Abstract
Background
This study aimed to explore the amplitude of low-frequency fluctuations (ALFF) for whole-brain in leukoaraiosis (LA) patients suffering from cognitive decline or impairment.
Material/Methods
Patients were selected by employing magnetic resonance imaging (MRI) technique. According to results of the clinical dementia rating and Montreal cognitive assessment (MoCA), patients were divided into 3 groups: LA patients diagnosed as vascular mild-cognitive impairment (LA-VaMCI, n=28), LA patients diagnosed as vascular-dementia (LA-VaD, n=18), and normal individuals (NC, n=28). Executive functions were evaluated by using the Stroop test and Trail Making Test (TMT). The higher scores in TMT test mean greater impairments. Changes for the ALFF were measured by using resting-state functional MRI (rs-fMRI) technique. Correlations between ALFF and cognition scores were analyzed.
Results
It was found that widespread differences in ALFF were present predominantly in the posterior cingulate cortex/precuneus (PCC/PCu) and in the right inferior temporal gyrus (ITG). Compared with the NC group, ALFF values in PCC/PCu were significantly decreased (F=3.273, P=0.022) and ALFF values were significantly increased (F=2.864, P=0.033) in temporal regions of the LA-VaD patients. ALFF values in LA-VaMCI patients were significantly increased in ITG compared to that in the NC group (F=1.064, P=0.042) and the LA-VaD group (F=2.725, P=0.037). Impairment in executive functions were positively correlated with average ALFF of the left PCu.
Conclusions
This research showed that LA patients exhibited abnormal intrinsic-brain activities. Furthermore, altered ALFF was positively correlated with executive function scores.
MeSH Keywords: Leukoaraiosis, Magnetic Resonance Imaging, Membrane Potentials, Mild Cognitive Impairment
Background
Leukoaraiosis (LA), also called white matter lesions, is a universal neuroimaging phenomenon that usually occurs in elderly people (more than 50% of LA occurs in elderly people) [1]. It has been shown that the progression of LA is correlated with cognitive decline or cognitive impairment [2]. LA is considered to be a risk factor for the cognitive damage, such as dysfunctional attention and impaired executive-function [2]. LA slowly and progressively damages brain white matter prior to the identifiable clinical manifestations. Potential mechanisms for LA damaged cognitive impairment or decline have been elusive until now. Nowadays, imaging technologies might be helpful to discover the associated mechanisms.
Resting state functional magnetic resonance imaging (fMRI) is widely used to investigate the pathophysiology of the brain [3,4]. The fMRI is also proven to be more effective at identifying functional pathology compared to task-fMRI techniques [5]. In particular, the amplitude low frequency fluctuations (ALFF) derived from the resting-state fMRI (rs-fMRI) signals are commonly applied to examine the intrinsic and spontaneous activities of the brain and exhibit valuable information for the pathological mechanism of neurological diseases [6,7]. Recent studies [8,9] have suggested that cognitive deficits can also be studied using rs-fMRI. For example, many studies have found abnormal ALFF in the Alzheimer’s disease [8] and mild cognitive impairment (MCI) [10]. However, research is lacking in explicit investigations on the relationship between cognitive deficits and rs-fMRI dysfunction in LA patients, although a limited study [11] indicated that decreased ALFF in the left middle temporal gyrus may aid early detection of cognitive impairment in LA patients.
As far as we know, there are no investigations that have investigated the intrinsic functional changes in LA patients with different severities of cognitive impairment. Due to the lack of existing positive evidence on different severities of cognitive impairment in LA patients, we hypothesized for this study regarding ALFF changes in LA with MCI patients and LA with vascular dementia patients. Finally, we hypothesized abnormal resting-state activities would be associated with neuro-cognitive functioning, in particular executive performance. This research aimed to apply the rs-fMRI method to detect changes in the ALFF of LA patients with different severities of cognitive impairment. Furthermore, the correlation between ALFF changes in LA patients and their cognitive performance was analyzed.
Material and Methods
Ethics committee approval and statement
We complied with the STROBE (strengthening the reporting of observational studies in epidemiology) statement and the checklist in reporting the present observational study (Supplementary Table 1). The experimental protocols in this study were approved by the Ethics Committees of Beijing Tiantan Hospital, Capital Medical University, China (ethical approval number: KYSB2016-166). Each participants with normal cognitive function gave written informed consent for participating in the present study and publishing associated results. For patients with cognitive impairment, written informed consent was acquired from their guardians.
Participants
Participants were recruited from the Beijing Tiantan Hospital affiliated to the Capital Medical University, between January 2015 and January 2017. Diagnosis of LA was made unanimously by 2 radiologists who independently evaluated the fluid-attenuated inversion recovery (FLAIR) MRIs without any knowledge of the participant’s clinical profile. Fifty patients who were diagnosed LA by both radiologists were included in this study. In addition, 28 age, gender, and education level matched healthy controls were recruited. The inclusion criteria for the LA patients were as follows: 1) patients aged between 50 and 85 years old, 2) patients showed LA on MRI scans according to the revised version of the scale of Fazekas [12], and 3) presence of a contactable informant throughout the study. The exclusion criteria were as follows: 1) cardiac or renal failure, cancer, or other severe systemic diseases; 2) unrelated neurological diseases such as epilepsy, traumatic brain injury, and multiple sclerosis, 3) chronic cerebral infarction or other lesions, 4) leukoencephalopathy of non-vascular origin, 5) dementia of non-vascular origin, 6) psychiatric diseases or drug addiction, 7) consciousness disruption or aphasia, and 7) inability or refusal to undergo brain MRI.
