Abstract
Cancer treatments may compromise the fertility of children, adolescents, and young adults, and treatment-related infertility represents an important survivorship issue that should be addressed at diagnosis and in follow-up to ensure optimal decision-making, including consideration of pursuing fertility preservation. Risk of infertility varies substantially with patient and treatment factors. The ability to accurately assess fertility risk for many patients is hampered by limitations of the current literature, including heterogeneity in patient populations, treatments, and outcome measures. In this article, we review and synthesize the available data to estimate fertility risks from modern cancer treatments for both children and adult cancer survivors to enable clinicians to counsel patients about future fertility.
Over the past decades, therapeutic advances have transformed the care of cancer patients, yielding substantial improvements in cure rates and survival. Children and young adults have gained particular benefit, given successes realized in the treatment of childhood acute lymphoblastic leukemia (ALL), Hodgkin lymphoma (HL), and testicular cancer, among other malignancies. These gains have come with many costs, and, of the long-term treatment sequelae, infertility is among the most important to patients. The most comprehensive definition of “cancer survivor” includes patients from diagnosis onwards and recognizes that management of survivorship issues, even those arising years after treatment, must start at diagnosis (1). Fertility is a survivorship issue that needs to be discussed before initiating therapy to allow for informed decision-making regarding treatment options, family planning, and fertility preservation strategies (2). Fertility should also be considered throughout follow-up, as needed, to address timing and safety of potential pregnancy. Proactively addressing this critical survivorship issue for those at risk for infertility is associated with lower regret and improved quality of life (3). Thus, clinicians should be able to counsel patients with accurate, up-to-date evidence about this critical issue.
Several recent reviews of fertility preservation strategies have been published (4–7) and guidelines crafted (8–10). However, to our knowledge, there is no recent evaluation regarding the risk of infertility associated with specific diseases and therapies among different age groups. This review synthesizes the literature and summarizes the current best estimates of fertility risk from modern day cancer treatment for children and adults to enable clinicians to counsel patients with the most up-to-date understanding of their risks and the potential indication for fertility preservation.
Methods
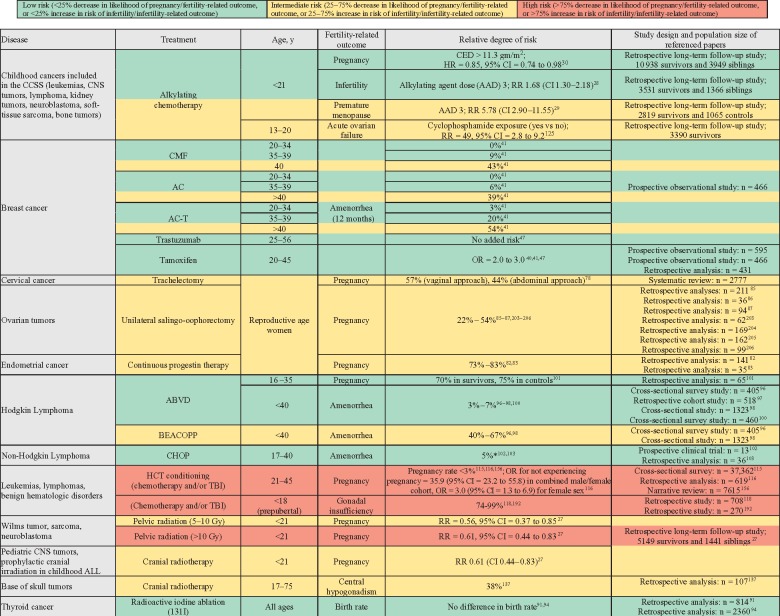
We performed a review of published articles describing the risks of cancer treatments to female and male fertility using the PubMed database. Search terms included, but were not limited to, names of malignancies (eg, breast cancer, leukemia), cancer therapies (eg, chemotherapies, hormone therapies, biologics, and radiation techniques), and outcomes (eg, pregnancies, birth, fertility, infertility, amenorrhea, azoospermia). We included only peer-reviewed articles written in English. No date cutoff was imposed. Reference lists were reviewed for additional relevant articles. All study designs were considered. Randomized trials and prospective observational studies were included preferentially over retrospective studies. For the purposes of this review, the primary outcome of interest is fertility (the ability to conceive a child), and we thus report pregnancy and live birth data whenever possible and use surrogates, such as amenorrhea, semen parameters, and laboratory markers of gonadal insufficiency, as alternatives when necessary (Figure 1). Findings are broken down by sex and age group (pediatric vs adult) and by cancer type for ease of reference. They are further grouped by general degree of risk (high, intermediate, and low) as detailed in Figures 2–4 and color-coded.
Figure 1.
Definitions of fertility-related outcomes.
Figure 2.
Risks to female fertility associated with cancer treatments. AAD = alkylating agent dose; ABVD = doxorubicin, bleomycin, vinblastine, dacarbazine; AC = doxorubicin, cyclophosphamide; AC-T = doxorubicin, cyclophosphamide, paclitaxel; ALL = acute lymphoblastic leukemia; BEACOPP = bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; CED = cyclophosphamide equivalent dose; CHOP = cyclophosphamide, doxorubicin, vincristine, prednisone; CI = confidence interval; CMF = cyclophosphamide, methotrexate, fluorouracil; CNS = central nervous system; HCT = hematopoietic cell transplantation; OR = odds ratio; RR = relative risk; TBI = total body irradiation.
Female Fertility
Data from prior prospective and retrospective trials inform the risks of infertility, yet limitations exist in assessing the likelihood that an individual patient will remain fertile. Ovarian reserve varies among women and is affected by additional factors, including genetic polymorphisms associated with age of menopause (11) and others in genes encoding drug metabolism enzymes (12, 13) may also affect risk of ovarian toxicity. Psychosocial factors, many of which are affected by cancer diagnosis and treatment, are also important determinants of reproductive choices and may have important implications when comparing fecundity of survivors to control populations (14).
Due to ease of measurement, amenorrhea is frequently reported as a primary outcome yet is an imperfect predictor of fertility. Female survivors who have spontaneous menses, particularly if irregular, may still have decreased ovarian reserve and reproductive potential (15–19). Abnormalities of traditional laboratory markers such as follicle-stimulating hormone, estradiol, and inhibin-B levels are late markers of ovarian aging (20). Anti-Mullerian hormone (AMH) is more strongly correlated with antral follicle count (21) and is an earlier predictor of decreased ovarian reserve (22). AMH is evaluable in both pre- and postmenarchal females (23) and detects diminished ovarian reserve among female cancer survivors (17, 18, 24, 25). AMH may therefore be a preferred laboratory assay for establishing a pretreatment baseline, assessing ovarian reserve among survivors, and determining who might benefit from fertility preservation services (26).
The following section details data regarding fertility risks in select populations, diseases, and treatments and is presented with a summary in Figure 2.
Childhood Cancers
Multiple reports from the Childhood Cancer Survivor Study (CCSS), a multi-institutional long-term follow-up study that included patients and matched sibling control subjects, have demonstrated associations between chemotherapy use, as measured by summed alkylating agent dose or cyclophosphamide equivalent dose (CED), and risk of clinical infertility, never achieving pregnancy, and premature menopause (27–30). A 2016 CCSS report, which included 10 938 patients treated between 1970 and 1999 and unexposed to pelvic or brain radiation, found that alkylating chemotherapy decreased the likelihood of pregnancy only at the highest quartile of CED exposure (>11.3 gm/m2, hazard ratio [HR] = 0.85, 95% CI = 0.74 to 0.98) , suggesting that most survivors treated with a variety of chemotherapy regimens alone are not at high risk of infertility (Figure 3) (30). Most studies support the impact of alkylating chemotherapy on infertility and surrogates, including acute ovarian failure (AOF) and premature menopause (19, 31–33), but not all have identified a detrimental effect (34, 35). The 2016 CCSS report did not identify an association between platinum agents (cisplatin, carboplatin) and the likelihood of pregnancy in female childhood cancer survivors (30). However, females with germ cell tumors were not included, and whether platinum administered in this setting (following unilateral oophorectomy) affects future fertility remains unknown. The CCSS represents the most robust source of data, and considering the body of literature, there is strong evidence that higher levels of exposure to alkylating chemotherapy have a greater effect on fertility and biological surrogates. Although the absence of statistical significance with lower exposures in CCSS is reassuring, modern day regimens that include compressed chemotherapy and/or higher CED levels have not been fully evaluated and it is therefore reasonable to use the lowest alkylator dose possible that is associated with the best cure rates (36).
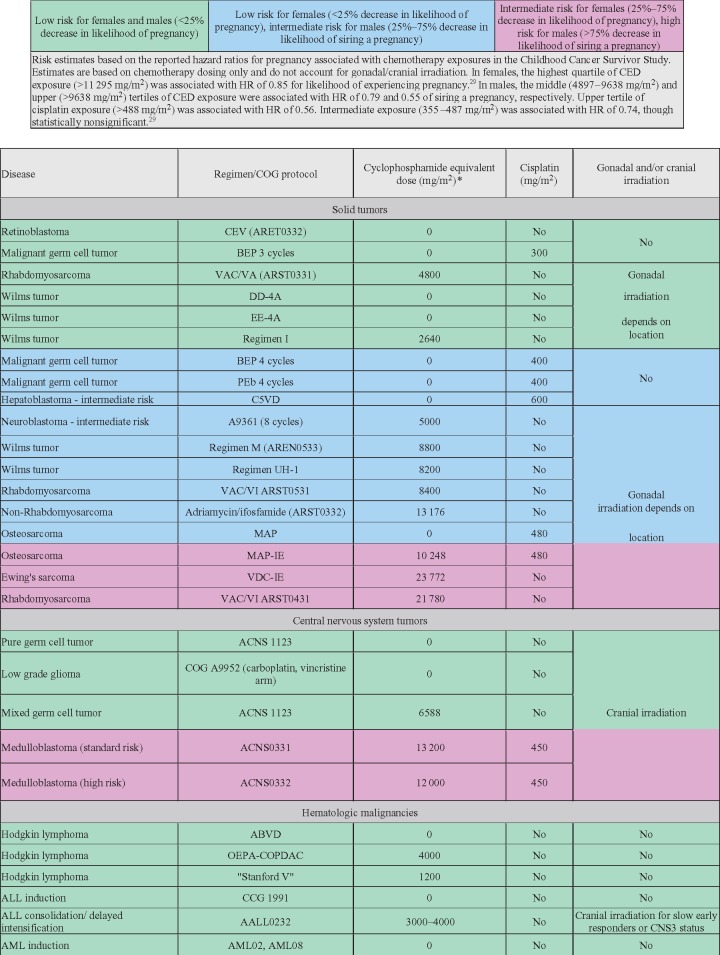
Figure 3.
Estimated risk to male and female fertility from select pediatric chemotherapy regimens using calculated cyclophosphamide equivalent dose (CED) and cisplatin exposures. *CED dose (mg/m2) = 1.0 (cyclophosphamide dose [mg/m2]) + 0.244 (ifosfamide dose [mg/m2]) + 0.857 (procarbazine dose [mg/m2]) + 14.286 (chlorambucil dose [mg/m2]) + 15.0 (carmustine dose [mg/m2]) + 16.0 (lomustine dose [mg/m2]) + 40 (melphalan dose [mg/m2]) + 50 (thiotepa dose [mg/m2]) + 100 (nitrogen mustard dose [mg/m2]) + 8.823 (busulfan dose [mg/m2]). HR = hazard ratio.
