Abstract
Kaposi’s sarcoma-associated herpesvirus (KSHV), a human gamma-herpesvirus, is etiologically linked to the development of several malignancies, mainly Kaposi’s sarcoma. Expressed as an early viral protein, KSHV ORF57 is essential for lytic replication and virion production. ORF57 selectively binds to a subset of viral RNA and affects nearly all aspects of viral RNA processing. To globally identify all viral and host RNA associated with KSHV ORF57 in the infected cells, we have utilized UV cross-linking and immunoprecipitation (CLIP) of KSHV57 combined with high-throughput RNA sequencing (CLIP-seq) to identify ORF57-binding RNA in BCBL-1 cells at genome-wide level. This unit provides step-by-step details on this new method that is applicable for any pathogen or host RNA-binding proteins by slight modification.
Keywords: KSHV, ORF57, UV cross-linking, RNA, high throughput sequencing
INTRODUCTION
KSHV or human herpesvirus 8 (HHV-8) is an opportunistic pathogen. Its infection in healthy individuals is asymptomatic, however in immunocompromised patients it leads to emergence of Kaposi’s sarcoma, a tumor of endothelial origin, and at least two lymphotropic malignancies: primary effusion lymphoma, and multicentric Castleman’s disease (Chang et al., 1994; Cesarman et al., 1995; Dupin et al., 2000). Like other herpesviruses, KSHV displays two distinguishable life cycles, latent and lytic replication. KSHV latency exhibits limited viral gene expression necessary for maintenance of episomal viral genome in infected cells. Latently infected cell can be reactivated into lytic infection that displays an orchestrated expression of all viral genes, leading to virion production (Nakamura et al., 2003).
KSHV ORF57 protein, also known as Mta (mRNA transcript accumulation factor), is a RNA-binding protein produced at the early stage of KSHV lytic infection (Majerciak and Zheng, 2009). ORF57 regulates viral gene expression at the posttranscriptional level affecting nearly all aspects of viral RNA processing including RNA stability, splicing, and translation (Massimelli et al., 2011; Sahin et al., 2010; Majerciak et al., 2008; Majerciak et al., 2014; Kang et al., 2011; Boyne et al., 2010). We and others have demonstrated that ORF57 formation of multiple ribonucleoprotein complexes with target RNA is essential to exert its various RNA functions (Majerciak et al., 2006; Majerciak et al., 2008; Massimelli et al., 2013; Kang et al., 2011; Nekorchuk et al., 2007; Sahin et al., 2010).
Covalently-linked RNA immunoprecipitation assay (CLIP), or RNA-IP, is an antibody-based technique used to study RNA-protein interactions in living cells. CLIP is based on covalent capturing of RNA in close contact with a RNA-binding protein (RBP). This is achieved by UV light cross-linking followed by immunopurification of the cross-linked RNA-RBP complex with RBP-specific antibody and concluded with extraction of pulled down RNA (Ule et al., 2003; Majerciak et al., 2006). It was previously use to identify the RNA target of numerous cellular proteins. Here, we present its use to identify ORF57-binding RNA, which could be optimized for other systems according to different proteins and antibodies. Initially, we applied anti-ORF57 CLIP in combination of low-throughput RT-PCR to identify ORF57 in association with KSHV ORF59, ORF50, K8 and LANA RNA in butyrate-activated JSC-1 cells (Majerciak et al., 2006; Majerciak et al., 2008). Subsequently, we partially digested the cross-linking ORF57-RNA complexes with RNase A/T1 and purified the ORF57-protected RNA fragments after removing ORF57 with proteinase K. The isolated RNA fragments were then converted to cDNA by linker ligation and RT-PCR. By cloning and sequencing of these cDNA fragments, we identified numerous viral RNA transcripts cross-linked with ORF57 (Kang et al., 2011; Massimelli et al., 2011). In this protocol, we combine our CLIP with high-throughput RNA sequencing (RNA-seq) to allow systematic analysis of ORF57-associated RNA sequences in living cells on genome-wide scale. The step-by-step protocol covers UV irradiation of host cells, immunoprecipitation of RNA-protein complexes, RNase digestion and isolation of the ORF57-protected RNA fragments, sequencing library construction and Illumina sequencing.
BASIC PROTOCOL 1: Covalently-linked immunoprecipitation (CLIP) assay to obtain KSHV ORF57-binding RNA
The transformed B-cell lines (BC-1, BC-2, BC-3, BCBL-1 and JSC-1 cells) isolated from patients with primary effusion lymphomas (PEL) have been widely used to study KSHV life cycle (Cesarman et al., 1995; Arvanitakis et al., 1996; Renne et al., 1996; Cannon et al., 2000). PEL cells, harboring a latent KSHV genome, could be reactivated to lytic phase by a treatment with various chemical inducers, such as sodium n-butyrate (Bu), 12-O-tetradecanoylphorbol-13-acetate (TPA or PMA), and valproic acid (VA). KSHV ORF57 protein is a viral early protein essential for virus replication (Bello et al., 1999; Majerciak and Zheng, 2009) and regulates gene expression at the posttranscriptional level by interaction with numerous viral RNA. To perform a comprehensive genome-wide analysis of ORF57-binding RNA, KSHV-positive BCBL-1 cells are treated with valproic acid (VA) to induce viral lytic replication. The reactivated cells are irradiated with UV light and the endogenous ORF57-RNA complexes are pulled down by a rabbit anti-ORF57 antibody (Majerciak et al., 2006; Kang et al., 2011; Majerciak et al., 2008). The pulled down ORF57-RNA are subjected to partial digestion with RNase A/T1 and the ORF57-protected RNA fragments are isolated after removal of ORF57 protein by proteinase K digestion. The resulting RNA are then used to prepare small RNA library, which is sequenced using the Illumina platform.
CAUTION: Based on Centers for Disease Control and Prevention (CDC) KSHV is classified as a Biosafety Level 2 (BSL-2) pathogen. Follow all appropriate guidelines and regulations for the use and handling of KSHV-infected cells and samples.
Materials
Primary effusion lymphoma BCBL-1 cells (Renne et al., 1996, NIH AIDS Reagent program, cat. no. 3233) are cultivated in complete RPMI 1640 medium [RPMI 1640 (ThermoFisher Scientific, cat. no. 22400–089) supplemented with 20% fetal bovine serum (Hyclone-GE Healthcare, cat. no. SH30070.03) and 1 × Penicillin-Streptomycin-Glutamine (ThermoFisher Scientific, cat. no. 10378–016)]. KSHV lytic infection is induced by addition of valproic acid (VA) at final concentration of 1 mM on culture medium for 24 hours. Valproic acid 0.1M (100 ×) stock solution is prepared by dissolving sodium salt of valproic acid (Sigma-Aldrich, cat. no. P4543) in molecular grade water and filtering through 0.22 μm filter (EMD Millipore, cat. no. SLGS033SB).
A custom polyclonal anti-ORF57 antibody prepared by rabbit immunization with KLH-linked synthetic peptide corresponding to aa 119–132 of ORF57 protein and followed by on-column affinity purification (Majerciak et al., 2006; Kang et al., 2011) is used for this protocol, together with rabbit IgG isotype (ThermoFisher Scientific, cat. no. 02–6102) used in parallel as an antibody negative control.