Clinical cognitive assessment
All participants were instructed to complete a comprehensive neuropsychological battery. Global mental functioning was assessed through the Chinese version of Mini Mental State Examination (MMSE) [13], the Clinical Dementia Rating scale (CDR) and the Beijing version of Montreal Cognitive Assessment (MoCA) [14], under the supervision of a physician. The subsequent neuropsychological battery was tested in 5 cognition domains: 1) memory, tested by the Auditory Verbal Learning (AVLT-delay recall) [15]; 2) visuospatial ability and attention, tested by the Symbol Digit Modalities Test (SDMT) [16]; 3) language, tested by the Category Verbal Fluency Test [17]; 4) processing speed, tested by the Stroop Test (Stroop B-time) [18] and the Trail Making Test A (TMTa) [19] and 5) executive function, tested by the Trail Making Test B-A (TMTba) and the Stroop Test (Stroop CB-time). These tests were completed in a strict order in accordance with the standard protocols in a quiet room. In this study, higher score in TMT test means greater impairment.
Based on the results of these cognitive tests, the participants were divided into 3 group: LA patients diagnosed as vascular mild-cognitive impairment (LA-VaMCI group, n=28), LA patients diagnosed as vascular-dementia (LA-VaD group, n=18), and the normal control participants (NC, n=28). The LA-VaMCI patients complied with the following criteria: 1) CDR=0.5, 2) 24< MMSE <27 with ≥6 years education, or 20< MMSE <24 with <6 years education, or 17< MMSE <21 with 0 years education; and 3) MoCA <26. The LA-VaD patients complied with the following criteria: 1) CDR ≥1; 2) MMSE ≤24 with ≥6 years education, or MMSE ≤20 with <6 years education, or MMSE ≤17 with 0 year of education; and 3) MoCA <26. The NC participants complied with the following criteria: 1) MRI showed normal brain structure; 2) CDR=0; 3) MMSE ≥27 with ≥6 years education, or MMSE ≥24 with <6 years education, or MMSE ≥21 with 0 years education; and 4) MoCA ≥26.
Data acquisition of brain MRI
MRI was conducted by using a Siemens Magnetom Verio 3T superconducting MRI system, which was provided by the Radiology Department of Beijing Tiantan Hospital, Beijing, China. A T2W-FLAIR sequence was applied to detect white matter hyperintensities (parameters: echo time 140 ms, repetition time 11 000 ms, inversion time 2600 ms, slice thickness 3–3.5 mm, interslice gap 0.5 mm). The parameters of the T1-weighted 3D magnetization applied as follows: repetition time (TR): 2300 ms, time inversion (TI): 1200 ms, echo time (TE): 3.28 ms, flip angle (FA): 9°, matrix size: 256×256, slice thickness: 1 mm, number of slices: 256, inter-slice gap: 0.5 mm. The blood oxygenation level-dependent (BOLD) rs-fMRI was conducted in axial plane by utilizing the EPI (echo-planar imaging) sequence of a few associated parameters, including TE: 30 ms, TR: 2000 ms, field of view: 256×256 mm2, FA=90°, NEX: 1, matrix size: 64×64, inter-slice gap: 0.5 mm, slice thickness: 3.7 mm, number of slices: 32, acquisition time: 480 s. A total of 240 image volumes were obtained from each participants. All of the patients were instructed to stay awake, relax and close their eyes. Meanwhile, during the resting fMRI scan, the participants were instructed to remain as motionless as possible. The noise was also reduced by using rubber earplugs, and the participant’s head was also fixed to minimize the potential motion artifacts by employing the foam cushions.
Processing and analysis of rs-fMRI image
The structural data analysis was conducted by using VBM8 toolbox (website: http://dbm.neuro.uni-jena.de/vbm.html). SPM8 package (website: http://www.fil.ion.ucl.ac.uk/spm) was used to analyze the data preprocessing. Slice-timing realignment and adjustment for the correction of the head-motion were also conducted. Four LA patients were excluded from the data processing because of the excessive head movement. The criterion for excessive head movement was that the head motion exceeded 3° rotation or 1.0 mm translocation in any direction throughout the course of the scan [20]. The EPI images were normalized spatially to the stereotaxic coordinates for standard MNI (Montreal Neurological Institute) template and re-sampled into a 3×3×3 mm3 voxel size. Then, they were smoothed by convoluting with an isotropic Gaussian kernel of 8 mm to reduce the spatial noise. In order to further exclude the possible effects for the confounding factors that were un-possible associated with specific regional correlation, several spurious variance sources were removed by using the linear-regression analysis, including the average signals from the white matter and the cerebrospinal fluid, 6 head motion parameters, and the global brain signal [21,22]. Finally, in order to remove the higher frequency noise and the lower frequency drift, we band-filtered the residual-time series within the frequency range from 0.01 Hz to 0.08 Hz [23].
The ALFF was analyzed and calculated using the REST software (www.restfmri.net) according to a previous study described [23]. Briefly, in the window of ALFF analysis of the REST software, the frequency band between 0.01 Hz to 0.08 Hz was selected. Then, the individual ALFF map was generated and was normalized with the individual’s global mean ALFF as done in the ReHo analysis. One-sided on-sample t tests were conducted to illustrate where in brain the standardized ALFF values were significantly larger than one.
