Abstract
Circulating tumor cells (CTCs) are particularly rare in non-metastatic breast cancer, and the clinical validity of CTC detection in that clinical setting was initially not well recognized. A cytological CTC detection device (CellSearch) fulfilling the CLIA requirements for analytical validity was subsequently developed and, in 2008, we reported the first study (REMAGUS02) showing that distant metastasis-free survival was shorter in early breast cancer patients with one or more CTCs. In the past 10 years, other clinical studies and meta-analyses have established CTC detection as a level-of-evidence 1 prognostic biomarker for local relapses, distant relapses, and overall survival. This review summarizes available data on CTC detection and the promises of this proliferation- and subtype-independent metastasis-associated biomarker in early breast cancer patients.
Circulating tumor cells (CTCs) are cancer cells that are detected in the patient’s blood. The development of robust and clinical study-friendly techniques has led to thorough investigation of CTCs as biomarkers in stageIV breast cancer, where CTCs can frequently be detected. About 70% of metastatic breast cancer patients exhibit no less than 1 CTC/7.5 mL of blood and 50% no less than 5 CTC/7.5 mL (1). As CTCs are rare in early breast cancer, technical and statistical concerns were initially raised about their validity as biomarkers. These initial theoretical concerns have been largely invalidated in several large studies that established CTC detection as a reliable and valuable biomarker of the metastatic process. This review, which reports data from preclinical models whenever relevant, primarily focuses on clinical findings in early breast cancer patients.
Detection Techniques
CTCs were first described in the late 19th century on autopsy examination (2). CTC detection techniques generally consist of isolating cells with epithelial markers against a background of mesenchymal-derived blood cells. The scarcity of CTCs, usually less than 1 CTC/10 mL of blood in nonmetastatic cancers, is not compatible with most fluorescence-activated cell-sorting techniques. Current CTC detection techniques usually combine two steps: primary enrichment followed by CTC detection and counting. The first step, CTC enrichment, is achieved either 1) by positive or negative immunoselection using various membrane antigens (epithelial-cell adhesion [EpCAM], MUC1, CD45…) or 2) by the physical properties of CTC, such as (example 1) or (example 2): size-based filtering, dielectrophoresis, and so forth. After enrichment, CTC detection and counting relies on cytology-based techniques that combine optical visualization of the cells with other markers, usually nuclear staining and epithelial antigen immunocytolabeling. Of note, epithelial mRNA expression-based CTC detection has demonstrated poor specificity (3). As systemic inflammation is a confounding factor in cancer biomarker detection and validation (4), these detection methods have been largely discontinued.
Two of the hundreds of CTC detection techniques developed over the past decade have gained clinical acceptance. The Epic platform relies on high-throughput imaging of all blood cells and therefore avoids the selection step (5). There are, however, no data on its validity in early breast cancer, as clinical development was conducted almost exclusively in prostate cancer. The CellSearch system (Janssen Diagnostics, Raritan, NJ, USA), developed in the early 2000s (6), first enriches EpCAM-positive cells. The enriched cells are then fluorescently labeled with a nucleic acid dye (DAPI) and monoclonal antibodies (CD45 and epithelial cytokeratins). Practically, buffer contains ferrofluid-based capture reagent and immunofluorescent reagents. A system-embedded data button in the reagent holder assists in kit lot tracking and reagent use monitoring, specially designed cartridges for optimal CTC isolation and analysis. The samples are transferred to the CellTracks Analyzer, a semi-automated immunofluorescent microscope for cell detection. In the final step, expert cytologists and/or technicians manually validate whether stained cells are CTCs or not. However, CTCs that do not express EpCAM, and CTCs that express EpCAM but not cytokeratins 8, 18, and 19 will not be detected by the CellSearch system. It is of note that detection of CTCs corresponds to the detection of epithelial cells in blood with no guaranteed certainty that the cells isolated are indeed of tumor origin. In the seminal study with CellSearch (6), 8 of 145 (5.5%) healthy subjects displayed 1 epithelial cell per 7.5 mL of blood, whereas none displayed less than 2 epithelial cells. Similarly, only 14 of 199 (7.5%) women with benign breast diseases or other nonmalignant diseases had 1 epithelial cell that could be counted as a CTC in a 7.5- mL blood sample. Interreader variability is often present in the final step of CTC detection, which involves image recognition by a trained technician or physician, especially in samples containing very few CTCs (7). An image analysis algorithm has been developed to fully automatize CTC counting and to improve interreader reproducibility (8). Currently, all large clinical studies performed in early breast cancer use the CellSearch platform with manual CTC counting.
