Abstract
Objectives
We investigated endoplasmic reticulum (ER) stress and cytokine expression in peripheral blood-derived macrophages and synovial tissue from HLA-B27+ SpA patients.
Methods
Macrophages from healthy donors, SpA and RA patients were polarized with IFN-γ or IL-10 and activated with lipopolysaccharide. Expression of ER stress markers (BiP, CHOP, ERdj4) and cytokines (IL-23, IL-12, TNF, IL-10) was measured by qRT-PCR. Expression of ER stress markers and cytokines in synovial tissue from SpA patients was evaluated by microarray analysis.
Results
Macrophages from HLA-B27+ SpA patients did not show elevated ER stress markers. However, the expression of IL-23 and IL-12 by peripheral blood-derived macrophages was higher in HLA-B27+ SpA in comparison with healthy donors. Synovial tissue from HLA-B27+ SpA patients showed higher expression of TNF compared with HLA-B27− SpA patients.
Conclusion
HLA-B27+ SpA patients showed increased expression of IL-23, IL-12 and TNF without evidence of ER stress.
Keywords: spondyloarthropathy, HLA-B27, endoplasmic reticulum stress
Key message
There is no evidence of endoplasmic reticulum stress in activated peripheral blood-derived macrophages from HLA-B27+ SpA patients.
Introduction
HLA-B27 has the highest contribution to the inherited risk of developing AS, and also plays a dominant role in the other SpA subtypes [1]. One of the theories for the pathogenic role of HLA-B27 is based on the tendency of HLA-B27 to misfold inside the endoplasmic reticulum (ER) and thereby generating ER stress. This so-called unfolded protein response (UPR) can inhibit protein translation, upregulate ER chaperone molecules such as Immunoglobulin heavy-chain-binding protein (BiP) and Endoplasmic reticulum–localized DnaJ 4 (ERdj4), and activate transcription factors such as CCAAT-enhancer-binding protein homologous protein (CHOP), followed by increased production of pro-inflammatory cytokines (in particular, IL-23).
Based on the accumulating evidence for the involvement of the IL-23/IL-17 axis in the pathogenesis of SpA [2], a number of studies have focused on the role of ER stress [3]. Induction of the UPR was shown in B27/hβ2m rats as a result of HLA-B27 overexpression and was characterized by a predominant increase in IL-23 production and Th17 activation [4]. Studies in humans showed enhanced expression of BiP and CHOP in SF-derived [5, 6] and peripheral blood-derived macrophages (PBDM) [7] from SpA patients. However, the question of whether ER stress is responsible for the IL-23/IL-17 activation remains unanswered, because recent reports on the relationship between IL-23 production and ER stress in HLA-B27+ SpA have conflicting findings [8, 9].
It was previously stated that IFN-γ contributes to the UPR in SpA by upregulating HLA-B27 [7]. However, we and others showed an alternative macrophage activation signature (with IL-10 polarized macrophages, MΦIL-10 as prototype) in SpA vs a classical macrophage signature (with IFN-γ polarized macrophages, MΦIFN-γ as prototype) in RA synovitis [10–12]. Therefore, the aim of this study was to investigate whether the UPR could explain an altered cytokine expression in MΦIL-10 and MΦIFN-γ derived from peripheral blood or in synovial tissue of HLA-B27+ SpA patients.
Materials and methods
Patients
Peripheral blood samples were obtained from 6 healthy donors (HD), 17 SpA (9 HLA-B27+ and 8 HLA-B27−) and 10 RA patients. Synovial tissue biopsies were obtained from 11 SpA patients (7 HLA-B27+ and 4 HLA-B27−). The patients fulfilled the Assessment of SpondyloArthritis international Society criteria for SpA and the ACR classification criteria for RA. All patients had active disease as defined by at least one swollen joint and/or inflammatory back pain, and none of the patients was treated with biologicals. All patients and HD gave written informed consent to participate to the study, as approved by the Medical Ethics Committee of the Academic Medical Centre/University of Amsterdam (reference number METC 2013_057).
Monocyte isolation and in vitro polarization and stimulation
Monocytes were isolated from peripheral blood by gradient centrifugation as previously described [10]. Freshly isolated monocytes were polarized for 4 days in the presence of human recombinant IFN-γ (50 ng/ml; R&D Systems, Abingdon, UK) or IL-10 (50 ng/ml; R&D Systems). Macrophages were activated with 500 ng/ml lipopolysaccharide (LPS) for an additional 4 h.