Vented T175 tissue culture flasks (Sarstedt, cat. no. 83.3912.002)
100 mm tissue culture dishes (Sarstedt, cat. no. 83.3902)
Trypan Blue Solution, 0.4% (ThermoFisher Scientific, cat. no. 15250–061)
Conical 15 ml polypropylene centrifuge tubes (Sarstedt, cat. no. 62.554.002)
Nuclease-free 1.7 ml microcentrifuge tubes (GeneMates, cat. no. C-3262–1)
MicroAmp Reaction Tube with Cap, 0.2 mL, autoclaved (ThermoFisher Scientific, cat. no. N8010612)
Phosphate-buffered saline (PBS, ThermoFisher Scientific, cat. no. 10010–0023)
TRIzol reagent (Life Technologies, cat. no. 15596–026)
Chloroform (Sigma-Aldrich, cat. no. C2432)
Isopropanol (Sigma-Aldrich, cat. no. I9516)
UltraPure 25:24:1 (v/v/v) phenol/chloroform/isoamyl alcohol (ThermoFisher Scientific, cat. no. 15593–031)
GlycoBlue Coprecipitant (15mg/ml, Ambion, cat. no. AM9516)
DEPC-treated ultrapure water (K.D Medical, cat. no. RGF-3050)
RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS, pH 7.4, Boston BioProducts, cat. no. BP-115)
IP buffer (50 mM HEPES pH 7.5, 200 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 10% glycerol, 0.1% NP-40) prepared in DEPC-water
Complete EDTA-free mini protease inhibitor cocktail tablets (Roche, cat. no. 04 693 159 001). The 10 × solution is prepared by dissolving 1 tablet in 1 ml of DEPC-treated water. The tablet could be also directly dissolved in 10 ml of lysis buffer.
Protein A beads (EMD Millipore, cat. no. 16–125)
RNase A/T1 mix (2 mg/ml of RNase A and 5000 U/ml of RNase T1, ThermoFisher Scientific, cat. no. EN0551)
Recombinant Shrimp Alkaline Phosphatase (rSAP, 1000U/ml, New England Biolabs, cat. no. M0371S)
Proteinase K (600 mAU/ml, EMD Millipore, cat. no. 71049)
Proteinase K buffer (1× IP buffer supplemented with 1% SDS)
Phase Lock Gel Light, 1.5 ml tubes (VWR, cat. no. 10052–164)
3M sodium acetate, pH 5.2 (Quality Biological, cat. no. 351-035-721EA)
70–75% (v/v) ethanol
100% (v/v) ethanol
Agilent RNA 6000 Pico Kit (Agilent Technologies, cat. no. 5067–1513)
Universal miRNA Cloning Linker (New England BioLabs, cat. no. S1315S)
50% PEG8000 (New England BioLabs, from the T4 RNA Ligase II kit)
RNaseOUT (ThermoFisher Scientific, cat. no. 10777–019)
T4 RNA Ligase 2, truncated KQ (200,000 units/ml, New England Biolabs, cat. no. M0373L)
Agencourt RNAClean beads (Beckman Coulter, cat. no. A29168)
Agencourt AMPure XP - PCR Purification beads (Beckman Coulter, cat. no. A63880)
dNTP Mix, 10 mM each (Bioline, cat. no. BIO-39044)
SuperScript III First-Strand Synthesis System (ThermoFisher Scientific, cat. no. 18080–051)
CircLigase ssDNA ligase (Epicentre, cat. no. CL4111K)
Phusion DNA polymerase kit (New England BioLabs, cat. no. M0530S)
Reverse transcription primer (IDT custom synthesis)
[5’-(Phos)-AGATCGGAAGAGCGTCGTGTAGGGAAAGAGTGTAGATCTCGGTGGTCGC-(SpC18)-CACTCA-(SpC18)-TTCAGACGTGTGCTCTTCCGATCTATTGATGGTGCCTACAG-3’]
riboPCR_F primer (5’-AATGATACGGCGACCACCGAGATCTACAC-3’, IDT custom synthesis)
Indexed primers
(5’-CAAGCAGAAGACGGCATACGAGATNNNNNNGTGACTGGAGTTCAGA-CGTGTGCTCTTCCG-3’) (‘NNNNNN’ denotes the index, of which each index has a unique sequence. Index 1 = CGTGAT; index 2 = ACATCG; index 3 = GCCTAA; IDT custom synthesis)
DNA Clean & Concentrator-5 Kit (Zymo Research, cat. no. D4003)
1N NaOH
5M NaCl
5× SSC (0.75M NaCl, 0.075M sodium citrate, Denhardts solution [0.1% Ficoll, 0.1% polyvinylpyrrolidone, 0.l% BSA])
Hyb buffer (5× SSC + 0.05% Tween-20)
E-Gel EX Agarose Gels, 2% (ThermoFisher Scientific, cat. no. G4020–02)
Novex® TBE Gels, 10%, 12 well (ThermoFisher Scientific, cat. no. EC62752BOX)
6 × DNA loading buffer (ThermoFisher Scientific, cat. no. R0611)
TrackIt™ 10 bp DNA Ladder (ThermoFisher Scientific, cat. no. 10488–019)
SYBR® Gold Nucleic Acid Gel Stain (10,000 × Concentrate in DMSO, ThermoFisher Scientific, cat. no. S-11494)
Costar Spin-X Centrifuge Tube Filters (Cole-Parmer, cat. no. WU-01937–38)
Gel elution buffer (0.3 M sodium acetate pH 5.2, 0.1% SDS)
Herracell CO2 incubator (ThermoFisher Scientific)
Universal 320R centrifuge (Hettich)
Microcentrifuge 5424R (Eppendorf)
Thermomixer R (Eppendorf)
IX70 inverted phase-contrast microscope (Olympus)
VortexGenie2 (Scientific Industries)
Sonic Dismembrator (Model 100, ThermoFisher Scientific)
UV Crosslinker (VWR)
Qubit Fluorometer (ThermoFisher Scientific)
Magnetic stand (MPC-S, ThermoFisher Scientific)
Agilent Bioanalyzer 2100 (Agilent Technologies)
Veriti 96-Well Thermal Cycler (ThermoFisher Scientific)
MiSeq and HiSeq 2500 Ultra-High-Throughput Sequencing Systems (Illumina)
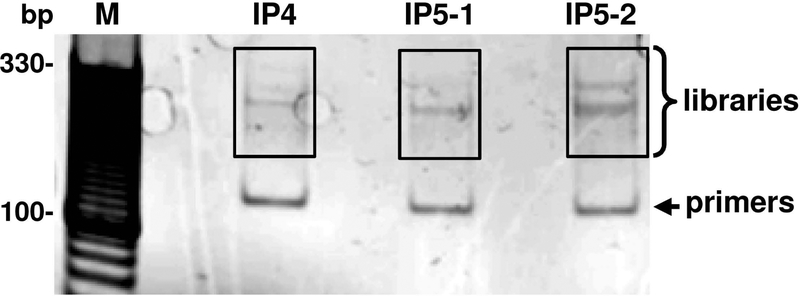
Cell culture and UV-crosslinking
1. Grow BCBL-1 cells (an atypical B lymphocyte line) in 40 ml of complete RMPI 1640 medium at 37 °C in humidified atmosphere containing 5% CO2 to cell density 1.0–1.5 × 106 cells/ml.
