ABSTRACT
Visceral adipose tissue (VAT) may play a critical role in atherosclerotic cardiovascular disease. The goal of this study was to determine the effect of human VAT-released pro‑inflammatory cytokines on monocyte adhesion to the endothelium. The cytokine effects on monocyte adhesion to the endothelial cells (ECs) were tested using adipose tissue-conditioned media (ATCM) prepared by culturing human VAT. The cytokines concentrations in ATCM, the cytokines expression and adhesion molecules in stimulated ECs were measured. The concentrations of IL-1β,TNF-α,MCP-1,IL-10,and RANTES measured in ATCM correlated positively with monocyte adhesiveness to ECs. Additionally, ATCM increased the adhesion molecules (ICAM-1, VCAM-1) gene expression. Selective inhibitors highlighted the importance of IL-1β and TNF-α in the process by a significant decrease in monocyte adhesion compared to ATCM preconditioning without inhibitors. Human VAT significantly increased monocyte adhesion to ECs. It was significantly influenced by IL-1β, TNF-α, MCP-1, IL-10, and RANTES, with IL-1β and TNF‑α having the strongest impact.
KEYWORDS: Atherosclerosis, cytokines, inflammation, endothelium, adipose tissue
Introduction
The driving factor in the development of atherosclerosis and its clinical complications could be subclinical inflammation of arteries and their surrounding structures. Based on previous studies, one of the main players in this process could be adipose tissue [1,2]. Therefore, a better understanding of the mechanisms that underlie the role of adipose tissue in vascular disease, that is, atherosclerosis, could be of key importance, especially when faced with the increasing prevalence of obesity worldwide. It has been already shown that the inflammatory processes provoked by modified low-density lipoprotein (LDL) particles in the vessel wall pathologically activate the endothelium and could lead to serious arterial dysfunction and even occlusion caused by the atherosclerotic process [3–6]. The key players in these processes are monocytes entering the subintimal space and activated to macrophages. However, the exact mechanisms of monocyte activation are still not fully understood. One of the main mediators could be pro-inflammatory cytokines, which lead to monocyte rolling, adhesion and extravasation from the bloodstream into lesion-prone areas of the arterial vasculature [7]. In this process, adipose tissue, its content and the activity of monocytes/macrophages could be of utmost importance. In this respect, visceral adipose tissue (VAT) seems to be the most dangerous [8,9]. Indeed, in our previous studies, we found substantially higher gene expression of pro-inflammatory cytokines and higher infiltration of pro-inflammatory, polarized macrophages in human VAT than in subcutaneous adipose tissue [10]. In this study, to further evaluate the role of pro-inflammatory cytokines produced by VAT, we analyzed their effect on monocyte adhesion to the endothelium.
The aims of the present study were to identify the VAT-released cytokines which could potentially affect the human endothelium and monocyte adhesiveness in vitro and, also, to identify the cell type being the main source of pro-inflammatory cytokines by comparing of adipose tissue-conditioned media (ATCM) and stromal vascular fraction-conditioned media (SVFCM). The study is unique in describing the effect of human adipose tissue-released products on the initial stages of atherosclerosis.
Results
The basic parameters obtained in the group of studied individuals such as age, smoking status, body mass index (BMI), plasma total cholesterol and C-reactive protein measured by a highly sensitive method (hs-CRP) were similar in men and women (Table 1). BMI was lower and the prevalence of smokers higher than in a 1% representative sample of the Czech population [11].
Table 1.
Basic physiological and clinical characteristics of the analysed groups of living kidney donors shown as mean ± SD.
| Total (n = 30) | Men (n = 11) | Women (n = 19) | |
|---|---|---|---|
| Age (years) | 46.2 ± 12.1 | 44.7 ± 4.6 | 47.1 ± 10.5 |
| Body mass index (kg/m2) | 26.1 ± 4.20 | 27.5 ± 3.38 | 25.2 ± 4.14 |
| Smoking (%) | 46.7 | 45.5 | 47.4 |
| Total cholesterol (mmol/L) | 5.41 ± 1.52 | 5.18 ± 2.47 | 5.57 ± 1.70 |
| hsCRP (mg/L) | 1.88 ± 2.36 | 1.28 ± 0.45 | 2.43 ± 3.15 |
Effects of ATCM on monocyte adhesion to the endothelium
The first main finding of our study was that preconditioning of human umbilical vein endothelial cells (HUVECs) using ATCM increased monocyte adhesion to the endothelium by a quarter compared to the control medium (50.8% ± 5.6 ATCM vs. 40.6% ± 5.4 negative control, respectively; p < 0.0001). At the same time, after the addition of tumor necrosis factor-α (TNF-α, 10 ng/ml) to the culture medium, adhesion of monocytes using ATCM was lower than in positive controls (60.0% ± 7.5, p < 0.0001) (Figure 1)
Figure 1.
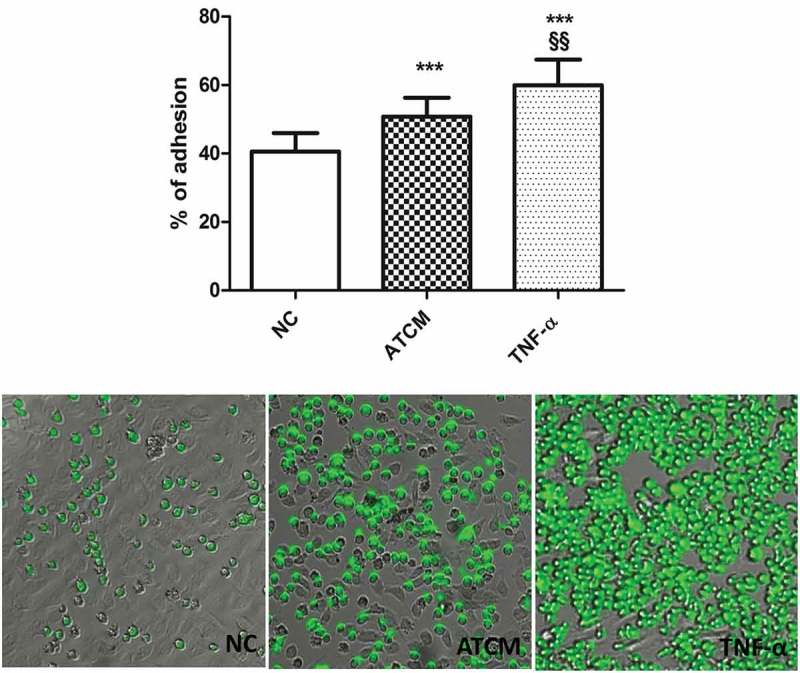
ATCM significantly increased (p < 0.0001) the level of cell adhesion compared to the negative control (NC).
