Abstract
In response to the rising cost of cancer drugs, the National Comprehensive Cancer Network (NCCN) recently developed a value framework, known as “Evidence Blocks,” to grade the efficacy, safety, evidence quality, evidence consistency, and affordability of treatments included in its clinical guidelines. The value scores were available for 55 of the 69 new cancer drugs approved by the US Food and Drug Administration from 2007 to 2016. Overall, the treatment costs for 95% of new cancer medicines in NCCN clinical guidelines were scored as “very expensive” or “expensive”. In multivariable ordered logistic regression models, there was no association between the affordability of new cancer drugs and efficacy and safety data available in clinical guidelines. Most guideline-recommended drugs were subject to annual list price increases exceeding inflation.
High cancer drug prices are a major public health concern (1). Over the past two decades, the inflation-adjusted price per life-year gained of new cancer drugs has more than tripled (2), and the financial burden of treatment has been associated with increased mortality (3) and nonadherence (4). In response, various value frameworks have been proposed, including by the American Society of Clinical Oncology (5), European Society for Medical Oncology (6), and others (7) to inform shared decision-making about cancer therapy choices. Application of these frameworks suggests that many new drug treatments do not provide clinically meaningful benefits (8). Recently, the National Comprehensive Cancer Network (NCCN) developed a value framework (“Evidence Blocks”) to accompany its clinical practice guidelines, which are among the most widely used in oncology (9). Unlike other frameworks, the NCCN Evidence Blocks explicitly include an “affordability” criterion alongside more traditional criteria such as a drug’s known benefits and risks; in addition, the NCCN Evidence Blocks aim to assess all evidence available in guidelines, including postmarketing studies. In this study, we sought to examine the association between the affordability criterion and the efficacy, safety, and quality of evidence underlying new drugs recommended in the NCCN clinical practice guidelines. We also analyzed temporal changes in list price increases of new drugs.
Using the Drugs@FDA database, we identified all new cancer drugs approved by the US Food and Drug Administration (FDA) between January 2007 and December 2016, and we matched approved indications to NCCN treatment guidelines. The NCCN Evidence Blocks are scored by guideline committees based on five components of guideline-recommended treatments—efficacy, safety, evidence quality, evidence consistency, and affordability—using a standardized scale from 1 (least favorable) to 5 (most favorable) (9). For example, for efficacy, a score of 5 signifies that the regimen/agent is highly effective (often providing long-term survival advantage or curative potential), while a score of 1 signifies that the regimen/agent provides symptomatic benefit only. For affordability, a score of 5 signifies that the affordability of the drug or regimen is considered high (ie, very inexpensive), while a score of 1 signifies that the drug or regimen is very expensive. Affordability is defined by the NCCN as an estimate of the overall total cost of a therapy, including drug cost, administration, infusions, supportive care, and toxicity monitoring and management.
We extracted the value scores and whether the drug had a “preferred” designation among the NCCN-recommended treatment options as of December 2017. To calculate inflation-adjusted price changes, we obtained wholesale acquisition costs from the RedBook (Truven Health Analytics) and Memorial Sloan Kettering’s DrugAbacus (7) and monthly inflation rates (CPI-U) from the US Department of Labor’s Bureau of Labor Statistics. Ordered logistic regression was used to assess the association between affordability and other value scores. All models also included a continuous variable for time trends and indicator variables for orphan drug designation (granted for drugs treating rare diseases affecting fewer than 200 000 people per year) and reimbursement type (Medicare Part B or other) (10). Statistical analyses were performed using Stata (version 12.0; StataCorp), and all P values were two-sided, with values of less than .05 considered statistically significant.
