Abstract
Background
To understand which breast cancer (BC) risk factors also increase the risk of fibroadenoma and investigate whether these factors have the same effect in BC patients with previous fibroadenoma.
Methods
Using multistate survival analysis on a large dataset (n = 58 322), we examined the effects of BC risk factors on transitions between three states: event-free, biopsy-confirmed fibroadenoma, and BC. Hazard ratios and corresponding 95% confidence intervals associated with covariate effects were estimated. Median follow-up time was 25.3 years.
Results
The mean ages at diagnosis of fibroadenoma and BC were 42.6 and 48.3 years, respectively. Participant characteristics known to increase the risk of BC were found to increase the risk of fibroadenoma (family history of BC and higher education). Participant characteristics known to confer protective effects for BC (older age at menarche, more children, and larger childhood body size) were found to reduce fibroadenoma risk. The effect sizes associated with the direct transitions from event-free to fibroadenoma and BC were generally not different for the covariates tested. Age at fibroadenoma diagnosis was associated with the transition from fibroadenoma to BC (hazard ratioper year increase = 1.07 [95% confidence interval = 1.03 to 1.12]).
Conclusion
We showed that biopsy-confirmed fibroadenomas shared many risk factors with BC. More work is needed to understand the relationships between fibroadenoma and BC to identify women who are at high risk of developing BC after a fibroadenoma diagnosis.
Benign breast diseases are very common and can evoke undue psychological distress and reduced quality of life among those diagnosed (1). Women with benign breast diseases experience elevated breast cancer risk (2) not restricted to the breast where the benign breast disease was detected. This suggests that the two breast disorders may share common causes, or a similar early ancestry, but develop in divergent paths at an early stage. Benign breast diseases are thus regarded as a known risk factor of breast cancer and diagnoses of benign lesions have been implemented in several breast cancer risk assessment models (2–4).
A common benign lesion is fibroadenoma, which is characterized by a nodule of fibrous tissue with epithelial elements (5). Fibroadenomas confer a moderate increased risk (∼2–3-fold) of later developing breast cancer (6). Although the increased breast cancer risk associated with other benign breast conditions such as atypical hyperplasia can diminish over time (7), the risk conferred by fibroadenoma is said to be persistent and to not vary over time (8). These lesions occur in about one in four women (9) and can account for more than two-thirds of all benign lesions in young women (10). The risk of fibroadenoma peaks at a relatively young age (20–30 years), after which the risk decreases and drops sharply at the time of menopause (6). Although fibroadenoma is prevalent among young women, it is common for the breast mass to shrink or regress with age (11). It was reported by Cerrato and Lebow that in young women the mass disappears spontaneously between 10% and 40% of the time (12). Greenberg et al. also noted, noted in a review on the management of breast fibroadenomas that as many as approximately 60% of all fibroadenoma cases observed were completely resolved after approximately 30 years (11). Consequently, the finding of fibroadenoma in older women is less common.
Breast fibroadenomas can be diagnosed through clinical examination (manual breast examination and palpation), imaging (mammography or ultrasound), and cytology (fine-needle aspiration cytology or core biopsy) (11,13). Due to the tendency of fibroadenomas to spontaneously regress with age, such breast lesions diagnosed in younger patients are often managed conservatively using a wait-and-see approach (14). Recent studies suggest that modern ultrasound is a reliable tool for the diagnosis of fibroadenoma in younger women referred with breast symptoms and that a pathology work-up is only necessary when there is overriding clinical concern (14–16). In contrast, fibroadenomas in older women are more often usually subjected to triple assessment and treated more aggressively (eg, surgical excision) (17). Although the peak incidence of fibroadenoma and breast cancer is differentially associated with age, fibroadenoma appears to share a number of risk factors with breast cancer (18,19). An increased risk of developing both breast diseases is observed among women of higher socioeconomic status and women who experienced early menarche (11,20). A family history of breast cancer in first-degree relatives has been reported by some investigators to be related to an increased risk of developing benign lesions (10,21). Conversely, body mass index (BMI) and the number of biological children have been shown to be negatively associated with both fibroadenoma and (premenopausal) breast cancer (11). Evidence of an association between age of menarche, age of menopause, and hormonal therapy, including oral contraceptives, have been less consistently shown for fibroadenoma (11).
The importance of characterizing the differences between benign and malignant breast disease is accentuated by the high prevalence of fibroadneoma and increasing burden of breast cancer. The epidemiology of fibroadenoma and the potential transition to breast cancer are still poorly understood. Using a multistate modeling approach, we wish to 1) understand if breast cancer risk factors also increase risk of fibroadenoma, and 2) investigate whether the associations of these risk factors with breast cancer differ in women who have been diagnosed with fibroadenoma. In cases without confirmation through a pathology examination, a definitive diagnosis cannot be established with certainty (22); hence, we limit our analysis to biopsy-confirmed fibroadenoma.
