Abstract
Background
Increasing evidence indicates that loss of muscle mass is associated with adverse outcomes in metastatic colorectal cancer. Here, we investigate which demographic, lifestyle- (smoking), tumor-, and treatment-related factors are associated with muscle loss in patients with metastatic colorectal cancer during first-line palliative systemic treatment.
Methods
Data from 300 patients with computed tomography scans both at start and after six initial cycles of capecitabine plus oxaliplatin and bevacizumab was used (CAIRO3). From computed tomography, muscle mass normalized for stature (skeletal muscle index [SMI]) was calculated. A priori-selected variables were tested using multivariable linear regression models (P values ≤.05). Two models were developed: Model 1 contained variables measured at start and Model 2 contained variables assessed after initial therapy.
Results
In Model 1, loss of SMI was statistically significantly associated with a higher initial SMI (−0.32%, 95% confidence interval [CI] = −0.45% to −0.19% per unit increase in initial SMI), smoking status (−2.74%, 95% CI = −5.29% to −0.19% for smokers), and interval of metastases (−3.02%, 95% CI = −5.50% to −0.53%) for metachronous vs synchronous metastases), and primary tumor resection was statistically significantly associated with a gain in SMI (2.17%, 95% CI = 0.13% to 4.21% for resection vs no resection). In Model 2, loss of SMI was statistically significantly associated with response to capecitabine plus oxaliplatin and bevacizumab (−2.48%, 95% CI = −4.33% to −0.62% for stable disease vs partial/complete response).
Conclusions
Our results highlight, given the association of sarcopenia and survival, that patients with higher SMI should not be ignored. In addition, smoking is a potentially modifiable factor associated with muscle loss. The association between smoking and muscle loss might relate to worse clinical outcomes in smokers with metastatic colorectal cancer.
Skeletal muscle loss, one of the main characteristics of sarcopenia (1) and a diagnostic criterion for cancer cachexia (2), is a common, albeit occult, phenomenon in many cancer types, including colorectal cancer (CRC). A recent meta-analysis found that the overall prevalence of sarcopenia in patients with different primary tumors exceeded 40%, including CRC with prevalence varying from 19% to 71% (3). Depletion of muscle mass has shown to be associated with poor clinical outcomes such as reduced responsiveness and tolerability to cancer treatment, quality of life, and survival (3–8). Although several studies investigated the associations between skeletal muscle loss and disease outcomes (3), only a few studies have investigated which characteristics are related to skeletal muscle loss in cancer patients.
We previously found that muscle loss is reversible, is more likely to occur during periods of systemic treatment, and may be influenced by the intensity of treatment regimens (9). Other studies investigating which factors modulate muscle mass in patients with cancer described that skeletal muscle loss is more prevalent during periods of progressive disease (7) and at the end of life (7,10,11). One study specifically focused on palliative patients during the last phase of life, and found that patients with male sex and increased systemic inflammation marker lost more muscle mass in the last 24 months of life compared with their counterparts (12). Recently, a study in non-small cell lung cancer patients found that a higher initial body mass index (BMI) and higher initial skeletal muscle mass (SMM) were factors related to more loss of muscle mass (13). Lastly, one study that included CRC patients who underwent elective surgery reported that loss of SMM was statistically significantly associated with type of surgical approach (higher in open vs laparoscopic) and tumor stage (higher in stage III–IV vs I–II) (10). To date, no studies of a comparable nature have been conducted in patients with metastatic colorectal cancer (mCRC).
Despite the evidence on the reversibility of SMM loss in patients with mCRC (9) and increasing knowledge on potential strategies to reverse sarcopenia (3), recent data suggest that most patients with CRC, particularly those with advanced tumors, are not able to maintain SMM during systemic treatment (7,9,11). Understanding the determinants that are associated with SMM loss during treatment will help to better identify patients who are at risk of losing muscle mass and may contribute to the development of interventions that aim to avoid muscle mass loss. Therefore, the aim of our study was to investigate which demographic, lifestyle-, tumor-, and treatment-related factors are associated with loss of muscle mass in mCRC patients during first-line palliative systemic treatment.