Quantitative real-time PCR
The adherent monocyte-derived macrophages were washed with PBS and subsequently lysed on the plate for RNA isolation using GeneElute Mammalian Total RNA Miniprep kit (Sigma-Aldrich, St. Louis, TX, USA) according to the manufacturer’s instructions. Quantitative real-time PCR was performed using StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The mRNA levels were normalized to those of the human housekeeping gene glyceraldehyde 3-phosphate dehydrogenase. Oligonucleotide primers were designed using the online tool for real-time PCR (TaqMan) primer design (Genscript): IL-10 (forward 5′-GATGCCTTCAGCAGAGTGAA-3′, reverse 5′-CCCAGGTAACCCTTAAAGTCC-3′), IL-12p35 (forward 5′-ACCAGGTGGAGTTCAAGACC-3′, reverse 5′-TGGCACAGTCTCACTGTTGA-3′), p-12/-23p40 (forward 5′-AAGGAGGCGAGGTTCTAAGC-3′, reverse 5′-TGGGTTCTTTCTGGTCCTTT-3′), IL-23p19 (forward 5′-TTCTCTGCTCCCTGATAGCC-3′, reverse 5′-CCTCAGGCTGCAGGAGTT-3′) and TNF (forward 5′-CCCATGTTGTAGCAAACCCT-3′, reverse 5′-TGAGGTACAGGCCCTCTGAT-3′) and the online tool qPrimerDepot of the National Institutes of Health (Bethesda, MA, USA): BiP (forward 5′-CATCACGCCGTCCTATGTCG-3′, reverse 5′-CGTCAAAGACCGTGTTCTCG-3′), CHOP (forward 5′-AGCCAAAATCAGAGCTGGAA-3′, reverse 5′-TGGATCAGTCTGGAAAAGCA-3′) and ERdj4 (forward 5′-AATGCAGATTGCAAAGATGAAA-3′, reverse 5′-CAGCTCTGTGGAGGAGCAG-3′). Primers were obtained from Invitrogen (Carlsbad, CA, USA).
Microarray analysis
RNA was obtained from synovial tissue biopsies, followed by cDNA synthesis and labelling using a two-colour microarray-based gene expression protocol according to the manufacturer’s instructions (Agilent, Santa Clara, CA, USA). Microarray data analysis was performed as previously described [13].
Statistics
Statistical analysis was performed using Prism software (GraphPad, La Jolla, CA, USA). Data were expressed as the mean (s.e.m.). A Kruskal–Wallis test followed by Dunn’s multiple comparison test was used for comparisons between the groups. A value of P < 0.05 was considered statistically significant.
Results
Expression of BiP is not increased in MΦIFN-γ and MΦIL-10 from HLA-B27+ SpA in comparison with HLA-B27− SpA, RA patients and HD
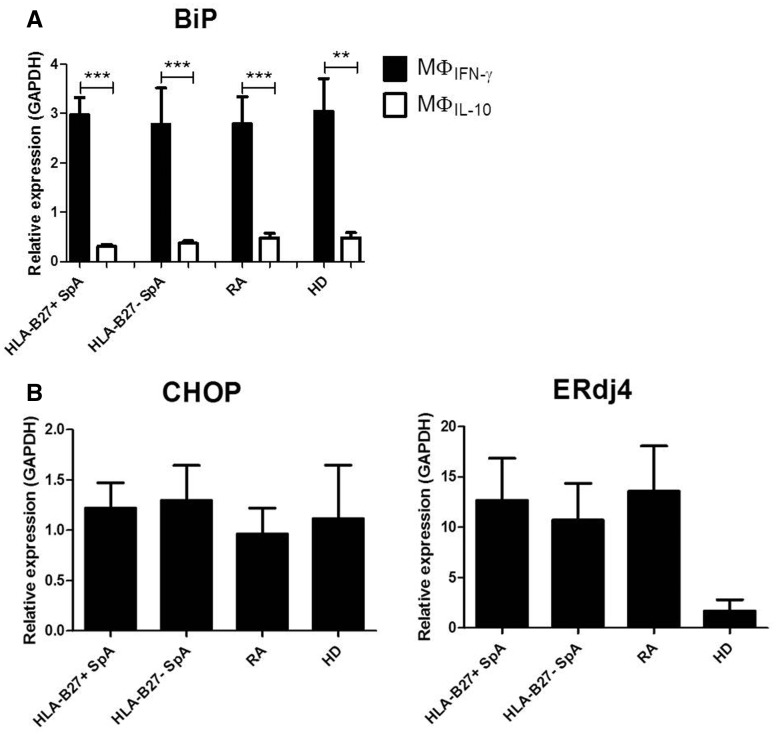
PBDM from HLA-B27+ SpA, HLA-B27− SpA, RA patients and HD were polarized with IFN-γ and IL-10 (MΦIFN-γ and MΦIL-10), followed by no stimulation or LPS stimulation. In the absence of LPS stimulation, BiP mRNA levels were very low (data not shown). As expected, in the presence of LPS the BiP mRNA levels were higher in MΦIFN-γ compared with MΦIL-10 (P < 0.01). However, for both MΦIFN-γ and MΦIL-10 there were no significant differences between HLA-B27+ SpA and HLA-B27− SpA, RA patients and HD (Fig. 1A).
