.
We found no statistically significant association between AIs and MD. The null association may be explained by the relatively low dense area at baseline and small decrease in dense area during follow-up in this group of mainly postmenopausal women (due to the pharmacodynamics of the drug). The six previous studies on AIs and density change are inconsistent (3), of which only two were conducted in the adjuvant setting (29,30). The inconsistency in results may be due to confounding because common side effects of AIs include myalgia, joint pain, and osteoporosis. Thus, many women taking AIs may be prescribed nonsteroidal anti-inflammatory drugs, bisphosphonates, calcium, and vitamin D, all of which have been inversely associated with breast cancer risk (31–35) and could be associated with MD (36–38). Both the side effects of AIs and additional drug prescription may be important to take into account when studying the association between AIs and density change in future studies.
Consistent with previous studies (26), women who experienced a greater density reduction were younger, more often premenopausal, and had a higher baseline dense area. We also observed a greater dense area reduction among women with higher BMI. However, we lack an explanatory hypothesis for this observation and thus cannot exclude the possibility that the association might be due to other unmeasured confounders.
Certain limitations of our study should be addressed. First, we were able to include only 36% of the participants because we needed both a baseline and follow-up mammogram to assess density change. Women who died before a follow-up mammography was performed (ie, within a maximum of 3 years of their breast cancer diagnosis) were therefore excluded, limiting generalizability to breast cancer patients with an especially poor prognosis. However, tumor characteristics, which are closely associated with survival, were not associated with MD reduction in our study. Thus, this selection is not likely to have threatened the internal validity of our study. Secondly, an investigation of MD as an early marker of therapy response should ideally focus on density change within the first year, which our study was insufficiently powered to do. This limitation is minimized by the fact that similar results were observed for year one, two, and three for all of the associations between adjuvant therapy and MD reduction (Supplementary Table 2, available online). Thirdly, detailed information on drug dispensation was available for only a subpopulation of women whose breast cancers were diagnosed from 2005. This limited sample size disabled us from analyzing possible effects of therapy switching (between tamoxifen and AIs), tamoxifen duration, and days since last tamoxifen use on density change and therefore needs to be addressed in future studies. Finally, misclassification of tamoxifen continuers is possible because dispensation of tamoxifen does not necessarily guarantee consumption of the medication. Such misclassification would dilute rather than create our observed association. However, despite these limitations, the Swedish Drugs Registry enabled us to study tamoxifen dispensation rather than intention to treat, thereby reducing the risk of exposure misclassification. Furthermore, measurement errors of change in MD were minimized thanks to the completely automated density measurements and alignment of images (21).
In conclusion, our study provides further evidence that MD is associated with both therapy response and compliance among tamoxifen users. The effect of tamoxifen on density reduction was already seen during the first follow-up mammogram, supporting the notion that MD change is an early marker of therapy response to tamoxifen. Assessment of density change may therefore be of great clinical benefit; failing to decrease in MD during tamoxifen treatment could be either a sign of poor compliance or poor treatment effect and a signal that a new treatment strategy is needed. Conversely, density reduction in tamoxifen users is a sign of therapy response and could also serve as an incentive to adhere to the medication. This would be of great value because endocrine treatment is prescribed for 5–10 years and compliance is poor due to side-effects. Mammography is already a well-established part of the clinical follow-up of breast cancer patients and there are now validated, automated density assessment tools. Thus, clinical implementation of density assessment as a routine part of breast cancer patient follow-up would be simple and come at a low cost.
Funding
This work was supported by the Stockholm County Council (grant no. K0138-2015), the Swedish Research Council (grant no. 2014 -2271), Swedish Cancer Society (grant no. CAN 2016/684), and FORTE (grant no. 2016-00081). This study was also supported by the Cancer Health Risk Prediction Center (www.crispcenter.org), a Linneus Centre (contract ID 70867902) financed by the Swedish Research Council. Jonas Bergh was supported by Stockholm County Council and the Swedish Cancer Society.
