Structured Summary
Background:
Universal skin and nasal decolonization reduces multidrug-resistant pathogens and bloodstream infections in intensive care units. The effect of universal decolonization on pathogens and infections in non-critical care units is unknown.
Methods:
A two-arm cluster-randomized trial was conducted in 53 hospitals in the Hospital Corporation of America Healthcare system. Hospitals were randomized and their participating non-critical care units assigned to either routine care or daily Chlorhexidine bathing for all patients plus mupirocin for known methicillin-resistant Staphylococcus aureus (MRSA) carriers. The trial involved a 12-month baseline (Mar 2013-Feb 2014) and 21-month intervention (Jun 2014-Feb 2016). Primary outcome was MRSA or vancomycin-resistant enterococcus (VRE) clinical cultures attributed to participating units, and secondary outcome was all-pathogen bloodstream infection. Proportional hazards models assessed differences in outcome reductions across arms, accounting for clustering by hospital.
Findings:
There were 189,081 patients in the baseline period and 339,902 patients (156,889 patients in the routine care group and 183,013 patients in the decolonization group) in the intervention period across 194 non-critical care units in 53 hospitals. Hazard ratios for MRSAA/RE clinical isolates during intervention vs. baseline periods were 0·87 (CI:0·79, 0·95) for routine care, and 0·79 (CI:0·73, 0·87) for decolonization (ρ=0·17), and 0·96 (CI:0·85, 1·08) and 0·90 (0·81, 1·01) for all-pathogen bloodstream infection, respectively (p=0·44). Adverse events were rare (1 in >7,000; 25/183,013) for Chlorhexidine and none were reported for mupirocin.
Interpretation:
Decolonization with universal Chlorhexidine bathing and targeted mupirocin for MRSA carriers did not reduce multidrug-resistant organisms or all-pathogen bloodstream infection in all non-critical care patients.
Trial Registration:
clinicaltrials.gov Identifier:
Introduction
Extensive reductions in healthcare-associated infections (HAIs) have been achieved in the United States, largely due to successful infection prevention efforts in intensive care units (ICUs).1 Investments in HAI reduction in ICUs have led to several multi-center ICU trials of infection prevention strategies.2–13 Notably, several recent trials of universal decolonization involving daily Chlorhexidine (CHG) bathing with and without nasal mupirocin prompted widespread adoption of this practice in ICUs, due to evidence that universal decolonization reduces device-associated bacteremia, all-cause bacteremia, and multidrug-resistant organisms (MDROs).7–13
Although the patient-specific risk is highest in ICUs, most HAIs occur outside the ICU setting because the populations are so much larger. Questions remain as to whether ICU-proven strategies should be applied across the entire hospital due to the potential for less impact and higher cost-to-benefit ratios.
We now report the results of a trial in non-critical care units evaluating an intervention similar to one that was found to reduce MDRO infection and bacteremia in ICUs.7
Methods
Study Design
The ABATE Infection Trial (Active Bathing to Eliminate Infection) was a 2-arm cluster-randomized trial of hospitals comparing routine bathing to decolonization in non-critical care units. The trial consisted of a 12-month baseline period from March 1, 2013-Feb 28, 2014, a 2-month phase-in period from April 1, 2014-May 31, 2014, and a 21-month intervention period from June 1, 2014-February 29, 2016. The two strategies included:
Routine Bathing: Non-critical care units in these hospitals continued to use their routine non-antiseptic disposable cloths for bed bathing, and liquid soap for showering at their usual frequency. This arm was considered standard-of-care. (See Routine Care Protocol in online supplement.)
Decolonization: Non-critical care units in these hospitals had routine soap exchanged for 4% rinse-off liquid CHG in the shower and 2% leave-on CHG disposable cloths for bed baths. Daily bathing or showering was encouraged. Post-showering application of 2% leave-on CHG to wounds and devices was included as part of protocol training. In addition, patients known to the hospital to be MRSA carriers (by reported history, prior culture result, or information from transferring facilities) received twice-daily nasal 2% mupirocin ointment for five days while on a participating unit because the combination of mupirocin plus CHG has been shown to effective for reducing colonization and infection due to MRSA.7,14–15 (See Decolonization Protocol in online supplement.)
Recruitment and Eligibility Criteria
Recruitment occurred in the last quarter of 2012 among the 158 hospitals in the Hospital Corporation of America (HCA) Healthcare system. HCA hospitals account for 5% of U.S. hospitalizations and nearly all are community hospitals. Eligibility criteria included hospitals with adult non-critical care medical, surgical, mixed medical/surgical, oncology, and step-down units. Bone marrow transplant, peri-partum care, psychiatry, pediatric, and acute rehabilitation units were excluded from being study units within participating hospitals. Participating hospitals were required to have stable infection prevention initiatives and products during the baseline period, and to agree to refrain from new initiatives conflicting with the trial.
Central IRB approval was obtained from Harvard Pilgrim Health Care with a waiver of informed consent. All participating hospitals formally ceded IRB oversight to the HPHC IRB, except for the designated medical center IRB (Chippenham & Johnston Willis Hospitals) that provided prisoner oversight for the trial. This trial was registered at clinicaltrials.gov (Identifier: ).
Cluster Randomization
The unit of randomization was the hospital, with all participating units in the hospital assigned to the same arm. Randomization occurred in November 2013, prior to the start of the intervention period, and used the first four months of baseline data to establish similar hospital pairs. Pairing was done by calculating the Mahalanobis distance between facilities across baseline values of equally-weighted key variables and choosing pairings with the minimum average within-pair distance.16 A single pseudo-random number uniformly distributed between 0 and 1 was generated for each pair. If it was less than 0.5, the arbitrary “first” member of the pair was assigned to Routine Care and the other to Decolonization. If it was greater than or equal to 0.5, then then assignments were reversed. The remaining unpaired hospital of the 53 participants was assigned as the “first” member of a pair with no match.
Key variables included:
annual admissions and attributable patient days, percent surgical patients, percent cardiac/orthopedic surgery patients, prevalence of MRSA or VRE, Romano comorbidity score,17 and baseline rates of MRSA and VRE clinical cultures and all-pathogen bacteremia attributable to participating units.
Implementation
On-site implementation for the Decolonization Arm was performed by hospital personnel responsible for local quality improvement processes, including infection prevention personnel and unit managers and directors. Usual communication channels and implementation methods for quality improvement initiatives were used, including computer-based training, daily electronic charting of bathing compliance in routine nursing documentation systems, and charting of each mupirocin administration in medication records. Decolonization Arm hospitals received staff and patient educational materials, static-cling posters for each patient’s room to encourage daily baths, and waterproof step-by-step instructions were posted in every shower. On-site training was provided for use of 2% CHG-impregnated cloths, with emphasis on comprehensive bathing, including cleansing of superficial wounds and devices within six inches of the body.18 In addition to the daily charting of bathing compliance, nursing leaders of participating units observed three CHG bed baths per quarter and obtained three patient self-assessments on bathing using a provided checklist to visually assess protocol adherence. These skills assessments were used to tailor further unit training on the protocol. All skin and prophylactic (non-treatment) wound products were confirmed to be CHG compatible, and adverse events were managed by treating physicians.
Investigators held arm-specific coaching calls monthly to discuss implementation, protocol adherence, and verify that new initiatives were disclosed for assessment of trial conflict. Hospital study champions from both arms received feedback reports to encourage adherence (avoidance of decolonization for the Routine Care Arm and application of decolonization for the Decolonization Arm). Both arms received reminders about documenting bathing, including feedback for missed nursing documentation, as well as special presentations that reviewed national best practice for infection prevention.
