Abstract
Background
Growing evidence suggests a role for cancer susceptibility genes such as BRCA2 and PALB2 in young-onset colorectal cancers. Using a cohort of young colorectal cancer patients, we sought to identify and provide functional evidence for germline pathogenic variants of DNA repair genes not typically associated with colorectal cancer.
Methods
We recruited 88 patients with young-onset colorectal cancers seen at a general oncology center. Whole-exome sequencing was performed to identify variants in DNA repair and colorectal cancer predisposition genes. Pathogenic BRCA2 and PALB2 variants were analyzed using immunoblot and immunofluorescence on patient-derived lymphoblastoid cells.
Results
In general, our cohort displayed characteristic features of young-onset colorectal cancers. Most patients had left-sided tumors and were diagnosed at late stages. Four patients had familial adenomatous polyposis, as well as pathogenic APC variants. We identified 12 pathogenic variants evenly distributed between DNA repair and colorectal cancer predisposition genes. Six patients had pathogenic variants in colorectal cancer genes: APC (n = 4) and MUTYH monoallelic (n = 2). Another six had pathogenic variants in DNA repair genes: ATM (n = 1), BRCA2 (n = 1), PALB2 (n = 1), NTHL1 (n = 1), and WRN (n = 2). Pathogenic variants BRCA2 c.9154C>T and PALB2 c.1059delA showed deficient homologous recombination repair, evident from the impaired RAD51 nuclear localization and foci formation.
Conclusion
A substantial portion of pathogenic variants in young-onset colorectal cancer was found in DNA repair genes not previously associated with colorectal cancer. This may have implications for the management of patients. Further studies are needed to ascertain the enrichment of pathogenic DNA repair gene variants in colorectal cancers.
Colorectal cancer (CRC) is one of the leading causes of cancer deaths worldwide (1). Disease incidence increases dramatically from age 50 years with approximately 10% of cases occurring earlier (2). However, this epidemiologic pattern is shifting, with incidence rising among adults less than age 50 years (3). Compared with the older population, young CRC patients tend to present at more advanced stages with poorer outcomes (4). Early detection, intervention, and prevention would help to reduce disease incidence and mortality (4,5). Current guidelines for early screening are targeted toward individuals with family history of CRC or carriers of germline pathogenic variants in genes with established CRC risk (6).
CRC has a heterogeneous genetic susceptibility profile (4). Up to 70% of all CRCs arise sporadically from somatic variants in low- to moderate-penetrance genes. Only 10% are attributed to germline pathogenic variants in CRC predisposition genes including the mismatch repair genes (3%), APC (1%), MUTYH biallelic (<1%), and MUTYH monoallelic (2%) (7). The remaining 20% may be contributed by germline variants in other moderate- to high-penetrance genes not typically associated with CRCs (8–10).
Recent genomic studies on CRC have identified pathogenic germline variants among DNA repair genes not typically associated with CRCs. Pathogenic variants of BRCA2 and PALB2 have been consistently seen among CRC patients and a threefold increased risk of developing early-onset CRC has also been reported in BRCA2 mutation carriers (7,11–13). However, the mechanism of CRC carcinogenesis has predominantly been overactivation of the Wnt signaling pathway secondary to disruption of tumor suppressors such as APC (14,15). It may be premature to conclude the relevance of DNA repair genes in CRC susceptibility given the limited population and functional evidence (7,11,12). A study on germline mutations of DNA repair genes among young-onset CRCs identified seven germline pathogenic variants in BRCA2 and PALB2 but only one of the tumors showed a loss of heterozygosity (LOH) for BRCA2 (12).
Notably, DNA damage repair processes are not exclusive and often interact on a molecular level (16). For instance, the homologous recombination (HR) proteins BRCA1 and BARD1 interact physically with MSH2 and MSH6 to regulate downstream processes in DNA mismatch repair (MMR) (16). Therefore, it is possible that defects in HR may contribute to CRC pathogenesis (17). Functional studies such as tumor LOH and HR repair analyses, as well as population studies, will be useful in clarifying the relevance of defective HR pathway in CRC development (7). We sought to fill this gap by profiling the spectrum of pathogenic variants in DNA repair and CRC predisposition genes and evaluate the functional impact of identified variants within an Asian cohort with young-onset CRC. In addition, to investigate if PALB2 and BRCA2 germline variants would indeed result in functional impairment, we evaluated our identified variants functionally.
Materials and Methods
Study Design
We recruited patients diagnosed with CRC before age 50 years on follow-up status at our general medical oncology center from November 2014 to December 2016. We excluded patients with MMR-deficient tumors given our study aim to investigate CRC predisposition in DNA repair genes beyond the known CRC susceptibility genes. Although some individuals with MMR-deficient tumors may have germline pathogenic variants in these genes, this proportion would be reasonably small given the majority of MMR-deficient tumors were accounted for by somatic or germline MMR gene mutations (18). Healthy individuals with no prior history of cancer were obtained from a local database of 831 Asian volunteers (median age = 40 years). Clinicopathological data on age, sex, personal and family (first-degree relatives) cancer history, tumor histology, American Joint Committee on Cancer (AJCC) staging, site, and immunohistochemistry staining for MMR proteins were retrieved from electronic medical records.
Whole-Exome Sequencing
Patient-derived genomic DNA was extracted using a QIAamp DNA mini kit (Qiagen, Hilden, Germany) and sequenced on an Illumina Hiseq 4000 (Illumina, Inc, San Diego, CA, USA). Reads were aligned to the human reference genome (hs37d5) as previously described and elaborated in Supplementary Methods (available online) (19). To prioritize candidate germline variants, we evaluated variants with minor allele frequency (MAF) below 0.01 for functional and genetic evidence of pathogenicity according to American College of Medical Genetics and Genomics (ACMG) criteria and curation tools from ClinGene. MAF was defined in reference to Exome Aggregation Consortium and 1000 Genomes databases and an in-house database of local control population, which provided a better reflection of polymorphisms in the Singaporean population, hence a more accurate estimate of allele frequency for evaluation of PM2 criterion (20,21). MAF of 0.03 was used for genes with autosomal recessive inheritance. Pathogenic variants were validated by Sanger sequencing using BigDye Terminator v3.1 (ABI, ThermoFisher Scientific Corporation, Waltham, MA, USA). Resulting chromatograms were analyzed using Mutation Surveyor (Softgenetics, State College, PA, USA). Tumor DNA, where available, was extracted to assess for LOH of pathogenic variants. Patient genomic DNA was also analyzed for copy number variations using digital multiplex ligation-dependent probe amplification (digitalMLPA) (Supplementary Materials, available online). Genetic testing was performed exclusively on affected patients; relatives were not included.