Structural image analysis
The loss of gray matter (GM) could affect the functional results. In order to identify the brain regions with the GM loss, the voxel-based morphometry (VBM) analysis was conducted for the structural images (website: http://www.fil.ion.ucl.ac.uk/spm). The FMRIB software Library (FSL3) Tools were used to analyze the GM volume differences among the 3 groups.
Statistical analysis
In this study, all of the data were analyzed by utilizing the SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). The chi-square test was employed to evaluate differences in gender distribution among the 3 groups. Kendall’s W test was used to evaluate the differences in education years among the 3 groups. The ANOVA test was employed to compare the difference in age, gender, education, cognitive test results (MMSE, MoCA), executive functions, processing speed, memory, language, and visual-spatial and attention among the groups.
After correcting for age and sex and using FDR-correction for multiple comparisons, the 2-way repeated-measures ANOVA was used to compare the ALFF values difference among the 3 groups. FDR-corrected post-hoc analysis was performed to correct the multiple comparisons for the P value. The Pearson’s correlation analysis was utilized to analyze the correlation between the ALFF results and the cognitive parameters in each group, controlling for age and years of education as confounding variables. Statistical significance was indicated when P was less than 0.05.
Results
Demographic information and cognitive characteristics
There were no significant differences for the age, sex, or education years among the 3 groups. Significant differences in episodic memory tests was observed with the NC group (having the highest scores in MMSE and MoCA) and the LA-VaD group (having the lowest scores in these tests). Significant differences were also found in executive functions, processing speed, and other parameters (Table 1).
Table 1.
Regions showing ALFF differences between the 3 groups.
| NC (n=28) | LA-VaMCI (n=28) | LA-VaD (n=18) | F/t/x2 | P values | |
|---|---|---|---|---|---|
| Age (years) | 58.35±6.82 | 59.28±6.12 | 60.28±11.65 | 0.89 | 0.678 |
| Gender (Male/Female) | 13/15 | 14/14 | 8/10 | 0.43 | 0.825 |
| Education(years) | 13.43±2.76 | 13.43±2.76 | 11.35±3.39 | 0.21 | 0.319 |
| MMSE | 29.46±1.07 | 24.96±1.48 | 20.53±1.77 | 216.32 | 0.000 |
| MoCA | 28.64±1.66 | 21.68±2.74 | 17.17±2.09 | 152.34 | 0.000 |
| CDR | 0 | 0.5 | 2 | ||
| Executive function | |||||
| StroopCB | 19.33±4.09 | 22.79±3.38 | 19.72±2.21 | 7.96 | 0.001 |
| TMTba | 49.32±2.14 | 43.40±2.97 | 51.29±1.71 | 69.297 | 0.000 |
| Processing speed | |||||
| StroopB-time | 44.54±4.54 | 68.25±3.65 | 76.52±1.78 | 476.03 | 0.000 |
| TMTa | 30.93±2.18 | 40.73±1.49 | 43.97±1.05 | 376.335 | 0.000 |
| Memory AVLT-delay recall | 6.25±1.35 | 3.96±0.58 | 1.76±0.90 | 107.46 | 0.000 |
| Language Category Verbal Fluency | 10.93±0.81 | 8.46±0.79 | 7.12±0.99 | 118.82 | 0.000 |
| Visual-spatial and attention SDMT | 49.25±4.73 | 38.21±5.74 | 32.41±2.15 | 75.36 | 0.000 |
| NC (n=28) | LA-VaMCI (n=28) | LA-VaD (n=18) | F/t/x2 | P values | |
| Age (years) | 58.35±6.82 | 59.28±6.12 | 60.28±11.65 | 0.89 | 0.678 |
| Gender (Male/Female) | 13/15 | 14/14 | 8/10 | 0.43 | 0.825 |
| Education(years) | 13.43±2.76 | 13.43±2.76 | 11.35±3.39 | 0.21 | 0.319 |
| MMSE | 29.46±1.07 | 24.96±1.48 | 20.53±1.77 | 216.32 | 0.000 |
| MoCA | 28.64±1.66 | 21.68±2.74 | 17.17±2.09 | 152.34 | 0.000 |
| CDR | 0 | 0.5 | 2 | ||
| Executive function | |||||
| StroopCB | 19.33±4.09 | 22.79±3.38 | 19.72±2.21 | 7.96 | 0.001 |
| TMTba | 49.32±2.14 | 43.40±2.97 | 51.29±1.71 | 69.297 | 0.000 |
| Processing speed | |||||
| StroopB-time | 44.54±4.54 | 68.25±3.65 | 76.52±1.78 | 476.03 | 0.000 |
| TMTa | 30.93±2.18 | 40.73±1.49 | 43.97±1.05 | 376.335 | 0.000 |
| Memory AVLT-delay recall | 6.25±1.35 | 3.96±0.58 | 1.76±0.90 | 107.46 | 0.000 |
| Language Category Verbal Fluency | 10.93±0.81 | 8.46±0.79 | 7.12±0.99 | 118.82 | 0.000 |
| Visual-spatial and attention SDMT | 49.25±4.73 | 38.21±5.74 | 32.41±2.15 | 75.36 | 0.000 |
Comparisons were performed at P<0.05, corrected for multiple comparisons using FDR-correction. x, y, z, coordinates of peak locations in the MNI space. MMSE – Mini Mental State Examination; MoCA – Montreal Cognitive Assessment; CDR – Clinical Dementia Rating; TMT – Trail Making Test; ALVT – Auditory Verbal Learning; SDMT – Symbol Digit Modalities Test; MNI – Montreal Neurological Institute; PCu – precuneus; ITG – inferior temporal gyrus.