Materials and Methods
Study Participants
The KARMA (www.karmastudy.org) study is a population-based cohort study comprising women participating in the screening program or attending clinical mammography at one of the four mammography units in Sweden (Södersjukhuset, Helsingsborg, Landskrona, and Lund hospitals) (see description of cohort in Ref. 23). All women invited for screening from January 2011 to March 2013, at the four hospitals, were invited to participate in the study. Additionally, women who had a clinical mammography at any of the participating mammography screening centers during this time were also invited. During the recruitment period, a total of 210 233 women were invited to participate in the KARMA study and 70 877 women (34%) joined the study (see description of cohort in Ref. 23).
Assessment of Fibroadenoma and Breast Cancer
Histologically verified fibroadenoma diagnoses (Systemized Nomenclature of Medicine – Clinical Terms (morphology code M90100 [fibroadenoma] and topography code T04 [breast]) from surgical biopsies (excisional or core) and fine-needle aspirations were retrieved (April 24, 2015) from the pathology medical records in Lund and Stockholm. Computerized medical records of biopsy reports were available in all of Sweden since the early 1990s; recording started in the 1980s, with retrospective registration of some older reports (24). If there were several diagnoses of fibroadenoma for the same woman, the first diagnosis was selected. The computerized search yielded 1085 KARMA participants (out of 14 341 unique individuals with pathology reports in the SymPathy system = 7.6%) with a biopsy record of fibroadenoma, diagnosed between 1979 and 2015.
Diagnoses of breast cancer (ICD7 code 170*) were retrieved through linkage with the Swedish Cancer Registry (October 16, 2015). The nationwide registry was founded in 1958 and has excellent coverage (∼99%) (25).
Assessment of Other Covariates
Information on education (elementary, intermediate, and university), first-degree family history of breast cancer (mother, sister, or daughter, yes/no), age at menarche in years (<13/≥13), body size at age 7 years (nine somatotype categories, collapsed into small, medium, and large), oral contraceptive use (never used, progestin-only “mini” pill, and combination pill), and number of children (0, 1, or ≥2) were obtained from the KARMA questionnaire. The variables ever oral contraceptive use and number of children were recoded (based on start year of use and birth year) to reflect status at baseline (start of follow-up at 1990; see below). For discrete variables, missing values were treated as separate categories.
Analytical Cohort
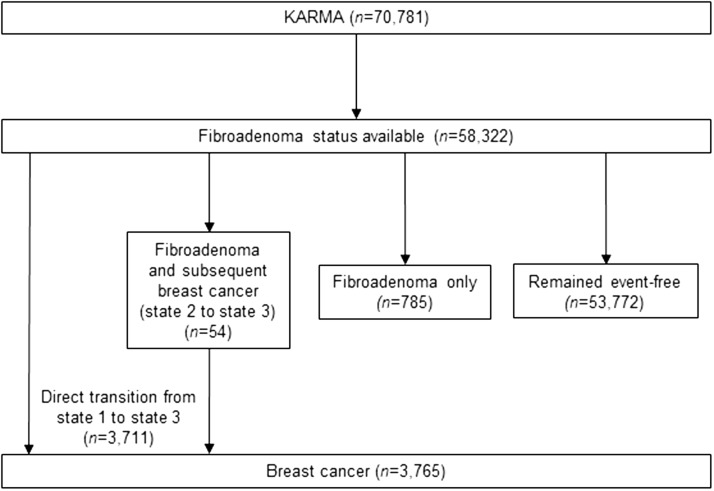
Figure 1 describes the analytical datasets used in this study. Computerized records of biopsy reports were available in all of Sweden after 1990, so we started the follow-up from January 1, 1990. Our analytical cohort was restricted to women who did not have a history of benign breast disease at KARMA study entry and who had a record in the SymPathy system from 1990 onwards (n = 58 322). Women were followed up from January 1, 1990, until death, breast cancer diagnosis, or end of follow-up (April 24, 2015, ie, latest date we had updated information from all data sources). Median follow-up time from January 1, 1990 was 25.3 years.
Figure 1.
Summary of analytical datasets. Three states were defined as “event-free,” “fibroadenoma,” and “breast cancer,” respectively.
Statistical Analysis
To assess whether fibroadenoma itself was a risk factor for breast cancer, fibroadenoma was coded as a time-dependent covariate in Cox regression models with chronological age as the underlying time scale and breast cancer as the event of interest. The proportional hazards assumption for model fit was checked using the cox.zph() function in R (Vienna, Austria). Although the covariate information was collected retrospectively (recalled and self-reported in questionnaire), we analyzed the data as if it were collected prospectively. In this analysis, we thus needed to rely on an assumption that the hazards of death with and without fibroadenoma are equal for there to be no survivorship bias when estimating hazard ratios (HRs) from our model.