Patient and disease factors appear to have little impact upon subsequent fertility outcomes among children. Age at diagnosis is an important predictor of fertility-related outcomes in some adult malignancies; however, data from the CCSS showed no association between age at diagnosis and future fertility (30). Although HL has been found to be an independent risk factor for premature menopause and is associated with diminished pretreatment ovarian reserve in adult women (29, 37), a large prospective cohort study of female childhood HL survivors found that rates of parenthood were comparable to the German general population through age 39 years, suggesting that the impact of HL and its treatment on fertility is not substantial until the majority of the reproductive years have passed (38). Furthermore, regimens that include alkylating chemotherapy, which are associated with lower AMH values relative to doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) (25), are being used less frequently in modern therapy, limiting the impact upon fertility.
Breast Cancer
Among adults, the most robust data regarding fertility risks are available for women with breast cancer. In a recent meta-analysis of five prospective, randomized trials evaluating gonadotropin-release hormone (GnRH) agonists during breast cancer chemotherapy, the rate of premature ovarian insufficiency in the control (chemotherapy alone) arms was 31%, demonstrating that many premenopausal women retain ovarian function (39). Importantly, older age is a well-established risk factor for treatment-related amenorrhea (TRA) following alkylating chemotherapy for breast cancer (40–47), with rates of 0–15%, 30%–50%, and about 70% among women age younger than 35 years, 36–40 years, and older than 40 years, respectively, and younger women who experience TRA are more likely to resume menses (40, 41, 47). Thus, when considering absolute rates of TRA, the age distribution of study populations must be considered.
Cyclophosphamide, included in most adjuvant regimens, appears to be the primary driver of TRA. In a randomized trial comparing the efficacy of a doxorubicin/docetaxel to regimens combining cyclophosphamide, doxorubicin, and a taxane, either sequentially (AC-T) or concurrently (TAC), in which 70% of participants were older than 40 years old, rates of TRA were lower with doxorubicin/docetaxel (38%) relative to AC-T (70%) and TAC (58%) (P < .001) (48). How much cyclophosphamide and how it is administered also appears to contribute to heterogeneity in early and late surrogates of ovarian toxicity. A prospective study of menstrual patterns, in which 46% of participants were over 40 years of age, revealed monthly bleeding immediately following chemotherapy in only 16% of premenopausal women treated with AC-based regimens, in which cyclophosphamide is delivered in four intravenous infusions, vs 48% with CMF, in which cyclophosphamide is taken orally daily for 14 days of six 28-day cycles (40). However, the proportion with monthly bleeding 24 months following chemotherapy rose to 50% among those who received AC, but declined to 30% among those treated with CMF.
Additional data inform the risks of TRA with taxanes, which are now frequently included in adjuvant regimens. AC-T has been associated with numerically higher rates of TRA than AC alone (29% vs 19% in a high-quality prospective study and an odds ratio [OR] of 1.59 [95% CI = 0.8 to 3.2] in a large retrospective study), though neither difference was statistically significant (41, 47). In a study of patients with small HER2+ tumors treated with paclitaxel/trastuzumab, TRA occurred in 18 of 64 (28%) premenopausal women, but in only 1 of 11 (9%) women age 40 years or younger vs 14 of 29 (48%) women aged over 45 years (49). Data on docetaxel/cyclophosphamide is limited to one prospective cohort study of women age 40 years and younger in which the rate of 12-month TRA with docetaxel/cyclophosphamide was 33% and similar to AC (46%) and AC-T (40%) (50). One study suggested that docetaxel could be more gonadotoxic than paclitaxel; AC followed by docetaxel was associated with statistically significantly higher risk of TRA than AC (OR = 9.4) and AC-T (OR = 7.2), though conclusions are limited by the small number of patients treated with AC followed by docetaxel (n = 17) (41). Although some reports have identified taxanes as an independent risk factor (41, 46), most have not (42, 43, 45, 51–54). Recognizing limitations in the literature, especially studies grouping paclitaxel and docetaxel, the available data suggest taxanes may contribute to TRA, but that the absolute effect appears to be small and the predominant predictors of amenorrhea for women receiving taxane-containing chemotherapy are age and exposure to alkylating chemotherapy.
Neither dose-dense schedule nor the addition of trastuzumab has been associated with TRA (47, 55). Platinum agents are increasingly being explored for triple negative breast cancer and it will be important to characterize their impact on TRA. Cisplatin, a DNA cross-linking agent, appears gonadotoxic in some settings, though published data are scarce (56, 57). Cisplatin was associated with reduced pregnancy rates in males, but not females, in the CCSS (30, 57, 58). In one small study of women diagnosed at age 45 years or younger with breast cancer (n = 165), the rate of 12-month TRA was only 6% among the 35 women treated with carboplatin-docetaxel (TP) (59). Given the limited data and plausible mechanism of gonadotoxicity, it is most appropriate to counsel patients that platinum agents, particularly cisplatin, may impair fertility.
Tamoxifen use following adjuvant chemotherapy is associated with a 2-fold increase in risk of TRA (40, 41, 47, 60), though no women age 40 years or younger treated with tamoxifen alone developed amenorrhea within a large prospective cohort study (50). Tamoxifen does not appear to have permanent effects on menstrual function or fertility but is teratogenic and must be held before and during pregnancy; thus, the need to delay childbearing can pose a risk to fertility through ovarian aging.
TRA has been associated with improved overall survival in breast cancer (61); thus, reversible means of obtaining this “chemoendocrine effect” may be desirable for women interested in future fertility. Ovarian suppression with GnRH agonists is increasingly being incorporated into the treatment of premenopausal women with hormone-sensitive disease (62, 63). Although chemotherapy has often been the default approach for young women, optimization of endocrine therapy with ovarian suppression may be a more prudent approach for some with lower risk disease and a means of preserving future fertility.
Gynecologic Malignancies
In the treatment of cervical cancer, the focus for women interested in future fertility has been on prevention of anatomical changes that impair childbearing. The risk of infertility with hysterectomy is 100%, although successful pregnancies have occurred with oocyte retrieval and use of a surrogate (64–66). Fertility-sparing procedures, such as vaginal or abdominal radical trachelectomy in which the cervix and upper vagina are removed, are now options for highly selected patients (67). Pregnancy rates among women attempting to conceive range from 25% to 95%, with most estimates above 40% (68–77). Whereas all women in one series required in vitro fertilization (IVF) after treatment, 16 of 17 patients who conceived in another series did so naturally (68, 75). Miscarriage rates range from 9% to 42% and rates of pregnancies with gestation beyond 37 weeks range from 14% to 55% (69–74). A recent systematic review found improved pregnancy rates associated with vaginal radical trachelectomy (57%) vs laparotomic abdominal radical trachelectomy (44%) (78). Candidates for fertility-sparing procedures generally do not receive adjuvant radiation or chemotherapy (67). However, neoadjuvant chemotherapy (cisplatin plus ifosfamide in squamous cell carcinoma or cisplatin plus doxorubicin in adenocarcinoma) has been used on an experimental basis to downstage patients who were not upfront candidates for fertility-sparing surgery and resulted in fertility preservation in 20 of 28 patients (71%), 10 of whom (50%) became pregnant in one series (79). Pregnancy data are not available for women receiving definitive concurrent chemoradiation. A small series demonstrated the feasibility of ovarian transposition before chemoradiation, with ovarian failure experienced by 1 of 7 women age 40 years or younger and 6 of 7 women aged over 40 years (80).
Although hysterectomy with bilateral salpingo-oophorectomy represents the standard approach to localized endometrial cancer, continuous progestin therapy is a fertility-sparing option for highly selected young women with endometrial hyperplasia or stage IA endometrial adenocarcinoma (81). Although reproductive outcomes data are limited, the two largest series demonstrated pregnancies in 51 of 70 patients (73%) and 10 of 12 patients (83%) who attempted pregnancy after achieving a complete remission (82, 83).
Management of epithelial ovarian cancer also includes removal of critical reproductive organs. For highly selected women, depending on extent and type of disease, fertility-sparing procedures with unilateral salpingo-oophorectomy and complete surgical staging may be performed (84). In one study of women with unilateral stage I invasive epithelial ovarian cancer, 182 of 186 women (96.8%) remained premenopausal postoperatively (85). Pregnancy rates among women attempting to conceive after unilateral salpingo-oophorectomy for EOC have ranged from 27% to 53% (85–88).
Gonadal-sparing surgery is the goal of management of ovarian cyst and tumors in adolescent and young adult women. Most ovarian tumors in girls and adolescents are benign. Malignant tumors are usually of germ cell origin. For tumor marker negative tumors, a fertility-sparing procedure can be performed. With positive tumor markers, a unilateral oophorectomy and staging procedure—in which peritoneal fluid is collected, lymph nodes and omentum are inspected, and biopsy reserved for suspicious sites—is performed to maintain fertility. Minimizing abdominopelvic surgery prevents fertility issues due to adhesions. Most ovarian germ cell tumors in adolescents and young adults are stage I and about 50% are cured with surgery. For women requiring further treatment, fertility preservation should be considered, given the effect of cisplatin on ovarian function remains unknown in this setting.
Thyroid Cancer
Thyroidectomy represents the primary treatment for localized, differentiated thyroid cancer and, with thyroid hormone replacement, is not gonadotoxic. Radioactive iodine ablation (131I) may also be used for differentiated thyroid cancer and leads to transient amenorrhea in up to 20% of patients (89). The average age of menopause is slightly lower following 131I (49.5 vs 51.0 years), but there is no difference in birth rate and 131I does not appear to have long-term effects on fertility (90–94).
Hodgkin Lymphoma
The majority of adult women diagnosed with HL are of reproductive age and, given their excellent cancer prognosis, fertility in survivorship may be particularly important (95). A robust literature demonstrates that TRA among HL survivors is affected by age, systemic therapy, and exposure to pelvic radiation (16, 96, 97). Rates of TRA are high among regimens that contain heavy alkylator exposure: cyclophosphamide, vincristine, procarbazine, prednisone, doxorubicin, bleomycin, vinblastine, dacarbazine (COPP/ABVD) (54.6%), bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednison (BEACOPP) (47%–56%), and dose-escalated BEACOPP (40%–67%) (96–98). ABVD is the standard initial chemotherapy in the United States for adults and is associated with very low rates of TRA (3%–7%) (96–100). Pregnancy rates following ABVD are similar to control subjects (70% vs 75%) (101). Thus, fertility preservation is usually not needed for women receiving ABVD alone. Patients are often instructed to delay pregnancy for 2 years beyond treatment (when most relapses occur); thus, fertility preservation measures could be considered if this delay would substantially reduce the remaining fertile period. Ongoing trials integrating targeted and immune-based therapies such as brentuximab and nivolumab into upfront HL therapy, both of which are unlikely to directly affect fertility, may further limit the need for gonadotoxic alkylating chemotherapy.
Non-Hodgkin Lymphoma
Non-Hodgkin lymphoma (NHL) encompasses indolent and aggressive diseases addressed with a variety of treatments, including chemotherapy, targeted therapies, and immune-based therapies. Some studies suggest relatively low fertility rates for female survivors, with successful pregnancies in 21% (15) and TRA in 41% (16). The total cyclophosphamide exposure with six cycles of cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP; 4500 mg/m2) is nearly twice that of adjuvant breast cancer regimens, and therefore the effects on amenorrhea and fertility are expected to be at least that of women of corresponding age treated for breast cancer. However, young women with breast cancer have low rates of amenorrhea, and other studies suggest more favorable fertility outcomes for NHL survivors with greater than 50% achieving pregnancy and TRA in under 10%, even with dose-intensified CHOP (cyclophosphamide 12 000 mg/m2) (102, 103). Older women are more likely to experience AOF (15). Women treated with rituximab, an anti-CD20 monoclonal antibody, are counseled to avoid pregnancy given concerns regarding teratogenicity and immunosuppression of offspring (104). No data are available regarding the impact of rituximab on female fertility, though the mechanism of action would not be expected to directly affect fertility. Treatment of relapsed NHL (and HL) with chemotherapy or autologous hematopoietic cell transplantation (HCT) is clearly associated with worse reproductive outcomes due largely to greater use of alkylator chemotherapy.
