Abstract
Objective
Adaptive designs can enable highly sophisticated and efficient early phase trials, but the clinical inference from these trials is surrounded by complexity, and currently there is a paucity but steadily increasing amount of use of these designs in all fields of medicine. We aim to review early phase trials in RA to discover those that have used adaptive designs and benchmark trial characteristics.
Methods
From an OVID search for journal articles reporting the results of early phase trials in rheumatology, 35 studies were found, with 9 subsequently excluded; 11 were added from manual searches and 19 from searching the references. Study characteristics were extracted from the 56 papers (describing 62 trials), including the number of arms, number of patients, the primary outcome and when it was measured.
Result
One early phase trial using an adaptive design was found. The benchmark early phase trial in RA is a phase II double-blinded randomized trial, with four arms (one control and three intervention), each with 34 patients, and ACR20 measured at 16 weeks as the primary outcome.
Conclusion
The one adaptive design reviewed here, and a simulation study found in the search, both indicate that adaptive designs can be applied to early phase trials in RA. We have described the benchmark, which the efficiency of early phase trials using an adaptive design needs to exceed. These efficient designs could drive down numbers required, time for data collection and thus cost. Changes have been suggested, but more needs to be done.
Keywords: rheumatoid arthritis, adaptive design, early phase trial, systematic review
Key messages
A paucity of adaptive designs is found in early stage RA trials.
Research into RA treatments must make use of adaptive designs.
Research into relevant, early time-point, patient-reported, RA outcome measures is required to aid these designs.
Introduction
Randomized controlled trials are the gold standard in evidence-based medicine. However, many clinical trials struggle to meet the recruitment target and time line [1]. This issue is not unique to rheumatology; randomized controlled trials are resource intensive in terms of time, personnel, finance and the available patient pool. Some of these obstacles might be mitigated by the use of adaptive trial designs, which have been developed to improve clinical trial efficiency. The application of adaptive designs in early phase trials has become highly pragmatic, efficient and pertinent. Both the US Food and Drug Administration and the European Medicines Agency accept adaptive designs but have issued guidance on aspects that require special consideration [2, 3]. However, oncology is the only area where adaptive designs are established [4, 5], and there is a relative paucity of their use across other fields of medicine, including rheumatology.
An adaptive design trial is one in which modifications are made at various time points, dependent on pre-specified outcomes collated from the data observed up to that point. The modifications, time points [or more often, a point after which n patients have been recruited or have sufficient outcome(s) data] and the outcomes are many and variable, even within specific disease areas. Modifications can include adaptations to the randomization schedule, sample size re-estimation (both blinded and unblinded) and changes to the inclusion/exclusion criteria and to the mix of drugs defined as the intervention. The various and multiple interim analyses in these designs can allow for early curtailment of the trial owing to futility, safety or non-inferiority, and certain designs allow for intervention arms (such as multi-arm, multi-stage [6]) to be dropped for these reasons. Other designs allow for an operationally seamless transition from phase II(a/b) to phase III [7]. These designs are far removed from trials with incorporated interim analyses, which are often less statistically rigorous [8].
Since their introduction, researchers have formulated increasingly inventive designs to bring about the desired purposes for undertaking these trials: flexibility, efficiency and value for money (ever more pertinent in the current political and economic global and academic climates). It is most important, however, to reduce any possible harm to patients by attempting to minimize the expected sample size required to prove (or otherwise) the hypothesis in question.
The methodology for these designs started with Gehan’s 1961 [9] single-arm design, Fleming’s [10] single-stage design of 1982, then Simon’s [11] two-stage design of 1989, with the notion of dual outcomes, for both efficiency and toxicity, introduced by Bryant and Day in 1995 [12]. Now we look to UK-based names, such as Royston et al. [13], Burnett et al. and Hills and Burnett [14, 15], Mander et al. and Wason and Mander [16, 17], and Magirr et al. [18]. In the USA, luminaries include Thall and Cook [19], who introduced the concept of Bayesian-adaptive designs, and Berry et al. [20], who have furthered that field, in addition to O’Quigley et al. [21].
Outcome Measures in Rheumatology (OMERACT) has established a virtual special interest group to examine how adaptive designs might be applied to rheumatology research, in particular addressing issues set out in the US Food and Drug Administration and European Medicines Agency guidance [2, 3]. We focus on early phase trials, because the US Food and Drug Administration and European Medicines Agency have accepted adaptive designs for early phase trials, but not for phase III trials. Therefore, the purpose of this systematic review was, first, to discover those early phase trials that have used adaptive designs in RA, and second, to describe the characteristics of early phase trials to determine the benchmark, which the efficiency of early phase trials using an adaptive design needs to exceed. The results from this study will form the evidence base that will underpin work of the OMERACT Adaptive Clinical Trial Design Special Interest Group, by providing the requirements for designing an early phase trial in RA on matters such as the number of arms, the number of patients, the primary outcome and when it is measured. To implement an adaptive design into any such trial, it will need to be shown that it can be superior in efficiency to the benchmark.
Methods
Search and eligibility criteria
The initial step for this systematic review was to search for journal articles reporting the results of early phase trials in rheumatology. This was undertaken in January 2015 using the OVID online database using the following search terms:
((rheu* OR arth*) AND (early phase or phase 1 or phase I or phase 2 or phase 2a or phase 2 b or phase II or phase IIa or phase IIb) AND trial).ti.
The search included no specific terms for adaptive designs, because the relevant articles should also be identifiable as early phase trials under the terms used. This search also included papers on PsA, OA and systemic JIA, but these were later removed because the objective of this systematic review focused on RA as a specific exemplar. Ethical approval was not obtained to undertake this systematic review, because it was not required.
Data collection
Data were collected on phase, randomization, blinding, whether the trial used an adaptive design (and, if so, what sort), time period for data collection, countries involved, amount of participation time, inclusion and exclusion criteria, the intervention(s) and the treatment schedules for control and intervention groups, the primary outcome, when the primary outcome was assessed, the total number, the number of arms and number in each arm, and any additional outcomes, plus any further notes. Results relating to the primary outcome were also collected and were summarized using forest plots created by RevMan5.3 [22] (with specific primary outcomes at specific time points grouped together).