Methods
Patient Population
For the current analysis, we used data from the CAIRO3 study (ClinicalTrials.gov number NCT00442637) (14). CAIRO3 is a randomized controlled phase III trial of the Dutch Colorectal Cancer Group on the effect of maintenance treatment with capecitabine plus bevacizumab vs observation in previously untreated mCRC patients who responded with stable disease or better (partial response [PR] or complete response [CR]) after six initial cycles with capecitabine plus oxaliplatin and bevacizumab (CAPOX-B). The main eligibility criteria for randomization in CAIRO3 were histological proof of CRC, unresectable metastatic disease, and World Health Organization performance status 0 or 1. For the current analyses, we used data from the first six cycles with CAPOX-B (later called “initial therapy”) to exclude the possible effect of disease progression on change in SMM. Patients with available computed tomography (CT) scans both at start and after six cycles of initial therapy were included. Primary approval for the CAIRO3 study was given by the Medical Ethical Committee Arnhem-Nijmegen and by local institutional review boards. Written informed consent was obtained from all participants, and research was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Skeletal Muscle Measurements
Skeletal muscle area was measured on abdominal CT scans that were routinely performed at the start and after six cycles of initial therapy. For the quantification of SMM, CT scans were acquired and analyzed by trained analysts using the software tool Slice-o-matic (version 5.0; Tomovision). Skeletal muscle area was measured on a single slice at the level of the third vertebra (L3), which is shown to highly correlate with total body SMM (r2 = 0.86) (15). Prespecified thresholds in Hounsfield units (−29 to 150) were used to identify the different muscle compartments (16,17). To reduce measurement error due to variation in the positioning of patients between consecutive CT scans, each second scan was rotated and fused with a rigid fusion method and L3 of the first scan as a bony landmark. To calculate the skeletal muscle index (SMI), the generally accepted regression equation below was used (15,18,19):
Data Collection
Data managers of The Netherlands Comprehensive Cancer Organisation collected sociodemographic and clinical data from medical records. To collect additional data from the initial treatment phase, medical records were reviewed to retrieve data on initial body weight and levels of leukocytes, thrombocytes, C-reactive protein (CRP), lactate dehydrogenase (LDH), and albumin. For this study, empirically selected variables collected at the start of initial therapy were sex, age, active smoking (yes vs no), primary tumor sidedness (left vs right), interval of metastases (metachronous vs synchronous), primary tumor resection (yes vs no), and prior adjuvant therapy (yes vs no). In addition, two variables that were assessed after initial therapy were selected, namely the occurrence of dose reductions during initial therapy (yes vs no) and the patient’s response to initial therapy (stable disease vs CR or PR). The presence of sarcopenia was determined by previously suggested sex-specific cutoff points, which were SMI less than 43 cm2/m2 for men with a BMI lower than 25 kg/m2, SMI less than 53 cm2/m2 for men with a BMI greater than 25 kg/m2, and SMI less than 41 cm2/m2 for women with any BMI (19). For the interval of metastases, we distinguished between synchronous and metachronous, with synchronous metastases being defined as distant metastases occurring within 6 months after diagnosis of the primary tumor and metachronous metastases occurring later than 6 months after diagnosis of the primary tumor.
Statistical Analysis
All characteristics were described as mean (SD) or median with interquartile range. To meet model assumptions, logarithmic transformations were applied on the initial blood values of leukocytes, thrombocytes, CRP, and LDH. Missing data (varying between 0 and 15% per variable, except for laboratory measures because these were not measured for the study and retrieved retrospectively) were imputed using the R-package Multiple Imputation by Chained Equations (20) when appropriate, resulting in multiple (n = 20) imputed datasets. All empirically selected factors were tested on their univariate association with change in SMI (%) and subsequently analyzed using multivariable linear regression models. Models were fitted on each imputed dataset, and Rubin’s rules were used to subsequently pool the estimates from each model into a single estimate (21). Finally, to distinguish between associations at start and after initial therapy, two multivariable models were created. Model 1 contained only variables measured at the start of initial therapy and shows how they are related to changes in muscle mass during initial therapy, and Model 2 contained variables measured after initial therapy and shows the cross-sectional associations with muscle mass changes at that time. Sex was included in both models given the increasing evidence on sex-specific differences in cancer-induced muscle wasting (22,23). The variable “initial presence of sarcopenia” was not included in the models because of multicollinearity, and “initial level of CRP” was not included because of the presence of selective missing values. All statistical tests were two sided, and significance of the results was interpreted based on confidence intervals (P ≤ .05). All analyses were performed in R studio version 1.0.143.