Leukemia
The backbone treatment for acute myeloid leukemia (other than acute promyelocytic leukemia) remains daunorubicin, an anthracycline, and cytarabine. Fertility risks associated with anthracyclines are poorly defined because anthracyclines have usually been co-administered with alkylators for more prevalent cancers. However, rates of amenorrhea with anthracyclines may be substantial even in the absence of alkylators (48). Treatment of ALL often includes alkylating agents and risk presumably varies by the regimen selected. Limited studies on menstrual function and fertility, often grouping patients with AML and ALL, have demonstrated rates of AOF with induction chemotherapy as low as 17% (15, 105), though AOF may underrepresent the impact of treatment on fertility (15). The greatest threat to fertility among women with leukemia is gonadotoxic conditioning before HCT, as exhibited by lower rates of infertility among women who receive consolidation chemotherapy (106). The urgency to initiate induction therapy generally precludes standard fertility preservation measures for women with acute leukemia, but opportunities may exist in the first complete remission or before induction therapy for those with high-risk myelodysplasia, especially because pregnancies can be achieved after HCT with banked embryos or oocytes (107).
The risk to fertility associated with long-term imatinib, a tyrosine kinase inhibitor (TKI) targeting bcr-abl, for chronic myeloid leukemia is not well studied. Offspring of women exposed to imatinib during pregnancy have demonstrated congenital abnormalities, and patients should practice reliable contraception while on TKI therapy, including imatinib or others (108–110). Despite case reports of impaired ovarian function (111) and premature ovarian failure (112), it is not clear whether imatinib or other TKIs affect fertility. Patients who discontinue are at risk for loss of response (113); thus, the benefit of continuing therapy during reproductive years represents a threat to fertility and consideration can be given to alternative strategies for having future biologic children, including use of a gestational carrier.
Hematopoietic Cell Transplant
Survivors who undergo HCT are at high risk for ovarian failure and infertility due to the treatment of their primary disease and the conditioning regimen, which generally includes gonadotoxic chemotherapy with or without radiation. However, many pregnancies have been identified following HCT, including autologous and nonmyeloablative and myeloablative allogeneic transplants for malignant and nonmalignant conditions (114–116). True risks of infertility and amenorrhea have been difficult to assess given heterogeneous patient populations and that not all survivors desire or attempt pregnancy (117). Of 708 postmenarchal women in one study, 110 (16%) recovered ovarian function and 32 (5%) subsequently became pregnant (118). Of 82 premenarchal girls, 23 (28%) developed normal gonadal function and 9 (11%) became pregnant (118). In another study of HCT survivors with a median age of 33 years and follow-up of 8 years, 8 of 292 (3%) female survivors became pregnant vs 72% of sibling control subjects (116). In the entire cohort (including men), survivors were dramatically less likely to report pregnancy (OR = 35.9, 95% CI = 23.2 to 55.8). Female sex was an independent risk factor for infertility among survivors (OR = 3.0, 95% CI = 1.3 to 6.9), though use of assisted reproductive technologies (ART), which may be have been more common among men given availability of sperm banking, was not recorded (116). Pregnancy rates were low following allogeneic and autologous HCT, and predictors of infertility included age 30 years or older (OR = 4.8, 95% CI = 2.1 to 10.7) and receipt of total body irradiation (TBI) (OR = 3.3, 95% CI = 1.5 to 7.3) (116). Although one prospective cohort study of HCT survivors with an average age at diagnosis of 33 years identified no pregnancies among 60 females 10 years after HCT, other studies have demonstrated pregnancy rates of 40%–65% and regular menstrual cycles in 63%–68% among autologous HCT survivors under age 40 years (119–121). One early prospective study demonstrated that regular menses resumed in only 6% of women transplanted for leukemia with cyclophosphamide and TBI conditioning regimens but in 74% of those transplanted for aplastic anemia (and 100% age <26 years) with cyclophosphamide only conditioning regimens, and both increasing age at diagnosis (relative risk [RR]per year 1.2, P = .002) and receipt of TBI (RR12 Gy vs none 8.3, P < .001) were associated with ovarian failure (122). Thus, although fertility may be severely impaired by transplant, risk varies considerably by age and conditioning regimen. All females undergoing HCT should be assumed to be at intermediate to high risk of ovarian failure and infertility, and additional research is needed to help risk-stratify women based on patient, disease, and treatment characteristics.
Radiotherapy
Radiation can diminish fertility when directed to reproductive organs or the structures that produce hormones necessary for reproduction. Mathematical modeling suggests the dose at which 50% of immature oocytes die is under 2 Gy (123). The exposure required to induce ovarian failure and infertility decreases with increased age, due to the normal decline in ovarian reserve and an increase in radiosensitivity of oocytes in growing follicles relative to primordial oocytes (124–127).
The CCSS demonstrated a dose-dependent effect of radiation on risk of ever being pregnant (5–10 Gy, RR = 0.56, 95% CI = 0.37 to 0.85; >10 Gy, RR = 0.18, 95% CI = 0.13 to 0.26) relative to sibling control subjects (27). Radiation exposures as low as 1 Gy are associated with increased risk of premature menopause (RR = 4.3, 95% CI = 1.2 to 15.5) and AOF (RR = 3.6, 95% CI = 1.9 to 7.2) (29, 125). More than 80% of girls with exposure greater than 20 Gy experienced AOF (125). TRA occurred in all 11 women within the Georgia Cancer Registry exposed to pelvic radiation for a variety of malignancies (16). A retrospective series also demonstrated TRA in only 5% of premenopausal women treated for colon cancer, but in 94% treated for rectal cancer, likely due at least in part to pelvic radiation (128). Scattered radiation from abdominal fields may reach the ovary, with one study showing a median exposure of 1 Gy, and should be considered when assessing the infertility risk from abdominal radiation (129).
Pelvic radiation may also affect the uterus, leading to atrophy of the myometrium and endometrium (130) and decreased uterine length and blood flow (131, 132). The degree to which uterine effects contribute is unclear, though patients treated with pelvic radiation are known to be at substantial risk for pregnancy complications (115).
Cranial irradiation affects fertility through the development of endocrinopathies involving the hypothalamic-pituitary-gonadal axis (133). Among children (male and female), prophylactic cranial irradiation (PCI) (18–24 Gy) for ALL is associated with a lower birth rate than chemotherapy alone (134, 135). The CCSS confirmed that hypothalamic/pituitary radiation doses of 22–27 Gy (HR = 0.67, 95% CI = 0.53 to 0.84) and over 30 Gy (RR = 0.61, 95% CI = 0.44 to 0.83) are associated with decreased fertility among females (27, 136). Whether the decreased radiation doses currently used for PCI and implementation of proton radiotherapy for CNS malignancies will mitigate fertility risk remains unknown (133). The 5-year risk of central hypogonadism in one study of patients treated with conformal radiation techniques for base of skull tumors was 38% among females, with onset ranging from 2 to 11 years after treatment (137); thus, survivors remain at risk for infertility years beyond diagnosis.
Male Fertility
Infertility among male cancer survivors is common yet has been the subject of less research than female infertility. Pregnancy and live birth data are sparse, and spermatogenesis parameters are frequently used as surrogates although generally felt to be more reliable than markers of ovarian reserve. Semen analysis methodology and reference ranges for semen volume, sperm concentration, total sperm number, morphology, and motility have been standardized (138, 139). Each parameter has been associated with time to pregnancy and/or probability of conception (139–143). Today, use of in vitro fertilization and intracytoplasmic sperm injection overcome most sperm defects and allow successful pregnancy. Semen analysis does not assess the cellular and biochemical processes required to bind, penetrate, and fertilize the oocyte (139) or genetic contributors to infertility (144); thus, a normal semen analysis does not guarantee fertility.
Although treatment usually represents the greatest threat to fertility of male survivors, the underlying malignancy may affect semen production and quality through poorly understood mechanisms. In testicular cancer, rates of oligospermia and azoospermia at presentation are 50% and 10%, respectively (145–148). Similarly, HL is associated with oligospermia and azoospermia before treatment (149–151). Interestingly, treatment of the underlying malignancy may yield improvements in spermatogenesis (152–155).
Radiation and chemotherapy have cytotoxic effects on testicular germinal epithelium including Sertoli cells, but to a lesser extent on Leydig cells, leading to frequent impairment of spermatogenesis without hypogonadism (117, 156). Sperm counts fall dramatically within 2 months following chemotherapy or radiation (157). Lack of early recovery does not necessarily portend permanent sterility. Recovery of spermatogenesis can occur up to 5 years after treatment (158), and spermatozoa have been successfully extracted via microdissection testicular sperm extraction in up to 37% of azoospermic males in whom it was attempted following chemotherapy in one series and 47% in another (159, 160).
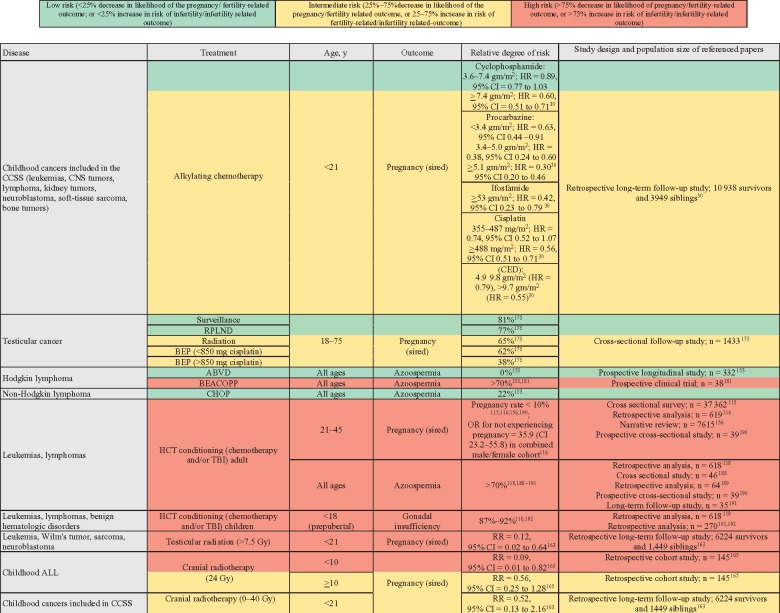
The next section details data regarding the risk to male infertility in select populations, diseases, and associated treatment modalities and is presented with a summary in Figure 4.
Figure 4.
Risks to male fertility associated with cancer treatments. ABVD = doxorubicin, bleomycin, vinblastine, dacarbazine; ALL = acute lymphoblastic leukemia; BEACOPP = bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; BEP = bleomycin, etoposide, cisplatin; CCSS = Childhood Cancer Survivor Study; CED = cyclophosphamide equivalent dose; CHOP = cyclophosphamide, doxorubicin, vincristine, prednisone; CNS = central nervous system; HCT = hematopoietic cell transplantation; PCI = prophylactic cranial irradiation; RPLND = retroperitoneal lymph node dissection; TBI = total body irradiation.