Study Outcomes
The primary study outcome was combined MRSA or VRE clinical cultures attributable to a participating unit. This included positive cultures from any body site with the exception of screening cultures, such as nasal surveillance cultures for MRSA or rectal surveillance cultures for VRE. There were two secondary outcomes, specified a priori. The first was multidrug-resistant Gram-negative rods (MDR-GNR) clinical cultures, and the second was all-pathogen bloodstream infection attributable to a participating unit. MDR-GNR was defined as the combination of extended-spectrum beta-lactamase producers (ESBLs), carbapenem-resistant enterobacteriaceae (CRE), acinetobacter species resistant to all third and fourth generation cephalosporins plus extended-spectrum penicillins with beta-lactamase inhibitors, and pseudomonas resistant to aztreonam, all third and fourth generation anti-pseudomonal cephalosporins, plus extended-spectrum penicillins with beta-lactamase inhibitors. Consistent with planned analyses, only the first event per patient was assessed for each outcome. We performed post-hoc analyses of the primary and bloodstream infection outcomes in four subgroups: 1) patients in hospitals with outcomes in the highest quartile of MRSA/VRE clinical culture rates in the baseline period, 2) patients with devices (central venous catheters (including accessed ports), midline catheters, or lumbar drains), 3) patients in dedicated oncology units, and 4) MRSA carriers, since those patients received mupirocin in addition to CHG based upon the trial protocol.
Data Collection and Outcome Assignment
Census, microbiology, pharmacy, supply chain, nursing documentation, and administrative data were obtained from the HCA centralized clinical data warehouse. For microbiologic outcomes (first per patient), pathogens were attributed to a participating unit if the collection date occurred >2 calendar days after unit admission through 2 days after unit discharge, consistent with CDC guidance for surveillance of hospital-associated infections.19 Skin commensals in blood cultures were only considered bloodstream infections if CDC criteria were met.20
Statistical Analysis
We powered the trial on the rarest outcome, all-pathogen bloodstream infection. With 53 hospitals and 21-months of follow-up, we had 89% power to detect a two-tailed 20% difference in the Decolonization Arm versus the Routine Care Arm. One year into the intervention period, the trial was elongated from 18 months to 21 months following reassessment of power based upon the full 12 months of baseline data compared to an initial assessment involving 4 months of baseline data.21 No data from the intervention period was accessed during this reassessment of power.
Main trial results were assessed using intent-to-treat, unadjusted analyses. Proportional hazards models with shared frailties were used to account for clustering within hospital.22–23 Trial success was determined by the significance of the arm-by-treatment period interaction, which assessed whether the difference in hazard ratio (HR) between the baseline and intervention periods differed significantly between the arms. As a conservative approach, we ignored the pair-matching performed in randomization in the data analysis.24 Phase-in period data were excluded from all analyses.
We additionally conducted adjusted and as-treated analyses. Adjusted models accounted for individual age, gender, race, MDRO history, Medicaid insurance, prior nursing home stay within 90 days of admission, Elixhauser comorbidity index, unit type (medical, surgical, mixed medical-surgical, oncology, and step-down), transplant hospital, and surgery during admission. All analyses were performed using SAS 9–3 (SAS Institute, Cary NC).
Role of the Funding Source
The funder of the study had no decision-making authority regarding study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author (SSH), programmer analyst (TRA), and statistician (KK) had full access to all the data and all authors were responsible for the decision to submit for publication.
Results
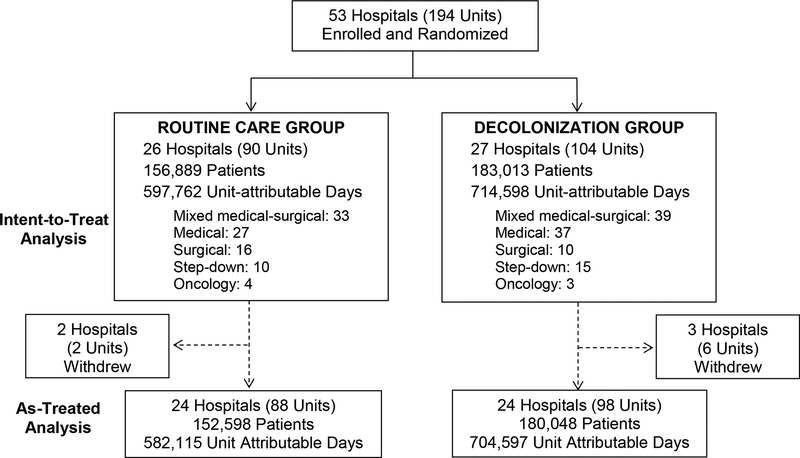
We randomized 53 hospitals in 14 states. Collectively, participating hospitals represented 64 medical, 26 surgical, 72 mixed medical-surgical, 7 oncology, and 25 step-down units (Figure 1). Five hospitals withdrew after the intervention started: two hospitals in the Routine Care Arm (due to competing interventions), and three hospitals in the Decolonization Arm (due to a competing intervention, closure of the only participating unit, and hospital divestiture from HCA). All available trial period data from the HCA centralized clinical data warehouse (including time after early exit from the trial for the three hospitals with ongoing data from previously participating units) was included in the intent-to-treat analyses, and accrued participation time is included in as treated analyses.
Figure 1. ABATE Infection Trial CONSORT Diagram.
CONSORT diagram showing the recruitment and randomization process for the ABATE Infection Trial. N indicates the number of patients in the intervention period in each arm with their associated unit-attributable days, defined as patient days occurring from 3 days in the participating unit through two days after unit discharge if still hospitalized.
There were 189,081 patients in the baseline period and 339,902 patients in the intervention period. Patient characteristics were highly similar across arms, but the Routine Care Arm had more patients with chronic pulmonary disease and a history of VRE, while the Decolonization Arm had more patients with medical devices (Table 1). The distribution of medical device types is found in Appendix A.
Table 1.