Results
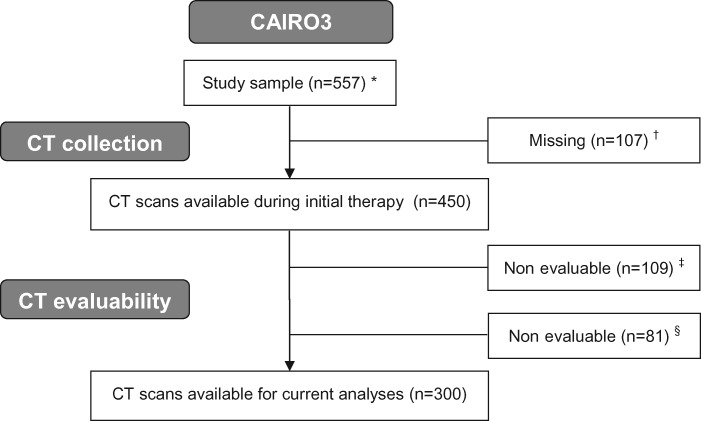
The flowchart of the selection of individuals for the current analyses is shown in Figure 1. In total, 557 patients from 64 participating hospitals in the Netherlands were originally included in the CAIRO3 study. Of the 450 patients for whom CT scans were available, 300 (66.7%) had evaluable CT scans both at start and after six cycles of initial therapy with CAPOX-B that were used for skeletal muscle measurements.
Figure 1.
Flowchart of the selection of patients for the current analyses. *One participating patient revoked informed consent. †No CT scans available from nine participating hospitals because of logistic reasons. ‡CT scan at start of initial therapy nonevaluable. Reasons: no CT abdomen available, incomplete depiction of skeletal muscle at L3, stoma through muscle layer at L3, scan of insufficient quality. §CT scan at randomization nonevaluable. Reasons: no CT abdomen available, incomplete depiction of skeletal muscle at L3, stoma through muscle layer at L3, scan of insufficient quality. CAIRO3 = Maintenance Treatment Versus Observation After Induction in Advanced Colorectal Carcinoma; CT = computed tomography.
Patient Population
Patient characteristics of our study population before and during initial therapy are shown in Table 1. A total of 63.0% were male and the mean age was 63.5 (8.7) years. Mean BMI at start of initial therapy was 25.9 (4.1) kg/m2 and mean initial SMI was higher in male patients compared with female patients (49.5 [7.9] cm2/m2 and 40.9 [6.8] cm2/m2, respectively). On average, patients lost 1.17% SMI during initial therapy. Furthermore, 16.8% were active smokers, which is slightly lower than the Dutch general population at that time because the proportion of smokers older than 16 years in 2007–2012 decreased from 30% to 25% (24). Regarding tumor and treatment-related characteristics, 74.9% had a primary tumor located at the left side of the colon, and 24.3% had metachronous metastases. Primary tumor resection before inclusion in the study had been performed in 59.3% of the patients, 34.0% received prior adjuvant chemotherapy, 33.0% responded with stable disease, and 47.0% received dose reductions during initial therapy.
Table 1.
