Abstract
Regulatory agencies across the world are facing the challenge of performing risk-based prioritization of thousands of chemicals in commerce. Here, we present an approach using the Threshold of Toxicological Concern (TTC) combined with heuristic high-throughput exposure (HTE) modelling to rank order chemicals for further evaluation. Accordingly, for risk-based prioritization, chemicals with exposures > TTC would be ranked as higher priority for further evaluation whereas substances with exposures < TTC would be ranked as lower priority. An initial proof of concept, using a dataset of 7986 substances with previously modeled median and upper 95% credible interval (UCI) total daily median exposure rates showed fewer than 5% of substances had UCI exposures > the Cramer Class III TTC (1.5 μg/kg-day). We extended the analysis by profiling the same dataset through the TTC workflow published by Kroes et al (2004) which accounts for known exclusions to the TTC as well as structural alerts. UCI exposures were then compared to the appropriate class-specific TTC. None of the substances categorized as Cramer Class I or Cramer Class II exceeded their respective TTC values and no more than 2% of substances categorized as Cramer Class III or acetylcholinesterase inhibitors exceeded their respective TTC values. The modeled UCI exposures for the majority of the 1853 chemicals with genotoxicity structural alerts did exceed the TTC of 0.0025 μg/kg-day, but only 79 substances exceeded this TTC if median exposure values were used. For substances for which UCI exposures exceeded relevant TTC values, we highlight possible approaches for consideration to refine the HTE : TTC approach. Overall, coupling TTC with HTE offers promise as a pragmatic first step in ranking substances as part of a risk-based prioritization approach.
Keywords: Threshold of Toxicological Concern (TTC), high throughput exposure (HTE), risk-based prioritization
1. Introduction
The last decade has seen an ever-increasing number of regulatory frameworks being implemented for chemical safety evaluation. One of the more prominent frameworks was the European Union (EU) Registration Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation that came into force in June 2007, which set forth minimum sets of information that needed to be submitted, by manufacturers and importers on the basis of tonnage triggers [1]. Frameworks similar to REACH have also been established in other regions including China [2], Korea [3] and Turkey [4]. In the United States (US), the primary chemicals management law for the last 40 years has been the Toxic Substances Control Act (TSCA). The Frank R. Lautenberg Chemical Safety 21st Century Act (LCSA) which was enacted in June 2016 [5] significantly revised this law. There are currently ∼80,000 substances on the TSCA inventory (see https://www.epa.gov/tsca-inventory/about-tsca-chemical-substance-inventory), which includes both substances that are currently active in commerce (~25,000) and those that are not actively used. United States Environmental Protection Agency (US EPA) has estimated there are approximately 15,000 non-polymeric chemicals produced in quantities greater than 10,000 pounds per year in commerce [6]. In addition, US EPA reviews pre-manufacturing dossiers for about 1,000 new substances each year [7], but not all new substances are commercialized. Regardless of jurisdiction, all these regulatory programs have many challenges in common. Most notably, the scope of substances in commerce equates to inventories comprising thousands of substances, many of which lack sufficient publicly available toxicity and exposure information to inform risk-based decision-making.
1.1. Background
Approaches for conducting risk-based prioritization are not new, and, in various forms, they represent an important component of regulatory frameworks in many countries, including Australia, Canada, US and the EU (summarized in Supplemental Material). Nevertheless, this is particularly challenging in the US since the TSCA requires the US EPA to perform risk-based prioritization of chemicals in commerce and then, for high-priority substances, develop risk evaluations that integrate toxicity data with exposure information derived from intended conditions of use [5].
Rather than perform extensive in vivo testing based on toxicological responses in animals, which, in terms of time and resources, is not even practical for thousands of substances, there has been a concerted move within the scientific and regulatory community towards investigating alternative approaches. These alternative approaches employ methods such as (quantitative) structure–activity relationships ((Q)SAR), high throughput screening (HTS) assays, toxicokinetics, physiological mechanisms and dose-dependent biological changes. These paradigm-changing approaches stem, in a large part, from the visions articulated in the 2007 NRC report “Toxicity Testing in the 21st Century: A Vision and a Strategy” [8], the 2012 NRC report “Exposure Science in the 21st Century: A Vision and A Strategy” [9-10] and most recently the 2017 NRC Report “Using 21st Century Science to Improve Risk-Related Evaluations” [11].
To actualize the vision of these NRC reports, several large research programs were initiated (see summary of a selection of these programs in the Supplemental Material). Many of these have mostly focused on applying in vitro assays (including batteries of tests) to measure biological activities of chemicals as indicators of biological pathway perturbations. It may also be possible to use the results as points of departure for chemical toxicity decision-making. In addition, there has been an acceleration of exposure science initiatives focused on deriving exposure estimates so that these advanced methods can generate exposure information for integrating with 21st century toxicity information to enable risk-based decision-making. One such example is the ExpoCast initiative by US EPA that has been instrumental in developing mechanistic and heuristic models for making high-throughput exposure (HTE) predictions that can be rapidly parameterized for thousands of chemicals. As previously demonstrated [12-15], the integration of hazard-related HTS results with HTE modeling should enable risk-based evaluations to be undertaken earlier in the chemical management decision process. For such risk-based evaluations, chemicals with estimated human exposures (based on HTE modeling) that approach or overlap doses of potential concern (e.g., oral equivalent dose calculated from HTS assays) would be prioritized to undergo further evaluation, and substances with HTE modeled exposures below those calculated from HTS assays would be lower priority. The ILSI-HESI RISK211 project [16] and its associated internet-based application tool provides a risk-based approach that can be tailored to support decisions for diverse risk contexts, from screening to in-depth evaluations. An example has recently been published using TTC within the RISK21 project for prioritizing potential drinking water contaminants based on theoretical exposures derived from water solubilities [17].
As promising as these efforts have been, there are challenges to overcome before such approaches become truly routine for the range of regulatory needs. Although HTS assays are less time and resource intensive to run than traditional in vivo studies, testing of thousands or tens of thousands of chemicals using HTS methods still represents a considerable practical undertaking. Richard et al. [18] described the challenges and tactical considerations of moving from a chemical structure-based inventory to a library of samples for testing in the ToxCast HTS assays. Some of those challenges include identifying substances that cannot be readily tested in an in vitro system. HTS testing may be hindered by technical limitations such as volatility, hydrophobicity, susceptibility to hydrolysis, all of which are important considerations when assessing the quality control of the samples, which in turn is critical for interpreting the experimental results. For example, false negative responses may occur if substances volatilize, and false positive results may occur if surfactants change the conformation of proteins.
There is also the question of appreciating the extent to which an existing suite of HTS assays, such as the ToxCast library, actually represent the landscape of biological activities of interest. High throughput transcriptomics (HTTR) may encompass a wider coverage of the biological landscape and represent an important first step to direct subsequent HTS assays that characterize specific mechanisms/pathways [19-21]. Existing HTS assays typically have limited metabolic capabilities, pertinent to certain chemical classes and toxicity effects. Progress is being made to take account of metabolism in in vitro systems, examples include incorporating metabolism into the ToxCast HTS assays [22-23]. As methods progress, one can envision at least in the short-term, the development of a “toolbox” comprising a variety of experimental techniques to address specific classes of substances or distinct decision contexts.
Computational approaches have also emerged, and there is growing interest to exploit these approaches prior to any experimental testing (in vitro or traditional toxicity testing. For example, predictive models, such as read-across, (Q)SAR, machine learning, and hybrid models are now being developed for routine predictions of many toxicity outcomes [24-27]. Machine learning and other SAR type approaches based on chemical descriptor information have also been applied to derive models capable of predicting ToxCast HTS assay outcomes themselves or understand how chemoinformatics can enrich the interpretation of HTS results [18, 27]. Predictive QSAR models can provide physicochemical properties such as LogKow (the log of the octanol-water partition coefficient), vapor pressure, pKa (acid dissociation constant), etc., which can also inform the testing results. The EPA Chemistry Dashboard is one resource that has incorporated a range of models to derive predictions for a number of physicochemical and environmental fate properties for chemicals in the DSSTox inventory (see https://comptox.epa.gov/dashboard [28]). Other tools include the OECD Toolbox [https://www.qsartoolbox.org/]. The Threshold of Toxicological Concern (TTC), as discussed below, is an example of a useful computational approach that can be employed as a pragmatic, fit-for-purpose screening tool.
