Abstract
Age-related neurological disorders continue to pose a significant societal and economic burden. Aging is a complex phenomenon that affects many aspects of the human body. Specifically, aging can have detrimental effects on the progression of brain diseases and endogenous stem cells. Stem cell therapies possess promising potential to mitigate the neurological symptoms of such diseases. However, aging presents a major obstacle for maximum efficacy of these treatments. In this review, we discuss current preclinical and clinical literature to highlight the interactions between aging, stem cell therapy, and the progression of major neurological disease states such as Parkinson’s disease, Huntington’s disease, stroke, traumatic brain injury, amyotrophic lateral sclerosis, multiple sclerosis, and multiple system atrophy. We raise important questions to guide future research and advance novel treatment options.
Keywords: Age-related brain disorders, neurodegenerative diseases, stroke, traumatic brain injury, cell-therapy
1. Introduction
Over the past century, advancement in medical care, technology and accessibility has led to an increase in average human life expectancy. However, with increased lifespan, the risk of age-related neurodegenerative diseases and neurological disorders have increased too (Reeve et al., 2014; Wyss-Coray, 2016). In combination with the growing aging population, the incident rate of neurological disorders will likely climb. Neurological disorders cause major economical and societal costs for patients, family members, and society as a whole (McGovern Institute, 2018). There are no cures for most neurological disorders and primary treatments only focus on managing symptoms and slowing down disease progression (McGovern Institute, 2018). Patients have to cope with years of low quality of life, associated stress on themselves and family members, all while dealing with the ever-rising cost of healthcare (McGovern Institute, 2018). Furthermore, the aging population is at much higher risk of these diseases than other groups (Harvard NeuroDiscovery Center, 2018). An estimated 12 million Americans will be affected by neurological disorders in the next three decades (Harvard NeuroDiscovery Center, 2018). Therefore, there is a great need for new effective treatment that has the potential to apply across a wide range of neurological disorders.
There are many cellular and molecular characteristics of aging mainly genomic instability, mitochondrial dysfunction, inflammation, protein homeostasis etc. (Lopez-Otin et al., 2013). Many of these hallmarks contribute a significant role in the pathology and the progression of neurological disorders (Lopez-Otin et al., 2013; Ransohoff, 2016; Wyss-Coray, 2016). For example, mitochondrial dysfunction not only reduces the overall bioenergetics of the cells but also causes an increase in reactive oxygen species (ROS) production. The excessive ROS production can trigger an inflammatory response. Similarly, accumulations of misfolded proteins such as β-amyloid and α-synuclein are hallmarks of neurodegenerative diseases (e.g. Alzheimer’s disease and Parkinson’s disease respectively). The loss of protein homeostasis also triggers the cellular damage response which eventually leads to inflammation. As a result, there is an overall increase in chronic low-grade inflammation (high level of pro-inflammatory cytokines and low level of anti-inflammatory cytokines) in the aging brain (Currais, 2015; Deleidi et al., 2015). This imbalance between pro- and anti-inflammatory cytokines alters the morphology of microglia into a primed state (Norden et al., 2015). In the primed state, the microglia are more susceptible to hyper-activation and also last longer. It has been hypothesized that primed microglia exacerbates the progression of diseases and it is an important link between traumatic brain injury (TBI) and neurodegenerative diseases (Norden et al., 2015).
Stem cells hold great promise for treating many neurological disorders. However, concerns about safety and efficacy limit the number of clinical trials utilizing stem cells for these disorders. One of the major concerns is the potential for tumorigenicity, caused by high proliferative capacity of stem cells. This is the primary reason for regulatory agencies’ reservation for stem cells to be widely accepted as a therapeutic option. While these concerns are founded, increasing pre-clinical data in various animal models, has shown the tremendous potential of stem cell therapy for the aging population with neurological disorders. Furthermore, there is a consensus that stem cells are not only replacing dying cells but are also regulating inflammation and immune responses, as well as secreting therapeutic cytokines and factors (Lindvall et al., 2012). Hence, using stem cells that have a low proliferation rate might still provide therapeutic outcomes.