Childhood Cancers
Within the CCSS, male survivors were statistically significantly less likely than sibling control subjects to sire a pregnancy, and chemotherapy appears to be a greater risk to fertility for males (HR = 0.63, 95% CI = 0.58 to 0.68) than females (HR = 0.87, 95% CI = 0.81 to 0.94) (30). Among males unexposed to radiation, exposure to individual alkylators, including cyclophosphamide (3.6–7.4 gm/m2, HR = 0.89, 95% CI = 0.77 to 1.03; >7.4 gm/m2, HR = 0.60, 95% CI = 0.51 to 0.71), ifosfamide (26–53 gm/m2, HR = 0.61, 95% CI = 0.36 to 1.01; >53 gm/m2, HR = 0.42, 95% CI = 0.23 to 0.79), and procarbazine (<3.3 gm/m2, HR = 0.63, 95% CI = 0.44 to 0.91; 3.3–5 gm/m2, HR = 0.38, 95% CI = 0.24 to 0.60; >5 gm/m2, HR = 0.30, 95% CI = 0.20 to 0.46), was associated with statistically significant decreases in male fecundity. Similar findings were demonstrated when analyzing by CED per 5-gm/m2 increment (HR = 0.82, 95% CI = 0.79 to 0.86). Although lacking statistical significance, potentially due to inadequate statistical power, low or moderate exposure to the other alkylators, including busulfan (<450 mg/m2, HR = 0.46, 95% CI = 0.15 to 1.42) and lomustine (>411 mg/m2, HR = 0.82, 95% CI = 0.26 to 2.60), may still have clinically significant effects on male fertility. This builds upon prior evidence for a dose-dependent increase in risk of infertility and impairment in spermatogenesis among male cancer survivors treated with alkylating chemotherapy (161–164). Among a cohort treated with alkylating chemotherapy and without radiation, 25% were azoospermic and 28% oligospermic, and CED was associated with azoospermia (OR = 1.22 per 1000 mg/m2, 95% CI = 1.11 to 1.34) (161). Importantly, the CCSS confirmed that cisplatin, a DNA crosslinking agent, is associated with decreased male fertility (<355 mg/m2, HR = 0.85, 95% CI = 0.56 to 1.27; 355–487 mg/m2, HR = 0.74, 95% CI = 0.52 to 1.07; > 488 mg/m2; HR = 0.56, 95% CI = 0.39 to 0.82) (30). Compared with sibling control subjects, male childhood ALL survivors appear to have normal fertility, presumably due to low alkylator exposure (165). Although early reports suggested that pubertal males are more susceptible to gonadotoxicity, the CCSS and St. Jude’s cohorts did not identify age as a risk factor among males (161, 163, 166).
Testicular Cancer
Although unilateral orchiectomy theoretically spares the unaffected testis, sperm concentration decreases post-procedure and azoospermia may occur among men who initially presented with oligospermia (167). Treatment of bilateral testicular cancer with bilateral orchiectomy yields sterility, but this presentation represents only 1% of testicular cancers and the majority are metachronous rather than synchronous (168). Retrograde ejaculation was previously a frequent complication of retroperitoneal lymph node dissection (169,170), but is now uncommon with nerve-sparing techniques (171–173).
Despite data suggesting that testicular cancer survivors are as likely to sire a pregnancy as age-matched control subjects (174), adjuvant therapy for testicular cancer affects fecundity in some patients. A prospective study of men treated with unilateral orchiectomy followed by surveillance or adjuvant therapy found that paternity rates without cryopreserved sperm were lower among men who received radiotherapy (65%) or cisplatin (<850 mg [62%]; >850 mg [38%]) than men who underwent surveillance (81%), but no difference was seen between retroperitoneal lymph node dissection (77%) and surveillance (175). Retrograde ejaculation was reported by 54 of 520 men (10%), 12 (22%) of whom conceived without cryopreserved sperm. Subsequent analyses of men receiving adjuvant chemotherapy demonstrated a dose-dependent effect, with post-treatment paternity rates of 100%, 83%, and 76% among men receiving two, three, and four cycles, respectively (176). Similarly, mean sperm counts recover as early as 12 months following two or fewer cycles and by 24 months following more than two cycles, and more men who receive high-dose cisplatin experience prolonged azoospermia (177, 178). Return to normospermia appears to be statistically significantly more likely with carboplatin than cisplatin (HR = 4.5, 95% CI = 2.6 to 7.8) (178).
Thyroid Cancer
131I is associated with a testicular radiation exposure of about 0.10 Gy. No data regarding effects on spermatogenesis or fertility are available (179). Given the gonadotoxicity of radiation doses less than 1 Gy (see Radiotherapy section below), fertility interest should be assessed routinely and sperm banking is prudent for patients interested in future fertility.
Hodgkin and Non-Hodgkin Lymphoma
Most male HL survivors will have normal fertility following treatment. Within a case-control study, 29.3% of survivors had biological children post-treatment relative to 32.4% of age-matched control subjects (OR = 0.84, 95% CI = 0.70 to 1.00) (14). Among 345 male survivors who attempted pregnancy, 217 (63%) conceived naturally and 49 (15%) with ART. Second line treatment (OR = 0.10, 95% CI = 0.04 to 0.24) and age older than 35 years (OR = 0.16, 95% CI = 0.07 to 0.36) were associated with lower fecundity. Alkylating chemotherapy was associated with a dose-dependent effect (OR = 0.34, 95% CI = 0.16 to 0.70 for <3 MOPP-equivalent cycles; OR = 0.04, 95% CI = 0.02 to 0.09 for >3 MOPP-equivalent cycles).
Over the past several years, therapy has shifted away from regimens heavy in alkylator exposure and towards ABVD. A prospective study that included HL and NHL found that no patients treated with ABVD were azoospermic 1 year post-treatment, with higher rates following CHOP (22%) and BEACOPP (75%) (153). Two years after treatment, sperm counts recovered to normal in 90% following ABVD and only 61% following CHOP. Other studies support the low rate of azoospermia with ABVD (4%) and high rates with BEACOPP (89%) (180–182). The variability in risk between regimens is substantial. Most adult HL patients in the United States are now treated with ABVD and their risk of infertility is low, whereas risk is intermediate or high for those receiving BEACOPP and regimens for aggressive NHL. All men should be encouraged to bank sperm at diagnosis, but opportunities to bank may still exist in early survivorship or in a relapsed setting before re-initiating therapy.
Leukemia
Limited data are available on risks to fertility among men receiving chemotherapy for acute leukemias. Patients with AML and ALL have been grouped together, despite differences in treatments, including in use of alkylating agents. One series of 13 patients with acute leukemias reported azoospermia in 46% (183), and two of five patients in another series treated with daunarubicin and cytarabine developed severe oligospermia (184). It seems likely that some patients experience at least transient azoospermia, even if some retain or recover spermatogenesis (185). Given the uncertainty, all male patients should be offered sperm banking before treatment, particularly given the effect of HCT on fertility.
Pregnancies have been identified in female partners of males on imatinib therapy for chronic myeloid leukemia, though data are insufficient to conclude whether imatinib is associated with congenital abnormalities in offspring of males (108). The risk of age-related loss of fertility due to delayed conception is less statistically significant for males because interrupting treatment for conception may be more feasible for males than females as only a washout period before conception might be recommended, whereas females are recommended to also hold treatment through the duration of a pregnancy. Although one study used a washout period of 1 month from imatinib (186), spermatogenesis occurs over an estimated 74 days and the minimum safe duration remains unknown (187).
Hematopoietic Cell Transplantation
Conditioning chemotherapy and radiation frequently lead to severe impairment of male fertility. Fewer than 30% of men recover spermatogenesis and normal testicular function following HCT, with the lower rates of recovery with greater gonadotoxic exposures (118, 188–192). The birth rate among male HCT survivors is statistically significantly lower than the general population, though pregnancies following heavily gonadotoxic conditioning regimens such as BuCy or BEAM and TBI have been reported (114–116, 156). In a retrospective study of 327 adult male HCT survivors with median follow-up of 7.7 years, 26 (8%) had sired a pregnancy, with similar rates following allogeneic and autologous transplants (116). Despite very low pregnancy rates, risk appears to vary by exposures. TBI, older age (>30 years), and chronic GVHD have been associated with impaired spermatogenesis (116, 117, 190). Higher rates of detectable sperm have been found following lower dose BuCy (cyclophosphamide 120 mg/kg) than conventional dosing (cyclophosphamide 200 mg/kg) (81% vs <20) (118, 193). Because no patient is at low risk, sperm banking should be recommended for all pubertal males before HCT.
Radiotherapy
The testes are sensitive to small radiation exposures, and the effect on spermatogenesis depends upon gonadal dose and radiation schedule (194). Oligospermia occurs following greater than 1 Gy of testicular radiation, and exposures of 4–6 Gy yield profound impairments in spermatogenesis (195). The testes may be exposed to dose scatter in abdominal and pelvic radiation for rectal cancer, testicular cancer, and other malignancies. Abdominal radiation following unilateral orchiectomy for testis cancer yielded exposure to the remaining testicle of 0.09 and 0.32 Gy with paraaortic and dog-leg fields, respectively, in one series, although testicular shields may reduce exposure (196, 197). Among men treated with radiation (0.28–0.9 Gy) following unilateral orchiectomy for testicular seminoma, sperm counts recover following treatment, most within 1 year (148). Radiation therapy for HL may be associated with testicular exposure of 0.06–0.7 Gy and lead to transient oligospermia, but spermatogenesis appears to normalize within 18 months (198). Similarly, among men treated for rectal cancer, exposures under 1.3 Gy lead to transient azoospermia in greater than 70%, but almost all patients recover spermatogenesis (199).
Gonadal exposure of 10 Gy in TBI yields azoospermia in more than 80% of patients, though concurrent alkylating chemotherapy may also contribute (118). Male childhood cancer survivors who receive more than 7.5 Gy are profoundly less likely to sire a pregnancy (HR = 0.12, 95% CI = 0.02 to 0.64), and one study found no pregnancies following more than 10 Gy for childhood ALL (163, 200).
High-dose PCI (>24 Gy) is associated with diminished fertility among adult male survivors of childhood ALL. One analysis demonstrated impaired fertility for survivors treated with 24 Gy at ages younger than 10 years (RR = 0.09, 95% CI = 0.01 to 0.82) and 10 years or older (RR = 0.54, 95% CI = 0.25 to 1.28) (165). Within the CCSS, a statistically nonsignificant trend was identified following 0–40 Gy of hypothalamic/pituitary radiation (HR = 0.52, 95% CI = 0.13 to 2.16) and more than 40 Gy (HR = 0.29, 95% CI = 0.06 to 1.28) (163). Modern protocols now tailor PCI use to risk (CNS leukemia, slower early responders) and use lower doses of 12–18 Gy, which do not appear to affect spermatogenesis (201, 202).
Conclusion
Young cancer survivors face wide variation in fertility risk attributable to age at diagnosis, disease, and treatment. Nevertheless, there is a need for additional biomarkers to improve prediction of impaired fertility, with emerging data suggesting that measures of ovarian reserve such as AMH may add value. Future studies will need to assess the risks of modern treatment regimens, including potential impact of targeted and immune-based therapies, and the role of ART on pregnancy rates in survivor populations. Recognizing the importance of survivorship issues, clinical trials should aim to incorporate patient-reported outcome measures to collect long-term fertility data. Understanding what is known and what is unknown about fertility risks is needed in order to counsel patients optimally regarding situations in which fertility preservation strategies may be needed and when patients can feel confident foregoing them. Newly diagnosed young patients with cancer and survivors may also benefit from the development and incorporation of counseling tools and guidelines for referral to oncofertility specialists.