When the point at which the primary outcome was assessed was not entirely clear, we applied the following strategy: where adverse events were the primary outcome, the amount of participation time was used; where multiple outcomes were listed, the worst case (longest duration) was taken.
Results
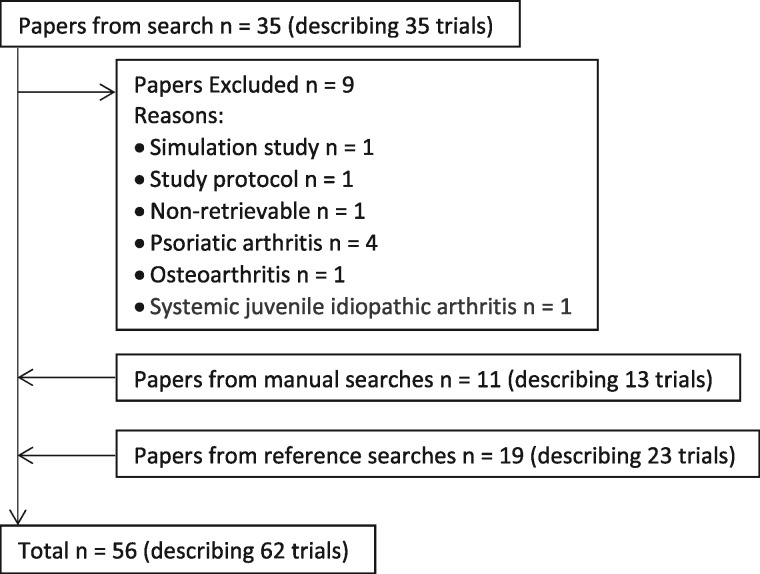
Based on abstracts, 35 papers were found in the OVID search, of which nine papers were excluded (supplementary Table S1, available at Rheumatology Advances in Practice online) [23–31]. Eleven papers were found through further manual searches, and 19 more came from searching the reference lists of papers already found for review, or to be used in the discussion. This gave a total of 56 papers (see supplementary material extracted data, available at Rheumatology Advances in Practice online) [32–87]. This information, and that relating to how, and which, papers came to be reviewed here is available is Fig. 1.
Fig. 1.
Flow of numbers of papers (and numbers of trials described) throughout the different search phases
In 54 of these papers, a single trial was reported, except for Choy et al. [35], in which three separate trials are reported, and for Namour et al. [64], in which 5 separate trials are reported. Thus, there are 56 papers reporting on 62 trials. Therefore, for each analysis, it is noted whether it is referring to the number of papers or the number of trials.
There was one example of an early phase trial using an adaptive design in the 62 trials, which was part A of Choy et al. [35].
The trials took place in a variety of different geographical regions, as shown in Table 1; the most common was the USA. Phases were listed as I, Ib, II, IIa, IIb, I/II and IIa/b, with phase II being the most frequent (supplementary Table S2, available at Rheumatology Advances in Practice online). All but five (plus a protocol that was combined with another to form a trial [68]) used randomization. Of the 62 trials, 49 were double-blinded, one was single-blinded (within Choy et al. [35]), seven were open-label and one used the term ‘masked’ [57]. The length of overall data collection time (from 20 of 56 papers) ranged from 3 to 61 months, with a mean of 19.9 (s.d.: 12.84) months and a median of 16.5 [interquartile range (IQR): 13.0; 23.5] months. The amount of trial participation time ranged from 0.86 to 76 weeks (from 61 of 62 trials), with a mean of 18.5 (s.d.: 13.77) weeks and a median of 16.0 (IQR: 9.0; 24.0) weeks.
Table 1.
Countries in which early phase clinical trials in RA are taking place (data from 39 of 56 papers)
| Country | n |
|---|---|
| USA | 15 |
| The Netherlands | 11 |
| UK | 9 |
| Belgium | 8 |
| Germany | 7 |
| Russia | 5 |
| Canada, Norway, Poland, Serbia/(former state of) Serbia and Montenegro | 4 |
| Austria, Australia, Finland, Hungary, Ukraine | 3 |
| Brazil, China, Czech Republic, Denmark, Japan, Mexico, New Zealand, Romania, Spain, Sweden | 2 |
| Argentina, Belarus, Chile, Colombia, Egypt, Estonia, France, Georgia, Greece, India, Israel, Italy, Lithuania, Malaysia, Philippines, Portugal, Republic of Korea, Slovakia, South Africa, Switzerland, Taiwan, Thailand, Turkey | 1 |
Numerous inclusion and exclusion criteria were applied across these papers (see supplementary material, available at Rheumatology Advances in Practice online). The main areas were for inclusion were as follows: fulfilled ACR [88] criteria; DAS28 [89], CRP, ESR and/or morning stiffness above specified values; number of swollen and tender joints above a specified value; failed previous DMARD treatment; length and stability of MTX treatment; and, age, generally >18 years, and sometimes limited to a maximum of 75 years. For exclusion, these were as follows: recent DMARD treatment; and stability of NSAID treatment.
Of the 62 trials, 14 did not include a control arm. Forty-six used some form of placebo, although two [53, 54] used background MTX throughout all arms in combination with placebo or the study treatment.
The most common primary outcome was ACR20 [90] (Table 2), with many secondary outcomes measured (usually reflecting the RA core set) [91]. The full list is available in the supplementary material, available at Rheumatology Advances in Practice online, but the most frequent were the individual components of DAS28 [89] and the ACR 50 and 70 response criteria.
Table 2.
Primary outcomes (data from 37 of 62 trials)
| Outcome | n (%) |
|---|---|
| ACR20 | 18 (48.6) |
| DAS28 | 6 (16.2) |
| Adverse events | 2 (5.4) |
| Area under curve formed from ACR20 at three time points | 1 (2.7) |
| ACR20 + ACR50 | 1 (2.7) |
| CRP + ESR | 1 (2.7) |
| Efficacy (based on clinical symptoms, signs and laboratory tests; <30% is ineffective, ≥30% to <50% is effective, and ≥50% is remarkable) | 1 (2.7) |
| Modified Paulus approach | 1 (2.7) |
| MRI erosion score | 1 (2.7) |
| Paulus20 | 1 (2.7) |
| Radiological score (Van der Heijde modified Sharp score) | 1 (2.7) |
| SJC | 1 (2.7) |
| Time exhibiting Paulus20, weeks | 1 (2.7) |
| TJC + SJC | 1 (2.7) |
SJC: swollen joint count; TJC: tender joint count.