Selection of CRC Predisposition and DNA Repair Genes
In sum, 20 CRC predisposition and 44 DNA repair genes associated with cancer susceptibility were evaluated. CRC predisposition genes include APC, AXIN2, BMPR1A, BRAF, CDH1, CHEK2, EPCAM, FLCN, GREM1, MLN1, MSH2, MSH6, MUTYH, PMS2, POLD1, POLE, PTEN, SMAD4, STK11 and TP53. DNA repair genes include ATM, ATR, BAP1, BARD1, BLM, BRCA1, BRCA2, BRIP1, CDKN2A, CDK4, DDB2, ERCC2, ERCC3, ERCC4, ERCC5, FANCA, FANCB, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, GEN1, MITF, MRE11A, NBN, NTHL1, PALB2, RAD50, RAD51, RAD51C, RAD51D, RAD54L, RECQL4, RET, RFWD3, SLX4, UBE2T, WRN, XPA, XPC, and XRCC2.
Statistical Analysis
Patient characteristics and sequencing results were summarized as mean (SD) or median (interquartile range) for continuous variables and proportions for categorical variables. Prevalence (95% confidence interval [CI]) of variants were estimated as a proportion of young-onset CRC patients. χ2, Fisher exact test, and one-way ANOVA were used to compare clinicopathological variables among carriers and noncarriers of pathogenic variants.
Cell Treatment
Patient-derived lymphoblastoid cells (LCLs) were treated with 10 µM etoposide for 1 hour, subsequently recovered by incubation in fresh medium for 1 hour or 6 hours, and then harvested for immunoblot and immunofluorescence analyses. Vehicle control contained an equivalent volume of dimethyl sulfoxide.
Immunoblot
Subcellular fractionation was performed using the nuclear/cytoplasmic separation protocol previously described (22). Fractionated proteins were electrophoresed on sodium-dodecyl-sulfate polyacrylamide gel and transferred to polyvinylidene difluoride membrane (Milipore, Sigma-Aldrich, St. Louis, MO, USA). Membranes were blocked, incubated overnight with primary antibody followed by secondary horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin (DakoCytomation, Glostrup, Denmark; P044701 and P044801, 1/10 000). Immunoreactivity was detected with enhanced chemilumescent horseradish peroxidase substrate (Advansta, Menlo Park, CA, USA) and quantified using ImageJ (NIH, MD, USA) software.
Immunofluorescence
Cells were fixed 15 minutes using 4% paraformaldehyde, permeabilized 5 minutes using 0.3% Triton-X 100, and blocked 30 minutes with 10% goat serum in phosphate-buffered saline before incubation at 37ºC for 30 minutes with primary antibodies. Slides were stained with AlexaFluor 488 or 594 conjugated secondary antibodies (ThermoFisher Scientific Corporation; A11008 and A11020, 1:1000) and mounted with Prolong Gold antifade reagent with 4′, 6-diamidino-2-phenylindole (Life Technologies, ThermoFisher Scientific Corporation). Images were acquired by confocal microscope (Zeiss LSM800). Cells containing five or more foci were scored positive for RAD51 foci formation.
Results
Clinicopathological Characteristics of Patients
Of the 88 young CRC patients, males and females were equally represented; the majority was Chinese (73.9%). Age at diagnosis averaged 41 years, ranging from 17 to 49 years. Most patients had left-sided colorectal cancer (80.7%) and were diagnosed at late stages (AJCC stage III and IV; 84.1%). Other than young age at diagnosis, high-risk phenotypic features necessitating clinical genetic testing were absent in most patients; more than half of the patients did not have first-degree relatives with cancer and only four patients had a personal history of multiple colonic polyps (Table 2). Adenocarcinoma, not otherwise specified, was the most common histological subtype. Full clinicopathological data are summarized in Table 1.
Table 2.
Clinicopathological characteristics of pathogenic variants
| Pathogenic variant | Sex | Race | Current age, y | Age at CRC diagnosis, y | Other personal history of cancers | Family history of cancers in first-degree relatives | MMR status |
|---|---|---|---|---|---|---|---|
| APC c.1884_1885delTT | Male | Indian | 34 | 29 | Familial adenomatous polyposis; multiple gastric and colonic polyps | Not recorded* | Not recorded |
| APC c.3921_3925del AAAAG | Female | Indian | 23 | 18 | Familial adenomatous polyposis; single gastric and multiple colonic polyps | Mother (adenomatous polyposis coli) | Not recorded |
| APC c.4615delT | Male | Chinese | 39 | 32 | Gardner syndrome, with synchronous desmoid tumor at 32yo; multiple gastric and colonic polyps | Mother (cervical cancer); siblings had negative findings on colonoscopy | Not recorded |
| APC c.3928A>T | Male | Chinese | 29 | 22 | Familial adenomatous polyposis; multiple gastric and colonic polyps | Paternal cousin (unknown cancer); no family history of FAP | Proficient |
| MUTYH c.857G>A | Male | Chinese | Deceased at 50 | 49 | None | Not recorded | Not recorded |
| MUTYH c.934-2A>G | Female | Chinese | 53 | 49 | None | Sister (uterine cancer, 60yo); brother (colon cancer, 63yo) | Proficient |
| ATM c.477_481delATCTC | Male | Chinese | 41 | 36 | None | None | Proficient |
| BRCA2 c.9154C>T | Male | Chinese | 52 | 46 | None | Father (Gastric cancer, 63yo) | Proficient |
| NTHL1 c.793G>A | Male | Chinese | 56 | 48 | None | Mother (colon cancer, 68yo) | Proficient |
| PALB2 c.1059delA | Male | Chinese | 48 | 46 | None | Father (lung cancer, 30yo); paternal uncle (colon cancer, 60yo); paternal aunt (breast cancer, 67yo); maternal uncle (melanoma, 40yo); maternal aunt (breast cancer) | Proficient |
| WRN c.499_500delAA | Female | Chinese | 48 | 44 | Krukenberg tumor, 44yo | Father (colon cancer, 75yo); paternal aunt (colon cancer, 60yo); maternal aunt (uterine cancer); maternal cousin (ovarian cancer) | Proficient |
| WRN c.499_500delAA | Male | Chinese | 55 | 49 | None | Father (stomach cancer); brother (colon cancer) | Proficient |
Not recorded in the patient’s electronic medical records. CRC = colorectal cancer.
Table 1.