TNF-α (10ng/ml) used as a positive control significantly increased the percentage of adhesion of HUVECs. *** p < 0.0001 compared to NC; §§ p < 0.001 compared to positive control, NC – negative control, ATCM – adipose tissue-conditioned media, TNF-α – tumor necrosis factor α.
The second main finding was that ATCM also increased monocyte adhesion proportionally to the concentrations of IL-1ß (IL-1ß, p < 0.0001, r = 0.82), TNF-α (p < 0.0001, r = 0.70), monocyte chemoattractant protein 1 (MCP-1, p < 0.0001, r = 0.69), and RANTES (CXCL5, p < 0.01, r = 0.54) (Figure 2). Quite unexpectedly, monocyte adhesion correlated positively with IL-10 concentration (p < 0.0001, r = 0.67). Finally, no correlation between monocyte adhesion and IL-4, IL-5 or CXCL5 concentrations was found.
Figure 2.
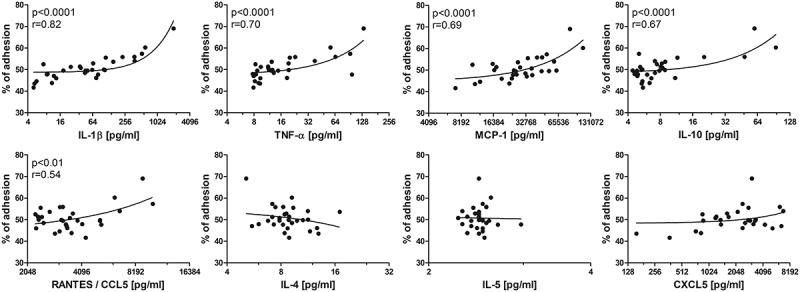
Correlation of the level of adhesion to cytokine concentrations in ATCM.
The X-axis has a log2 scale. IL-1β/4/5/10 – interleukin 1β/4/5/10, TNF-α – tumour necrosis factor α, MCP-1 – monocyte chemoattractant protein-1 (CCL2), RANTES – CCL5 – regulated the activation, normal T cell expression and secretion.
In addition, stimulation of endothelial cells (ECs) by IL-1ß (200 pg/ml) and TNF-α (30 pg/ml) at concentrations much lower than usually used in in vitro models using ATCM significantly increased monocyte adhesion to the endothelium (Figure 3).
Figure 3.
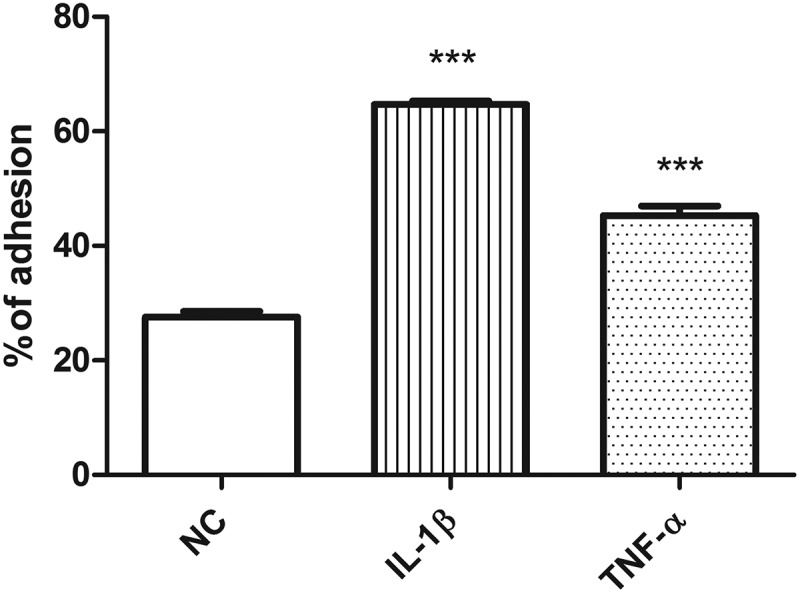
Stimulation of endothelial cells by IL-1ß and TNF-α at concentrations measured in ATCM.
IL-1ß (200 pg/ml) and TNF-α (30 pg/ml). NC – negative control, IL-1ß – interleukin 1ß, TNF-α – tumor necrosis factor α, * p < 0.05, *** p < 0.001.
Gene expression in endothelial cells influenced by ATCM
Preconditioning of ECs by ATCM significantly increased gene expression of the adhesion molecules vascular cell adhesion protein 1 (VCAM-1, 1.60-fold, p < 0.01) and intercellular cell adhesion protein 1 (ICAM-1, 4.07-fold, p < 0.01), which are important for the adhesion process (Figure 4). Moreover, ATCM increased pro-inflammatory IL-6 (2.64-fold, p < 0.0001) and decreased transforming growth factor β (TGF-β, 0.59-fold, p < 0.0001) gene expression in ECs more than in the negative control. The gene expression of MCP-1 and selectin E (SelE) was not significantly influenced in the whole group due to the high individual differences in the case of the SelE molecule.
Figure 4.