Fig. 1.
Endoplasmic reticulum stress marker expression by macrophages from HLA-B27+ SpA compared with HLA-B27− SpA, RA and healthy donors
Graphs represent mRNA expression of BiP by MΦIFN-γ and MΦIL-10 (A), and respectively mRNA expression of CHOP and ERdj4 by MΦIFN-γ (B), after lipopolysaccharide stimulation. The mRNA levels were measured by qRT-PCR, normalized to the expression of GAPDH. Bars represent the mean (s.e.m.). **P < 0.01, ***P < 0.001.
Expression of CHOP and ERdj4 is not increased in MΦIFN-γ from HLA-B27+ SpA patients in comparison with HLA-B27– SpA, RA patients and HD
Given that IFN-γ is known to upregulate the expression of HLA-B27, thus increasing the risk for ER stress, we additionally measured the mRNA levels of CHOP and ERdj4 in LPS-stimulated MΦIFN-γ and again found no significant differences between activated MΦIFN-γ from HLA-B27+ SpA compared with HLA-B27− SpA, RA patients and HD (Fig. 1B).
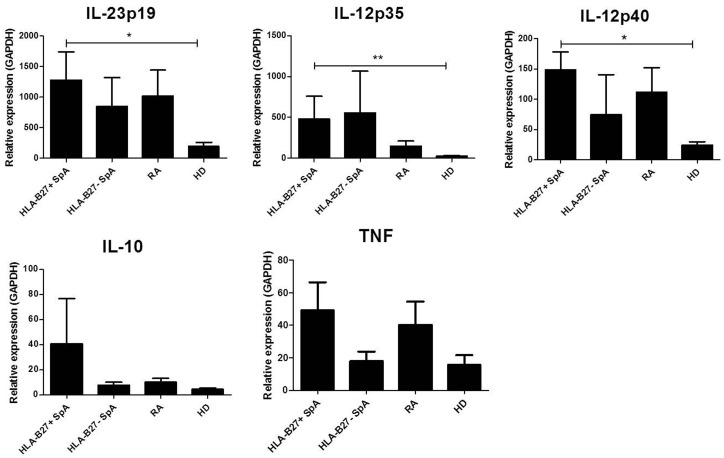
Expression of IL-23 and IL-12 is increased in MΦIFN-γ from HLA-B27+ SpA patients in comparison with HD
In the absence of LPS stimulation, MΦIL-10 expressed significantly higher levels of IL-23 compared with MΦIFN-γ, but the overall expression of all measured cytokines was low, and there were no differences between the arthritis patients and HD. Upon LPS stimulation, the cytokine expression of MΦIL-10 remained low (data not shown). In contrast, MΦIFN-γ showed a strong increase in the expression of all cytokines after LPS activation. MΦIFN-γ from HLA-B27+ SpA patients expressed significantly higher levels of IL-23p19 (P < 0.05), IL-12p40 (P < 0.05) and IL-12p35 (P < 0.01) compared with HD. There was no significant difference between IL-23 and IL-12 mRNA levels between MΦIFN-γ from HLA-B27+ SpA and HLA-B27− SpA or RA patients (Fig. 2). The levels of TNF and IL-10 expression were similar in LPS-activated MΦIFN-γ from all groups.
Fig. 2.
Cytokine expression by MΦIFN-γ from HLA-B27+ SpA compared with HLA-B27– SpA, RA and healthy donors
Graphs represent mRNA expression of IL-23p19, IL-12p35, IL-12p40, IL-10 and TNF by MΦIFN-γ after lipopolysaccharide stimulation. The mRNA levels were measured by qRT-PCR, normalized to the expression of GAPDH. Bars represent the mean (s.e.m.). *P < 0.05, **P < 0.01.