ABATE Infection Trial Population Characteristics
| Baseline 12 months N = 189,081 |
Intervention 21 months N = 339,902 |
|||||
|---|---|---|---|---|---|---|
| Variable | Routine Care | Decolonization | Routine Care | Decolonization | ||
| % | % | % | % | |||
| Admissions (N) | 87,277 | 101,804 | 156,889 | 183,013 | ||
| Unit Attributable Patient Days a | 320,938 | 382,932 | 597,762 | 714,598 | ||
| Hospital Stay in Days (Median (IQR)) | 5·0 (4·0–7·0) | 5·0 (4·0–8·0) | 5·0 (4·0–8·0) | 5·0 (4 0–8 0) | ||
| Participating Unit Stay in Days (Median (IQR)) | 4·0 (3 0–6 0) | 4·0 (3·0–7·0) | 4·0 (3·0–6·0) | 4·0 (3 0–7 0) | ||
| Age in Years (Mean (SD)) | 62·6 (182) | 62·9 (18·4) | 62·3 (182) | 62·6 (186) | ||
| Female b | 54·1 | 54·8 | 53·9 | 54·8 | ||
| Race c | ||||||
| White | 70·2 | 64·0 | 68·4 | 61·2 | ||
| Hispanic c | 12·4 | 19·0 | 14·4 | 21·1 | ||
| Black | 13·2 | 11·7 | 13·7 | 12·3 | ||
| Asian | 1·0 | 1·7 | 1·3 | 2·3 | ||
| Other/Unknown | 3·3 | 3·7 | 2·2 | 3·1 | ||
| Insurance | ||||||
| Medicare | 57·1 | 57·0 | 56·1 | 56·6 | ||
| Commercial | 21·3 | 23·6 | 19·7 | 21·7 | ||
| Medicaid | 9·2 | 7·9 | 11·3 | 9·3 | ||
| Other | 12·3 | 11·1 | 10·7 | 1·00 | ||
| Unknown | 0·1 | 0·3 | 2·2 | 2·3 | ||
| Comorbidities | ||||||
| Diabetes | 28·5 | 29·6 | 29·4 | 30·3 | ||
| Chronic Pulmonary Disease | 20·9 | 18·1 | 20·9 | 17·7 | ||
| Anemia | 21·1 | 22·9 | 20·4 | 22·4 | ||
| Renal Failure | 14·2 | 14·5 | 13·9 | 14·7 | ||
| Obesity | 14·0 | 14·9 | 13·8 | 15·2 | ||
| Congestive Heart Failure | 9·2 | 8·3 | 9·1 | 8·4 | ||
| Peripheral Vascular Disease | 6·5 | 6·9 | 6·0 | 6·3 | ||
| Liver Disease | 3·3 | 3·7 | 3·2 | 3·6 | ||
| Rheumatologic Disease | 2·9 | 3·2 | 2·8 | 2·9 | ||
| Cancer | 4·7 | 5·3 | 4·4 | 4·9 | ||
| History of MRSA d | 2·1 | 1·9 | 1·9 | 1·6 | ||
| History of VRE d | 0·7 | 0·5 | 0·6 | 0·3 | ||
| History of MDR-GNR d | 2.0 | 2.0 | 1.9 | 2.0 | ||
| Surgery During Admission | 21·1 | 22·7 | 20·9 | 22·4 | ||
| ICU Stay Prior To Study Unit Stay | 8·2 | 7·4 | 8·1 | 7·9 | ||
| Skilled Nursing Facility Stay within 90 Days Prior | 2·0 | 2·0 | 2·1 | 2·0 | ||
| Patients with Devices e | 11·0 | 12·8 | 9·8 | 12·8 | ||
| Unit Type | ||||||
| Mixed Medical-Surgical | 37·4 | 37·5 | 37·1 | 38·2 | ||
| Medical | 34·5 | 37·8 | 34·3 | 37·8 | ||
| Surgery | 16·2 | 10·8 | 16·8 | 10·8 | ||
| Step-down | 10·0 | 11·9 | 9·7 | 11·2 | ||
| Oncology | 1·9 | 2·0 | 2·0 | 2·1 | ||
Pathogens were attributed to a participating unit if the collection date occurred >2 days after unit admission through 2 days after unit discharge
Missing for 49 patients
Method of collection of race data changed between baseline and intervention periods. In the baseline period, respondents were asked to select one response from a list of race categories, which included “Hispanic.” In the intervention period, Hispanic ethnicity was asked separately from race and respondents were allowed to select up to two race categories.
Defined using available (from March 2013) screening and clinical cultures prior to day 2 of the ABATE participating unit stay
Defined as central venous catheters (including accessed ports), midline catheters, or lumbar drains. See Appendix A for details.
The intervention achieved substantial separation of treatment across arms. In the Decolonization Arm, median compliance with CHG bathing or showering across participating hospitals was 79% (interquartile range (IQR):66%−79%), reflecting a median number of 28,184 assessments (IQR:22,734–37,479). Among those who used CHG, sampling across hospitals found 78% (7669/9843) were bed baths versus showers. Median compliance with mupirocin was 88% (IQR:81%−91%), reflecting a median number of 1,803 assessments (IQR: 1,166–2,639) among MRSA carriers in participating hospitals. In the Routine Care Arm, CHG use for bathing or showering was rare across participating hospitals (median 1%, IQR 0–2%), reflecting a median of 12,325 assessments (IQR:8,166–18,408). Use of mupirocin among MRSA carriers was similarly rare (median 1%, IQR 0–3%), reflecting a median number of 785 assessments (IQR:410–1019). Use in the Routine Care Arm was usually for pre-operative purposes.
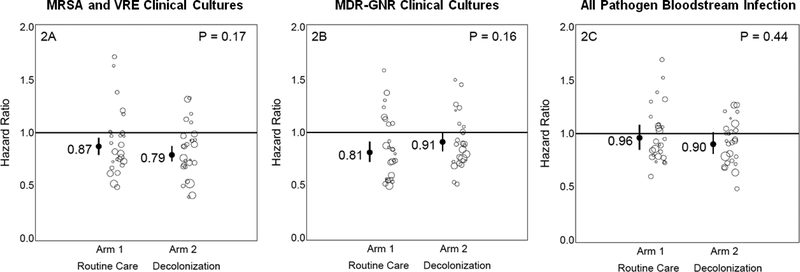
For the primary outcome of unit-attributable MRSA- or VRE-positive clinical cultures (Figure 2), both arms improved significantly from baseline values. No difference was seen in the relative hazard of the intervention vs. baseline period for the Routine Care Arm (HR=0·87 (95% Confidence Interval (CI):0·79·0·95)) versus the Decolonization Arm (HR=0·79 (0·73·0·87)), (P=0·17). The secondary outcomes were also not significantly different between intervention and baseline periods for MDR-GNR clinical cultures (Routine Care (HR 0·81 (0·72·0·91)) versus Decolonization Arm (HR 0·91 (0·82·1·00)), P=0–16) or all-pathogen bloodstream infection (Routine Care (HR 0·96 (0·85·1·08)) versus Decolonization Arm (HR 0·90 (0·80·1 01)),P=0·44) (Figure 2). Adjusted and as treated results were similar (Table 2). The number of outcome events per trial outcome and their associated rates per 1,000 patient days-at-risk are shown in Appendix B.
Figure 2. Outcomes in Total Trial Population.
Graphic showing impact of trial interventions on trial outcomes in the overall trial population. Arm-specific hazard ratios and confidence intervals from proportional hazards models (intent-to-treat unadjusted) accounting for clustering by hospitals are shown for MRSA or VRE clinical cultures (2A), MDR-GNR clinical cultures (2B), and all-pathogen bloodstream infection (2C). Bubble plots of hazard ratios (predicted random effects or exponentiated frailties) from individual hospitals relative to their arm effects are shown adjacent to arm-specific hazard ratios and confidence intervals. The size of the bubble is proportional to the number of patients contributing data to the trial.
NOTE: Change in figure due to addition of middle panel. Hard to track changes.
Table 2.