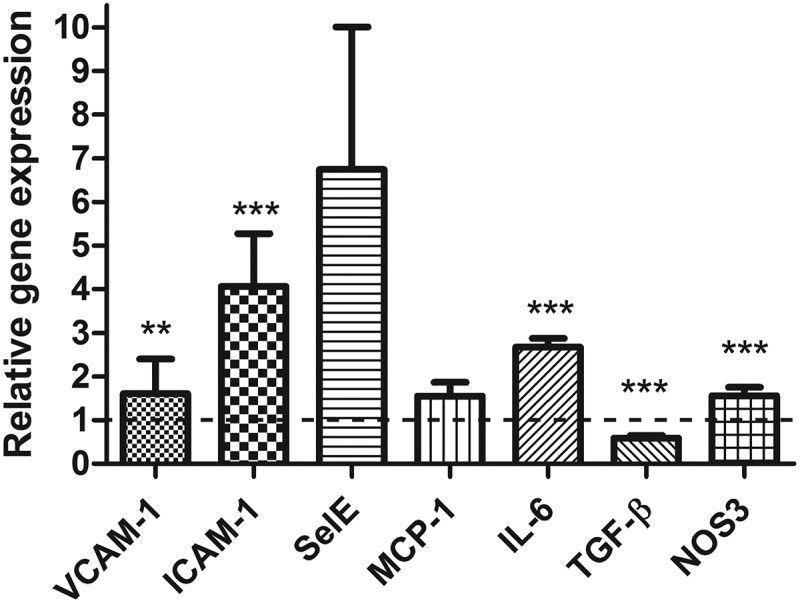
Effect of ATCM on relative gene expression in endothelial cells compared to the negative control.
** p < 0.01, *** p < 0.001, SelE p = 0.089. ICAM-1 – intercellular adhesion molecule 1, VCAM-1 – vascular cell adhesion molecule 1, SelE – selectin E, MCP-1 – monocyte chemoattractant protein-1 (CCL2), IL-6 – interleukin 6, TGF-β – transforming growth factor β, NOS3 – nitric oxide synthase type 3
Effect of selective cytokine inhibition
To evaluate the real role of individual cytokines in the process of endothelial activation and monocyte adhesion to the endothelium, selective cytokine inhibitors were used.
Inhibition of IL-1ß and TNF-α significantly decreased monocyte adhesion ACTM-activated ECs whereas no effects of MCP-1 and RANTES inhibitors were demonstrated (Figure 5).
Figure 5.
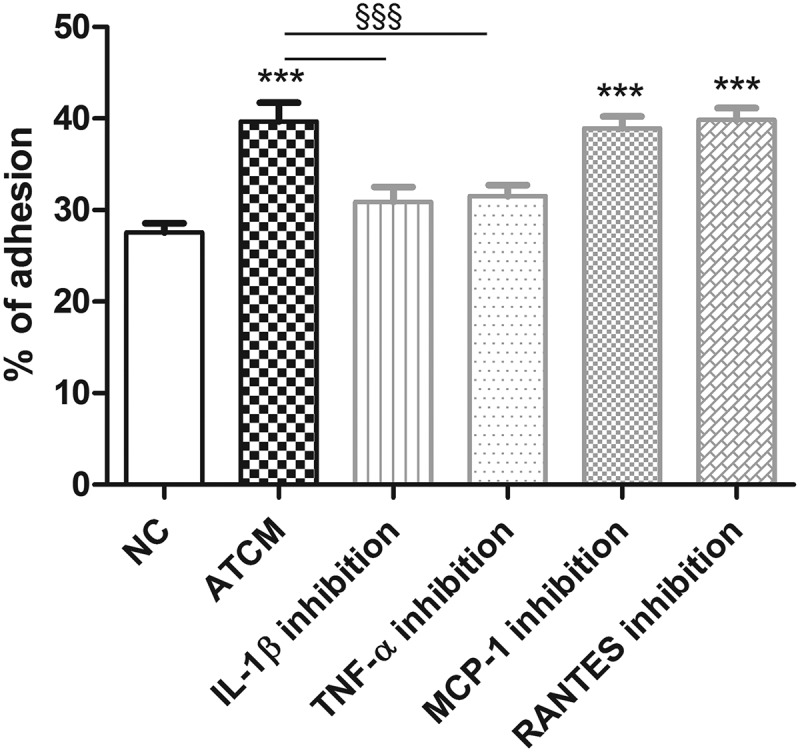
Effect of inhibition of selected cytokines during stimulation of endothelial cells. NC – negative control, ATCM – adipose tissue-conditioned media, IL-1ß – interleukin 1ß, TNF-α – tumor necrosis factor α, MCP-1 – monocyte chemoattractant protein-1 (CCL2), RANTES – CCL5 – regulated the activation, normal T cell expression and secretion, *** p < 0.001 compared to NC, §§§ p < 0.001 compared to ATCM stimulation.
The increase in adhesion molecules (VCAM-1, ICAM-1, and SelE) induced by ATCM stimulation was partially abolished by selective IL-1ß, TNF-α and MCP-1 inhibitors. The effect shown to be the strongest was that on SelE gene expression, as all inhibitors significantly decreased its gene expression with the most prominent effect being that of the TNF-α inhibitor (3.17 ± 0.71 vs. 13.4 ± 2.52, p < 0.001) (Figure 6). TNF-α highly significantly decreased ICAM-1 gene expression (1.34 ± 0.91 vs. 3.34 ± 0.68, p < 0.01) and a minor, but also significant, the effect of IL-1ß was shown (1.21 ± 0.33 vs. 3.03 ± 0.71, p < 0.05). Gene expression of VCAM-1 was significantly decreased when IL-1ß had been inhibited (0.77 ± 0.18 vs. 3.34 ± 0.68, p < 0.05); while TNF-α (p = 0.09) and MCP-1 (p = 0.19) inhibition did not reach the level of significance, a trend could be clearly seen.
Figure 6.