Aging affects both endogenous and exogenous stem cells. The proliferation potential of various stem cell niches in the body declines with age. The reduction in the proliferative capacity of stem cells has tremendous effect on the maintenance of the body. For example, many studies have reported that there is a decrease in cell cycle activity of hematopoietic stem cells in aged mice compared to young mice (Flores and Blasco, 2010; Janzen et al., 2006; Rossi et al., 2007). This leads to a decrease in hematopoiesis causing diminished immune response, increased incidence of myeloid malignancies, and anemia (Lopez-Otin et al., 2013). Similarly, many studies observed decline in functions with age in bone marrow derived mesenchymal stem cells (BM-MSCs) both in vitro and in vivo (Baker et al., 2015). Neural stem cells, found in neurogenic niches such as the subgranular zone (SGZ) and subventricular zone (SVZ), also decrease in proliferation and maturation in the aged brain due to an unfavorable microenvironment and accumulated DNA damage (DeCarolis et al., 2015; Rolando and Taylor, 2014). These findings support the idea that the age of both the stem cell donor and recipient matter for transplantation. In fact, many studies have demonstrated that donor age negatively affect many characteristics of stem cells such as differentiation, expansion, immunogenicity, and reprograming efficiency of stem cells (Aksoy et al., 2014; Choudhery et al., 2014; Trokovic et al., 2015; Wu et al., 2014). Conversely, the aging brain might negatively affect the efficacy of transplanted stem cells due to a hostile microenvironment (Conboy et al., 2015; Della Porta et al., 2014; Katsimpardi et al., 2014; Sinha et al., 2014). In addition, several co-morbities may emerge as a person ages (cardiovascular disease, arthritis, colitis), which may affect the inflammatory response to injury, as well as influence the differentiation potential and therapeutic outcome of a stem cell graft. In the same token, standard treatment of these co-morbidities may also impact stem cell therapy. Indeed, therapeutic use of steroids in arthritic aged populations could alter BBB permeability or endothelial tight junction, and in turn promote anti-inflammatory response in the CNS (Yan et al., 2017). Similarly, a selectively compromised BBB following mannitol treatment in stroke may allow subsequent penetration of stem cells to the brain parenchyma (Tajiri et al., 2016). Taking into account all these mitigating aging-related factors will likely improve the functional outcomes of stem cell therapy for neurological disorders.
As mentioned above, the current treatment regimens for many neurological disorders pertain mainly to managing symptoms and slowing down disease progression. New therapies that might stop or reverse the pathology trajectory would be of great importance to both physicians and patients. This review focuses on the potential use of stem cells for neurological disorders, mainly Parkinson’s disease (PD), Huntington’s disease (HD), stroke, TBI, amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and multiple system atrophy (MSA) with an emphasis on their relation to aging. In subsequent sections, we highlight relevant literature in both pre-clinical and clinical settings and raise relevant translational questions that may help to advance the field toward clinical use of stem cells for neurological disorders.
2. Inflammation, Stem Cells, and Aging
The neuroinflammatory response is known to play a role in the progression of a variety of neurodegenerative disorders. Although it is a natural process by which the body attempts to clear the brain of injured cell debris, inflammation can cause further cell death in TBI and stroke if prolonged. In response to altered homeostasis, components of the innate immune system, such as phagocytic microglia and infiltrating neutrophils, participate in pro-inflammatory cytokine secretion to induce increased permeability of the blood-brain barrier and the recruitment of other immune cells (Ransohoff et al., 2015). The adaptive immune system also contributes to inflammation, consisting of antibody-producing B cells and several types of T cells, but it is important to note that B and T cells act at the periphery (Ransohoff et al., 2015).
Stem cell grafts exert powerful immunomodulatory effects in the CNS despite few differentiate into the injured cell phenotype (Hirano, 1990). Mesenchymal stem cells have been shown to rescue neurons after exposure to oxygen-glucose deprivation by the inhibition of inflammatory cytokine tumor necrosis factor (TNF)-α (Huang et al., 2014). Similarly, bone marrow-derived mesenchymal stem cells host an endogenous population of T-regulatory cells which have anti-inflammatory effects such as the suppression of interleukin-6 and TNF-α secretion (Neal et al., 2018). Moreover, stem cells possess an anti-inflammatory secretome of growth factors and cytokines that facilitate brain repair after injury (Drago et al., 2013).
