Abstract
Species diversity in the genus Ulva remains understudied worldwide. Using molecular analyses we investigated the species composition, diversity, distribution, and relative frequencies of the genus Ulva along the entire coast of Jeju Island, off the southern tip of Korea. Species identification was performed for 215 samples collected from 23 sites, based on comprehensive phylogenetic and model-based species delimitation analyses using the sequences of two molecular markers, chloroplast elongation factor Tu (tufA) and nuclear rDNA internal transcribed spacer (ITS). We identified 193 specimens as nine Ulva species, 14 specimens as Blidingia spp., and eight samples undetermined, based on the combined analysis of tufA and ITS phylogenies. Two model-based approaches generally supported nine groups of Ulva species. Previously documented species complex, such as U. ohnoi−U. spinulosa and U. procera−U. linza showed discordant relationships between the two phylogenies. The occurrence of U. torta on Jeju Island was first observed, despite its existence on the mainland previously reported. Ulva australis [16 of 23 sites; 34.4% (relative frequency)], U. ohnoi (16; 21.9%), and U. procera (11; 14%) were found to be the predominant species. Our study highlights that molecular analysis is critical for species delimitation in the genus Ulva and provides fundamental information for an understanding of green-tide assemblages on the “biological hotspot” coastal ecosystem, Jeju Island in Korea. This study will also help to monitor and manage local green tides at the areas that are currently encountering rapid climate changes.
Introduction
The marine green macroalgal genus Ulva, especially notorious for green tide formation, is comprised of approximately 100 species, of which 18 species have so far been recorded from Korea [1–4]. Although species of Ulva have a simple multicellular thallus structure, they show a wide range of complex shapes due to phenotypic plasticity and differences in morphogenesis [5, 6]. Therefore, morphology based species identification in the genus Ulva often involves taxonomic errors due to intraspecific variation [4–7]. In Korea, previous taxonomic classifications for Ulva have mostly consisted of morphological investigations, therefore the reported number of species might be over- or under-estimated [2, 3, 8, 9]. Relatively little attention has been paid to molecular-based ecological surveys for species identification of the genus Ulva from this country [but see 4, 10]. There is a need to accurately identify native and non-native species of Ulva because some have been implicated in causing green tides [10]. A thorough documentation of the biodiversity of the Ulvalean native flora using molecular tools is essential to understanding and managing algal growth as well as marine ecology in Korea.
In recent years, green algal massive growth forming “green mats” have been frequently detected all year round along the shorelines on Jeju Island, off the southernmost region of Korea (Fig 1) [11]. Such events have been reported from the southern coast of the mainland [12, 13]. These phenomena have been considered as one of the largest problems in maintaining “healthy” coastal ecosystems in Jeju Island [10], being well known as a biological hotspot [14] and also as a popular holiday destination. Nevertheless, what species are major constituents for such “green carpets”, and the species diversity and distribution of the genus Ulva at an entire-island scale remain largely unknown.
Fig 1. Green algal Ulva mats on the shoreline of the Hado Beach (site 13B) on Jeju Island in Korea.
Photos were taken on July 2013.
In the present study, we aim to determine the species composition and diversity, geographic distribution, and relative frequencies of Ulva species along the entire coast of Jeju Island, a pristine habitat and hotspot for biodiversity. To this end, we conducted three different phylogenetic analyses [neighbor-joining (NJ), maximum-likelihood (ML) and Bayesian inference (BI)] on a total of 215 samples collected from 23 sites using two sets of molecular markers, including nuclear (nu) DNA, internal transcribed spacer 1 (ITS1), 5.8S ribosomal RNA, ITS2 region (ITS1-5.8S-ITS2; ITS), and chloroplast (cp) DNA encoded elongation factor Tu (tufA). Additionally, two model-based species delimitation approaches, such as Automatic Barcode Gap Discovery (ABGD) [15] and Generalized Mixed Yule Coalescent (GMYC) [16] were conducted to validate the results of our phylogenetic analyses. One of the goals of this study is to verify and discuss the accuracy and reliability of these two markers for the species delimitation of Ulva. The results of our study will provide fundamental information on the green-tide forming Ulva species and lay the groundwork for future research on managing and mitigating local green tides in the “biological hotspot” coastal ecosystem, Jeju Island, in Korea.
Materials and methods
Study area and sample collection
No specific permission to collect samples was required at the study sites, and the field research did not involve endangered or protected species. This study was conducted along the coastline of Jeju Island, which is located approximately 150–200 km off the southern coast of the Korean Peninsula (Fig 2). Jeju Island is known as one of the fastest warming regions worldwide; the sea surface temperature around this region has risen by 1.6°C–2.1°C over the last 100 years [17]. This island has been suggested as an ecosystem supporting high biodiversity, particularly for marine benthic species [14]. The tidal regime is semi-diurnal with a maximum tidal range of about 2.7 m during spring tides (Tide Tables for the Coast of Korea, National Oceanographic Research Institute of Korea).
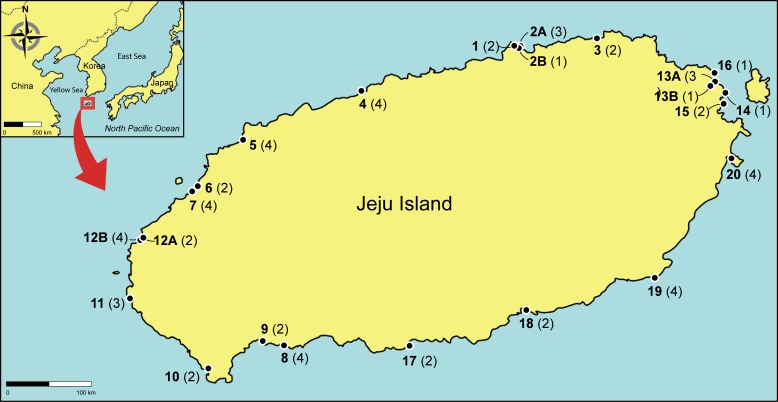
Fig 2. Map of 23 collection sites along the coast of Jeju Island in Korea.
The collection site numbers are given in bold. Those numbers cross-reference to Table 1 and S1 Table. Names of these sampling sites are given in S1 Table. The number of species found in each site, based on our molecular analyses is shown within parentheses.
We collected 215 specimens, morphologically identified as Ulva species, from 23 sites along the entire coast of Jeju Island in April 2015 (Table 1; Fig 2; S1 Table). All samples were collected at 2–3 meter intervals of each other within sites, and these sampling distances were maintained across sites. More than 2 individuals per morphotypes were collected from each of the 23 sites by considering different species based on morphology. However, we could not perform detailed morphological assessments of the collected specimens, partly due to the fact that they were not always in intact shape. Detailed information for collection sites and number of species are shown in S1 Table.