Biology
During the metastatic process, malignant cells can acquire the capacity to separate from the initial tumor, circulate in the bloodstream, and relocate at a distant site. CTC detection reveals that aggressive tumors release thousands of malignant cells into the bloodstream each day, but a minority of cells (0.1%) survive the various stress factors and form distant metastases (9,10). CTCs have a short survival time in the bloodstream, estimated to range from 1 to 3 hours (11). In early breast cancer, little is known about the biology of CTC release by the primary tumor. In neoadjuvant and adjuvant studies, a moderate association of CTC detection with positive lymph nodes has been reported, but not with any of the other usual prognostic factors, nor with tumor subtype (12, 13). Similar findings were obtained with disseminated tumor cells (DTCs), which are cancer cells that have stopped circulating and have extravasated in distant organs such as bone marrow (BM). DTC detection requires a BM puncture and relies on the same principles as CTC detection (14), with a detection rate of 15%–30% in nonmetastatic breast cancer patients (15–17), although detection techniques are not usually entirely superimposable (18). The largest studies that have investigated the correlation between CTC and DTC detection in breast cancer patients are summarized in Table 1. A statistically significant concordance between CTC and DTC detections has been reported in many studies; however, that concordance is largely driven by “double-negative” (ie, CTC- and DTC-negative) patients, whereas “double-positive” patients are rare. Otherwise, DTC-based experimental (19,20) and clinical (21) studies have suggested that tumor cells can disseminate early, even before the breast tumor has become invasive. A single study on 73 patients with either ductal or lobular carcinoma in situ reported that three patients (4.1%) had 1 CTC per 22.5 mL of blood (CellSearch) (22).
Table 1.
Correlation between bone marrow disseminated tumor cells and circulating tumor cells detection in breast cancer*
| References | No. of patients | Stage | Tech. | Conc. % | Correlation P | Detection rate, % |
Prognostic impact |
||
|---|---|---|---|---|---|---|---|---|---|
| CTC | DTC | CTC | DTC | ||||||
| Pierga et al. (2004) (50) | 114 | I–IV | ICC | 66 | <.001 | 24 | 59 | n.s. | DFS |
| Wiedswang et al. (2006) (51) | 341 | I–III | ICC + IMS | 81 | n.s. | 10 | 14 | DFS OS | DFS OS |
| Benoy et al. (2006) (52) | 148 | I–IV | RT-PCR | 68 | n.s. | 15 | 28 | n.s. | OS |
| Fehm et al. (2009) (53) | 414 | I–III | ICC/RT-PCR | 72 | .05 | 13 | 24 | n.a. | n.a. |
| Daskalaki et al. (2009) (54) | 165 | I–II | RT-PCR | 94 | <.001 | 55 | 58 | OS | OS |
| Banys et al. (2012) (55) | 209 | I–III | ICC/RT-PCR | 74 | .03 | 21 | 15 | n.a. | n.a. |
| Molloy et al. (2011) (56) | 733 | I–II | ICC/RT-PCR | 80 | .01 | 8 | 12 | DFS OS | DFS OS |
| Schindlbeck et al. (2013) (40) | 202 | I–IV | ICC/CellS | 71 | .002 | 20 | 28 | OS | n.s. |
| Hartkopf et al. (2014) (57) | 178 | IV | ICC/CellS | 61 | n.s. | 52 | 36 | DFS OS | OS |
Only studies with >100 patients have been included. CellS = CellSearch; conc = concordance rate; CTC = circulating tumor cells; DFS = disease-free survival; DTC = disseminated tumor cell; ICC = immunocytostaining; IMS = immunomagnetic selection; n.a. = not available; n.s. = not statistically significant; OS = overall survival; pts = patients; RT-PCR = reverse transcription polymerase chain reaction; tech = techniques used.
Neoadjuvant Studies
The dynamics of CTC count in the context of neoadjuvant chemotherapy (ie, before initial tumor surgery) aims to determine whether a tumor has initiated micrometastatic spread at distant sites and potentially to measure the early tumor response to systemic treatment. We pooled individual data from all studies that used CellSearch during neoadjuvant chemotherapy in a meta-analysis (Table 2).
Table 2.