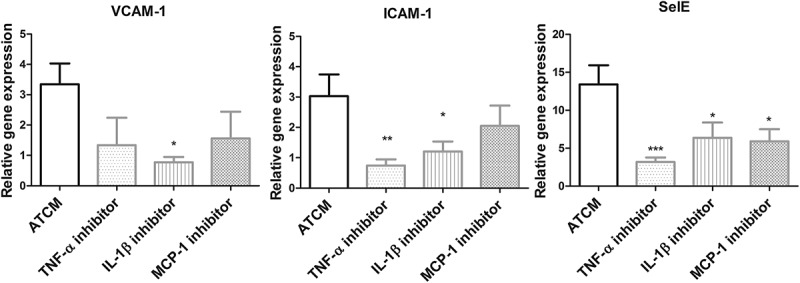
Relative gene expression of adhesion molecules with the addition of TNF-α, IL-1ß and MCP-1 inhibitor compared to negative control.
Inhibition of selected cytokines decreased gene expression of adhesion molecules – VCAM-1, ICAM-1 and SelE compared to negative control (unstimulated endothelial cells). VCAM-1 – vascular cell adhesion molecule 1; ICAM-1 – intercellular adhesion molecule 1; SelE – selectin E; IL-1ß – interleukin 1ß; TNF α – tumor necrosis factor α; MCP-1 – monocyte chemoattractant protein-1 (CCL2), * p < 0.05, ** p < 0.01, *** p < 0.0001.
Comparison of ATCM and SVFCM in cytokine production
When comparing SVFCM and ATCM, no difference was found in IL-1β level, and a significantly higher concentration was found for TNF-α (Table 2). By contrast, the concentrations of IL-6, MCP-1, RANTES, and IL-10 were significantly higher in ATCM than in SVFCM. The IL-6, IL-10, MCP-1, and RANTES concentrations in ATCM were significantly higher than those in SVFCM for the same subject.
Table 2.
Comparison of cytokine concentrations in ATCM and SVFCM shown as mean ± SD. IL-1β/6/10 – interleukin 1β/6/10; TNF-α – tumour necrosis factor α; MCP-1 – monocyte chemoattractant protein-1 (CCL2); RANTES – regulated the activation, normal T cell expression and secretion (CCL5).
| ATCM | SVFCM | p value | |
|---|---|---|---|
| IL-1β (pg/mL) | 605.8 ± 148.7 | 699.4 ± 124.3 | 0.7072 |
| TNF-α (pg/mL) | 266.8 ± 33.00 | 695.7 ± 125.1 | 0.0159 |
| IL-10 (pg/mL) | 127.2 ± 10.4 | 84.10 ± 5.73 | 0.0008 |
| IL-6 (ng/mL) | 142.8 ± 21.87 | 16.71 ± 3.31 | 0.0007 |
| MCP-1 (ng/mL) | 39.21 ± 5.26 | 8.40 ± 1.65 | 0.0006 |
| RANTES (ng/mL) | 4.23 ± 0.66 | 2.003 ± 0.09 | 0.0079 |
Potential impact of extreme values
Among all genes being investigated, the largest difference between the highest and lowest quartiles was found for the expression of VCAM-1 (4.36 ± 3.1 vs. 1.01 ± 0.8, p = 0.011) (Table 3). On the one hand, no significant difference in the mean values of MCP-1 expression between ATCM and negative control was found. On the other hand, we observed a significant difference between the highest and lowest quartiles (3.19 ± 2.6 vs. 0.80 ± 0.04, p = 0.022). No similar relationships were found for other genes. This is consistent with the positive correlation between the gene expression of VCAM-1 (p < 0.05, r = 0.44) and MCP-1 (p < 0.05, r = 0.38) involved in monocyte adhesion to ECs. No differences between the means were noted, and interquartile comparisons for SelE gene expression were between the ATCM and negative control groups despite the highest mean value due to variations in individual values. Nevertheless, our analysis of the whole group showed a significant correlation between the relative gene expression of SelE and monocyte adhesion (p < 0.0001, r = 0.73). The differences between ATCM and negative control observed in the extreme quartiles of TGF-β reached borderline significance (0.40 ± 0.9 vs. 0.60 ± 0.2, p = 0.063). Although an increased relative gene expression of ICAM-1 and IL-6 in ATCM-treated ECs was documented, no significant differences were observed between their extreme quartiles and monocyte adhesion.
Table 3.
Comparisons of gene expression analysis of the level of monocyte adhesion in the extreme quartiles. VCAM-1 – vascular cell adhesion molecule 1; ICAM-1 – intercellular adhesion molecule-1; SelE – selectin E; TGF-β – transforming growth factor β; IL-6 – interleukin 6; MCP-1 – monocyte chemoattractant protein-1 (CCL2); NOS3 – nitric oxide synthase type 3.
| Upper quartile | Lower quartile | p value | |
|---|---|---|---|
| VCAM-1 | 4.36 ± 3.1 | 1.01 ± 0.8 | 0.011 |
| SelE | 19.45 ± 29.5 | 0.33 ± 0.2 | 0.088 |
| ICAM-1 | 7.89 ± 10.7 | 1.37 ± 0.83 | 0.107 |
| TGF-β | 0.60 ± 0.2 | 0.40 ± 0.2 | 0.063 |
| IL-6 | 2.00 ± 1.1 | 2.84 ± 1.0 | 0.130 |
| MCP-1 | 3.19 ± 2.6 | 0.80 ± 0.04 | 0.022 |
| NOS3 | 1.22 ± 0.3 | 1.34 ± 0.6 | 0.619 |
When including cardiovascular risk factors (sex, age, menopause, BMI, smoking, hs-CRP concentration) in the analyses, no effect on the level of monocyte adhesion to ECs was shown. However, when comparing the extreme quartiles of the level of monocyte adhesion to ECs, we observed a moderately lower mean BMI than in the highest quartile (25.8 ± 2.96 kg/m2 vs. 28.9 ± 2.97 kg/m2, p = 0.064). An opposite relationship was found for age (lower extreme quartile 51.1 ± 10.5 years vs. the upper extreme quartile 42.9 ± 13.7, p = 0.198). However, this finding may have been influenced by the relatively small number of subjects included in the study.