VBM analysis
Because LA mainly damages the cerebral white matter, and our findings showed that no significant differences in regional cerebral gray matter volume were found between normal controls and the LA patients. This result was consistent with the description of leukoaraiosis is a pathology of the cerebral whiter matter.
ALFF analysis
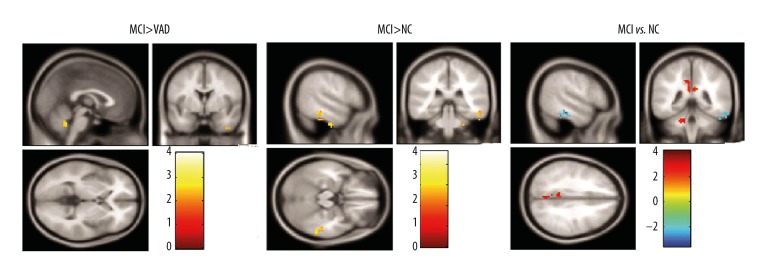
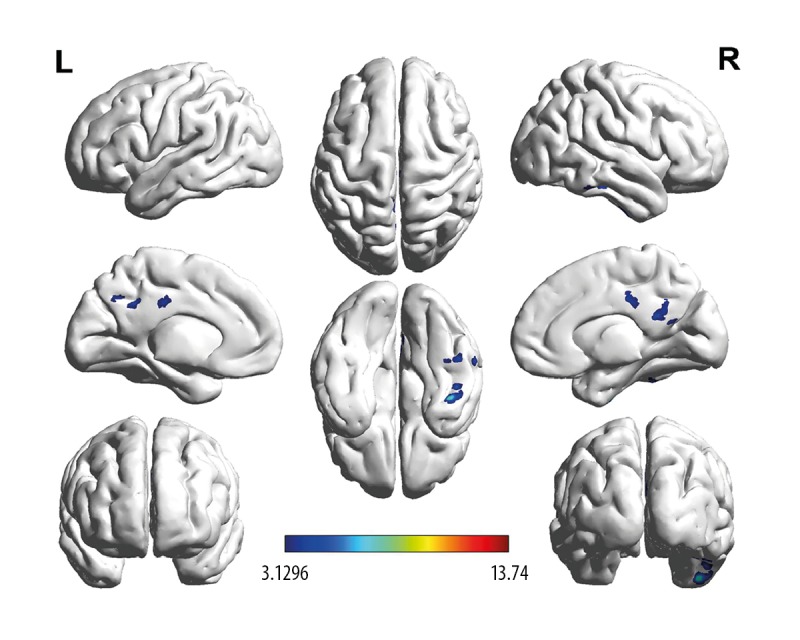
The ANOVA statistical analysis showed that brain regions with a significant difference in ALFF values mainly included the left precuneus (PCu_L, F=3.273, P=0.022) and the right inferior temporal gyrus (ITG), as shown in Figure 1 and Table 2. Compared to the NC group, the LA groups showed enhanced ALFF values in the right ITG (F=1.064, P=0.042) and decreased ALFF in PCu_L (Figures 2 and 3, Table 2, F=2.864, P=0.033). The LA-VaMCI group showed significantly enhanced values of ALFF in the right ITG compared to the LA-VaD group (Figures 2 and 3, Table 2, F=2.725, P=0.037).
Figure 1.
Comparisons for the ALFF signals in LA-VaMCI patients, LA-VaD patients, and NC individuals. Results were corrected by FDR-correction method (cluster size >145, P<0.05). Color-bars illustrated t value in LA-VaMCI patients, LA-VaD patients, and the NC individuals. Warm-color illustrated the enhanced ALFF values and the cool-color illustrated the reduced ALFF values. ALFF – amplitude of low-frequency fluctuations; LA – leukoaraiosis; VaMCI – vascular mild-cognitive impairment; VaD – vascular-dementia, NC – normal control.
Table 2.
Demographic and neuropsychological characteristics of the study participants.
| ROIs | Regions | Number of voxels | Peak F | MNI coordinate (mm) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 1 | Right ITG | 797 | 9.39 | 42 | −9 | −42 |
| Increased ALFF in LA | ||||||
| 2 | Left PCu | 207 | 8.33 | −6 | −45 | 24 |
| Decreased ALFF in LA | ||||||
MNI – Montreal Neurological Institute Coordinate System; ITG – inferior temporal gyrus; ALFF – amplitude of low-frequency fluctuations; PCu – precuneus; LA – leukoaraiosis.
Figure 2.
Brain regions showing different ALFF signals between groups. Color-bars illustrated t value in LA-VaMCI patients, LA-VaD patients, and the NC individuals. Warm-color illustrated the enhanced ALFF values and the cool-color illustrated the reduced ALFF values. ALFF – amplitude of low-frequency fluctuations; LA – leukoaraiosis; VaMCI – vascular mild-cognitive impairment; VaD – vascular-dementia; NC – normal control.
Figure 3.