Patient characteristics
| Characteristics | All patients (n = 88) | Patients without pathogenic variants (n = 76) | Patients with pathogenic CRC predisposition gene variants (n = 6) | Patients with pathogenic DNA repair gene variants (n = 6) | P |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 44 (50.0) | 35 (46.1) | 4 (66.7) | 5 (83.3) | .149 |
| Female | 44 (50.0) | 41 (53.9) | 2 (33.3) | 1 (16.7) | |
| Race | |||||
| Chinese | 65 (73.9) | 55 (72.4) | 4 (66.7) | 6 (100.0) | .017 |
| Malay | 11 (12.5) | 11 (14.5) | 0 (0.0) | 0 (0.0) | |
| Indian | 4 (4.5) | 2 (2.6) | 2 (33.3) | 0 (0.0) | |
| Others | 8 (9.1) | 8 (10.5) | 0 (0.0) | 0 (0.0) | |
| Age of diagnosis, y | |||||
| Mean ± SE | 41.4 ± 8.0 | 41.8 ± 7.3 | 33.2 .6 ± 5.4 | 44.8 ± 1.9 | .019* |
| Range | 17 to 49 | 17 to 49 | 18 to 49 | 36 to 49 | |
| CRCs in first-degree relatives | |||||
| Present | 13 (18.6) | 8 (13.8) | 2 (33.3) | 3 (50.0) | .059 |
| Absent | 57 (81.4) | 50 (86.2) | 4 (66.7) | 3 (50.0) | |
| Missing | 18 | 18 | 0 | 0 | |
| Breast cancers in first-degree relatives | |||||
| Present | 4 (5.7) | 4 (6.9) | 0 (0.0) | 0 (0.0) | .645 |
| Absent | 66 (94.3) | 54 (93.1) | 6 (100.0) | 6 (100.0) | |
| Missing | 18 | 18 | 0 | 0 | |
| Ovarian cancers in first-degree relatives | |||||
| Present | 1 (1.4) | 1 (1.7) | 0 (0.0) | 0 (0.0) | .900 |
| Absent | 69 (98.6) | 57 (98.3) | 6 (100.0) | 6 (100.0) | |
| Missing | 18 | 18 | 0 | 0 | |
| Any cancers in first-degree relatives | |||||
| Present | 32 (45.7) | 23 (39.7) | 4 (66.7) | 5 (83.3) | .416 |
| Absent | 38 (54.3) | 35 (60.3) | 2 (33.3) | 1 (16.7) | |
| Missing | 18 | 18 | 0 | 0 | |
| Location of CRC | |||||
| Right sided | 17 (19.3) | 16 (21.1) | 0 (0.0) | 1 (16.7) | .447 |
| Left sided | 71 (80.7) | 60 (78.9) | 6 (100.0) | 5 (83.3) | |
| Histological subtype | |||||
| Adenocarcinoma | 5 (5.7) | 3 (3.9) | 1 (16.7) | 1 (14.3) | .387 |
| Mucinous adenocarcinoma | 8 (9.1) | 7 (9.2) | 0 (0.0) | 1 (14.3) | |
| Adenocarcinoma, NOS | 75 (85.2) | 66 (86.8) | 5 (83.3) | 4 (66.7) | |
| Tumor differentiation | |||||
| Well | 5 (5.7) | 4 (5.3) | 1 (16.7) | 0 (0.0) | .724 |
| Moderate | 65 (73.9) | 55 (72.4) | 5 (83.3) | 5 (83.3) | |
| Poor | 6 (6.8) | 6 (7.9) | 0 (0.0) | 1 (16.7) | |
| Not specified | 12 (13.6) | 11 (14.5) | 0 | 0 | |
| AJCC disease stage | |||||
| I | 1 (1.1) | 0 (0.0) | 1 (16.7) | 0 (0.0) | .022† |
| II | 13 (14.8) | 11 (14.5) | 1 (16.7) | 1 (16.7) | |
| III | 47 (53.4) | 40 (52.6) | 3 (50.0) | 4 (66.7) | |
| IV | 27 (30.7) | 25 (32.9) | 1 (16.7) | 1 (16.7) | |
| MMR status (IHC) | |||||
| MMR-deficient | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .272 |
| MMR-proficient | 69 (100.0) | 60 (100.0) | 3 (100.0) | 6 (100.0) | |
| Missing | 19 | 16 | 3 | 0 | |
| ECOG at diagnosis | |||||
| 0 | 64 (73.6) | 53 (70.7) | 6 (100.0) | 5 (83.3) | .585 |
| 1 | 21 (2.4) | 20 (26.7) | 0 (0.0) | 1 (16.7) | |
| 2 | 2 (2.3) | 2 (2.7) | 0 (0.0) | 0 (0.0) | |
| Missing | 1 | 1 | 0 | 0 | |
| CEA at diagnosis | |||||
| 5 or less | 44 (50.0) | 36 (47.4) | 4 (66.7) | 4 (66.7) | .462 |
| More than 5 | 44 (50.0) | 40 (52.6) | 2 (33.3) | 2 (33.3) | |
Post hoc analysis showed statistically significant differences when comparing patients with pathogenic CRC predisposition gene variants against those without any pathogenic variants (P = .026) and those with pathogenic DNA repair gene variants (P = .027). AJCC = American Joint Committee on Cancer; CEA = carcinoembryonic antigen test; CRC = colorectal cancer; ECOG = Eastern Cooperative Oncology Group Performance Status; IHC = immunohistochemistry; MMR = mismatch repair; NOS = not otherwise specified.
Statistically significant difference was observed between patients with pathogenic CRC predisposition gene variants and those without any pathogenic variants (P = .004).
Variant Pathogenicity in Colorectal Predisposition Genes
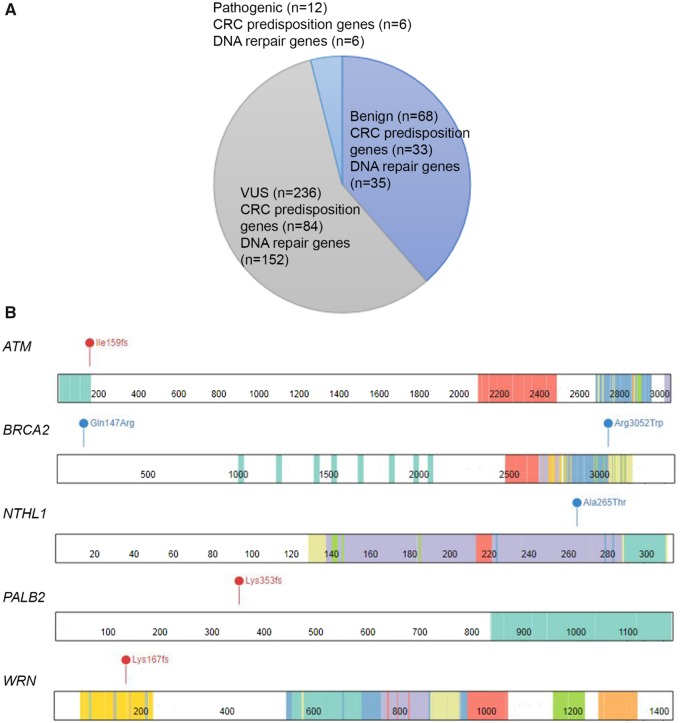
Using whole-exome sequencing, we identified 316 germline variants fulfilling the MAF criteria described earlier (Figure 1A), of which 12 were classified pathogenic. No copy number variation was identified in the entire cohort. Of the pathogenic variants identified from whole-exome sequencing, six occurred in known CRC predisposition genes: three truncating and one missense APC variants, and two missense MUTYH monoallelic variants (Table 2). All four APC variants were identified in four FAP patients with both gastric and colonic polyposis. Three patients developed rectal cancer without extracolonic involvement; the remaining patient subsequently developed a synchronous desmoid tumor and sigmoid colon cancer. Overall, compared to noncarriers, patients with pathogenic variants in CRC predisposition genes presented disease at an earlier age (mean age of 33 vs 42 years, P = .026) and at an earlier stage (33.4% at stage I and II vs 14.5%, P = .004) (Table 1).