Discussion
Our results show that human VAT-released cytokines directly increase monocyte adhesion in vitro. The level of monocyte adhesion positively correlates with the concentrations of IL-1β, TNF-α, MCP-1, RANTES and IL-10 measured in ATCM. These results imply that human VAT is an important source of pro-inflammatory cytokines, which possibly affects local inflammation within adipose tissue as well as systemic low-grade inflammation. In addition, we confirmed that it is the cells of stromal vascular fraction (SVF) that are the main source of pro-inflammatory cytokines.
This finding is consistent with the previously reported pro-atherogenic effects of pro-inflammatory cytokines in experimental models [12–15]. These effects have also been demonstrated to play an important role in the development of obesity-related diseases [16–20]. Our results are in agreement with the work of Henrichot et al. [15] focusing on the effect of ATCM on chemotaxis and effect of IL-8 and MCP-1. However, according to our results, the effect of MCP-1 on cell adhesion was not that strong as shown for chemotaxis [15]. These relationships between cytokines and monocyte adhesion to the endothelium were also found in our human study with a group of normal weight individuals – healthy LKDs.
The unexpected positive correlation between IL-10 concentration and monocyte adhesion could be explained by the synergy of IL-10 with another component of ATCM. This is supported by our finding that, when assessing separately the effect of IL-10 on monocyte adhesion using an identical model, it tended to increase the level of adhesion only moderately (data not presented). However, IL-10 definitely did not decrease the level of adhesion, as reported in an earlier study by Mostafa Mtairag et al. [21]. Data from other studies also indicate that while IL-10 is generally considered an anti-inflammatory cytokine, it decreases EC adhesion in experimental models [21,22]; there is also evidence that IL-10 might have a negative influence on the endothelium [23].
ATCM precondition led to pro-inflammatory changes in EC gene expression in vitro. We focused on the adhesive molecules VCAM-1, ICAM-1, and SelE, which play important roles, especially in the initial steps of leukocyte adhesion to the endothelium and are mostly expressed after a pro-inflammatory stimulus [24]. ATCM significantly increased ICAM-1 and VCAM-1 gene expression. Our data on the effect of these adipose tissue-produced cytokines on gene expression are in agreement with their important role in the adhesion process [25].
In addition to changes in the gene expression of adhesion molecules, which affect monocyte behavior, ATCM significantly increased the expression of pro-inflammatory IL-6 and nitric oxide synthase (NOS3), thereby affecting EC behavior. In this setting, the production of molecules increased the adhesiveness and, additionally, also that of pro-inflammatory molecules in ECs. These two effects act synergistically. At the same time, the decreased gene expression of anti-inflammatory TGF-β [26–29] potentiated pro-inflammatory changes in ECs in our study. These data documenting a combination of endothelial activation and increased production of adhesive molecules are in agreement with previous studies [29–31]. Moreover, the increased expression of IL-6 in ECs might locally influence macrophage polarization towards pro-atherogenic macrophages [32] in synergy with other pro-inflammatory cytokines (IL-1β and TNF-α) produced mainly by macrophages infiltrated into VAT [33,34]. This process may directly contribute to the development of atherosclerosis by increasing the number of monocytes trapped within the vessel wall, similar to the process of macrophage infiltration into adipose tissue.
To analyze the importance of all the cytokines studied, we inhibited ATCM adhesion by selective inhibition of IL-1β, TNF-α, MCP-1 and RANTES. Inhibition of IL-1β and TNF-α significantly decreased monocyte adhesion compared to stimulation by ATCM alone, whereas no effect of inhibition of MCP-1 and RANTES was shown suggesting a major role of IL-1β and TNF-α released from VAT in this process. When inhibiting IL-1β, TNF-α and MCP-1, the relative gene expression of the adhesion molecules in ECs decreased compared to stimulation by ATCM.
To document that even low concentrations of cytokines in ATCM are capable of changing the level of adhesion, a separate experiment using mean IL‑1β and TNF-α concentrations in ATCM was performed. This control study showed that IL‑1β and TNF-α, at concentrations as low as those in ATCM, significantly increased monocyte adhesion.
When comparing the cytokine concentrations in ATCM and SVFCM from the same adipose tissue in a separate experiment with eight subjects, the IL-6, IL-10, MCP-1 and RANTES concentrations in ATCM were much higher than in SVFCM. The implication is that these four cytokines are produced predominantly by adipocytes. Similar production of IL-1β and only a slightly higher concentration of TNF-α were found in SVFCM suggesting that these cytokines are produced mainly by SVF. In agreement with earlier data [34–37], the main producers might be macrophages. The possibility of cytokine diffusion from adipose tissue must be considered whereas the concentrations of cytokines after SVFCM incubation represent their production in these isolated cells. It supports our opinion that these two cytokines play the most important role in monocyte adhesion. They also elicit the closest relationship between their concentrations and macrophage adhesion when analyzing the effect of adhesion of individual ATCM. In addition, the adhesion and cytokine concentrations of IL-1β and TNF-α in SVFCM alone correlated significantly with monocyte adhesion despite the low number of samples, whereas no relation was found for the concentrations of MCP-1, RANTES and IL-6.
When studying a possible effect of cardiovascular risk factors (age, sex, BMI, smoking and plasma hs-CRP concentration) on monocyte adhesion, none of these parameters was effective. Strictly speaking, our data did not demonstrate any relation of ATCM-influenced adhesion to sex, age, smoking and pro-inflammatory status. Only when comparing two extreme quartiles of the level of adhesion did the difference in BMI reach borderline significance (p = 0.063). One might speculate that the relative changes in BMI in our healthy subjects were not strong enough (16.9–33.3) to detect a significant effect of adipose tissue in our model. The same applies to all other risk factors. This is in agreement with our previous results, as we also did not find an effect of BMI on macrophage polarization in VAT [38] and pro-inflammatory gene expression in VAT [39] in the LKD group.