Patient characteristics during initial therapy (n = 300)*
| Characteristic | No. | Missing | Descriptives† |
|---|---|---|---|
| Demographics | |||
| Sex | 300 | 0% | |
| Male | 189 (63.0%) | ||
| Female | 111 (37.0%) | ||
| Age, y | 300 | 0% | 63.5 (8.7) |
| Initial BMI, kg/m2 | 287 | 4.3% | 25.9 (4.1) |
| Initial SMI, cm2/m2 | 300 | 0% | |
| Total group | 46.3 (8.6) | ||
| Male | 49.5 (7.9) | ||
| Female | 40.9 (6.8) | ||
| Initial presence of sarcopenia‡ | 294 | 2.0% | |
| Yes | 149 (50.7%) | ||
| No | 145 (49.3%) | ||
| Smoking status | 256 | 14.7% | |
| Yes | 43 (16.8%) | ||
| No | 213 (83.2%) | ||
| Initial level of leukocytes, 109/L | 198 | 34.0% | 8.4 [6.9–10.3] |
| Initial level of thrombocytes, 103/mm3 | 271 | 9.7% | 339.0 [259.5–435.0] |
| Initial level of CRP, mg/L | 77 | 74.3% | 17.0 [6.0–58.0] |
| Initial level of LDH, U/L | 212 | 29.3% | 308.0 [197.8–487.0] |
| Initial level of albumin, g/L | 158 | 47.3% | 39.6 [37.0–43.0] |
| Tumor characteristics | |||
| Primary tumor sidedness | 299 | 0.3% | |
| Left | 224 (74.9%) | ||
| Right | 75 (25.1%) | ||
| Interval of metastases | 300 | 0% | |
| Metachronous | 73 (24.3%) | ||
| Synchronous | 227 (75.7%) | ||
| Treatment-related characteristics | |||
| Primary tumor resection | 300 | 0% | |
| Yes | 178 (59.3%) | ||
| No | 122 (40.7%) | ||
| Prior adjuvant therapy | 300 | 0% | |
| Yes | 102 (34.0%) | ||
| No | 198 (66.0%) | ||
| Best response to initial therapy | 300 | 0% | |
| Stable disease | 99 (33.0%) | ||
| Complete or partial response | 201 (67.0%) | ||
| Dose reduction during initial therapy | 300 | 0% | |
| Yes | 141 (47.0%) | ||
| No | 159 (53.0%) |
Descriptives are presented as count (percentage), mean (SD), or median [interquartile range]. BMI = body mass index; CRP = C-reactive protein; LDH = lactate dehydrogenase; SMI = skeletal muscle index.
In case of missing data, the descriptive statistics of complete cases are presented.
Sarcopenic status based on sex-specific cutoff points described by Martin et al. (19).
Characteristics Associated with SMI Change
In univariate linear regression analyses (Table 2), factors statistically significantly associated with SMI change were initial BMI, initial SMI, smoking status, initial level of thrombocytes, interval of metastases, and patients’ response to initial therapy. Of these six variables, a higher (log-transformed) initial level of thrombocytes was statistically significantly associated with increased SMI, meaning that a 1% increase in initial thrombocyte levels was associated with a 0.09% (95% confidence interval [CI] = 0.03% to 0.15%) gain in SMI. For the other five statistically significantly associated variables, we found mean changes in SMI of −0.38% (95% CI = −0.60% to −0.17%) per unit increase in initial BMI, −0.32% (95% CI = −0.42% to −0.22%) per unit increase in initial SMI, −2.64% (95% CI = −5.17% to −0.12%) for active smokers, −3.61% (95% CI = −5.64% to −1.58%) for patients with metachronous metastases, and −2.56% (95% CI = −4.43% to −0.69%) for patients with stable disease during initial therapy.
Table 2.