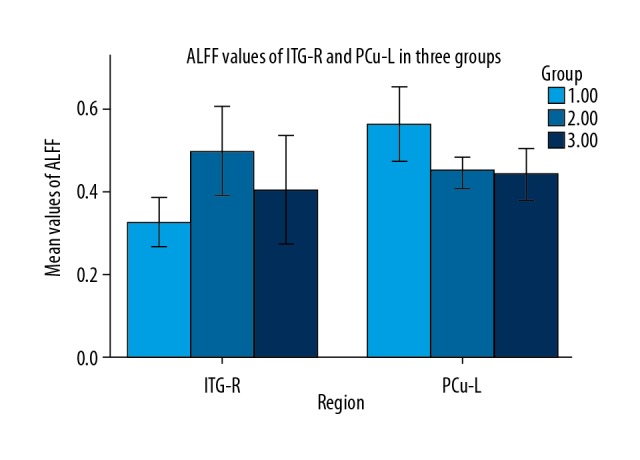
ALFF values of PCu_L and ITG_R. The different brain-regions, ALFF values were demonstrated on horizontal axis and vertical axis, respectively. Light gray represented the recordings for NC group. Dark gray represented the recordings for the LA-VaD group. Black represented the recordings for the LA-VaMCI group. ALFF – amplitude of low-frequency fluctuations; PCu_L – left precuneus; ITG_R – right inferior temporal gyrus; NC – normal control; LA – leukoaraiosis; VaMCI – vascular mild-cognitive impairment; VaD – vascular-dementia.
Relations between ALFF and execution function
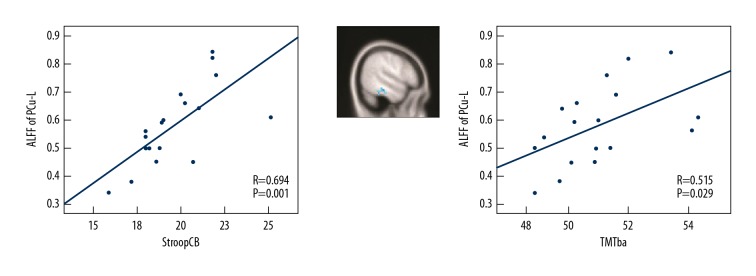
The regression analyses were conducted based on the criteria outlined in materials and methods section. In the LA patients (LA-VaMCI patients and LA-VaD patients), these included assessing the relations between ALFF values of the PCu_L and cognitive indexes including executive functions (StroopCB and TMTba), processing speed (StroopB-time and TMTa), visual-spatial and attention (SDMT), language (category verbal fluency) and memory (AVLT-delay recall). As shown in Figure 4, executive function scores as measured by StroopCB and TMTba in the LA groups had a positive relationship with ALFF signals in the left precuneus (P=0.01 and r=0.694 for StroopCB and P=0.029 and r=0.515 for TMTba). However, there were no significant correlations between ALFF signals in the left precuneus and processing speed (P=0.062 and r=0.342 for StroopB-time and P=0.065, r=0.412 for TMTa) or memory (P=0.105, r=0.297 for AVLT-delay recall) or language (P=0.081 and r=0.482 for category verbal fluency) or visual-spatial and attention (P=0.065 and r=0.376 for SDMT). Meanwhile, if the ALFF was positively correlated with higher or lower impairment of executive functions needs to be clarified.
Figure 4.
The correlation analysis between cognitive performance of LA patients and the associated ALFF values. The “Left” illustrated the partial correlation between the Stroop CB scores and the ALFF values. The Stroop CB scores were positively correlated with mean ALFF values for PCu_L (r=0.694, P=0.001). The “Middle” illustrated the regions showing different ALFF values between the NC individuals and the LA patients (LA>NC, P<0.05). The “Right” illustrated the partial correlation between the ALFF values and the TMTba scores. The mean ALFF values were positively correlated with the TMTba scores for PCu_L (r=0.515, P=0.029). In this part, the influences of gender, educative years, age, the GM volume were modified as the covariates. In the image, each dot represented a data deriving from one patient or individual. LA – leukoaraiosis; ALFF – amplitude of low-frequency fluctuations; PCu_L – left precuneus; NC – normal individuals; GM – gray matter.
Discussion
Increasing evidence has suggested that LA may serve as a neuroimaging biomarker for potential cognitive decline [24]. To explore the underlying neural mechanism of how LA affects cognition, we investigated the ALFF changes in LA patients with cognitive impairment by comparing these values with those of normal controls. We found ALFF differences in the right ITG and the PCC/PCu. In addition, we also found that ALFF values of LA patients are positively correlated with their cognitive decline. The aforementioned results hint that brain regions might be the most influenced areas of LA patients. Meanwhile, the altered spontaneous ALFF values are possible indicators for cognitive impairment of these patients. ALFF values in the aforementioned regions might act as potential values in understanding cognitive decline in LA patients.
Previous resting state studies have proven that ALFF may be effective in examining spontaneous neuronal activities of the cortex [25]. The present study found that ALFF values in the left PCu of LA patients were significantly decreased compared with healthy controls post correcting GM volume’s effects. The PCu is considered the hub of fronto-parietal central executive network [26], which is helpful in manipulating information and actively maintaining the process of learning and working memory. According to Gusnard anatomy of default network partitions, the PCu, located in the back of the cerebral cortex, is the most active region among the default network nodes and plays an important role in memory networks [27]. Previous studies have shown in Alzheimer’s disease that the decreased functional activities of posterior cingulate cortex were positively correlated with the score of delayed recall of auditory verbal learning test. In this research, we discovered that the decreased ALFF in left PCu was correlated with the decline of the StroopCB time. The previous studies also showed that the ALFF abnormalities could be considered as a potential biomarker for the early diagnosis or prognostic marker for several diseases, such as Alzheimer’s disease [28], leukoaraiosis [29], and vascular dementia [30]. According the above backgrounds, the ALFF illustrated abnormalities with increased or decreased values, in different studies [8,10,11,28–30]. These differences might be caused by the differences in disease stages and the severity of diseases, which should be clarified in future studies.