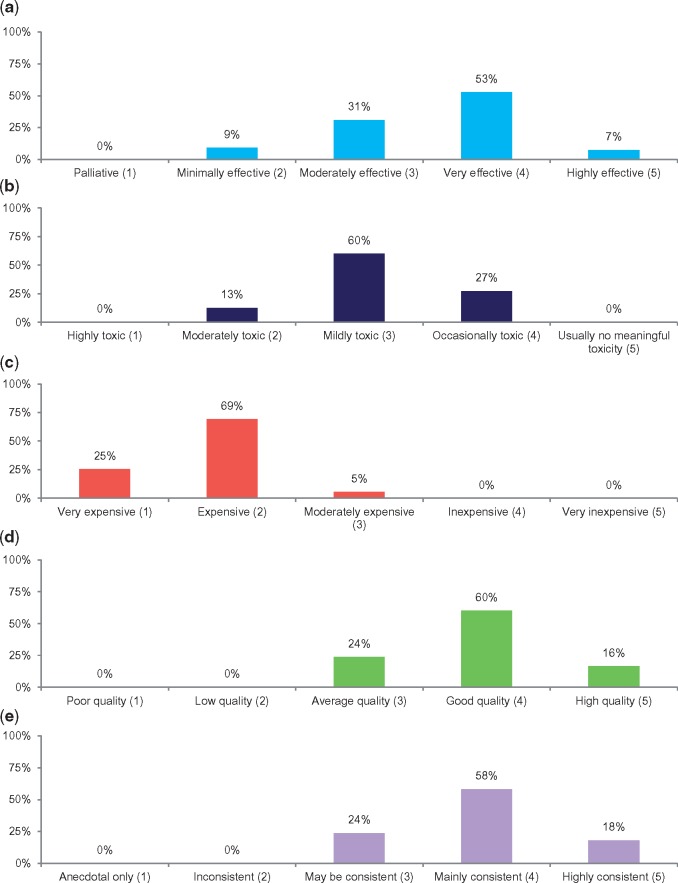
Fifty-five of the 69 new cancer drugs approved by the FDA between 2007 and 2016 were recommended and scored in the NCCN guidelines (Supplementary Tables 1 and 2, available online). Among these drugs, the mean values were 3.6 (efficacy), 3.1 (safety), 3.9 (evidence quality), 3.9 (evidence consistency), and 1.8 (affordability). Fourteen (25%) drugs were scored as “very expensive,” and 38 (69%) as “expensive” (Figure 1). Similar proportions of drugs with vs without a preferred guideline position were scored as “very expensive” or “expensive” (94% vs 95%). In multivariable analyses, approval year was the only statistically significant predictor of affordability, with affordability scores declining over time (odds ratio [OR] = 0.59, 95% confidence interval [CI] = 0.45 to 0.79, P < .001). There was no evidence of an association between the scores for affordability with efficacy, safety, evidence quality, or evidence consistency (Table 1). Overall, the median inflation-adjusted annual list price increase was 3% (interquartile range = 1%–8%).
Figure 1.
National Comprehensive Care Network (NCCN) Evidence Block scores for new cancer drugs approved by the Food and Drug Administration between 2007 and 2016. A–E) The efficacy, safety, affordability, evidence quality, and evidence consistency domains, respectively, of the NCCN Evidence Blocks. Corresponding numerical scores are listed parenthetically on the x-axes. NCCN = National Comprehensive Cancer Network.
Table 1.
Predictors of NCCN Evidence Blocks score for affordability from multivariable ordered logistic regression model
| Characteristic | OR (95% CI) | P |
|---|---|---|
| Approval year | 0.59 (0.45 to 0.79) | <.001 |
| NCCN score: Efficacy* | ||
| 2 | Ref. | |
| 3 | 0.89 (0.17 to 4.62) | .89 |
| 4 | 0.12 (0.01 to 1.34) | .09 |
| 5 | 0.03 (0.00 to 1.00) | .05 |
| NCCN score: Safety* | ||
| 2 | Ref. | |
| 3 | 0.23 (0.02 to 2.25) | .21 |
| 4 | 0.37 (0.03 to 4.28) | .42 |
| NCCN score: Evidence Quality* | ||
| 3 | Ref. | |
| 4 | 0.38 (0.07 to 2.05) | .21 |
| 5 | –† | .71 |
| NCCN score: Evidence Consistency* | ||
| 3 | Ref. | |
| 4 | 4.58 (0.34 to 61.7) | .25 |
| 5 | –† | .86 |
| Orphan drug designation | ||
| Yes | 0.94 (0.16 to 5.56) | .94 |
| No | Ref. | |
| Payable by Medicare Part B | ||
| Yes | 0.37 (0.07 to 1.92) | .24 |
| No | Ref. | |
| Preferred guideline position | ||
| Yes | 1.04 (0.21 to 5.10) | .96 |
| No | Ref. |
There were no drugs that received scores of 1 for Efficacy, 1 or 5 for Safety, 4 or 5 for Affordability, 1 or 2 for Evidence Quality, and 1 and 2 for Evidence Consistency. Similar results were obtained in bivariate ordered logistic regression modeling (to account for potential overfitting). CI = confidence interval; NCCN = National Comprehensive Cancer Network; OR = odds ratio.