Table 1. Species composition, diversity and relative frequency of the genus Ulva and Blidingia spp. along the coast of Jeju Island in Korea, based on our molecular phylogenetic analyses.
| Species | Nr. of specimens (%) | Nr. of site | Sampling sites |
|---|---|---|---|
| U. australis (= U.pertusa)a | 74 (34.4) | 16 | 1,2a,3,4,5,6,7,8,10,12a,12b,13a,15,16,19,20 |
| U. compressa | 4 (1.9) | 2 | 6,15 |
| U. californica | 13 (6.0) | 6 | 1,4,9,13A, 15,20, |
| U. flexuosa | 8 (3.7) | 5 | 2a, 11, 12B, 19, 20 |
| U. laetevirens (= U.rigida)a | 4 (1.9) | 2 | 11,19 |
| U. ohnoi (U. spinulosa)b | 47 (21.9) | 16 | 1,2a,2b,5,7,8,9,10,12a,12b,13b,14,17,18,19,20 |
| U. procera (U. linza)b | 30 (14.0) | 11 | 2a,3,4,5,6,7,8,11,13a,17,20 |
| U. torta | 4 (1.9) | 2 | 12b,8 |
| U. arasakii | 9 (4.2) | 3 | 4,5,7 |
| Blidingia spp. | 14 (6.5) | 7 | 4,5,7,9,11,13a,16 |
| Discordant species | 8 (3.7) | 6 | 2a,3,4,7,8, 15 |
| Total | 215 | 23 |
The analyses were performed for the sampled 215 specimens from 23 sites using two molecular markers (chloroplast DNA tufA and nuclear DNA ITS). Discordant species (e.g., unidentified) were defined when two gene phylogenies suggested different species.
aSynonyms
bSpecies complexes
Genomic DNA extraction, PCR, and sequencing
Collected specimens were washed with freshwater, patted dry, and raked to remove epiphytes if present. Leaf tissue was dried at 60°C for 24 h and pulverized using TissueLyserII (QIAGEN, USA). Powdered tissue samples were transferred to a 1.5-ml microcentrifuge tube with silica gel and stored at −20°C until genetic analysis. Genomic DNA was extracted using a DNeasy Plant Mini Kit (QIAGEN), according to the manufacturer’s protocol. Genomic DNA was quantified using Qubit 2.0 Fluorometer (Invitrogen, USA).
Polymerase chain reaction (PCR) was performed to amplify cpDNA tufA (813 bp) and nuDNA ITS regions (732 bp) in a reaction volume of 20 μl. The primer TufGF4: 5'-GGN GCN GCN CAA ATG GAY GG-3' and tufAR: 5'-CCT TCN CGA ATM GCR AAW CGC-3' were used for the tufA [18] and the primer 18S150F: 5'-TCT TTG AAA CCG TAT CGT GA-3' and ENT26SA: 5'-GCT TAT TGA TAT GCT TAA GTT CAG CGG GT-3' were used for the ITS [19]. PCR conditions were as follows: an initial denaturation at 94°C for 1 min; followed by 35 cycles of 30 s at 94°C, 30 s at 50°C–54°C, and 1–2 min at 72°C; and a final extension step of 72°C for 10 min. The PCR products were visualized on 1% agarose gels, and PCR products were purified and then sequenced in both directions by Macrogen Inc. Sequencing (Korea) using an ABI PRISM 3130xl genetic analyzer (Applied Biosystems, USA). All obtained sequences were deposited in GenBank nucleotide sequence database under accession Nos. (tufA: MK992043-MK992249, ITS: MN069870-MN070070).
Phylogenetic analyses for species identification
The capacity of species identification by phylogenetic analysis depends on the accuracy of reference sequences. Sequences designation for species in GenBank database could be inaccurate due to taxonomical errors resulting from high levels of morphological variation aroused by phenotype plasticity, which is quite common in the genus Ulva [5, 6]. Thus, we set several criteria to select the reference sequences for Ulva species to avoid false positive species identification and exclude ambiguous sequences from the GenBank database. To choose reference sequences for our phylogenetic analyses, we retrieved 437 tufA gene sequences of Ulva species available from the database and reconstructed a NJ tree along with three sequences of Blidingia spp. (order Ulvales, family Kornmanniaceae) as an outgroup using MEGA7.0.14 [20] under the complete deletion option with 1000 bootstrap replicates. A total of 44 sequences representing 19 Ulva species were finally chosen as reference sequences by considering the selection criteria that one or two sequences were selected if conspecific sequences form a strong monophyly, and sequences were discarded if they show ambiguous phylogenetic positions, such as clustering with distantly related species.
A total of 262 sequences containing 215 sequences from the study specimens with 47 reference sequences (44 for 19 Ulva species, plus 3 for Blidingia spp.) were used to reconstruct the phylogenetic tree of the tufA gene (S1 Table). All sequences were aligned using ClustalW multiple sequences alignment package [21], which is implemented in BioEdit 7.1.9 [22]. We conducted a NJ analysis with 1000 bootstrap replicates in MEGA. ML analysis was conducted under the GTR+G model with 1000 bootstrap replicates using RAxML Web-Servers [23] and BI, implemented in MrBayes 3.2 [24], was performed with HKY+G+I chosen as the best-fit substitution model by AICc with jModeltest 2.1.7 [25]. The MCMC run was set for 10,000,000 generations with four chains, and the first 25% of the samples were discarded as the burn-in.
For the ITS region, a total of 243 sequences containing 210 sequences from the sampled specimens (after excluding five specimens with a poor quality of sequences) with 33 reference sequences (30 for 27 Ulva species, plus three for Blidingia spp.) retrieved from the GenBank database were used for phylogenetic analyses (S1 Table). Sequence alignments and phylogenetic analyses based on NJ, ML and BI methods were performed as for tufA phylogeny. Ambiguously aligned regions for the ITS, due to high levels of nucleotide diversity, were excluded for the phylogenetic analyses. The HKY+G substitution model was chosen for the 732 bp of the ITS region.
Species were first determined based on phylogeny of each gene separately, when the study specimens formed a monophyly (Table 1; S1 Table). For each gene, we chose a “species” delimitated by a majority of inferences, in cases of discordance found among three phylogenetic inferences (NJ, ML, and BI). We defined species as “discordant species” or “unidentified” when two gene phylogenies suggested different species. When more than one species in the reference sequences showed monophyletic relationships with the study specimens, we chose those as “species”, which had the lowest sequence divergence (K2P) with the study specimens.
Model-based species delimitation analyses
We performed two different algorithmic species delimitation methods, ABGD and GMYC, to validate the results of the phylogeny-based species identification (Table 2; Figs 3 and 4). The ABGD analyses were run on the web-interface (https://wwwabi.snv.jussieu.fr/public/abgd/abgdweb.html) with default settings for both markers with an exception of the Kimura K80 distance model and three relative gap widths (X) (X = 1.0, 1.5, 2.0) applied. Values for the prior intraspecific diversity (P) was set from 0.001 to 0.1 (S2 Table).
Table 2. The number of groups (clusters) for each species obtained from ABGD (A) and GMYC (B) analyses inferred from chloroplast (tufA) and nuclear (ITS) markers.