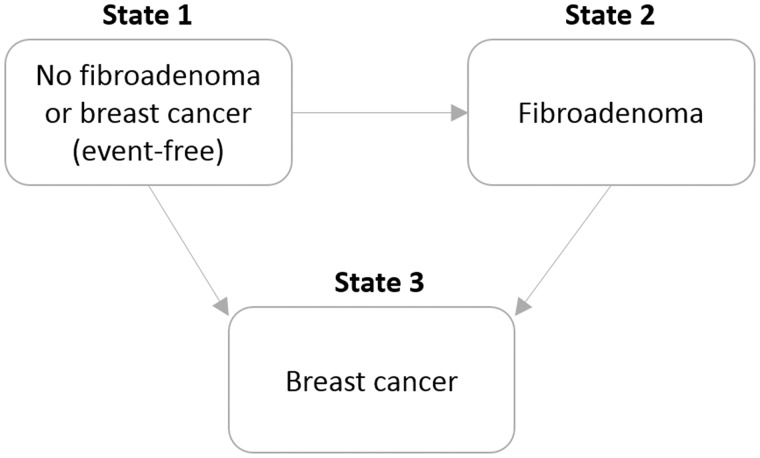
Using multistate survival analysis [an illness-death model (26,27)], we then explored the effects of breast cancer risk factors on transitions between the states event-free (state 1), fibroadenoma (state 2 / intermediate state), and breast cancer (state 3). An illness-death model without recovery (clock-reset approach, nonhomogeneous semi-Markov model) was fitted using the “mstate” package in R (28) (Figure 2). This analysis is important because intermediate events may change the natural history of the disease development, so that the role of some risk factors may not be the same after the intermediate event. Breast cancer is treated as the absorbing state (ie, fibroadenomas diagnosed after a breast cancer diagnosis were not considered). All subjects start in the event-free state. Possible courses (until end of follow-up) for each woman include: 1 → 1 (the woman remained event-free until the end of the study); 1 → 3 (a direct transition from state 1 into breast cancer was observed); 1 → 2 (the woman developed fibroadenoma and not breast cancer); and 1 → 2 → 3 (the woman developed fibroadenoma and subsequently breast cancer). Covariate effects were allowed to vary freely across distinct transitions [incorporating covariates in multistate models through transition intensities may explain differences in the course of the disease across individuals (26)]. Chronological age was chosen as the underlying time scale in all analyses because it provides the most flexible control for age effects (29). Time in the event-free state (ie, age at fibroadenoma diagnosis) was included as a covariate for the transition from fibroadenoma to breast cancer. To test whether covariate effects can be assumed to be identical across transitions (ie, the same across transitions or transition-specific), interactions between covariates and transitions were tested using the likelihood ratio test (“lmtest” package in R) (27). If a covariate has different effects for transitions 1 → 3 and 1 → 2, this would imply that the covariate has a different role in the two outcomes. If a covariate has different effects for transitions 1 → 3 and 2 → 3, this would imply that fibroadenoma modifies the effect of the covariate. Because the data were not prospective, but were analyzed as if it were, estimation of the actual transition probabilities in these multistate models is subject to survivorship bias. We therefore report only HRs and corresponding 95% confidence intervals (CIs) (for the possible transitions 1 → 2, 1 → 3, and or 2 → 3), which will be unbiased as long as the covariate under study is conditionally independent (conditional on adjustment variables) of the hazard of dying of breast cancer or other causes (when in any of the three states).
Figure 2.
Multistate model used.
All analyses were performed in R (v. 3.3.1) (30). All statistical tests were two-sided, with an alpha level of 0.05.
Ethical Approval
This study was approved by the regional Ethical Review Board in Stockholm (2010/958-31/1). All participants gave informed consent to the retrieval of data from medical records and national registers, and answered a detailed questionnaire on background and lifestyle risk factors.
Results
Characteristics of the Study Population
Participant characteristics are described in Table 1. The mean age at start of follow-up of 58 322 KARMA participants included in this study was 32.6 years. Around half the women attended university (50.8%). One in eight women (12.5%) reported a family history of breast cancer. A large majority of all women reported use of combination birth control pills, which are oral contraceptives that contain both estrogen and progestin (63.8%). The use of the minipill, also known as the progestin-only birth control pill, was less common (13.3%). At the start of follow-up, 45.2% of the women did not have any children. The mean age at diagnosis of fibroadenoma and breast cancer was 42.6 and 48.3 years, respectively.
Table 1.