Table 3.
Comparison of studies on pathogenic variants in CRC patients
| Matthew et al. (7) | Pearlman et al. (11) | Saud et al. (12) | Stoffel et al. (5) | Our study | |
|---|---|---|---|---|---|
| n = 1058 (%) | n = 450 (%) | n = 680 (%) | n = 430 (%) | n = 88 (%) | |
| Age of diagnosis (mean ± SD), y | 55.7 ± 12.6 | 42.5 | 68.8 ± 10.3 | 40.0 | 41.4 ± 0.8 |
| Study setting | Gastrointestinal cancer institute | General oncology clinics | General oncology clinics | Cancer genetics clinics | General oncology clinic |
| Genetic studies | Next-generation sequencing | Next-generation sequencing | Next-generation sequencing | Next-generation sequencing | Next-generation sequencing digitalMLPA |
| Functional studies | None | Tumor LOH | Tumor LOH | None | Tumor LOH; homologous recombination assay (RAD51) |
| CRCs in first-degree relatives | 138 (13.0) | 33 (45.8) | 164 (25.0) | 111 (25.8) | 13 (18.6) |
| Breast cancers in first-degree relatives | 138 (13.0) | 8 (11.1) | 85 (19.0) | NA | 4 (5.7) |
| Ovarian cancers in first-degree relatives | 23 (2.2) | 2 (2.8) | 19 (4.3) | NA | 1 (1.4) |
| Any cancers in first-degree relatives | 870 (82.2) | 53 (11.8) | 395 (59.0) | NA | 33 (36.3) |
| MMR-deficient tumors/MSI-H | 83 (14.5); 486 missing data | 48 (10.7); no missing data | 92 (16.0); 113 missing data | 41 (20.1); 226 missing data | 0 (0.0); 19 missing data |
| CRC predisposition genes tested | APC, BMPR1A, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, MUTYH, PMS2, PTEN, SMAD4, STK11, TP53 | APC, BMPR1A, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, MUTYH, PMS2, PTEN, SMAD4, STK11, TP53 | APC, BMPR1A, CHEK2, MLH1, MSH2, MSH6, MUTYH, PMS2, POLD1, POLE, PTEN, SMAD4, STK11, TP53 | APC, AXIN2, BMPR1A, BRAF, CDH1, CHEK2, EPCAM, KRAS, MLH1, MSH2, MSH6, MUTYH, PMS2, POLD1, POLE, PTEN, SMAD4, STK11, TP53 | APC, AXIN2, BMPR1A, BRAF, CDH1, CHEK2, EPCAM, FLCN, GREM1, MLN1, MSH2, MSH6, MUTYH, PMS2, POLD1, POLE, PTEN, SMAD4, STK11, TP53 |
| DNA repair genes tested | ATM, BARD1, BRCA1, BRCA2, BRIP1, CDK4, CDKN2A, CHEK2, NBN, PALB2, RAD51C, RAD51D | ATM, BARD1, BRCA1, BRCA2, BRIP1, CDK4, CDKN2A, CHEK2, NBN, PALB2, RAD51C, RAD51D | ATM, ATR, BAP1, BARD1, BLM, BRCA1, BRCA2, BRIP1, DDB2, ERCC2, ERCC3, ERCC4, ERCC5, FANCA, FANCB, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, GEN1, MRE11, NBN, NTHL1, PALB2, PCNA, POLH, RAD51, RAD51C, RAD51D, RAD54L, RECQL4, SLX4, UBE2T, WRN, XPA, XPC, XRCC3 | AKT1, ALK, ARID1A, ATR, AURKA, BAP1, BARD1, BRCA1, BRCA2, BRIP1, CASP8, CDKN2A, CTNNB1, ERBB3, FAM123B, FAT1, FBXW7, FGFR3, GALNT12, GREM1, HNF1A, HOXB12, HRAS, IGF1, IGF2, IGF2R, MEN1, MET, MSH3, MYC, NBN, NOTCH1, PALB2, PIK3CA, PTCH1, PTPN11, RAD51, SDHB, SMAD2, SMAD3, SMAD7, SOX9, SYNE1, TERT, TET2, TGFRB2, VHL | ATM, ATR, BAP1, BARD1, BLM, BRCA1, BRCA2, BRIP1, CDKN2A, CDK4, DDB2, ERCC2, ERCC3, ERCC4, ERCC5, FANCA, FANCB, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, GEN1, MITF, MRE11A, NBN, NTHL1, PALB2, RAD50, RAD51, RAD51C, RAD51D, RAD54L, RECQL4, RET, RFWD3, SLX4, UBE2T, WRN, XPA, XPC, XRCC2 |
| DNA used | Germline DNA | Germline DNA and tumor DNA | Germline DNA | Germline DNA | Germline DNA and tumor DNA |
| Pathogenic variants in CRC predisposition genes | MLH1 (n=13), MSH2 (n=7), MSH6 (n=6), PMS2 (n=7), MUTYH biallelic (n=3), APC (n=19), MUTYH monoallelic (n=18), CHEK2 (n=2) | MLH1 (n=13), MSH2 (n=16), MSH6 (n=2), PMS2 (n=5), MUTYH biallelic (n=4), APC (n=10), MUTYH monoallelic (n=7), SMAD4 (n=1) | MSH2 (n=1), MSH6 (n=1), PMS2 (n=2), APC (n=10), MUTYH monoallelic (n=11), CHEK2 (n=4), TP53 (n=2) | MLH1 (n=24), MSH2 (n=25), MSH6 (n=5), PMS2 (n=2), MUTYH biallelic (n=6), APC (n=8), MUTYH monoallelic (n=1), SMAD4 (n=2), CHEK2 (n=1) | APC (n=4), MUTYH monoallelic (n=2) |
| Pathogenic variants in DNA repair genes | ATM (n=10), BARD1 (n=1), BRCA1 (n=3), BRCA2 (n=8), BRIP1 (n=3), CDKN2A (n=1), NBN (n=2), PALB2 (n=2), TP53 (n=1) | ATM (n=4), BRCA1 (n=2), BRCA2 (n=4), CDKN2A (n=1) and PALB2 (n=2) | ATM (n=5), BAP1 (n=1), BARD1 (n=1), BLM (n=3), BRCA1 (n=1), BRCA2 (n=4), BRIP1 (n=2), ERCC2 (n=2), ERCC3 (n=1), ERCC4 (n=1), FANCC (n=1), FANCE (n=1), FANCL (n=1), GEN1 (n=2), MRE11 (n=2), PALB2 (n=3), POLH (n=1), RECQL4 (n=2), SLX4 (n=1), XPA (n=1), XRCC3 (n=1) | BRCA1 (n=1), TP53 (n=1) | ATM (n=1), BRCA2 (n=1), NTHL1 (n=1), PALB2 (n=1), WRN (n=2) |
| Features of patients with pathogenic CRC predisposition gene variants* | Younger age† | Younger age | Younger age | Right-sided colon involvement† | Younger age† |
| Right-sided colon involvement† | Right-sided colon involvement | Family history of breast cancers† | MSI tumors† | Earlier stage of diagnosis† | |
| Earlier stage of diagnosis† | Earlier stage of diagnosis | MSI tumors | Family history of CRCs† | ||
| MSI tumors† | MSI tumors | Earlier stage of diagnosis† | |||
| Personal and family history of multiple CRCs† | Personal and family history of multiple CRCs* | ||||
| Features of patients with pathogenic DNA repair gene variants* | None | None | None | None | None |
Compared to noncarriers of germline pathogenic variants. CRC= colorectal cancer; digitalMLPA = digital multiplex ligation-dependent probe amplification; MMR= mismatch repair; MSI-H = microsatellite instability-high.