In conclusion, we have documented that cytokines released from human VAT increase monocyte adhesion to the endothelium and lead to pro-inflammatory changes detected as pro-inflammatory gene expression in preconditioned ECs. Furthermore, we have demonstrated that the most potent pro-inflammatory cytokines in this respect were IL-1β and TNF-α.
Patients and methods
Donors of adipose tissue
Visceral adipose tissue was obtained intraoperatively from LKDs during kidney transplantation (from the area outside Gerota’s fascia). All healthy LKDs were informed about the study in detail, and they signed informed consent forms prior to inclusion in the study. The study was approved by the Institute’s Ethics Committee.
Conditioned media
Adipose tissue was transferred to the laboratory within 15 min, and all visible blood vessels and fibrous tissue were removed with it to be subsequently minced into small pieces (approx. 2 mm3); the remaining traces of blood were washed out by PBS (Dulbecco’s Phosphate Buffered Saline, w/o Calcium, w/o Magnesium, Biosera, Nuaille, France).
Adipose tissue-conditioned media (ATCM)
Adipose tissue-conditioned media (ATCM) were prepared by incubation of cleansed and minced VAT (n = 30) in culture media (1 ml per 1 g of adipose tissue) (EBM-2, Lonza, with 0.2% fatty acid-free bovine serum albumin, Sigma Aldrich, A1595, SLBP3641V, St. Louis, MO, USA) in a thermostat for 24 hours (5% CO2, 37°C). The ACTM were separated from tissue using a 150 µm strainer, followed by a 22 µm syringe filter, and kept in a ‒80°C freezer until use.
Stromal vascular fraction-conditioned media (SVFCM)
The stromal vascular fractions (SVFs) of eight VAT samples were isolated for the preparation of SVFCM to be compared to ATCM. Approximately 2 g of the VAT cleaned from all visible blood vessels and fibrous tissue was exposed to collagenase (c = 0.002 g/ml, Sigma-Aldrich), and then repeatedly filtered and purified. SVFCM were prepared in a fashion similar to ATCM (incubated in 1 ml of a culture medium per SVF isolated from 1 g of VAT).
Model of monocyte adhesion to the endothelium
The level of monocyte adhesion was evaluated based on the differences in fluorescence intensity, reported as percentages, according to Bae et al. [40]. In particular, fully grown HUVECs (Pooled, Lonza, lot. 369,948) in a 96-well plate were incubated in hexaplicate with 5% ATCM or SVFCM in a culture medium (EBM-2 with 2% FBS) (EBM-2, Lonza) for 24 hours. Adhesion data using conditioned media stimulation were compared to a negative control without the addition of conditioned media. In a separate experiment, selective inhibitors were used to evaluate the role of the individual cytokines released from VAT (AF 12,198 – IL-1β inhibitor, c = 1µM, Cat. No. 1793; C 87 TNF-α inhibitor, c = 10µM, Cat. No. 5484; BMS CCR2 22 – MCP-1 inhibitor, Cat. No. 3129, c = 1µM; DAPTA – RANTES inhibitor, c = 10 nM, Cat. No. 2423; Tocris, R&D Systems). Subsequently, calcein acetoxymethyl (calcein-AM, Molecular Probes)-labelled monocytes (THP-1, 105 cells/well) were added for 30 mins and allowed to adhere to the EC monolayer. After measuring baseline fluorescence intensity, non-adhering monocytes were washed out with PBS three times, with final fluorescence intensity measured using a microplate reader. TNF-α (10 ng/ml, R&D Systems) was used as a positive control.
THP-1 (ECACC 88,081,201) cells were cultured in RPMI-1640 () with 10% fetal bovine serum (FBS) (Gibco™, Thermo Fisher Scientific), 2 mM L-glutamine (Sigma Aldrich) and penicillin-streptomycin (Sigma Aldrich). Human umbilical vein endothelial cells (HUVECs) were cultured using an EGM-2 BulletKit (CC-3156 & CC-4176, Lonza).
Measurements of the cytokine ATCM concentration
The cytokine concentrations of IL-1ß, MCP-1, TNF-α, MCP-1, IL-4, IL-5, IL-10, CXCL5 and RANTES in the conditioned media were measured by Luminex performance assays (R&D Systems Inc.). Plates were read using a Luminex 100 analyzer (PerkinElmer Life Inc.,) and xPONENT software (Luminex). The concentrations of IL-1ß varied from 5.15 to 528.39 pg/ml, those of TNF-α from 7.76 to 133.68 pg/ml, MCP-1 from 7.28 to 111.16 ng/ml, RANTES from 2266.1 to 10,221.4 pg/ml, IL-10 from 4.43 to 60.0 pg/ml, IL-4 from 5.12 to 17.0 pg/ml, IL-5 from 2.31 to 2.69 pg/ml and those of CXCL5 from 159.52 to 6579.96 pg/ml.
C-reactive protein (hs-CRP) was measured by a high-sensitivity immunoturbidimetric assay method (Cobas Mira Plus, Roche).