Characteristics of the analytical cohort (n = 58 322) from the Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA)
| Characteristic | No. (%) | Mean (SD) age at diagnosis, y |
|---|---|---|
| Age at start of follow-up, mean (SD), y | 32.6 (10.0) | |
| Highest education | ||
| Elementary | 7633 (13.1) | |
| Intermediate | 17 922 (30.7) | |
| University | 29 653 (50.8) | |
| Other | 3114 (5.3) | |
| Family history of breast cancer | ||
| No | 47 799 (82.0) | |
| Yes | 7290 (12.5) | |
| Age at menarche, mean (SD), y | 13.1 (1.5) | |
| Body size at age 7 years | ||
| Small | 29 471 (50.5) | |
| Medium | 20 098 (34.5) | |
| Large | 7090 (12.2) | |
| Ever oral contraceptive use | ||
| Progestin-only “mini” pill | 7733 (13.3) | |
| Combination pill | 37 186 (63.8) | |
| Number of children | ||
| 0 | 26 364 (45.2) | |
| 1 | 8127 (13.9) | |
| ≥2 | 22 311 (38.3) | |
| Breast conditions | ||
| Fibroadenoma | 839 (1.4) | 42.6 (8.7) |
| Invasive breast cancer | 3765 (6.5) | 48.3 (9.3) |
Fibroadenoma Is Associated With Breast Cancer Risk
Women with a diagnosis of fibroadenoma had a 74% higher rate of being diagnosed with breast cancer than women without a diagnosis (unadjusted HR = 1.74 [95% CI = 1.33 to 2.27]). The proportional hazards assumption was not violated (P = .218), suggesting that the increase in breast cancer risk conferred by fibroadenoma did not vary over time.
Fibroadenoma and Breast Cancer Share Common Risk Factors
Table 2 shows how participant characteristics were associated with the risks of transitions from healthy to fibroadenoma and breast cancer. Among the factors associated with higher HRs for transitions to both breast diseases from an event-free state (no fibroadenoma and no breast cancer) were higher education and family history of breast cancer. Later age at menarche, larger childhood body size, and more children were associated with lower HRs for direct transitions to both breast diseases from an event-free state. Oral contraceptive use at start of follow-up was not associated with the direct transitions to both diseases. The effect sizes associated with the transition from event-free to fibroadenoma (1→2) and from event-free to breast cancer (1→3) were not found to be different for any of the factors studied. For oral contraceptive use, effect sizes were different between transitions 1→2 and 2→3 (P = .001) and 1→3 and 2→3 (P = .002). For childhood body size, although a difference in effect size between 1→2 and 2→3 was observed (P = .035), only the medium category was statistically significant (P = .012) (Table 2).
Table 2.
Estimated effects associated with breast cancer risk factors in Cox semi-Markov models (adjusted for sojourn time in event-free state)*
| Event-free to breast cancer (state 1 to state 3) (n = 58 322, nevent = 3711) |
Event-free to fibroadenoma (state 1 to state 2) (n = 58 322, nevent = 839) |
Fibroadenoma to breast cancer (state 2 to state 3) (n = 817, nevent = 54) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk factors | HR | L95 | U95 | P | HR | L95 | U95 | P | P(1→3, 1→2) | HR | L95 | U95 | P | P(1→3, 2→3) | P(1→2, 2→3) |
| Education | .130 | .463 | .798 | ||||||||||||
| Elementary | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||||||||
| Intermediate | 1.26 | 1.14 | 1.39 | <.001 | 1.48 | 1.16 | 1.90 | .002 | 1.99 | 0.71 | 5.57 | .189 | |||
| University | 1.21 | 1.11 | 1.33 | <.001 | 1.56 | 1.23 | 1.97 | <.001 | 2.13 | 0.82 | 5.58 | .122 | |||
| Family history of breast cancer | .545 | .302 | .418 | ||||||||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||||||||
| Yes | 1.62 | 1.49 | 1.76 | <.001 | 1.53 | 1.27 | 1.83 | <.001 | 1.16 | 0.61 | 2.22 | .653 | |||
| Age at menarche | .787 | .609 | .565 | ||||||||||||
| <13 y | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||||||||
| ≥13 y | 0.91 | 0.85 | 0.98 | .011 | 0.89 | 0.77 | 1.03 | .124 | 1.06 | 0.59 | 1.90 | .840 | |||
| Body size at age 7 y | .284 | .035 | .099 | ||||||||||||
| Small | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||||||||
| Medium | 0.92 | 0.86 | 0.99 | .029 | 0.80 | 0.69 | 0.94 | .006 | 0.35 | 0.16 | 0.79 | .012 | |||
| Large | 0.77 | 0.69 | 0.86 | <.001 | 0.74 | 0.58 | 0.93 | .012 | 0.72 | 0.30 | 1.73 | .467 | |||
| Oral contraceptives | .430 | .002 | .001 | ||||||||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||||||||
| Progestin-only “mini” pill | 1.06 | 0.95 | 1.18 | .279 | 1.02 | 0.83 | 1.26 | .814 | 3.11 | 1.64 | 5.90 | .001 | |||
| Combination pill | 1.04 | 0.96 | 1.12 | .329 | 0.93 | 0.80 | 1.09 | .362 | 2.34 | 1.11 | 4.91 | .025 | |||
| Number of biological children | .842 | .583 | .505 | ||||||||||||
| 0 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||||||||
| 1 | 0.77 | 0.69 | 0.85 | <.001 | 0.76 | 0.61 | 0.94 | .002 | 0.93 | 0.39 | 2.21 | .867 | |||
| ≥2 | 0.65 | 0.60 | 0.71 | <.001 | 0.62 | 0.53 | 0.73 | <.001 | 0.95 | 0.47 | 1.90 | .877 | |||
HR = hazard ratio; L95 and U95 = lower and upper 95% confidence intervals. P(transition 1, transition 2) denotes two-sided P value from likelihood ratio test testing whether covariate effects can be assumed to be identical across transitions (ie, the same across transitions or transition-specific)
The associations did not change appreciably in a multivariable model where all the studied breast cancer risk factors were included (Table 3). Covariates found to be associated with the transition from fibroadenoma to breast cancer were body size at age 7 years (HRmedium vs small = 0.31 [95% CI = 0.13 to 0.72]) and oral contraceptive use (HRminipill vs no use = 3.11 [95% CI =1.62 to 5.94] and HRcombination pill vs no use = 2.48 [95% CI = 1.16 to 5.30]).