Funding
The authors have no funding sources to report.
Notes
Affiliations of authors: Dana-Farber Cancer Institute (PDP, ASL, AHP); Dana-Farber Cancer Institute and Boston Children’s Hospital (ALF, AMF, PEM, LRD); Brigham and Women’s Hospital (ESG); Boston Children’s Hospital and Brigham and Women’s Hospital (MRL).
References
- 1. Marzorati C, Riva S, Pravettoni G.. Who is a cancer survivor? A systematic review of published definitions. J Cancer Educ. 2017;32(2):228–237. [DOI] [PubMed] [Google Scholar]
- 2. Ethics Committee of the American Society of Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: an Ethics Committee opinion. Fertility and Sterility. 2018;110(6):380–386. [DOI] [PubMed] [Google Scholar]
- 3. Letourneau JM, Ebbel EE, Katz PP.. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118(6):1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jeruss JS, Woodruff TK.. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360(9):902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dillon KE, Gracia CR.. Pediatric and young adult patients and oncofertility. Curr Treat Options Oncol. 2012;13(2):161–173. [DOI] [PubMed] [Google Scholar]
- 6. Lambertini M, Del Mastro L, Pescio MC.. Cancer and fertility preservation: international recommendations from an expert meeting. BMC Med. 2016;14(1):1–16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH.. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol. 2015;3(7):556–567. [DOI] [PubMed] [Google Scholar]
- 8. Font-Gonzalez A, Mulder RL, Loeffen EAH.. Fertility preservation in children, adolescents, and young adults with cancer: quality of clinical practice guidelines and variations in recommendations. Cancer. 2016;122(14):2216–2223. [DOI] [PubMed] [Google Scholar]
- 9. Peccatori FA, Azim HA Jr, Orecchia R.. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi160–vi170. [DOI] [PubMed] [Google Scholar]
- 10. Practice Committee of American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2013;100(5):1214–1223. [DOI] [PubMed] [Google Scholar]
- 11. van Dorp W, van den Heuvel-Eibrink MM, Stolk L.. Genetic variation may modify ovarian reserve in female childhood cancer survivors. Hum Reprod. 2013;28(4):1069–1076. [DOI] [PubMed] [Google Scholar]
- 12. Su HI, Sammel MD, Velders L.. Association of cyclophosphamide drug-metabolizing enzyme polymorphisms and chemotherapy-related ovarian failure in breast cancer survivors. Fertil Steril. 2010;94(2):645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reimer T, Kempert S, Gerber B, Thiesen HJ, Hartmann S, Koczan D.. SLCO1B1*5 polymorphism (rs4149056) is associated with chemotherapy-induced amenorrhea in premenopausal women with breast cancer: a prospective cohort study. BMC Cancer. 2016;16(337):337.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Kaaij MA, Heutte N, Meijnders P.. Parenthood in survivors of Hodgkin lymphoma: an EORTC-GELA general population case-control study. J Clin Oncol. 2012;30(31):3854–3863. [DOI] [PubMed] [Google Scholar]
- 15. Letourneau JM, Ebbel EE, Katz PP.. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer. 2012;118(7):1933–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacobson MH, Mertens AC, Spencer JB, Manatunga AK, Howards PP.. Menses resumption after cancer treatment-induced amenorrhea occurs early or not at all. Fertil Steril. 2016;105(3):765–772 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Partridge AH, Ruddy KJ, Gelber S, et al. Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril. 2010;94(2):638–644. [DOI] [PubMed] [Google Scholar]
- 18. Gracia CR, Sammel MD, Freeman E, et al. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012;97(1):134–140.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN.. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab. 2003;88(11):5307–5314. [DOI] [PubMed] [Google Scholar]
- 20. Burger HG, Dudley EC, Hopper JL, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J lin Endocrinol Metab. 1999;84(11):4025–4030. [DOI] [PubMed] [Google Scholar]
- 21. Fanchin R. Serum anti-Mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18(2):323–327. [DOI] [PubMed] [Google Scholar]
- 22. Kevenaar ME, Meerasahib MF, Kramer P, et al. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology. 2006;147(7):3228–3234. [DOI] [PubMed] [Google Scholar]
- 23. Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370–385. [DOI] [PubMed] [Google Scholar]
- 24. Bath LE. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum Reprod. 2003;18(11):2368–2374. [DOI] [PubMed] [Google Scholar]
- 25. Decanter C, Morschhauser F, Pigny P, Lefebvre C, Gallo C, Dewailly D.. Anti-Mullerian hormone follow-up in young women treated by chemotherapy for lymphoma: preliminary results. Reprod Biomed Online. 2010;20(2):280–285. [DOI] [PubMed] [Google Scholar]
- 26. Lunsford AJ, Whelan K, McCormick K, McLaren JF.. Antimullerian hormone as a measure of reproductive function in female childhood cancer survivors. Fertil Steril. 2014;101(1):227–231. [DOI] [PubMed] [Google Scholar]
- 27. Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2009;27(16):2677–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barton SE, Najita JS, Ginsburg ES, et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2013;14(9):873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98(13):890–896. [DOI] [PubMed] [Google Scholar]
- 30. Chow EJ, Stratton KL, Leisenring WM, et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2016;17(5):567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thomas-Teinturier C, Allodji RS, Svetlova E, et al. Ovarian reserve after treatment with alkylating agents during childhood. Hum Reprod. 2015;30(6):1437–1446. [DOI] [PubMed] [Google Scholar]
- 32. Akar B, Doğer E, Çakıroğlu Y, Çorapçıoğlu F, Sarper N, Çalışkan E.. The effect of childhood cancer therapy on ovarian reserve and pubertal development. Reprod Biomed Online. 2015;30(2):175–180. [DOI] [PubMed] [Google Scholar]
- 33. Thomas-Teinturier C, El Fayech C, Oberlin O, et al. Age at menopause and its influencing factors in a cohort of survivors of childhood cancer: earlier but rarely premature. Hum Reprod. 2013;28(2):488–495. [DOI] [PubMed] [Google Scholar]
- 34. Chiarelli AM, Marrett LD, Darlington G.. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964-1988 in Ontario, Canada. Am J Epidemiol. 1999;150(3):245–254. [DOI] [PubMed] [Google Scholar]
- 35. Byrne J, Mulvihill JJ, Myers MH, et al. Effects of treatment on fertility in long-term survivors of childhood or adolescent cancer. N Engl J Med. 1987;317(21):1315–1321. [DOI] [PubMed] [Google Scholar]
- 36. van Dorp W, Mulder RL, Kremer LCM, et al. Recommendations for premature ovarian insufficiency surveillance for female survivors of childhood, adolescent, and young adult cancer: a report from the international late effects of childhood cancer guideline harmonization group in collaboration with the pancaresurfup consortium. J Clin Oncol. 2016;34(28):3440–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lawrenz B, Fehm T, von Wolff M, et al. Reduced pretreatment ovarian reserve in premenopausal female patients with Hodgkin lymphoma or non-Hodgkin-lymphoma--evaluation by using antimullerian hormone and retrieved oocytes. Fertil Steril. 2012;98(1):141–144. [DOI] [PubMed] [Google Scholar]
- 38. Brämswig JH, Riepenhausen M, Schellong G.. Parenthood in adult female survivors treated for Hodgkin's lymphoma during childhood and adolescence: a prospective, longitudinal study. Lancet Oncol. 2015;16(6):667–675. [DOI] [PubMed] [Google Scholar]
- 39. Lambertini M, Moore HCF, Leonard RCF, et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient–level data. J Clin Oncol. 2018;36(19):1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol. 2006;24(7):1045–1051. [DOI] [PubMed] [Google Scholar]
- 41. Sukumvanich P, Case LD, Van Zee K, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: a prospective study. Cancer. 2010;116(13):3102–3111. [DOI] [PubMed] [Google Scholar]
- 42. Fornier MN, Modi S, Panageas KS, Norton L, Hudis C.. Incidence of chemotherapy-induced, long-term amenorrhea in patients with breast carcinoma age 40 years and younger after adjuvant anthracycline and taxane. Cancer. 2005;104(8):1575–1579. [DOI] [PubMed] [Google Scholar]
- 43. Han HS, Ro J, Lee KS, et al. Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Res Treat. 2009;115(2):335–342. [DOI] [PubMed] [Google Scholar]
- 44. Lower EE, Blau R, Gazder P, Tummala R.. The risk of premature menopause induced by chemotherapy for early breast cancer. J Womens Health Gen Bas Med. 1999;8(7):949–954. [DOI] [PubMed] [Google Scholar]
- 45. Okanami Y, Ito Y, Watanabe C, et al. Incidence of chemotherapy-induced amenorrhea in premenopausal patients with breast cancer following adjuvant anthracycline and taxane. Breast Cancer. 2011;18(3):182–188. [DOI] [PubMed] [Google Scholar]
- 46. Yoo C, Yun MR, Ahn JH, et al. Chemotherapy-induced amenorrhea, menopause-specific quality of life, and endocrine profiles in premenopausal women with breast cancer who received adjuvant anthracycline-based chemotherapy: a prospective cohort study. Cancer Chemother Pharmacol. 2013;72(3):565–575. [DOI] [PubMed] [Google Scholar]
- 47. Abusief ME, Missmer SA, Ginsburg ES, Weeks JC, Partridge AH.. The effects of paclitaxel, dose density, and trastuzumab on treatment-related amenorrhea in premenopausal women with breast cancer. Cancer. 2010;116(4):791–798. [DOI] [PubMed] [Google Scholar]
- 48. Ganz PA, Land SR, Geyer CE Jr, et al. Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial. J Clin Oncol. 2011;29(9):1110–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ruddy KJ, Guo H, Barry W, et al. Chemotherapy-related amenorrhea after adjuvant paclitaxel-trastuzumab (APT trial). Breast Cancer Res Treat. 2015;151(3):589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poorvu PD, Gelber SI, Rosenberg SM, et al. Treatment-related amenorrhea among young women one year following diagnosis of early-stage breast cancer. J Clin Oncol. 2015;33:Suppl Abstract 9523. [Google Scholar]
- 51. Najafi S, Djavid GE, Mehrdad N, et al. Taxane-based regimens as a risk factor for chemotherapy-induced amenorrhea. Menopause. 2011;18(2):208–212. [DOI] [PubMed] [Google Scholar]
- 52. Tham YL, Sexton K, Weiss J, Elledge R, Friedman LC, Kramer R.. The rates of chemotherapy-induced amenorrhea in patients treated with adjuvant doxorubicin and cyclophosphamide followed by a taxane. Am J Clin Oncol. 2007;30(2):126–132. [DOI] [PubMed] [Google Scholar]
- 53. Minisini AM, Menis J, Valent F, et al. Determinants of recovery from amenorrhea in premenopausal breast cancer patients receiving adjuvant chemotherapy in the taxane era. Anticancer Drugs. 2009;20(6):503–507. [DOI] [PubMed] [Google Scholar]
- 54. Reh A, Oktem O, Oktay K.. Impact of breast cancer chemotherapy on ovarian reserve: a prospective observational analysis by menstrual history and ovarian reserve markers. Fertil Steril. 2008;90(5):1635–1639. [DOI] [PubMed] [Google Scholar]
- 55. Lambertini M, Ceppi M, Cognetti F, et al. Dose-dense adjuvant chemotherapy, treatment-induced amenorrhea and overall survival in premenopausal breast cancer patients: a pooled analysis of the MIG1 and GIM2 phase III studies. Eur J Cancer. 2017;71:34–42. [DOI] [PubMed] [Google Scholar]
- 56. Meirow D, Nugent D.. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7(6):535–543. [DOI] [PubMed] [Google Scholar]
- 57. Maneschi F, Benedetti-Panici P, Scambia G, Salerno G, D'Agostino G, Mancuso S.. Menstrual and hormone patterns in women treated with high-dose cisplatin and bleomycin. Gynecol Oncol. 1994;54(3):345–348. [DOI] [PubMed] [Google Scholar]
- 58. Low JJ, Perrin LC, Crandon AJ, Hacker NF.. Conservative surgery to preserve ovarian function in patients with malignant ovarian germ cell tumors. Cancer. 2000;89(2):391–398. [PubMed] [Google Scholar]
- 59. Sharma P, Rock L, Kimler BF, et al. Chemotherapy-induced amenorrhea (CIA) risk associated with taxane/platinum-based chemotherapy in young (≤45 years) breast cancer patients. J Clin Oncol. 2014;32(suppl) (9592): [Google Scholar]
- 60. Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N.. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17(8):2365–2370. [DOI] [PubMed] [Google Scholar]
- 61. Swain SM, Jeong J, Geyer CE Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362(22):2053–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Francis PA, Pagani O, Fleming GF, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Giacalone P, Laffargue F, Benos P, Dechaud H, Hedon B.. Successful in vitro fertilization-surrogate pregnancy in a patient with ovarian transposition who had undergone chemotherapy and pelvic radiation. Fertil Steril. 2001;76(2):388–389. [DOI] [PubMed] [Google Scholar]
- 65. Zinger M, Liu JH, Husseinzadeh N, Thomas MA.. Successful surrogate pregnancy after ovarian transposition, pelvic irradiation, and hysterectomy. J Reprod Med. 2004;49(2):573–574. [PubMed] [Google Scholar]
- 66. Steigrad S, Hacker NF, Kolb B.. In vitro fertilization surrogate pregnancy in a patient who underwent radical hysterectomy followed by ovarian transposition, lower abdominal wall radiotherapy, and chemotherapy. Fertil Steril. 2005;83:1547–1549. [DOI] [PubMed] [Google Scholar]
- 67.Cervical Cancer Version 1. 2017. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Accessed December 29, 2016.