Statistical significance (P < .05) was observed.
Table 4.
Pathogenicity classification using ACMG criteria
| Variant | Population data | Predictive data | Functional data | Segregation data | De novo data | Allelic data | Reputable database* | References |
|---|---|---|---|---|---|---|---|---|
| Pathogenic/likely pathogenic | ||||||||
| APC c.1884_1885del | PM2 | PVS1 | NA | NA | NA | NA | NA | |
| APC c.3921_3925del | PM2 | PVS1 | PM1 | NA | NA | NA | PP5 | |
| APC c.4615delT | PM2 | PVS1 | NA | NA | NA | NA | NA | |
| APC c.3928A>T | PM2 | PVS1 | PM1 | NA | NA | NA | NA | |
| MUTYH c.857G>A | PM2 | PP3 | PS3 | NA | NA | NA | PP5 | |
| MUTYH c.934-2A>G | BS1 | PVS1 | PS3 | NA | NA | NA | PP5 | |
| ATM c.477_481delATCTC | PM2 | PVS1 | NA | NA | NA | NA | PP5 | |
| BRCA2 c.9154C>T | PM2 | PP3 | PS3 | NA | NA | NA | PP5 | |
| NTHL1 c.793G>A | PM2 | PP3 | PM1 | NA | NA | NA | NA | |
| PALB2 c.1059delA | PM2 | PVS1 | NA | NA | NA | NA | PP5 | |
| WRN c.499_500delAA | PM2 | PVS1 | PM1 | NA | NA | NA | NA | |
| VUS | ||||||||
| BRCA2 c.440A>G | PM2 | NA | NA | NA | NA | NA | NA | (45) |
*Variants were assessed for pathogenicity using ClinVar archives, Mastermind search engine by Genomenon, and genetic databases such as the Leiden Open Variation Database (LOVD), BRCA Exchange, InSiGHT, and ARUP MEN2 database. VUS = variants of uncertain significance.
Figure 1.
A) Variants identified in this study. B) Locations of pathogenic DNA repair variants. Red represents frameshift mutations; blue represens missense mutations. BRCA2 c.440A>G (p.Q147R) is a VUS included in the functional studies. CRC = colorectal cancer; VUS = variants of uncertain significance.
Variant Pathogenicity in DNA Repair Genes
The remaining half of the pathogenic variants occurred in DNA repair genes, affecting six patients. Clinically, carriers of pathogenic variants in DNA repair genes were similar to noncarriers (Table 1). These variants included one truncating ATM variant, one missense BRCA2 variant, one missense NTHL1 variant, one truncating PALB2 variant, and two truncating WRN variants (Table 2). Notably, two of the six variants occurred in BRCA2 and PALB2. BRCA2 and PALB2 form a protein complex essential for RAD51 nuclear localization, which is critical for the repair process (31). As both BRCA2 and PALB2 pathogenic variants occurred in domains key to proper RAD51 localization (Figure 1B), we hypothesized that these mutations would impair RAD51 nuclear localization and foci formation. To validate our hypothesis, we performed immunoblotting and immunofluorescence assays for BRCA2 c.9154C>T and PALB2 c.1059delA variants.
Functional Studies
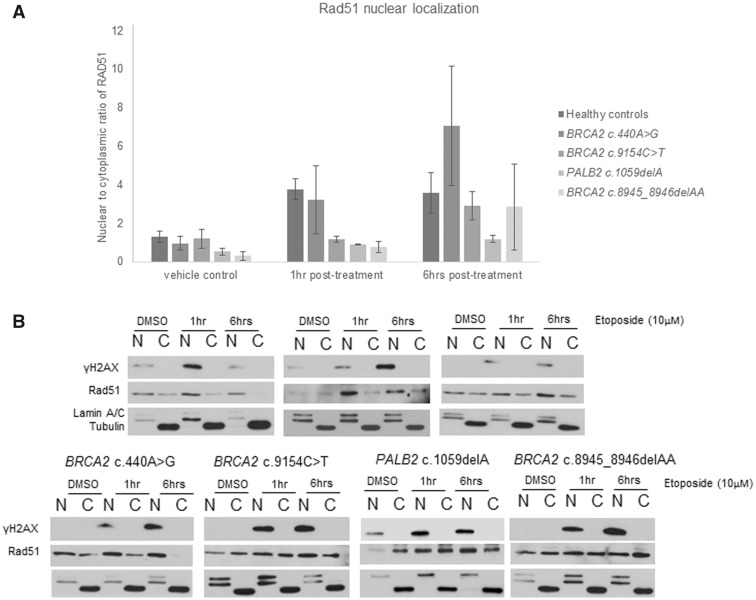
To assess the functional impact of BRCA2 c.9154C>T and PALB2 c.1059delA, we induced double-stranded DNA breaks and measured the HR efficiency by quantifying the RAD51 nuclear localization and foci formation on patient-derived LCLs. Compared to healthy controls, BRCA2 and PALB2 mutation carriers showed impaired RAD51 nuclear localization and foci formation (Figures 2 and 3). To further support the tumorigenesis role of these variants, we assessed for tumor LOH but did not observe LOH of PALB2 (Supplementary Figure 1, available online). LOH data was unavailable for BRCA2 c.9154C>T due to insufficient tumor sample.