Table 3.
Estimated effects associated with breast cancer risk factors in Cox semi-Markov models, multivariable adjusted (all studied breast cancer risk factors were included in the model)*
| Event-free to breast cancer (n = 58 322, nevent = 3711) |
Event-free to fibroadenoma (n = 58 322, nevent = 839) |
Fibroadenoma to breast cancer (n = 839, nevent = 54) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk factors | HR | L95 | U95 | P | HR | L95 | U95 | P | HR | L95 | U95 | P |
| Education (intermediate vs elementary) | 1.22 | 1.11 | 1.35 | <.001 | 1.38 | 1.07 | 1.78 | .013 | 2.44 | 0.85 | 7.00 | .098 |
| Education (university vs elementary ) | 1.16 | 1.06 | 1.27 | .001 | 1.42 | 1.11 | 1.81 | .005 | 2.47 | 0.92 | 6.69 | .074 |
| Family history of breast cancer (yes vs no) | 1.61 | 1.48 | 1.75 | <.001 | 1.54 | 1.28 | 1.85 | <.001 | 1.05 | 0.56 | 1.97 | .878 |
| Age at menarche (≥13 y vs <13 y) | 0.90 | 0.84 | 0.97 | .007 | 0.89 | 0.76 | 1.03 | .111 | 1.11 | 0.60 | 2.04 | .743 |
| Body size at age 7 y (medium vs small) | 0.91 | 0.84 | 0.98 | .011 | 0.78 | 0.67 | 0.92 | .002 | 0.31 | 0.13 | 0.72 | .006 |
| Body size at age 7 y (large vs small) | 0.75 | 0.67 | 0.84 | <.001 | 0.73 | 0.57 | 0.92 | .009 | 0.86 | 0.36 | 2.09 | .742 |
| Mini pill (yes vs no) | 1.05 | 0.94 | 1.16 | .405 | 1.01 | 0.82 | 1.24 | .927 | 3.11 | 1.62 | 5.94 | .001 |
| Combination pill (yes vs no) | 1.03 | 0.95 | 1.11 | .500 | 0.92 | 0.79 | 1.08 | .325 | 2.48 | 1.16 | 5.30 | .019 |
| Number of biological children (1 vs 0) | 0.83 | 0.75 | 0.93 | .001 | 0.80 | 0.64 | 0.99 | .036 | 0.95 | 0.37 | 2.45 | .909 |
| Number of biological children (≥2 vs 0) | 0.71 | 0.65 | 0.77 | <.001 | 0.66 | 0.56 | 0.78 | <.001 | 1.07 | 0.50 | 2.27 | .864 |
| Time spent in event-free state (ie, age at fibroadenoma diagnosis, y) | 1.07 | 1.03 | 1.12 | <.001 | ||||||||
HR = hazard ratio; L95 and U95 = lower and upper 95% confidence intervals; P values are two-sided.
Age at fibroadenoma diagnosis was associated with the transition from fibroadenoma to breast cancer (HRper year increase = 1.07 [95% CI =1.03 to 1.12]).
Discussion
In our study, we confirmed that biopsy-confirmed fibroadenoma is a risk factor for breast cancer. There was substantial agreement between the risk factors for fibroadenoma and breast cancer. Participant characteristics known to increase risk of breast cancer were also found to increase the risk of fibroadenoma (family history of breast cancer and higher education). Furthermore, participant characteristics known to confer protective effects for breast cancer (older age at menarche, more children, and larger childhood body size) were also found to reduce fibroadenoma risk. The effect sizes associated with the direct transitions from event-free to fibroadenoma and breast cancer were not different for all the covariates tested (ie, breast cancer risk factors affect risk of developing fibroadenoma to the same degree). Age at fibroadenoma diagnosis, body size at age 7 years, and ever use of oral contraceptives were risk factors for the transition from fibroadenoma to breast cancer.
Despite their potential, multistate modeling methods are not as frequently used as other survival analysis techniques (26,31). The method is not widely implemented on epidemiologic data. The few breast cancer studies that have previously employed multistate models studied the associations between prognostic factors and different breast cancer outcomes (local recurrence, distant disease, and breast cancer-related death) (32–34). To our knowledge, this is the first application of a multistate model approach to examine the associations between risk factors and two breast diseases—fibroadenoma (itself a risk factor for breast cancer and a potential intermediate event) and breast cancer—simultaneously.