Research in structural neuro-imaging has also illustrated that the PCu is mainly located in the medial aspect of the parietal lobe and participates in distinct functional networks [31], which is in agreement with our findings in terms of the location of this brain region. The posterior cingulate cortex (PCC) is an important region for the core executive function of the frontal lobe [32]. The executive dysfunctions of LA patients observed in the present study may arise from disruptions in the afferents of the PCC.
Inferior temporal gyrus (ITG) is considered a central portion of the language formulation area region and is known as a tertiary visual association cortex [33,34], which also involves in the visual perception, language, and the memory [35,36]. Meanwhile, the ITG also participates in the emotion regulation and the sub-serves cognition processing, and even the default mode network [36–39]. It has been reported that both Alzheimer’s disease and MCI patients illustrated the reduced cortical thickness in temporal lobe, which plays a crucial role in memory preservation [40]. In this study, the results showed that the ALFF values in right inferior temporal lobe of LA groups were significantly increased with a more significant increase in LA-MCI patients. Therefore, the present study extended the previously reported findings and suggests that the default mode network plays critical roles in the LA pathophysiology. One possible mechanism is that the distal cortex compensates the dysfunction of lesioned white matter [41,42]. Though an association between decreased cerebral cortical thickness and increased LA volume has been reported [43], the present study did not show a significant decrease in the thickness of the temporal lobe. More specifically, the neuro-imaging data support the linking of temporal lobe to the alexithymia [44,45]. Evidence from cross-sectional studies suggests a link between cerebral age-related white matter changes and depressive symptoms in older people [46]. For this reason, we considered that the relationship between depressive symptoms in LA patients and ITG should be studied in the future.
The present research also had a few limitations. First, the sample size of this study was relatively small. In further studies, a larger sample should be used to confirm our findings. Second, ALFF values which reflect the spontaneous low-frequency fluctuations of neural activity in a certain voxel, were not dynamic due to the cross-sectional investigation. Third, although there was no difference in GM volumes, the structural differences among the 3 groups could still have affected the measurement of rs-fMRI signals. Fourth, this study did not provide details for the prevalence of vascular risk factors and comorbidities, such as hypertension, diabetes, dyslipidemia, and myocardial infarction. However, LA is usually associated with these vascular risk factors. Diabetes, hypertension, atherosclerotic carotid artery diseases, cerebral hemodynamics impairment, in turn, are associated with neuro-cognitive functions [47–49]. In future studies, we would evaluate the associations between cerebral hemodynamic status, LA entities and ALFF and observed the relationships between vascular risk factors and neuro-cognitive functions. Fifth, the cerebral connectivity data, which has also been correlated with the cognitive functions, were not available. In future studies, we would evaluate the cerebral connectivity data and analyze relationship with cognitive functions. Sixth, the grade of the severity of LA was not evaluated in this study. The group with “vascular dementia” might have greater LA. Therefore, the severity of LA would be graded in future studies. Seventh, this study did not involve patients with LA but without cognitive impairment. In future studies, we would include LA patients without cognitive impairment for clarification.
Conclusions
This study investigated spontaneous and aberrant activities of the LA patients with cognitive impairment by evaluating the ALFF values utilizing rs-fMRI. The significant decrease of ALFF values was discovered in the left PCu, and increased in the right ITG. The ALFF values of the left PCu illustrated a positive correlation with executive function scores, suggesting that spontaneously aberrant activities of the aforementioned brain regions may aid in understanding the characteristics of cognitive impairment of LA patients.
Supplementary Table
Supplementary Table 1.
STROBE statement – checklist of items that should be included in reports of case-control studies.
| Item No | Recommendation | |
|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract [2] |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found [2] | ||
| Introduction | ||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported [4] |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses [5] |
| Methods | ||
| Study design | 4 | Present key elements of study design early in the paper [5,6] |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection [5,6] |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls [5,6] |
| (b) For matched studies, give matching criteria and the number of controls per case | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable [6–10] |
| Data sources/ measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group [6–10] |
| Bias | 9 | Describe any efforts to address potential sources of bias [N/A] |
| Study size | 10 | Explain how the study size was arrived at [5,6] |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why [10] |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding [10] |
| (b) Describe any methods used to examine subgroups and interactions [N/A] | ||
| (c) Explain how missing data were addressed [N/A] | ||
| (d) If applicable, explain how matching of cases and controls was addressed [N/A] | ||
| (e) Describe any sensitivity analyses [N/A] | ||
| Results | ||
| Participants | 13* | (a) Report numbers of individuals at each stage of study – e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed [N/A] |
| (b) Give reasons for non-participation at each stage [N/A] | ||
| (c) Consider use of a flow diagram [N/A] | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders [11] |
| (b) Indicate number of participants with missing data for each variable of interest [N/A] | ||
| Outcome data | 15* | Report numbers in each exposure category, or summary measures of exposure [11,12] |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included [N/A] |
| (b) Report category boundaries when continuous variables were categorized [N/A] | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period [N/A] | ||
| Other analyses | 17 | Report other analyses done – e.g., analyses of subgroups and interactions, and sensitivity analyses [N/A] |
| Discussion | ||
| Key results | 18 | Summarize key results with reference to study objectives [12,13] |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias [15] |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence [15] |
| Generalizability | 21 | Discuss the generalizability (external validity) of the study results [16] |
| Other information | ||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based [17] |
Give information separately for cases and controls.
An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at http://www.strobe-statement.org.