2. For induction, determine cell number and viability using Trypan blue exclusion (see APPENDIX A4.A, Stevenson 2006) or an automated cell counter and dilute cells with fresh, pre-warmed, complete RPMI 1640 medium to obtain 40 ml of cell suspension with 4 × 105 cells/ml . Add 400 μl of 100 mM stock solution of VA for the final concentration of 1 mM and cultivate cells for additional 24 hours.
3. After induction, take 20 ul of cell suspension for cell number calculation and the cell density should be about 1.5 × 106/ml. Distribute the remaining cell suspension equally into three separate 15-ml conical tubes (~13 ml/tube).
4. Spin cell suspension at 1,000 × g for 10 min. Discard the supernatant.
5. Wash cells with 10 ml of PBS.
6. Repeat the step 4.
7. Resuspend the cells in each tube in 5 ml of ice-cold PBS and transfer the cell suspension into three 100-mm tissue culture dishes.
8. Place the dishes on ice bed, remove the lid and expose to 254 nm UV light with dose of 480 mJ/cm2.
Note: Adherent cells should be grown on tissue culture plates, not flasks, and crosslinked directly after rinsing the cells with residual amount of PBS to prevent cell drying. After crosslinking aspirate PBS and lyse the cells directly with RIPA buffer (Step 11). Use a scraper for cell detachment if necessary.
9. After the UV crosslinking, transfer cell suspension into a 15-ml conical tube and rinse the dishes with additional 5 ml PBS to collect all remaining cells.
10. Centrifuge the cell suspension at 1,000 × g for 10 min and discard the supernatant.
11. Lyse the cells by adding 1 ml of 1 × RIPA buffer supplemented with 1 × Complete EDTA-free protease-inhibitor cocktail to the cell pellet. Transfer cell lysate into a new 1.7-ml microcentrifuge tube and incubate on ice for 30 min.
12. After the incubation, sonicate each sample with 10 short (1–2 seconds long) pulses using a sonicator with a power setting at level 4.
13. Spin the cell lysates for 10 min at 20,000 × g at 4 °C. Carefully transfer the supernatant from each tube into a new 15-ml conical tube. Combine all cell lysates originated from three dishes to obtain total 3 ml of the cleared cell lysates.
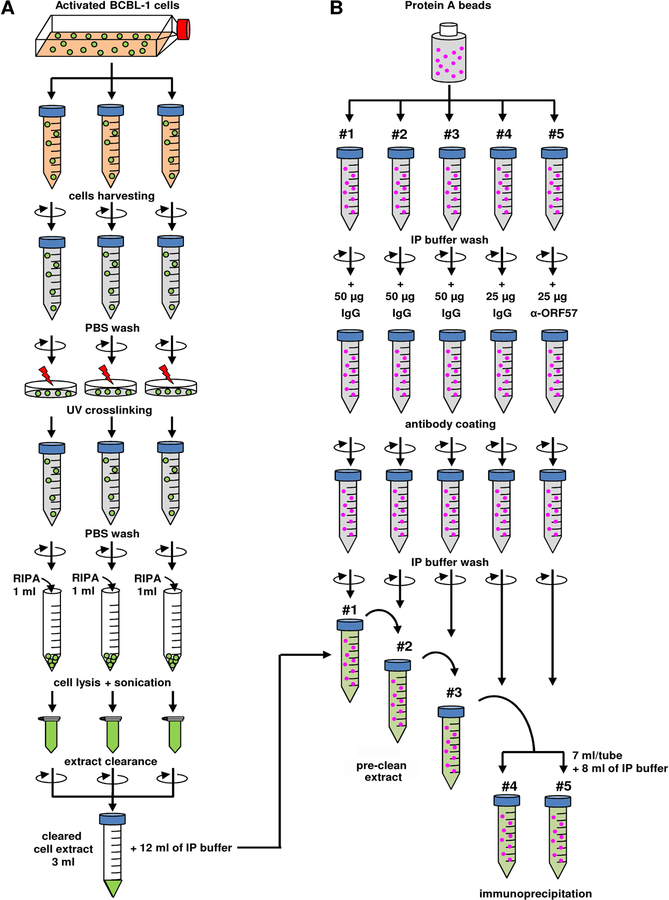
Note: See flowchart at Figure 1A for cell extract preparation. The prepared cell lysate could be used directly for IP or kept in −80 °C.
Figure 1. Flowchart of cell extract preparation (A) and setting of immunoprecipitation (B).
Immobilize protein-A beads with rabbit anti-ORF57 antibody or rabbit IgG
14. Add 400 μl (50 % slurry) of protein A beads into 5 individual 15-ml conical tubes containing 4 ml of IP buffer. Note: Three of five tubes will be used for cell lysate pre-clean, and the remaining two, one for specific antibody and one for antibody control (see flowchart on Figure 1B).
15. Spin five tubes with the beads for 5 min and discard the supernatant. Resuspend the beads in each tube in 4 ml of IP buffer followed by 5 min centrifugation. Repeat this step for 3 times.
Note: All centrifugations in this and the following steps are performed by Eppendorf Model 5702 microcentrifuge with an A-4–38 bucket rotor at 3,000 × g.
16. After the last spin, resuspend the beads in each tube with 4 ml of IP buffer.
17. Coat the beads with an antibody by adding 50 μg of rabbit control IgG into tubes #1–3 (for pre-clean), 25 μg of rabbit control IgG into tube #4 (IP negative control), and 25 μg of rabbit anti-ORF57 antibody into tube #5. Rotate overnight at 4 °C.
18. Spin the tubes with antibody-coated beads for 5 min, discard the supernatant, and wash the beads 3 more times, each time with 4 ml of IP buffer as described in Step 15. Keep the beads in the IP buffer on ice until cell lysates are ready.
Pre-cleaning of cell lysates and IP with rabbit anti-ORF57
19. Discard IP buffer from tube #1 (from Step 18) containing beads immobilized with 50 μg of rabbit control IgG and add 3 ml of cell lysate prepared from Step 13 and diluted with 12 ml of IP buffer. Rotate the tube at 4 °C for 1 hour.
20. Spin the tube 1 and transfer the supernatant into tube #2. Rotate the tube at 4 °C for 1 hour. Repeat the same with tube #3 for total 3 rounds of pre-cleaning.
Note: These pre-cleaning steps of cell lysates are important to remove all non-specific interactions with beads or IgG. A non-specific antibody for the pre-cleaning should be the same species origin and the same subtype as the specific antibody against the target protein. In this protocol, a rabbit anti-ORF57 antibody was used. Thus, normal rabbit IgG was used as a non-specific control antibody for the pre-cleaning. If such a control antibody is not available, use uncoated beads for the pre-cleaning.