Our findings on risk factors of fibroadenoma were mostly in agreement with the existing literature and known risk factors of breast cancer. Education, which is an aggregate of several breast cancer risk factors related to parity, lifestyle, and attendance to and compliance to screening, is known for its positive associations with breast cancer (19). Bertelsen et al. (21) found an approximately 1.5-fold increased risk of developing epithelial benign breast disease (including fibroadenoma) among first-degree relatives of young breast cancer patients compared with women in the general population. Berkey et al. reported that among young women with benign breast diseases (BBD) (∼70% of which were fibroadenomas), a family history of breast cancer further increased breast cancer risk (10). Our results on the similar associations between family history and two breast diseases support the presence of a shared inherited component. The prevailing evidence for age at menarche is less conclusive, but late age at menarche has generally been associated with decreased risk of fibroadenoma [reviewed previously (35)]. In studies on early-life risk factors, women who reported larger childhood body size were consistently shown to have a lower incidence of fibroadenoma, and this association remained statistically significant after adjustment for current BMI (36,37). Nulliparity is a known risk factor for both breast cancer and fibroadenoma (19,38).
Multistate modeling offers a flexible tool for the study of covariate effects on the various transition rates (39). Through simultaneous analyses of the transitions and adjustments for intermediate events, a multistate model shows the pathways of associations across subsequent disease states that are not directly visible with separate Cox regressions. These models may bring out important biological insights that may otherwise be ignored (39). For example, a number of studies conducted in the 1970s and 1980s showed that oral contraceptive use, especially estrogen plus progestin preparations, decreased the incidence of BBD (including fibroadenoma), so much so that the protective effect against BBD has been regarded as a noncontraceptive health benefit of oral contraceptives (40–42). However, the reduced risk associated with BBD contradicts the known link between oral contraceptives and increased breast cancer risk. Such opposing effects may be partly explained by the fact that hormones in oral contraceptive pills can exert mitogenic properties on transformed cells and at the same time inhibit tumorigenesis in normal or nontransformed cells (43). However, we did not observe associations between oral contraceptive use and direction transitions to fibroadenoma or breast cancer. An explanation for the null result could be that the associated risks of developing both breast diseases may be restricted to recent use of oral contraceptives (44). More modern formulations of oral contraceptive pills also contain substantially reduced doses of both estrogen and progestin compared with those used in the 1970s, which could result in the diminished effects seen for fibroadenoma and breast cancer (40). It should be noted that the assumption of transition-specific covariate effects can potentially result in an overfit of the model, particularly in the processes with rare event counts (ie, transition from fibroadenoma to breast cancer) (32). The finding that oral contraceptive use was associated with the transition from fibroadenoma to breast cancer is noteworthy but in need of replication by other studies. Apart from fibroadenoma itself being a risk factor for breast cancer, the age at which the fibroadenoma was diagnosed was also a risk factor for breast cancer. Our results showed that older women who developed biopsy-confirmed fibroadenomas were more likely to later develop breast cancer. An elevated risk of concurrent malignant disease in older women diagnosed with fibroadenoma has also been reported previously (45). Our finding supports that, while conservative management of fibroadenoma may be recommended for younger women, a more aggressive approach for older women may be warranted.
The main strength of this study is the large sample size and links to the cancer registry and medical records (pathology reports in SymPathy), making direct comparisons of risk factors between fibroadenoma and breast cancer possible through multistate models. The information captured by the KARMA questionnaire is based on recalled and self-reported information, which can contribute to bias. There are certain constraints on external validity, because our study cohort is not prospective, but analyzed as if it were. All women had to be alive during KARMA study recruitment between 2011 through 2013, leading to survivorship bias. The HR estimates associated with covariate effects are valid under the assumption that the covariates under study are independent of the hazard rates of death. Although we believe that any bias will be small (few individuals in the general population would have died from exposure to the risk factors studied), future work on a prospective cohort is needed to validate our results. Further work is also needed to study the effects of other breast cancer risk factors, such as alcohol use and BMI trajectory. Participants of the KARMA study were part of a cohort who attended either screening or clinical mammography and could thus be more health conscious or economically well-to-do than the general population. However, mammography screening is part of the national healthcare system in Sweden, primarily funded through taxation, and all residents are essentially offered equal access. It should also be noted that only biopsy-confirmed fibroadenomas were included in this study. Biopsy confirmation of fibroadenoma may be more common among older women; hence, care is needed if the results were to be generalized to a population that includes fibroadenomas diagnosed at a younger age.
Fibroadenoma is not typically a life-threatening disease, and most women will not later develop cancer. However, we showed that biopsy-confirmed fibroadenomas shared many risk factors with breast cancer. More work is needed to understand the relationships between fibroadenoma and breast cancer to identify women who are at high risk of developing breast cancer after a fibroadenoma diagnosis.