CTC in neoadjuvant therapy: published studies with CellSearch and meta-analysis*
| References | No. of patients | Stage | Blood screened, mL | CTC detection rate, % |
Correlation CTC and pCR | Prognostic impact |
Detail of prognostic impact OS HR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|
| Before NCT | After NCT | DFS | OS | ||||||
| Studies | |||||||||
| 115 | II–III | 7.5 | 23 | 17 | No | Yes | Yes | n.a. | |
|
213 | I–III | 7.5 | 21 | 10 | No | Yes | Yes | n.a. |
|
51 | I–III | 22.5 | 11 | 16 | No | — | — | n.a. |
|
95 | I–III | 7.5 | 18 | — | No | — | — | n.a. |
|
57 |
|
7.5 | — | — | No | Yes | Yes | n.a. |
|
77 | III (T4d) | 7.5 | 54 | — | No | No | No | n.a. |
|
63 | III (T4d) | 7.5 | — | 27 | No | Yes | No | n.a. |
|
137 | III (T4d) | 7.5 | 35 | 7 | No | Yes | Yes | n.a. |
|
34 | I–III | 7.5 | — | — | No | Yes | — | n.a. |
| Meta-analysis | |||||||||
|
2156 | I–III |
|
25 | 17 | No | Yes | Yes | No CTC detected:
|
CI = confidence interval; CTC = circulating tumor cells; DFS = disease-free survival; HR = hazard ratio; OS = overall survival; n.a. = not available; NCT = neoadjuvant treatment; pCR = pathological complete response; pts . patients;
Using less than 1 CTC per 7.5 mL of blood as the positivity threshold, the CTC detection rate in most neoadjuvant studies was 20%–22% in patients before the start of neoadjuvant chemotherapy (23,24). However, two studies conducted in 137 inflammatory (T4d) breast cancers reported a much higher detection rate (39%) (25). The recent international meta-analysis (IMENEO study) based on more than 2000 patients from 16 centers observed a statistically significant association between CTC and T stage (P < .001), which was mostly driven by high CTC counts in T4d tumors and hormone-receptor negativity (P = .04). After excluding T4d tumors from the analysis, CTC positivity was independent from any baseline clinical or pathological characteristics. CTC positivity rates were 21.4% and 24.2% in node-negative and node-positive breast cancers, respectively (P = .22). The meta-analysis reported a statistically significant decrease in CTC count at the end of neoadjuvant chemotherapy compared to baseline (P < .001) (13). No statistically significant correlation was observed between changes in CTC counts during therapy or persistence of CTCs after chemotherapy that obtained a pathological complete response (23,24,26–28). CTC counts before neoadjuvant chemotherapy were found to be a strong and independent prognostic indicator for distant-metastasis-free survival (hazard ration [HR] = 3.73, 95% confidence interval [CI] = 2.82 to 4.90), overall survival (HR = 3.93, 95% CI = 2.81 to 5.45), and locoregional relapses (HR = 3.02, 95% CI = 1.88 to 4.75) (13). Interestingly, the impact on survival was directly related to the number of CTCs detected, supporting the idea of using CTC counts as a quantitative marker. It is important to note that although most known breast cancer prognostic factors are closely associated with tumor proliferation and/or subgroup and/or pathological complete response, CTC positivity has a different profile and does not overlap with other known prognostic factors.
Adjuvant Studies
Adjuvant therapies aim at eradicating any residual tumor cells after surgery, and metastasis-associated biomarkers such as CTC detection may be of value in tailoring the use of these adjuvant therapies. Some studies reporting on CTC detection before surgery found a detection rate of 10%–30% (≥1 CTC per 7.5 mL, CellSearch), whereas a few studies compared CTC detection before and after the surgical removal of the primary breast tumor (29–33) (Table 3). Apart from CellSearch-based studies, unusually high concentrations of CTC have been reported in one study using laser scanning cytometry (35). All studies suggested that CTC detection in that setting is a prognostic indicator for distant disease-free survival and/or overall survival. The randomized trial SUCCESS-A included more than 2000 patients at intermediate or high risk of relapse and demonstrated that, after median follow-up of 35 months, CTC positivity before and after adjuvant chemotherapy was an independent prognostic factor, with poor distant-free survival (HR = 2.28, 95% CI = 1.48 to 3.50) and overall survival (HR = 3.95, 95% CI = 2.13 to 7.32) (34). Among CTC-positive patients, those with at least 5 CTC per 30 mL exhibited the worst prognosis. Janni et al. (12) conducted a pooled analysis of individual data from 3173 patients and suggested that the presence of CTCs was an independent predictor of poor disease-free survival (HR = 1.82, 95% CI = 1.47 to 2.26), distant disease-free survival (HR = 1.89, 95% CI = 1.49 to 2.40), breast cancer-specific survival (HR = 2.04, 95% CI = 1.52 to 2.75), and overall survival (HR = 1.97, 95% CI = 1.51 to 2.59). However, among the recruited population, 8% of patients received neoadjuvant chemotherapy. In addition to the prognostic impact of CTC detection, a recent retrospective analysis of the SUCCESS-A trial and of the Surveillance, Epidemiology, and End Results database suggested that, with statistically significant interaction tests, CTC-positive patients benefit from adjuvant radiation therapy in terms of relapse-free survival and/or overall survival, whereas CTC-negative patients do not (36). These retrospective studies were underpowered and should be considered as hypothesis-generating; moreover, they did not analyze results according to radiation fields. These observations match those previously reported in a cohort of early breast cancer patients who underwent bone marrow DTC detection (37) in which DTC positivity was found to be a predictive factor for adjuvant-extended locoregional lymph node irradiation, even after 10years of follow-up (38). Taken together, these three cohorts could provide the rationale for a clinical utility trial in which adjuvant radiation therapy is modulated by detection of CTCs.