During the aging process, immune cells begin to work aberrantly, hindering critical homeostatic pathways related to brain regeneration and repair. For example, the fragmentation of microglia increases with age, leading to senescent microglia and the generation of a pathological immune response (Safaiyan et al., 2016). In response to systemic inflammation, microglia from middle-aged mice show increased secretion of pro-inflammatory cytokines compared to juvenile mice (Nikodemova et al., 2016). The exaggerated immune response associated with aging causes further cell death. In neonates, stem cells exhibit greater proliferative and immunosuppressive capacity (Batsali et al., 2017; Kim et al., 2013). To this end, the neonatal brain establishes an environment more conducive for both endogenous and exogenous stem cells to achieve therapeutic effects than the older brain after injury (Yasuhara et al., 2006b, 2006c). Differences in the basal immune system between the young and old brain must be taken into consideration when designing and engineering stem cell therapies across the spectrum of developmental and aging neurological disorders.
3. Stem Cell Applications for Aging and Neurological Disorders
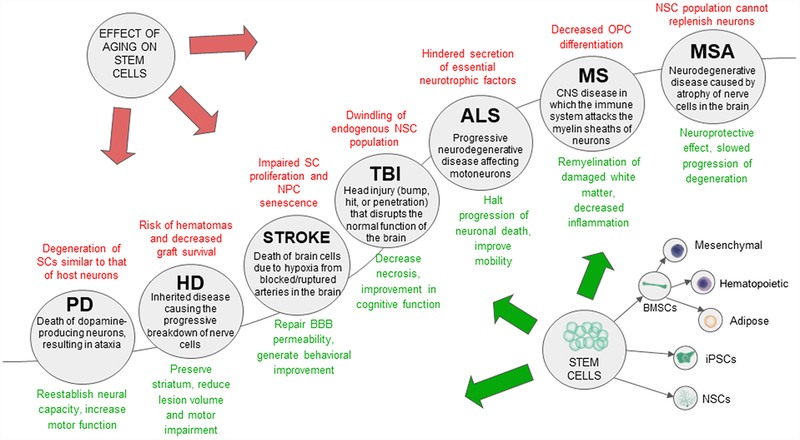
With recent advancement in life sciences technologies, novel tools are available to investigate the potential of cell therapy for many neurological disorders mentioned above both in vitro and in vivo (Morimoto et al., 2013; Bhattacharya and Stubblefield, 2013; Tajiri et al., 2014b; Liang et al., 2016). In order to develop safe and effective cell therapy methods that can be translated to clinical application, it is important to understand the complex underlying pathologies of these diseases, particularly with aging as a contributing factor. Aging is not only a risk factor for many central nervous system (CNS) disorders, but also contributes to their progression. Figure 1 summarizes the effects of aging in neurological diseases, endogenous stem cells, and transplantation of exogenous stem cells. The following sections will focus on the effects of aging on CNS disorders and the potential use of stem cells for treating their underlying causes.
Figure 1: The effect of aging on stem cell therapy in age-related disorders.
Stem cell therapy demonstrates a potentially neuroprotective effect on age related diseases listed in the illustration. However, aging could diminish the therapeutic effects of stem cells within these diseases, potentially leading to graft failure and other risks.
3.1. Parkinson’s Disease
PD is one of the most prevalent neurodegenerative diseases. Several treatments for the early-stages of PD help manage early symptomology (Schuepbach et al., 2013), but progressed forms present more complicated motor abnormalities that are unmanageable (Lindvall et al., 2012). In addition, aging has been shown to present morphological changes in a Parkinsonian brain (Vermilyea et al., 2017). The function of the nigrostriatal dopamine (DA) system declines as the host progresses in age (Vermilyea et al., 2017). Stem cells have been implicated as an efficacious treatment for early PD, with the advantages of preventing the disease from progressing to more severe stages (Schuepbach et al., 2013). Past experimentation has shown that transplanting stem cells into the brain allows them to assimilate in dopaminergic deficient regions and re-establish neural capacity through the mechanism of differentiation and/or releasing of neurotrophic factors (Lindvall et al., 2012). Similarly, studies have shown transplanted intracerebral stem cells to acquire local astroglial phenotypes or reprogram host cells to display astroglial characteristics to promote the emergence of local dopaminergic neurons (Altarche-Xifro et al., 2016; Niclis et al., 2017). Accordingly, transplanted stem cells show significant benefits that may implicate stem cells as an early treatment for PD or for use as a combination treatment with standard dopaminergic treatments (Table 1).