Funding
KARMA was financed by the Märit and Hans Rausing’s Initiative Against Breast Cancer and the Kamprad Family Foundation. This work was supported by the Swedish Research Council (grant number: 2014-2271); Swedish Cancer Society (grant number: CAN 2016/684); and FORTE (grant number: 2016-00081). JL is a recipient of a National Research Foundation Singapore Fellowship (NRF-NRFF2017-02) and an award from Ollie och Elof Ericssons Stiftelse för Vetenskaplig Forskning. KH received funding from the Swedish Research Council (2016-01245) and the Swedish Cancer Society (2017/287).
Notes
Affiliations of authors: Department of Human Genetics, Genome Institute of Singapore, Singapore, Singapore (JL, PJH); Department of Surgery, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore (JL); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (JL, KH, ME, PH, KC); Department of Pathology and Cytology, Karolinska University Hospital, Stockholm, Sweden (EDR); Department of Biosciences and Nutrition, Karolinska Institutet, Stockholm, Sweden (LSL); Department of Oncology, Södersjukhuset, Stockholm, Sweden (PH).
Conflict of interest: The authors declare no potential conflicts of interest.
Author contributions: KC and JL designed the study. ME prepared the data. KH and PJH guided the statistical analysis. EDR, LSL and PH provided subject knowledge. JL and PJH performed the analyses and wrote the article. All authors critically reviewed and approved of the article.
We thank Hanis Mariyah Mohd Ishak for statistical support and careful proofreading of the article.
References
- 1. Keyzer-Dekker CM, van Esch L, de Vries J, et al. An abnormal screening mammogram causes more anxiety than a palpable lump in benign breast disease. Breast Cancer Res Treat. 2012;134(1):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353(3):229–237. [DOI] [PubMed] [Google Scholar]
- 3. Tice JA, Miglioretti DL, Li CS, et al. Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer. J Clin Oncol. 2015;33(28):3137–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tyrer J, Duffy SW, Cuzick J.. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–1130. [DOI] [PubMed] [Google Scholar]
- 5. Dent DM, Cant PJ.. Fibroadenoma. World J Surg. 1989;13(6):706–710. [DOI] [PubMed] [Google Scholar]
- 6. Dupont WD, Page DL, Parl FF, et al. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med. 1994;331(1):10–15. [DOI] [PubMed] [Google Scholar]
- 7. Dupont WD, Page DL.. Relative risk of breast cancer varies with time since diagnosis of atypical hyperplasia. Hum Pathol. 1989;20(8):723–725. [DOI] [PubMed] [Google Scholar]
- 8. Krieger N, Hiatt RA.. Risk of breast cancer after benign breast diseases. Variation by histologic type, degree of atypia, age at biopsy, and length of follow-up. Am J Epidemiol. 1992;135(6):619–631. [DOI] [PubMed] [Google Scholar]
- 9. Guray M, Sahin AA.. Benign breast diseases: classification, diagnosis, and management. Oncologist. 2006;11(5):435–449. [DOI] [PubMed] [Google Scholar]
- 10. Berkey CS, Tamimi RM, Rosner B, et al. Young women with family history of breast cancer and their risk factors for benign breast disease. Cancer. 2012;118(11):2796–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greenberg R, Skornick Y, Kaplan O.. Management of breast fibroadenomas. J Gen Intern Med. 1998;13(9):640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cerrato F, Labow B.. Diagnosis and management of fibroadenomas in the adolescent breast. Semin Plast Surg. 2013;27(1):23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Foxcroft L, Evans E, Hirst C.. Newly arising fibroadenomas in women aged 35 and over. Aust N Z J Surg. 1998;68(6):419–422. [DOI] [PubMed] [Google Scholar]
- 14. Smith GE, Burrows P.. Ultrasound diagnosis of fibroadenoma—is biopsy always necessary? Clin Radiol. 2008;63(5):511–515; discussion 516–517. [DOI] [PubMed] [Google Scholar]
- 15. Yue D, Swinson C, Ravichandran D.. Triple assessment is not necessary in most young women referred with breast symptoms. Ann R Coll Surg Engl. 2015;97(6):466–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Neill AC, Shine S, Coffey L, et al. Audit on breast ultrasound in women under 25 years. Ir J Med Sci. 2013;182(2):287–289. [DOI] [PubMed] [Google Scholar]
- 17. Deschenes L, Jacob S, Fabia J, et al. Beware of breast fibroadenomas in middle-aged women. Can J Surg. 1985;28(4):372–374. [PubMed] [Google Scholar]
- 18. La Vecchia C, Parazzini F, Franceschi S, et al. Risk factors for benign breast disease and their relation with breast cancer risk. Pooled information from epidemiologic studies. Tumori. 1985;71(2):167–178. [DOI] [PubMed] [Google Scholar]
- 19. Parazzini F, La Vecchia C, Franceschi S, et al. Risk factors for pathologically confirmed benign breast disease. Am J Epidemiol. 1984;120(1):115–122. [DOI] [PubMed] [Google Scholar]