Footnotes
Source of support: Supported by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201836), National Key Technology Research and Development Program of China ( 2018YFC2002300, 2018YFC 2002302, 2015BAI12B02), the National Natural Science Foundation of China (NSFC: 81371201, 31600933) National Key Technology Research and Development Program of the Ministry of Science and Technology of The People’s Republic of China (2015BAI12B04) and Hubei Province Health and Family Planning Scientific Research Project (Grant No. WJ2017M208, WJ2017F057)
Conflict of interest
None.
References
- 1.Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: An update. Nat Rev Neurol. 2015;11:157–65. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- 2.Herrmann LL, Le MM, Ebmeier KP. White matter hyperintensities in late life depression: A systematic review. J Neurol Neurosurg Psych. 2008;79:619–24. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- 3.Abudureheman Y, Wang J, Liu W. Comparison of intravoxel incoherent motion diffusion-weighted magnetic resonance (MR) imaging to T1 mapping in characterization of hepatic alveolar echinococcosis. Med Sci Monit. 2017;23:6019–25. doi: 10.12659/MSM.903929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 5.Fleisher AS, Sherzai A, Taylor C, et al. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer’s disease risk groups. Neuroimage. 2009;47:1678–90. doi: 10.1016/j.neuroimage.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Zou QH, Zhu CZ, Yang Y, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137–41. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Yan C, Zhao C, et al. Spatial patterns of intrinsic brain activity in mild cognitive impairment and Alzheimer’s disease: A resting-state functional MRI study. Hum Brain Mapp. 2011;32:1720–40. doi: 10.1002/hbm.21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen X, Wu X, Li R, et al. Alzheimer’s disease-related changes in regional spontaneous brain activity levels and inter-region interactions in the default mode network. Brain Res. 2013;1509:58–65. doi: 10.1016/j.brainres.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Han Y, Wang J, Zhao Z, et al. Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: A resting-state fMRI study. Neuroimage. 2011;55:287–95. doi: 10.1016/j.neuroimage.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Yang J, Yin X, et al. Abnormal intrinsic brain activity patterns in leukoaraiosis with and without cognitive impairment. Behav Brain Res. 2015;292:409–13. doi: 10.1016/j.bbr.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am J Roentgenol. 1987;149:351–56. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 13.Katzman R, Zhang M, Ouang-Ya-Qu, et al. A Chinese version of the mini-mental state examination; Impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol. 1988;41:971–78. doi: 10.1016/0895-4356(88)90034-0. [DOI] [PubMed] [Google Scholar]
- 14.Tinica G, Mocanu V, Zugun-Eloae F, et al. Clinical and histological predictive risk factors of atrial fibrillation in patients undergoing open-heart surgery. Exp Ther Med. 2015;10:2299–304. doi: 10.3892/etm.2015.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards. Stroke. 2006;37:2220–41. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg SJ, Ryan JJ, Prifitera A. Rey Auditory-Verbal Learning Test performance of patients with and without memory impairment. J Clin Psychol. 1984;40:785–87. doi: 10.1002/1097-4679(198405)40:3<785::aid-jclp2270400325>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Sheridan LK, Fitzgerald HE, Adams KM, et al. Normative Symbol Digit Modalities Test performance in a community-based sample. Arch Clin Neuropsychol. 2006;21:23–28. doi: 10.1016/j.acn.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Mok EH, Lam LC, Chiu HF. Category verbal fluency test performance in Chinese elderly with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2004;18:120–24. doi: 10.1159/000079190. [DOI] [PubMed] [Google Scholar]
- 19.Koss E, Ober BA, Delis DC, et al. The Stroop Color-Word Test indicator of dementia severity. Int J Neurosci. 1984;24:53–61. doi: 10.3109/00207458409079534. [DOI] [PubMed] [Google Scholar]
- 20.Gordon NG. The Trail Making Test in neuropsychological diagnosis. J Clin Psychol. 1972;28:167–69. doi: 10.1002/1097-4679(197204)28:2<167::aid-jclp2270280212>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 21.Yu Y, Zhou X, Wang H, et al. Small-world brain network and dynamic functional distribution in patients with subcortical vascular cognitive impairment. PLoS One. 2015;10:e0131893. doi: 10.1371/journal.pone.0131893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–78. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song XW, Dong ZY, Long XY, et al. REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sporns O, Tononi G, Edelman GM. Theoretical neuroanatomy: Relating anatomical and functional connectivity in graphs and cortical connection matrices. Cereb Cortex. 2000;10:127–41. doi: 10.1093/cercor/10.2.127. [DOI] [PubMed] [Google Scholar]
- 25.The LADIS Study Group. Poggesi A, Pantoni L, Inzitari D, et al. 2001–2011: A decade of the LADIS (Leukoaraiosis And DISability) Study: What have we learned about white matter changes and small-vessel disease? Cerebrovasc Dis. 2011;32:577–88. doi: 10.1159/000334498. [DOI] [PubMed] [Google Scholar]
- 26.Zuo XN, Martino AD, Kelly C, et al. The oscillating brain: Complex and reliable. Neuroimage. 2010;49:1432–45. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trend Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Liang P, Xiang J, Liang H, et al. Altered amplitude of low-frequency fluctuations in early and late mild cognitive impairment and Alzheimer’s disease. Curr Alzheimer Res. 2014;11:389–98. doi: 10.2174/1567205011666140331225335. [DOI] [PubMed] [Google Scholar]
- 29.Cheng R, Qi H, Liu Y, et al. Abnormal amplitude of low-frequency fluctuations and functional connectivity of resting-state functional magnetic resonance imaging in patients with leukoaraiosis. Brain Behav. 2017;7:e00714. doi: 10.1002/brb3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C, Li C, Yin X, et al. Abnormal intrinsic brain activity patterns in patients with subccortical ischemic vascular dementia. PLoS One. 2014;9:387880. doi: 10.1371/journal.pone.0087880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 32.Margulies DS, Vincent JL, Kelly C, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA. 2009;106:20069–74. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerrouche N, Herholz K, Mielke R, et al. 18FDG PET in vascular dementia: Differentiation from Alzheimer’s disease using voxel-based multivariate analysis. J Cereb Blood Flow Metab. 2006;26:1213–21. doi: 10.1038/sj.jcbfm.9600296. [DOI] [PubMed] [Google Scholar]