2. Using Threshold of Toxicological Concern (TTC) with Chemical Specific High Throughput Exposure Estimates to Prioritize Thousands of Substances
A practical first step of a risk-based prioritization process could be to couple the well-established Threshold of Toxicological Concern (TTC) with chemical-specific exposure estimates derived from HTE modeling. The advances made in the development of HTE modelling provides an opportunity to use TTC-based exposure thresholds to prioritize or rank thousands of substances and to identify substances that would be the first candidates for further evaluation. Such evaluation could rely on approaches such as HTTR, HTS, or traditional animal toxicity testing. The approach presented here focuses on evaluating the utility of the TTC approach for a set of 7968 chemicals for which HTE modelling had been used to derive exposure ranges consistent with the results of NHANES2 biomonitoring of a sample of the US population [29]. Although the focus of this paper is on the use of TTC for risk-based priority setting of chemicals in commerce for regulatory purposes, screening level risk evaluations using the TTC can also be performed for food contact materials, impurities in pharmaceuticals, pesticides, personal care products [30].
2.1. Threshold of Toxicological Concern
TTC is a level of exposure that is considered to be of no appreciable risk to human health despite the absence of chemical-specific toxicity data [31]. A recent state-of-the science review by EFSA/WHO [30] concludes that TTC is a fit-for-purpose approach that has broad applicability as a risk assessment tool. As analytical detection approaches are improved, it is conceivable that more contaminants will be observed in the environment, for which a pragmatic means of assessing their associated health risks is needed. TTC does not supplant toxicity testing or chemical-specific risk assessment where required. The TTC concept has been incorporated into some regulatory frameworks; these include evaluation of food additives (i.e., flavor chemicals and food contact chemicals) and genotoxic impurities in pharmaceuticals [32-33]. For a comprehensive history of the TTC concept and its application to chemicals in food, the reader is referred to the International Life Sciences Institute (ILSI) monograph [34], the State of the Science review by EFSA/WHO [30], and other reviews [35]. Here we present a brief overview of the structural TTCs and their application within the Kroes et al. [36] workflow to put this application into context and highlight chemical-specific exclusions. Health Canada [37], Australia’s National Industrial Chemicals Notification and Assessment Scheme (NICNAS) [38] and Food Standards Australia New Zealand (FSANZ) [39] are using or considering TTC as a tool for prioritization and risk-based screening efforts. More recently, evaluating the utility of the TTC was noted as a possible activity in US EPA’s Draft Strategic Plan to Promote the Development and Implementation of Alternative Test Methods [40].
Kroes et al. [36] presents a tiered TTC approach that establishes several human exposure thresholds over four orders of magnitude, ranging from 0.15 μg /d (0.0025 μg /kg-day) to 1800 μg /day (30 μg /kg-day). The exposure limit established for each TTC tier is based on an evaluation of existing toxicity data for chemicals in each tier. It is noted that some chemicals have been excluded from TTC because they are not represented in the toxicity databases supporting TTC (e.g., metals or metal containing compounds, proteins) or because standard risk assessment approaches are not appropriate (2,3,7,8-dibenzo-p-dioxin (TCDD) and its analogues, and steroids). However, it is generally recognized that the majority of organic compounds are within the chemical domain of TTC [30] and those lacking toxicity data can be assigned to the appropriate TTC tier based on an evaluation of chemical structure.
Kroes et al. [36] determined the lowest TTC tier to be 0.15 μg /d (0.0025 μg/kg-day); this category is assigned to any compound with structural alerts for genotoxicity/mutagenicity. The exposure limit for this tier is based on an evaluation of the distribution of carcinogenic potencies of 730 chemical carcinogens (expressed by the carcinogenic potency, TD50, the chronic dose-rate in mg/kg body wt/day which would induce tumors in half the test animals at the end of a standard lifespan for the species – see https://toxnet.nlm.nih.gov/cpdb/); these were plotted and transformed into a distribution of exposures calculated to represent an estimated lifetime cancer risk of one in a million based on linear low-dose extrapolation. Based on this distribution, an exposure of 0.15 μg /day (0.0025 μg /kg-day) was determined to be protective for most carcinogens. Three groups of carcinogens (aflatoxin-like compounds, azoxy- and N-nitroso-compounds; termed the ‘cohort of concern’) have been excluded because many compounds in these classes are characterized as high potency, genotoxic carcinogens.
For compounds without structural alerts, there are a series of noncancer TTC tiers, which are based on the three structural classes defined by the Cramer et al. [41] decision tree. The decision tree comprises 33 questions which utilizes recognized pathways for metabolic deactivation and activation, data on toxicity, and the presence of a substance as a component of traditional foods or as an endogenous metabolite. The three Cramer structural classes have been defined elsewhere [31] as follows:
Cramer Class 1 are substances of simple chemical structure with known metabolic pathways and are of low potential toxicity.
Cramer Class II contains substances that are intermediate -- they possess structures that are less innocuous than in the Class 1 but do not have a positive indication of toxicity which are characteristic of Class III.
Cramer Class III contain structural features that permit no strong initial impression of safety, or may even suggest significant toxicity.
Derivation of TTC values for each of the Cramer Classes stems from the work of Munro et al. [42]. Munro and colleagues compiled a database of NOELs for 613 substances that had been tested in repeat-dose oral toxicity studies including sub-chronic, chronic, reproductive and developmental toxicity. Where there were multiple NOELs for a given substance, the lowest one was selected (there were a total of 2941 NOELs for the 613 substances). The substances were then assigned to the appropriate Cramer structural class, and cumulative distributions of the logarithms of NOELs were plotted separately for each of the structural classes. Where necessary, adjustments were also made to extrapolate subchronic NOELs to chronic, and LOELs to NOELs. The 5th percentile NOEL was estimated for each structural class and this was in turn converted to the TTC limit by applying the conventional default safety/uncertainty factor of 100 (10X to account for extrapolation of animals to humans and 10X for human variability) [42]. Thus, TTC values are similar in many respects to health guidance values such as a Tolerable Daily Intake, or a Chronic Minimal Risk Level (MRL) both of which represent daily exposure levels that occur over a lifetime, even to sensitive subpopulations (e.g. children, without producing an appreciable degree of health risk) [43]. The TTC values established were 1800 μg/person/day for Cramer Class I, 540 μg/person/day for Cramer Class II, and 90 μg/person/day for Cramer Class III substances, assuming a 60kg3 body weight. In evaluating the distribution of NOELs, special consideration was given to chemicals shown to be neurotoxicants, immunotoxicants and teratogens. It was concluded that except for neurotoxicants (specifically organophosphate pesticides (OPs) and carbamates), such compounds would still be adequately represented by the TTC approach for systemic toxicity endpoints. For organophosphates and carbamates, a human exposure threshold of 18 μg/person/day was derived. This threshold was not intended to replace the normal regulatory assessments and controls for OPs and carbamates used as pesticides, but could be used to evaluate their risk should a non-approved or unregulated organophosphate/carbamate be detected as a contaminant. It is noted that the NOELs for the OPs and carbamates were not removed from the original Cramer Class III distribution, and many publications have suggested that this distribution should be re-evaluated without these compounds since an untested OP or carbamate would not be assigned to Cramer Class III. Munro et al. [31] suggested that the new limit for Cramer Class III would be at least 180 μg/day. Most regulatory agencies have not yet adopted this higher value, suggesting that it would be more prudent to wait until an update of the entire decision tree is available, rather than updating through an incremental process [30].
Traditionally, the TTC values have been expressed in units of μg/person/day based on a default body weight of 60 kg. More recently, it has been recommended that the TTC values be expressed in units of μg /kg-day to facilitate the use of these values for different populations including infants and children [30]. The corresponding TTC limits are 30 μg/kg-day for Cramer Class I, 9 μg/kg-day for Cramer Class II, 1.5 μg/kg-day for Cramer Class III substances, and 0.3 μg/kg-day for OPs and carbamates. The human exposure thresholds (TTC values) established by Kroes et al. [36] are summarized in Table 1.
Table 1.
Daily oral exposure TTC values for all applicable chemicals below which there would be no appreciable risk to human health.
| Type of substance | μg/person/day (μg/kg-day for 60 kg adult) |
|---|---|
| Alerts for potential genotoxic carcinogenicity | Kroes: 0.15 (0.0025 μg/kg-day) ICH: 1.5 (0.025 μg/kg-day)a |
| 4Acetylcholinesterase inhibitors (AChEI) (Organophosphates (OPs)/carbamates) | 18 (0.3 μg/kg-day) |
| Cramer Class III | 90 (1.5 μg/kg-day)b |
| Cramer Class II | 540 (9.0 μg/kg-day) |
| Cramer Class I | 1800 (30 μg/kg-day) |
higher threshold (1.5 μg/person per day) is used for pharmaceutical impurities that are mutagenic or that are suspected of being mutagenic (International Conference on Harmonization (ICH) [44]).