- 20. Dorjgochoo T, Deming SL, Gao Y-T, et al. History of benign breast disease and risk of breast cancer among women in China: a case–control study. Cancer Causes Control. 2008;19(8):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bertelsen L, Mellemkjaer L, Balslev E, et al. Benign breast disease among first-degree relatives of young breast cancer patients. Am J Epidemiol. 2008;168(3):261–267. [DOI] [PubMed] [Google Scholar]
- 22. Takei H, Iino Y, Horiguchi J, et al. Natural history of fibroadenomas based on the correlation between size and patient age. Jpn J Clin Oncol. 1999;29(1):8–10. [DOI] [PubMed] [Google Scholar]
- 23. Gabrielson M, Eriksson M, Hammarström M, et al. Cohort profile: the Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA). Int J Epidemiol. 2017; doi:10.1093/ije/dyw357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jarrick S, Lundberg S, Welander A, et al. Clinical validation of immunoglobulin A nephropathy diagnosis in Swedish biopsy registers. Clin Epidemiol. 2017;9:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33. [DOI] [PubMed] [Google Scholar]
- 26. Meira-Machado L, de Uña-Álvarez J, Cadarso-Suárez C, et al. Multi-state models for the analysis of time-to-event data. Stat Methods Med Res. 2009;18(2):195–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eulenburg C, Mahner S, Woelber L, et al. A systematic model specification procedure for an illness-death model without recovery. Plos One. 2015;10(4):e0123489.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Wreede LC, Fiocco M, Putter H.. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed. 2010;99(3):261–274. [DOI] [PubMed] [Google Scholar]
- 29. Korn EL, Graubard BI, Midthune D.. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145(1):72–80. [DOI] [PubMed] [Google Scholar]
- 30. R Core Team. R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing; 2016. https://www.R-project.org/.
- 31. Meira-Machado L, Roca-Pardiñas J.. p3state.msm: analyzing survival data from an illness-death model. J Stat Softw. 2011;38(3): [Google Scholar]
- 32. Eulenburg C, Schroeder J, Obi N, et al. A comprehensive multistate model analyzing associations of various risk factors with the course of breast cancer in a population-based cohort of breast cancer cases. Am J Epidemiol. 2016;183(4):325–334. [DOI] [PubMed] [Google Scholar]
- 33. Putter H, van der Hage J, de Bock GH, et al. Estimation and prediction in a multi-state model for breast cancer. Biom J. 2006;48(3):366–380. [DOI] [PubMed] [Google Scholar]
- 34. Meier-Hirmer C, Schumacher M.. Multi-state model for studying an intermediate event using time-dependent covariates: application to breast cancer. BMC Med Res Methodol. 2013;13(1):80.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Silvera SAN, Rohan TE.. Benign proliferative epithelial disorders of the breast: a review of the epidemiologic evidence. Breast Cancer Res Treat. 2008;110(3):397–409. [DOI] [PubMed] [Google Scholar]
- 36. Baer HJ, Schnitt SJ, Connolly JL, et al. Early life factors and incidence of proliferative benign breast disease. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2889–2897. [DOI] [PubMed] [Google Scholar]
- 37. Frazier AL, Rosenberg SM.. Preadolescent and adolescent risk factors for benign breast disease. J Adolesc Health. 2013;52(5 suppl):S36–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Minami Y, Ohuchi N, Taeda Y, et al. Risk factors for benign breast disease according to histopathological type: comparisons with risk factors for breast cancer. Jpn J Cancer Res. 1998;89(2):116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sartwell PE, Arthes FG, Tonascia JA.. Benign and malignant breast tumours: epidemiological similarities. Int J Epidemiol. 1978;7(3):217–221. [DOI] [PubMed] [Google Scholar]
- 40. The ESHRE Capri Workshop Group. Noncontraceptive health benefits of combined oral contraception. Hum Reprod Update. 2005;11(5):513–525. [DOI] [PubMed] [Google Scholar]
- 41. Vessey M, Yeates D.. Oral contraceptives and benign breast disease: an update of findings in a large cohort study. Contraception. 2007;76(6):418–424. [DOI] [PubMed] [Google Scholar]
- 42. Kubba AA. The benefits of oral contraceptives. J R Soc Health. 1985;105(2):73–74. [DOI] [PubMed] [Google Scholar]
- 43. Cibula D, Gompel A, Mueck AO, et al. Hormonal contraception and risk of cancer. Hum Reprod Update. 2010;16(6):631–650. [DOI] [PubMed] [Google Scholar]
- 44. Beaber EF, Buist DS, Barlow WE, et al. Recent oral contraceptive use by formulation and breast cancer risk among women 20 to 49 years of age. Cancer Res. 2014;74(15):4078–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shabtai M, Saavedra-Malinger P, Shabtai EL, et al. Fibroadenoma of the breast: analysis of associated pathological entities—a different risk marker in different age groups for concurrent breast cancer. Isr Med Assoc J. 2001;3(11):813–817. [PubMed] [Google Scholar]