Expression of TNF is increased in synovial tissue from HLA-B27+ SpA in comparison with HLA-B27− SpA without increased expression of ER stress markers
In order to correlate our findings in PBDM with expression of ER stress markers and cytokines at the tissue level, we performed a supplemental microarray analysis of synovial tissue biopsies from SpA patients. There was no significant difference in the expression of BiP, CHOP, ERdj4 and X-box binding protein 1 (XBP1) between HLA-B27+ and HLA-B27− SpA. However, synovial tissue of HLA-B27+ SpA patients showed significantly more expression of TNF than HLA-B27− SpA patients. The levels of IL-23 and IL-10 were similar in both groups (supplementary Table S1, available at Rheumatology Advances in Practice online).
As it was previously shown that autophagy (and not the UPR) regulates cytokine expression in the gut of HLA-B27+ SpA patients [14], we also evaluated the expression of autophagy genes HSPA8, HSP90AA1, ATG5, ATG12, ATG16L1 and MAP1LC3A in synovial tissue of SpA patients. The expression of HSPA8 in HLA-B27+ SpA patients was significantly higher compared with HLA-B27− SpA patients. On the contrary, there was a significantly lower expression of ATG5 in HLA-B27+ SpA compared with HLA-B27− SpA (supplementary Table S1, available at Rheumatology Advances in Practice online).
Discussion
In this study, we aimed to assess the potential impact of ER stress on cytokine expression by PBDM in human HLA-B27+ SpA. Experimental studies have indicated that HLA-B27 misfolding can lead to ER stress and thereby altered TLR-induced cytokine production by myeloid cells. Of particular interest is the upregulation of IL-23, because there is a genetic association of AS with genes involved in type IL-17 immune responses and microbial sensing and genes coding for ER aminopeptidases [15]. The data gathered from in vitro [8, 9, 16, 17] and animal models [4, 18] suggest that aberrant IL-23 signalling plays a key role in the pathogenesis of SpA. A number of previous in vitro [16] and animal studies [4] made use of strong chemical agents or non-physiological HLA-B27 overexpression for the induction of ER stress. Studies in which more physiological stimulation of cells was used showed conflicting results. Two recent studies questioned the relevance of ER stress-induced IL-23 production in human SpA as: (i) not only IL-23 but also other pro- and anti-inflammatory cytokines (including IL-10) were upregulated in HLA-B27+ SpA [8], and (ii) there was no preferential increase in the expression of ER stress markers by macrophages from HLA-B27+ SpA patients [8, 19].
Our experiments with MΦIFN-γ and MΦIL-10 reproduce, in part, the findings of Zeng et al. [8] in MΦM-CSF, as our data indicate a selective increase in IL-23 and IL-12 expression by MΦIFN-γ from HLA-B27+ SpA vs HD rather than a global increase of pro- and anti-inflammatory cytokines. This discrepancy might relate to the previously highlighted functional difference between differentially polarized macrophages. The lack of BiP and cytokine upregulation in MΦIL-10 after LPS stimulation provides further confirmation of the functional differences between macrophage subsets.
In agreement with Zeng et al. [8] and Neerinckx et al. [19], we could not find clear evidence of enhanced ER stress in PBDM or synovial tissue from HLA-B27+ SpA patients. These data should be interpreted with caution, however, because we cannot exclude the possibility that this approach is not sensitive enough to detect discrete levels of ER stress and/or that other factors besides HLA-B27 or IFN-γ are required to lead to significant ER stress [16]. Furthermore, additional mechanisms are probably involved in IL-23 regulation besides the UPR. Among these mechanisms, autophagy is an innate immune response, which was earlier reported to regulate IL-23 expression in the gut [15], but not in the synovial tissue or PBDM of SpA patients [20]. Our microarray analysis of synovial tissue from a small cohort of SpA patients shows higher expression of HSPA8 and lower expression of ATG5 in HLA-B27+ SpA compared with HLA-B27− SpA. This finding suggests a differential involvement of macroautophagy and chaperone-mediated autophagy in HLA-B27+ SpA. However, owing to the limited cohort size and the absence of non-inflammatory control tissue, we cannot draw clear conclusions based on these observations. Furthermore, in contrast to the gut microenvironment, the absence of bacterial interactions could explain a differential expression of autophagy genes in synovial compared with gut tissue. Future analysis of selected synovial cell populations should further elucidate the role of autophagy in synovial inflammation.
In conclusion, we show that IFN-γ-polarized and LPS-stimulated PBDM of HLA-B27+ SpA patients express higher levels of IL-23 and IL-12 compared with HD, without evidence of increased ER stress. Likewise, additional analysis of synovial tissue from HLA-B27+ SpA fails to demonstrate increased levels of ER stress in comparison with HLA-B27 SpA patients.