Table 1: Parkinson’s Disease.
In this table, pre-clinical studies from between 1995 and 2018 which specifically investigated stem cell therapy with intracerebral transplantation are referenced. Although many PD clinical trials are underway, data is not yet published and is therefore not included in this table. In summary, these milestone discoveries indicate that transplantation of NSCs, ESCs, and iPSC-derived DA neurons ameliorates Parkinsonian dyskinesia and promotes neurogenesis in rat models of PD.
| Authors | Model | Cell Type | Cell Quantity | Outcomes |
|---|---|---|---|---|
| Nikkhah et al., 1995 | 6-OHDA-lesioned rats | E14 rat fetal-derived DA rick cells | Two injections of 4×104 | Neurogenesis |
| Yasuhara et al., 2006a | 6-OHDA-lesioned rats | Human fetal-derived NSC | 2×106 cells | Neuroprotection, Neurogenesis, Behavioral amelioration |
| Kriks et al., 2011 | 6-OHDA-lesioned mice, rats and monkeys | Human ESC and iPSC-derived DA neurons | 1.5×105 for mice, 2.5×105 for rats, 7.5×106 for monkeys. | Neuroprotection, Neurogenesis |
| Kirkeby et al., 2012 | 6-OHDA-lesioned rats | Human ESC and fetal-derived NSC | Two injections of 1.5×105 cells at d10, 3×105 cells at d16 | Neurogenesis, Behavioral amelioration |
| L’episcopo et al., 2018 | MPTP-injected mice | Mouse NSC | 1×105 cells | Neuroprotection, Immunomodul ati on |
6-OHDA – 6-hydroxydopamine; DA – dopamine; NSC – neural stem cells; ESC – embryonic stem cells; iPSC – induced pluripotent stem cells; PD – Parkinson’s disease
Monkeys and rodents are the most used animals in experimental studies for PD models through the application of 6-hydroxydopamine or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Ambasudhan et al., 2014). Although intravenous and intra-arterial administrations have been the preferred methods because of their minimally invasive properties (Glover et al., 2012; Lescaudron et al., 2014), peripheral transmissions result in a declined capability of homing mechanisms to differentiated cells (i.e., DA neurons for PD) that express a low capacity for migration. Although PD patients typically have a partial decline of blood brain barrier (BBB) integrity, cells delivered peripherally may still be prevented from reaching the targeted areas in the brain. In the past, ventral mesencephalon embryonic DA neurons were employed via intracerebral transplantation, but microtransplantation techniques have been developed to minimize the traumatic effects of more invasive techniques (Nikkhah et al., 1995).
Although stem cell procurement presents an array of ethical issues, researchers have been able to derive stem cells from sources that are not as controversial (Ghosh et al., 2014). Induced pluripotent stem cells (iPSCs) may allow for sufficient amounts of DA neurons and present other beneficial characteristics such as cell reprogramming, which converts precursor cells into a stem cell lineage. Activation of the sonic hedgehog (Shh) pathway in combination with WNT signaling allows iPSCs to contribute to neuron survival and control restoration of amphetamine-induced rotational behavior in mice and rat models of PD (Kriks et al., 2011). Neural progenitors were transplanted and subsequently enhanced neural cell differentiation and promoted cell survival in the host brain 10 days after intrastriatal injection (Kirkeby et al., 2012). Neither tumorigenesis nor necrosis were present in the engraftment area six weeks later suggesting an absence of graft rejection (Kirkeby et al., 2012). A recent study has also demonstrated the therapeutic potential of neural stem cells (NSCs) transplanted into an aged PD brain. The grafted NSCs restored midbrain DA neuronal function (L’Episcopo et al., 2018). A collection of studies introduces the impact of iPSCs on older individuals, suggesting that the deletion of all senescent epigenetic markers can revert stem cells back to a rejuvenated state resembling that of a young person (Lopez-Leon et al., 2017). Moreover, older iPSCs can differentiate into cells expressing younger characteristics (Lopez-Leon et al., 2017). Researchers identified that, in order for the deletion of aged epigenomes to take place, dedifferentiation of the pluripotency stage is needed (Lopez-Leon et al., 2017). Thus, cell differentiation presents rejuvenating potential in old neurotoxic cells. Similar studies were conducted using Cj-iPSCs derived from adult marmoset fibroblasts (Lopez-Leon et al., 2017). After performing in vitro cell differentiation methods, results suggested that marmoset stem cells are able to target and regenerate the dopaminergic system (Lopez-Leon et al., 2017). Researchers are continuously uncovering more properties of stem cells’ regenerative potential in the aging Parkinsonian brain. Although recent studies have been successful, there is not sufficient data to begin clinical trials (Ghosh et al., 2014).