X: relative gap width, P: prior intraspecific divergence.
| Species identified by phylogenetic analyses (ML, BI, ML) | (A) ABGD | (B) GMYC | ||||
|---|---|---|---|---|---|---|
| tufA | tufA | ITS | ITS | tufA | ITS | |
| X = 1.0 | X = 1.5 | X = 1.0 | X = 1.5 | |||
|
P = 0.0017 |
P = 0.0077 |
P = 0.0077 |
P = 0.0129 |
|||
| U. australis (= U.pertusa)a | 1 | 1 | 1 | 1 | 1 | 1 |
| U. arasakii | 1 | 1 | 1 | 1 | 1 | |
| U. compressa | 1 | 1 | 1 | 1 | 1 | |
| U. californica | 2 | 1 | 1 | 1 | 1 | 1 |
| U. flexuosa | 1 | 1 | ||||
| U. torta | 2 | 1 | 1 | 1 | ||
| U. procera (U. linza)b | 5 | 1 | 1 | 1 | 1 | |
|
U. laetevirens (= U.rigida)a |
1 | 1 | 1 | 1 | 1 | 1 |
| U. ohnoi (U. spinulosa)b | 2 | 1 | 1 | 1 | ||
| Blidingia spp. | 4 | 4 | 3 | 3 | not included | not included |
| Non sampled species groups (sequences from GenBank database only) | 11 | 7 | 11 | 11 | 4 | - |
| Total number of groups (clusters) | 31 groups | 17 groups | 22 groups | 22 groups | 13 clusters | 3 clusters |
| Total number of groups (clusters) of Ulva specimens analyzed in this study | 16 groups | 6 groups | 8 groups | 8 groups |
9 clusters |
3 clusters |
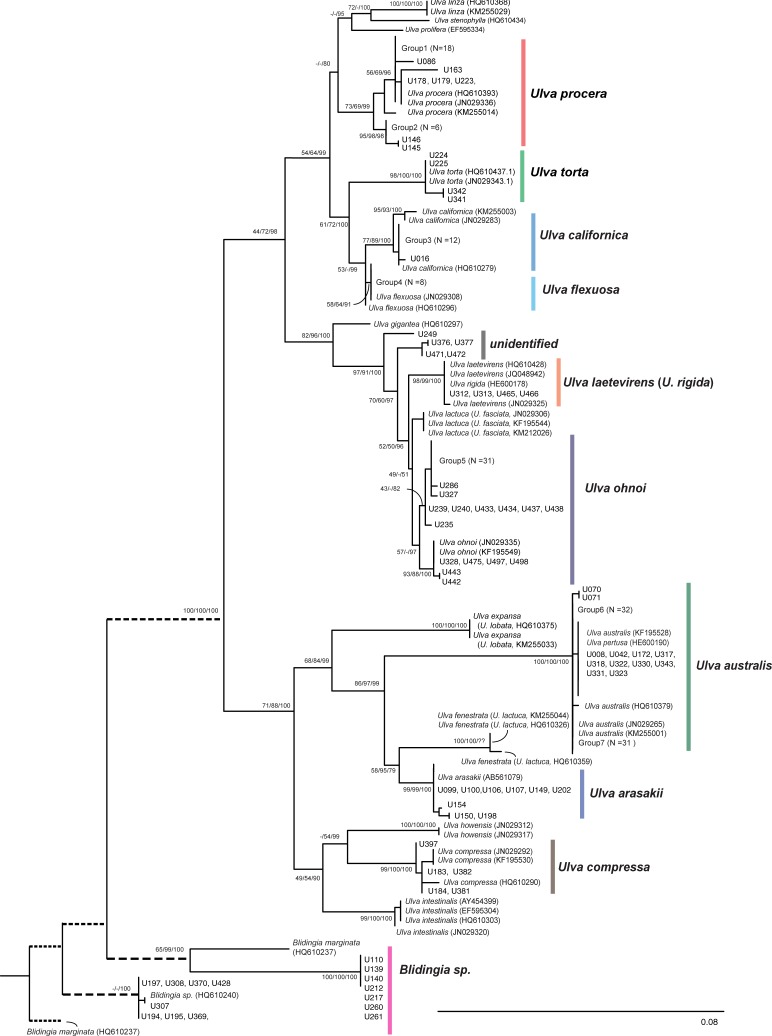
Fig 3. Maximum likelihood (ML) phylogram based on 262 tufA sequences of Ulva and Blidingia species.
Numbers indicate bootstrap values for maximum likelihood and neighbor-joining, and Bayesian posterior probabilities, respectively. The truncated branches are displayed with dashed lines. Sample IDs (e.g., U001) for each specimen collected in this study (cross-reference to S1 Table) are shown and GenBank accession numbers are included for reference sequences. A large number of specimens found within a particular clade are marked as “Group” with the total number of specimens [e.g., Group6 (N = 32)]. Species names of U. lactuca, U. expansa, and U. fenestrata are updated based on recently revised taxonomic status using genetic analysis of the holotype specimens [30]. Originally attributed names from GenBank are shown in parenthesis.
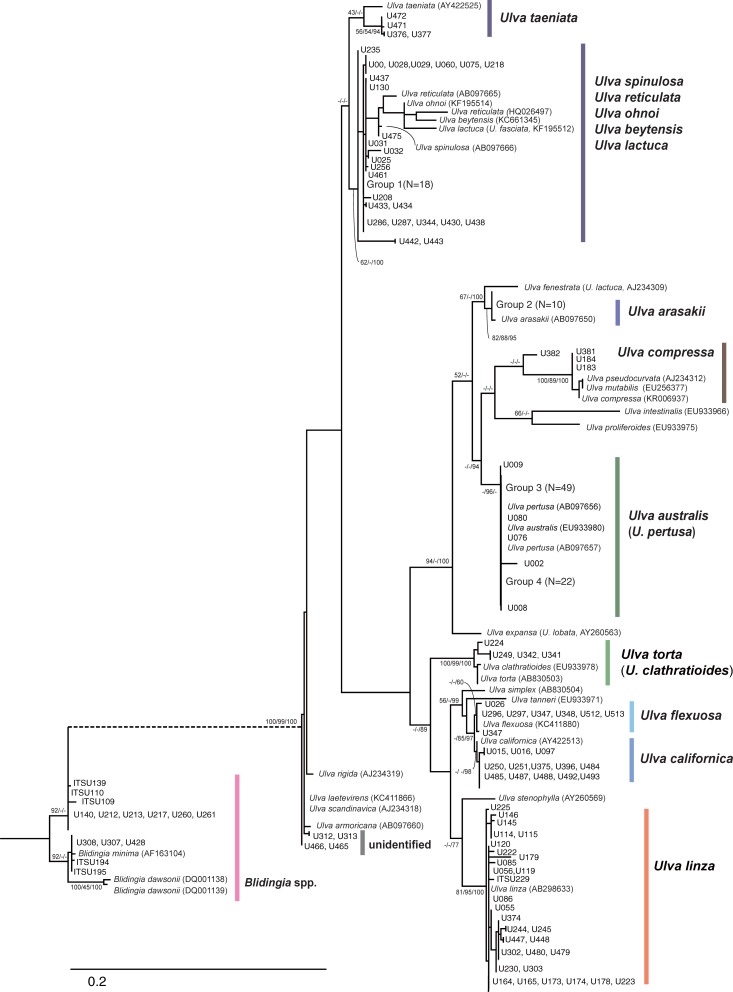
Fig 4. Maximum likelihood (ML) phylogram based on 243 ITS sequences of Ulva and Blidingia species.