21. Remove 1 ml of the pre-cleaned cell lysates for input control. The input could be stored at −80 °C until needed.
22. Divide the remaining 14 ml of pre-cleaned lysates from step 20 equally (7 ml/tube) into tubes #4 and #5 respectively containing anti-ORF57 and the control rabbit IgG-immobilized beads. Add 8 ml of IP buffer to each tube to total volume of 15 ml.
23. Rotate the tubes at 4 °C overnight.
24. Spin the tubes for 5 min and discard the supernatant.
25. Resuspend the beads in 4 ml of IP buffer, spin for 5 min. Repeat this step one more time.
26. Resuspend the beads in each tube in 10 ml of IP buffer. Take 1 ml of the bead suspension from each tube for Western blot analysis of the immunoprecipitated KSHV ORF57 protein (Figure 2A).
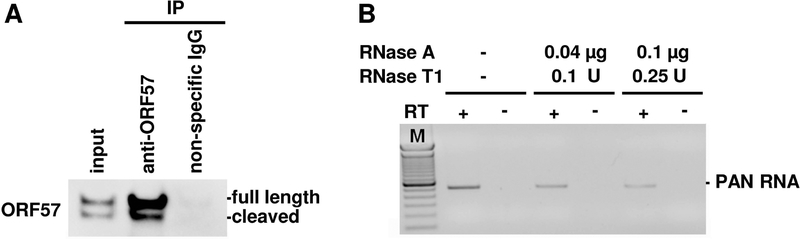
Figure 2. Optimization of ORF57 immunoprecipitation and RNase digestion.
(A) ORF57-RNA complexes were immunoprecipitated by a rabbit anti-ORF57 antibody from cells extract of BCBL-1 cells treated with valproic acid for 24 h (input), normal rabbit IgG served as a negative control. The levels of ORF57 in input (1%) and immunoprecipitates (3%) in CLIP experiment were detected by Western blot with mouse anti-ORF57 antibody detecting both full-length ORF57 protein (upper band) and its caspase-cleavage product (lower band).
(B) To determine an optimal RNase digestion condition, the immunoprecipitated ORF57 complexes were incubated with various amounts of RNase A/T1 mix for 5 sec at room temperature and followed by proteinase K treatment and RNA extraction. The RNase A/T1 digestion efficiency was checked by RT-PCR for the remaining KSHV PAN RNA, a known ORF57 target.
Note: This step is important for determination of IP efficiency and specificity by comparing the immunoprecipitated protein level by the specific antibody over the control IgG.
On-beads digestion and dephosphorylation of immunoprecipitated RNA.
27. Spin the remaining 9 ml of the bead suspension for 5 min and discard the supernatant.
28. Resuspend the beads in 1.5 ml of IP buffer containing RNase A/T1 mixture (1:2,000 dilution) buffer and digest the immumoprecipitated RNA by incubation at room temperature for 5 sec.
Note: RNase A and T1 are single-stranded RNA endoribonucleases. RNase A cleaves RNA at the 3´ end of pyrimidine residues, whereas RNase T1 specifically degrades RNA at G residues. While this step could be perform with either RNase A or T1 alone, we recommend A/T1 mixture to improve digestion efficiency and reduce cutting bias. The RNase concentration and time of digestion should be optimized individually for each RNA binding protein. Figure 2B shows gradual decrease of PAN (a polyadenylated nuclear noncoding RNA of KSHV), a known ORF57 target, due to digestion with various concentration of RNase A/T1as monitored by RT-PCR. Alternatively, RNA digestion could be analyzed by Agilent 2100 Bioanalyzer and the optimized digestion should give the digested products in a size range of ~50–200 nts as shown in Figure 3 for ORF57-CLIPed, RNase-digested RNA products.
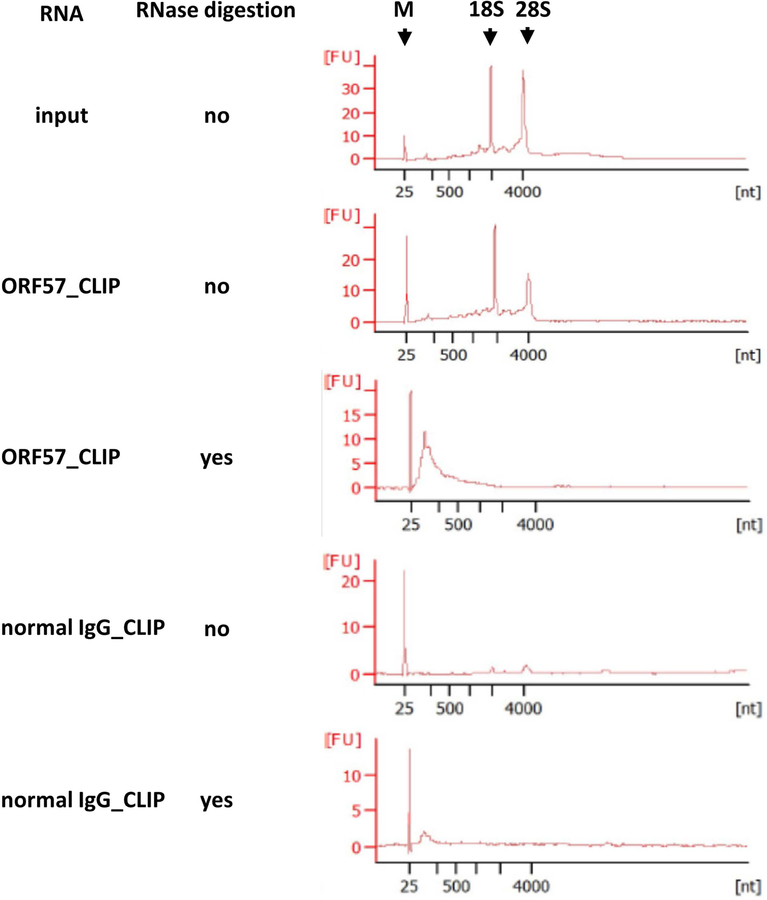
Figure 3. Profile of ORF57-CLIPed RNA analyzed by Agilent Bioanalyzer 2100.
The total RNA from input, ORF57 CLIPs or IgG CLIPs without or with RNase (0.1 μg RNase A and 0.25 U RNase T1) digestion was analyzed by Agilent Bioanalyzer 2100 using RNA 6000 Pico Kit. The first peak is a 20-bp molecular marker (M). Two additional peaks in the input and undigested ORF57-CLIP correspond to 18S and 28S ribosomal RNA (rRNA). ORF57-CLIPed, RNase-digested RNA products are shown as a broad peak in size of ~50–200 nts. The “x” axis shows RNA size in nucleotides [nt] and “y” axis shows the RNA concentration expressed as fluorescent units [FU].
29. Add 4 ml of fresh IP buffer to each tube and immediately spin for 5 min. Discard the supernatant. Resuspend the beads in 4 ml of IP buffer and spin the bead resuspension for 5 min.
Note: The purpose of this step is to remove the digested RNA products that are not protected by ORF57 on the beads.
30. Discard the supernatant and resuspend beads in 500 μl of IP buffer containing rSAP (0.01U/μl). Transfer beads suspension into a microcentrifuge tube, place in thermomixer, and incubate for 30 min at 37 °C with gentle mixing at 300 rpm.