Proportional Hazard Model Results, Total Trial Population and Subpopulation Groups
| Hazard Ratios (95 % Confidence Interval) | |||
|---|---|---|---|
| Routine Care | Decolonization | P-Value | |
| Total Non-Critical Care Population | |||
| Intent-to-Treat, Unadjusted | N= 244,166 | N=284,817 | |
| MRSA or VRE Clinical Cultures | 0·87 (0·79–0·95) | 0·79 (0·73–0·87) | 0·17 |
| All-Pathogen Bloodstream Infection | 0·96 (0·85–1·08) | 0·90 (0·81–1·01) | 0·44 |
| MDR-GNR Clinical Cultures | 0·81 (0·72–0·91) | 0·91 (0·82–1·00) | 0·16 |
| Intent-to-Treat, Adjusted a | N=244,166 | N=284,817 | |
| MRSA or VRE Clinical Cultures | 0·89 (0·81–0·97) | 0·84 (0·77–0·91) | 0·34 |
| All-Pathogen Bloodstream Infection | 0·97 (0·86–1·09) | 0·92 (0·82–1·03) | 0·53 |
| MDR-GNR Clinical Cultures | 0·85 (0·76–0·95) | 0·93 (0·84–1·03) | 0·27 |
| As-Treated, Unadjusted | N=239,875 | N=281,696 | |
| MRSA or VRE Clinical Cultures | 0·87 (0·80–0·96) | 0·79 (0·72–0·86) | 0·11 |
| All-Pathogen Bloodstream Infection | 0·95 (0·84–1·08) | 0·91 (0·81–1·01) | 0·55 |
| MDR-GNR Clinical Cultures | 0·82 (0·73–0·92) | 0·91 (0·82–1·00) | 0·18 |
| Post-Hoc Subpopulation Groups | |||
| Hospitals with High Baseline Rates b | |||
| MRSA or VRE Clinical Cultures | N=65,767 0·68 (0·58–0·79) |
N=57,550 0·69 (0·59–0·82) |
0·81 |
| All-Pathogen Bloodstream Infection | N=58,273 0·86 (0·70–1·07) |
N=96,885 0·81 (0·68–0·96) |
0·62 |
| Patients with Devicesc | N=24,950 | N=36,475 | |
| MRSA or VRE Clinical Cultures | 1·17 (1·00–1·37) | 0·80 (0·69–0·92) | 0·0004 d |
| MRSA Clinical Cultures Only | 1·17 (0·99–1·39) | 0·87 (0·74–1·02) | 0·0127 |
| VRE Clinical Cultures Only | 1·26 (0·85–1·86) | 0·58 (0·44–0·78) | 0·0020 d |
| All-Pathogen Bloodstream Infection | 1·13 (0·96–1·33) | 0·82 (0·71–0·94) | 0·0035 d |
| Patients in Oncology Units | N=4,723 | N=5,800 | |
| MRSA or VRE Clinical Cultures | 0·68 (0·33–1·40) | 0·61 (0·31–1·20) | 0·83 |
| All-Pathogen Bloodstream Infection | 0·84 (0·42–1·66) | 0·78 (0·36–1·70) | 0·90 |
| Patients with a History of MRSA | N=4,756 | N=4,841 | |
| MRSA Clinical Cultures | 0·86 (0·74–0·99) | 0·92 (0·80–1·06) | 0·50 |
| All-Pathogen Bloodstream Infection | 0·96 (0·71–1·31) | 0·93 (0·70–1·25) | 0·89 |
Adjusted models accounted for individual age, gender, race, Medicaid insurance, MDRO history, prior nursing home stay within 90 days of admission, Elixhauser comorbidity index, unit type (medical, surgical, mixed medical-surgical, oncology, and step-down), transplant hospital, and surgery during admission. Regarding MDRO history, the outcome of MRSA and VRE clinical cultures was adjusted for MRSA and VRE history; the outcome of MDR-GNR clinical cultures was adjusted for MDR-GNR history; and the outcome of all-pathogen bloodstream infection accounted for history of MRSA, VRE and MDR-GNR.
Top quartile hospitals based upon baseline rates of each outcome
Defined as central venous catheters (including accessed ports), midline catheters, or lumbar drains
Remains significant after correcting for multiple comparisons.
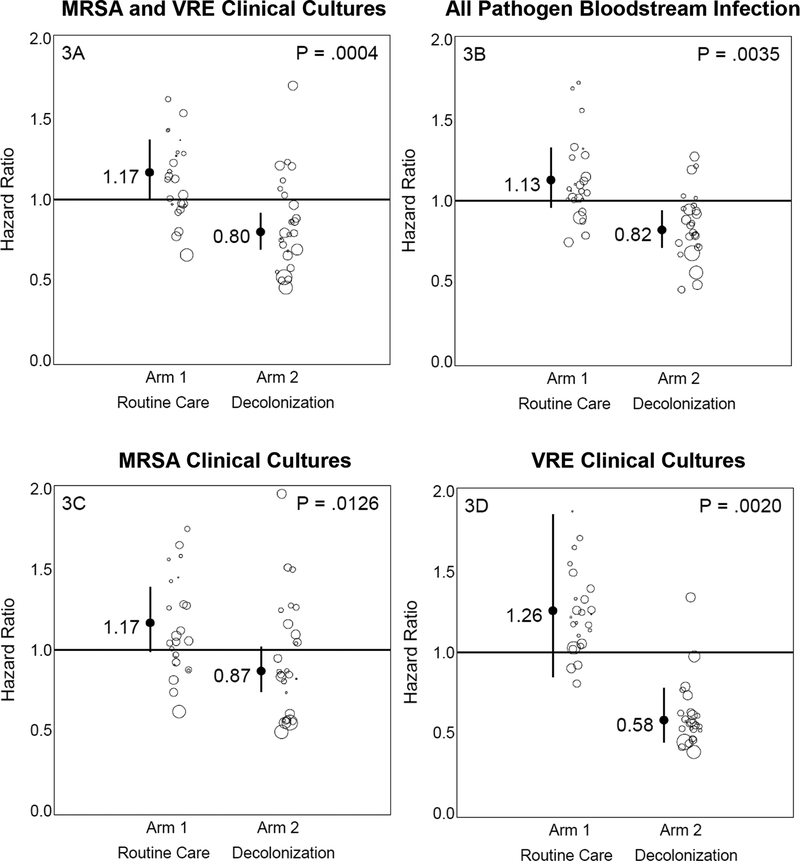
Post-hoc subgroup analyses (Table 2) were conducted to assess if a higher risk subpopulation would benefit from the intervention when the overall non-ICU population did not. Patients with devices experienced a significant 31% greater reduction in all-cause bacteremia and a significant 37% greater reduction in MRSA or VRE clinical cultures when compared to the control group. When evaluating MRSA alone and VRE alone, there was a 30% reduction in MRSA clinical cultures and a 68% reduction in VRE clinical cultures (Figure 3). These findings remained significant after accounting for multiple comparisons. Patients who had medical devices accounted for 10% (15,372/156,889) of the routine care intervention period population, but were responsible for 37% (446/1,209) of MRSA or VRE cultures and 56% (413/740) of bloodstream infections. Pathogen types are found in Appendix C.
Figure 3. Outcomes in Patients with Devices.
Graphic showing impact of trial interventions on trial outcomes in the post-hoc subpopulation of patients with devices. Arm-specific hazard ratios and confidence intervals from proportional hazards models (intent-to-treat, unadjusted) accounting for clustering by hospitals are shown for MRSA or VRE clinical cultures (3A), all-pathogen bloodstream infection (3B), MRSA clinical cultures only (3C), and VRE clinical cultures only (3D). Results remained significant after adjusting for multiple comparisons. Bubble plots of hazard ratios (predicted random effects or exponentiated frailties) from individual hospitals relative to their arm effects are shown adjacent to arm-specific hazard ratios and confidence intervals. The size of the bubble reflects the relative number of patients contributing data to the subpopulation.
NOTE: Change in figure due to extending p-values out to 4 digits. Hard to track changes.