Numbers indicate bootstrap values for maximum likelihood and neighbor-joining, and Bayesian posterior probabilities, respectively. The truncated branches are displayed with dashed lines. Sample IDs (e.g., U001) for each specimen collected in this study (cross-reference to S1 Table) are shown and GenBank accession numbers are included for reference sequences. A large number of specimens found within a clade are marked as “Group” with the total number of specimens. Species names of U. lactuca, U. expansa, and U. fenestrata are updated based on recently revised taxonomic status using genetic analysis of the holotype specimens [30]. Originally attributed names from GenBank are shown in parenthesis.
Prior to model-based GMYC analyses, sequences were collapsed into unique haplotypes using DnaSP v5 [26]. For the GMYC analyses, ultrametric trees for each tufA and ITS were generated using Bayesian inference implemented in BEAST v.2.5.2 [27]. Branch lengths were estimated assuming strict clock and Yule models as a tree prior. Markov Chains Monte Carlo (MCMC) was run for five million generations, sampling 1000 trees. Runs were inspected for convergence using Tracer v. 1.7 [28] and trees were summarized with a 25% burn-in. GMYC analyses were run with a single threshold using SPLITS package in R [29].
Results
Sequence analyses
A total of 262 tufA (including 47 reference sequences) and 243 ITS sequences (33 reference sequences) were analyzed for species delimitation of 215 specimens collected across the Jeju Island coast (Table 1; Figs 3 and 4; S1 Table). The tufA sequence alignment had a length of 813 bp with 285 variable nucleotide sites, of which 261 were parsimony-informative sites and 24 were singleton sites. The ITS region alignment included 732 bp with 261 variable sites, of which 202 were parsimony-informative sites and 59 were singleton sites. Estimates of the average sequence divergence (K2P) were 8.8% (±0.7%) for tufA and 6.4% (±0.8%) for ITS. We also estimated pairwise distance (K2P) among reference sequences retrieved from the GenBank database for Ulva species. Overall mean distance among Ulva species for tufA (44 sequences) and ITS (30 sequences) were 5.9% (±0.6%) and 8.7% (±0.9%), respectively. When we found a very low level of sequence divergence or no genetic variation between species, we considered them as the same species (i.e., synonym) in cases of both genes.
Species diversity, composition, and relative frequencies based on phylogenetic analyses
In a total of 215 specimens, we identified 193 specimens as nine Ulva species, 14 specimens as Blidingia spp., and eight samples undetermined (e.g., discordance between two phylogenies), based on the comprehensive phylogenetic analyses using tufA and ITS (Table 1; Figs 3 and 4). In the tufA gene tree, nine clades were clustered with reference sequences of U. procera, U. torta, U. californica, U. flexuosa, U. laetevirens, U. ohnoi, U. australis, U. arasakii, and U. compressa. Estimates of average intraspecific divergence for the nine Ulva species ranged from 0.03% [U. flexuosa (N = 10)] to 0.54% [U. procera (N = 34)]. The highest level of interspecific divergence (11%) was found between U. australis and U. torta, whereas the lowest interspecific divergence was found between U. laetevirens and U. rigida (0.0%), U. australis and U. pertusa (0.1%), and U. fasciata (U. lactuca [30]) and U. ohnoi (0.7%).
ITS phylogeny revealed the same number of nine Ulva species, but species composition was slightly different from tufA. Specimens clearly identified as U. ohnoi (N = 47) from the tufA phylogeny formed monophyletic groups with six different species: U. ohnoi, U. reticulata, U. beytensis, U. fasciata (U. lactuca [30]), U. spinulosa, and U. taeniata in ITS phylogeny (Fig 4). Although internal relationships among these six species were not clearly resolved in all three phylogenetic analyses, the lowest divergence (0%) of specimens in the clade was found with U. spinulosa (Fig 4). Specimens identified as U. procera (N = 30) in the tufA phylogeny appeared as U. linza in the ITS phylogeny (Figs 3 and 4).
For the ITS marker, estimates of average intraspecific divergence ranged from 0% [U. arasakii (N = 11), U. australis (N = 77), U. flexuosa (N = 8), U. pertusa (N = 2), U. torta (N = 5), and U. californica (N = 14)] to 0.9% [U. ohnoi (N = 14)], whereas the largest interspecific divergence was 9.8% between U. mutabilis and U. reticulata for all species analyzed in the ITS phylogeny. Lack of interspecific divergence (0%) was found between U. americana and U. laetevirens, U. mutabilis and U. pseudocurvata, U. laetevirens and U. scandinavica, U. clathratioides and U. torta, U. australis and U. pertusa, and U. americana and U. scandivnavica.
Additionally, eight specimens showed discordant species identities between two different gene trees. Three specimens were identified as Ulva species in one marker and Blidingia spp. in the other marker (U109: U. procera vs. Blidingia sp.; U197: Blidingia sp. vs. U. arasakii; U213: U. ohnoi vs. Blidingia sp.). Five specimens were identified as different Ulva species between the phylogenies (U050, 391: U. ohnoi vs. U. australis; U075: U. australis vs. U. spinulosa; U225: U. torta vs. U. linza; U382: U. australis vs. U. compressa).
Considering a combined analysis of two phylogenies based on three different methods (NJ, ML, and BI) and interspecific sequence divergence estimates, nine Ulva species were finally identified from the 215 collected specimens (Table 1). The three most abundant species were U. australis (= U. pertusa) (34.4%, N = 74), U. ohnoi (U. spinulosa for ITS) (21.9%, N = 47), and U. procera (U. linza for ITS) (14.0%, N = 30) that were observed from 16, 16, and 11 out of the 23 sampling sites, respectively. The least abundant species included U. compressa (1.9%, N = 4), U. laetevirens (1.9%, N = 4), and U. torta (1.9%, N = 4), which were all detected from 2 of the 23 sites. Only 58 specimens (27%) were matched with species identification based on morphology by eye when collected in the field (S1 Table). Even 14 specimens identified as Blidingia spp. in the phylogeny were recognized as Ulva species at the time of collection.
Model-based species delimitation analyses
The number of Ulva species identified by phylogenetic analyses lied within the range of the number of species groups or clusters estimated from the ABGD and GMYC analyses. In ABGD analyses, the number of species groups varied depending on P and X values. The number of groups identified ranged from one (when P = 0.1000) to 53 (when P = 0.0010) for tufA, while from one (when P = 0.1000) to 52 (when P = 0.0010) for ITS from the whole data set (215 specimens) (S2 Table). The number of groups, showing a minimum difference (i.e., one) between the results of the initial partition (IP) and recursive partition (RP), was 17 (IP) and 18 (RP) for the tufA gene dataset (when P = 0.0077, X = 1.5). Similarly, ITS dataset suggested 22 (IP) and 23 (RP) groups of the minimum difference between two partitions (when P = 0.0129, X = 1.5) (Table 2; S2 Table). The number of groups was reduced by excluding Blidingia spp. and unidentified species (Table 2). Given the minimum difference in the group number between IP and RP (i.e., one), number of groups only for Ulva specimens was suggested as six for tufA (when P = 0.0077, X = 1.5) and eight for ITS (when P = 0.0129, X = 1.5) (Table 2).