- 68. Johansen G, Lonnerfors C, Falconer H, Persson J.. Reproductive and oncologic outcome following robot-assisted laparoscopic radical trachelectomy for early stage cervical cancer. Gynecol Oncol. 2016;141(1):160–165. [DOI] [PubMed] [Google Scholar]
- 69. Shepherd JH, Spencer C, Herod J, Ind TE.. Radical vaginal trachelectomy as a fertility-sparing procedure in women with early-stage cervical cancer-cumulative pregnancy rate in a series of 123 women. BJOG: Int J O&G. 2006;113(6):719–724. [DOI] [PubMed] [Google Scholar]
- 70. Kasuga Y, Nishio H, Miyakoshi K, et al. Pregnancy outcomes after abdominal radical trachelectomy for early-stage cervical cancer: a 13-year experience in a single tertiary care center. Int J Gynecol Cancer. 2016;26(1):163–168. [DOI] [PubMed] [Google Scholar]
- 71. Plante M, Gregoire J, Renaud MC, Roy M.. The vaginal radical trachelectomy: an update of a series of 125 cases and 106 pregnancies. Gynecol Oncol. 2011;121(2):290–297. [DOI] [PubMed] [Google Scholar]
- 72. Kim CH, Abu-Rustum NR, Chi DS, et al. Reproductive outcomes of patients undergoing radical trachelectomy for early-stage cervical cancer. Gynecol Oncol. 2012;125(3):585–588. [DOI] [PubMed] [Google Scholar]
- 73. Pareja R, Rendon GJ, Sanz-Lomana CM, Monzon O, Ramirez PT.. Surgical, oncological, and obstetrical outcomes after abdominal radical trachelectomy - a systematic literature review. Gynecol Oncol. 2013;131(1):77–82. [DOI] [PubMed] [Google Scholar]
- 74. Speiser D, Mangler M, Kohler C, et al. Fertility outcome after radical vaginal trachelectomy: a prospective study of 212 patients. Int J Gynecol Cancer. 2011;21(9):1635–1639. [DOI] [PubMed] [Google Scholar]
- 75. Tokunaga H, Watanabe Y, Niikura H, et al. Outcomes of abdominal radical trachelectomy: results of a multicenter prospective cohort study in a Tohoku Gynecologic Cancer Unit. Int J Clin Oncol. 2015;20(4):776–780. [DOI] [PubMed] [Google Scholar]
- 76. Wethington SL, Cibula D, Duska LR, et al. An international series on abdominal radical trachelectomy: 101 patients and 28 pregnancies. Int J Gynecol Cancer. 2012;22(7):1251–1257. [DOI] [PubMed] [Google Scholar]
- 77. Okugawa K, Kobayashi H, Sonoda K, et al. Oncologic and obstetric outcomes and complications during pregnancy after fertility-sparing abdominal trachelectomy for cervical cancer: a retrospective review. Int J Clin Oncol. 2017;22(2):340–346. [DOI] [PubMed] [Google Scholar]
- 78. Bentivegna E, Maulard A, Pautier P, Chargari C, Gouy S, Morice P.. Fertility results and pregnancy outcomes after conservative treatment of cervical cancer: a systematic review of the literature. Fertil Steril. 2016;106(5):1195–1211.e5. [DOI] [PubMed] [Google Scholar]
- 79. Robova H, Halaska MJ, Pluta M, et al. Oncological and pregnancy outcomes after high-dose density neoadjuvant chemotherapy and fertility-sparing surgery in cervical cancer. Gynecol Oncol. 2014;135(2):213–216. [DOI] [PubMed] [Google Scholar]
- 80. Huang KG, Lee CL, Tsai CS, Han CM, Hwang LL.. A new approach for laparoscopic ovarian transposition before pelvic irradiation. Gynecol Oncol. 2007;105(1):234–237. [DOI] [PubMed] [Google Scholar]
- 81.Uterine Neoplasms Version 1. 2017. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed December 29, 2016.
- 82. Park J-Y, Seong SJ, Kim T-J, et al. Pregnancy outcomes after fertility-sparing management in young women with early endometrial cancer. Obstet Gynecol. 2013;121(1):136–142. [DOI] [PubMed] [Google Scholar]
- 83. Hahn HS, Yoon SG, Hong SR, et al. Conservative treatment with progestin and pregnancy outcomes in endometrial cancer. Int J Gynecol Cancer. 2009;19(6):1068–1073. [DOI] [PubMed] [Google Scholar]
- 84.Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer Version 1. 2016. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 29, 2016.
- 85. Satoh T, Hatae M, Watanabe Y, et al. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: a proposal for patient selection. J Clin Oncol. 2010;28(10):1727–1732. [DOI] [PubMed] [Google Scholar]
- 86. Ditto A, Martinelli F, Lorusso D, Haeusler E, Carcangiu M, Raspagliesi F.. Fertility sparing surgery in early stage epithelial ovarian cancer. J Gynecol Oncol. 2014;25(4):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hu J, Zhu LR, Liang ZQ, et al. Clinical outcomes of fertility-sparing treatments in young patients with epithelial ovarian carcinoma. J Zhejiang Univ Sci B. 2011;12(10):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Eskander RN, Randall LM, Berman ML, Tewari KS, Disaia PJ, Bristow RE.. Fertility preserving options in patients with gynecologic malignancies. Am J Obstet Gynecol. 2011;205(2):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vini L, Hyer S, Al-Saadi A, Pratt B, Harmer C.. Prognosis for fertility and ovarian function after treatment with radioiodine for thyroid cancer. Postgrad Med J. 2002;78(916):92–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ceccarelli C, Bencivelli W, Morciano D, Pinchera A, Pacini F.. 131I therapy for differentiated thyroid cancer leads to an earlier onset of menopause: results of a retrospective study. J Clin Endocrinol Metab. 2001;86(8):3512–3515. [DOI] [PubMed] [Google Scholar]
- 91. Dottorini ME, Lomuscio G, Mazzucchelli L, Vignati A, Colombo L.. Assessment of female fertility and carcinogenesis after Iodine-131 therapy for differentiated thyroid carcinoma. J Nucl Med. 1995;36(1):21–27. [PubMed] [Google Scholar]
- 92. Sawka AM, Lakra DC, Lea J, et al. A systematic review examining the effects of therapeutic radioactive iodine on ovarian function and future pregnancy in female thyroid cancer survivors. Clin Endocrinol (Oxf). 2008;69(3):479–490. [DOI] [PubMed] [Google Scholar]
- 93. Lee SL. Complications of radioactive iodine treatment of thyroid carcinoma. J Natl Compr Canc Netw. 2010;8(11):1277–1286. [DOI] [PubMed] [Google Scholar]
- 94. Anderson C, Engel SM, Weaver MA, Zevallos JP, Nichols HB.. Birth rates after radioactive iodine treatment for differentiated thyroid cancer. Int J Cancer. 2017;141(11):2291–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.SEER Cancer Statistics Review, 1975–2013. 2016. Accessed October 27, 2016.