Participating hospitals reported 196 quality improvement interventions to trial investigators. Of these, 129 (66%) were unrelated to the trial while 67 (34%) directly competed with trial outcomes. Hospitals chose not to implement 64 of the 67 competing quality improvement interventions. Three hospitals dropped from the trial to pursue the remaining three conflicting interventions. In addition, HCA released guidance to all hospitals in the health system to initiate universal ICU decolonization with daily CHG and nasal mupirocin three months prior to the start of the baseline period. Results from a sensitivity analysis restricted to patients without a preceding ICU stay prior to entering a participating unit were similar to results from the full trial population.
There were 25 adverse events, all involving Chlorhexidine. All were associated with mild pruritus or rash, and all resolved rapidly upon discontinuation. These occurred among 183,013 patients in units assigned to Chlorhexidine. There were no reported adverse events among 2,908 patients with an MRSA history in units assigned to receive mupirocin.
Discussion
There has been widespread adoption of CHG bathing with or without nasal decolonization in ICUs across the United States and other countries in response to cluster-randomized clinical trials demonstrating marked reductions in central-line associated bloodstream infections and allcause bloodstream infections, as well as reductions in MRSA carriage and transmission. The success of this strategy in ICUs has raised questions about whether the benefit could be extended to other populations, such as non-critically ill hospitalized patients, post-discharge patients, or patients in nursing homes.25–27
The ABATE Infection Trial found that universal CHG bathing for all patients outside the ICU plus mupirocin for MRSA carriers did not significantly reduce clinical cultures with MDROs or bloodstream infections compared to routine care. This 53-hospital pragmatic trial with over half-a-million patients in the baseline and intervention periods combined was well-powered to detect a 20% difference in these outcomes. Instead, we found an 8% reduction in MRSA or VRE clinical cultures and a 6% reduction in bloodstream infection, neither of which was statistically significant or clinically meaningful for a broad-based intervention strategy.
This trial highlights the importance of a randomized control group as both arms showed significant improvement over baseline values for the primary outcome. The reason for improvement is not known since initiation of new infection prevention quality improvement efforts was closely monitored. We did, however, allow and expect hospitals to conduct campaigns to improve adherence to existing best practice. It is possible that the routine care arm was more adept at ensuring best practice or invested more effort into such improvement campaigns because they did not have to adopt a new intervention, but this would only affirm that universal CHG bathing and targeted nasal mupirocin for MRSA carriers do not provide improvement over current best practice for the general non-critical care patient population.
Because universal decolonization has been established as best practice in ICU patients,7–12 the lack of effect in general medical and surgical patients deserves explanation. One possible explanation is that patients often perform their own bathing and showering in non-ICU units and the application may be less robust than during fully-assisted ICU care. Nevertheless, we note that nearly 80% of patients using CHG had disposable cloths used for bathing, which generally implies some level of staff assistance and higher residual concentrations of CHG on the skin.28 Furthermore, in comparison to ICU patients, general inpatients have fewer devices, are less likely to undergo procedures, are better able to self-care and maintain personal hygiene, and thereby have a lesser degree of modifiable infection risks. Their length-of-stay in participating units was also short, only a median of 4 days. It is possible that elongated follow up beyond discharge may have identified more preventable cases. It is also possible that a longer application of the intervention beyond discharge or a greater adherence to the protocol could have provided greater protection. Nevertheless, when accounting for the fact that we did not require bathing on the discharge day, the 71% CHG adherence reflected a robust adoption across many facilities, especially when checklists for appropriate body application and cleansing of medical devices were being applied.
Importantly, we identified in a post-hoc analysis that a high-risk subgroup that significantly benefitted from the intervention and was responsible for a large and disproportionate fraction of trial outcomes. In patients with devices that we could identify from administrative records, including central lines, midline catheters, and lumbar drains, decolonization decreased MRSA or VRE cultures, and bloodstream infections by one-third. This reduction is even more impactful when one considers that patients with devices represented approximately 10% of the total population, but accounted for 37% of MRSA or VRE cultures, and 56% of all bloodstream infections.
The mechanism of decolonization has been well established for CHG. It reduces body surface bioburden of potentially pathogenic microbes and has strong biological plausibility to reduce infection in the setting of a break in skin integrity due to medical devices.28–31 Application of CHG prior to central line insertion, during dressing changes, and for routine bathing in ICUs has been shown to be superior to other agents and is now established as best practice.2,3,8,11 This trial’s observation of benefit of decolonization in non-critically ill patients with devices is consistent with these ICU findings of CLABSI reduction.8,11,32 In fact, some U.S. hospitals adopted CHG bathing in all patients with central lines when guidance from national societies recommended this strategy to reduce CLABSI in ICUs.32
We did not find a significant reduction in MRSA and VRE clinical cultures or bloodstream infection among patients in oncology units. However, this assessment was limited to only seven units, and oncology patients, who often have central lines, contributed to the benefit found in those with medical devices. Larger, more dedicated oncology assessments, may be necessary to disentangle whether Chlorhexidine exerts a benefit among immunocompromised patients independent of the protection applied to those with medical devices.
It should be noted that the benefit reported in patients with devices was in the context of a strategy that provided CHG bathing to all patients. Thus, it is not known whether CHG bathing and nasal decolonization given in a targeted fashion only to patients with devices would achieve the same point estimate reduction in MRSA, VRE, or all-cause bloodstream infection as found in the ABATE Infection Trial. While the majority of that reduction was likely due to direct application of these products to patients’ skin, it has been shown that CHG reduces shedding into the environmental and onto healthcare worker hands.33–34 We cannot estimate the proportion of benefit that may have been gained through indirect herd protection (e.g. protection from cross-colonization) from universal bathing. In addition, this benefit was achieved using a real-world pragmatic roll out of this intervention in community hospitals with no research staff on-site. Thus, the benefit seen in this population is likely to be generalizable to other hospitals.
This trial has several limitations. First, the studied population consisted of patients in general medical and surgical units in community hospitals where <3% had a known history of MRSA or VRE. A population with a higher prevalence or risk of MDROs or infection could have yielded a different outcome. Second, although we have daiy nursing documentation of whether CHG bathing or showering occurred, we have limited data on the quality of the application. Compared to ICUs, where decolonization has been found to be highly effective in reducing MDROs and bloodstream infection, bathing in the non-ICU is commonly performed by nursing assistants rather than nurses. In addition, patients often opt for their own application of both disposable bathing cloths and soap in showers, and thus, the quality of application to the skin is likely highly variable. Third, the benefit found in the subpopulation of patients with devices was determined post-hoc and the trial was not originally designed for this evaluation. While the findings remain significant after adjusting for multiple comparisons, the level of evidence from a post-hoc analysis differs substantially from the key pre-specified outcomes of a clinical trial and should be weighed in the context of the overall evidence on this topic. In addition, any application of CHG to this or other subpopulations warrants periodic assessment for the emergence of antiseptic resistance over time.
In conclusion, universal daily CHG bathing plus nasal decolonization for MRSA carriers was an efficient and effective strategy for reducing MRSA, VRE, and all-cause bloodstream infection in patients with devices outside of ICUs, but not in the general non-ICU population.