Factors associated with changes in SMI during initial therapy
| Variables | Univariate analysis |
Multivariable model 1 |
Multivariable model 2 |
|||
|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | |
| Measured at start of initial therapy | ||||||
| Sex, female vs male | 1.67 | −0.16 to 3.50 | −1.52 | −3.60 to 0.57 | 1.75 | −0.06 to 3.57 |
| Age, y | −0.02 | −0.12 to 0.08 | −0.05 | −0.15 to 0.06 | — | — |
| Initial BMI, kg/m2 | −0.38 * | −0.60 to −0.17 | −0.05 | −0.29 to 0.19 | — | — |
| Initial SMI, cm2/m2 | −0.32 * | −0.42 to −0.22 | −0.32 * | −0.45 to −0.19 | — | — |
| Smoking status, yes vs no | −2.64 * | −5.17 to −0.12 | −2.74 * | −5.29 to −0.19 | — | — |
| Initial level of leukocytes,*†109/L | 0.06 | −0.01 to 0.12 | 0.03 | −0.05 to 0.11 | — | — |
| Initial level of thrombocytes,†*103/mm3 | 0.09 * | 0.03 to 0.15 | 0.03 | −0.05 to 0.11 | — | — |
| Initial level of LDH,† U/L | 0.003 | −0.03 to 0.04 | −0.01 | −0.05 to 0.02 | — | — |
| Initial level of albumin, g/L | 0.01 | −0.23 to 0.24 | 0.10 | −0.19 to 0.40 | — | — |
| Primary tumor sidedness, left vs right | 0.34 | −1.71 to 2.39 | 1.09 | −0.93 to 3.11 | — | — |
| Interval of metastases, metachronous vs synchronous | −3.61 * | −5.64 to −1.58 | −3.02 * | −5.50 to −0.53 | — | — |
| Primary tumor resection, yes vs no | 0.23 | −1.58 to 2.04 | 2.17 * | 0.13 to 4.21 | — | — |
| Prior adjuvant therapy, yes vs no | −0.43 | −2.31 to 1.44 | 0.06 | −1.79 to 1.91 | — | — |
| Measured after initial therapy | ||||||
| Best response to initial therapy, stable disease vs CR or PR | −2.56 * | −4.43 to −0.69 | — | — | −2.48 * | −4.33 to −0.62 |
| Dose reduction during initial therapy, yes vs no | −0.92 | −2.69 to 0.86 | — | — | −0.95 | −2.71 to 0.81 |
Statistically significant association (P ≤ .05). Results are presented as regression coefficients (β), representing the average percentage change in SMI during initial treatment per unit (or per percentage for leukocytes, thrombocytes, and LDH) increase of the corresponding variable, including 95% confidence intervals (95% CI). Model 1 contains only variables measured at start of initial therapy, and model 2 contains variables measured after initial therapy. BMI = body mass index; CR = complete response; LDH = lactate dehydrogenase; PR = partial response; SMI = skeletal muscle index.
Analyzed as log-transformed variable.
In multivariable adjusted linear regression models with all factors measured at the start of initial therapy (Table 2, Model 1), we found that initial SMI, smoking status, and interval of metastases were still statistically significantly associated with SMI loss. The effect sizes remained comparable to univariate analyses because we found mean changes in SMI of −0.32% (95% CI = −0.45% to −0.19%) per unit increase in initial SMI, of −2.74% (95% CI = −5.29% to −0.19%) for active smokers, and −3.02% (95% CI = −5.50% to −0.53%) for patients with metachronous metastases. Primary tumor resection was statistically significantly associated with a 2.17% (95% CI = 0.13% to 4.21%) gain in SMI. In multivariable analysis, no statistically significant associations were found for initial level of thrombocytes and initial BMI.
When additional variables assessed after the course of initial therapy were studied in a multivariable model (Table 2, Model 2), we found that response to initial therapy was statistically significantly associated with SMI loss: Patients with a stable disease during initial therapy lost 2.48% (95% CI = −4.33% to −0.62%) more SMI compared with patients responding with a PR or CR.
Discussion
In this study, we investigated possible associations between demographic, lifestyle- (ie, smoking), tumor-, and treatment-related factors and changes in muscle mass during six cycles of first-line palliative systemic treatment in mCRC patients. Our main findings were that a higher initial SMI, active smoking at start of initial therapy, and metachronous metastases were factors independently associated with SMI loss, whereas having had a primary tumor resection before initial therapy was statistically significantly associated with a gain in SMI. The tumor’s response to treatment also appeared to be a factor statistically significantly associated with SMI loss, because patients with a stable disease lost statistically significantly more SMI compared with patients responding with PR or CR.
The observed association between higher initial levels of SMI and SMI loss during first-line palliative systemic treatment is in line with a previous study conducted in patients with advanced non-small cell lung cancer (13). This study aimed to identify (non-tumor-related) factors that modulate changes in body composition and found that SMM deterioration during anticancer treatment occurred in patients with a higher BMI and greater SMM. Interestingly, in our analysis we found that initial level of BMI, when adjusted for initial SMI, was not independently associated with SMI loss during treatment. The univariate association between initial BMI and SMI loss seems to be explained by initial SMI, because in Model 1—including both initial BMI and SMI—only the initial level of SMI remains statistically significantly associated with SMI loss. Because higher SMI at start of initial therapy was associated with increased muscle loss, we emphasize that in clinical practice, attention should also be given to patients presenting with a higher SMI and interventions should not be offered only to sarcopenic patients.