Figure 2.
A) Nuclear localization of RAD51 expressed as a ratio of nuclear (N) to cytoplasmic (C) RAD51 levels. Cells with BRCA2 c.9154C>T, PALB2 c.1059delA, and BRCA2 c.8945_8946delAA show impaired RAD51 nuclear localization at the first hour posttreatment, whereas those with BRCA2 c.440A>G displayed normal RAD51 nuclear localization. Triplicates were performed for patients with pathogenic variants. Three healthy controls were used and duplicates were done per control. BRCA2 c.8945_8946delAA was included as a positive control. Independent t test was used to compare RAD51 nuclear localization between variants and healthy controls. A single asterisk (*) refers to P < .05, a double (**) to P < .005. B) Representative blot showing changes in nuclear and cytoplasmic RAD51 levels at 1 hour and 6 hours following etoposide treatment. In the healthy controls, nuclear RAD51 was higher than the cytoplasmic RAD51 following treatment. In contrast, the nuclear RAD51 remained similar to the cytoplasmic RAD51 in BRCA2 c.9154C>T, PALB2 c.1059delA, and BRCA2 c.8945_8946delAA. DMSO = dimethyl sulfoxide.
Figure 3.
A) Immunofluorescence analysis of RAD51 foci formation (represented as green foci) following etoposide treatment. Compared to healthy controls, RAD51 foci formation was impaired for BRCA2 c.9154C>T, PALB2 c.1059delA, and BRCA2 c.8945_8946delAA. B) Percentage of cells with more than 5 RAD51 foci. Impaired RAD51 foci formation was noted for BRCA2 c.9154C>T, PALB2 c.1059delA, and BRCA2 c.8945_8946delAA at 6 hours following treatment. Triplicates were performed for patients with pathogenic variants. Duplicates were done per healthy control. BRCA2 c.8945_8946delAA was included as a positive control. Independent t test was used to compare RAD51 nuclear localization between variants and healthy controls. A single asterisk (*) refers to P < .05; a double (**) refers to P < .005. DMSO = dimethyl sulfoxide.
Among the 236 variants of uncertain significance (VUS), eight occurred in BRCA2 and PALB2. Of these, we were only able to obtain LCL of BRCA2 c.440A>G, which occurred in a site with no known function (Figure 1B). As the variant was enriched among patients (MAF < 0.005; OR = 2.34, 95% CI = 0.49 to 11.18) and located near the PALB2-binding domain responsible for PALB2-RAD51 interaction (Figure 1B), we evaluated this variant functionally but found no evidence of perturbed RAD51 foci formation or tumor LOH (Figures 2 and 3; Supplementary Figure 1, available online).
Discussion
To the best of our knowledge, this was the first study of pathogenic DNA repair gene variants in Asian patients with young-onset CRCs. Similar to previous studies, we found most patients presented advanced-stage diseases in their forties (2,11,23). Another characteristic feature was a predilection for the distal colon and rectum, which would manifest as early symptoms of changes in bowel habit and constipation (24). Unfortunately, because of the patients’ relatively young age, clinicians tended to dismiss these nonspecific symptoms, hence delaying diagnosis and treatment (24).
Exceptions to this trend were patients with high-risk phenotypic features such as personal or family history of hereditary CRC syndromes or pathogenic variants in CRC predisposition genes. Studies on young-onset CRCs, including ours, observed that these patients were often diagnosed at a younger age and earlier disease stages compared with their peers who lack those features (5,7,11,12). This pattern of diagnosis might be related to the close surveillance and prompt treatment received by at-risk patients, which often translated to improved survival outcomes (25). These benefits could be extended to more patients, given that young-onset CRCs had a heritable component beyond CRC predisposition genes.
Previously, pathogenic variants identified among CRC patients predominantly involved CRC predisposition genes, partly because of use of limited gene panels (5,10,11). Broader use of pan-cancer panels resulted in increasing discovery of pathogenic variants in DNA repair genes and an enrichment of pathogenic PALB2 variants was observed in a study on CRC patients (5,7,11,12). It is conceivable that HR deficiency contributed to CRC tumorigenesis, given the indispensable role of HR in repair of highly damaging double-stranded DNA breaks (26).
In accordance with the Knudson two-hit hypothesis, deficient repair process and eventual tumorigenesis resulted from the inactivation of a wild-type allele in an individual with a germline pathogenic variant (27). Although tumor LOH has not been reported in patients with PALB2 and BRCA2 pathogenic variants, these variants could still be relevant in CRC tumorigenesis. Here, we demonstrated that BRCA2 and PALB2 monoallelic pathogenic variants were associated with impaired RAD51 foci formation. Likewise, previous studies established haploinsufficiency for both BRCA2 and PALB2 where a monoallelic pathogenic variant sufficed to directly impair function or promote the inactivation of the wild-type allele (28,29). In addition, tumor LOH was absent in most PALB2 germline mutation carriers and most PALB2 heterozygous cancers displayed high HR deficiency scores (12,30). An impaired HR might predispose the patient to worsening genomic instability and eventual tumorigenesis (26). Clinically, patients with pathogenic variants in DNA repair genes did not share any characteristic phenotype suggestive of a distinct CRC subset, unlike those with pathogenic variants in the CRC predisposition genes who tended to be younger with more right-sided colon involvement and earlier disease stages (5,7,11,12). To ascertain whether germline pathogenic variants in HR genes were truly drivers of CRCs and whether these CRCs shared a distinct tumor phenotype, more studies on tumor LOH and mutational signatures in various HR-deficient CRCs will be needed.
Besides HR, there were other prominent DNA repair pathways such as nonhomologous end joining and base excision repair (29,31,32). Nonhomologus end joinging was the alternative repair process for double-stranded DNA breaks under regulation by checkpoint kinases such as ATM (32). Germline variants of ATM were associated with autosomal recessive ataxia telangiectasia and autosomal dominant cancers, namely breast cancer. More recently, pathogenic variants in ATM were also found to be enriched in CRC patients (7,11,12). However, the precise CRC risks for ATM pathogenic variants were unclear and ATM was not considered a CRC predisposition gene by the National Comprehensive Cancer Network guidelines (12,33–35).