Research in Context
Evidence Before This Study
Several cluster-randomized clinical trials in intensive care units (ICUs) have led to the widespread adoption of universal ICU decolonization involving daily Chlorhexidine bathing with or without nasal ointments to prevent bloodstream infections and MRSA.7,8,10 These trials have also led to national guidance in the United States for use of daily Chlorhexidine bathing for reducing device-associated ICU infections, specifically central line-associated bloodstream infections.32 There is only modest quasi-experimental evidence that assesses decolonization in non-ICU settings.
We searched PubMed for “Chlorhexidine bathing” (MeSH Terms) and “hospital,” excluding “intensive care unit”, or “ICU” and found four quasi-experimental studies. One study found a 64% reduction in hospital-associated MRSA and VRE infection compared to historical controls after 14 months of daily bathing with Chlorhexidine (CHG) in four general medical units at an academic center.26 Another study found a 55% reduction in hospital-associated MRSA and a 36% reduction in hospital-associated VRE after CHG bathing for 7 months in a crossover study of four general medical units at an academic center.27 A third study involving 19-months of a non-randomized stepped-wedge design of hospital-wide CHG bathing reported a 29% reduction in Clostridium difficile infection with thrice weekly CHG bathing and a 59% reduction in C. difficile infection with daily CHG bathing.25 The last study involved three uncommon chronic care hospital units where one unit was randomly assigned to bathe patients daily with CHG for 12 months resulting in a 71% reduction in MRSA incidence among 122 persons.35 This last study was the only one where the reported benefit was not significant. All but one study used 2% norinse CHG cloths. Reported adherence with CHG bathing in these studies was approximately 60%.
Added Value of This Study
The Active BAThing to Eliminate (ABATE) Infection Trial is the first large-scale cluster-randomized trial to evaluate whether universal CHG bathing for all non-critical care patients plus targeted mupirocin for MRSA-positive patients reduced MDROs and all-cause bloodstream infection. It achieved CHG compliance (70%) that was higher than the quasi-experimental studies reported above. We found that universal decolonization did not reduce infection in the overall population, but, in post-hoc analyses, was associated with significant reductions in allcause bloodstream infection, and MRSA and VRE in patients with medical devices.
Implications of All the Available Evidence
The ABATE Infection Trial added the evaluation of over 500,000 adult patients in 53 randomized hospitals to identify benefits attributable to daily CHG bathing and targeted mupirocin use in general medical and surgical units. While prior single-center quasi-experimental studies found broad infection reduction benefit with daily CHG use across inpatients in academic hospitals, the ABATE Infection Trial did not find a benefit to either MDROs or bloodstream infections in non-ICU patients. This is in contrast to clear benefit found in several trials for ICU patients.
This trial also contributes to the evidence base for whether decolonization should be used for all inpatients with devices, not just those in ICUs. The current U.S. ICU guidance to use daily CHG bathing for prevention of central line-associated infections, has led many hospitals to adopt daily CHG bathing for all inpatients with central lines and other devices, although evidence in non-ICU patients has been lacking. The post-hoc analysis in the ABATE Infection Trial found that non-ICU patients with medical devices experienced a significant 37% reduction in MRSA and VRE and a significant 31% reduction in all-cause bloodstream infection. Importantly, patients with medical devices constituted only 10% of the inpatient population, but were responsible for 37% of inpatient MRSA and VRE cultures and 56% of all-cause bloodstream infections. Despite these findings, because the ABATE Infection Trial involved universal decolonization in all inpatients, the value of decolonizing only patients with devices is still not known.
Supplementary Material
Acknowledgements
We thank the following for their invaluable contribution: Laurie Brewer, Jane Englebright, Deborah Lilly, Bradley Ninness, Russell Poland, Lorraine Rognstad, Deepak Sharma, and David Vulcano, and Dottie White from HCA, and Grace Lee from Harvard Medical School/Harvard
Pilgrim Health Care Institute (currently at Stanford Children’s Hospital). We also thank Sage Products Medical Science Liaisons for providing bathing demonstrations to the decolonization arm.
We especially recognize and thank the unit staff and leadership, infection prevention programs, nurse educators, microbiology laboratories, and administrative leadership at all of the participating hospitals for their commitment and dedication to this trial: Blake Medical Center (Bradenton, FL), Chippenham Johnston Willis Medical Center (Richmond, VA), Cartersville Medical Center (Cartersville, GA), Clear Lake Regional Medical Center (Webster, TX), Coliseum Northside Hospital (Macon, GA), Colleton Medical Center (Walterboro, SC), Corpus Christi Medical Center (Corpus Christi, TX), Eastside Medical Center (Snellville, GA), Garden Park Medical Center (Gulfport, MS), Hendersonville Medical Center (Hendersonville, TN), Henrico Doctors’ Hospital (Richmond, VA), John Randolph Medical Center (Hopewell, VA), Kingwood Medical Center (Kingwood, TX), Las Colinas Medical Center (Irving, TX), Las Palmas Medical Center (El Paso, TX), Lee’s Summit Medical Center (Lee’s Summit, MO), LewisGale Hospital -Alleghany (Low Moor, VA), Medical Center of Plano (Plano Texas), Methodist Hospital (San Antonio, Texas), Methodist Specialty and Transplant Hospital (San Antonio, TX), Methodist Stone Oak Hospital (San Antonio, TX), Methodist Texsan Hospital (San Antonio, TX), MountainView Hospital (Las Vegas, NV), North Hills Hospital (North Richland Hills, TX), North Suburban Medical Center (Thornton, CO), Northeast Methodist Hospital (Live Oak, TX), Orange Park Medical Center (Orange Park, FL), Overland Park Regional Medical Center (Overland Park, KS), Palms West Hospital (Loxahatchee, FL), Parkland Medical Center (Derry, NH), Parkridge East Hospital (Chattanooga, TN), Parkridge Medical Center (Chattanooga, TN), Plaza Medical Center of Fort Worth (Fort Worth, TX), Portsmouth Regional Hospital (Portsmouth, NH), Regional Medical Center of Acadiana (Lafayette, LA), Research Medical Center (Kansas City, MO), Reston Hospital Center (Reston, VA), Rio Grande Regional Hospital (McAllen, TX), South Bay Hospital (Sun City Center, FL), St. David’s Medical Center (Austin, TX), St. Petersburg General Hospital (St. Petersburg, FL), Summit Medical Center (Hermitage, TN), Sunrise Hospital and Medical Center (Las Vegas, NV), Timpanogos Regional Hospital (Orem, UT), TriStar Horizon Medical Center (Dickson, TN), TriStar Southern Hills Medical Center (Nashville, TN), Valley Regional Medical Center (Brownsville, TX), West Florida Hospital (Pensacola, FL), West Hills Hospital & Medical Center (West Hills, CA), West Palm Hospital (West Palm Beach, FL).
This project was funded by the National Institutes of Health Common Fund and administered by the National Institute of Allergy and Infectious Diseases (UH2/UH3 AT007769–01 (Huang)). The findings and conclusions expressed in this article are those of the authors and do not necessarily represent the official position of the National Institutes of Health or the Centers for Disease Control and Prevention.
Funding:
This project was funded by the National Institutes of Health (United States).
