Abstract
Pembrolizumab, a monoclonal antibody against the immune checkpoint receptor–programmed cell death protein 1, has proven clinical efficacy in melanoma and other solid tumors. It increases the body’s immune response against the tumor cells. However, because of an uninhibited immune system, immune-mediated adverse effects can arise. Though most adverse effects from pembrolizumab involve the gastrointestinal tract, skin, and endocrine system, rheumatologic manifestations are not very well defined. We describe two cases of severe inflammatory arthritis and tenosynovitis, which are rare adverse effects of pembrolizumab. Increased awareness of this manifestation is imperative to establish the diagnosis and initiate timely treatment.
Keywords: Arthritis, checkpoint blockade, immune checkpoint inhibitors, pembrolizumab
Immune checkpoint inhibition with an anti–programmed cell death protein 1 (PD-1) antibody such as pembrolizumab has improved outcomes in advanced melanoma and non–small cell lung cancer.1 Although clinical trials have documented its impressive efficacy, unwanted immune-related adverse effects (AEs), resembling autoimmune diseases, have emerged and are generating widespread interest in the medical community. These immune-related AEs typically involve the skin, intestines, and endocrine system.2 Herein, we describe two cases of inflammatory polyarthritis and tenosynovitis that developed after treatment with pembrolizumab.
CASE PRESENTATION
Case 1
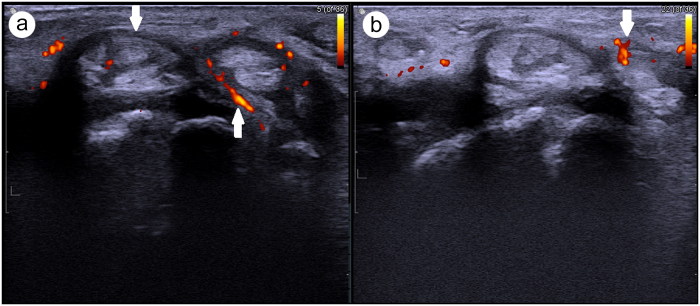
A 70-year-old white woman with stage 4 adenocarcinoma of the lung was being treated with pembrolizumab at a dose of 2 mg/kg intravenously every 3 weeks. After receiving eight cycles of pembrolizumab, she developed severe pain and swelling in her wrists, shoulders, and ankles. Laboratory evaluation showed an elevated erythrocyte sedimentation rate of 60 mm/h and C-reactive protein level of 166 mg/L (normal <10.9 mg/L). Anti-nuclear antibody, anti-SSA/SSB antibody, rheumatoid factor, anti-citrullinated peptide antibody, anti-neutrophil cytoplasmic antibody, complement 3, complement 4, and hepatitis B and C tests were negative. Plain radiographs of her hands and feet showed mild degenerative changes in multiple distal interphalangeal joints. Musculoskeletal ultrasound showed markedly active tenosynovitis in the bilateral dorsal, medial, and lateral compartment tendons (Figure 1). She was treated with a short course of indomethacin for 1 week and prednisone taper with a starting dose of 15 mg daily. Hydroxychloroquine (200 mg twice daily) was added at 6 weeks as a steroid-sparing drug. She showed a significant response to the therapy, with reduced signs of inflammation in her joints. At 6-month follow-up, she remained asymptomatic while she continued pembrolizumab.
Figure 1.
Transverse ultrasound of the dorsal (a) right wrist and (b) left wrist showing thickening and edema of tendons with increased blood flow on Doppler, indicating active tenosynovitis (white arrow).