GMYC model was not favored over the null model for tufA (P = 0.15) and ITS (P = 0.42), which implies a wide range of confidence interval (95% CI) in the number of Maximum Likelihood (ML) clusters from one to 14 for tufA and from one to seven for ITS (S2 Table). GMYC single-threshold analyses generated 13 clusters from Ulva species for tufA, whereas only three clusters were identified in ITS dataset. Non-sampled species, which included only from the GenBank database, were identified as four clusters. All collected Ulva specimens analyzed in this study were finally grouped into nine Ulva species clusters, which was consistent with the findings of the species identification based on the phylogenetic analyses (Table 2; Fig 2; S3 Table).
Discussion
We find a total of 9 Ulva species and Blidingia spp. distributed across 23 sites in Jeju Island, based on a combined analysis of cpDNA tufA and nuDNA ITS-based phylogenies. TufA gene appears to be a more appropriate marker in differentiating nine different Ulva species relative to ITS region since the ITS phylogeny could not provide enough resolution or high support for several species groups, such as U. ohnoi with other closely related species and U. australis itself (Figs 3 and 4). Furthermore, ITS marker in GMYC analyses suggested three clusters of Ulva species (Table 2) and could not recover further distinct groups, which was clearly found as a monophyly (e.g., U. torta) in both phylogenetic trees (Figs 3 and 4).
Seven of nine Ulva species were identified as the same species based on two different phylogenies, whereas two species, U. ohnoi and U. procera, determined in the tufA phylogeny were identified as U. spinulosa and U. linza, respectively in the ITS phylogeny (Figs 3 and 4). Ulva ohnoi complex as we found in the ITS phylogeny (Fig 4) has been repeatedly reported in previous studies [31, 32]. Recent evidence for ongoing speciation between U. ohnoi and U. reticulata (which belongs to the U. ohnoi complex) through postzygotic isolation has been documented [33]. Although we could not determine distinct species precisely for the 47 specimens in the ITS phylogeny (Table 1), U. ohnoi or U. ohnoi complex including U. spinulosa would be acceptable based on the tufA phylogeny as well as the lowest level of average sequence divergence (0.5%) detected between U. ohnoi and U. spinulosa. Distinct species delimitation for U. procera and U. linza has also been long debated under Ulva linza-procera-prolifera (LPP) complex, which has been suggested by morphological and molecular analyses [31, 34, 35]. Thus, we regarded the 30 specimens showing a U. procera–U. linza discordance between two phylogenies as a single species group instead of two distinct species (Table 1).
Our analyses reveal that three species, U. australis (34% of 215 samples; 70% of 23 sites detected), U. ohnoi (22%; 70%), and U. procera (14%; 48%) are the predominant species along the Jeju Island coast. The occurrences of 5 species, U. compressa, U. flexuosa, U. laetevirens, U. torta, and U. arasakii in Jeju Island are first reported in this study, although they have previously been recorded from the mainland [3, 4, 10]. The two species, U. procera and U. flexuosa, have been designated as introduced species by the Ministry of Oceans and Fisheries of the Korean government [36]. Reference sequences retrieved from the GenBank database for U. procera whose type locality is Sweden [37] were obtained from the specimens collected from Canada: New Brunswick (HQ610393), Australia: Victoria (JN029336), and USA: California (KM255014). Also U. flexuosa having a type locality of the Mediterranean Sea [38] was used for the reference sequences from the specimens collected from Australia: New South Wales (JN029308) and Canada: British Columbia (HQ610296). A recent molecular study of the type specimen of U. australis suggests that its introduction from northeastern Asia (e.g., Japan) to Australia occurred via direct shipping by the middle of 19th century [39]. Thus, dispersal of the two species, U. procera and U. flexuosa from those regions (e.g., Europe, North America, Australia) to Jeju Island is plausible via transportation of ballast waters, as seen for U. ohnoi and U. fasciata (U. lactuca [30]) between Japanese waters and the Mediterranean Sea [40] and U. australis between Japanese waters and Australia [39].
We find that there are no sites where all 9 Ulva species co-occurred and no more than four species were observed within each site (Table 1; Fig 2). However, more abundant species tended to distribute geographically more widely. These findings indicate that some species, such as U. australis, U. ohnoi, and U. procera, are predominant and widely distributed across the Jeju Island coast. Ulva australis is known to be the most common species involved not only macroalgal assemblages, but also green tides in Korea and also in Japan [41, 42]. This species is massively recruited during the late summer-autumn season and has sheet-like blades enabling high surface area [SA] to volume [V] ratio, which is favorable for absorbing inorganic nutrients in water columns [13]. The growth rate of U. australis is facilitated by high inorganic nitrogen concentrations (NH4+ and NO3- + NO2-) in the surface water [43], and this species remains in a relatively high biomass during the winter period [42]. These characteristics can allow U. australis to last for more than five months after a bloom [13]. Thus, U. australis out-competes other Ulva species for space and nutrients. Ulva ohnoi is the most commonly-recorded species for green tides in various geographical regions [31, 32, 44–46]. This species shows a high affinity for nutrients, a high growth rate, and a broad range of tolerance to environmental conditions [45, 47, 48]. In particular, U. ohnoi shows high growth rates even in high water temperatures during summer as this species has a subtropical or tropical origin [31, 49]. This species has huge free-floating blades of approximately 4.0 m length by 4.0 m width (SRP, personal observation) suitable for fast uptake of nutrients. Seasonal succession of dominance between U. australis (or other Ulva species) and U. ohnoi might occur along the coasts of Jeju Island [46, 50], suggesting the lasting of serious local green algal massive growth over the summer period.
Ulva linza (U. procera for tufA) is a representative green-tide forming macroalgae in Korea, China, and Japan [7, 12, 51]. In contrast to U. australis and U. ohnoi, U. linza has thin and tubular blades (low SA to V ratio), which helps to mitigate wave-induced stresses [13]. Ulva linza shows rapid growth rates under low salinity and high nutrient conditions [13, 48]. Due to its ecological and physiological characteristics, initial blooms of U. linza occur in brackish waters and subsequently move from estuaries into offshore areas [52]. Accordingly, U. linza can be an important source for more serious green-tide blooms.
The species diversity and composition that we observed in Jeju Island might be attributed to species’ ecological characteristics such as survival, habitat, growth rate, and adaptive capacity (e.g., responding to environmentally stressful conditions such as salinity and desiccation) [53, 54]. Less abundant species such as U. compressa, U. laetevirens, and U. torta might have lower growth rates or weaker competitive abilities (e.g., nutrient uptake rate) compared with others. Worldwide, U. compressa occurring in brackish environments or in the upper intertidal zone may have adapted to low salinity and high desiccation [7, 12, 55–57]. As shown in previous studies, this species is found in only two sites with an extensive intertidal zone in this study. However, U. compressa would show low biomass and coverage in the field as this species persists for less than two months and is exposed to high herbivore pressure due to its morphology [13].