Table 3.
Circulating tumor cells in adjuvant therapy: main published studies and meta-analysis*
| References | No. of patients | Stage | Blood screened, mL | Detection rate, % |
Prognostic impact |
||
|---|---|---|---|---|---|---|---|
| Pre. ACT | Post. ACT | DFS HR (95% CI) | OS HR (95% CI) | ||||
| Studies | |||||||
| Krishnamurthy et al. (33) (2010) | 92 | I–III | 7.5 | 31 | — | — | — |
| Franken et al. (31) (2012) | 404 | I–III | 7.5 | 18 | — |
|
to |
| Lucci et al. (32) (2012) | 302 | I–III | 7.5 | 24 | — |
|
|
| Karhade et al. (30) (2014) | 113 | I–III (triple-negative) | 7.5 | 25 | — |
|
|
| Rack et al. (34) (2014) | 2026 | I–III | 30 | 21 | 22 |
|
|
| Van Dalum et al. (29) (2015) | 403 | I–III | 30 | 19 | 15 |
|
|
| Pooled-analysis | |||||||
| Janni et al. (12) (2016) | 3173 | I–III | 7.5 | 20 | — |
|
|
ACT = adjuvant chemotherapy; CI = confidence interval; DFS = disease-free survival; CI = confidence interval; HR = hazard ratio; n.a. = not available; OS = overall survival; pts = patients.
The TREAT-CTC Trial
The TREAT-CTC trial was the first attempt to demonstrate, albeit indirectly, the clinical utility of CTC detection in early breast cancer patients. Based on the repeated observation—that CTC positivity defines a subgroup of patients at higher risk of relapse—this interventional trial was set up to study whether adding a new therapy would help reduce the relapse rate in CTC-positive patients.
In 2010, at the time of the study design, trastuzumab was considered to be worth investigating in the adjuvant setting of HER2-negative breast cancer. This was based on unexpected results in the pivotal adjuvant trial, which included some HER2-negative patients (39), and on preclinical data suggesting that HER2 expression (even in the absence of amplification) may facilitate cancer cell dissemination and metastasis. Another report, using a reverse transcription polymerase chain reaction–based detection technique, also suggested that trastuzumab might contribute to reducing CTC levels in HER2-negative metastatic breast cancer patients (40). The TREAT-CTC trial was therefore designed to include HER2-negative patients after the completion of adjuvant chemotherapy and with a positive CTC test at of least 1 CTC per 7.5 mL (CellSearch). These high-risk patients were then randomly assigned to observation and administration of six cycles of trastuzumab. The primary objective of the study was to report that the CTC detection rate decreased after administration of trastuzumab because survival endpoints would have required a considerably larger number of patients.
The results of the TREAT-CTC trial have recently been published (41): 1317 HER2-negative patients were screened for CTCs at the end of adjuvant chemotherapy, 95 (7.2%) of whom were found to be CTC-positive. Sixty-three CTC-positive patients were randomly assigned to observation or administration of trastuzumab. Study accrual was stopped for futility by an independent committee after the CTC count was found not to have decreased in the trastuzumab arm. The TREAT-CTC trial therefore concluded that 1) CTC-based screening is feasible in the adjuvant setting of early breast cancer, 2) CTC-positive patients do have a higher risk of relapse, and 3) trastuzumab has no effect on CTCs in HER2-negative breast cancer. Interestingly, the inefficiency of trastuzumab in HER2-negative breast cancer patients was confirmed in the NSABP B-47 trial (42), which included 3270 patients who were not selected for CTC positivity.
Follow-up Studies
Few studies have looked at the detection rate of CTCs and their clinical impact in the follow-up period. In this setting, the aim of CTC detection is to isolate subgroups at high risk of later relapse. In the SUCCESS-A study, CTCs were detected at 2 and 5 years after primary diagnosis in 96 (16.7%) and 47 (8.2%) of the 574 patients, respectively. No association with tumor characteristics or type of primary therapy was found (43). Results at 2 years have been recently published: CTCs were detected in 18.2% of patients (median = 1 cell, range = 1–99 cells per 7.5 mL blood) at 2 years and were associated with a 3.9-fold increased risk of death and a 2.3-fold higher recurrence risk in multivariable models that included clinicopathologic features and CTC status at baseline; sensitivity analysis showed this effect only in HER-2-negative disease (44). Another report from this same study found that among 206 subjects enrolled in the SUCCESS study with follow-up information and known CTC status at 5 years, 7.8% were CTC-positive at 5 years (median = 1 cell, range = 1–53 cells per 7.5 mL blood) and was associated with a 6-fold increase in recurrence (45).