Notes
Affiliations of authors: Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (LE, WH, ME, KH, PH, KC); Department of Oncology-Pathology, Cancer Center Karolinska, Department of Oncology, Radiumhemmet, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden (LE, JB); Department of Oncology, South General Hospital, Stockholm, Sweden (PH).
The authors declare no potential conflicts of interest.
Supplementary Material
Reference
- 1. Boyd NF, Martin LJ, Yaffe MJ, et al. Mammographic density: a hormonally responsive risk factor for breast cancer. J Br Menopause Soc. 2006;12(4):186–193. [DOI] [PubMed] [Google Scholar]
- 2. Cuzick J, Warwick J, Pinney E.. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103(9):744–752. [DOI] [PubMed] [Google Scholar]
- 3. Shawky MS, Martin H, Hugo HJ, et al. Mammographic density: a potential monitoring biomarker for adjuvant and preventative breast cancer endocrine therapies. Oncotarget. 2017;8(3):5578–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mullooly M, Pfeiffer RM, Nyante SJ, et al. Mammographic density as a biosensor of tamoxifen effectiveness in adjuvant endocrine treatment of breast cancer: opportunities and implications. J Clin Oncol. 2016;34(18):2093–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghosh K, Brandt KR, Reynolds C, et al. Tissue composition of mammographically dense and non-dense breast tissue. Breast Cancer Res Treat. 2012;131(1):267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huo CW, Chew G, Hill P, et al. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 2015;17:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alowami S, Troup S, Al-Haddad S, et al. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003;5(5):R129–R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gabrielson M, Chiesa F, Paulsson J, et al. Amount of stroma is associated with mammographic density and stromal expression of oestrogen receptor in normal breast tissues. Breast Cancer Res Treat. 2016;158(2):253–261. [DOI] [PubMed] [Google Scholar]
- 9. Vachon CM, Sasano H, Ghosh K, et al. Aromatase immunoreactivity is increased in mammographically dense regions of the breast. Breast Cancer Res Treat. 2011;125(1):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chew GL, Huo CW, Huang D, et al. Increased COX-2 expression in epithelial and stromal cells of high mammographic density tissues and in a xenograft model of mammographic density. Breast Cancer Res Treat. 2015;153(1):89–99. [DOI] [PubMed] [Google Scholar]
- 11. Knight JA, Blackmore KM, Fan J, et al. The association of mammographic density with risk of contralateral breast cancer and change in density with treatment in the WECARE study. Breast Cancer Res. 2018;20(1):23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen JH, Nie K, Bahri S, et al. Decrease in breast density in the contralateral normal breast of patients receiving neoadjuvant chemotherapy: MR imaging evaluation. Radiology. 2010;255(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen JH, Pan WF, Kao J, et al. Effect of taxane-based neoadjuvant chemotherapy on fibroglandular tissue volume and percent breast density in the contralateral normal breast evaluated by 3T MR. NMR Biomed. 2013;26(12):1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Formenti SC, Demaria S.. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10(7):718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holm J, Humphreys K, Li J, et al. Risk factors and tumor characteristics of interval cancers by mammographic density. J Clin Oncol. 2015;33(9):1030–1037. [DOI] [PubMed] [Google Scholar]
- 16. Holm J, Eriksson L, Ploner A, et al. Assessment of breast cancer risk factors reveals subtype heterogeneity. Cancer Res. 2017;77(13):3708–3717. [DOI] [PubMed] [Google Scholar]
- 17. Gabrielson M, Eriksson M, Hammarstrom M, et al. Cohort profile: the Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA). Int J Epidemiol. 2017;46(6):1740–1741g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Emilsson L, Lindahl B, Koster M, et al. Review of 103 Swedish healthcare quality registries. J Intern Med. 2015;277(1):94–136. [DOI] [PubMed] [Google Scholar]