31. After RNA dephosphorylation, spin tubes at 3,000 × g for 5 min and discard the supernatant. Wash the beads briefly with 1 ml of IP buffer by spin at 3,000 × g for 5 min, discard the supernatant and proceed to next step.
Proteinase K digestion and RNA purification
32. Resuspend beads in 800 μl of pre-warmed proteinase K solution, place in thermomixer and incubate for 40 min at 37 °C with mixing at 300 rpm.
Note: To prepare proteinase K solution, dilute proteinase K stock solution in proteinase K dilution buffer to final concentration of 0.2 mg/ml. Pre-warm the prepared working solution for 20 min at 37 °C.
33. Divide each digested sample into two 1.7-ml microcentrifuge tubes (450 μl/tube).
34. Extract RNA by adding equal volume (450 μl) of phenol:chloroform:isoamyl alcohol (25:24:1 v/v/v) into each tube, mix rigorously by vortexing and transfer the mixture into a Phase Lock Light Gel tube.
Note: Before use, spin empty Phase Lock Light Gel tubes for 1 min at 20,000 × g to bring the gel into the bottom of the tubes.
35. Spin tubes for 10 min at 20,000 × g at 4 °C. Transfer aqueous layer from each tube into a new 1.7-ml tube.
36. Precipitate RNA by adding 45 μl of 3M sodium acetate (1/10 of sample volume), 2μl of GlycoBlue coprecipitant and 1 ml (2.5 × sample volume) of 100% cold ethanol into each tube. Mix well and precipitate at −80 °C overnight.
Note: We recommend adding GlycoBlue Coprecipitant into precipitation reaction to increase RNA yield. Its blue color also allows better visualization of RNA pellet.
37. Spin the precipitation samples at 20,000 × g for 15 min at 4 °C, carefully discard the supernatant, and rinse the pellet in each tube with 1 ml of 70% cold ethanol.
38. Spin samples at 20,000 × g for 5 min at 4 °C and discard all supernatant. Air-dry pellets for 5–10 min at room temperature.
39. Dissolve each pellet in 10 μl of DEPC water
40. Determine RNA size and concentration by Agilent 2100 Bioanalyzer using the Total RNA 6000 Pico Kit (see Figure 3).
Preparation of sequence library and Illumina sequencing
Linker ligation to RNA
41. Mix 5 μl of RNA (1–10 ng) from step 40 with 5 μl (50 ng) of 2 μM pre-adenylated linker (Universal miRNA Cloning Linker, 5’-rAppCTGTAGGCACCATCAAT–NH2-3’). Denature for 90 seconds at 80 °C, and then cool to room temperature.
42. Add the following reagents to the mixture:
| 10 × T4 RNA ligase buffer | 2 μ1 |
| 50% PEG8000 (from T4 RNA ligase kit) | 6 μ1 |
| RNaseOUT | 1 μ1 |
| T4 RNA Ligase 2, truncated KQ (200 U/μl) | 1 μ1 |
43. Incubate at room temperature for 2.5 hours.
Clean-up of linker-ligated RNA
44. Vortex the Agencourt RNAClean beads until they are well dispersed, then add 36 μl of beads suspension to each tube containing linker-ligated RNA. Gently pipette the entire volume up and down 10 times to mix thoroughly. Incubate the sample/beads mixtures at room temperature for 15 min.
45. Place the tube on a magnetic stand at room temperature for 5 min to make sure that all of the beads are bound to the side of the tube.
46. Remove and discard all of the supernatant from each tube.
47. With the tube remaining on the magnetic stand, add 200 μl of freshly prepared 70% ethanol to each tube without disturbing the beads.
48. Incubate the tube at room temperature for 30 seconds, then remove and discard all of the supernatant from each tube.
49. Let the tube stand at room temperature for 15 min to dry the beads and then remove the tube from the magnetic stand.
50. Add 11 μl of RNase-free water to each tube. Gently pipette the entire volume up and down 10 times to mix thoroughly.
51. Incubate the tube at room temperature for 2 min.
52. Place the tube on the magnetic stand at room temperature for 5 min.
53. Transfer 10 μl of the supernatant, i.e. eluted RNA, to a clean tube.
Reverse transcription
54. Mix 10 μl of RNA from Step 53 with 2 μl of 1.25 μM reverse transcription primer (5’-(Phos)-AGATCGGAAGAGCGTCGTGTAGGGAAAGAGTGTAGATCTCGGTGGTCGC-(SpC18)-CACTCA-(SpC18)-TTCAGACGTGTGCTCTTCCGATCTATTGATGGTGCCTACAG-3’). Denature for 2 min at 80 °C and then place on ice.
Note: The designation (Phos) indicates 5’phosphorylation and -(SpC18)- indicates a hexa-ethyleneglycol spacer. This primer can be custom synthesized with a commercial service provider such as IDT.
55. Add the following reagents:
| 5 × first strand buffer | 4 μ1 |
| dNTP mix, (10 mM each) | 1 μ1 |
| 0.1 MDTT | 2 μ1 |
| RNaseOUT | 1 μ1 |
| Superscript III (200 U/μl) | 1 μ1 |
56. Incubate for 60 min at 48 °C.
57. Hydrolyze the RNA by adding 2.2 μl of 1N NaOH, incubate at 98 °C for10 min.
Clean-up of first strand cDNA
58. Vortex Agencourt AMPure XP PCR Purification beads until they are well dispersed, then add 36 μl of beads to each tube containing cDNA. Gently pipette the entire volume up and down 10 times to mix thoroughly.
59. Incubate the sample/beads mixtures at room temperature for 15 min.
60. Place the tube on a magnetic stand at room temperature for 5 min to make sure that all of the beads are bound to the side of the tube.
61. Remove and discard all of the supernatant from each tube.
62. With the tube remaining on the magnetic stand, add 200 μl of freshly prepared 80% ethanol to each tube without disturbing the beads.
63. Incubate the tube at room temperature for 30 seconds, then remove and discard all of the supernatant from each tube.
64. Let the tube stand at room temperature for 15 min to dry the bead and then remove the tube from the magnetic stand.
65. Add 16 μl of RNase-free water to each tube. Gently pipette the entire volume up and down 10 times to mix thoroughly.
66. Incubate the tube at room temperature for 2 min.
67. Place the tube on a magnetic stand at room temperature for 5 min.
68. Transfer 15 μl of the supernatant to a clean tube.
Circularization
69. Mix the following reagents:
| First strand cDNA from step 68 | 15 μ1 |
| 10 × CircLigase buffer | 2 μ1 |
| 1 mM ATP | 1 μ1 |
| 50 mM MnCl2 | 1 μ1 |
| CircLigase (100 U/μ1) | 1 μ1 |
70. Incubate for 1 hour at 60 °C, then heat-inactivate the enzyme at 80 °C for 10 min.
71. Increase the sample volume by adding 70 μl of water, then add 6 μl of 5 M NaCl, 150 μl of isopropanol and incubate on dry ice for 30 min, followed by spin for 30 min at 20,000 × g at 4 °C, remove all the liquid and air dry for 10 min and dissolve the pellet in 20 μl of water.
