Abstract
A heritable condition is the identified cause of cancer in 5% to 10% of women with breast cancer and in 25% of women with ovarian cancer. It is critical to identify patients at risk for inherited genetic mutations to implement risk-reducing screening and interventions; however, reports in the medical literature indicate that an alarming number of patients with inherited genetic mutations do not receive recommended genetic counseling, testing, or interventions. In order to improve outcomes for these high-risk patients, barriers to genetic testing and counseling must be identified. We analyzed approximately 200 patients seen at our institution with breast or ovarian cancer who met criteria of the National Comprehensive Cancer Network for genetic counseling and testing. Of these patients, almost 70% had appropriate genetic testing and counseling. Review of the remaining 30% revealed that the largest obstacle to receiving genetic testing and/or counseling was lack of referral from the treating oncologist. Of the patients diagnosed with a pathogenic heritable mutation, most underwent appropriate risk-reducing procedures and surveillance. Thus, the initial referral to genetic counseling is the most significant barrier for at-risk patients at our institution and likely in this population at large. Additional study is needed to identify ways to improve appropriate use of genetic testing and counseling.
Keywords: Barriers to utilization, BRCA mutation, genetic counseling, hereditary breast and ovarian cancer
The National Comprehensive Cancer Network (NCCN) guidelines outline criteria for who should be offered genetic counseling and testing based on patients’ personal and/or family history of breast or ovarian cancer.1 About 5% to 10% of breast cancer cases and up to 25% of ovarian cancer cases are a result of an inherited gene mutation. Thus, thousands of patients each year may be predisposed for another primary cancer and have relatives who may also carry this gene mutation.2,3 Genetic counseling specializes in identifying individuals at genetic risk for cancer, ordering genetic testing for patients and family members, and recommending further screening and risk reduction strategies based on each person’s cancer risk.4 Unfortunately, genetic counseling is underutilized in oncology. One study found that only 8% of women who met NCCN guidelines for genetic counseling for hereditary breast cancer actually completed this counseling 1 year after physician referral.5 A similar report found that <50% of 1711 early stage patients with breast cancer with indications for genetic risk received counseling.6 However, we have yet to identify barriers to use of genetic counseling. Thus, we conducted a retrospective chart review to determine whether our patients with breast or ovarian cancer who met NCCN criteria received the appropriate genetic counseling, testing, and/or risk reduction management and we sought to identify barriers to patients getting this treatment.
METHODS
The Baylor Charles A. Sammons at Dallas Cancer Registry was used to identify patients suspected of being at risk for hereditary breast or ovarian cancers. We identified 211 patients diagnosed with breast or ovarian cancer between July 1, 2013, and June 30, 2016. All patients met at least one of the following NCCN criteria: (1) women aged ≤45 years diagnosed with breast cancer (including ductal carcinoma in situ); (2) women aged ≤60 diagnosed with triple-negative breast cancer; (3) men of any age diagnosed with breast cancer; (4) patients diagnosed with one breast cancer at age ≤50 years with a second primary breast cancer at any age; and/or (5) women diagnosed with ovarian cancer at any age. Using electronic health records (EHRs), we identified patients who underwent genetic counseling with or without genetic testing. For those who had a pathogenic germline mutation, further EHR review was performed to determine whether they underwent appropriate heightened surveillance or prophylactic surgeries, as recommended by the NCCN guidelines.1 Patients were excluded if they were diagnosed in our facility but received care at an outside center, they were lost to follow-up, or they presented to our institution once for a second opinion. We also excluded patients who died within 3 months of their cancer diagnosis and thus did not have reasonable time to receive appropriate genetic counseling and testing. Approval for the study was obtained from the Baylor Scott and White Research Institute Institutional Review Board.
Patient demographic information including sex, race/ethnicity, medical insurance status, age at diagnosis, tumor histology, tumor stage, hormone receptor status, type of genetic test used, genetic mutation status, use of medical management recommendations, and genetic discussion modality was collected for the 211 patients in the study. Data were analyzed for utilization of genetic counseling/testing results in our institution and to identify areas for improvement.