Supplementary Material
Acknowledgements
D.L.B. is supported by a VICI grant from The Netherlands Organization for Scientific Research (NWO) and by a Consolidator grant from the European Research Council (ERC).
Supplementary data
Supplementary data are available at Rheumatology Advances in Practice online.
References
- 1. Bowness P. HLA-B27. Annu Rev Immunol 2015;33:29–48. [DOI] [PubMed] [Google Scholar]
- 2. Yeremenko N, Paramarta J, Baeten D.. The interleukin-23/interleukin-17 immune axis as a promising new target in the treatment of spondyloarthritis. Curr Opin Rheumatol 2014;26:361–370. [DOI] [PubMed] [Google Scholar]
- 3. Smith JA. The role of the unfolded protein response in axial spondyloarthritis. Clin Rheumatol 2016;35:1425–1431. [DOI] [PubMed] [Google Scholar]
- 4. DeLay ML, Turner MJ, Klenk EI. et al HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum 2009;60:2633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gu J, Rihl M, Marker-Hermann E. et al Clues to pathogenesis of spondylarthropathy derived from synovial fluid mononuclear cell gene expression profiles. J Rheumatol 2002;29:2159–64. [PubMed] [Google Scholar]
- 6. Dong W, Zhang Y, Yan M. et al Upregulation of 78-kDa glucose-regulated protein in macrophages in peripheral joints of active ankylosing spondylitis. Scand J Rheumatol 2008;37:427–34. [DOI] [PubMed] [Google Scholar]
- 7. Feng Y, Ding J, Fan CM, Zhu P.. Interferon-γ contributes to HLA-B27-associated unfolded protein response in spondyloarthropathies. J Rheumatol 2012;39:574–82. [DOI] [PubMed] [Google Scholar]
- 8. Zeng L, Lindstrom MJ, Smith JA.. Ankylosing spondylitis macrophage production of higher levels of interleukin-23 in response to lipopolysaccharide without induction of a significant unfolded protein response. Arthritis Rheum 2011;63:3807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rezaiemanesh A, Mahmoudi M, Amirzargar AA. et al Ankylosing spondylitis M-CSF-derived macrophages are undergoing unfolded protein response (UPR) and express higher levels of interleukin-23. Mod Rheumatol 2017;27:862–7. [DOI] [PubMed] [Google Scholar]
- 10. Ambarus CA, Noordenbos T, de Hair MJ, Tak PP, Baeten DL.. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res Ther 2012;14:R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vandooren B, Noordenbos T, Ambarus C. et al Absence of a classically activated macrophage cytokine signature in peripheral spondylarthritis, including psoriatic arthritis. Arthritis Rheum 2009;60:966–75. [DOI] [PubMed] [Google Scholar]
- 12. Smith JA, Barnes MD, Hong D. et al Gene expression analysis of macrophages derived from ankylosing spondylitis patients reveals interferon-γ dysregulation. Arthritis Rheum 2008;58:1640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeremenko N, Noordenbos T, Cantaert T. et al Disease-specific and inflammation-independent stromal alterations in spondylarthritis synovitis. Arthritis Rheum 2013;65:174–85. [DOI] [PubMed] [Google Scholar]
- 14. Ciccia F, Accardo-Palumbo A, Rizzo A. et al Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation. Ann Rheum Dis 2014;73:1566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. International Genetics of Ankylosing Spondylitis Consortium (IGAS). Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet 2013;45:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goodall JC, Wu C, Zhang Y. et al Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc Natl Acad Sci USA 2010;107:17698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prevosto C, Goodall JC, Hill Gaston JS.. Cytokine secretion by pathogen recognition receptor-stimulated dendritic cells in rheumatoid arthritis and ankylosing spondylitis. J Rheumatol 2012;39:1918–28. [DOI] [PubMed] [Google Scholar]
- 18. Sherlock JP, Joyce-Shaikh B, Turner SP. et al IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat Med 2012;18:1069–76. [DOI] [PubMed] [Google Scholar]
- 19. Neerinckx B, Carter S, Lories RJ.. No evidence for a critical role of the unfolded protein response in synovium and blood of patients with ankylosing spondylitis. Ann Rheum Dis 2014;73:629–30. [DOI] [PubMed] [Google Scholar]
- 20. Neerinckx B, Carter S, Lories R.. IL-23 expression and activation of autophagy in synovium and PBMCs of HLA-B27 positive patients with ankylosing spondylitis. Response to: ′Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation′ by Ciccia et al. Ann Rheum Dis 2014;73:e68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.