Apart from ATM, we identified monoallelic variants in NTHL1 and WRN. The NTHL1 gene was involved in base excision repair, and was recently validated as an autosomal recessive CRC susceptibility gene after NTHL1 biallelic variants were found to cause CRCs (36–38). A homozygous nonsense variant in NTHL1 (p.Gln90*) was detected among seven CRC patients from three unrelated families whereas compound heterozygous NTHL1 variants were found in a patient with multiple primary tumors, including CRC (38). For WRN, which encoded for a helicase with exonuclease function, preliminary studies hinted at the possibility of WRN-BRCA1 interaction in HR pathway (39). Pathogenic variants in WRN were predominantly truncating and WRN somatic hypermethylation was also observed in various tumors, including colon tumors (40). However, the increased risk of neoplasia was only seen in biallelic WRN mutations (41). Similar to NTHL, the unclear role of WRN monoallelic variants in young-onset CRCs will require further evaluation.
The cumulative risk of young-onset CRC associated with MUTYH monoallelic variants was modest and statistically insignificant (0.8%, 95% CI = 0.5% to 1.3%), unlike those of biallelic MUTYH variants (24.8%, 95% CI = 7.7% to 57.1%) (42). Moreover, the frequency of MUTYH monoallelic variants in CRC patients (1.6% to 2.2%) was similar to that of controls (1.7%) (7,11,12,43). Currently, CRC screening guidelines do not require carriers of MUTYH monoallelic variants to undergo more intensive screening than the general population (44).
In addition to identifying pathogenic variants, we functionally evaluated a VUS in BRCA2 (c.440A>G). This variant was previously reported in a Chinese family with multiple breast cancers where it was found in all three sisters with breast cancers and absent in the father and another unaffected sister (45). However, we did not observe any functional evidence for pathogenicity consistent with the low in silico prediction score for pathogenicity (REVEL score = 0.338). Hence we concluded that this variant was unlikely to be pathogenic.
However, our study has several limitations. We had a small sample size of 88 patients. Our study was thus underpowered to perform statistical inference of the odds ratio, which was required as part of the ACMG curation criteria (20). Nonetheless, we identified similar clinical and genetic features as the larger studies. In addition, our cohort had similar characteristics of young-onset CRCs as the earlier studies. Another limitation was that we were unable to perform tumor LOH analysis for the MUTYH pathogenic variants, which would have been useful in confirming their pathogenicity status.
To reliably interpret germline variants, both genetic and functional evidence are required. Currently, there is a lack of functional validation of pathogenic variants in the DNA repair genes. Most were limited to tumor LOH studies. Although presence of biallelic inactivation offered evidence for the variant as a driver mutation, monoallelic variants could still have an impact on tumorigenesis. Direct assessment of protein function would be essential to determine the pathogenicity of a variant in question, especially for genes such as BRCA2 and PALB2, which exhibited haploinsufficiency and did not require LOH to exert deleterious functional effects (31,32). Complementing tumor LOH studies, our patient-based RAD51 assays could directly evaluate the function of the variant protein.
A substantial portion of patients with young-onset CRC harbored germline pathogenic variants. Excluding those with hereditary CRC syndromes, most patients with pathogenic variants did not have high-risk phenotypic features. Hence, germline genetic testing should preferably be performed for all young-onset CRC patients. Screening of germline variants in HR genes such as BRCA2 and PALB2 might improve the yield of germline genetic testing. These genes contributed a considerable portion of the pathogenic variants identified among young-onset CRCs and many had established risk management guidelines (46). Furthermore, pathogenic variants in these genes do exert functional consequences and are potentially tumorigenic. Although these genes have yet to be acknowledged as CRC predisposition genes, the evidence is growing. Larger population studies and genome-wide association studies will provide the much-needed conclusion on their relevance in CRC tumorigenesis.
Funding
This work was supported by the National Medical Research Council (CSA) (NMRC/CSA-INV/0017/2017) and Singhealth Foundation Research Grant (SHF/PRISM002/2015) to JN and Duke-NUS (AM-ETHOS01/FY2017/26-A26) to MRT.
Notes
Affiliations of authors: Duke-NUS Medical School, Singapore 169857, Singapore (MRT, JCAJ, JN); Division of Medical Oncology, National Cancer Center, Singapore 169610, Singapore (JBC, STC, SHC, NDBI, EC, WHL, JN); Department of Pathology, Singapore General Hospital, Singapore 169608, Singapore (SMFBSAA, KHL); Singhealth Duke-NUS Institute of Precision Medicine (PRISM), Singapore 169856, Singapore (SD, PT, WKL); Cancer & Stem Cell Biology Program, Duke-NUS Medical School, Singapore 169857, Singapore (PT, WKL); Cancer Science Institute of Singapore, National University Singapore, Singapore 117599, Singapore (PT); Division of Molecular and Cellular Research, National Cancer Center, Singapore 169610, Singapore (IBHT); Genome Institute of Singapore, Agency for Science, Technology and Research, Singapore 138672, Singapore (IBHT); Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore 639798, Singapore (JN); Institute of Molecular and Cellular Biology, Agency for Science, Technology and Research, Singapore 138673, Singapore (JN).
The authors declare no relevant conflict of interest.
This study was approved by the Singhealth Centralized Institutional Review Board (IRB 2010/426/B) with signed informed consent from participants.
We thank our patients and research participants for their contribution to the study. We would also like to acknowledge MRC-Holland for the reagents and analysis support in performing digital multiplex ligation-dependent probe amplification. We would like to acknowledge St. Jude PeCan Data Portal for the web application in generating the lolliplots.
Supplementary Material
References
- 1. National Registry of Diseases Office. Singapore Cancer Registry Annual Registry Report 2015 2017. https://www.nrdo.gov.sg/docs/librariesprovider3/Publications-Cancer/cancer-registry-annual-report-2015_web.pdf? sfvrsn=6. Accessed May 4, 2018.