Appendix A. Distribution of Medical Devices Among Trial Participants
| Variable | Baseline (12 months) |
Intervention (21 months) |
||
|---|---|---|---|---|
| Routine Care | Decolonization | Routine Care | Decolonization | |
| N (%) | N (%) | N (%) | N (%) | |
| Patients | 87,277 | 101,804 | 156,889 | 183,013 |
| Patients with Devices a | 9,578 (11·0) | 13,057 (12·8) | 15,372 (9·8) | 23,417 (12·8) |
| Central Venous Catheters | ||||
| PICC b | 6,357 (7·3) | 9,112 (9·0) | 9,699 (6·2) | 14,087 (7·7) |
| Non-PICC | 2,803 (3·2) | 4,494 (4·4) | 3,985 (2·5) | 7,056 (3·9) |
| Dialysis Catheter | 867 (1·0) | 1,248 (1·2) | 1,749 (1·1) | 2,571 (1·4) |
| Midline Catheter | 304 (0·3) | 393 (0·4) | 1,088 (0·7) | 3,109 (1·7) |
| Epidural Catheter | 46 (0·1) | 68 (0·1) | 81 (0·1) | 178 (0·1) |
Sum of all devices exceeds total patients with devices because patients may have more than one device.
PICC = peripherally inserted central catheter
Appendix B. Trial Outcomes Among Total Trial Population and Post-Hoc Subpopulation Groups
| Routine Care | Decolonization | |||
|---|---|---|---|---|
| Baseline | Intervention | Baseline | Intervention | |
| N =87,277 | N = 101,804 | N = 156,889 | N = 183,013 | |
| MRSA or VRE Clinical Cultures | ||||
| Total Trial Population Events/At-Risk Days (Ratesa) | ||||
| Intent-to-Treat, Unadjusted | 756/316,391 (2·39) |
1,209/588,916 (2·05) |
838/376,808 (2·22) |
1,224/705,283 (1·74) |
| As-Treated, Unadjusted | 756/316,391 (2·39) |
1,184/573,476 (2·06) |
836/376,275 (2·22) |
1,199/695,510 (1·72) |
| Post-Hoc Subpopulation Groups Events/At-Risk Days (Rates) | ||||
| Hospitals with High Baseline Rates b | 311/84,274 (3·69) |
381/154,892 (2·46) |
269/80,154 (3·36) |
321/139,468 (2·30) |
| Patients with Devices c | 229/65,976 (3·47) |
446/111,423 (4·00) |
329/86,022 (3·82) |
486/171,516 (2·83) |
| Patients in Oncology Units | 13/6,708 (1·94) |
17/13,147 (1·29) |
16/8,405 (1·90) |
17/14,743 (1·15) |
| Patients with a History of MRSA | 320/7,712 (41·49) |
471/14,355 (32·81) |
326/8,923 (36·53) |
455/14,479 (31·42) |
| All Pathogen Bacteremia | ||||
| Total Trial Population Events/At-Risk Days (Rates) | ||||
| Intent-to-Treat, Unadjusted | 407/317,556 (1·28) |
740/590,514 (1·25) |
488/378,102 (1·29) |
828/706,212 (1·17) |
| As-Treated, Unadjusted | 407/317,556 (1·28) |
716/575,057 (1·25) |
488/377,565 (1·29) |
824/696,244 (1·18) |
| Post-Hoc Subpopulation Groups Events/At-Risk Days (Rates) | ||||
| Hospitals with High Baseline Rates b | 134/74,083 (1·81) |
228/143,653 (1·59) |
211/122,088 (1·73) |
322/229,808 (1·40) |
| Patients with Devices c | 218/65,833 (3·31) |
413/111,070 (3·72) |
309/86,108 (3·59) |
492/170,862 (2·88) |
| Patients in Oncology Units | 13/6,689 (1·94) |
22/13,074 (1·68) |
11/8,404 (1·31) |
15/14,717 (1·02) |
| Patients with a History of MRSA | 66/9,267 (7·12) |
109/16,640 (6·55) |
77/10,616 (7·25) |
109/16,811 (6·48) |
| MDR-GNR Clinical Cultures | ||||
| Total Trial Population Events/At-Risk Days (Rates) | ||||
| Intent-to-Treat, Unadjusted | 481/316,970 (1·52) |
741/591,934 (1·25) |
584/377,821 (1·55) |
1,003/705,364 (1·42) |
| As-Treated, Unadjusted | 481/316,970 (1·52) |
729/576,367 (1·26) |
584/377,284 (1·55) |
993/695,387 (1·43) |
Per 1,000 unit-attributable patient days at-risk for the event (days after first event removed)
Top quartile hospitals based upon baseline rates of each outcome
Defined as central venous catheters (including accessed ports), midline catheters, or lumbar drains
Appendix C. Trial Outcomes by Pathogen Group During Baseline (12 months) and Intervention (21 months) Periodsa
| Routine Care | Decolonization | |||
|---|---|---|---|---|
| Baseline N =87,277 |
Intervention N = 101,804 |
Baseline N = 156,889 |
Intervention N = 183,013 |
|
| Events/At-Ris Days (Rateb) | ||||
| MRSA Clinical Cultures | 662/317,143 (2·09) |
1,049/590,407 (1·78) |
671/378,600 (1·77) |
1,023/707,487 (1·45) |
| VRE Clinical Cultures | 102/320,118 (0·32) |
184/596,056 (0·31) |
183/380,996 (0·48) |
222/712,158 (0·31) |
| MDR-GNR Clinical Cultures | At Risk Days = 316,970 | At Risk Days = 591,934 | At Risk Days = 377,821 | At Risk Days = 705,364 |
| ESBL | 219 (0·69) | 403 (0·68) | 300 (0·79) | 547 (0·78) |
| CRE | 97 (0·31) | 125 (0·21) | 93(0·25) | 192 (0·27) |
| MDR Pseudomonas | 136 (0·43) | 178 (0·30) | 146 (0·39) | 206 (0·29) |
| MDR Acinetobacter | 29 (0·09) | 35 (0·06) | 45(0·12) | 58 (0·08) |
| All-Pathogen Bloodstream Infection | At Risk Days = 317,556 | At Risk Days = 590,514 | At Risk Days = 378,102 | At Risk Days = 706,212 |
| Gram-positive | 252 (0·79) | 476 (0·81) | 317 (0·84) | 474 (0·67) |
| Skin commensal c | 47 (0·15) | 85 (0·14) | 70 (0·19) | 95 (0·13) |
| Non-commensal | 205 (0·65) | 391 (0·66) | 248 (0·66) | 389 (0·55) |
| Gram-negative | 128 (0·40) | 196 (0·33) | 141 (0·37) | 298 (0·42) |
| Candida species | 27 (0·09) | 68 (0·12) | 30 (0·07) | 56 (0·08) |
N indicates number of patients
Per 1,000 unit-attributable patient days at-risk for the event (days after first event removed). Note that the sum of the MRSA and VRE clinical cultures shown in this table exceed the reported number of patients with combined MRSA or VRE clinical cultures in Appendix B because patients may have both cultures.
Bloodstream infection based upon CDC criteria for skin commensals (requires two cultures within two calendar days)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Sage Products and Molnlycke contributed antiseptic Chlorhexidine product to this trial. Investigators are also conducting other studies in which contributed antiseptic product is provided to participating hospitals and nursing homes from Stryker (Sage Products) (KK, LH, MHC, MKH, RAW, SSH), 3M (AG, LH, SSH), Clorox (CS, ES, JH, JM, JP, KH, KK, LH, LS, MHC, MKH, RAW, RP, SSH, TRA), Xttrium (LH, SSH), and Medline (CS, ES, JH, JM, JP, KH, KK, LH, LS, MHC, MKH, RAW, RP, SSH, TRA). Companies contributing product have no role in the design, conduct, analysis, or publication of the ABATE Infection Trial or other studies conducted by these investigators. All other authors have no disclosures.