The time at which the primary outcome was measured ranged from 2 to 26 weeks (from 37 of 62 trials), with a mean of 13.6 (s.d.: 7.32) weeks and a median 12.0 (IQR: 9.0; 20.0) weeks.
The total number of patients ranged from 12 to 509 (from all 62 trials), with a mean of 133.8 (s.d.: 132.55) and a median of 73.0 (IQR: 35.3; 222.5). The number of arms ranged from 2 to 9 (from all 62 trials), with a mean of 4.3 (s.d.: 2.06) and a median of 4.0 (IQR: 2.8; 6.0). The number of patients per arm ranged from 3.13 to 252.00 (from all 62 trials), with a mean of 34.01 (s.d.: 39.041) and a median of 23.42 (IQR: 8.44; 51.46).
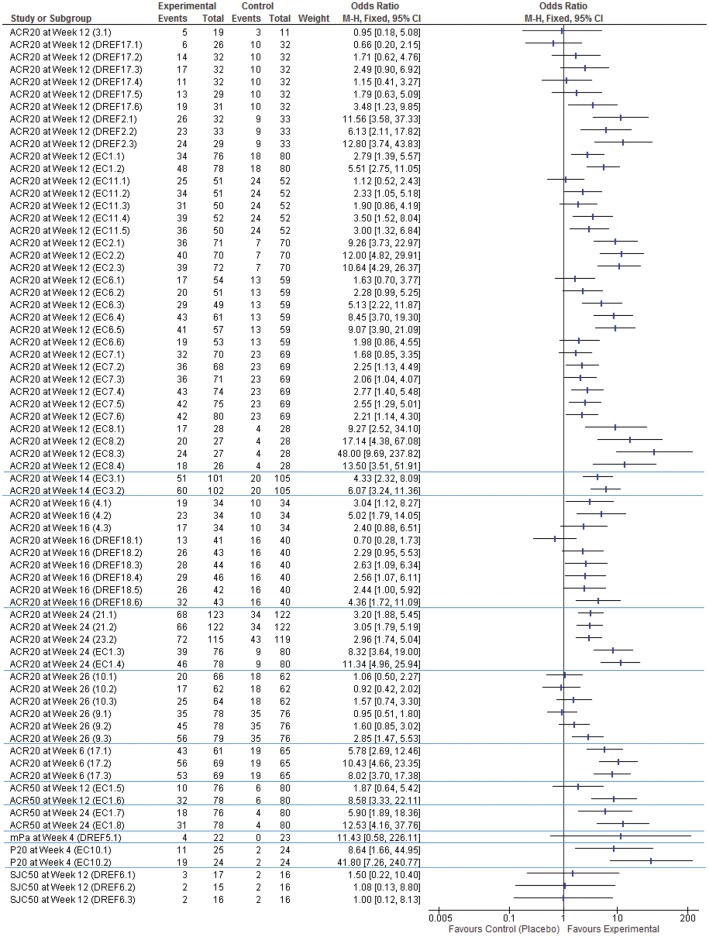
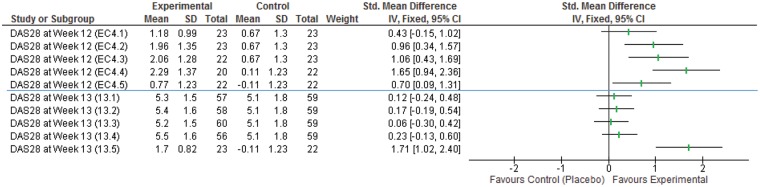
The forest plots (Figs 2 and 3) show that the odds ratios and effect sizes found in these papers are highly varied, but these results are either positive or indicate null findings (i.e. no evidence of a difference). There are no published negative results available.
Fig. 2.
Forest plot of dichotomous outcomes (ACR20, ACR50, modified Paulus approach, Paulus20 and 50% decrease in swollen joint count from baseline)
mPa: Paulus approach (see supplementary material, available at Rheumatology Advances in Practice online for detail); P20: Paulus20; SJC50: 50% decrease in swollen joint count from baseline. Numbers in brackets are [Search Number].[Intervention Number], as noted in the supplementary material, available at Rheumatology Advances in Practice online.
Fig. 3.
Forest plot of continuous outcomes (DAS28)
Number in brackets are [Search Number].[Intervention Number], as noted in the supplementary material, available at Rheumatology Advances in Practice online.
Discussion
There are many key issues that should be addressed for adaptive design trials before use for the rheumatic diseases (Table 3). There was only one example of an early phase trial using an adaptive design in the 62 trials, which was part A of Choy et al. [35]. From the papers reviewed here, the benchmark early phase trial in RA is a phase II double-blinded randomized trial, with four arms (one control and three intervention) containing ∼134 patients (34 in each arm), and a primary outcome of ACR20 [90] measured at 16 weeks. (The mean points to 13.6 weeks, but given that measurements are generally taken every 4 weeks, this rounds up to 16 weeks). It should be noted, however, that this is a very general set of values taken from widely varying research, some of which was inherently biased to have certain criteria by the very nature of the interaction between the intervention and the population sampled.
Table 3.