Sparano et al. (46) performed a per-protocol secondary analysis of the prospective NCT00433511 clinical trial, which accrued patients without clinical evidence of recurrence between 4.5 and 7.5 years after primary surgical treatment of HER2-negative stage II–III breast cancer followed by adjuvant systemic therapy (47). In this late recurrence substudy, the results indicated that 26 of 547 patients (4.8%, 95% CI = 3.1% to 6.9%) had positive CTC assay results. Of 18 patients, 7 (38.9%, 95% CI = 17.3% to 64.3%) with hormone receptor-positive disease and a positive CTC assay result had a recurrence. In multivariate models including clinical covariates, a positive CTC assay result was associated with a 13.1-fold higher risk of recurrence (HR = 13.1, 95% CI = 4.7 to 36.3) in the hormone receptor-positive population. None of the eight patients with hormone receptor-negative disease and a positive assay result had a recurrence (0%, 95% CI = 0% to 37%). The limitation of the study was a short median follow-up at 1.6 years after CTC assay.
CTC Clearance as a Clinical Trial Endpoint: Statistical Issues
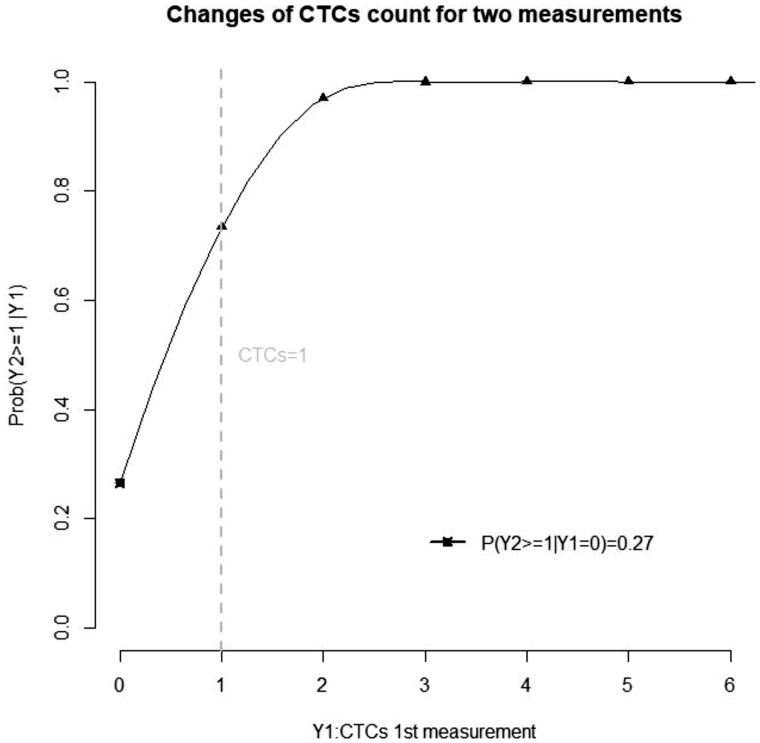
Because CTC positivity is strongly associated with early breast cancer outcome, using CTC clearance after an experimental therapeutic intervention is a tempting endpoint for any future trial. However, the Poisson law, which rules the detection of rare events, makes the use of such an endpoint complex. As an illustration, we performed a statistical analysis to study the interpretation of CTC changes between successive measurements (see Supplementary Data No. 1, available online). This statistical modeling suggested that, in the context of scarce events, a decline in the number of CTCs may not reflect the treatment effect. As shown on Figure 1, this issue was mostly seen for patients with 1 CTC at baseline, whereas CTC clearance appears more relevant as a trial endpoint for patients with at least 2 CTCs at baseline.
Figure 1.
Positivity rate of a second circulating tumor cell CTC) count according to the first CTC count. This graph displays the probability of (Y2) being above the cut-off (CTCs = 1) as a function of a prior CTC count (Y1), in the absence of any efficient therapeutic intervention.
Conclusions
From a biological perspective, the detection of CTCs has opened a window onto the metastatic process in early breast cancer patients. Although detection of CTCs is a rare event, its clinical validity as a prognostic marker has been repeatedly confirmed and has reached the highest level of evidence. The clinical utility of CTC detection, however, remains to be investigated in prospective trials, potentially focusing on adjuvant radiation therapy, systemic therapy, and/or extended hormone therapy, taking into account the recent development of other blood-borne biomarkers such as circulating tumor DNA (48). Potential trial designs have been proposed elsewhere to demonstrate the clinical utility of CTCs (49). Ideally, improvements in detection techniques and in the downstream molecular characterization of the CTCs isolated will ultimately lead to the development of tailored drugs targeting the breast cancer metastatic process.
Funding
This work was supported by the Institut Curie SIRIC2 (grant INCa-DGOS-INSERM_12554).