Gene expression in activated endothelial cells
Total RNA was isolated from HUVECs treated with 5% ATCM or SVFCM in EBM-2 with 2% fetal bovine serum in parallel-run samples. Samples of the experiment with the addition of cytokine inhibitors (IL-1β, TNF-α, and MCP-1) to ATCM stimulation were examined in a separate run. The RNeasy Plus mini kit (Qiagen) was used for RNA isolation. A total of 300 ng isolated RNA was used for reverse transcription. After DNase I (Sigma Aldrich) treatment to eliminate DNA from the RNA preparations, cDNA was generated according to the manufacturer’s instructions using the High Capacity RNA-to-cDNA Master Mix Kit (Life Technologies). Levels of gene expression of the cytokines and adipokines of interest were subsequently determined on the Corbett Life Science Rotor Gene 3000 system (Qiagen) using the 5x HOT FIREPol® EvaGreen® qPCR Mix Plus (Solise BioDyne). The nucleotide sequences of the primer pairs of MCP-1 (CCL2, C-C motif chemokine ligand 2, forward 5ʹ-agaagctgtgatcttcaagacc-3ʹ, reverse 5ʹ-agctgcagattcttgggttg-3ʹ), IL-6 (forward 5ʹ-agcagcaaagaggcactggca-3ʹ, reverse 5ʹ-tgaggaacaagccagagctgtgc-3ʹ), nitric oxide synthase 3 (NOS3, forward 5′-gtggctgtctgcatggacct-3′, reverse 5′-ccacgatggtgactttggct-3′), transforming growth factor beta 1 (TGF-β, forward 5′-tcgccagagtggttatctttt-3′, reverse 5′-tagtgaacccgttgatgtcc-3′), selectin E (SelE, forward 5ʹ-tcacagtgtttcgacagctg-3ʹ, reverse 5ʹ-gcaaatgcatggagggttgt-3ʹ), intercellular adhesion molecule 1 (ICAM-1, forward 5ʹ-tcttcctcggccttcccata-3ʹ, reverse 5ʹ-catttggggccatggtacct-3ʹ), and vascular cell adhesion molecule 1 (VCAM-1, forward 5ʹ-cctggaggaaggcagttctg-3ʹ, reverse 5ʹ-ccaggctggaagaagcagaa-3ʹ). Glyceraldehyde 3-phosphate dehydrogenase (forward 5′-agggctgcttttaactctggt-3′; reverse 5′-ccccacttgattttggaggga-3′) levels were used as endogenous controls for normalization. Relative gene expression was calculated using the ΔΔCt method [41] and compared to the respective adipose tissue.
Data analysis
Statistical analyses were performed with Prism biostatistics software, version 5 (GraphPad Prism). Levels of adhesion were compared by one-way ANOVA followed by post hoc Bonferroni’s multiple comparison test. Outliers were detected using Dixon’s Q test. The t-test or Wilcoxon sign-ranked repeated measures test were performed for relative gene expression analysis according to the Shapiro-Wilk test for normality. The comparison of paired samples (SVFCM and ATCM) was analyzed by the paired t-test. Linear regression analysis was used for correlation relationships. Probability values of less than 0.05 were considered significant.
Funding Statement
The project was supported by the Charles University Grant Agency, project GA UK No. 592216, and Ministry of Health, Czech Republic for the development of research organization 00023001 (Institute for Clinical and Experimental Medicine, Prague, Czech Republic) for institutional support. Grantová Agentura, Univerzita Karlova [592216]; Ministerstvo Zdravotnictví Ceské Republiky [00023001].
Acknowledgments
The datasets used and/or analyzed during the current study are available from the corresponding author on request.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was reported by the authors.
Author contributions
SC designed the study, processed and collected samples, performed study supervision, acquisition and interpretation of data, and drafted the manuscript. HK participated in sample processing and performance of the experiments. JF, FT and LJ performed recruitment of subjects and adipose tissue removal and contributed to critical revision of the manuscript. RP and IKL participated in developing the study concept and design, interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. All authors have read and approved the final version of the manuscript.
References
- [1].Alexopoulos N, Katritsis D, Raggi P.. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis. 2014. March;233(1):104–112. [DOI] [PubMed] [Google Scholar]
- [2].Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17(4):332–341. [DOI] [PubMed] [Google Scholar]
- [3].Libby P. Inflammation in atherosclerosis. Nature. 2002. December 26;420(6917):868–874. [DOI] [PubMed] [Google Scholar]
- [4].Gradinaru D, Borsa C, Ionescu C, et al. Oxidized LDL and NO synthesis-biomarkers of endothelial dysfunction and ageing. Mech Ageing Dev. 2015November;151:101–113. [DOI] [PubMed] [Google Scholar]
- [5].Morishita R, Ishii J, Kusumi Y, et al. Association of serum oxidized lipoprotein(a) concentration with coronary artery disease: potential role of oxidized lipoprotein(a) in the vasucular wall. J Atheroscler Thromb. 2009;16(4):410–418. [DOI] [PubMed] [Google Scholar]
- [6].Zhao WW, Wu CH, Chen XP. Cryptotanshinone inhibits oxidized LDL-induced adhesion molecule expression via ROS dependent NF-B pathways. Cell Adhes Migr. 2016;10(3):248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liao JK. Linking endothelial dysfunction with endothelial cell activation. J Clin Invest. 2013. February;123(2):540–541. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gast KB, Den Heijer M, Smit JWA, et al. Individual contributions of visceral fat and total body fat to subclinical atherosclerosis: the NEO study. Atherosclerosis. 2015. August;241(2):547–554. [DOI] [PubMed] [Google Scholar]
- [9].Kranendonk ME, van Herwaarden JA, Stupkova T, et al. Inflammatory characteristics of distinct abdominal adipose tissue depots relate differently to metabolic risk factors for cardiovascular disease: distinct fat depots and vascular risk factors. Atherosclerosis. 2015. April;239(2):419–427. [DOI] [PubMed] [Google Scholar]
- [10].Kralova Lesna I, Kralova A, Cejkova S, et al. Characterisation and comparison of adipose tissue macrophages from human subcutaneous, visceral and perivascular adipose tissue. J Transl Med. 2016. July 11;14(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cifkova R, Skodova Z, Bruthans J, et al. Longitudinal trends in major cardiovascular risk factors in the Czech population between 1985 and 2007/8. Czech MONICA and Czech post-MONICA. Atherosclerosis. 2010. August;211(2):676–681. . [DOI] [PubMed] [Google Scholar]