Methodological issues with adaptive design trials in rheumatology
| Methodological issues | Dissemination of interim results, especially if not fully blinded or incorporate some subjective element/analyst access to unblinded interim results and how they may influence investigators managing the trial (who must remain unequivocally objective), i.e. operational bias |
| Decision-making around early stopping should not be based solely on a statistically significant primary analysis, but should also include results of subgroup analyses and careful assessment of the adequacy of the safety database | |
| Whether patients who have yet to reach the interim time point should be included in these analyses or not, especially if this influences the potential decision-making | |
| Results based on P-values alone | |
| Control of the type I error rate | |
| Interpretation of study results when the study design has changed as a result of interim analyses | |
| Rejection of a global null hypothesis across all stages, which may not be sufficient or methodologically sound | |
| Involvement of sponsor personnel in interim decision-making | |
| Differential population for recruitment before and after modification, which will affect treatment effect | |
| Making hypothesis claims from results of interim analyses | |
| Interim analyses/adaptation choices provide multiple opportunities to show a successful treatment effect (with greater likelihood of doing so than if no such analyses existed), thus introducing inherent multiplicity bias | |
| The potential to select a modification as a result of an interim analysis that, by random chance, is more favourable than the true value, thus creating bias that will lead to an overestimate of the true treatment effect | |
| Limiting the opportunity to reflect on the data, including safety issues, and thus limiting the design of future well-thought-through research | |
| An increase in pressure to make assumptions, even when only limited prior information exists | |
| Exploratory adaptive design study flaws, which could lead to sub-therapeutic dose selection in subsequent (adequate and well-controlled) trials |
It appears that the standard is to use composite measures, such as ACR20 [90] or DAS28 [89], measured at a time point of 16 weeks. For adaptive designs to be implemented properly in this field and to provide the improvements in efficiency desired, we require a highly discriminate outcome measure, which can be assessed at early time points for interim analyses implemented in these designs. Analysing the response characteristic of commonly used outcome measures over time will be vital. Given the small sample, missing data imputation might also have an important role.
A paper found in the search but not reviewed, in that it was a simulation study, was by Thygesen et al. [27]. Here, the authors undertook a set of simulations around a Bayesian dose-finding procedure applied to an adaptive seamless phase I/II trial, specifically in RA. This was a bivariate procedure to look at both safety and efficacy, and the set-up was thus that the hypothetical trial collected data on these when the patient received the treatment every 4 weeks. For safety, information on all adverse events was collected, and if any of these matched a pre-defined list, the event was considered a dose-limiting event. For efficacy, CRP and ACR20 were also recorded. The outcomes were thus:
(i) Occurrence of a dose limiting event within 4 weeks of treatment (yes or no)—a measure of safety; (ii) Success with respect to ACR20 at 4 weeks and a 25% reduction in CRP at 4 weeks relative to baseline (yes or no)—an early indicator of efficacy; (iii) Success with respect to ACR20 at 16 weeks (yes or no)—a more reliable indicator of efficacy [27].
This suggests that it is possible to look at outcomes, such as CRP and ACR20, at a relatively early time point. Equally, in part A of Choy et al. [35], we see that the primary outcome was the change in DAS28 from baseline to day 14. At this point, and having been measured every week, the safety, tolerability, efficacy, pharmacokinetics and pharmacodynamics were assessed. Also, in a paper [40] using the Paulus20 outcome [92], efficacy was assessed at week 4. However, in the majority of the papers reviewed here, it seems that researchers are rarely keen to use ACR20 or DAS28 outcomes at a time point that would be suitable for an adaptive design. Vital research is required to investigate early, potentially patient-reported, outcomes that are predictive of eventual ACR20 or DAS28 results so that sample size and trial design may be adjusted.
It is also important that both papers show that these designs do not require large numbers in comparison with the benchmark trial we have reported with 134 patients. Part A of Choy et al. [35] required 64 patients, and the various simulations of Thygesen et al. [27] required numbers as low as 74. Although there is not enough evidence here to say for certain, this is indicative of the notion that adaptive designs tend to require fewer patients than standard trial designs.
Another relevant factor here is the overly restrictive inclusion and exclusion criteria, which makes for highly stringent recruitment barriers. This then drives up the length of time required for data collection, which was almost 2 years on average. To collect data on only ∼134 patients in this time shows that the throughput of patients into these trials is simply too slow.
It is also worth noting that all the papers reviewed here provided only positive or no-evidence-of-a-difference results (Figs 2 and 3), which, along with the fact that most of this research is done in the private sector, explains the small number of papers available. Publication bias based on the main trial outcome [93] has already been shown to be a disappointing barrier to furthering rheumatological research, which undoubtedly means that time, money and other resources are wasted on research that is already known to be fruitless.
In addition to the methodological papers laid out in the Introduction [9–21], there are numerous examples of adaptive designs in practice in the field of oncology, many of which are summarized in reviews [5, 94]. These have mostly made use of group sequential methods [95, 96]. Part A of Choy et al. [35], was a randomized, double-blind placebo-controlled Bayesian-adaptive dose-finding study. This used trial simulations of the Bayesian-adaptive pharmacokinetic/pharmacodynamic design to estimate the sample size and a Bayesian-adaptive dose-finding algorithm to identify subsequent doses after the starting dose [97].
In a 2015 editorial in The Journal of Rheumatology, Pope et al. [1] wrote:
Over the last 2 decades, we have seen advances in the clinical management of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, systemic sclerosis, vasculitis, and systemic lupus erythematosus. Yet trial designs and entry criteria required for drug development have not kept pace with medical care and thus no longer reflect patients seen in typical rheumatology practices in Canada, the United States and Western Europe [1].
In addition to this editorial, the Canadian Rheumatology Research Consortium has also suggested changes [98], which focus on both inclusion and exclusion criteria and study design.
We already know that updates can be made in the field of rheumatology. Five years before his paper defining the ACR20, 50 and 70 outcomes [90], Felson et al. [99] had published on the need for standardized outcomes and reporting. Also, a review in 2009 [100] showed how far trial design for RA had advanced in the previous decade, which noted the already existing debate [101, 102]. We are therefore hopeful that researchers in rheumatology and clinical trialists can start a change of the tide. The key objectives of the OMERACT Adaptive Clinical Trial Design Virtual Special Interest Group are to identify and address key barriers to adaptive design for clinical trials in RA and to improve clinical efficiency, which will benefit patients, researchers and funders.