- 96. Behringer K, Breuer K, Reineke T, et al. Secondary amenorrhea after Hodgkin's lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin's Lymphoma Study Group. J Clin Oncol. 2005;23(30):7555–7564. [DOI] [PubMed] [Google Scholar]
- 97. De Bruin ML, Huisbrink J, Hauptmann M, et al. Treatment-related risk factors for premature menopause following Hodgkin lymphoma. Blood. 2008;111(1):101–108. [DOI] [PubMed] [Google Scholar]
- 98. Behringer K, Mueller H, Goergen H, et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J Clin Oncol. 2013;31(2):231–239. [DOI] [PubMed] [Google Scholar]
- 99. Boltezar L, Pintaric K, Jezersek Novakovic B.. Fertility in young patients following treatment for Hodgkin's lymphoma: a single center survey. J Assist Reprod Genet. 2016;33(3):325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. van der Kaaij MA, Heutte N, Meijnders P, et al. Premature ovarian failure and fertility in long-term survivors of Hodgkin's lymphoma: a European Organisation for Research and Treatment of Cancer Lymphoma Group and Groupe d'Etude des Lymphomes de l'Adulte Cohort Study. J Clin Oncol. 2012;30(3):291–299. [DOI] [PubMed] [Google Scholar]
- 101. Hodgson DC, Pintilie M, Gitterman L, et al. Fertility among female Hodgkin lymphoma survivors attempting pregnancy following ABVD chemotherapy. Hematol Oncol. 2007;25(1):11–15. [DOI] [PubMed] [Google Scholar]
- 102. Dann EJ, Epelbaum R, Avivi I, et al. Fertility and ovarian function are preserved in women treated with an intensified regimen of cyclophosphamide, adriamycin, vincristine and prednisone (Mega-CHOP) for non-Hodgkin lymphoma. Hum Reprod. 2005;20(8):2247–2249. [DOI] [PubMed] [Google Scholar]
- 103. Elis A, Tevet A, Yerushalmi R, et al. Fertility status among women treated for aggressive non-Hodgkin's lymphoma. Leuk Lymphoma. 2006;47(4):623–627. [DOI] [PubMed] [Google Scholar]
- 104. Chakravarty EF, Murray ER, Kelman A, Farmer P.. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117(5):1499–1506. [DOI] [PubMed] [Google Scholar]
- 105. Fanning SR, Jin T, Hilden JM, et al. Chemotherapy-related amenorrhea (CRA) among premenopausal patients treated for acute leukemia. Blood. 2006;108(11):3315. [Google Scholar]
- 106. Watson M, Wheatley K, Harrison GA, et al. Severe adverse impact on sexual functioning and fertility of bone marrow transplantation, either allogeneic or autologous, compared with consolidation chemotherapy alone. Cancer. 1999;86(7):1231–1239. [DOI] [PubMed] [Google Scholar]
- 107. Lipton JH, Virro M, Solow H.. Successful pregnancy after allogeneic bone marrow transplant with embryos isolated before transplant. J Clin Oncol. 1997;15(11):3347–3349. [DOI] [PubMed] [Google Scholar]
- 108. Ault P, Kantarjian H, O'Brien S, et al. Pregnancy among patients with chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2006;24(7):1204–1208. [DOI] [PubMed] [Google Scholar]
- 109. Pye SM, Cortes J, Ault P, et al. The effects of imatinib on pregnancy outcome. Blood. 2008;111(12):5505–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Abruzzese E, Trawinska MM, Perrotti AP, De Fabritiis P.. Tyrosine kinase inhibitors and pregnancy. Mediterr J Hematol Infect Dis. 2014;6(1):2014028.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zamah AM, Mauro MJ, Druker BJ, et al. Will imatinib compromise reproductive capacity?. Oncologist. 2011;16(10):1422–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Christopoulos C, Dimakopoulou V, Rotas E.. Primary ovarian insufficiency associated with imatinib therapy. N Engl J Med. 2008;358(10):1079–1080. [DOI] [PubMed] [Google Scholar]
- 113. Mahon F-X, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–1035. [DOI] [PubMed] [Google Scholar]
- 114. Loren AW, Chow E, Jacobsohn DA, et al. Pregnancy after hematopoietic cell transplantation: a report from the late effects working committee of the Center for International Blood and Marrow Transplant Research (CIBMTR). Biol Blood Marrow Transplant. 2011;17(2):157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Salooja N, Szydlo RM, Socie G, et al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet. 2001;358(9278):271–276. [DOI] [PubMed] [Google Scholar]
- 116. Carter A, Robison LL, Francisco L, et al. Prevalence of conception and pregnancy outcomes after hematopoietic cell transplantation: report from the Bone Marrow Transplant Survivor Study. Bone Marrow Transplant. 2006;37(11):1023–1029. [DOI] [PubMed] [Google Scholar]
- 117. Joshi S, Savani BN, Chow EJ, et al. Clinical guide to fertility preservation in hematopoietic cell transplant recipients. Bone Marrow Transplant . 2014;49(4):477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sanders JE, Hawley J, Levy W, et al. Pregnancies following high-dose cyclophsophamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87(7):3045–3052. [PubMed] [Google Scholar]
- 119. Hammond C, Abrams JR, Syrjala KL.. Fertility and risk factors for elevated infertility concern in 10-year hematopoietic cell transplant survivors and case-matched controls. J Clin Oncol. 2007;25(23):3511–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Akhtar S, Youssef I, Soudy H, Elhassan TA, Rauf SM, Maghfoor I.. Prevalence of menstrual cycles and outcome of 50 pregnancies after high-dose chemotherapy and auto-SCT in non-Hodgkin and Hodgkin lymphoma patients younger than 40 years. Bone Marrow Transplant. 2015;50(12):1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lasica M, Taylor E, Bhattacharyya P, et al. Fertility in premenopausal women post autologous stem cell transplant with BEAM conditioning. Eur J Haematol. 2016;97(4):348–352. [DOI] [PubMed] [Google Scholar]
- 122. Sanders JE, Buckner CD, Amos D, et al. Ovarian function following marrow transplantation for aplastic anemia or leukemia. J Clin Oncol. 1988;6(5):813–818. [DOI] [PubMed] [Google Scholar]
- 123. Wallace WHB, Thomson AB, Kelsey TW.. The radiosensitivity of the human oocyte. Hum Reprod. 2003;18(1):117–121. [DOI] [PubMed] [Google Scholar]
- 124. Wallace WH, Anderson RA, Irvine DS.. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6(4):209–218. [DOI] [PubMed] [Google Scholar]
- 125. Chemaitilly W, Mertens AC, Mitby P, et al. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab. 2006;91(5):1723–1728. [DOI] [PubMed] [Google Scholar]
- 126. Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF.. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7(10):1342–1346. [DOI] [PubMed] [Google Scholar]
- 127. Ogilvy-Stuart AL, Shalet SM.. Effect of radiation on the human reproductive system. Environ Health Perspect. 1993;101(Suppl 2):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wan J, Gai Y, Li G, Tao Z, Zhang Z.. Incidence of chemotherapy- and chemoradiotherapy-induced amenorrhea in premenopausal women with stage II/III colorectal cancer. Clin Colorectal Cancer. 2015;14(1):31–34. [DOI] [PubMed] [Google Scholar]
- 129. Sudour H, Chastagner P, Claude L, et al. Fertility and pregnancy outcome after abdominal irradiation that included or excluded the pelvis in childhood tumor survivors. Int J Radiat Oncol Biol Phys. 2010;76(3):867–873. [DOI] [PubMed] [Google Scholar]
- 130. Teh WT, Stern C, Chander S, Hickey M.. The impact of uterine radiation on subsequent fertility and pregnancy outcomes. Biomed Res Int. 2014;2014:482968.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Critchley HO, Wallace WH, Shalet SM, Mamtora M, Higginson J, Anderson DC.. Abdominal irradiation in childhood; the potential for pregnancy. BR J Obstet Gynaecol. 1992;99(5):392–394. [DOI] [PubMed] [Google Scholar]
- 132. Larsen EC, Schmiegelow K, Rechnitzer C, Loft A, Muller J, Nyboe Andersen A.. Radiotherapy at a young age reduces uterine volume of childhood cancer survivors. Acta Obstet Gynecol Scand. 2004;83(1):96–102. [DOI] [PubMed] [Google Scholar]
- 133. Wo JY, Viswanathan AN.. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys. 2009;73(5):1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Nygaard R, Clausen N, Siimes MA, et al. Reproduction following treatment for childhood leukemia: a population-based prospective cohort study of fertility and offspring. Med Pediatr Oncol. 1991;19(6):459–466. [DOI] [PubMed] [Google Scholar]
- 135. Byrne J, Fears TR, Mills JL, et al. Fertility in women treated with cranial radiotherapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;42(7):589–597. [DOI] [PubMed] [Google Scholar]
- 136. Green DM, Nolan VG, Kawashima T, et al. Decreased fertility among female childhood cancer survivors who received 22-27 Gy hypothalamic/pituitary irradiation: a report from the Childhood Cancer Survivor Study. Fertil Steril. 2011;95(6):1922–1927, 7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Pai HH, Thornton A, Katznelson L, et al. Hypothalamic/pituitary function following high-dose conformal radiotherapy to the base of skull: demonstration of a dose-effect relationship using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2001;49(4):1079–1092. [DOI] [PubMed] [Google Scholar]
- 138. World Health Organization. World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. 5th ed Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 139. Wang C, Swerdloff RS.. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102(6):1502–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bonde JP, Ernst E, Jensen TK, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352(9135):1172–1177. [DOI] [PubMed] [Google Scholar]
- 141. Slama R, Eustache F, Ducot B, et al. Time to pregnancy and semen parameters: a cross sectional study among fertile couples from four European cities. Hum Reprod. 2002;17(2):503–515. [DOI] [PubMed] [Google Scholar]
- 142. Zinaman MJ, Brown CC, Selevan SG, Clegg ED.. Semen quality and human fertility: a prospective study with health couples. J Androl. 2000;21(1):145–153. [PubMed] [Google Scholar]
- 143. Buck Louis GM, Sundaram R, Schisterman EF, et al. Semen quality and time to pregnancy: the Longitudinal Investigation of Fertility and the Environment Study. Fertil Steril. 2014;101(2):453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Vozdova M, Oracova E, Kasikova K, et al. Balanced chromosomal translocations in men: relationships among semen parameters, chromatin integrity, sperm meiotic segregation and aneuploidy. J Assist Reprod Genet. 2013;30(3):391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pont J, Albrecht W.. Fertility after chemotherapy for testicular germ cell cancer. Fertil Steril. 1997;68(1):1–5. [DOI] [PubMed] [Google Scholar]
- 146. Fossa SD, Aass N, Molne K.. Is routine pre-treatment cryopreservation of semen worthwhile in the management of patients with testicular cancer? Br J Urol. 1989;64(5):524–529. [DOI] [PubMed] [Google Scholar]
- 147. Horwich A, Nicholls EJ, Hendry WF.. Seminal analysis after orchiectomy in stage I teratoma. Br J Urol. 1988;62(1):79–81. [DOI] [PubMed] [Google Scholar]
- 148. Berthelsen JG. Sperm counts and serum follicle-stimulating hormone levels before and after radiotherapy and chemotherapy in men with testicular germ cell cancer. Fertil Steril. 1984;41(2):281–286. [DOI] [PubMed] [Google Scholar]
- 149. Rueffer U, Breuer K, Josting A, et al. Male gonadal dysfunction in patients with Hodgkin's disease prior to treatment. Ann Oncol. 2001;12(9):1307–1311. [DOI] [PubMed] [Google Scholar]
- 150. van der Kaaij MA, Heutte N, Echten AJV, et al. Sperm quality before treatment in patients with early stage Hodgkin's lymphoma enrolled in EORTC-GELA lymphoma group trials. Haematologica. 2009;94(12):1691–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Viviani S, Ragni G, Santoro A, et al. Testicular dysfunction in Hodgkin's disease before and after treatment. Eur J Cancer. 1991;27(11):1389–1392. [DOI] [PubMed] [Google Scholar]