Less abundant species such as U. compressa, U. laetevirens, and U. torta were observed only at sites in the proximity of the harbor in this study. This implies that they might be recently introduced to Jeju Island from elsewhere, and there has been insufficient time for them to reach population stability there. This hypothesis is plausible, given that this is the first report of those three species occurring in Jeju Island. Although a total of 17 species of Ulva have been known in the marine algal flora of Korea [2, 4], the existence of only 7 species: U. pertusa, U. linza (U. procera), U. fasciata (currently identified as U. lactuca [30]), U. californica, U. conglobata, U. lactuca (currently identified as U. fenestrata [30]), and U. japonica (currently named as Umbraulva japonica [58]) have been reported in Jeju Island to date [3, 10]. Recently, U. torta whose type locality is Germany [38, 59] was first reported on the eastern coast of Korea [1]. We further demonstrate its habitation on the coast of Jeju Island, albeit relatively rare.
Alternatively, different species composition and varying extents of species diversity among the 23 sites might be partly explained by the patterns of green algal blooming in the Yellow Sea in the northwestern parts of Jeju Island. Ulva species are major macroalgae leading to green-tide blooming, especially around the northwestern Pacific regions, including China, Japan, and Korea [10, 60–62]. In particular, Jeju Island is one of the places suffering from severe green tides every year in Korea. Free-floating species, such as U. compressa, U. flexuosa, and LPP (linza-procera-prolifera) complex have been suggested to be main species responsible for the Yellow Sea green tides [63–65]. We found that the third most common species in Jeju Island was U. procera (U. linza). This species has been identified as a key species causing green tides in the open water near the southwestern coasts of Korea, which is the southeastern part of the Yellow Sea green-tide patch [10]. Thus, the Yellow Sea green tides might possibly lead to the observed relatively high proportions of U. procera on the Jeju Island coast. However, this hypothesis needs to await future research.
The species diversity and composition in the green-tide Ulva communities on Jeju Island might vary in space and time. It has been reported that the most common species changes from U. pertusa in spring and summer to U. ohnoi in winter in Mikawa Bay, Japan [50]. The study specimens were sampled during a spring-summer season, and therefore further investigations with additional samples collected at a different season (e.g., fall-winter) will allow us to understand seasonal changes in dominant Ulva species and thus help to predict the intensity and range of local green tides on Jeju Island in the future.
The largest degrees of interspecific genetic distances for tufA (11%) and ITS (9.8%) genes found in this study are within the previously reported ranges (2.1%–13.3%) of Ulva species, as suggested in [10]. Moreover, we found less than 1% average interspecific divergence in several species pairs, such as between U. ohnoi and U. fasciata (currently identified as U. lactuca [30]) (0.7%) in tufA, as suggested in a previous study [66]. Interspecific distance in tufA between U. laetevirens and U. rigida showed 0% average sequence divergence. For some species, we have considered them as different species rather than synonyms based on our phylogenetic tree-based criteria (see above). For example, U. flexuosa and U. californica are considered as different species, since they form separate monophyletic groups despite the low divergence of 1% and 0.7% for tufA and ITS, respectively. In GMYC analyses, these two species were clustered separately for tufA while the same cluster for ITS (Table 2). However, the species delimitation (including taxonomical synonym) for Ulva should be treated with caution, particularly when a few molecular markers show identical sequences or very low levels of sequence divergence between the study objects. Recent whole-mitogenome sequencing of U. pertusa, known as a taxonomic synonym of U. australis [67], showed distinct interspecific variation, such as 4.7 kb size difference and variable tandem duplication mutations. Thus, vigorous analysis at a large genomic scale is required to accurately define “true” species or taxonomical synonyms in the genus Ulva.
Our phylogenetic tree-based species delimitation for the genus Ulva using two different marker-sets reveals an identical number of species, although tree topologies of some species (e.g., U. ohnoi−U. spinulosa, U. procera−U. linza) are incongruent between the two phylogenies. Furthermore, our algorithmic species delimitation approaches, particularly GMYC supported the number of species groups for Ulva. These results highlight the reliability and accuracy of our tree-based criteria for species delimitation of Ulva, which has been shown to demonstrate taxonomic complexities, such as several cases of synonyms. Our own morphology-based species identification by eye in the field showed surprisingly high levels of misidentification, emphasizing the importance of molecular-based species determination. It is well known that valid taxonomical characters are difficult to apply to species identification for Ulva, due to the extremely high levels of phenotypic plasticity in response to various environmental factors [5, 6]. Nevertheless, detailed morphological assessments along with genetic analysis will provide intriguing insights into the interspecific differences in the levels of phenotypic diversity among different Ulva species. Our study shows that the molecular-based phylogenetic and model-based approaches using two different molecular markers (i.e., chloroplast and nuclear DNA) can be applied to efficient and reliable species identification for monitoring changes in species composition and relative abundances of the green-tide Ulva masses in large coastal areas. Also, the approaches and the results reported in this study can help to assess fluctuations in species composition and seasonal population dynamics at the sites which are currently experiencing rapid environment changes, such as Jeju Island, Korea.
Supporting information
(CSV)
(XLSX)
P, Prior intraspecific divergence (P); IP, Initial partition; RP, Recursive partition; Ng, Number of groups of the full data set (Ng).
(XLSX)
(XLSX)
Acknowledgments
We are grateful to members of the Molecular Ecology and Evolution Laboratory of Sangji University and also those of the Estuarine and Coastal Ecology Laboratory of Jeju National University for helping to collect samples in the field.
Data Availability
All obtained sequences were deposited in GenBank nucleotide sequence database under accession Nos. (tufA: MK992043-MK992249, ITS: MN069870-MN070070).
Funding Statement
This study was supported by the Ministry of Oceans and Fisheries, Korea (Project title: Long-term changes in structure and function in the marine ecosystems of Korea) to SRP and HJL, a National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2016R1D1A1B03934959) to HJL and also by a grant (NRF-2017R1C1B1010741) from the Basic Science Research Program, through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science, ICT and Future Planning to JHKang. The funder provided support in the form of salaries for author (JHKim), but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of this author are articulated in the ‘author contributions’ section. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.An JW, Nam KW. First Record of Ulva torta (Ulvales, Chlorophyta) in Korea. Korean J Environ Biol. 2017;35(3):329–34. [Google Scholar]
- 2.Kim H-S, Boo S-M, Lee IK, Sohn C-H. National list of species of Korea: marine algae: National Institute of Biological Resources; 2013. [Google Scholar]
- 3.Lee IK, Kang JW. A check list of marine algae in Korea. Korean J Phycol. 1986;1:311–25. [Google Scholar]
- 4.Lee SH, Kang PJ, Nam KW. New record of two marine ulvalean species (Chlorophyta) in Korea. J Ecol Environ. 2014;37(4):379–85. [Google Scholar]
- 5.Guidone M, Thornber C, Wysor B, O'Kelly CJ. Molecular and morphological diversity of Narragansett Bay (RI, USA) Ulva (Ulvales, Chlorophyta) populations. J Phycol. 2013;49(5):979–95. 10.1111/jpy.12108 [DOI] [PubMed] [Google Scholar]
- 6.Wichard T, Charrier B, Mineur F, Bothwell JH, Clerck OD, Coates JC. The green seaweed Ulva: a model system to study morphogenesis. Front Plant Sci. 2015;6:72 10.3389/fpls.2015.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa T, Ohki K, Kamiya M. Differences of spatial distribution and seasonal succession among Ulva species (Ulvophyceae) across salinity gradients. Phycologia. 2013;52:637–51. [Google Scholar]
- 8.Lee YP. Marine algae of Jeju Academy Publishing Co, Seoul, Korea: (in Korean). 2008. [Google Scholar]
- 9.Lee YP, Kang SY. A catalogue of the seaweeds in Korea Jeju National University Press, Jeju, Korea: 2002. [Google Scholar]
- 10.Kang EJ, Kim J-H, Kim K, Choi H-G, Kim KY. Re-evaluation of green tide-forming species in the Yellow Sea. Algae. 2014;29(4):267. [Google Scholar]
- 11.Baek ER. Current status and eco-physiological responses of green tides in Jeju Island, Korea. MSc Thesis, Jeju National University, Korea. 2017:53p.