2.2. High throughput exposure (HTE) modelling
Wambaugh and colleagues [29] developed a rapid heuristic high throughput exposure (HTE) model that enables prediction of potential human exposure to the many thousands of substances for which little or no empirical exposure data are available. This HTE model was calibrated by comparison to NHANES urinary data that reflects total exposure (all routes/sources) [29]. The NHANES biomonitoring data are spot samples and reflect the level of the chemical (or its metabolite) at a point in time. Biomarker concentrations are impacted by chemical half-life, unknown time of exposure, and unknown or uncertain magnitude, frequency, and duration of exposure, etc. In spot biomonitoring sampling, while measurements of compounds with long half-lives will be indicative of long-term exposures, for compounds with short half-lives, the lower and upper concentration percentiles measured may not be informative of long-term average biomarker concentrations for individuals. Instead, the lower end of the distribution may comprise samples taken at longer times since exposure events and the upper end may reflect samples collected closer in time after exposure events [45]. That said, the research of Aylward et al. [46] concluded that, for substances with short half-lives, central tendency measures in NHANES “may be more informative of longer-term average biomarker concentrations on a population basis,” and the NHANES study design is sufficient to allow estimation of the central tendency of the exposure rate for compounds with short-half-lives. Therefore, the HTE model of the NHANES data allows inferences about the central tendency of the US populations and demographic groups (i.e., population median exposure rate), but does not inform higher or lower exposed subgroups of individuals (e.g. workers or individuals exposed to local sources of exposure). The HTE model characterizes a population median exposure rate that is augmented by five predictive “heuristics” that correspond to greater or lesser chemical exposure rates. However, the importance of these predictors does not vary significantly between demographic groups and therefore any actual differences in exposure between the groups are only captured in the median predicted exposure rate, which is the same across all chemicals for a given demographic group.
The extent to which portions of the population will be exposed at a higher level depends on how each chemical is specifically used and the situations relevant to the individuals (e.g., occupational vs. general population). In the Aylward et al. [46] analysis of the NHANES biomarker concentrations the ratio between the 95th percentile highest exposed individuals and the median was as large as 50.15. Simulation studies by Isaacs et al. predict that the ratio of the 99th highest exposed potential to the median could be as much as a million (personal communication). Therefore, while the HTE model of the NHANES data allows inferences about the median (and the upper confidence intervals of the median exposures) of the U.S. population and different demographic groups, the data does not provide modeled exposures for higher exposed subpopulations of individuals. This limitation does not prevent the use of the findings for prioritizations since chemicals with higher median doses for the overall population are likely to have higher doses for individuals in the population. However, the limitation does prevent the demonstration that a chemical predicted to have negligible risk to highly exposed subpopulations.
3. Materials and Methods
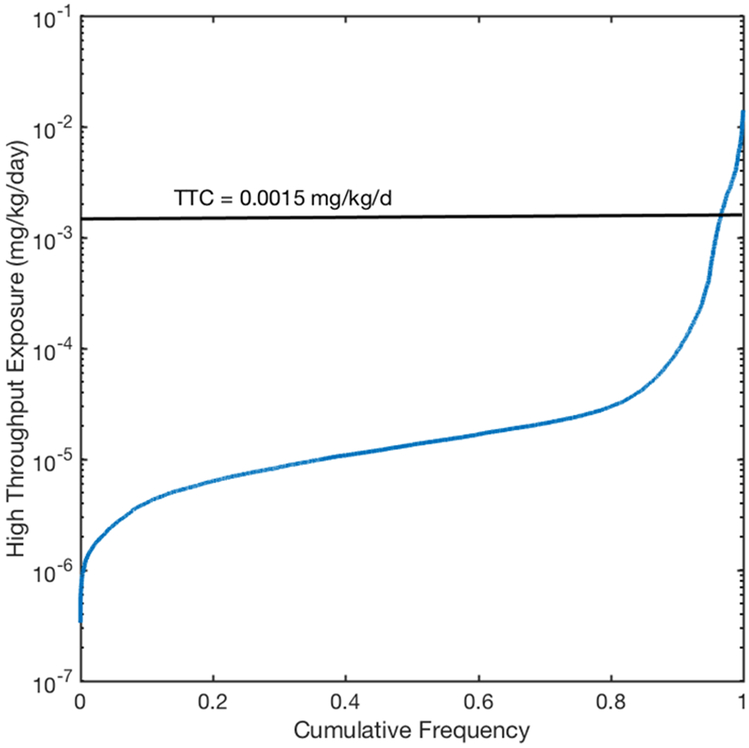
The dataset that was evaluated in this approach was taken from Wambaugh et al [29] who filtered the Tox21 library to reflect substances with similar uses to those in NHANES. To test the feasibility of this approach, we initially assumed none of the 7986 substances in the dataset were automatically excluded from the TTC approach or contained structural alerts that raised a concern for potential genotoxicity. Then we compared the conservative Cramer Class III TTC value of 1.5 μg/kg-day to the previously calculated median and upper 95% credible interval (UCI) of total daily median exposure rates for 7968 chemicals ([47]; Figure 1). As discussed in greater detail in the Results section 4.1, using the ratio of exposure to TTC (i.e., HTE: TTC) as a metric for risk-based prioritization, fewer than 5% of chemicals had an UCI level of exposure that exceeded the TTC.
Figure 1.
Risk-Based screening of 7986 chemicals by comparison of HTE-derived upper 95% credible interval (UCI) of total daily median exposures to the Cramer Class III TTC value of 1.5 μg/kg-day. The labeled black horizontal line shows the TTC of 1.5 μg/kg-day.
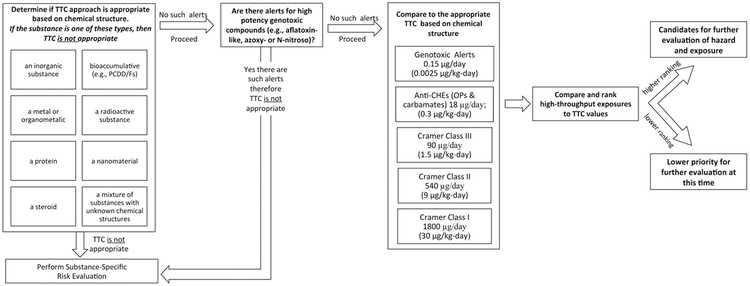
The initial evaluation showed the approach of using the ratio of exposure to TTC (HTE: TTC) appeared feasible (see section 4.1) and we processed the same dataset through the Kroes et al. [36] TTC workflow. The objective was to determine what impact these exclusions and other TTC values had on the ability of the TTC approach to be used as part of a screening level risk-based prioritization (as outlined in Figure 2). As indicated in Figure 2, if a criterion for exclusion was met, a chemical would be excluded from the TTC approach and instead, be subjected to a substance-specific risk evaluation. For completeness, we included this tranche in the workflow in Figure 2, although we did not conduct any such substance-specific assessments as part of this study. Substances that were excluded were set aside, and then, for the remaining chemicals, we applied the specific TTC values for Cramer classes I, II, and III, AChEIs and substances with genotoxicity structural alerts to the respective datasets for risk-based prioritization.
Figure 2.
Proposed Workflow for Using the TTC Approach as Part of a Risk-based Prioritization approach
Although the workflow could have relied in theory on the Kroes et al. [36] decision tree as encoded within Toxtree v2.5 [48], we found that the module did not lend itself to batch processing as a dose needed to be specified upfront on a per substance basis. If this step was bypassed using the “silent” checkbox, the entire workflow was not correctly processed to completion (see Supplementary information). Based on this observation, we created separate filters to mimic the exclusions as depicted in Figure 2 (see Supplementary information). Chemical structures were extracted from DSSTox within the EPA CompTox dashboard (https://comptox.epa.gov/dashboard [28]). Inorganics, bioaccumulative substances, steroids were identified using the Kroes et al. [36] workflow module contained within Toxtree v2.5 (Ideaconsult Ltd), the OECD Toolbox’s ‘structure type’ profiler and Leadscope structural features (www.leadscope.com). OPs were identified by parsing out the SMiles ARbitrary Target Specification (SMARTS) flag in the Kroes workflow module and creating a separate rule in Toxtree whereas carbamates were identified as structural features using Leadscope’s structural feature hierarchy. Genotoxic alerts (including alerts that would trigger for high potency carcinogens) were identified using the in vitro mutagenicity alerts in the ISS6 profiler within the OECD Toolbox v3.4 (https://www.qsartoolbox.org/). Cramer structural classes were identified using the Cramer module within Toxtree v2.5 [49]. The results from each of the respective filters were then combined within a KNIME pipeline (KNIME 2.12.2; https://www.knime.com/knime-analytics-platform) to process the entire structure file. We applied the specific TTC values for Cramer classes I, II, and III, AChEIs and substances with genotoxicity structural alerts to the respective datasets for risk-based prioritization (assuming a 60-kg adult). The predicted median and 95th percentile UCI exposure values from Wambaugh et al. [29] were joined for individual chemicals from each TTC category (i.e., Cramer I, II, III, AChEIs, Genotoxicity) by CAS number using the left_join function in the dplyr package with RStudio (R version 3.4.1 “Single Candle”). In the exposure values spreadsheet, some CAS numbers were corrupted (approximately 80 substances) and could not be matched programmatically to individual chemicals by the R program. Therefore, the exposure values for these substances were entered manually.