Conclusion
Our search discovered a single example of an early phase trial that used an adaptive design in RA. We have described the benchmark, which the efficiency of early phase trials using an adaptive design needs to exceed. Research into treatments for RA should make use of adaptive designs if there is a desire to move forward in the world, or else it will begin to lag behind other clinical research efforts [5]. Beyond the academic elegance and need for statistical a priori definitions, these designs can reduce numbers in terms of participants and resources and can lead to trials that are operationally connected.
Whether we create, test and use objective early-time-point outcomes for use in adaptive designs may well be the important question. We must look for innovative ways of measuring outcomes in RA at much earlier time points. Also, we would need to look at which of these outcomes is more discriminatory.
Funding: The Cardiff Regional Experimental Arthritis Treatment and Evaluation (CREATE) Centre is joint funded by Health and Care Research Wales (HCRW) and Arthritis Research UK (ARUK). The Parker Institute, Bispebjerg and Frederiksberg Hospital, is supported by a core grant from the Oak Foundation (OCAY-13-309).
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Pope JE, Thorne JC, Haraoui BP. et al. Arthritis clinical trials at a crossroad. J Rheumatol 2015;42:14–7. [DOI] [PubMed] [Google Scholar]
- 2. U.S. Department of Health and Human Services FaDA, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Adaptive Design Clinical Trials for Drugs and Biologics 2010. 2017. https://www.fda.gov/downloads/drugs/guidances/ucm201790.pdf (25 July 2017, date last accessed).
- 3. (CHMP) CFMPFHU. Reflection Paper on Methodological Issues in Confirmatory Clinical Trials Planned with an Adaptive Design 2007. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003616.pdf (25 July 2017, date last accessed).
- 4. Dimairo M, Boote J, Julious SA, Nicholl JP, Todd S.. Missing steps in a staircase: a qualitative study of the perspectives of key stakeholders on the use of adaptive designs in confirmatory trials. Trials 2015;16:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hatfield I, Allison A, Flight L, Julious SA, Dimairo M.. Adaptive designs undertaken in clinical research: a review of registered clinical trials. Trials 2016;17:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Royston P, Barthel FM, Parmar MK, Choodari-Oskooei B, Isham V.. Designs for clinical trials with time-to-event outcomes based on stopping guidelines for lack of benefit. Trials 2011;12:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stallard N, Todd S.. Seamless phase II/III designs. Stat Methods Med Res 2011;20:623–34. [DOI] [PubMed] [Google Scholar]
- 8. Bowalekar S. Adaptive designs in clinical trials. Perspect Clin Res 2011;2:23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gehan EA. The determination of the number of patients required in a preliminary and a follow-up trial of a new chemotherapeutic agent. J Chronic Dis 1961;13:346–53. [DOI] [PubMed] [Google Scholar]
- 10. Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics 1982;38:143–51. [PubMed] [Google Scholar]
- 11. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10. [DOI] [PubMed] [Google Scholar]
- 12. Bryant J, Day R.. Incorporating toxicity considerations into the design of two-stage phase II clinical trials. Biometrics 1995;51:1372–83. [PubMed] [Google Scholar]
- 13. Royston P, Parmar MK, Qian W.. Novel designs for multi-arm clinical trials with survival outcomes with an application in ovarian cancer. Stat Med 2003;22:2239–56. [DOI] [PubMed] [Google Scholar]
- 14. Burnett AK, Hills RK, Hunter AE. et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia 2013;27:75–81. [DOI] [PubMed] [Google Scholar]
- 15. Hills RK, Burnett AK.. Applicability of a “Pick a Winner” trial design to acute myeloid leukemia. Blood 2011;118:2389–94. [DOI] [PubMed] [Google Scholar]
- 16. Mander AP, Wason JM, Sweeting MJ, Thompson SG.. Admissible two-stage designs for phase II cancer clinical trials that incorporate the expected sample size under the alternative hypothesis. Pharm Stat 2012;11:91–6. [DOI] [PubMed] [Google Scholar]
- 17. Wason JM, Mander AP.. Minimizing the maximum expected sample size in two-stage phase II clinical trials with continuous outcomes. J Biopharm Stat 2012;22:836–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Magirr D, Stallard N, Jaki T.. Flexible sequential designs for multi-arm clinical trials. Stat Med 2014;33:3269–79. [DOI] [PubMed] [Google Scholar]
- 19. Thall PF, Cook JD.. Dose-finding based on efficacy–toxicity trade-offs. Biometrics 2004;60:684–93. [DOI] [PubMed] [Google Scholar]
- 20. Berry SM, Spinelli W, Littman GS. et al. A Bayesian dose-finding trial with adaptive dose expansion to flexibly assess efficacy and safety of an investigational drug. Clin Trials 2010;7:121–35. [DOI] [PubMed] [Google Scholar]
- 21. O’Quigley J, Pepe M, Fisher L.. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics 1990;46:33–48. [PubMed] [Google Scholar]
- 22.Review Manager (RevMan) [computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- 23. Kavanaugh A, Menter A, Mendelsohn A. et al. Effect of ustekinumab on physical function and health-related quality of life in patients with psoriatic arthritis: a randomized, placebo-controlled, phase II trial. Curr Med Res Opin 2010;26:2385–92. [DOI] [PubMed] [Google Scholar]
- 24. McInnes IB, Sieper J, Braun J. et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis 2014;73:349–56. [DOI] [PubMed] [Google Scholar]
- 25. Mease P, Genovese MC, Gladstein G. et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum 2011;63:939–48. [DOI] [PubMed] [Google Scholar]
- 26. Saikawa I, Sugioka Y, Hotokebuchi T. et al. [Recombinant human erythropoietin (KRN5702) therapy for autologous blood transfusion in patients with rheumatoid arthritis undergoing joint replacement surgery–a multicenter phase II clinical trial]. Ryumachi 1994;34:583–93. [PubMed] [Google Scholar]