- 2. Rho YS, Gilabert M, Polom K, et al. Comparing clinical characteristics and outcomes of young-onset and late-onset colorectal cancer: an international collaborative study. Clin Colorectal Cancer. 2017;16(4):334–342. [DOI] [PubMed] [Google Scholar]
- 3. Koblinski J, Jandova J, Nfonsam V.. Disparities in incidence of early- and late-onset colorectal cancer between Hispanics and Whites: a 10-year SEER database study. Am J Surg. 2018;215(4):581–585. [DOI] [PubMed] [Google Scholar]
- 4. Deen KI, Silva H, Deen R, et al. Colorectal cancer in the young, many questions, few answers. World J Gastrointest Oncol. 2016;8(6):481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stoffel EM, Koeppe E, Everett J, et al. Germline genetic features of young individuals with colorectal cancer. Gastroenterology. 2017;154(4):897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high risk assessment: breast and ovarian .J Natl Compr Canc Netw. 2017;15:9-20. [DOI] [PubMed] [Google Scholar]
- 7. Yurgelun MB, Kulke MH, Fuchs CS, et al. Cancer susceptibility gene mutations in individuals with colorectal cancer. J Clin Oncol. 2017;35(10):1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85. [DOI] [PubMed] [Google Scholar]
- 9. Stigliano V, Sanchez-Mete L, Martayan A, et al. Early-onset colorectal cancer: a sporadic or inherited disease? World J Gastroenterol. 2014;20(35):12420–12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tariq K, Ghias K, Tariq K, Ghias K.. Colorectal cancer carcinogenesis: a review of mechanisms. Cancer Biol Med. 2016;13(1):120.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pearlman R, Frankel WL, Swanson B, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 2017;3(4):464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. AlDubayan SH, Giannakis M, Moore ND, et al. Inherited DNA-repair defects in colorectal cancer. Am J Hum Genet. 2018;102(3):401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Phelan C, Iqbal J, Lynch H, et al. Incidence of colorectal cancer in BRCA1 and BRCA2 mutation carriers: results from a follow-up study. Br J Cancer. 2014;110(2):530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sorscher S. Mutation frequencies in patients with early-onset colorectal cancer. JAMA Oncol. 2017;3(11):1585.. [DOI] [PubMed] [Google Scholar]
- 15. Daniels MS, Bannon SA, Mork ME.. Frequency of germline BRCA1/2 mutations in unselected patients with colorectal cancer. J Clin Oncol. 2017; 35(22):2588. [DOI] [PubMed] [Google Scholar]
- 16. Sharma B, Preet Kaur R, Raut S, Munshi A.. BRCA1 mutation spectrum, functions, and therapeutic strategies: the story so far. Curr Probl Cancer. 2018; 42(2):189–207. [DOI] [PubMed] [Google Scholar]
- 17. Wang Q, Zhang H, Guerrette S, et al. Adenosine nucleotide modulates the physical interaction between hMSH2 and BRCA1. Oncogene. 2001;20(34):4640–4649.. [DOI] [PubMed] [Google Scholar]
- 18. Haraldsdottir S, Hampel H, Tomsic J, et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. 2014;147(6):1308–1316.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan SH, Lim WK, Ishak NDB, et al. Germline mutations in cancer predisposition genes are frequent in sporadic sarcomas. Sci Rep. 2017;7(1):10660.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Biesecker LG, Harrison SM.. The ACMG/AMP reputable source criteria for the interpretation of sequence variants. Genet Med. 2018. doi:10.1038/gim.2018.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suzuki K, Bose P, Leong-Quong RY,et al. REAP: a two minute cell fractionation method. BMC Res Notes. 2010;3(1):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siegel RL, Jemal A, Ward EM.. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1695–1698. [DOI] [PubMed] [Google Scholar]
- 24. You Y, Xing Y, Feig BW, et al. Young-onset colorectal cancer: is it time to pay attention? Arch Intern Med. 2012;172(3):287–289. [DOI] [PubMed] [Google Scholar]
- 25. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. [DOI] [PubMed] [Google Scholar]
- 26. Khanna KK, Jackson SP.. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27(3):247.. [DOI] [PubMed] [Google Scholar]
- 27. Xu C-F, Solomon E.. Mutations of the BRCA1 gene in human cancer. Semin Cancer Biol. 1996;7(1):33–40. [DOI] [PubMed] [Google Scholar]
- 28. Nikkilä J, Parplys AC, Pylkäs K, et al. Heterozygous mutations in PALB2 cause DNA replication and damage response defects. Nat Commun. 2013;4(1):2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zámborszky J, Szikriszt B, Gervai JZ, et al. Loss of BRCA1 or BRCA2 markedly increases the rate of base substitution mutagenesis and has distinct effects on genomic deletions. Oncogene. 2017;36(6):746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hartley T, Cavallone L, Sabbaghian N, et al. Mutation analysis of PALB2 in BRCA1 and BRCA2-negative breast and/or ovarian cancer families from Eastern Ontario, Canada. Hered Cancer Clin Pract. 2014;12(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodgers K, McVey M.. Error-prone repair of DNA double-strand breaks. J Cell Physiol. 2016;231(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou Y, Lee J-H, Jiang W, et al. Regulation of the DNA damage response by DNA-PKcs inhibitory phosphorylation of ATM. Mol Cell. 2017;65(1):91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ahmed M, Rahman N.. ATM and breast cancer susceptibility. Oncogene. 2006;25(43):5906.. [DOI] [PubMed] [Google Scholar]
- 34. Dominguez-Valentin M, Nakken S, Tubeuf H, et al. Identification of genetic variants for clinical management of familial colorectal tumors. BMC Med Genet. 2018;19(1):26.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chung DC. Genetic testing and early onset colon cancer. Gastroenterology. 2018;154(4):788–789. [DOI] [PubMed] [Google Scholar]
- 36. Broderick P, Dobbins SE, Chubb D, et al. Validation of recently proposed colorectal cancer susceptibility gene variants in an analysis of families and patients—a systematic review. Gastroenterology. 2017;152(1):75–77.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rivera B, Castellsagué E, Bah I, et al. Biallelic NTHL1 mutations in a woman with multiple primary tumors. N Engl J Med. 2015;373(20):1985–1986. [DOI] [PubMed] [Google Scholar]
- 38. Weren RDA, Ligtenberg MJL, Kets CM, et al. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat Genet. 2015;47(6):668. [DOI] [PubMed] [Google Scholar]
- 39. Drikos I, Sachinidis A, Vassi I, et al. The role of BRCA1 and BRCA2 genes in the appearance of pediatric and adolescent disorders. J Neoplasm. 2017;2(2):17. [Google Scholar]
- 40. Kawasaki T, Ohnishi M, Suemoto Y, et al. WRN promoter methylation possibly connects mucinous differentiation, microsatellite instability and CpG island methylator phenotype in colorectal cancer. Mod Pathol. 2008;21(2):150. [DOI] [PubMed] [Google Scholar]
- 41. Lauper JM, Krause A, Vaughan TL, et al. Spectrum and risk of neoplasia in Werner syndrome: a systematic review. PLOS One. 2013;8(4):e59709.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Win AK, Dowty JG, Cleary SP, et al. Risk of colorectal cancer for carriers of mutations in MUTYH, with and without a family history of cancer. Gastroenterology. 2014;146(5):1208–1211.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma X, Zhang B, Zheng W.. Genetic variants associated with colorectal cancer risk: comprehensive research synopsis, meta-analysis, and epidemiological evidence. Gut. 2014;63(2):326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kwong A, Wong LP, Chan KYK, et al. Characterization of the pathogenic mechanism of a novel BRCA2 variant in a chinese family. Fam Cancer. 2008;7(2):125–133. [DOI] [PubMed] [Google Scholar]
- 46. Patel SG, Ahnen DJ.. Colorectal cancer in the young. Curr Gastroenterol Rep. 2018;20(4):15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.