Notes
Affiliations of authors: Department of Medical Oncology, Institut Curie, Paris and Saint Cloud, France (LT, LC, JYP, FCB); Inserm U900, Institut Curie, Saint Cloud, France (AM, AL); Circulating Tumor Biomarkers Laboratory, Institut Curie, Inserm CIC 1428, PSL Research University, Paris, France (LC, CP, JYP, FCB); UVSQ, Paris Saclay University, Saint Cloud, France (LC, FCB); Université Paris Descartes, Paris, France (JYP); Conservatoire national des arts et métiers, Paris, France (AL).
FC Bidard’s research has been supported by grants from Janssen Diagnostics.
Supplementary Material
References
- 1. Bidard F-C, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406–414. [DOI] [PubMed] [Google Scholar]
- 2. Ashworth T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869;14:146. [Google Scholar]
- 3. Kowalewska M, Chechlinska M, Nowak R.. Carcinoembryonic antigen and cytokeratin 20 in peritoneal cells of cancer patients: are we aware of what we are detecting by mRNA examination? Br J Cancer. 2008;98(2):512–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chechlinska M, Kowalewska M, Nowak R.. Systemic inflammation as a confounding factor in cancer biomarker discovery and validation. Nat Rev Cancer. 2010;10(1):2–3. [DOI] [PubMed] [Google Scholar]
- 5. Werner SL, Graf RP, Landers M, et al. Analytical validation and capabilities of the epic CTC platform: enrichment-free circulating tumour cell detection and characterization. J Circ Biomark. 2015;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–6904. [DOI] [PubMed] [Google Scholar]
- 7. Ignatiadis M, Riethdorf S, Bidard F-C, et al. International study on inter-reader variability for circulating tumor cells in breast cancer. Breast Cancer Res BCR. 2014;16(2):R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ligthart ST, Coumans FAW, Bidard F-C, et al. Circulating tumor cells count and morphological features in breast, colorectal and prostate cancer. PloS One. 2013;8(6):e67148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Méhes G, Witt A, Kubista E, et al. Circulating breast cancer cells are frequently apoptotic. Am J Pathol. 2001;159(1):17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wirtz D, Konstantopoulos K, Searson PC.. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. 2011;11(7):512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meng S, Tripathy D, Frenkel EP, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10(24):8152–8162. [DOI] [PubMed] [Google Scholar]
- 12. Janni WJ, Rack B, Terstappen L, et al. Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin Cancer Res. 2016;22(10):2583–2593. [DOI] [PubMed] [Google Scholar]
- 13. Bidard F-C, Michiels S, Riethdorf S, et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J Natl Cancer Inst. 2018;110(6):560–567. [DOI] [PubMed] [Google Scholar]
- 14. Vincent-Salomon A, Bidard FC, Pierga JY.. Bone marrow micrometastasis in breast cancer: review of detection methods, prognostic impact and biological issues. J Clin Pathol. 2008;61(5):570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gebauer G, Fehm T, Merkle E, et al. Epithelial cells in bone marrow of breast cancer patients at time of primary surgery: clinical outcome during long-term follow-up. J Clin Oncol. 2001;19(16):3669–3674. [DOI] [PubMed] [Google Scholar]
- 16. Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353(8):793–802. [DOI] [PubMed] [Google Scholar]
- 17. Bidard F-C, Vincent-Salomon A, Gomme S, et al. Disseminated tumor cells of breast cancer patients: a strong prognostic factor for distant and local relapse. Clin Cancer Res. 2008;14(11):3306–3311. [DOI] [PubMed] [Google Scholar]
- 18. Schindlbeck C, Andergassen U, Jueckstock J, et al. Disseminated and circulating tumor cells in bone marrow and blood of breast cancer patients: properties, enrichment, and potential targets. J Cancer Res Clin Oncol. 2016;142(9):1883–1895. [DOI] [PubMed] [Google Scholar]
- 19. Hüsemann Y, Geigl JB, Schubert F, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13(1):58–68. [DOI] [PubMed] [Google Scholar]
- 20. Podsypanina K, Du Y-C, Jechlinger M, et al. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321(5897):1841–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sänger N, Effenberger KE, Riethdorf S, et al. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int J Cancer. 2011;129(10):2522–2526. [DOI] [PubMed] [Google Scholar]
- 22. Ignatiadis M, Rothé F, Chaboteaux C, et al. HER2-positive circulating tumor cells in breast cancer. PLoS One. 2011;6(1):e15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bidard F-C, Belin L, Delaloge S, et al. Time-dependent prognostic impact of circulating tumor cells detection in non-metastatic breast cancer: 70-month analysis of the REMAGUS02 study. Int J Breast Cancer. 2013;2013:130470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riethdorf S, Müller V, Zhang L, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010;16(9):2634–2645. [DOI] [PubMed] [Google Scholar]