- 27. Thygesen H, Dragalin V, Whitehead A, Whitehead J.. A bivariate Bayesian dose-finding procedure applied to a seamless phase I/II trial in rheumatoid arthritis. Pharm Stat 2012;11:476–84. [DOI] [PubMed] [Google Scholar]
- 28. Utset TO, Auger JA, Peace D. et al. Modified anti-CD3 therapy in psoriatic arthritis: a phase I/II clinical trial. J Rheumatol 2002;29:1907–13. [PubMed] [Google Scholar]
- 29. Williams NH, Amoakwa E, Belcher J. et al. Activity Increase Despite Arthritis (AIDA): phase II randomised controlled trial of an active management booklet for hip and knee osteoarthritis in primary care. Br J Gen Pract 2011;61:e452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williams NH, Amoakwa E, Burton K. et al. Activity Increase Despite Arthritis (AIDA): design of a Phase II randomised controlled trial evaluating an active management booklet for hip and knee osteoarthritis [ISRCTN24554946. ]. BMC Fam Pract 2009;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Woo P, Wilkinson N, Prieur AM. et al. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res Ther 2005;7:R1281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allen M, Oberle K, Grace M, Russell A.. Elk velvet antler in rheumatoid arthritis: phase II trial. Biol Res Nurs 2002;3:111–8. [DOI] [PubMed] [Google Scholar]
- 33. Antoni C, Kalden JR.. Combination therapy of the chimeric monoclonal anti-tumor necrosis factor alpha antibody (infliximab) with methotrexate in patients with rheumatoid arthritis. Clin Exp Rheumatol 1999;17:S73–7. [PubMed] [Google Scholar]
- 34. Bao C, Chen S, Gu Y. et al. Leflunomide, a new disease-modifying drug for treating active rheumatoid arthritis in methotrexate-controlled phase II clinical trial. Chin Med J (Engl) 2003;116:1228–34. [PubMed] [Google Scholar]
- 35. Choy EH, Bendit M, McAleer D. et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of an anti- oncostatin M monoclonal antibody in rheumatoid arthritis: results from phase II randomized, placebo-controlled trials. Arthritis Res Ther 2013;15:R132.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choy EH, Hazleman B, Smith M. et al. Efficacy of a novel PEGylated humanized anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomized, dose-escalating trial. Rheumatology (Oxford) 2002;41:1133–7. [DOI] [PubMed] [Google Scholar]
- 37. Choy EH, Isenberg DA, Garrood T. et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum 2002;46:3143–50. [DOI] [PubMed] [Google Scholar]
- 38. den Broeder A, van de Putte L, Rau R. et al. A single dose, placebo controlled study of the fully human anti-tumor necrosis factor-alpha antibody adalimumab (D2E7) in patients with rheumatoid arthritis. J Rheumatol 2002;29:2288–98. [PubMed] [Google Scholar]
- 39. Doyle MK, Rahman MU, Frederick B. et al. Effects of subcutaneous and intravenous golimumab on inflammatory biomarkers in patients with rheumatoid arthritis: results of a phase 1, randomized, open-label trial. Rheumatology 2013;52:1214–9. [DOI] [PubMed] [Google Scholar]
- 40. Elliott MJ, Maini RN, Feldmann M. et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor α (cA2) versus placebo in rheumatoid arthritis. Lancet 1994;344:1105–10. [DOI] [PubMed] [Google Scholar]
- 41. Emery P, Fleischmann R, Filipowicz-Sosnowska A. et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum 2006;54:1390–400. [DOI] [PubMed] [Google Scholar]
- 42. Fleischmann R, Cutolo M, Genovese MC. et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690, 550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum 2012;64:617–29. [DOI] [PubMed] [Google Scholar]
- 43. Genovese MC, Bojin S, Biagini IM. et al. Tabalumab in rheumatoid arthritis patients with an inadequate response to methotrexate and naive to biologic therapy: a phase II, randomized, placebo-controlled trial. Arthritis Rheum 2013;65:880–9. [DOI] [PubMed] [Google Scholar]
- 44. Genovese MC, Fleischmann R, Furst D. et al. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised Phase IIb study. Ann Rheum Dis 2014;73:1607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haringman JJ, Gerlag DM, Smeets TJ. et al. A randomized controlled trial with an anti-CCL2 (anti-monocyte chemotactic protein 1) monoclonal antibody in patients with rheumatoid arthritis. Arthritis Rheum 2006;54:2387–92. [DOI] [PubMed] [Google Scholar]
- 46. Huizinga TW, Fleischmann RM, Jasson M. et al. Sarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheum Dis 2014;73:1626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Isaacs JD, Manna VK, Rapson N. et al. CAMPATH-1H in rheumatoid arthritis—an intravenous dose-ranging study. Br J Rheumatol 1996;35:231–40. [DOI] [PubMed] [Google Scholar]
- 48. Kavanaugh A, Genovese M, Baughman J. et al. Allele and antigen-specific treatment of rheumatoid arthritis: a double blind, placebo controlled phase 1 trial. J Rheumatol 2003;30:449–54. [PubMed] [Google Scholar]
- 49. Krausz S, Boumans MJ, Gerlag DM. et al. Brief report: a phase IIa, randomized, double-blind, placebo-controlled trial of apilimod mesylate, an interleukin-12/interleukin-23 inhibitor, in patients with rheumatoid arthritis. Arthritis Rheum 2012;64:1750–5. [DOI] [PubMed] [Google Scholar]
- 50. Kremer JM, Cohen S, Wilkinson BE. et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690, 550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 2012;64:970–81. [DOI] [PubMed] [Google Scholar]
- 51. Kremer JM, Dougados M, Emery P. et al. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve-month results of a phase iib, double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:2263–71. [DOI] [PubMed] [Google Scholar]
- 52. Lemmel EM, Franke M, Gaus W. et al. Results of a phase-II clinical trial on treatment of rheumatoid arthritis with recombinant interferon-gamma. Rheumatol Int 1987;7:127–32. [DOI] [PubMed] [Google Scholar]