We then compared the HTE-derived UCI total daily median exposures to the appropriate class-specific TTC values. For risk-based prioritization, using the HTE: TTC approach, it was proposed that chemicals with exposures greater than the TTC would be ranked higher for further evaluation relative to substances with exposures less than the TTC.
4. Results
The results of the feasibility evaluation of the HTE: TTC approach are presented in Section 4.1. In Section 4.2, we present the results of applying the TTC workflow to the full set of 7968 substances to assign substances into the different TTC categories. The results of applying the HTE: TTC approach for risk-based prioritization using the assigned specific TTC values and HTE-derived UCI total daily median exposure estimates are presented in Section 4.3.
4.1. Initial Evaluation for Proof of Concept
To first investigate this approach, we assumed none of the substances were automatically excluded from the TTC approach or contained structural alerts that raised a concern for potential genotoxicity. We then compared the Cramer Class III TTC value of 1.5 μg/kg-day to the previously calculated median and upper 95% credible interval (UCI) of daily exposures for 7968 chemicals ([47]; Figure 1). Of the 7986 chemicals in the Wambaugh et al. [29] HTE dataset, only 273 (fewer than 5%) were found to have UCI daily exposures estimates that exceeded the Cramer Class III TTC value of 1.5 μg/kg-day (Supplemental Material); this was also the case for all demographic groups – data not shown. In addition, we compared the Cramer Class III TTC value to the substances with the highest predicted median exposures from the SHEDS-HT exposure modeling analysis of 2507 organic chemicals associated with consumer products and agricultural pesticides [50]. Similar to the results obtained from the Wambaugh et al. [29] HTE modeling, for median intakes, only a small fraction - 14 of 2507 substances (< 0.5%) exceeded the Cramer Class III TTC (see Supplemental Material).
4.2. Applying the TTC workflow to evaluate exposure to substances using class-specific TTC values
Chemical structures were available for 7699 of the 7968 substances in the dataset. Individual filters were used to exclude dioxin-like substances, metals, mixtures, salts, high potency carcinogens, and steroids from the set. The number of chemicals falling into each TTC category are summarized in Table 2. Overall, 904 substances out of the starting 7966 structures were found not applicable for the TTC approach based on the aforementioned filters, and would therefore require a more in-depth, chemical-specific evaluation. The remaining set of 6795 substances applicable for consideration of the TTC were then evaluated further using the workflow in Figure 2 to determine which TTC value was most appropriate. The large number of steroids identified and excluded (211) was surprising and suggests that the custom filter may be too conservative in its removal of substances containing a steroid backbone for potential endocrine activity.
Table 2.
Results of filtering the dataset based on structures to categorize substances for application of structural category-specific TTC values.
| TTC category | Number of chemicals |
|---|---|
| Total dataset | 7968 |
| Dataset with available structures | 7699 |
| TTC is not appropriate | 904 |
| TTC is appropriate * | 6795 |
| Substances with structural alerts for genotoxicity | 1853 |
| AChEIs | 102 |
| Cramer class III | 3214 |
| Cramer class II | 332 |
| Cramer class I | 1294 |
Appropriate indicates that these substances nominally fall within the “applicability domain” or “scope” of the TTC approach
4.3. Risk-based screening using the appropriate class-specific TTC value for each chemical
For risk-based screening, we compared the HTE-modeled UCI total daily median exposures for each chemical to the appropriate TTC value. The results are summarized in Table 3. Overall, these findings indicate coupling the TTC approach with HTE modeling is a feasible approach as a pragmatic first step as part of risk-based prioritization for chemical safety evaluations.
Table 3.
Risk-Based Prioritization Results Based on the HTE: TTC approach
| TTC category | Number of chemicals |
TTC (μg/kg-day for 60 kg adult) |
Percentage of Substances Exceeding the TTC |
|
|---|---|---|---|---|
| UCI Exposure Value (number of chemicals) |
Median Exposure Value (number of chemicals) |
|||
| Cramer class III | 3214 | 1.5 μg/kg-day | 2% (58)a | 0 |
| Cramer class II | 332 | 9.0 μg/kg-day | 0 | 0 |
| Cramer class I | 1294 | 30 μg/kg-day | 0 | 0 |
| AChEIs | 102 | 0.3 μg/kg-day | 1% (1) | 0 |
| Genotoxic alerts | 1853 | Kroes 0.0025 μg/kg-day | 94% (1740) | 4% (79) |
| ICH 0.025 μg/kg-day | 18% (333) | 1% (19) | ||
Note: if the TTC Cramer Class III value of 3 μg/kg-day were to be used (the re-calculated Cramer Class III TTC developed by Munro et al., [31] following removal of organophosphates from the dataset) then the predicted UCI modeled exposures for less than 1% of the 3214 substances (specifically 28 chemicals) would be exceeding this TTC.
Of the 1294 substances in Cramer Class I, none exceeded the corresponding TTC value. This was also the case for all 332 substances categorized as Cramer Class II. For Cramer Class III, 58 substances out of 3214 exceeded the TTC value and one anti-cholinesterase inhibitor out of the 102 substances identified as such exceeded its TTC. For the 1853 chemicals with structural alerts for potential genotoxicity, the UCI modeled exposure for the vast majority exceeded the TTC of 0.0025 μg/kg-day, whereas, using median exposure values, only 79 exceeded this TTC. Furthermore, using the ICH genotoxic TTC [44] based on a lifetime cancer risk of 1 in 100,000, the 95th percentile modeled exposure for 18% of chemicals exceeded this TTC and for median exposure values, only 1% would exceed this TTC. (Note, the ICH genotoxic TTC corresponding to a lifetime risk of 1 in 100,000 falls within EPA’s risk range of 10−4 to 10−6 for carcinogens [51], the World Health organization guidelines [52] and California’s Proposition 65 regulations (https://oehha.ca.gov/media/downloads/faqs/p65fact.pdf).
5. Discussion
The results indicate the HTE : TTC approach could be adopted as a pragmatic first step in the risk-based prioritization of thousands of substances. Indeed the HTE: TTC approach is based on transparent, reproducible and published methods, does not favor data rich substances over data poor substances, explicitly includes known domains of applicability that cover a wide range of chemical structures (and can screen out substances that fall outside domains of applicability); covers a broad suite of toxicity endpoints (including developmental, reproductive toxicities and carcinogenicity); and enables evaluation of a wide range of chemical exposures.
5.1. Refinements to the HTE : TTC approach
As promising as the approach was found in this study, additional work is still merited to evaluate the robustness of the TTC assignments based on the specific software implementation. Preliminary work using the specific Kroes workflow in Toxtree and parsing out some of the decision tree questions as separate filters (described in the supplementary information) shows that the exact numbers of chemicals falling into each of the TTC bins does vary from the analysis reported here, but this did not markedly impact the number of chemicals exceeding the HTE estimates in each TTC category. Further work is planned to compare the assignments made by the approach in this study relative to other published datasets e.g. Kroes et al., [36]; Yang et al., [53].
Enhancements for underlying TTC approach itself could also be considered. A number of activities that could be taken into account have been discussed by EFSA/WHO [30], Boobis et al., [54] and Hartung [35]. For example, progress is being made in extending the structural coverage of the database underpinning the structural TTC approach. A number of investigators have re-evaluated the TTC limits established by Munro et al [42] and Kroes et al. [36] in light of additional data; these re-evaluations have consistently shown the existing TTC limits to be protective (reviewed by EFSA/WHO, [30]). One of the most extensive evaluations was conducted by Yang et al. [53], who created a database of No-/Lowest-Observed-Adverse-Effect Level (NOAEL/LOAEL) values from studies of 552 cosmetics-related chemicals, including ingredients, contaminants, and packaging chemicals. When combined with the historical TTC dataset compiled by Munro et al. [42], the combined dataset was increased from 613 chemicals to 966 chemicals. The derived human exposure TTC values from the larger dataset were slightly higher (~ 1.5-fold) than those published by Munro et al. [42] and Kroes et al. [36] for Cramer Classes I and III; too few chemicals were available for derivation of a Classes II TTC value. Yang et al [53] concluded that the TTC values in current use were also protective for chemicals associated with cosmetics.