PCR amplification of final library
72. Add the following reagents:
| Circularized cDNA from step 71 | 10 μ1 |
| 5 × HF buffer (from the Phusion DNA polymerase kit, NEB) | 10 μ1 |
| 10 μM riboPCR_F | 0.5 μ1 |
| 10 μM indexed primer | 0.5 μ1 |
| 10 mM dNTPs | 1 μ1 |
| Phusion DNA polymerase | 0.5 μ1 |
| 23.5 μ1 water to total 50 μ1 |
73. Run the PCR with the following program:
98 °C for 30 seconds
15 cycles of 98 °C for 10 seconds, 65 °C for 10 seconds and 72 °C for 30 seconds
72 °C for 5 min
4 °C hold
74. Check 5μl of amplification products on a 2% agarose E-Gel EX gel.
Size selection of the libraries
75. Cleanup PCR reaction using DNA Clean & Concentrator-5 Kit, elute with 8 μl of water.
76. Pre-run a pre-cast Novex 10% TBE gel for 15–30 min at 80V and wash the wells.
77. Mix DNA sample with 6 × DNA loading buffer and load on the gel. Run samples at 80V for 20 min then increase the voltage to 120V for approximately 2 hours until the first dye run out of the bottom of the gel. 10bp DNA ladder is used as size marker.
78. Stain the gel with 30 ml of 1 × SybrGold solution for 5 min.
79. Cut the gel band corresponding to 200–350 nt with a clean new razor blade (Figure 4). Shred gel slice into small pieces and transfer to a 1.5 ml tube containing 400 μl of gel elution buffer.
Figure 4. Agarose gel electrophoresis of the constructed ORF57 CLIP-seq libraries.
CLIP-seq libraries were prepared from three independent RNA samples immunoprecipitated by ORF57 antibody. After PCR amplification, the final libraries were resolved on an E-Gel Ex 2% agarose gel. The lower bands are amplification products of self-circularized primers. The 200–350 bp region (highlighted in white boxes) was excised from the gel for high-throughput sequencing. M = 10 bp marker.
80. Elute DNA by rotating the tubes gently at 4 °C overnight.
81. Spin tubes for 2 min at 20,000 × g and transfer supernatant into Spin-X filter. Spin down again at 20000 × g for 2 min.
82. Remove filter cartridge and add 1μl of GlycoBlue and 2.5 volumes of 100% ethanol to the flow-through. Precipitate eluted DNA by 1–2 hours incubation on dry ice.
83. Spin down at 20,000 × g for 30 min at 4°C and carefully remove the supernatant.
84. Wash the pellet with 750 μl of 75% ethanol, spin at 20,000 × g for 5 min and carefully remove all of the supernatant. Allow pellet to air dry for 5–10 min and dissolve the DNA in 20 μl of DNase-free water.
Illumina sequencing of the CLIP-seq libraries
85. Quantified the resulting CLIP-seq libraries in step 84 by Qubit fluorometer.
86. Adjust all libraries to the same concentration (e.g., 2 nM).
87. Pool CLIP-seq libraries together by equal volume.
88. Denature in 0.1M NaOH at room temperature for 5 min by mixing 10 μl of 2 nM pooled libraries, 9 μl TE, 1 μl of 2M NaOH.
89. Dilute the denatured libraries in Hyb buffer (5× SSC + 0.05% Tween-20) to a final concentration of 12 pM
90. Load the dilute to Miseq for low-coverage sequencing. Library quality can be evaluated by (1) percentage of raw reads with insert great than 18 nt (e.g. typically greater than 75%) and (2) mapping rate of the adaptor-trimmed reads to the reference genome (typically greater than 80%).
91. If the libraries pass the quality control, large-scale data acquisition is achieved using an Illumina HiSeq2500 platform with a 2 × 50 bp modality. The same library pool can be used and the final loading concentration is approximately 10–12 pM. We typically obtain 20–40 million paired-reads for each CLIP-seq library or up to 10 CLIP-seq libraries per Hiseq2500 lane. The actual sequencing depth depends on the binding specificity of the RBP of interest. Raw image data are preprocessed to generate sequence reads in fastq format.
Note: Other instruments can also be used based on availability.
This step is typically handled at core facility and/or commercial service providers. The end user only needs to submit quantified CLIP-seq libraries, whereas all other steps (pooling, denaturation and sequencing) will be performed by sequencing provider. Consult NextGen sequencing professionals for guidelines regarding to sample submission, which might subject to change pertinent to each sequencing provider.
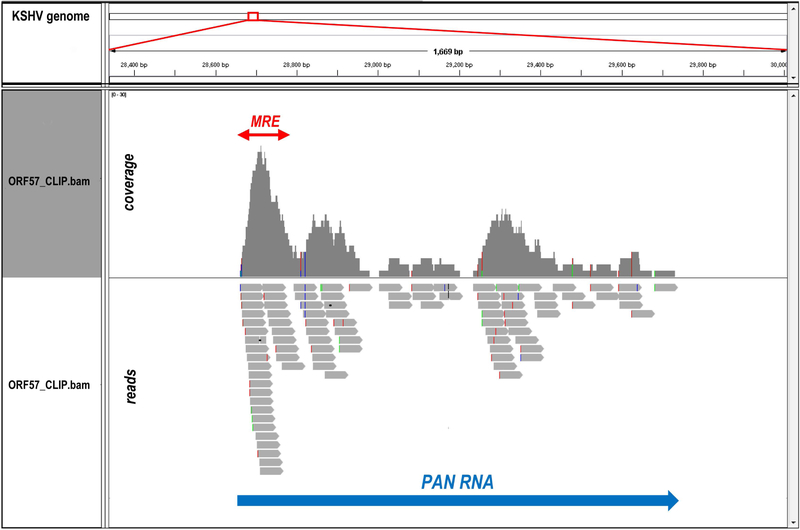
92. Map the raw sequencing reads to the corresponding genome(s) by BWA aligner (Li and Durbin, 2009) allowing a maximum of two mismatches. For our study, we sequentially aligned the reads to KSHV genome (GenBank Acc. No. U75698.1) and human genome (UCSC version hg19). All uniquely mapped KSHV-specific reads were used for further analyses. Integrated Genome Viewer (IGV, Broad Institute, free download at https://www.broadinstitute.org/software/igv/) was used for data visualization (Figure 5).
Figure 5. Identification of ORF57 binding site in KSHV PAN RNA by ORF57 CLIP-seq.
The reads obtained in ORF57 CLIP-seq were mapped to KSHV genome (GenBank Acc. No. U75698.1) and visualized by Integrated Genome Viewer (IGV). The snapshot shows the reads distribution within KSHV PAN locus (large blue arrow) from one representative library. Colored vertical lines in reads and their coverage profile are the nucleotides with sequencing errors. The previously identified ORF57 (Mta)-responsive element (MRE) at the PAN RNA 5’ end (Massimelli et al., 2011) is marked by a red arrow.