- 20. Eriksson M, Czene K, Pawitan Y, et al. A clinical model for identifying the short-term risk of breast cancer. Breast Cancer Res. 2017;19(1):29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eriksson M, Li J, Leifland K, et al. A comprehensive tool for measuring mammographic density changes over time. Breast Cancer Res Treat. 2018;169(2):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maskarinec G, Nagata C, Shimizu H, et al. Comparison of mammographic densities and their determinants in women from Japan and Hawaii. Int J Cancer. 2002;102(1):29–33. [DOI] [PubMed] [Google Scholar]
- 23. Haars G, van NPA, van GCH, et al. Measurements of breast density: no ratio for a ratio. Cancer Epidemiol Biomarkers Prev. 2005;14(11 pt 1):2634–2640. [DOI] [PubMed] [Google Scholar]
- 24. Li J, Humphreys K, Eriksson L, et al. Mammographic density reduction is a prognostic marker of response to adjuvant tamoxifen therapy in postmenopausal patients with breast cancer. J Clin Oncol. 2013;31(18):2249–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burton A, Maskarinec G, Perez-Gomez B, et al. Mammographic density and ageing: a collaborative pooled analysis of cross-sectional data from 22 countries worldwide. PLoS Med. 2017;14(6):e1002335.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nyante SJ, Sherman ME, Pfeiffer RM, et al. Prognostic significance of mammographic density change after initiation of tamoxifen for ER-positive breast cancer. J Natl Cancer Inst. 2015;107(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nyante SJ, Sherman ME, Pfeiffer RM, et al. Longitudinal change in mammographic density among ER-positive breast cancer patients using tamoxifen. Cancer Epidemiol Biomarkers Prev. 2016;25(1):212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aktepe F, Kapucuoğlu N, Pak I.. The effects of chemotherapy on breast cancer tissue in locally advanced breast cancer. Histopathology. 1996;29(1):63–67. [DOI] [PubMed] [Google Scholar]
- 29. Engmann NJ, Scott CG, Jensen MR, et al. Longitudinal changes in volumetric breast density with tamoxifen and aromatase inhibitors. Cancer Epidemiol Biomarkers Prev. 2017;26(6):930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vachon CM, Ingle JN, Suman VJ, et al. Pilot study of the impact of letrozole vs. placebo on breast density in women completing 5 years of tamoxifen. Breast. 2007;16(2):204–210. [DOI] [PubMed] [Google Scholar]
- 31. Gronich N, Rennert G.. Beyond aspirin-cancer prevention with statins, metformin and bisphosphonates. Nat Rev Clin Oncol. 2013;10(11):625–642. [DOI] [PubMed] [Google Scholar]
- 32. Thun MJ, Jacobs EJ, Patrono C.. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9(5):259–267. [DOI] [PubMed] [Google Scholar]
- 33. Abbas S, Linseisen J, Slanger T, et al. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer—results of a large case-control study. Carcinogenesis. 2008;29(1):93–99. [DOI] [PubMed] [Google Scholar]
- 34. Takkouche B, Regueira-Mendez C, Etminan M.. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J Natl Cancer Inst. 2008;100(20):1439–1447. [DOI] [PubMed] [Google Scholar]
- 35. Hidayat K, Chen GC, Zhang R, et al. Calcium intake and breast cancer risk: meta-analysis of prospective cohort studies. Br J Nutr. 2016;116(1):158–166. [DOI] [PubMed] [Google Scholar]
- 36. Hack CC, Stoll MJ, Jud SM, et al. Correlation of mammographic density and serum calcium levels in patients with primary breast cancer. Cancer Med. 2017;6(6):1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maskarinec G, Urano Y, Gill J, et al. Nonsteroidal anti-inflammatory drugs (NSAIDs) and mammographic density. Breast Cancer Res Treat. 2008;112(1):133–139. [DOI] [PubMed] [Google Scholar]
- 38. Yaghjyan L, Colditz GA, Drake B.. Vitamin D and mammographic breast density: a systematic review. Cancer Causes Control. 2012;23(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.