- 25. Pierga J-Y, Bidard F-C, Autret A, et al. Circulating tumour cells and pathological complete response: independent prognostic factors in inflammatory breast cancer in a pooled analysis of two multicentre phase II trials (BEVERLY-1 and -2) of neoadjuvant chemotherapy combined with bevacizumab. Ann Oncol. 2017;28(1):103–109. [DOI] [PubMed] [Google Scholar]
- 26. Mego M, Cierna Z, Janega P, et al. Relationship between circulating tumor cells and epithelial to mesenchymal transition in early breast cancer. BMC Cancer. 2015;15:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pierga J-Y, Petit T, Delozier T, et al. Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 study. Lancet Oncol. 2012;13(4):375–384. [DOI] [PubMed] [Google Scholar]
- 28. García-Sáenz JA, Martín M, Maestro ML, et al. Circulating tumour cells in locally advanced breast cancer. Clin Transl Oncol. 2009;11:544–547. [DOI] [PubMed] [Google Scholar]
- 29. van Dalum G, van der Stam GJ, Tibbe AGJ, et al. Circulating tumor cells before and during follow-up after breast cancer surgery. Int J Oncol. 2015;46(1):407–413. [DOI] [PubMed] [Google Scholar]
- 30. Karhade M, Hall C, Mishra P, et al. Circulating tumor cells in non-metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2014;147(2):325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franken B, de Groot MR, Mastboom WJB, et al. Circulating tumor cells, disease recurrence and survival in newly diagnosed breast cancer. Breast Cancer Res. 2012;14(5):R133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lucci A, Hall CS, Lodhi AK, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13(7):688–695. [DOI] [PubMed] [Google Scholar]
- 33. Krishnamurthy S, Cristofanilli M, Singh B, et al. Detection of minimal residual disease in blood and bone marrow in early stage breast cancer. Cancer. 2010;116(14):3330–3337. [DOI] [PubMed] [Google Scholar]
- 34. Rack B, Schindlbeck C, Jückstock J, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014;106(5) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pachmann K, Camara O, Kavallaris A, et al. Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J Clin Oncol. 2008;26(8):1208–1215. [DOI] [PubMed] [Google Scholar]
- 36. Goodman CR, Seagle B-L, Friedl TWP, et al. Association of circulating tumor cell status with benefit of radiotherapy and survival in early-stage breast cancer. JAMA Oncol. 2018;4(8):e180163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bidard F-C, Kirova YM, Vincent-Salomon A, et al. Disseminated tumor cells and the risk of locoregional recurrence in nonmetastatic breast cancer. Ann Oncol. 2009;20(11):1836–1841. [DOI] [PubMed] [Google Scholar]
- 38. Mignot F, Loirat D, Dureau S, et al. Disseminated tumor cells predict efficacy of regional nodal irradiation in early stage breast cancer. Int J Radiat Oncol Biol Phys. 2019;103(2):389–396. [DOI] [PubMed] [Google Scholar]
- 39. Paik S, Kim C, Wolmark N.. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358(13):1409–1411. [DOI] [PubMed] [Google Scholar]
- 40. Georgoulias V, Bozionelou V, Agelaki S, et al. Trastuzumab decreases the incidence of clinical relapses in patients with early breast cancer presenting chemotherapy-resistant CK-19mRNA-positive circulating tumor cells: results of a randomized phase II study. Ann Oncol. 2012;23(7):1744–1750. [DOI] [PubMed] [Google Scholar]
- 41. Ignatiadis M, Litière S, Rothe F, et al. Trastuzumab versus observation for HER2 non amplified early breast cancer with circulating tumor cells (EORTC 90091-10093, BIG 1-12, Treat CTC): a randomized phase 2 trial. Ann Oncol. 2018;29(8):1777–1783. [DOI] [PubMed] [Google Scholar]
- 42. Fehrenbacher L, Cecchini R, Geyer C, et al. Abstract GS1-02: NSABP B-47 (NRG oncology): phase III randomized trial comparing adjuvant chemotherapy with adriamycin (A) and cyclophosphamide (C) → weekly paclitaxel (WP), or docetaxel (T) and C with or without a year of trastuzumab (H) in women with node-positive or high-risk node-negative invasive breast cancer (IBC) expressing HER2 staining intensity of IHC 1+ or 2+ with negative FISH (HER2-Low IBC). Cancer Res. 2018;78:GS1–G02. [Google Scholar]
- 43. Bauer ECA, Schochter F, Widschwendter P, et al. Prevalence of circulating tumor cells in early breast cancer patients 2 and 5 years after adjuvant treatment. Breast Cancer Res Treat. 2018;171(3):571–580. [DOI] [PubMed] [Google Scholar]
- 44. Trapp E, Janni W, Schindlbeck C, et al. Presence of circulating tumor cells in high-risk early breast cancer during follow-up and prognosis. J Natl Cancer Inst. 2019;111(4):380–387. [DOI] [PubMed] [Google Scholar]