Regarding tumor-related factors, we found that patients with metachronous metastases lost on average 3.0% more muscle mass during initial therapy compared with patients with synchronous metastases. This might be explained by prolonged exposure to tumor-induced metabolic changes that contribute to muscle wasting before start of palliative systemic treatment (25). Moreover, we found that patients responding with a stable disease lost on average 2.5% more muscle mass compared with patients who achieved a PR or CR. This finding adds to a previous study in which progression of disease was associated with increased muscle wasting (7) by showing that patients, next to a survival benefit, may also physically benefit from a good response to treatment. This consolidates the potential role of tumor load on cancer-related muscle wasting. However, causal inferences on treatment-related variables remain elusive because we cannot exclude the possibility of reversed causality in our analysis.
Although smoking has been established as a risk factor for the development of sarcopenia (26), previous studies did not include smoking status in their analyses, which is likely because of poor data collection on smoking behavior in clinical settings, including trials. In CAIRO3, smoking status was known for 85.3% of the patients, allowing us to include this factor in our models. Indeed, we found that active tobacco smokers lost on average 2.7% more SMI during initial therapy compared with nonsmokers. Potential molecular mechanisms involved in muscle wasting due to smoking are thought to be induced by particular free radicals and carcinogenic components of tobacco smoke (ie, aldehydes, reactive oxygen species and reactive nitrogen species) (26,27). A recent meta-analysis including 62 278 CRC patients showed that smoking at the time of diagnosis and, to a lesser extent former smoking, is associated with a poorer survival compared with never smokers (28). In addition, it is known that smoking cessation has a positive impact on CRC prognosis (29). Here we show, for the first time to our knowledge, that smoking at the start of first-line systemic treatment is associated with increased SMI loss in mCRC patients, suggesting that SMI might be a mediator in the association between smoking and survival. Future research should investigate whether quitting smoking after diagnosis is positively associated with muscle mass. A recent perspective noted that, despite existing recommendations to offer effective evidence-based cessation treatment to all patients with cancer who smoke, clinicians often ignore these cessation treatments (30). To improve future cessation support for cancer patients, it should become an integrated component in cancer care, and resources to refer patients for such support as well as clinician education should be enhanced (30,31).
To maintain muscle mass in patients with higher initial SMI and to potentially improve muscle mass in patients with lower initial levels of SMI, various interventions have been described (3,32). It has been shown that physical activity interventions have the potential to reverse sarcopenia in cancer patients (33). Current interventions are mainly focused on improving dietary intake and increasing physical activity to counteract muscle protein catabolism. Additionally, several pharmacologic approaches are being studied, of which orexigenic agents such as ghrelin and anamorelin hold the most evidence (34). However, it is suggested that a multimodal approach including both nutritional support (high energy, high protein, and omega-3 fatty acids) and exercise programs will synergistically contribute to preservation of muscle mass and possibly lead to improved outcomes (3,35).
This analysis also has a number of limitations. Because systemic inflammation is a process that plays a statistically significant role in cancer cachexia (36,37), it is of interest when studying factors related to muscle wasting. In previous studies conducted in patients with CRC, elevated initial CRP levels and neutrophil to lymphocyte ratios were associated with skeletal muscle loss (10,12,38). Because CRP levels were determined only by clinical indication, we could not impute missing values and did not include this variable in our models. Unfortunately, other markers for inflammation, as well as data on nutritional intake, alcohol consumption, physical activity levels, corticosteroid use (eg, dexamethasone), and comorbidities were not available at the start of initial therapy, and thus residual confounding cannot be ruled out.