After the first successful transplantation of stem cells in 1987, many PD patients have volunteered to be part of these clinical trials. Although human fetal tissues have shown to increase motor function, due to ethical issues, many scientists have refrained from using these tissues (Tarazi et al., 2014). Currently, clinical trials for evaluating safety are ongoing. In the United Kingdom, project TRANSEURO is investigating the benefits of grafting allogeneic dopaminergic neuroblasts derived from fetal ventral mesencephalic tissue into PD patients to recuperate the lost DA neurons (). Intracerebral transplantation, although invasive, allows for direct targeting of specific areas that promotes normal neuronal function within the brain (Fangerau et al., 2014). On a similar note, transplanted cells are more likely to be lost in the peripheral systems, and the BBB will not be crossed to migrate long distances (Fangerau et al., 2014). This method of direct microinjection increases the likelihood of neural restoration while delivering a viable amount of stem cells. Intracerebral microinjection instruments, in combination with an accurate three-dimensional array of stereotactic surgery, will likely decrease surgical trauma and successfully introduce the stem cells to the targeted area.
Age is directly correlated with negative changes in the brain’s morphology, but the effects of aging on stem cells’ ability to promote differentiation and activate restorative mechanisms demand further investigation. As previously stated, ventral midbrain tissues from terminated human fetuses have been shown to increase motor function for several years after transplantation (Ambasudhan et al., 2014). Yet, it is worth noting that the maturity of embryonic donors influences the DA neuron survival rate in PD therapy (Freeman et al., 1995). The upper age limit for mesencephalic DA neurons is 56–65 days post conception depending on the particular graft. It seems likely that grafts harvested after this window are not viable due to axotomy transpiring during the engraftment process (Freeman et al., 1995). After the graft is placed in the brain, cell behavior can elucidate their efficacy (Freeman et al., 1995). The recipient age and health status are as equally important as the donor age. In a study, fetal neural allografts were examined 16 years post-transplantation. Inspection revealed the newly differentiated neurons to have tau pathology, a protein present in several neurodegenerative diseases. This finding suggests that aberrant tau proteins are manifest in newly differentiated cells arising from grafted tissues despite these cells being free of such pathology before engraftment (Cisbani et al., 2017). This suggests that age-related pathologies may also negatively affect the newly-grafted stem cells. This complicated relationship between aging and stem cells must be taken into consideration in order to have successful cell therapy for PD.
3.2. Huntington’s Disease
Due to its particular inheritance pattern, HD can be predicted through genetic testing as early as in utero (Emerich et al., 2006). Symptoms of HD typically emerge around the age of 40–50 after which point functional decline progresses with aging, resulting in death at a mean of 15–20 years later (Lee and Kim, 2006). It has been suggested that aging is correlated with the accumulation of protein aggregates due to protein misfolding and the decline of the antioxidant defense system with a resulting increase in oxidative stress, processes associated with the development of HD (Butterfield et al., 2001; Oh et al., 2014). Even though symptoms of HD arise during middle age for most, neural degeneration begins at least 15 years prior to motor symptom development as white matter degradation and striatal atrophy characteristic of HD are already detectable by MRI at this point (Fink et al., 2015). Ten percent of cases exhibit an onset prior to age 20 and are classified as Juvenile HD (JHD) (Fink et al., 2015). The emergence of HD is associated with an increase in the number of CAG repeats on the huntingtin gene where over 38 repeats is expressed as adult onset while over 60 repeats transmits JHD (Fink et al., 2015).