It is also worth noting that the limitations the HTE model of the NHANES data allows inferences about the median (and the upper confidence intervals of the median exposures) of the U.S. population and different demographic groups. The data does not provide modeled exposures for higher exposed subpopulations of individuals.
6. Possible next steps for substances prioritized by the HTE: TTC approach
Whilst further evaluation of all substances will ultimately be required, in Table 4, we summarize possible next steps for substances identified as higher priority. All possible actions should be formulated keeping the specific decision context in mind.
Table 4.
Possible next steps for prioritized substances a
|
The decision context should guide the selection of options, therefore these possible next steps should be considered illustrative, not exhaustive.
One possible activity would be to refine the exposure analysis for higher prioritized substances (item 1 in Table 4). While the heuristic HTE model of Wambaugh et al. [29] provides estimates of population-wide representative exposures in the U.S. (i.e., the same type of exposure estimate as NHANES biomonitoring data), this HTE model does not produce scenario-specific individual exposure values such as high-end consumer scenarios or occupational exposure. Therefore, one potential next step could be to use the Targeted Risk Assessment tool as used in REACH or adapting other appropriate models, such as EPA’s Consumer Exposure Model [58], to allow for screening-level, scenario-based, deterministic exposure assessments. The refined exposure estimates could be used with appropriate TTC values in a refined risk-based prioritization approach or with actual toxicity data to yield a chemical-specific, scenario-specific, screening level risk evaluation.
A number of next steps could be envisioned and implemented to refine potential hazards (Item 2 in Table 4). For systemic endpoints, including, but not limited to, chronic toxicity, immunotoxicity, neurotoxicity, and reproductive and developmental toxicity, since only approximately 1-2% of the substances were considered of higher priority for further evaluation, obtaining and evaluating any relevant existing toxicity data for each chemical could be a practical undertaking. If a chemical’s relevant toxicity dataset contained information on most of the toxicity endpoints of interest, chemical specific NOAELs or points of departure (PODs) could be derived. In cases where little or no reliable existing toxicity data exist for a substance, a read-across approach could be used.
If actual data on bioactivity are needed, then testing using in vitro HTS/transcriptomics methods could be undertaken utilizing in vitro to in vivo extrapolation (IVIVE) to convert in vitro PODs to equivalent oral doses and subsequently integrating with HTE (or a refined exposure assessment) to derive bioactivity-based chemical-specific risk-based screening evaluations (e.g., [12, 15]). In cases where gaps in toxicity datasets are still of concern, and if these are determined to be data needs, a tiered in vivo testing approach could be designed (e.g., [59-60]).
In our analysis, the set of substances categorized as having structural alerts for potential genotoxic carcinogenicity were found to have the highest proportion of predicted exposures that exceeded the category-specific TTC values. This is not surprising, given the significant conservatism inherent to the derivation of the TTC value (i.e., linear low-dose extrapolation) and the wide range of potencies for carcinogens (i.e., over six orders of magnitude). Williams and colleagues [61] demonstrated that there is high confidence in specific in silico mutagenicity prediction models for predicting negatives. Thus, it is highly unlikely that the HTE: TTC ratio approach would underestimate the identification of potential genotoxic carcinogens.
This approach could lead to false positives because of the database and assumptions used in deriving this specific TTC. This TTC value (0.0025 μg/kg-day) for substances having structural alerts for potential genotoxicity-induced cancer was derived by assuming no threshold and then using linear extrapolation to derive a dose equivalent to an individual excess lifetime cancer risk of 1×10−6. Boobis et al. [54] provide detailed recommendations for reviewing and updating TTC values for potential carcinogens. The authors stress [54] that compounds carcinogenic by a mode of action (MOA) other than DNA-reactive mutagenicity will be adequately covered by the existing systemic toxicity Cramer classification procedures. They recommend first reviewing the carcinogen database to identify and segregate substances that are carcinogenic by a DNA-reactive mutagenic MOA from those that act by a non-genotoxic mode of action and then developing a carcinogen TTC to be applied for likely DNA-reactive mutagenic agents, while also emphasizing that compounds that may be carcinogenic by a MOA other than DNA-reactive mutagenicity are adequately covered by higher TTC values. Concomitantly, to implement this approach, screening tools to identify structural alerts of those compounds likely to be DNA-reactive mutagenic carcinogens will need to be updated (or developed de novo). Implementation of these improvement activities would be beneficial to refine this specific TTC category.
Absent of such refinements in this specific TTC, what are some potential next steps in pursuing chemicals prioritized based on the HTE: TTC approach where the TTC value is based on structural alerts for potential genotoxicity? A logical first step would be to obtain the existing relevant data of genotoxicity for each of these substances, since the TTC screening approach is only based on the presence of structural alerts. If available, chemical specific results of in vitro or in vivo studies could be collated for carcinogenic endpoints. Such considerations were proposed by Felter et al. [62] and Cheeseman et al. [63] by refining TTC values based on the availability of in vitro bacterial mutagenicity data. Then evaluations would be conducted to determine whether the existing data were supportive of a potential mutagenic MOA. In cases where data needs are identified, tiered toxicity testing approaches should be considered [59, 64-66].
6.1. Does the HTE: TTC approach address potential exposures, hazards and risks to children?
Increasingly, assessments are including specific evaluation of potential health risks to sensitive subpopulations, including children. This raises the question of whether the HTE: TTC approach addresses potential exposures, hazards and risks relevant to children. Children are considered in the sense that TTC values are similar in many respects to health guidance values such as a Tolerable Daily Intake, Chronic Minimal Risk Level (MRL) all of which represent daily exposure levels that occur over a lifetime, even to sensitive subpopulations (e.g. children) without producing an appreciable degree of health risk [43, 67]. In addition, TTC values themselves also encompass toxicity endpoints related to concerns for children’s health. TTC values are based on effects on all organ systems in the in vivo animal experimental systems used. Typically, these include consideration of toxicity in all major organ systems, such as the nervous system, digestive system, circulatory system, respiratory system, reproductive system, etc. Laufersweiler et al., [68] independently analyzed developmental and reproductive toxicity datasets and showed that the TTC values “provide a conservative value for protection of human health with respect to reproductive and developmental endpoints.” Furthermore, EFSA/WHO’s review [30] concluded that systemic toxicity TTC values (Cramer Classes I, II, and III) are sufficiently protective for adverse effects on development, a toxicity of particular concern for children’s health.
Typically, comparisons to TTC values should be made to average daily lifetime exposures. However, if specific exposures to children were of concern, the priority substances identified by the HTE: TTC approach could be subjected to screening-level scenario based deterministic exposure assessments using appropriate models that explicitly include chemical product use scenarios for children. In addition, for exposures that are limited to discrete periods, an assumption of lifetime exposure may be an overestimate and adjustments to the TTC values could be made (e.g., see the recommendations of Felter et al., [62]). Then, the derived exposure estimates could be compared to the appropriate less-than-lifetime adjusted TTC value for each specific chemical to yield a chemical-specific, scenario-specific screening level risk evaluation.
7. Conclusions
Overall, this analysis shows the utility of the TTC method and illustrates how integrating HTE estimates with TTC values could be employed as a pragmatic first step in a risk-based prioritization approach. Given that considerable attention has been focused globally on the development and implementation of non-animal testing methods for toxicity evaluation, and that the TTC approach requires no further animal testing, opportunities to expand the understanding of, and confidence in the application of the TTC approach by the global regulatory science community are warranted. In addition, this analysis documents the value of harnessing predictive exposure modeling for use in risk-based prioritization. The ongoing efforts to refine and improve such exposure modeling are critical research activities, and there is a pressing need to generate more accurate predictions of exposures to chemicals in commerce. The goal is to extend such exposure prediction models beyond population-wide predictions to enable exposure modeling results of the thousands of chemicals in commerce to be confidently used in assessments that go beyond prioritization.
Supplementary Material
Highlights.