This observational study was performed in a large homogenous group of mCRC patients with stable disease or a better response during initial therapy. The exclusion of patients with progression of disease removed the possible effect of disease progression on change in SMI from our analysis and allowed us to investigate which other factors play a role in muscle wasting. Another strength of this study was that the data originated from a Dutch nationwide randomized clinical trial in which high-quality data on patient-, tumor-, and treatment-related characteristics were available. Lastly, the use of abdominal CT scans is a well-acknowledged, accurate, and precise quantification method to measure body composition (15,19), which is favorable when comparing results to the current literature.
To conclude, our data indicate that SMI loss during first-line palliative systemic treatment for mCRC was associated with lifestyle-related as well as tumor- and treatment-related factors. We found that higher initial levels of SMI, active smoking, metachronous metastases, and treatment response with stable disease were associated with SMI loss, whereas the absence of the primary tumor is associated with a gain in SMI. We speculate that muscle mass might be a mediator in the association between active smoking and poor survival. Hence, our results further support efforts of oncologists and supportive care nurses to facilitate in smoking cessation to improve outcomes including, but not limited to, muscle mass preservation.
Funding
The original CAIRO3 study was sponsored by the Dutch Colorectal Cancer Group, and the current work is supported by the Province of Utrecht, the Netherlands.
Notes
Affiliations of authors: Department of Medical Oncology, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands (JWGD, SAK, MK); Department of Epidemiology, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands (JWGD, SAK, MJO, PHMP, AMM); Department of Medical Oncology, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands (CJAP).
Conflict of interest: none declared.
We thank all patients and staff at each of the participating hospitals.
References
- 1. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. [DOI] [PubMed] [Google Scholar]
- 3. Bozzetti F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol. 2017;28(9):2107–2118. [DOI] [PubMed] [Google Scholar]
- 4. Malietzis G, Aziz O, Bagnall NM, et al. The role of body composition evaluation by computerized tomography in determining colorectal cancer treatment outcomes: a systematic review. Eur J Surg Oncol. 2015;41(2):186–196. [DOI] [PubMed] [Google Scholar]
- 5. Kurk S, Peeters P, Stellato R, et al. Impact of sarcopenia on dose limiting toxicities in metastatic colorectal cancer patients (mCRC pts) receiving palliative systemic treatment. Ann Oncol. 2017;28(suppl 5):544–545. [Google Scholar]
- 6. Kurk S, Peeters PHM, Stellato RK, et al. Impact of skeletal muscle index (SMI) loss during palliative systemic treatment (Tx) on time to progression and overall survival (OS) in metastatic colorectal cancer (mCRC) patients. J Clin Oncol. 2017;35(15_suppl):10087. [Google Scholar]
- 7. Prado CM, Sawyer MB, Ghosh S, et al. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr. 2013;98(4):1012–1019. [DOI] [PubMed] [Google Scholar]
- 8. Baracos VE. Cancer-associated cachexia and underlying biological mechanisms. Annu Rev Nutr. 2006;26(1):435–461. [DOI] [PubMed] [Google Scholar]
- 9. Kurk SA, Peeters PHM, Dorresteijn B, et al. Impact of different palliative systemic treatments on skeletal muscle mass in metastatic colorectal cancer patients. J Cachexia Sarcopenia Muscle. 2018;9(5):909–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malietzis G, Currie AC, Johns N, et al. Skeletal muscle changes after elective colorectal cancer resection: a longitudinal study. Ann Surg Oncol. 2016;23(8):2539–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MAE, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol. 2016;34(12):1339–1344. [DOI] [PubMed] [Google Scholar]
- 12. Wallengren O, Iresjo BM, Lundholm K, et al. Loss of muscle mass in the end of life in patients with advanced cancer. Support Care Cancer. 2015;23(1):79–86. [DOI] [PubMed] [Google Scholar]
- 13. Atlan P, Bayar MA, Lanoy E, et al. Factors which modulate the rates of skeletal muscle mass loss in non-small cell lung cancer patients: a pilot study. Support Care Cancer. 2017;25(11):3365–3373. [DOI] [PubMed] [Google Scholar]
- 14. Simkens LH, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385(9980):1843–1852. [DOI] [PubMed] [Google Scholar]
- 15. Shen W, Punyanitya M, Wang ZM, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333–2338. [DOI] [PubMed] [Google Scholar]
- 16. Prado CMM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–635. [DOI] [PubMed] [Google Scholar]
- 17. Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014;210(3):489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mourtzakis M, Prado CMM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006. [DOI] [PubMed] [Google Scholar]
- 19. Martin L, Birdsell L, MacDonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–1547. [DOI] [PubMed] [Google Scholar]
- 20. van Buuren S, Groothuis-Oudshoorn K. . mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. [Google Scholar]
- 21. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987. [Google Scholar]
- 22. Anderson LJ, Liu H, Garcia JM.. Sex differences in muscle wasting. Adv Exp Med Biol. 2017;1043:153–197. [DOI] [PubMed] [Google Scholar]
- 23. Montalvo RN, Counts BR, Carson JA.. Understanding sex differences in the regulation of cancer-induced muscle wasting. Curr Opin Support Palliat Care. 2018;12(4):394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Statistics Netherlands (CBS). StatLine: Health, Lifestyle, Health Care Use and Supply, Causes of Death; from 1900 2017. https://opendata.cbs.nl/statline/#/CBS/en/dataset/37852eng. Accessed December, 2018.