Not estimable.
Our study has several strengths and limitations. This report uses data from NCCN’s cancer value framework, which may become increasingly accessed by physicians and patients given the broad use of NCCN guidelines in clinical practice. A limitation is that we focused on drugs that were included and scored in NCCN guidelines, so while our study included 80% of new FDA-approved cancer drugs during the study period, these findings may not be generalizable to other therapies. As the NCCN continues to integrate the Evidence Blocks across its clinical guidelines, scores may be available for more drugs in the future. Finally, additional predictors of drug affordability, such as patients’ insurance status, may influence the interpretation of NCCN’s affordability scores for certain patients.
In conclusion, treatment costs for 95% of new cancer medicines in the NCCN clinical guidelines were scored as “very expensive” or “expensive,” and most drugs were subject to annual list price increases exceeding inflation—raising questions about the ability of patients to access drugs recommended in NCCN clinical guidelines. We also found that the affordability of new cancer drugs was not associated with the efficacy and safety data available in clinical guidelines. However, under current law, Medicare and Medicaid generally cover all FDA-approved cancer drugs, as well as unapproved uses listed in drug compendia. Policies to better align drug expenditures with value could allow public payers to reduce spending on low-value therapies so that limited resources may be redirected to treatments that offer patients better outcomes.
Funding
This work was supported by the Laura and John Arnold Foundation (no grant number). PORTAL is also supported by grants from the Harvard Program in Therapeutic Science and the Engelberg Foundation.
Notes
Affiliations of authors: Program on Regulation, Therapeutics, and Law (PORTAL), Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Boston, MA (TJH, ASK, BG); Institute of Cancer Policy, King's College London, London, UK (BG).
The funding organizations had no role in the design or conduct of the study, in the collection, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript. ASK has received unrelated research support from the FDA Office of Generic Drugs and Division of Health Communication (2013–2016) outside the submitted work. BG reports no conflicts of interest. TJH reports prior employment by Blackstone and Bain Capital, which have invested in health care companies.
Supplementary Material
References
- 1. Kesselheim AS, Avorn J, Sarpatwari A.. The high cost of prescription drugs in the United States: Origins and prospects for reform. JAMA. 2016;3168:858–871. [DOI] [PubMed] [Google Scholar]
- 2. Memorial Sloan Kettering Cancer Center. Monthly and median costs of cancer drugs at the time of FDA approval 1965–2016. J Natl Cancer Inst. 2017;1098:djx173. [DOI] [PubMed] [Google Scholar]
- 3. Ramsey SD, Bansal A, Fedorenko CR et al. , . Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;349:980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL.. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;324:306–311. [DOI] [PubMed] [Google Scholar]
- 5. Schnipper LE, Davidson NE, Wollins DS et al. , . Updating the American Society of Clinical Oncology value framework: Revisions and reflections in response to comments received. J Clin Oncol. 2016;3424:2925–2934. [DOI] [PubMed] [Google Scholar]
- 6. Cherny NI, Sullivan R, Dafni U et al. , . A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol. 2015;268:1547–1573. [DOI] [PubMed] [Google Scholar]
- 7.Memorial Sloan Kettering Cancer Center. Evidence Based Drug Pricing Project. http://www.drugabacus.org/. Accessed March 20, 2018.
- 8. Tibau A, Molto C, Ocana A et al. , . Magnitude of clinical benefit of cancer drugs approved by the US Food and Drug Administration. J Natl Cancer Inst. 2018;1105:djx232. [DOI] [PubMed] [Google Scholar]
- 9. Carlson RW, Jonasch E.. NCCN Evidence Blocks. J Natl Compr Canc Netw. 2016;14(5 Suppl):616–619. [DOI] [PubMed] [Google Scholar]
- 10. Prasad V, Wang R, Afifi SH, Mailankody S.. The rising price of cancer drugs—a new old problem? JAMA Oncol. 2017;2017;32:277–278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.