Case 2
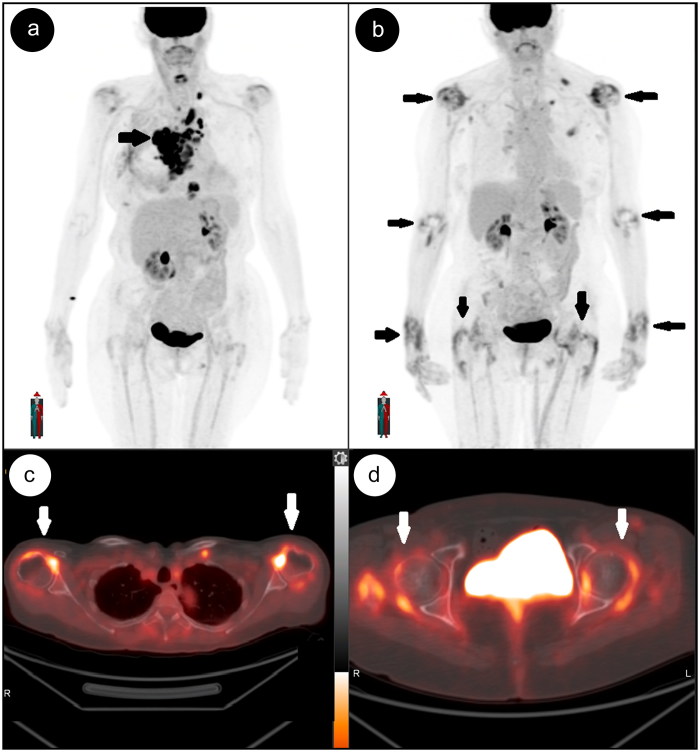
A 68-year-old white woman presented with symptoms of pain and swelling in the wrists, elbows, shoulders, feet, ankles, and hips. She had a past history of seronegative inflammatory arthritis in these joints but was in remission. After failing several therapies for Hodgkin’s lymphoma, the treatment was switched to pembrolizumab and her joint symptoms recurred after receiving seven cycles. Laboratory evaluation showed an erythrocyte sedimentation rate of 2 mm/h and a C-reactive protein level of 36 mg/L. Anti-SSA/SSB antibodies, rheumatoid factor, and anti-cyclic citrullinated peptide antibodies were negative. Plain radiographs of her hands and feet were normal; however, musculoskeletal ultrasound showed severe tenosynovitis of the extensor carpi ulnaris. Grade II to III synovial hypertrophy and hyperemia were noted in the bilateral dorsal wrists, multiple bilateral metacarpophalangeal joints, and proximal interphalangeal joints. A fluorodeoxyglucose positron emission tomography scan performed for cancer surveillance showed multifocal areas of increased uptake surrounding the joints of the shoulders, elbows, hands, and hips (Figure 2). Pembrolizumab was discontinued and she was treated with 30 mg of prednisone daily, followed by addition of hydroxychloroquine (400 mg daily) and sulfasalazine (1 g twice daily) as steroid-sparing agents, with good clinical improvement in synovitis.
Figure 2.
(a) Baseline positron emission tomography maximum intensity projection showing multiple metabolically active lesions in the chest consistent with known lymphoma. (b) Follow-up maximum intensity projection image and (c, d) axial fused positron emission tomography-computed tomography images of shoulder and hip joints after treatment with pembrolizumab demonstrate complete resolution of lymphoma; however, there are new areas of periarticular uptake involving both shoulders and the elbows, wrists, and knee joints (black and white arrows).
DISCUSSION
Cancer therapy has radically improved in the past decade with development of immune checkpoint treatments.3 Despite good clinical outcomes, their use might be limited because of immune-related AEs. The most common AEs associated with pembrolizumab have been reported to be fatigue, itching, diarrhea, and rash.4 Inflammatory arthritis and tenosynovitis associated with pembrolizumab are unusual. In the earlier clinical trials, arthralgia occurred in 7% of patients; however, no arthritis or tenosynovitis was reported.5 Most cases of arthritis with pembrolizumab have emerged as incidental case reports. A systematic review of published cases (n = 251) noted only 10 cases of pembrolizumab-related immune-related AE and, of these, two patients experienced polyarthritis.6 Another recent systematic review and meta-analysis reported arthritis with anti-programmed death-ligand 1 agents at a rate below 1%, indicating its rarity.7 The optimal treatment of immune-related AEs remains uncertain and should be determined on a case-by-case basis. Temporary discontinuation of pembrolizumab may be needed. Consensus recommendations on management of arthritis include use of corticosteroids, nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, and anticytokine therapy for severe cases.8
In conclusion, our report highlights rare immune-related AEs from pembrolizumab treatment. Clinicians should be aware of the possible development of inflammatory polyarthritis and tenosynovitis. As our familiarity with immune checkpoint blockade agents continues to increase, collaboration with oncologists is needed for identification of immune-related AEs and planning of the optimal treatment strategy for the patient.9
References
- 1.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21:24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michot JM, Bigenwald C, Champiat S, et al. . Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Day D, Hansen AR. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs. 2016;30:571–584. doi: 10.1007/s40259-016-0204-3. [DOI] [PubMed] [Google Scholar]
- 5.Ribas A, Puzanov I, Dummer R, et al. . Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One. 2016;11:e0160221. doi: 10.1371/journal.pone.0160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxi S, Yang A, Gennarelli RL, et al. . Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793. doi: 10.1136/bmj.k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puzanov I, Diab A, Abdallah K, et al. . Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Champiat S, Lambotte O, Barreau E, et al. . Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27:559–574. doi: 10.1093/annonc/mdv623. [DOI] [PubMed] [Google Scholar]