An approach of using TTC and HTE to facilitate risk-based prioritization is presented
Used the Kroes et al. (2004) workflow to identify the number of chemicals within respective TTC classes
Compared the predicted exposures with the relevant TTC values
The appropriate TTC value was found to be protective for most TTC categories
Possible refinements to the approach are outlined
8. Acknowledgements and Declaration of Interests
The authors had complete control over the design, conduct, interpretation, and reporting of the analyses included in this manuscript. The contents of this manuscript are solely the responsibility of the authors and do not necessarily reflect the views or policies of their employers. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. An initial version of this analysis was presented as a poster at the 2016 Society of Toxicology annual meeting and at the 2017 annual meeting of the American Society for Cellular and Computational Toxicology. The authors’ affiliations are as shown on the cover page. R. Becker is employed by ACC (https://www.americanchemistry.com/), an industry trade association that represents a diverse set of companies engaged in the business of chemistry; for a list of ACC members see https://www.americanchemistry.com/Membership/MemberCompanies/. ACC works to improve environmental, health and safety performance through Responsible Care®, common sense advocacy designed to address major public policy issues, and health and environmental research and product testing. S. Felter is employed full-time by Procter and Gamble Company, a consumer goods corporation. G. Patlewicz, J. Wambaugh, R. Becker and S. Felter worked on this paper as part of their normal job responsibilities. T. Simon is an independent consultant, and his work on this analysis was funded under a contract with ACC. We thank D. Dreier, who served as a contractor to ACC, for assistance in merging the spreadsheets. None of the authors have appeared in any legal proceedings related to the contents of the paper.
Abbreviations
- (AOPs)
Adverse Outcome Pathways
- (FSANZ)
Food Standards Australia New Zealand
- (HTE)
High Throughput Exposure
- (HTS)
high throughput screening
- (ILSI)
International Life Sciences Institute
- (HESI)
Health and Environmental Sciences Institute
- (RISK21)
Risk Assessment in the 21st Century
- (LCSA)
Frank R. Lautenberg Chemical Safety 21st Century Act
- (LogKow)
log of the octanol-water partition coefficient
- (LOAEL)
Lowest-Observed-Adverse-Effect Level
- (MOA)
mode of action
- (NHANES)
National Health and Nutrition Examination Survey is a survey research program conducted by the National Center for Health Statistics within the Centers for Disease Control and Prevention (CDC)
- (NICNAS)
National Industrial Chemicals Notification and Assessment Scheme
- (NOAEL)
No-Observed-Adverse-Effect Level
- (OECD)
Organisation for Economic Co-operation and Development
- (REACH)
Registration Evaluation, Authorisation and Restriction of Chemicals
- (TTC)
Threshold of Toxicological Concern
- (TSCA)
Toxic Substances Control Act
- (US EPA)
United States Environmental Protection Agency
- (UCI)
Upper 95% credible interval
- ((Q)SAR)
(quantitative) structure–activity relationships
- (pKa)
acid dissociation constant
- (SMARTS)
SMiles ARbitrary Target Specification
Footnotes
International Life Sciences Institute (ILSI) Health and Environmental Sciences Institute (HESI) Risk Assessment in the 21st Century (RISK21) – see http://risk21.org/
NHANES = National Health and Nutrition Examination Survey is a survey research program conducted by the National Center for Health Statistics within the Centers for Disease Control and Prevention (CDC)
It is noted that typically 70kg is assumed for an adult body weight, at the time when TTCs were first derived, a default of 60kg was used.
For the remainder of the paper we refer to the OPs and carbamates set as AChEI based on the underlying data that was used by Kroes et al [36] to underpin them as distinct from the Cramer structural classes
ISS = Istituto Superiore di Sanità
9. References
- [1].European Commission (EC), Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Off. J. Eur. Union L396/1 (2006). [Google Scholar]
- [2].MEP (Chinese Ministry of Environmental Protection), 2010. The Measures for Environmental Administration of New Chemical Substances (China MEP Order 7). http://www.chemsafetypro.com/Topics/China/China_REACH_MEP_Order_7_New_Substance_Notification.html [accessed 21 January 2018]. [Google Scholar]
- [3].Korea, 2016. National Assembly in Korea, Revised Korea REACH - The Act on the Registration and Evaluation of Chemicals, Chemical Inspection and Regulation Service. (2016). http://www.cirs-reach.com/news-and-articles/revised-korea-reach---the-act-on-the-registration-and-evaluation-of-chemicals.html [accessed 21 January 2018]. [Google Scholar]
- [4].Turkey, 2017. Draft By-Law on Registration, Evaluation, Authorization and Restriction of Chemicals. http://files.chemicalwatch.com/KKD%C4%B0K%20ingilizce.pdf [accessed 21 January 2018]. [Google Scholar]
- [5].US EPA, 2017. Assessing and Managing Chemicals under TSCA; The Frank R. Lautenberg Chemical Safety for the 21st Century Act. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/frank-r-lautenberg-chemical-safety-21st-century-act [accessed 21 January 2018].
- [6].US EPA, 2008. Overview: Office of Pollution Prevention and Toxics Laws and Programs. https://archive.epa.gov/oppt/pubs/oppt101_tscalaw_programs_2008.pdf [accessed 21 January 2018].
- [7].US EPA, 2017. Reviewing New Chemicals under the Toxic Substances Control Act (TSCA); Statistics for the New Chemicals Review Program under TSCA. https://www.epa.gov/reviewing-new-chemicals-under-toxic-substances-control-act-tsca/statistics-new-chemicals-review [accessed 21 January 2018].
- [8].NRC. 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy. National Academy Press, Washington DC. [Google Scholar]
- [9].NRC, 2012. Exposure Science in the 21st Century: A Vision and A Strategy. National Academy Press, Washington DC. [PubMed] [Google Scholar]
- [10].Cohen Hubal EA, Richard A, Aylward L, Edwards S, Gallagher J, Goldsmith MR, Isukapalli S, Tornero-Velez R, Weber E, Kavlock R, Advancing exposure characterization for chemical evaluation and risk assessment, J. Toxicol. Environ. Health B Crit. Rev. 13 (2010) 299–313. 10.1080/10937404.2010.483947. [DOI] [PubMed] [Google Scholar]
- [11].NRC, 2017. Using 21st Century Science to Improve Risk-Related Evaluations. National Academy Press, Washington DC. [PubMed] [Google Scholar]
- [12].Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K, Clewell HJ 3rd, Dix DJ, Andersen ME, Houck KA, Allen B, Judson RS, Singh R, Kavlock RJ, Richard AM, Thomas RS, Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment, Toxicol. Sci 125 (2012) 157–174. 10.1093/toxsci/kfr254. [DOI] [PubMed] [Google Scholar]
- [13].Shin HM, Ernstoff A, Arnot JA, Wetmore BA, Csiszar SA, Fantke P, Zhang X, McKone TE, Jolliet O, Bennett DH, Risk-based high-throughput chemical screening and prioritization using exposure models and in vitro bioactivity assays, Environ. Sci. Technol. 49 (2015) 6760–6771. 10.1021/acs.est.5b00498. [DOI] [PubMed] [Google Scholar]
- [14].Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Setzer WR, Houck KA, Strope CL, Cantwell K, Judson RS, LeCluyse E, Clewell HJ, Thomas RS, Andersen ME, Incorporating high-throughput exposure predictions with dosimetry-adjusted in vitro bioactivity to inform chemical toxicity testing. Toxicol. Sci. 148 (2015) 121–136. 10.1093/toxsci/kfv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bell SM, Chang X, Wambaugh JF, Allen DG, Bartels M, Brouwer KLR, Casey WM, Choksi N, Ferguson SS, Fraczkiewicz G, Jarabek AM, Ke A, Lumen A, Lynn SG, Paini A, Price PS, Ring C, Simon TW, Sipes NS, Sprankle CS, Strickland J, Troutman J, Wetmore BA, Kleinstreuer NC, In vitro to in vivo extrapolation for high throughput prioritization and decision making, Toxicol. In Vitro. 47 (2018) 213–227. 10.1016/j.tiv.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Embry MR, Bachman AN, Bell DR, Boobis AR, Cohen SM, Dellarco M, Dewhurst IC, Doerrer NG, Hines RN, Moretto A, Pastoor TP, Phillips RD, Rowlands JC, Tanir JY, Wolf DC, Doe JE, Risk assessment in the 21st century: roadmap and matrix, Crit. Rev. Toxicol. 44 Suppl 3 (2014) 6–16. 10.3109/10408444.2014.931924. [DOI] [PubMed] [Google Scholar]