- 53. Maini RN, Breedveld FC, Kalden JR. et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor α monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998;41:1552–63. [DOI] [PubMed] [Google Scholar]
- 54. Maini RN, Taylor PC, Szechinski J. et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum 2006;54:2817–29. [DOI] [PubMed] [Google Scholar]
- 55. Marcora SM, Chester KR, Mittal G, Lemmey AB, Maddison PJ.. Randomized phase 2 trial of anti-tumor necrosis factor therapy for cachexia in patients with early rheumatoid arthritis. Am J Clin Nutr 2006;84:1463–72. [DOI] [PubMed] [Google Scholar]
- 56. Mease P, Strand V, Shalamberidze L. et al. A phase II, double-blind, randomised, placebo-controlled study of BMS945429 (ALD518) in patients with rheumatoid arthritis with an inadequate response to methotrexate. Ann Rheum Dis 2012;71:1183–9. [DOI] [PubMed] [Google Scholar]
- 57. Moreland L, Gugliotti R, King K. et al. Results of a phase-I/II randomized, masked, placebo-controlled trial of recombinant human interleukin-11 (rhIL-11) in the treatment of subjects with active rheumatoid arthritis. Arthritis Res 2001;3:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moreland LW, Alten R, Van den Bosch F. et al. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose-finding, double-blind, placebo-controlled clinical trial evaluating CTLA-4Ig and LEA29Y eighty-five days after the first infusion. Arthritis Rheum 2002;46:1470–9. [DOI] [PubMed] [Google Scholar]
- 59. Moreland LW, Bucy RP, Tilden A. et al. Use of a chimeric monoclonal anti-CD4 antibody in patients with refractory rheumatoid arthritis. Arthritis Rheum 1993;36:307–18. [DOI] [PubMed] [Google Scholar]
- 60. Moreland LW, McCabe DP, Caldwell JR. et al. Phase I/II trial of recombinant methionyl human tumor necrosis factor binding protein PEGylated dimer in patients with active refractory rheumatoid arthritis. J Rheumatol 2000;27:601–9. [PubMed] [Google Scholar]
- 61. Moreland LW, Pratt PW, Mayes MD. et al. Double-blind, placebo-controlled multicenter trial using chimeric monoclonal anti-CD4 antibody, cM-T412, in rheumatoid arthritis patients receiving concomitant methotrexate. Arthritis Rheum 1995;38:1581–8. [DOI] [PubMed] [Google Scholar]
- 62. Moreland LW, Schiff MH, Baumgartner SW. et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med 1999;130:478–86. [DOI] [PubMed] [Google Scholar]
- 63. Moreland LW, Sewell KL, Trentham DE. et al. Interleukin-2 diphtheria fusion protein (DAB486IL-2) in refractory rheumatoid arthritis. A double-blind, placebo-controlled trial with open-label extension. Arthritis Rheum 1995;38:1177–86. [DOI] [PubMed] [Google Scholar]
- 64. Namour F, Vanhoutte FP, Beetens J. et al. Pharmacokinetics, safety, and tolerability of GLPG0259, a mitogen-activated protein kinase-activated protein kinase 5 (MAPKAPK5) inhibitor, given as single and multiple doses to healthy male subjects. Drugs R D 2012;12:141–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ng CM, Bruno R, Combs D, Davies B.. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol 2005;45:792–801. [DOI] [PubMed] [Google Scholar]
- 66. Nishimoto N, Yoshizaki K, Maeda K. et al. Toxicity, pharmacokinetics, and dose-finding study of repetitive treatment with the humanized anti-interleukin 6 receptor antibody MRA in rheumatoid arthritis. Phase I/II clinical study. J Rheumatol 2003;30:1426–35. [PubMed] [Google Scholar]
- 67. Seymour M, Petavy F, Chiesa F. et al. Ultrasonographic measures of synovitis in an early phase clinical trial: a double-blind, randomised, placebo and comparator controlled phase IIa trial of GW274150 (a selective inducible nitric oxide synthase inhibitor) in rheumatoid arthritis. Clin Exp Rheumatol 2012;30:254–61. [PubMed] [Google Scholar]
- 68. Strand V, Lipsky PE, Cannon GW. et al. Effects of administration of an anti-CD5 plus immunoconjugate in rheumatoid arthritis. Results of two phase II studies. The CD5 Plus Rheumatoid Arthritis Investigators Group. Arthritis Rheum 1993;36:620–30. [DOI] [PubMed] [Google Scholar]
- 69. Tak PP, Thurlings RM, Rossier C. et al. Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum 2008;58:61–72. [DOI] [PubMed] [Google Scholar]
- 70. Takeuchi T, Harigai M, Tanaka Y. et al. Golimumab monotherapy in Japanese patients with active rheumatoid arthritis despite prior treatment with disease-modifying antirheumatic drugs: results of the phase 2/3, multicentre, randomised, double-blind, placebo-controlled GO-MONO study through 24 weeks. Ann Rheum Dis 2013;72:1488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tanaka Y, Suzuki M, Nakamura H. et al. Phase II study of tofacitinib (CP-690, 550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res 2011;63:1150–8. [DOI] [PubMed] [Google Scholar]
- 72. Tohru A, Hiroshi A, Takamasa K. et al. Clinical evaluation of ketoprofen in rheumatoid arthritis–early phase II study by multi-clinical trial. New drug research group. Ryumachi 1973;13:256–60. [PubMed] [Google Scholar]
- 73. van de Putte LB, Rau R, Breedveld FC. et al. Efficacy and safety of the fully human anti-tumour necrosis factor α monoclonal antibody adalimumab (D2E7) in DMARD refractory patients with rheumatoid arthritis: a 12 week, phase II study. Ann Rheum Dis 2003;62:1168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. van der Lubbe PA, Dijkmans BA, Markusse HM, Nässander U, Breedveld FC.. A randomized, double-blind, placebo-controlled study of CD4 monoclonal antibody therapy in early rheumatoid arthritis. Arthritis Rheum 1995;38:1097–106. [DOI] [PubMed] [Google Scholar]
- 75. van Holten J, Pavelka K, Vencovsky J. et al. A multicentre, randomised, double blind, placebo controlled phase II study of subcutaneous interferon beta-1a in the treatment of patients with active rheumatoid arthritis. Ann Rheum Dis 2005;64:64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. van Vollenhoven RF, Kinnman N, Vincent E, Wax S, Bathon J.. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum 2011;63:1782–92. [DOI] [PubMed] [Google Scholar]