COMMENTARY
Background Information
Posttranscriptional regulation of gene expression occurs through a variety of mechanisms such as RNA capping, splicing, polyadenylation, export, and turnover. The dynamic binding of RBPs onto RNA is one of the principal components of posttranscriptional gene regulation involved in all aspects of RNA processing (Gerstberger et al., 2014). In living cells, the interaction between a RBP and its target RNA is often dynamic and requires specific physiological and biochemical microenvironment. As a result, to reconstitute physiological RNA-protein interactions in cells by in vitro assay would be difficult. The conclusion derived from in vitro assays in the absence of other cellular proteins in a physiological condition might be misleading. Therefore, isolation of native RNA-protein complexes formed in living cells would be fundamental to determine biological roles of individual RBPs. RNA-CLIP has been widely utilized to isolate and identify RNA molecules specifically associated with individual RBPs in vivo (Kang et al., 2011; Ule et al., 2003; Jensen and Darnell, 2008; Majerciak et al., 2006; Majerciak et al., 2008). In contrast to formaldehyde which also crosslinks protein–protein complexes, UV irradiation selectively crosslinks the RBPs closely associated RNA. As results, UV crosslinking allows to distinguish the direct RNA-protein interactions from the indirect interactions mediated by protein-protein interactions.
Introduction of photoactivatable ribonucleoside (PAR) analogues, such as 4-thiouridine (4-SU) or 6-thioguanosine (6-SG) into nascent RNA transcripts in living cells, in CLIP protocol (PAR-CLIP), led to higher crosslinking efficiency and further increased resolution (Hafner et al., 2010). RNA-CLIP was recently revolutionized by the introduction of high-throughput sequencing method (HITS-CLIP or RNA CLIP-seq) for identification and analysis of the CLIPed RNA (Darnell, 2010). However, all RNA CLIP-based methods require an antibody specific to the RBP of interest. Interaction of ORF57 with target RNA has been shown to be indispensable for ORF57 activity. In contrast to majority of RBPs, ORF57 does not contain any classical RNA-binding motif although its binding to RNA appears to be related to the arginine-rich region at the N-terminus (Majerciak et al., 2006; Li, Verma et al., 2012). The selective target recognition by ORF57 requires different co-factors for cooperative RNA binding (Majerciak and Zheng, 2009; Majerciak et al., 2011; Massimelli et al., 2011; Majerciak et al., 2014; Tunnicliffe et al., 2014).
Critical Parameters and Troubleshooting
The use of a high-quality antibody is critical for successful RNA CLIP-seq. If such an antibody is not available, introduction of an epitope tag (such as FLAG, HA, c-myc, etc.) into the ectopically expressed protein could be an alternative. The selected antibody should be first tested for their suitability for immunoprecipitation and specificity to the selected RNA target. If necessary, the specificity could be improved with increased washing stringency by altering salt concentration in a wash buffer. Depending on antibody origin and subtype, protein A beads may be replaced with protein G beads (EMD Millipore, cat. no. 16–266) without any protocol modification.
Use of sufficient cell lysate is critical for generation of comprehensive library. The cell lysate from approximately 2 × 107 cells was used for each library preparation in our study. The amount of cell lysate for individual RNA CLIPs should be empirically determined. To obtain an ideal length of RNA fragments for subsequent analysis, optimal RNase digestion condition should be determined empirically before construction of the library. Considering the sequence bias attributed to RNases used, we prefer a mixture of RNase A/T1 for RNA partial digestion. RNase concentration should be adjusted to obtain the digested RNA fragments in size of 50–200 nts. Low concentration of the RNases results in longer RNA fragments, which will increase the difficulty to identify the specific RNA-binding sites of ORF57. On the other hand, over-digestion with high dose of the RNases results in short RNA fragments, which may increase the specific mapping of the obtained reads to the virus or host genome. To control for the correct size of CLIPed RNA fragments, we performed RNase digestion of the RNA-protein complexes after immunoprecipitation rather than in the cell lysate before IP. After removing proteins from the RNA-protein complexes, the CLIPed RNA was purified and analyzed by Agilent 2100 Bioanalyzer. Based on the results, the RNase concentration was adjusted to obtain the digested RNA fragments in the required size range.
Ligation of a 3’ linker and circularization are two critical steps for library preparation. For linker ligation, the molar ratio between the linker and RNA should be around 10:1. Similarly, too much excess of the reverse transcription primer may lead to high background due to self-ligation. The final concentration of both primers therefore needs to be optimized accordingly with the input RNA amount. Furthermore, the size selection step is important for removing amplification products without insert. It also serves as a quality control step to check the insert size, which in bulk part should reflects the size of RNase A/T1 treated RNA fragments. The final libraries need to be quantified by Qubit or other fluorescence based methods. We typically load 10 pM final libraries for the Miseq instrument and 12 pM for the Hiseq-2500 platform (v3 chemistry). Paired-end sequencing is preferred if one want to map both end of the immunoprecipitated RNA fragments.
Anticipated Results
KSHV-positive BCBL-1 cells support both latent and lytic virus infection. When the UV cross-linked RNA-protein complexes in living cells were pulled down with an anti-ORF57 antibody, the IP efficiency of ORF57 protein was detected by Western blotting, with no detection of ORF57 in an IP control of normal rabbit IgG (Figure 2A). UV cross-linking of ORF57 to its closely associated RNA is the only way for us to detect a specific RNA transcript bound by ORF57 by RT-PCR. In contrast, the cells without UV irradiation before the cell lysate preparation for IP would not render a positive detection by RT-PCR on the target RNA of ORF57 after anti-ORF57 IP (Kang et al., 2011).
The RNA-protein complexes pulled down with anti-ORF57 antibody were briefly digested with RNase A/T1 to remove the protein-free parts of RNA within the complexes. After dephosphorylation and proteinase K digestion, RNA fragments purified and analyzed by Agilent 2100 Bioanalyzer should be in size of 50~200 nt (Figure 3).
The mappability of the CLIP-seq libraries largely depends on the specificity of the antibody for the RBP of interest. In terms of data analysis, if two or more reads are exactly the same in sequence, only one read is kept to remove potential duplications due to PCR amplification. The remaining reads are then mapped to the reference genome(s) of interest. Downstream analysis only use the sequence reads that can be mapped to a unique location to identify the sequence motifs enriched in the CLIP library. Using the current protocol, we obtained ~105 reads uniquely-mapped to KSHV genomes. The reads distribution at the 5’ end of PAN RNA (Figure 5), a known ORF57 target, was consistent with the previously identified ORF57 binding site (Massimelli et al., 2011; Sei and Conrad, 2011).
Time Considerations
The sufficient time should be devoted to prepare the required number of cells. This may take 1–2 weeks. The protein A or G agarose beads should be coated with antibody overnight one day ahead the cell lysate pre-cleaning. The RNA-protein complexes are immunoprecipitated overnight. The RNA digestion, dephosphorylation, proteinase K digestion, and RNA purification will take 1–2 days. Library construction and Illumina sequencing may take another two weeks. The best pause point during the library construction is the PCR amplification step, which can be carried out overnight. If the final libraries are not sequenced promptly, they should be kept in low-binding tubes with addition of 0.1% Tween-20. Altogether, the entire protocol would take minimal two weeks, but could be up to 4 weeks.