- [17].Wolf DC, Bachman A, Barrett G, Bellin C, Goodman JI, Jensen E, Moretto A, McMullin T, Pastoor TP, Schoeny R, Slezak B, Wend K, Embry MR, Illustrative case using the RISK21 roadmap and matrix: prioritization for evaluation of chemicals found in drinking water, Crit Rev Toxicol. 46 (2016) 43–53. 10.3109/10408444.2015.1082973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Richard AM, Judson RS, Houck KA, Grulke CM, Volarath P, Thillainadarajah I 3, Yang C, Rathman J, Martin MT, Wambaugh JF, Knudsen TB, Kancherla J, Mansouri K, Patlewicz G, Williams AJ, Little SB, Crofton KM, Thomas RS, ToxCast Chemical Landscape: Paving the Road to 21st Century Toxicology, Chem. Res. Toxicol. 29 (2016) 1225–1251. 10.1021/acs.chemrestox.6b00135. [DOI] [PubMed] [Google Scholar]
- [19].Thomas R, 2017. Analysis of Transcriptomic Dose Response Data in the Context of Chemical Risk https://cfpub.epa.gov/si/si_public_file_download.cfm?p_download_id=531624 [accessed 21 January 2018]. [Google Scholar]
- [20].Buesen R, Chorley BN, da Silva Lima B, Daston G, Deferme L, Ebbels T, Gant TW, Goetz A, Greally J, Gribaldo L, Hackermüller J, Hubesch B, Jennen D, Johnson K, Kanno J, Kauffmann HM, Laffont M, McMullen P, Meehan R, Pemberton M, Perdichizzi S, Piersma AH, Sauer UG, Schmidt K, Seitz H, Sumida K, Tollefsen KE, Tong W, Tralau T, van Ravenzwaay B, Weber RJM, Worth A, Yauk C, Poole A, Applying ‘omics technologies in chemicals risk assessment: Report of an ECETOC workshop, Regul Toxicol Pharmacol. 91 Suppl 1 (2017) S3–S13, doi: 10.1016/j.yrtph.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Haider S, Mansouri K, Black MB, McMullen PD, Pendse S. 2018. Pathway Analysis and Mode-of-Action Prediction Based on Computational Modeling of High Throughput Toxicogenomics (Abstract No. 2492). Presented at SOT 2018, San Antonio, March 2018. [Google Scholar]
- [22].DeGroot DE, Thomas RS, Simmons SO 2016. Alginate Immobilization of Metabolic Enzymes (AIME) for High‐Throughput Screening Assays (Abstract No. 3757). The Toxicologist: Late-Breaking Supplement http://www.toxicology.org/events/am/AM2016/docs/2016_LB_Supplement.pdf [Google Scholar]
- [23].Moreau M, Phillips M, Haider S, Nicolas C, Mansouri K, Mallick P, Pendse S, Clewell R, Clewell H, Yoon M. 2018. Towards Incorporation of the Metabolite Exposure in High-Throughput In Vitro to In Vivo Extrapolation (HT-IVIVE) (Abstract 3188) Presented at SOT 2018, San Antonio, March 2018. [Google Scholar]
- [24].Shah I, Liu J, Judson RS, Thomas RS, Patlewicz G, Systematically evaluating read-across prediction and performance using a local validity approach characterized by chemical structure and bioactivity information, Regul. Toxicol. Pharmacol 79 (2016) 12–24. 10.1016/j.yrtph.2016.05.008. [DOI] [PubMed] [Google Scholar]
- [25].Pradeep P, Mansouri K, Patlewicz G, Judson RS, A systematic evaluation of analogs and automated read-across prediction of estrogenicity: A case study using hindered phenols, Computational Toxicology 4 (2017) 22–30. 10.1016/j.comtox.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Patlewicz GY, Fitzpatrick J, Current and future perspectives on the development, evaluation and application of in silico approaches for predicting toxicity. Chem. Res. Toxicol. 29 (2016) 438–451. [DOI] [PubMed] [Google Scholar]
- [27].Zhu H, Bouhifd M, Donley E, Egnash L, Kleinstreuer N, Kroese ED, Liu Z, Luechtefeld T, Palmer J, Pamies D, Shen J, Strauss V, Wu S, Hartung T, 2016. Supporting read-across using biological data, ALTEX 33 (2016) 167–182. 10.14573/altex.1601252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC, Patlewicz G, Shah I, Wambaugh JF, Judson RS, Richard AM, The CompTox Chemistry Dashboard: a community data resource for environmental chemistry, J. Cheminform 9 (2017) 61 10.1186/s13321-017-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wambaugh JF, Wang A, Dionisio KL, Frame A, Egeghy P, Judson R, Setzer RW, High throughput heuristics for prioritizing human exposure to environmental chemicals, Environ. Sci. Technol. 48 (2014) 12760–12767. 10.1021/es503583j. [DOI] [PubMed] [Google Scholar]
- [30].EFSA/WHO (European Food Safety Authority and World Health Organization). 2016. Review of the Threshold of Toxicological Concern (TTC) approach and development of new TTC decision tree. EFSA Supporting publication 2016: EN-1006. http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2016.EN-1006/epdf [accessed 21 January 2018]. [Google Scholar]
- [31].Munro IC, Renwick AG, Danielewska-Nikiel B, The Threshold of Toxicological Concern (TTC) in risk assessment, Toxicol. Lett. 180 (2008) 151–156. [DOI] [PubMed] [Google Scholar]
- [32].EMA (European Medicines Agency). 2006. Guideline on the Limits of Genotoxic Impurities. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002903.pdf [accessed 21 January 2018].
- [33].FDA (Food and Drug Administration). 2015. M7 Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM347725.pdf [accessed 21 January 2018].
- [34].Barlow S, Threshold of toxicological concern: a tool for assessing substances of unknown toxicity present at low levels in the diet ILSI Concise Monograph Series, ISBN 1-57881-188-0 (2005), 2105 ILSI Press, Washington DC and Brussels. [Google Scholar]
- [35].Hartung T, Thresholds of Toxicological Concern – setting a threshold for testing below which there is little concern, ALTEX 34 (2017) 331–351. [DOI] [PubMed] [Google Scholar]
- [36].Kroes R, Renwick AG, Cheeseman M, Kleiner J, Mangelsdorf I, Piersma A, Schilter B, Schlatter J, van Schothorst F, Vos JG, Würtzen G; European branch of the International Life Sciences Institute, Structure-based Thresholds of Toxicological Concern (TTC): Guidance for application to substances present at low levels in the diet, Food Chem. Toxicol. 42 (2004) 65–83. [DOI] [PubMed] [Google Scholar]
- [37].Health Canada. 2016. Science Approach Document Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Health Canada. https://www.ec.gc.ca/ese-ees/326E3E17-730A-4878-BC25-D07303A4DC13/HC%20TTC%20SciAD%20EN%202017-03-23.pdf [accessed 21 January 2018].
- [38].NICNAS (Australian Government, Dept. of Health, National Industrial Chemicals Notification and Assessment Scheme, Inventory Multi-tiered Assessment and Prioritisation (IMAP) framework). 2016. https://www.nicnas.gov.au/chemical-information/imap-assessments/what-is-imap. [accessed 21 January 2018].
- [39].FSANZ (Food Standards Austraila and New Zeland). 2016. Chemical Migration from Packaging into Food. http://www.foodstandards.gov.au/code/proposals/Documents/P1034%20Packaging%201CFS%20SD3%20Risk%20Profile%20Mar2016.pdf [accessed 21 January 2018].
- [40].US Environmental Protection Agency. 2018. Draft Strategic Plan to Promote the Development and Implementation of Alternative Test Methods. March 7, 2018. https://www.regulations.gov/contentStreamer?documentId=EPA-HQ-OPPT-2017-0559-0584&contentType=pdf [accessed 6 June 2018]
- [41].Cramer GM, Ford RA, Hall RL, Estimation of toxic hazard - a decision tree approach. Food Cosmet. Toxicol. 16 (1978) 255–276. [DOI] [PubMed] [Google Scholar]
- [42].Munro IC, Ford RA, Kennepohl E, Sprenger JG, Correlation of a structural class with noobserved-effect levels: a proposal for establishing a threshold of concern, Food Chem Toxicol. 34, (1996) 829–867. [DOI] [PubMed] [Google Scholar]
- [43].WHO/IPCS 2006. Principles for evaluating health risks in children associated with exposure to chemicals. Environmental Health Criteria 237 http://www.who.int/ipcs/publications/ehc/ehc237.pdf [Google Scholar]
- [44].ICH (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use) 2014. Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk. (ICH; ); Geneva) http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Multidisciplinary/M7/M7_R1_Addendum_Step_4_2017_0331.pdf [accessed 21 January 2018]. [Google Scholar]
- [45].Aylward LL, Kirman CR, Adgate JL, McKenzie LM, Hays SM, Interpreting variability in population biomonitoring data: role of elimination kinetics, J Exp Sci Environ Epidemiol. 22 (2012) 398–408. [DOI] [PubMed] [Google Scholar]
- [46].Aylward LL, Kirman CR, Schoeny R, Portier CJ, Hays SM, Evaluation of biomonitoring data from the CDC National Exposure Report in a risk assessment context: perspectives across chemicals, Environ Health Perspect. 121 (2013) 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Becker RA, Wambaugh J, Patlewicz G, Felter S, Simon T, Integrating the Threshold of Toxicological Concern (TTC) with High Throughput Exposure Assessment for Risk-Based Screening of Several Thousand Commodity Chemicals, The Toxicologist, Abstract 2677, (2016) 394 https://www.toxicology.org/pubs/docs/Tox/2016Tox.pdf. [Google Scholar]
- [48].Toxtree. https://eurl-ecvam.jrc.ec.europa.eu/laboratories-research/predictive_toxicology/qsar_tools/toxtree.