RESULTS
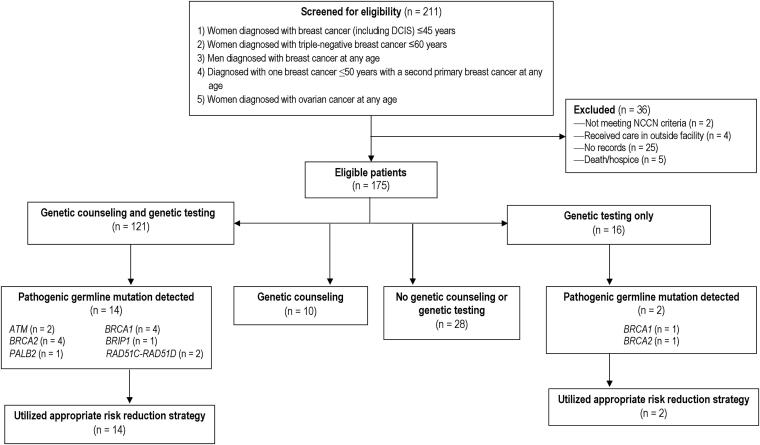
A total of 211 patient records were evaluated from the patient population identified in the cancer registry. Thirty-six patients were deemed ineligible for the study: 4 who received outside care, 5 who died prior to planned genetic counseling, 2 who did not meet NCCN criteria for testing, and 25 for whom we could not find records (Figure 1). A total of 175 patients were deemed eligible based on our inclusion criteria. As shown in Table 1, the patients were overwhelming female (98.3%). At the time of diagnosis, 52% were ≤45 years old, and 13.1% were ≥70 years old. Most patients (65.7%) were Caucasian, and most (80%) had private health insurance. Multigene panel testing through various commercial genetic laboratories was used for 65.1% of patients, and testing only for BRCA1 and BRCA2 mutations was performed for 13.1%. In our cohort, 21% did not undergo any type of genetic testing (Table 1).
Figure 1.
Flow diagram depicting patient screening, inclusion/exclusion, and results of analysis for primary findings. DCIS indicates ductal carcinoma in situ; NCCN, National Comprehensive Cancer Network.
Table 1.
Demographic characteristics of 175 patients eligible for genetic testing
| n | % | |
|---|---|---|
| Gender | ||
| Female | 172 | 98.3% |
| Male | 3 | 1.7% |
| Ethnicity | ||
| African American | 36 | 20.6% |
| Asian | 3 | 1.7% |
| Latino/a | 19 | 10.9% |
| White | 115 | 65.7% |
| Other/unknown | 2 | 1.1% |
| Insurance status | ||
| Private | 140 | 80.0% |
| Medicaid | 4 | 2.3% |
| Medicare or Veterans Affairs | 25 | 14.3% |
| No insurance | 6 | 3.4% |
| Age at diagnosis (years) | ||
| ≤45 | 91 | 52.0% |
| 46–49 | 12 | 6.9% |
| 50–59 | 31 | 17.7% |
| 60–69 | 18 | 10.3% |
| ≥70 | 23 | 13.1% |
| Genetic test type | ||
| No test performed | 38 | 21.7% |
| BRCA 1/2 testing only | 23 | 13.1% |
| Multigene panel | 114 | 65.1% |
| Genetic test outcome | ||
| Not tested | 38 | 21.7% |
| Negative | 104 | 59.4% |
| Variant of unknown significance only | 17 | 9.7% |
| Pathogenic mutation | 16 | 9.1% |
| Genetic discussion modality | ||
| Formal counseling (genetic counselor) | 124 | 70.9% |
| Physician-directed discussion | 7 | 4.0% |
| No counseling | 44 | 25.1% |
Sixteen patients (9.1%) had a pathogenic gene mutation associated with hereditary breast and/or ovarian cancer. In these 16 patients, we identified 2 ATM mutations, 5 BRCA1 mutations, 5 BRCA2 mutations, 1 BRIP1 mutation, 1 PALB2 mutation, 1 RAD51C mutation, and 1 RAD51D mutation (Table 2). Fourteen patients with pathogenic mutations received genetic counseling, either by a genetic counselor or a physician. Among those who underwent testing, 104 did not have an identifiable pathogenic gene mutation associated with hereditary breast or ovarian cancer, and 17 had a variant of unknown significance (Table 3).
Table 2.
Outcomes of patients with pathogenic mutations
| Patient number | Criteria met | Genetic mutation | Genetic counseling | Risk reduction mastectomy | Risk reduction salpingo-oophorectomy | Heightened surveillance (breast, skin, pancreas) | Familial risk counseling |
|---|---|---|---|---|---|---|---|
| 1 | Ovarian cancer | ATM | PDC | NA | * | X | ND |
| 2 | Ovarian cancer | ATM | GCC | NA | * | X | X |
| 3 | Breast cancer, ≤60 years TNBC | BRCA1 | GCC | * | ND | X | X |
| 4 | Ovarian cancer | BRCA1 | GCC | X | * | NA | X |
| 5 | Breast cancer, ≤60 years TNBC | BRCA1 | GCC | * | ND | X | X |
| 6 | Breast cancer, ≤45 years | BRCA1 | GCC | * | ND | X | X |
| 7 | Breast cancer, ≤45 years | BRCA1 | ND | * | X | NA | ND |
| 8 | Breast cancer, ≤45 years | BRCA2 | GCC | * | ND | X | X |
| 9 | Breast cancer, ≤45 years | BRCA2 | GCC | * | X | X | X |
| 10 | Breast cancer, ≤45 years | BRCA2 | ND | * | X | ND | ND |
| 11 | Breast cancer, ≤45 years | BRCA2 | GCC | * | X | X | X |
| 12 | Breast cancer, ≤60 years TNBC | BRCA2 | GCC | * | X | X | X |
| 13 | Ovarian cancer | BRIP1 | PDC | NA | * | NA | ND |
| 14 | Breast cancer, ≤45 years | PALB2 | GCC | * | NA | NA | X |
| 15 | Ovarian cancer | RAD51C | PDC | * | X | NA | ND |
| 16 | Breast cancer, ≤60 years TNBC | RAD51D | GCC | NA | * | NA | X |
*Procedure completed as part of initial cancer treatment.