- 25. Fearon KC, Glass DJ, Guttridge DC.. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153–166. [DOI] [PubMed] [Google Scholar]
- 26. Steffl M, Bohannon RW, Petr M, et al. Relation between cigarette smoking and sarcopenia: meta-analysis. Physiol Res. 2015;64(3):419–426. [DOI] [PubMed] [Google Scholar]
- 27. Silander E, Nyman J, Hammerlid E.. An exploration of factors predicting malnutrition in patients with advanced head and neck cancer. Laryngoscope. 2013;123(10):2428–2434. [DOI] [PubMed] [Google Scholar]
- 28. Walter V, Jansen L, Hoffmeister M, et al. Smoking and survival of colorectal cancer patients: systematic review and meta-analysis. Ann Oncol. 2014;25(8):1517–1525. [DOI] [PubMed] [Google Scholar]
- 29. Ordóñez-Mena JM, Walter V, Schöttker B, et al. Impact of prediagnostic smoking and smoking cessation on colorectal cancer prognosis: a meta-analysis of individual patient data from cohorts within the CHANCES consortium. Ann Oncol. 2018;29(2):472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Croyle RT, Morgan GD, Fiore MC.. Addressing a core gap in cancer care—the NCI Moonshot Program to help oncology patients stop smoking. N Engl J Med. 2019;380(6):512–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Warren GW, Dibaj S, Hutson A, et al. Identifying targeted strategies to improve smoking cessation support for cancer patients. J Thorac Oncol. 2015;10(11):1532–1537. [DOI] [PubMed] [Google Scholar]
- 32. Argilés JM, López-Soriano FJ, Stemmler B, et al. Novel targeted therapies for cancer cachexia. Biochem J. 2017;474(16):2663–2678. [DOI] [PubMed] [Google Scholar]
- 33. Adams SC, Segal RJ, McKenzie DC, et al. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Breast Cancer Res Treat. 2016;158(3):497–507. [DOI] [PubMed] [Google Scholar]
- 34. Bai Y, Hu YX, Zhao YH, et al. Anamorelin for cancer anorexia-cachexia syndrome: a systematic review and meta-analysis. Support Care Cancer. 2017;25(5):1651–1659. [DOI] [PubMed] [Google Scholar]
- 35. Konishi M, Ishida J, von Haehling S, et al. Nutrition in cachexia: from bench to bedside. J Cachexia Sarcopenia Muscle. 2016;7(2):107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seelaender M, Batista M Jr, Lira F, et al. Inflammation in cancer cachexia: to resolve or not to resolve (is that the question?). Clin Nutr. 2012;31(4):562–566. [DOI] [PubMed] [Google Scholar]
- 37. Tan BHL, Ross JA, Kaasa S, et al. Identification of possible genetic polymorphisms involved in cancer cachexia: a systematic review. J Genet. 2011;90(1):165–177. [DOI] [PubMed] [Google Scholar]
- 38. Feliciano EMC, Kroenke CH, Meyerhardt JA, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. 2017;3(12):e172319.. [DOI] [PMC free article] [PubMed] [Google Scholar]