- 34.Dien J, Brian ES, Molfese DL, et al. Combined ERP/fMRI evidence for early word recognition effects in the posterior inferior temporal gyrus. Cortex. 2013;49:2307–21. doi: 10.1016/j.cortex.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gattass R, Lima B, Soares JG, Ungerleider LG, et al. Controversies about the visual areas located at the anterior border of area V2 in primates. Vis Neurosci. 2015;32:E019. doi: 10.1017/S0952523815000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noppeney U, Price CJ. Retrieval of visual, auditory, and abstract semantics. Neuroimage. 2002;15:917–26. doi: 10.1006/nimg.2001.1016. [DOI] [PubMed] [Google Scholar]
- 37.Bouyukliev I, Simonis J. Anatomic subdivisions in human temporal cortical neuronal activity related to recent verbal memory. Nat Neurosci. 2002;5:64–71. doi: 10.1038/nn785. [DOI] [PubMed] [Google Scholar]
- 38.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–58. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damoiseaux JS, Rombouts SARB, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faivre A, Rico A, Zaaraoui W, et al. Assessing brain connectivity at rest is clinically relevant in early multiple sclerosis. Mult Scler. 2012;18:1251–58. doi: 10.1177/1352458511435930. [DOI] [PubMed] [Google Scholar]
- 43.Hawellek DJ, Hipp JF, Lewis CM, et al. Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc Natl Acad Sci USA. 2011;108:19066–71. doi: 10.1073/pnas.1110024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sang WS, Lee JM, Im K, et al. Cortical thinning related to periventricular and deep white matter hyperintensities. Neurobiol Aging. 2012;6:S368. doi: 10.1016/j.neurobiolaging.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Aust S, Alkan HE, Koelsch S, et al. How emotional abilities modulate the influence of early life stress on hippocampal functioning. Soc Cogn Affect Neurosci. 2014;9:1038–45. doi: 10.1093/scan/nst078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng Y, Ma X, Tang Q. Brain response during visual emotional processing: An fMRI study of alexithymia. Psychiatry Res. 2013;213:225–29. doi: 10.1016/j.pscychresns.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Williamson W, Lewandowski AJ, Forkert ND, et al. Association of cardiovascular risk factors with MRI indices of cerebrovascular structure and function and white matter hyperintensities in young adults. JAMA. 2018;320:665–73. doi: 10.1001/jama.2018.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lattanzi S, Brigo F, Vernieri F, et al. Visit-to-visit variability in blood pressure and Alzheimer’s disease. J Clin Hypertens (Greenwich) 2018;20:918–24. doi: 10.1111/jch.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lattanzi S, Carbonari L, Pagliariccio G, et al. Neurocognitive functioning and cerebrovascular reactivity after carotid endarterectomy. Neurology. 2018;90:e307–15. doi: 10.1212/WNL.0000000000004862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
STROBE statement – checklist of items that should be included in reports of case-control studies.
| Item No | Recommendation | |
|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract [2] |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found [2] | ||
| Introduction | ||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported [4] |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses [5] |
| Methods | ||
| Study design | 4 | Present key elements of study design early in the paper [5,6] |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection [5,6] |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls [5,6] |
| (b) For matched studies, give matching criteria and the number of controls per case | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable [6–10] |
| Data sources/ measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group [6–10] |
| Bias | 9 | Describe any efforts to address potential sources of bias [N/A] |
| Study size | 10 | Explain how the study size was arrived at [5,6] |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why [10] |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding [10] |
| (b) Describe any methods used to examine subgroups and interactions [N/A] | ||
| (c) Explain how missing data were addressed [N/A] | ||
| (d) If applicable, explain how matching of cases and controls was addressed [N/A] | ||
| (e) Describe any sensitivity analyses [N/A] | ||
| Results | ||
| Participants | 13* | (a) Report numbers of individuals at each stage of study – e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed [N/A] |
| (b) Give reasons for non-participation at each stage [N/A] | ||
| (c) Consider use of a flow diagram [N/A] | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders [11] |
| (b) Indicate number of participants with missing data for each variable of interest [N/A] | ||
| Outcome data | 15* | Report numbers in each exposure category, or summary measures of exposure [11,12] |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included [N/A] |
| (b) Report category boundaries when continuous variables were categorized [N/A] | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period [N/A] | ||
| Other analyses | 17 | Report other analyses done – e.g., analyses of subgroups and interactions, and sensitivity analyses [N/A] |
| Discussion | ||
| Key results | 18 | Summarize key results with reference to study objectives [12,13] |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias [15] |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence [15] |
| Generalizability | 21 | Discuss the generalizability (external validity) of the study results [16] |
| Other information | ||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based [17] |
Give information separately for cases and controls.
An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at http://www.strobe-statement.org.