GCC indicates genetic counselor counseling; NA, not applicable; ND, not done; PDC, physician-directed counseling; TNBC, triple-negative breast cancer; X, procedure completed as part of risk-reduction strategy.
Table 3.
Distribution of genetic discussion modality by patient’s genetic testing and/or results
| Genetic discussion modality | No genetic testing | Negative genetic testing | VUS | Pathogenic mutation detected |
|---|---|---|---|---|
| Formal counseling (genetic counselor) | 9 | 88 | 16 | 11 |
| Physician-directed discussion | 1 | 2 | 1 | 3 |
| No counseling | 28 | 14 | 0 | 2 |
| Total | 38 | 104 | 17 | 16 |
VUS indicates variant of unknown significance.
Of the study’s 175 eligible patients, 69% underwent genetic counseling and testing, 5% underwent genetic counseling only, 9% underwent genetic testing only, and 16% did not pursue genetic testing or counseling (Figure 1). Overall, 124 patients (71%) received genetic counseling from a certified genetic counselor, 7 patients (4%) received physician-directed discussions regarding their genetic risk, and 44 patients (25%) did not receive any genetic counseling (Figure 1, Table 3).
DISCUSSION
We showed that appropriate use of genetic counseling and testing as recommended by NCCN guidelines occurred for ∼70% of our patients, which exceeds reported rates.6 This difference from earlier studies may be due to our institution’s well-established genetic counseling department, which was created in 1998. In addition, our genetic counselors play an active role in multidisciplinary tumor boards and conferences, where they are involved in patient care discussions. Increasing accessibility and roles of genetic counselors to community oncologists and their patients would likely expand their utilization. When certified genetic counselors were added to on-site staff, there was increased counselor use and testing.7
Most patients who did not undergo genetic counseling were not referred by their physician. Only two patients declined genetic counseling and/or testing because of personal preference and/or cost. Lack of timely physician referrals was the single most important barrier to use of certified genetic counselors and testing. This finding prompted our program to re-examine efforts toward physician education on NCCN guidelines for genetic testing for patients with breast or ovarian cancer. One means was a “pocket guide” created for referring physicians with all indications warranting referral for genetic counseling and testing (Online Supplement). We have ongoing plans for increased cooperation between physicians and genetic counselors, including use of EHRs for referrals. With better use of current NCCN guidelines, oncologists and other providers will be able to ensure that more at-risk patients and their families receive suitable risk management.
Previous studies showed that patient race affected genetic counseling use, with African Americans receiving more counseling than Caucasian patients.6 In contrast, we did not identify any differences based on race in genetic counseling or testing. However, we did see a trend when data were adjusted for age; most patients who did not undergo genetic counseling were over the age of 50. Katz et al showed that younger women (<50 years of age) were more likely to have a genetic discussion than were those >70 years of age.6 The occurrence of cancer in younger patients raises suspicion for a heritable condition, prompting immediate referral to genetic counseling, whereas older patients are thought to develop more sporadic cancers, making them less likely to receive genetic testing. However, if older patients still meet NCCN guidelines, they should be assessed for additional cancer risk and familial risk for several types of cancer. We would caution practicing oncologists and genetic counselors to be aware of this subset of at-risk individuals, despite tendencies to attribute malignancy to age.
Most of our patients were privately insured or had Medicare; few were uninsured. There was no difference in genetic counseling based on insurance. This may be due to patient financial assistance programs offered by our genetic counseling group and commercial genetic testing laboratories. Our findings were in contrast to other studies, where patients receiving Medicaid received less genetic counseling than privately insured patients.6 Financial barriers exist for cancer patients who need genetic counseling/testing nationally; however, funding assistance programs may be the best way to obtain correct testing for patients unable to afford out-of-pocket costs.