- 12.Park SR. Seasonal patterns and recruitment dynamics of green tide-forming Ulva species along the intertidal rocky shores of the southern coast of Korea. Ocean Sci J. 2014;49(4):383–90. [Google Scholar]
- 13.Park SR, Kang YH, Lee HJ, Ko YW, Kim JH. The importance of substratum and elevation in recruitment and persistence of ulvoid algal blooms on rocky intertidal shores of the southern Korean coast. Bot March 2014;51(1):55–66. [Google Scholar]
- 14.Kim S, Frontalini F, Martins V, Lee W. Modern benthic foraminiferal diversity of Jeju Island and initial insights into the total foraminiferal diversity of Korea. Mar Biodivers. 2016;46:337–54. [Google Scholar]
- 15.Puillandre N, Lambert A, Brouillet S, Achaz G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol. 2012;21(8):1864–77. 10.1111/j.1365-294X.2011.05239.x [DOI] [PubMed] [Google Scholar]
- 16.Pons J, Barraclough TG, Gomez-Zurita J, Cardoso A, Duran DP, Hazell S, et al. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst Biol. 2006;55(4):595–609. 10.1080/10635150600852011 [DOI] [PubMed] [Google Scholar]
- 17.Takatsuki Y, Kuragano T, Shiga T, Bungi Y, Inoue H, Fujiwara H, et al. Long-term trends in sea surface temperature adjacent to Japan. Sokko Jiho. 2007;74:S33–S87. [Google Scholar]
- 18.Saunders GW, Kucera H. An evaluation of rbcL, tufA, UPA, LSU and ITS as DNA barcode markers for the marine green macroalgae. Cryptogamie Algol. 2010;31:487–528. [Google Scholar]
- 19.Blomster J, Maggs CA, Stanhope MJ. Molecular and morphological analysis of Enteromorpha intestinalis and E. compressa (Chlorophyta) in the British Isles. J Phycol. 1998;34(2):319–40. [Google Scholar]
- 20.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall TA, editor BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 23.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML Web-servers. Syst Biol. 2008;75:758–71. [DOI] [PubMed] [Google Scholar]
- 24.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–42. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9(8):772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25(11):1451–2. 10.1093/bioinformatics/btp187 [DOI] [PubMed] [Google Scholar]
- 27.Bouckaert R, Heled J, Kuhnert D, Vaughan T, Wu CH, Xie D, et al. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput Biol. 2014;10(4):e1003537 10.1371/journal.pcbi.1003537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst Biol. 2018;67(5):901–4. 10.1093/sysbio/syy032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Team RC. R: A language and environment for statistical computing [Computer software manual]. Vienna, Austria. 2016.
- 30.Hughey JR, Maggs CA, Mineur F, Jarvis C, Miller KA, Shabaka SH, et al. Genetic analysis of the Linnaean Ulva lactuca (Ulvales, Chlorophyta) holotype and related type specimens reveals name misapplications, unexpected origins, and new synonymies. J Phycol. 2019. [DOI] [PubMed] [Google Scholar]
- 31.Hiraoka M, Shimada S, Uenosono M, Masuda M. A new green‐tide‐forming alga, Ulva ohnoi Hiraoka et Shimada sp. nov.(Ulvales, Ulvophyceae) from Japan. Phycol Res. 2004;52(1):17–29. [Google Scholar]
- 32.O’Kelly CJ, Kurihara A, Shipley TC, Sherwood AR. Molecular assessment of Ulva spp. (Ulvophyceae, Chlorophyta) in the Hawaiian Islands. J Phycol. 2010;46(4):728–35. [Google Scholar]
- 33.Monotilla AP, Nishimura T, Adachi M, Tanii Y, Largo DB, Hiraoka M. Examination of prezygotic and postzygotic isolating barriers in tropical Ulva (Ulvophyceae, Chlorophyta): evidence for ongoing speciation. J Phycol. 2018;54(4):539–49. 10.1111/jpy.12755 [DOI] [PubMed] [Google Scholar]
- 34.Brodie J, Maggs CA, John DM, Blomster J. Green seaweeds of Britain and Ireland British Phycological Society, London: 2007:80–103. [Google Scholar]
- 35.Shimada S, Nagano M, Hiraoka M, Ichihara K, Mineur F, Zhu W. Phylogeographic analysis of the genus Ulva (Ulvales, Chlorophyta), including bloom sample in Qingdao, China. Coast Mar Sci. 2010;34:117–22. [Google Scholar]
- 36.Bureau MEP. Report on 2011-year Marine ecosystem research on disturbed biological organisms and management projects Ministry of Oceans and Fisheries, Daejeon: 2012:13p. [Google Scholar]
- 37.Hayden HS, Blomster J, Maggs CA, Silva PC, Stanhope MJ, Waaland JR. Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. European J Phycol. 2003;38(3):277–94. [Google Scholar]
- 38.Guiry M, Guiry G. World-wide electronic publication, National University of Ireland, Galway: AlgaeBase;2012. Available from: http://www.algaebase.org. [Google Scholar]
- 39.Hanyuda T, Kawai H. Genetic examination of the type specimen of Ulva australis suggests that it was introduced to Australia. Phycol Res. 2018;66(3):238–41. [Google Scholar]
- 40.Flagella MM, Andreakis N, Hiraoka M, Verlaque M, Buia MC. Identification of cryptic Ulva species (Chlorophyta, Ulvales) transported by ballast water. J Biol Res. 2010;13:47. [Google Scholar]
- 41.Kim KY, Choi TS, Kim JH, Han T, Shin HW, Garbary DJ. Physiological ecology and seasonality of Ulva pertusa on a temperate rocky shore. Phycologia. 2004;43(4):483–92. [Google Scholar]
- 42.Yoshida G, Uchimura M, Hiraoka M. Persistent occurrence of floating Ulva green tide in Hiroshima Bay, Japan: seasonal succession and growth patterns of Ulva pertusa and Ulva spp.(Chlorophyta, Ulvales). Hydrobiologia. 2015;758(1):223–33. [Google Scholar]
- 43.Kwon HK, Kang H, Oh YH, Park SR, Kim G. Green tide development associated with submarine groundwater discharge in a coastal harbor, Jeju, Korea. Sci Rep. 2017;7(1):6325 10.1038/s41598-017-06711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirkendale L, Saunders GW, Winberg P. A molecular survey of Ulva (Chlorophyta) in temperate Australia reveals enhanced levels of cosmopolitanism. J Phycol. 2013;49(1):69–81. 10.1111/jpy.12016 [DOI] [PubMed] [Google Scholar]