- [12].Ait-Oufella H, Taleb S, Mallat Z, et al. Recent advances on the role of cytokines in atherosclerosis. Arterioscl Throm Vas. 2011. May;31(5):969–979. [DOI] [PubMed] [Google Scholar]
- [13].Lin JT, Kakkar V, Lu XJ. Impact of MCP-1 in atherosclerosis. Curr Pharm Design. 2014;20(28):4580–4588. [DOI] [PubMed] [Google Scholar]
- [14].Virani SS, Nambi V, Hoogeveen R, et al. Relationship between circulating levels of RANTES (regulated on activation, normal T-cell expressed, and secreted) and carotid plaque characteristics: the Atherosclerosis Risk in Communities (ARIC) carotid MRI study. Eur Heart J. 2011. February;32(4):459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Henrichot E, Juge-Aubry CE, Pernin AS, et al. Production of chemokines by perivascular adipose tissue - A role in the pathogenesis of atherosclerosis? Arterioscl Throm Vas. 2005. December;25(12):2594–2599. [DOI] [PubMed] [Google Scholar]
- [16].Hajer GR, van Haeften TW, Visseren FLJ. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008. December;29(24):2959–2971. . [DOI] [PubMed] [Google Scholar]
- [17].Caer C, Rouault C, Le Roy T, et al. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci Rep-Uk. 2017. June 7;7. doi: 10.1038/s41598-017-02660-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Braunersreuther V, Steffens S, Arnaud C, et al. A novel RANTES antagonist prevents progression of established atherosclerotic lesions in mice. Arterioscl Throm Vas. 2008. June;28(6):1090–1096. [DOI] [PubMed] [Google Scholar]
- [19].Dalmas E, Venteclef N, Caer C, et al. T cell-derived IL-22 amplifies IL-1 beta-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes. 2014. June;63(6):1966–1977. [DOI] [PubMed] [Google Scholar]
- [20].Hivert MF, Sullivan LM, Fox CS, et al. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocr Metab. 2008. August;93(8):3165–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mtairag E, Chollet-Martin S, Oudghiri M, et al. Effects of interleukin-10 on monocyte/endothelial cell adhesion and MMP-9/TIMP-1 secretion. Cardiovasc Res. 2001. March;49(4):882–890. [DOI] [PubMed] [Google Scholar]
- [22].Verma SK, Garikipati VNS, Krishnamurthy P, et al. IL-10 accelerates re-endothelialization and inhibits post-injury intimal hyperplasia following carotid artery denudation. Plos One. 2016. 25;11(1):Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gimeno MJ, Pascual G, Garcia-Honduvilla N, et al. Modulatory role of IL10 in endothelial cell damage and platelet adhesion. Histol Histopathol. 2003. July;18(3):695–702. [DOI] [PubMed] [Google Scholar]
- [24].Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscl Throm Vas. 2007. November;27(11):2292–2301. [DOI] [PubMed] [Google Scholar]
- [25].Sans M, Panes J, Ardite E, et al. VCAM-1 and ICAM-1 mediate leukocyte-endothelial cell adhesion in rat experimental colitis. Gastroenterology. 1999. April;116(4):874–883. [DOI] [PubMed] [Google Scholar]
- [26].Hung MJ, Cherng WJ, Hung MY, et al. Interleukin-6 inhibits endothelial nitric oxide synthase activation and increases endothelial nitric oxide synthase binding to stabilized caveolin-1 in human vascular endothelial cells. J Hypertens. 2010. May;28(5):940–951. [DOI] [PubMed] [Google Scholar]
- [27].Sena CM, Pereira AM, Seica R. Endothelial dysfunction - A major mediator of diabetic vascular disease. Bba-Mol Basis Dis. 2013. December;1832(12):2216–2231. [DOI] [PubMed] [Google Scholar]
- [28].Gamble JR, Khewgoodall Y, Vadas MA. Transforming growth-factor-beta inhibits E-selectin expression on human endothelial-cells. J Immunol. 1993. May 15;150(10):4494–4503. [PubMed] [Google Scholar]
- [29].Walshe TE, Dole VS, Maharaj ASR, et al. Inhibition of VEGF or TGF-beta signaling activates endothelium and increases leukocyte rolling. Arterioscl Throm Vas. 2009. August;29(8):1185–U70. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Watson C, Whittaker S, Smith N, et al. IL-6 acts on endothelial cells to preferentially increase their adherence for lymphocytes. Clin Exp Immunol. 1996. July;105(1):112–119. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bozic M, Alvarez A, de Pablo C, et al. Impaired vitamin D signaling in endothelial cell leads to an enhanced leukocyte-endothelium interplay: implications for atherosclerosis development. Plos One. 2015. August 31;10(8). DOI: 10.1371/journal.pone.0136863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Luckett-Chastain L, Calhoun K, Schartz T, et al. IL-6 influences the balance between M1 and M2 macrophages in a mouse model of irritant contact dermatitis. J Immunol. 2016. May 1;196:17. [Google Scholar]
- [33].Kitagawa T, Yamamoto H, Hattori T, et al. Tumor necrosis factor-alpha gene expression in epicardial adipose tissue is related to coronary atherosclerosis assessed by computed tomography. J Atheroscler Thromb. 2018;25(3):269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bing C. Is interleukin-1 beta a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte. 2015. April 3;4(2):149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vicennati V, Vottero A, Friedman C, et al. Hormonal regulation of interleukin-6 production in human adipocytes. Int J Obesity. 2002. July;26(7):905–911. [DOI] [PubMed] [Google Scholar]
- [36].Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010. February;10(2):89–102. [DOI] [PubMed] [Google Scholar]
- [37].Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003. December;112(12):1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kralova Lesna I, Petras M, Cejkova S, et al. Cardiovascular disease predictors and adipose tissue macrophage polarization: is there a link? Eur J Prev Cardiol. 2018. February;25(3):328–334. [DOI] [PubMed] [Google Scholar]
- [39].Cejkova S, Kralova Lesna I, Fronek J, et al. Pro-inflammatory gene expression in adipose tissue of patients with atherosclerosis. Physiol Res. 2017. September 22;66(4):633–640. [DOI] [PubMed] [Google Scholar]
- [40].Bae JS, Kim YU, Park MK, et al. Concentration dependent dual effect of thrombin in endothelial cells via Par-1 and Pi3 kinase. J Cell Physiol. 2009. June;219(3):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9). DOI: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]