Although a cure for HD remains undiscovered, stem cell therapy may temporarily combat the functional effects of HD progression consequential of aging, thus improving quality of life for patients (Table 2). Pursuing a means to minimize the rate of functional decline in HD patients, many experimental therapies have worked with HD models to graft cells into the striatum after lesion development by cause of neurotoxins such as quinolinic acid (QA) and preserve the structural and functional integrity of the striatum (Emerich et al., 2006). Similar to the functional effects observed in transplants of iPSCs (Mu et al., 2014) and olfactory ensheathing cells (OECs) (Emerich et al., 2006), introducing choroid plexus (CP) into QA lesions allowed the cells to manipulate both the brain’s homeostatic processes and the release of trophic factors (Emerich et al., 2006). Transplants of CP into QA-lesioned rat striata not only result in reduced lesion volume and motor impairment, but also mitigate excitotoxic damage to neuronal groups (Borlongan et al., 2007). In effect, this treatment preserves glial cells’ endogenous production of chemokines and neurotrophic factors from the defective huntingtin allele (Baig, 2014). Engrafting CP into the putamen and caudate also displays neuroprotective effects in HD primate models, reducing lesion volume five-fold with respect to the control group (Emerich et al., 2006). Evidence suggests that OECs may undergo somatic cell nuclear transfer to induce their differentiation into glia, allowing for transplantation that avoids an immune response (Mu et al., 2014). Eliminating the need for neurosurgical techniques, the proposed method for the introduction of OECs suggests grafting the cells onto the cribriform plate of the ethmoid bone (Baig, 2014). In current rodent models of HD, iPSC grafts are able to survive and migrate to the impaired striatum (Baig, 2014). Once transplanted, the iPSCs also demonstrate the ability to differentiate into glial cells and neurons (Baig, 2014). The recently suggested “biobridge” mechanism attests to the capacity for stem cells to promote both the movement of endogenous cells to the damaged area and their release of integral neurotrophic factors (Lee et al., 2017). Transplantation of iPSCs is able to decrease degeneration of the striatum, improve functional recovery, and potentially form a microenvironment that promotes the initiation and proliferation of glia, avoiding excitotoxic damage to striatal γ-aminobutyric acid (GABAergic) neurons (Borlongan et al., 2007).
Table 2: Huntington’s Disease.
In this table, all clinically relevant in PubMed from 2000–2018 using intracerebral transplant for Huntington’s Disease are cited. In summary, these milestone studies showed that CP cells, iPSCs, and ESCs transplantation promotes reduction of lesion size, migration and proliferation in the striatum in animal models of HD. In humans with HD, fetal tissue implantation results in improved cognitive and motor function, but the cells do not survive long term or become permanently engrafted within the striatum.
| Authors | Model | Cell Type | Cell Quantity | Outcomes |
|---|---|---|---|---|
| Emerich et al., 2006 | Primate intracerebral | CP | 50,000 cells per mL | Reduction of lesion volume by 5-fold |
| Mu et al., 2014 | Rodent Intrastriatal | iPSCs | 1,000,000 cells | Migration of iPSCs to striatum and differentiation into glial cells |
| Borlongan et al., 2007 | Rodent Intracerebral | CP | 50,000 cells per mL | Attraction of glial cells and proliferation in the striatum |
| Bachoud-Levi et al., 2000 | Human intrastriatal | Fetal striatal tissue | Not Specified | Improved cognitive and motor function in daily activities |
| Keene et al., 2007 | Human intracerebral | Fetal tissue | Not Specified | Grafts exhibited limited to no HD characteristics |
| Bachoud-Levi et al., 2006 | Human intracerebral | Fetal Neuronal Tissue | Not Specified | Grafts showed unhealthy morphology and no survival in the caudate region |
| Jiang et al., 2011 | Rodent intrastriatal | Embryonic cells | 130,000 cells/μl | Greater levels of striatal-like neurons in graft sites |
HD – Huntington’s Disease; ESC – embryonic stem cells; iPSC – induced pluripotent stem cells; CP – choroid plexus
The current outcomes of grafting stem cells into HD patients are inconsistent. Use of intrastriatal grafts comprised of human fetal striatal tissue has shown improved daily cognitive and motor functions in three of five total patients (Bachoud-Levi et al., 2000). Enhanced functionality in transplantation HD patients relative to untreated HD patients is further supported as images display heightened metabolic activity at the graft (Bachoud-Levi et al., 2000). Another study has found that grafts examined in two patients post-mortem 74 and 79 months following fetal neural transplant present no signs of pathological HD (Keene et al., 2007). Despite the success of the studies described above, insufficient integration of the grafts into the hosts restricts further improvement in clinical models than it has previously in animal models (Keene et al., 2007). Recent results show that a decade after the initial transplantation, grafts in three HD patients did not maintain healthy neuron structure and yielded no survival in the caudate region (home to the most severe astrogliosis and neuronal degeneration in cases of HD) (Cicchetti et al., 2009). The failure to ameliorate the destruction of the caudate region demonstrates the region-limited benefits of stem cell grafting in HD patients (Cicchetti et al., 2009). Even so, graft survival in patients 18 months and 6 years following transplantation advocates for additional fetal cell engraftment trials (Cicchetti et al., 2009).