- 152. Hendry WF, Stedronska J, Jones CR, Blackmore CA, Barrett A, Peckham MJ.. Semen analysis in testicular cancer and Hodgkin's disease: pre- and post-treatment findings and implications for cryopreservation. Br J Urol. 1983;55(6):769–773. [DOI] [PubMed] [Google Scholar]
- 153. Bujan L, Walschaerts M, Brugnon F, et al. Impact of lymphoma treatments on spermatogenesis and sperm deoxyribonucleic acid: a multicenter prospective study from the CECOS network. Fertil Steril. 2014;102(3):667–674.e3. [DOI] [PubMed] [Google Scholar]
- 154. Fosså SD, Theodorsen L, Norman N, Aabyholm T.. Recovery of impaired pretreatment spermatogenesis in testicular cancer. Fertil Steril. 1990;54(3):493–496. [DOI] [PubMed] [Google Scholar]
- 155. Jacobsen KD, Theodorsen L, Fossa SD.. Spermatogenesis after unilateral orchiectomy for testicular cancer in patients following surveillance policy. J Urol. 2001;165(1):93–96. [DOI] [PubMed] [Google Scholar]
- 156. Socie G, Salooja N, Cohen A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101(9):3373–3385. [DOI] [PubMed] [Google Scholar]
- 157. Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril. 2013;100(5):1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Meistrich ML, Wilson G, Brown BW, De Cunha MF, Lipschultz LI.. Impact of cyclophosphamide on long-term reduction in sperm count in men treated with combination chemotherapy for ewing and soft tissue sarcomas. Cancer. 1992;70(11):2703–2712. [DOI] [PubMed] [Google Scholar]
- 159. Hsiao W, Stahl PJ, Osterberg EC, et al. Successful treatment of postchemotherapy azoospermia with microsurgical testicular sperm extraction: the Weill Cornell experience. J Clin Oncol. 2011;29(12):1607–1611. [DOI] [PubMed] [Google Scholar]
- 160. Shin T, Kobayashi T, Shimomura Y, et al. Microdissection testicular sperm extraction in Japanese patients with persistent azoospermia after chemotherapy. Int J Clin Oncol. 2016;21(6):1167–1171. [DOI] [PubMed] [Google Scholar]
- 161. Green DM, Liu W, Kutteh WH, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. Lancet Oncol. 2014;15(11):1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wasilewski-Masker K, Seidel KD, Leisenring W, et al. Male infertility in long-term survivors of pediatric cancer: a report from the childhood cancer survivor study. J Cancer Surviv. 2014;8(3):437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Green DM, Kawashima T, Stovall M, et al. Fertility of male survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2010;28(2):332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61(1):53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Byrne J, Fears TR, Mills JL, et al. Fertility of long-term male survivors of acute lymphoblastic leukemia diagnosed during childhood. Pediatr Blood Cancer. 2004;42(4):364–372. [DOI] [PubMed] [Google Scholar]
- 166. Rivkees SA, Crawford JD.. The relationship of gonadal activity and chemotherapy-induced gonadal damage. JAMA. 1988;259(14):2123–2125. [PubMed] [Google Scholar]
- 167. Petersen PM, Skakkebaek NE, Rorth M, Giwercman A.. Semen quality and reproductive hormones before and after orchiectomy in men with testicular cancer. J Urol. 1999;161(3):822–826. [PubMed] [Google Scholar]
- 168. Che M, Tamboli P, Ro JY, et al. Bilateral testicular germ cell tumors: twenty-year experience at M. D. Anderson Cancer Center. Cancer. 2002;95(6):1228–1233. [DOI] [PubMed] [Google Scholar]
- 169. Fossa SD, Ous S, Abyholm T, Loeb M.. Post-treatment fertility in patients with testicular cancer. Br J Urol. 1985;57(2):204–209. [DOI] [PubMed] [Google Scholar]
- 170. Nijman JM, Schraffordt Koops H, Oldhoff J, Kremer J, Jager S.. Sexual function after bilateral retroperitoneal lymph node dissection for nonseminomatous testicular cancer. Arch Androl. 1987;18(3):255–267. [DOI] [PubMed] [Google Scholar]
- 171. Bhayani SB, Ong A, Oh WK, Kantoff PW, Kavoussi LR.. Laparoscopic retroperitoneal lymph node dissection for clinical stage I nonseminomatous germ cell testicular cancer: a long-term update. Urology. 2003;62(2):324–327. [DOI] [PubMed] [Google Scholar]
- 172. Kenney PA, Tuerk IA.. Complications of laparoscopic retroperitoneal lymph node dissection in testicular cancer. World J Urol. 2008;26(6):561–569. [DOI] [PubMed] [Google Scholar]
- 173. Permpongkosol S, Lima GC, Warlick CA, et al. Postchemotherapy laparoscopic retroperitoneal lymph node dissection: evaluation of complications. Urology. 2007;69(2):361–365. [DOI] [PubMed] [Google Scholar]
- 174. Kim C, McGlynn KA, McCorkle R, et al. Fertility among testicular cancer survivors: a case-control study in the U.S. J Cancer Surviv. 2010;4(3):266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Brydoy M, Fossa SD, Klepp O, et al. Paternity following treatment for testicular cancer. J Natl Cancer Inst. 2005;97(21):1580–1588. [DOI] [PubMed] [Google Scholar]
- 176. Brydoy M, Fossa SD, Klepp O, et al. Paternity and testicular function among testicular cancer survivors treated with two to four cycles of cisplatin-based chemotherapy. Eur Urol. 2010;58(1):134–140. [DOI] [PubMed] [Google Scholar]
- 177. Bujan L, Walschaerts M, Moinard N, et al. Impact of chemotherapy and radiotherapy for testicular germ cell tumors on spermatogenesis and sperm DNA: a multicenter prospective study from the CECOS network. Fertil Steril. 2013;100(3):673–680. [DOI] [PubMed] [Google Scholar]
- 178. Lampe H, Horwich A, Norman J, Nicholls J, Dearnaley DP.. Fertility after chemotherapy for testicular germ cell cancer. J Clin Oncol. 1997;68(1):1–5. [DOI] [PubMed] [Google Scholar]
- 179. Ceccarelli C, Canale D, Vitti P.. Radioactive iodine (131I) effects on male fertility. Curr Opin Urol. 2008;18(6):598–601. [DOI] [PubMed] [Google Scholar]
- 180. Kulkarni SS, Sastry PS, Saikia TK, Parikh PM, Gopal R, Advani SH.. Gonadal function following ABVD therapy for Hodgkin's disease. Am J Clin Oncol. 1997;20(4):354–357. [DOI] [PubMed] [Google Scholar]
- 181. Sieniawski M, Reineke T, Nogova L, et al. Fertility in male patients with advanced Hodkin lymphoma treated with BEACOPP: a report of the German Hodgkin Study Group (GHSG). Blood. 2008;111(1):71–76. [DOI] [PubMed] [Google Scholar]
- 182. Pryzant RM, Meistrich ML, Wilson G, Brown B, McLaughlin P.. Long-term reduction in sperm count after chemotherapy with and without radiation therapy for non-Hodgkin's lymphomas. J Clin Oncol. 1993;11(2):239–247. [DOI] [PubMed] [Google Scholar]
- 183. Bahadur G, Ozturk O, Muneer A, et al. Semen quality before and after gonadotoxic treatment. Hum Reprod. 2005;20(3):774–781. [DOI] [PubMed] [Google Scholar]
- 184. Lemez P, Urbanek V.. Cryopreservation of sperm from patients with leukemia: is it worth the effort? Cancer. 1999;86(10):2184–2185. [DOI] [PubMed] [Google Scholar]
- 185. Faderl S, Leukemias KH, Faderl S, Kantarjian H, Leukemias. Principles and Practice of Therapy, First edition. West Sussex, UK: Blackwell Publishing Ltd; 2011. [Google Scholar]
- 186. Mukhopadhyay A, Dasgupta S, Kanti Ray U, Gharami F, Bose CK, Mukhopadhyay S.. Pregnancy outcome in chronic myeloid leukemia patients on imatinib therapy. Ir J Med Sci. 2015;184(1):183–188. [DOI] [PubMed] [Google Scholar]
- 187. Heller CH, Clermont Y.. Kinetics of the germinal epithelium in man. Recent Prog Horm Res. 1964;20:545–575. [PubMed] [Google Scholar]
- 188. Jacob A, Barker H, Goodman A, Holmes J.. Recovery of spermatogenesis following bone marrow transplantation. Bone Marrow Transplant. 1998;22(3):277–279. [DOI] [PubMed] [Google Scholar]
- 189. Anserini P, Chiodi S, Spinelli S, et al. Semen analysis following allogeneic bone marrow transplantation. Additional data for evidence-based counselling. Bone Marrow Transplant. 2002;30(7):447–451. [DOI] [PubMed] [Google Scholar]
- 190. Rovo A. Spermatogenesis in long-term survivors after allogeneic hematopoietic stem cell transplantation is associated with age, time interval since transplantation, and apparently absence of chronic GvHD. Blood. 2006;108(3):1100–1105. [DOI] [PubMed] [Google Scholar]
- 191. Savani BN, Kozanas E, Shenoy A, Barrett AJ.. Recovery of spermatogenesis after total-body irradiation. Blood. 2006;108(13):4292.. [DOI] [PubMed] [Google Scholar]
- 192. Mertens AC, Ramsay NKC, Kouris S, Neglia JP.. Patterns of gonadal dysfunction following bone marrow transplantation. Bone Marrow Transplant. 1998;22(4):345–350. [DOI] [PubMed] [Google Scholar]
- 193. Grigg AP, McLachlan R, Zajac J, Sjer J.. Reproductive status in long-term bone marrow transplant survivors receiving busulfan-cyclophosphamide (120 mg/kg). Bone Marrow Transplant. 2000;26(10):1089–1095. [DOI] [PubMed] [Google Scholar]
- 194. Wallace WH, Thomson AB, Saran F, Kelsey TW.. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62(3):738–744. [DOI] [PubMed] [Google Scholar]
- 195. Rowley MJ, Leach DR, Warner GA, Heller CG.. Effect of graded doses of ionizing radiation on the human testis. Radiat Res. 1974;59(3):665–678. [PubMed] [Google Scholar]
- 196. Jacobsen KD, Olsen DR, Fossa K, Fossa SD.. External beam abdominal radiotherapy in patients with seminoma stage I: field type, testicular dose, and spermatogenesis. Int J Radiat Oncol Biol Phys. 1997;38(1):95–102. [DOI] [PubMed] [Google Scholar]
- 197. Ravichandran R, Binukumar JP, Kannadhasan S, Shariff MH, Ghamrawy KE.. Testicular shield for para-aortic radiotherapy and estimation of gonad doses. J Med Phys. 2008;33(4):158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kinsella TJ, Trivette G, Rowland J, et al. Long-term follow-up of testicular function following radiation therapy for early-stage Hodgkin's disease. J Clin Oncol. 1989;7(6):718–724. [DOI] [PubMed] [Google Scholar]
- 199. Centola GM, Keller JW, Henzler M, Rubin P.. Effect of low-dose testicular irradiation on sperm count and fertility in patients with testicular seminoma. J Androl. 1994;15(6):608–613. [PubMed] [Google Scholar]
- 200. Jahnukainen K, Heikkinen R, Henriksson M, Cooper TG, Puukko-Viertomies L-R, Mäkitie O.. Semen quality and fertility in adult long-term survivors of childhood acute lymphoblastic leukemia. Fertil Steril. 2011;96(4):837–842. [DOI] [PubMed] [Google Scholar]
- 201. Larsen EC, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from Children’s Oncology Group Study AALL0232. J Clin Oncol. 2016;34(20):2380–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Green DM, Zhu L, Wang M, et al. Effect of cranial irradiation on sperm concentration of adult survivors of childhood acute lymphoblastic leukemia: a report from the St. Jude Lifetime Cohort Study. Hum Reprod. 2017;32(6):1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Boran N, Cil AP, Tulunay G, et al. Fertility and recurrence results of conservative surgery for borderline ovarian tumors. Gynecol Oncol. 2005;97(3):845–851. [DOI] [PubMed] [Google Scholar]
- 204. Zanetta G, Bonazzi C, Cantu MG, et al. Survival and reproductive function after treatment of malignant germ cell ovarian tumors. J Clin Oncol. 2001;19(4):1015–1020. [DOI] [PubMed] [Google Scholar]
- 205. Fauvet R, Poncelet C, Boccara J, Descamps P, Fondrinier E, Daraï E.. Fertility after conservative treatment for borderline ovarian tumors: a French multicenter study. Fertil Steril. 2005;83(2):284–290. quiz 525–6. [DOI] [PubMed] [Google Scholar]
- 206. Zanetta G, Chiari S, Rota S, et al. Conservative surgery for Stage I ovarian carcinoma in women of childbearing age. Br J Obstet Gynaecol. 1997;104(9):1030–1035. [DOI] [PubMed] [Google Scholar]