- 45. Janni W, Rack BK, Fasching P, et al. Persistence of circulating tumor cells in high risk early breast cancer patients five years after adjuvant chemotherapy and late recurrence: results from the adjuvant SUCCESS A trial. J Clin Oncol. 2018;36(suppl 15). [Google Scholar]
- 46. Miller KD, O’Neill A, Gradishar W, et al. Double-blind phase III trial of adjuvant chemotherapy with and without bevacizumab in patients with lymph node-positive and high-risk lymph node-negative breast cancer (E5103). J Clin Oncol. 2018;36(25):2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sparano J, O’Neill A, Alpaugh K, et al. Association of circulating tumor cells with late recurrence of estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(12):1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schwarzenbach H, Pantel K.. Circulating DNA as biomarker in breast cancer. Breast Cancer Res. 2015;17(1):136.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sparano JA, Henry NL.. Surveillance after treatment of localized breast cancer: time for reappraisal? J Natl Cancer Inst. 2019;111(4):339–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pierga J-Y, Bonneton C, Vincent-Salomon A, et al. Clinical significance of immunocytochemical detection of tumor cells using digital microscopy in peripheral blood and bone marrow of breast cancer patients. Clin Cancer Res. 2004;10(4):1392–1400. [DOI] [PubMed] [Google Scholar]
- 51. Wiedswang G, Borgen E, Schirmer C, et al. Comparison of the clinical significance of occult tumor cells in blood and bone marrow in breast cancer. Int J Cancer. 2006;118(8):2013–2019. [DOI] [PubMed] [Google Scholar]
- 52. Benoy IH, Elst H, Philips M, et al. Real-time RT-PCR detection of disseminated tumour cells in bone marrow has superior prognostic significance in comparison with circulating tumour cells in patients with breast cancer. Br J Cancer. 2006;94(5):672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fehm T, Hoffmann O, Aktas B, et al. Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res. 2009;11(4):R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Daskalaki A, Agelaki S, Perraki M, et al. Detection of cytokeratin-19 mRNA-positive cells in the peripheral blood and bone marrow of patients with operable breast cancer. Br J Cancer. 2009;101(4):589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Banys M, Krawczyk N, Becker S, et al. The influence of removal of primary tumor on incidence and phenotype of circulating tumor cells in primary breast cancer. Breast Cancer Res Treat. 2012;132(1):121–129. [DOI] [PubMed] [Google Scholar]
- 56. Molloy TJ, Bosma AJ, Baumbusch LO, et al. The prognostic significance of tumour cell detection in the peripheral blood versus the bone marrow in 733 early-stage breast cancer patients. Breast Cancer Res. 2011;13(3):R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hartkopf AD, Stefanescu D, Wallwiener M, et al. Tumor cell dissemination to the bone marrow and blood is associated with poor outcome in patients with metastatic breast cancer. Breast Cancer Res Treat. 2014;147(2):345–351. [DOI] [PubMed] [Google Scholar]
- 58. Pierga J-Y, Bidard F-C, Mathiot C, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res. 2008;14(21):7004–7010. [DOI] [PubMed] [Google Scholar]
- 59. Bidard F-C, Mathiot C, Delaloge S, et al. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol. 2010;21(4):729–733. [DOI] [PubMed] [Google Scholar]
- 60. Azim HA, Rothé F, Aura CM, et al. Circulating tumor cells and response to neoadjuvant paclitaxel and HER2-targeted therapy: a sub-study from the NeoALTTO phase III trial. Breast. 2013;22(6):1060–1065. [DOI] [PubMed] [Google Scholar]
- 61. Hall C, Karhade M, Laubacher B, et al. Circulating tumor Cells after neoadjuvant chemotherapy in stage I-III triple-negative breast cancer. Ann Surg Oncol. 2015;22 Suppl 3:S552–558. [DOI] [PubMed] [Google Scholar]
- 62. Onstenk W, Kraan J, Mostert B, et al. Improved Circulating Tumor Cell Detection by a Combined EpCAM and MCAM CellSearch Enrichment Approach in Patients with Breast Cancer Undergoing Neoadjuvant Chemotherapy. Mol Cancer Ther. 2015;14(3):821–827. [DOI] [PubMed] [Google Scholar]
- 63. Mego M, Giordano A, De Giorgi U, et al. Circulating tumor cells in newly diagnosed inflammatory breast cancer. Breast Cancer Res. 2015;17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ueno T, Masuda N, Kamigaki S, et al. A multicenter phase II trial of neoadjuvant letrozole plus low-dose cyclophosphamide in postmenopausal patients with estrogen receptor-positive breast cancer (JBCRG-07): therapeutic efficacy and clinical implications of circulating endothelial cells. Cancer Med. 2018;7(6):2442–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.