- 77. Westhovens R, Keyser FD, Rekalov D. et al. Oral administration of GLPG0259, an inhibitor of MAPKAPK5, a new target for the treatment of rheumatoid arthritis: a phase II, randomised, double-blind, placebo-controlled, multicentre trial. Ann Rheum Dis 2013;72:741–4. [DOI] [PubMed] [Google Scholar]
- 78. Yocum DE, Solinger AM, Tesser J. et al. Clinical and immunologic effects of a PRIMATIZED anti-CD4 monoclonal antibody in active rheumatoid arthritis: results of a phase I, single dose, dose escalating trial. J Rheumatol 1998;25:1257–62. [PubMed] [Google Scholar]
- 79. Zandbelt MM, Houbiers JG, van den Hoogen FH. et al. Intranasal administration of recombinant human cartilage glycoprotein-39. A phase I escalating cohort study in patients with rheumatoid arthritis. J Rheumatol 2006;33:1726–33. [PubMed] [Google Scholar]
- 80. Zhuang Y, Xu Z, Frederick B. et al. Golimumab pharmacokinetics after repeated subcutaneous and intravenous administrations in patients with rheumatoid arthritis and the effect of concomitant methotrexate: an open-label, randomized study. Clin Ther 2012;34:77–90. [DOI] [PubMed] [Google Scholar]
- 81. Genovese MC, Kinnman N, de La Bourdonnaye G, Pena Rossi C, Tak PP.. Atacicept in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor antagonist therapy: results of a phase II, randomized, placebo-controlled, dose-finding trial. Arthritis Rheum 2011;63:1793–803. [DOI] [PubMed] [Google Scholar]
- 82. Koffeman EC, Genovese M, Amox D. et al. Epitope-specific immunotherapy of rheumatoid arthritis: clinical responsiveness occurs with immune deviation and relies on the expression of a cluster of molecules associated with T cell tolerance in a double-blind, placebo-controlled, pilot phase II trial. Arthritis Rheum 2009;60:3207–16. [DOI] [PubMed] [Google Scholar]
- 83. Kremer JM, Bloom BJ, Breedveld FC. et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690, 550 versus placebo. Arthritis Rheum 2009;60:1895–905. [DOI] [PubMed] [Google Scholar]
- 84. Landewe RB, Houbiers JG, Van den Bosch F. et al. Intranasal administration of recombinant human cartilage glycoprotein-39 as a treatment for rheumatoid arthritis: a phase II, multicentre, double-blind, randomised, placebo-controlled, parallel-group, dose-finding trial. Ann Rheum Dis 2010;69:1655–9. [DOI] [PubMed] [Google Scholar]
- 85. Silverman MH, Strand V, Markovits D. et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: data from a phase II clinical trial. J Rheumatol 2008;35:41–8. [PubMed] [Google Scholar]
- 86. van Vollenhoven RF, Houbiers JG, Buttgereit F. et al. The selective estrogen receptor α agonist Org 37663 induces estrogenic effects but lacks antirheumatic activity: a phase IIa trial investigating efficacy and safety of Org 37663 in postmenopausal female rheumatoid arthritis patients receiving stable background methotrexate or sulfasalazine. Arthritis Rheum 2010;62:351–8. [DOI] [PubMed] [Google Scholar]
- 87. Cohen SB, Dore RK, Lane NE. et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum 2008;58:1299–309. [DOI] [PubMed] [Google Scholar]
- 88. Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 89. Prevoo ML, van’t Hof MA, Kuper HH. et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 90. Felson DT, Anderson JJ, Boers M. et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. [DOI] [PubMed] [Google Scholar]
- 91. Boers M, Tugwell P, Felson DT. et al. World Health Organization and International League of Associations for Rheumatology core endpoints for symptom modifying antirheumatic drugs in rheumatoid arthritis clinical trials. J Rheumatol 1994;21:86–9. [PubMed] [Google Scholar]
- 92. Paulus HE, Egger MJ, Ward JR, Williams HJ the Cooperative Systematic Studies of the Rheumatic Diseases Group . Analysis of improvement in individual rheumatoid arthritis patients treated with disease-modifying antirheumatic drugs, based on the findings in patients treated with placebo. Arthritis Rheum 1990;33:477–484. [DOI] [PubMed] [Google Scholar]
- 93. van Lent M, Overbeke J, Out HJ.. Role of editorial and peer review processes in publication bias: analysis of drug trials submitted to eight medical journals. PLoS One 2014;9:e104846.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mistry P, Dunn JA, Marshall A.. A literature review of applied adaptive design methodology within the field of oncology in randomised controlled trials and a proposed extension to the CONSORT guidelines. BMC Med Res Methodol 2017;17:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Armitage P. Statistical methods in medical research. Oxford: Blackwell Scientific, 1971. [Google Scholar]
- 96. Armitage P. Sequential medical trials, 2nd edn.New York: Wiley, 1975. [Google Scholar]
- 97. Rousseau A, Sabot C, Delepine N. et al. Bayesian estimation of methotrexate pharmacokinetic parameters and area under the curve in children and young adults with localised osteosarcoma. Clin Pharmacokinet 2002;41:1095–104. [DOI] [PubMed] [Google Scholar]
- 98. Karsh J, Keystone EC, Haraoui B. et al. Canadian recommendations for clinical trials of pharmacologic interventions in rheumatoid arthritis: inclusion criteria and study design. J Rheumatol 2011;38:2095–104. [DOI] [PubMed] [Google Scholar]
- 99. Felson DT, Anderson JJ, Meenan RF.. Time for changes in the design, analysis, and reporting of rheumatoid arthritis clinical trials. Arthritis Rheum 1990;33:140–9. [DOI] [PubMed] [Google Scholar]
- 100. Strand V, Sokolove J.. Randomized controlled trial design in rheumatoid arthritis: the past decade. Arthritis Res Ther 2009;11:205.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Boers M. Add-on or step-up trials for new drug development in rheumatoid arthritis: a new standard? Arthritis Rheum 2003;48:1481–3. [DOI] [PubMed] [Google Scholar]
- 102. Strand V. Counterpoint from the trenches: a pragmatic approach to therapeutic trials in rheumatoid arthritis. Arthritis Rheum 2004;50:1344–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.