Although HD and JHD exhibit similar degenerative symptoms, different approaches must be employed in the development of effective stem cell therapies (Fink et al., 2015). JHD patients not only exhibit a more severe phenotype than HD patients, but also experience a more rapid progression of the disease (Fink et al., 2015; Squitieri et al., 2011). Due to its accelerated development, treatment options that are in consideration for HD, such as neuroprotection through the use of genetically engineered MSCs, are not likely to be viable options for JHD due to excessive neuronal loss relatively early in the course of the disease (Fink et al., 2015). Although investigation into simple replacement of medium spiny neurons (cells that characterize the primary neural degradation of HD) has the potential to generate hope for long-term recovery in the early stages of HD, treatment prospects diminish as aging progresses (Gogel et al., 2011). Cell grafts have been shown to differentiate when introduced to host tissue, providing provisional benefits to the deteriorating area. However, these implants atrophy faster than the original tissue (Gogel et al., 2011). Furthermore, if introduced into patients who have progressed to advanced stages of HD, cell grafts may be more likely to lead to hematomas and exhibit low survival rates in severely degraded regions (Gogel et al., 2011).
Although the functional benefits of stem cell transplantation can be recognized in HD patients for several years, they do not present a cure and the effects of aging continue to overshadow treatment prospects (Bachoud-Levi et al., 2006). The concerns that arise in response to the use of fetal cell grafts as a treatment for HD echo issues regarding cell graft use in PD patients. The constrained migratory ability of differentiated cells (i.e. GABAergic for HD) limits the effectiveness of minimally invasive transplantation, thus reducing the practicality of their use in treatment (Yasuhara et al., 2006). Furthermore, peripherally grafted cells may have difficulty crossing a healthy BBB to reach the failing striatum (Ghosh et al., 2014). Despite its invasiveness, these concerns defend intracerebral transplantation as the most viable cell transplant method for both PD and HD. Micro-transplantation, originally developed in the treatment of PD, is a method of intracerebral transplantation that may be used to mitigate harmful side effects of the procedure in HD patients. The technique’s use of multiple graft sites and variable numbers of cells per site has the potential to influence the quantity of developing neurons (Jiang et al., 2011). By increasing the number of graft sites used with micro-transplantation, the graft-host interface is expanded and more cells are exposed to endogenous trophic factors, resulting in a higher yield of striatal-like neurons (Jiang et al., 2011). Optimizing the transplantation procedure is critical in order to apply cell-based grafts to clinical HD (Jiang et al., 2011).
To add to the limited on-going clinical research of stem cell therapy to treat HD and achieve clinical application, progressive modifications in motor and cognitive functions, brain imaging, and blood and spinal fluid markers in both early and chronic HD patients must be given thorough attention (Jiang et al., 2011). These markers of efficacy may be beneficial in measuring the effects of intrastriatal transplantation of mesenchymal stem cells (MSCs) in large-scale clinical studies (). The advantages of delivering GABAergic cells via an intracerebral route as a treatment for HD could reduce the dosage required to transplant them peripherally, akin to the treatment of PD with dopaminergic cells, presenting a potential procedure by which to rehabilitate the deteriorated striatal region in HD patients (Choi et al., 2018; Kim et al., 2008). As in PD, the use of stem cells in the treatment of HD remains most effective in the form of intracerebral transplantation.