- [49].Patlewicz G, Jeliazkova N, Safford RJ, Worth AP, Aleksiev B, An evaluation of the implementation of the Cramer classification scheme in the Toxtree software, SAR QSAR Environ. Res. 19 (2008) 495–524. [DOI] [PubMed] [Google Scholar]
- [50].Isaacs KK, Glen WG, Egeghy P, Goldsmith MR, Smith L, Vallero D, Brooks R, Grulke CM, Özkaynak H, SHEDS-HT: An integrated probabilistic exposure model for prioritizing exposures to chemicals with near-field and dietary sources, Environ. Sci. Technol. 48 (2014) 12750–12759. [DOI] [PubMed] [Google Scholar]
- [51].US EPA. 2000. Methodology for Deriving Ambient Water Quality Criteria for the Protection of Human Health. https://nepis.epa.gov/Exe/ZyPDF.cgi/20003D2R.PDF?Dockey=20003D2R.PDF. [accessed 21 January 2018]. [Google Scholar]
- [52].WHO (World Health Organization). 2008. Guidelines for drinking-water quality Third Edition, Volume 1 Recommendations. World Health Organization, Geneva: http://apps.who.int/iris/bitstream/10665/204411/1/9789241547611_eng.pdf?ua=1[accessed 21 January 2018]. [Google Scholar]
- [53].Yang C, Barlow SM, Muldoon Jacobs KL, Vitcheva V, Boobis AR, Felter SP, Arvidson KB, Keller D, Cronin MTD, Enoch S, Worth A, Hollnagel HM, Thresholds of Toxicological Concern for cosmetics-related substances: New database, thresholds, and enrichment of chemical space, Food Chem. Toxicol. 109 (2017) 170–193. [DOI] [PubMed] [Google Scholar]
- [54].Boobis A, Brown P, Cronin MTD, Edwards J, Galli CL, Goodman J, Jacobs A, Kirkland D, Luijten M, Marsaux C, Martin M, Yang C, Hollnagel HM,. Origin of the TTC values for compounds that are genotoxic and/or carcinogenic and an approach for their re-evaluation, Crit. Revi. Toxicol 47 (2017) 705–727. [DOI] [PubMed] [Google Scholar]
- [55].Dionisio KL, Frame AM 2, Goldsmith MR, Wambaugh JF, Liddell A, Cathey T, Smith D, Vail J, Ernstoff AS, Fantke P, Jolliet O, Judson RS, Exploring consumer exposure pathways and patterns of use for chemicals in the environment, Toxicol. Rep 2 (2015) 228–237. 10.1016/j.toxrep.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Phillips KA, McEachran A, Sobus J, Isaacs K, Application of Functional Use Predictions to Aid in Structure Identification of Chemicals in House Dust. American Chemical Society Spring Meeting, San Francisco, CA, April 02 - 06, 2017 https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=335940 [accessed 21 January 2018]. [Google Scholar]
- [57].Sobus JR, Wambaugh JF, Isaacs KK, Williams AJ, McEachran AD, Richard AM, Grulke CM, Ulrich EM, Rager JE, Strynar MJ, Newton SR, Integrating tools for non-targeted analysis research and chemical safety evaluations at the US EPA, J. Expo. Sci. Environ Epidemiol (2017) 10.1038/s41370-017-0012-y.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].US EPA. 2017. Consumer Exposure Model (CEM) Version 2.0 User’s Guide. https://www.epa.gov/tsca-screening-tools/consumer-exposure-model-cem-version-20-users-guide [accessed 21 January 2018].
- [59].Plunkett LM, Kaplan AM, Becker RA, An enhanced tiered toxicity testing framework with triggers for assessing hazards and risks of commodity chemicals, Regul. Toxicol. Pharmacol. 58 (2010) 382–294. [DOI] [PubMed] [Google Scholar]
- [60].Doe JE, Boobis AR, Blacker A, Dellarco V, Doerrer NG, Franklin C, Goodman JI, Kronenberg JM, Lewis R, Mcconnell EE, Mercier T, Moretto A, Nolan C, Padilla S, Phang W, Solecki R, Tilbury L, van Ravenzwaay B, Wolf DC, A Tiered Approach to Systemic Toxicity Testing for Agricultural Chemical Safety Assessment, Crit. Rev. Toxicol. 36 (2006) 37–68. [DOI] [PubMed] [Google Scholar]
- [61].Williams RV, Amberg A, Brigo A, Coquin L, Giddings A, Glowienke S, Greene N, Jolly R, Kemper R, O’Leary-Steele C, Parenty A, Spirkl HP, Stalford SA, Weiner SK, Wichard J, It’s difficult, but important, to make negative predictions. Regul. Toxicol. Pharmacol. 6 (2016) 79–86. [DOI] [PubMed] [Google Scholar]
- [62].Felter S, Lane RW, Latulippe ME, Llewellyn GC, Olin SS, Scimeca JA, Trautman TD, Refining the threshold of toxicological concern (TTC) for risk prioritization of trace chemicals in food, Food Chem. Toxicol 47 (2009) 2236–2245. 10.1016/j.fct.2009.06.018. [DOI] [PubMed] [Google Scholar]
- [63].Cheeseman MA, Machuga EJ, Bailey AB, A tiered approach to threshold of regulation, Food Chem. Toxicol. 37 (1999) 387–412. [DOI] [PubMed] [Google Scholar]
- [64].Dearfield KL, Gollapudi BB, Bemis JC, Benz RD, Douglas GR, Elespuru RK, Johnson GE, Kirkland DJ, LeBaron MJ, Li AP, Marchetti F, Pottenger LH, Rorije E, Tanir JY, Thybaud V, van Benthem J, Yauk CL, Zeiger E, Luijten M, Next generation testing strategy for assessment of genomic damage: A conceptual framework and considerations, Environ. Mol. Mutagen. 58 (2017) 264–283. 10.1002/em.22045. [DOI] [PubMed] [Google Scholar]
- [65].Brusick D, Aardema M, Kier L, Kirkland D, Williams G, Genotoxicity Expert Panel review: weight of evidence evaluation of the genotoxicity of glyphosate, glyphosate-based formulations, and aminomethylphosphonic acid, Crit Rev Toxicol. 46(supp1) (2016) 56–74. [DOI] [PubMed] [Google Scholar]
- [66].Johnson GE, Soeteman-Hernández LG, Gollapudi BB, Bodger OG, Dearfield KL, Heflich RH, Hixon JG, Lovell DP, MacGregor JT, Pottenger LH, Thompson CM, Abraham L, Thybaud V, Tanir JY, Zeiger E, van Benthem J, White PA, Derivation of point of departure (PoD) estimates in genetic toxicology studies and their potential applications in risk assessment, Environ Mol Mutagen. 55 (2014) 609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].US EPA. 2017. Integrated Risk Information System (IRIS) Glossary. https://ofmpub.epa.gov/sor_internet/registry/termreg/searchandretrieve/glossariesandkeywordlists/search.do?details=&glossaryName=IRIS%20Glossary. [accessed 21 January 2018].
- [68].Laufersweiler MC, Gadagbui B, Baskerville-Abraham IM, Maier A, Willis A, Scialli AR, Carr GJ, Felter SP, Blackburn K, Daston G, Correlation of chemical structure with reproductive and developmental toxicity as it relates to the use of the threshold of toxicological concern, Regul Toxicol Pharmacol. 62 (2012) 160–182. 10.1016/j.yrtph.2011.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.