Declaration of Interests
Sage Products and Molnlycke contributed antiseptic Chlorhexidine product to this trial. Investigators are also conducting other studies in which contributed antiseptic product is provided to participating hospitals and nursing homes from Stryker (Sage Products) (KK, LH, MHC, MKH, RAW, SSH), 3M (LH, SSH), Clorox (CS, ES, JH, JM, JP, KH, KK, LH, LS, MHC, MKH, RAW, RP, SSH, TRA), Xttrium (LH, SSH), and Medline (CS, ES, JH, JM, JP, KH, KK, LH, LS, MHC, MKH, RAW, RP, SSH, TRA). Investigator-initiated grant funds were received from Clorox (MKH, MHC, LS, KH, RP). Companies contributing product or providing grant funds have no role in the design, conduct, analysis, or publication of the ABATE Infection Trial or other studies conducted by these investigators. All other authors have no disclosures.
References
- 1.National and state healthcare associated infections. Centers for Disease Control and Prevention. 2016. https://www.cdc.qov/HAI/pdfs/proqress-report/hai-proqress-report.pdf
- 2.Maki DG, Ringer M, Alvarado CJ. Prospective randomised trial of povidone-iodine, alcohol, and Chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet. 1991. August 10;338(8763):339–43. [DOI] [PubMed] [Google Scholar]
- 3.Mimoz O, Lucet JC, Kerforne T et al. Skin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): an open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet. 2015;386(10008):2069–2077. [DOI] [PubMed] [Google Scholar]
- 4.Raad I, Darouiche R, Dupuis J, et al. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. The Texas Medical Center Catheter Study Group. Ann Intern Med. 1997. August 15;127(4):267–74. [DOI] [PubMed] [Google Scholar]
- 5.de Smet AM, Kluytmans JA, Cooper BS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009. January 1;360(1):20–31. [DOI] [PubMed] [Google Scholar]
- 6.Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013. October 16;310(15): 1571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Climo MW, Yokoe DS, Warren DK, et al. Effect of daily Chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang SS, Septimus E, Hayden MK, et al. Effect of body surface decolonisation on bacteriuria and candiduria in intensive care units: an analysis of a cluster-randomised trial. Lancet Infect Dis. 2016; 16(1):70–79. [DOI] [PubMed] [Google Scholar]
- 10.Milstone AM, Elward A, Song X, et al. Daily Chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet. 2013;381(9872):1099–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Septimus E, Hickok J, Moody J, et al. Closing the Translation Gap: Toolkit-based Implementation of Universal Decolonization in Adult Intensive Care Units Reduces Central Line-associated Bloodstream Infections in 95 Community Hospitals. Clin Infect Dis. 2016;63(2):172–7. [DOI] [PubMed] [Google Scholar]
- 12.Huang SS, Septimus E, Avery TR, et al. Cost savings of universal decolonization to prevent intensive care unit infection: implications of the REDUCE MRSA trial. Infect Control Hosp Epidemiol. 2014. October;35 Suppl 3:S23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derde LPG, Cooper BS, Goossens H et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. Lancet Infect Dis.2014;14(1):31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–55. [DOI] [PubMed] [Google Scholar]
- 15.Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014. July;35(7):772–96. [DOI] [PubMed] [Google Scholar]
- 16.Mahalanobis PC. On the generalised distance in statistics. Proceedings of the National Institute of Sciences of India. 1936;2(1):49–55. [Google Scholar]
- 17.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. [DOI] [PubMed] [Google Scholar]
- 18.ABATE Infection Trial: Patient bathing video, https://vimeo.com/164608558. Last accessed June 19, 2018.
- 19.Centers for Disease Control and Prevention. National Healthcare Safety Network. Patient Safety Component Manual, https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanualcurrent.pdf. Last accessed on April 28, 2018.
- 20.CDC/NHSN surveillance definition of healthcare-associated infection and criteria for specific types of infections in the acute care setting. http://www.cdc.gov/nhsn/PDFs/pscManual/17pscNoslnfDefcurrent.pdf. Last accessed April 28, 2018. [DOI] [PubMed]
- 21.Kleinman K, Huang SS. Calculating power by bootstrap, with an application to cluster-randomized trials. eGEMs (Generating Evidence & Methods to Improve Patient Outcomes) 2017. February; 4(1): 1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes RH, Moulton LH. Cluster Randomized Trials, page 207 CRC Press, New York: 2009 [Google Scholar]
- 23.Ripatti S, Palmgren J. Estimation of Multivariate Frailty Models Using Penalized Partial Likelihood. Biometrics. 2000;56:1016–1022. [DOI] [PubMed] [Google Scholar]
- 24.Diehr P, Martin DC, Koepsell T, Cheadle A. Breaking the matches in a paired t-test for community interventions when the number of pairs is small. Stat Med. 1995;14(13):1491–504. [DOI] [PubMed] [Google Scholar]
- 25.Rupp ME, Cavalieri RJ, Lyden E, et al. Effect of hospital-wide Chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol. 2012;33(11):1094–100. [DOI] [PubMed] [Google Scholar]
- 26.Kassakian SZ, Mermel LA, Jefferson JA, Parenteau SL, Machan JT. Impact of Chlorhexidine bathing on hospital-acquired infections among general medical patients. Infect Control Hosp Epidemiol. 2011;32(3):238–43. [DOI] [PubMed] [Google Scholar]
- 27.Lowe CF, Lloyd-Smith E, Sidhu B, et al. Reduction in hospital-associated methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus with daily Chlorhexidine gluconate bathing for medical inpatients. Am J Infect Control. 2017;45(3):255–259. [DOI] [PubMed] [Google Scholar]
- 28.Rhee Y, Palmer LJ, Okamoto K, et al. ; Centers for Disease Control and Prevention Epicenter Program. Differential Effects of Chlorhexidine Skin Cleansing Methods on Residual Chlorhexidine Skin Concentrations and Bacterial Recovery. Infect Control Hosp Epidemiol. 2018;39(4):405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin MY, Lolans K, Blom DW, et al. ; Centers for Disease Control and Prevention Epicenter Program. The effectiveness of routine daily Chlorhexidine gluconate bathing in reducing Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae skin burden among long-term acute care hospital patients. Infect Control Hosp Epidemiol. 2014;35(4):440–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edmiston CE Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% Chlorhexidine gluconate-impregnated cloth with 4% Chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89–96. [DOI] [PubMed] [Google Scholar]
- 31.Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207(2):233–9. [DOI] [PubMed] [Google Scholar]
- 32.Marschall J, Mermel LA, Fakih M, et al. ; Society for Healthcare Epidemiology of America. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753–71. [DOI] [PubMed] [Google Scholar]
- 33.Vernon MO, Hayden MK, Trick WE, Hayes RA, Blom DW, Weinstein RA; Chicago Antimicrobial Resistance Project (CARP). Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med. 2006. February 13;166(3):306–12. [DOI] [PubMed] [Google Scholar]
- 34.Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with Chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009. June;37(6): 1858–65. [DOI] [PubMed] [Google Scholar]
- 35.Amirov CM, Binns MA, Jacob LE, Candon HL. Impact of Chlorhexidine bathing on methicillin-resistant Staphylococcus aureus incidence in an endemic chronic care setting: A randomized controlled trial. Am J Infect Control. 2017;45(3):298–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.