Outcomes of patients with identified heritable mutations who underwent genetic counseling were in line with recommendations. They were advised to undergo risk-reducing procedures, heightened surveillance, and familial risk assessment. When patients were divided based on whether they received counseling from a certified genetic counselor or nongeneticist physician, both groups had similar compliance with recommendations. However, there was a difference in whether the topics of family planning and family member risk were addressed. More often, physician-directed counseling did not address these two important components. Certified genetic counselors may be better able to follow variant of unknown significance updates and notify patients and providers of important changes in guidelines and genetic testing. Thus, every patient with indications for genetic evaluation should see a certified genetic counselor to ensure full assessment of risk to patient and family, in addition to or in place of talks with their physician.
One limitation of this study was the relatively small sample size. In addition, we could not identify patients meeting NCCN criteria for genetic testing/counseling based on family history. This information is not in the EHR, so we likely missed many patients suitable for genetic testing and counseling. We think that patients meeting criteria based on family history are at greatest risk, because family history is often incomplete due to patient knowledge or clinician documentation. To support this, we had no patients with a CHEK2 mutation despite the statistical likelihood of having one. Based on penetrance and inheritance patterns of CHEK2 mutations, family history may have identified these patients if family history were part of the inclusion criteria. A different approach to analysis may be needed to identify this group of often overlooked patients.
Another limitation of our study is that we based our analysis on NCCN guidelines published in October 2017. Our study population included patients diagnosed between July 2013 and June 2016. The guidelines at the time of their presentation may not match the 2017 NCCN guidelines we used. Nevertheless, physicians and genetic counselors must play an ongoing role in performing updated surveillance risk reduction strategies, because guidelines are frequently revised. In addition, no data were collected on timeliness of genetic testing and counseling for these patients. With development of new drugs for neoadjuvant treatment of patients with BRCA mutation breast cancer, timely identification of germline mutations is increasingly important.8
In summary, despite a well-established, accessible genetic counseling program, many high-risk patients do not receive referrals for appropriate evaluation. Further study is needed to set up best practices to ensure consistent detection of at-risk patients for genetic testing and counseling. Quality improvement projects have begun to increase physician awareness of NCCN guidelines and referrals to genetic counselors. Nationwide, use of Internet-based counseling would improve genetic counselor access; this is an ideal method for long-distance, real-time discussions, resulting in improved timeliness of referral to genetic counseling and receipt of testing results. Detection of a heritable mutation early in a patient’s treatment course is vital to planning and patient outcomes. The role of genetic counselors is an increasingly important component of successful oncologic care, and we need to overcome institutional barriers to their use.
Supplementary Material
SUPPLEMENT MATERIALS
Indications for Referrals for Genetic Counseling: Hereditary Cancer Risk Program. Dallas, TX: Baylor Scott & White Health, 2019. This supplement is available at https://doi.org./10.1080/08998280.2019.1612702.
References
- 1.Daly MB, Pilarski R, Berry M, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9–20. doi: 10.6004/jnccn.2017.0003. [DOI] [PubMed] [Google Scholar]
- 2.Nelson HD, Huffman LH, Fu R, Harris EL; US Preventive Services Task Force . Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2005;143:362–379. doi: 10.7326/0003-4819-143-5-200509060-00012. [DOI] [PubMed] [Google Scholar]
- 3.Kurian AW, Ward KC, Hamilton AS, et al. Uptake, results, and outcomes of germline multiple-gene sequencing after diagnosis of breast cancer. JAMA Oncol. 2018;4:1066–1072. doi: 10.1001/jamaoncol.2018.0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berliner JL, Fay AM. Risk assessment and genetic counseling for hereditary breast and ovarian cancer: recommendations of the National Society of Genetic Counselors. J Genet Couns. 2007;16:241–260. doi: 10.1007/s10897-007-9090-7. [DOI] [PubMed] [Google Scholar]
- 5.Kne A, Zierhut H, Baldinger S, et al. Why is cancer genetic counseling underutilized by women identified as at risk for hereditary breast cancer? Patient perceptions of barriers following a referral letter. J Genet Couns. 2017;26:697–715. doi: 10.1007/s10897-016-0040-0. [DOI] [PubMed] [Google Scholar]
- 6.Katz SJ, Ward KC, Hamilton AS, et al. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J Clin Oncol. 2018;36:1218–1224. doi: 10.1200/JCO.2017.76.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pederson HJ, Hussain N, Noss R, et al. Impact of an embedded genetic counselor on breast cancer treatment. Breast Cancer Res Treat. 2018;169:43–46. doi: 10.1007/s10549-017-4643-4. [DOI] [PubMed] [Google Scholar]
- 8.Litton JK, Scoggins M, Ramirez DL, et al. A feasibility study of neoadjuvant talazoparib for operable breast cancer patients with a germline BRCA mutation demonstrates marked activity. NPJ Breast Cancer. 2017;3:49. doi: 10.1038/s41523-017-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.