Acknowledgments
This work is supported by the Intramural Research Program of National Cancer Institute and National Heart, Lung, and Blood Institute of NIH (USA).
Literatures cited
- Arvanitakis L, Mesri EA, Nador RG, Said JW, Asch AS, Knowles DM, and Cesarman E 1996. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 88:2648–2654. [PubMed] [Google Scholar]
- Bello LJ, Davison AJ, Glenn MA, Whitehouse A, Rethmeier N, Schulz TF, and Barklie CJ 1999. The human herpesvirus-8 ORF 57 gene and its properties. J.Gen.Virol 80:3207–3215. [DOI] [PubMed] [Google Scholar]
- Boyne JR, Jackson BR, Taylor A, Macnab SA, and Whitehouse A 2010. Kaposi’s sarcoma-associated herpesvirus ORF57 protein interacts with PYM to enhance translation of viral intronless mRNAs. EMBO J. 29:1851–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JS, Ciufo D, Hawkins AL, Griffin CA, Borowitz MJ, Hayward GS, and Ambinder RF 2000. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi’s sarcoma herpesvirus-containing supernatant. J. Virol 74:10187–10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman E, Chang Y, Moore PS, Said JW, and Knowles DM 1995. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N.Engl.J.Med. 332:1186–1191. [DOI] [PubMed] [Google Scholar]
- Cesarman E, Moore PS, Rao PH, Inghirami G, Knowles DM, and Chang Y 1995. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 86:2708–2714. [PubMed] [Google Scholar]
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, and Moore PS 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 266:1865–1869. [DOI] [PubMed] [Google Scholar]
- Darnell RB 2010. HITS-CLIP: panoramic views of protein-RNA regulation in living cells. Wiley.Interdiscip.Rev.RNA 1:266–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin N, Diss TL, Kellam P, Tulliez M, Du MQ, Sicard D, Weiss RA, Isaacson PG, and Boshoff C 2000. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 95:1406–1412. [PubMed] [Google Scholar]
- Gerstberger S, Hafner M, and Tuschl T 2014. A census of human RNA-binding proteins. Nat.Rev.Genet 15:829–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M Jr., Jungkamp AC, Munschauer M, Ulrich A, Wardle GS, Dewell S, Zavolan M, and Tuschl T 2010. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141:129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB and Darnell RB 2008. CLIP: crosslinking and immunoprecipitation of in vivo RNA targets of RNA-binding proteins. Methods Mol.Biol 488:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JG, Majerciak V, Uldrick TS, Wang X, Kruhlak M, Yarchoan R, and Zheng ZM 2011. Kaposi’s sarcoma-associated herpesviral IL-6 and human IL-6 open reading frames contain miRNA binding sites and are subject to cellular miRNA regulation. J.Pathol 225:378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JG, Pripuzova N, Majerciak V, Kruhlak M, Le SY, and Zheng ZM 2011. Kaposi’s Sarcoma-Associated Herpesvirus ORF57 Promotes Escape of Viral and Human Interleukin-6 from MicroRNA-Mediated Suppression. J.Virol 85:2620–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DJ, Verma D, and Swaminathan S 2012. Binding of cellular export factor REF/Aly by Kaposi’s sarcoma-associated herpesvirus (KSHV) ORF57 protein is not required for efficient KSHV lytic replication. J.Virol 86:9866–9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H and Durbin R 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 25:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerciak V, Lu M, Li X, and Zheng ZM 2014. Attenuation of the suppressive activity of cellular splicing factor SRSF3 by Kaposi sarcoma-associated herpesvirus ORF57 protein is required for RNA splicing. RNA. 20:1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerciak V, Uranishi H, Kruhlak M, Pilkington GR, Massimelli MJ, Bear J, Pavlakis GN, Felber BK, and Zheng ZM 2011. Kaposi’s sarcoma-associated herpesvirus ORF57 interacts with cellular RNA export cofactors RBM15 and OTT3 to promote expression of viral ORF59. J.Virol 85:1528–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerciak V, Yamanegi K, Allemand E, Kruhlak M, Krainer AR, and Zheng ZM 2008. Kaposi’s sarcoma-associated herpesvirus ORF57 functions as a viral splicing factor and promotes expression of intron-containing viral lytic genes in spliceosome-mediated RNA splicing. J.Virol 82:2792–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerciak V, Yamanegi K, Nie SH, and Zheng ZM 2006. Structural and functional analyses of Kaposi sarcoma-associated herpesvirus ORF57 nuclear localization signals in living cells. J.Biol.Chem 281:28365–28378. [DOI] [PubMed] [Google Scholar]
- Majerciak V and Zheng ZM 2009. Kaposi’s sarcoma-associated herpesvirus ORF57 in viral RNA processing. Front.Biosci 14:1516–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerciak V and Zheng ZM 2015. KSHV ORF57, a Protein of Many Faces. Viruses. 7:604–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimelli MJ, Kang JG, Majerciak V, Le SY, Liewehr DJ, Steinberg SM, and Zheng ZM 2011. Stability of a Long Noncoding Viral RNA Depends on a 9-nt Core Element at the RNA 5’ End to Interact with Viral ORF57 and Cellular PABPC1. Int.J.Biol.Sci 7:1145–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimelli MJ, Majerciak V, Kruhlak M, and Zheng ZM 2013. Interplay between polyadenylate-binding protein 1 and Kaposi’s sarcoma-associated herpesvirus ORF57 in accumulation of polyadenylated nuclear RNA, a viral long noncoding RNA. J.Virol 87:243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Lu M, Gwack Y, Souvlis J, Zeichner SL, and Jung JU 2003. Global changes in Kaposi’s sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator. J.Virol 77:4205–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekorchuk M, Han Z, Hsieh TT, and Swaminathan S 2007. Kaposi’s Sarcoma-Associated Herpesvirus ORF57 Protein Enhances mRNA Accumulation Independently of Effects on Nuclear RNA. Export. J.Virol 81:9990–9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, and Ganem D 1996. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat.Med 2:342–346. [DOI] [PubMed] [Google Scholar]
- Sahin BB, Patel D, and Conrad NK 2010. Kaposi’s sarcoma-associated herpesvirus ORF57 protein binds and protects a nuclear noncoding RNA from cellular RNA decay pathways. PLoS.Pathog 6:e1000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sei E and Conrad NK 2011. Delineation of a core RNA element required for Kaposi’s sarcoma-associated herpesvirus ORF57 binding and activity. Virology 419:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B 2006. Common Bacterial Culture Techniques and Media. Curr. Protoc.Microbio 00:4A:A.4A.1–A.4A.8. [DOI] [PubMed] [Google Scholar]
- Tunnicliffe RB, Hautbergue GM, Wilson SA, Kalra P, and Golovanov AP 2014. Competitive and cooperative interactions mediate RNA transfer from herpesvirus saimiri ORF57 to the mammalian export adaptor ALYREF. PLoS.Pathog 10:e1003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ule J, Jensen KB, Ruggiu M, Mele A, Ule A, and Darnell RB 2003. CLIP identifies Nova-regulated RNA networks in the brain. Science 302:1212–1215. [DOI] [PubMed] [Google Scholar]