- 45.Melton JT III, Collado-Vides L, Lopez-Bautista JM. Molecular identification and nutrient analysis of the green tide species Ulva ohnoi M. Hiraoka & S. Shimada, 2004 (Ulvophyceae, Chlorophyta), a new report and likely nonnative species in the Gulf of Mexico and Atlantic Florida, USA. Aquat Invasions. 2016;11:225–37. [Google Scholar]
- 46.Yabe T, Ishii Y, Amano Y, Koga T, Hayashi S, Nohara S, et al. Green tide formed by free-floating Ulva spp. at Yatsu tidal flat, Japan. Limnology. 2009;10(3):239–45. [Google Scholar]
- 47.Pedersen MF, Borum J. Nutrient control of estuarine macroalgae: growth strategy and the balance between nitrogen requirements and uptake. Mar Ecol Prog Ser. 1997;161:155–63. [Google Scholar]
- 48.Taylor R, Fletcher RL, Raven JA. Preliminary studies on the growth of selected ‘green tide’algae in laboratory culture: effects of irradiance, temperature, salinity and nutrients on growth rate. Bot March 2001;44(4):327–36. [Google Scholar]
- 49.Lawton RJ, Mata L, de Nys R, Paul NA. Algal bioremediation of waste waters from land-based aquaculture using Ulva: selecting target species and strains. PLoS One. 2013;8(10):e77344 10.1371/journal.pone.0077344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawai H, Shimada S, Hanyuda T, Suzuki T. Species diversity and seasonal changes of dominant Ulva species (Ulvales, Ulvophyceae) in Mikawa Bay, Japan, deduced from ITS2 rDNA region sequences. Algae. 2007;22(3):221–8. [Google Scholar]
- 51.Zhang X, Xu D, Mao Y, Li Y, Xue S, Zou J, et al. Settlement of vegetative fragments of Ulva prolifera confirmed as an important seed source for succession of a large‐scale green tide bloom. Limnol Oceanogr. 2011;56(1):233–42. [Google Scholar]
- 52.Kang EJ, Kim J-H, Kim K, Kim KY. Adaptations of a green tide forming Ulva linza (Ulvophyceae, Chlorophyta) to selected salinity and nutrients conditions mimicking representative environments in the Yellow Sea. Phycologia. 2016;55(2):210–8. [Google Scholar]
- 53.Gao S, Shen S, Wang G, Niu J, Lin A, Pan G. PSI-driven cyclic electron flow allows intertidal macro-algae Ulva sp.(Chlorophyta) to survive in desiccated conditions. Plant Cell Physiol. 2011;52(5):885–93. 10.1093/pcp/pcr038 [DOI] [PubMed] [Google Scholar]
- 54.Luo MB, Liu F. Salinity-induced oxidative stress and regulation of antioxidant defense system in the marine macroalga Ulva prolifera. J Exp Mar Biol Ecol. 2011;409:223–8. [Google Scholar]
- 55.Holzinger A, Herburger K, Kaplan F, Lewis LA. Desiccation tolerance in the chlorophyte green alga Ulva compressa: does cell wall architecture contribute to ecological success? Planta. 2015;242(2):477–92. 10.1007/s00425-015-2292-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leskinen E, Alstrom-Rapaport C, Pamilo P. Phylogeographical structure, distribution and genetic variation of the green algae Ulva intestinalis and U. compressa (Chlorophyta) in the Baltic Sea area. Mol Ecol. 2004;13(8):2257–65. 10.1111/j.1365-294X.2004.02219.x [DOI] [PubMed] [Google Scholar]
- 57.Rybak AS. Species of Ulva (Ulvophyceae, Chlorophyta) as indicators of salinity. Ecol Indic. 2018;85:253–61. [Google Scholar]
- 58.Bae EH, Lee IK. Umbraulva, a new genus based on Ulva japonica (Holmes) Papenfuss (Ulvaceae, Chlorophyta). Algae. 2001;16(3):217–31. [Google Scholar]
- 59.Silva PC, Basson PW, Moe RL. Catalogue of the benthic marine algae of the Indian Ocean: University of California Press; 1996. [Google Scholar]
- 60.Liu D, Keesing JK, Xing Q, Shi P. World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Mar Pollut Bull. 2009;58(6):888–95. 10.1016/j.marpolbul.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 61.Liu X, Wang Z, Zhang X. A review of the green tides in the Yellow Sea, China. Mar Environ Res. 2016;119:189–96. 10.1016/j.marenvres.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 62.Son YB, Choi BJ, Kim YH, Park YG. Tracing floating green algae blooms in the Yellow Sea and the East China Sea using GOCI satellite data and Lagrangian transport simulations. Remote Sens Environ. 2015;156:21–33. [Google Scholar]
- 63.Duan W, Guo L, Sun D, Zhu S, Chen X, Zhu W, et al. Morphological and molecular characterization of free-floating and attached green macroalgae Ulva spp. in the Yellow Sea of China. J Appl Phycol. 2012;24(1):97–108. [Google Scholar]
- 64.Tian X, Huo Y, Chen L, He J, Zhang J, Jia R, et al. Molecular detection and analysis of green seaweeds from Rudong coasts in Jiangsu Province. Chinese Sci Bull. 2011;56(4–5):309–17. [Google Scholar]
- 65.Zhao J, Jiang P, Liu Z, Wei W, Lin H, Li F, et al. The Yellow Sea green tides were dominated by one species, Ulva (Enteromorpha) prolifera, from 2007 to 2011. Chinese Sci Bull. 2013;58(19):2298–302. [Google Scholar]
- 66.Couceiro L, Cremades J, Barreiro R. Evidence for multiple introductions of the Pacific green alga Ulva australis Areschoug (Ulvales, Chlorophyta) to the Iberian Peninsula. Bot March 2011;54(4):391–402. [Google Scholar]
- 67.Liu F, Melton JT III, Bi Y. Mitochondrial genomes of the green macroalga Ulva pertusa (Ulvophyceae, Chlorophyta): novel insights into the evolution of mitogenomes in the Ulvophyceae. J Phycol. 2017;53(5):1010–9. 10.1111/jpy.12561 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
(XLSX)
P, Prior intraspecific divergence (P); IP, Initial partition; RP, Recursive partition; Ng, Number of groups of the full data set (Ng).
(XLSX)
(XLSX)
Data Availability Statement
All obtained sequences were deposited in GenBank nucleotide sequence database under accession Nos. (tufA: MK992043-MK992249, ITS: MN069870-MN070070).