3.3. Stroke
In spite of development and advancements made within the field of medical science, stroke remains notorious for being a major cause of death and disability worldwide (Guzik and Bushnell, 2017). Animal models are used to explore the ever-expanding field of therapeutics, due to stroke’s significant contribution to mortality in the United States (Mozaffarian et al., 2015). Organized stroke unit care and reperfusion comprise 85% of ischemic stroke therapy. In light of acute stroke therapy, selected patients with large artery occlusions may receive intravenous thrombolysis or, more recently, endovascular mechanical thrombectomy treatment (Goyal et al., 2016). Systemic thrombolytics show a significant beneficial effect; however, they are used less often due to a small window of opportunity for administration following stroke. Therefore, it is imperative to develop novel strategies that do not require such a time constraint. Stem cell-based treatment may be the answer for widening the window of opportunity (Table 3). Stem cell techniques in animal models are used to closely mimic human infarcts that occur as the result of ischemia in terms of structure, size of affected area, and pathological manifestation. A widely used stroke induction strategy used in animal models occurs by way of middle cerebral artery occlusion (MCAO) for modeling the damage invoked by ischemic stroke seen in humans. In addition to animal modeled MCAO techniques, endothelin-1 injection and photothrombosis are also used to produce ischemic lesions that mimic human infarcts (Casals et al., 2011).
Table 3: Stroke.
In this table, all pre-clinical studies from 2004 to 2018 which focused on stem cell therapy for stroke are referenced. Moreover, the clinical trials presented were selected due to their 1) recency and 2) availability of published results. In summary, pre-clinical investigation in animal models of stroke show that treatment with various stem cell types improved sensorimotor functioning and reduced infarct volume. In humans, stem cell treatment was shown to be safe and effective in improving left ventricular function and NIHSS scores in stroke patients.
| Author | Model | Cell type | Cell Quantity | Outcomes |
|---|---|---|---|---|
| Borlongan et al., 2010 | MCAO | Menstrual blood cells | 4×105 cells | Sensorimotor improvement |
| Kurozumi et al., 2005 | MCAO | Human BM-MSC | 5 × 105 cells | Reduction of infarct-volume and Sensorimotor improvement |
| Fukuda et al., 2004 | MCAO | Human BM-MSC | low-dose 1 × 106 cells high-dose 6 × 106 cells |
Sensorimotor improvement |
| Borlongan et al., 2004c | MCAO | CP cells | 50–55 microcapsules | Sensorimotor improvement |
| Hara et al., 2007 | MCAO | NT2N cells | 2× 105 cells | Sensorimotor improvement |
| Tajiri, et al., 2012 | MCAO | human NSCs | 5 × 103 cells 1 × 104 cells 2 × 104 cells |
Reduction of infarct-volume and Sensorimotor improvement |
| Borlongan et al., 2005 | MCAO | human bone-marrow-derived CD133+ cells | 1 × 104 cells | Sensorimotor improvement |
| Nelson et al., 2002 | Human Stroke Patients | human NT2N | 2 × 106 cells | Safe (no adverse effects) |
| Bang et al., 2016 | Human Stroke Patients | MSC | 1 × 108 cells | improved left ventricular function |
| Kalladka et al., 2016 | Human Stroke Patients | Human neural stem cells 2 × 106 Intracerebral | Human neural stem cells 2 × 106 Intracerebral | improvement in NIHSS score |
BM-MSCs – bone marrow-derived mesenchymal stem cells; MCAO – middle cerebral artery occlusion; NT2N - Ntera2/D1 neuron-like; NIHSS – National Institutes of Health Stroke Scale; NSC – neural stem cells; CP – choroid plexus
Selection of stem cells in transplantation may approximate the success of stroke therapy. It is known that debilitative stroke has a highly abrupt onset, thus, off-the-shelf cryopreservable stem cell sources provide an advantage to research both ethically and logistically. These may be used for future treatment in opposition to freshly harvested stem cells, primarily in regard to treating ischemia (Rodrigues et al., 2011). Cryopreserved stem cells may either be thawed or transplanted instantly, providing a larger window for therapy while also lessening the time lapse between stroke onset and medical therapy (Borlongan et al., 1998). Additionally, cells that do not elicit host immune response are advantageous due to their adherence to safety criteria. A viable cell type for use in stroke therapy is that of autologous origin, because it is less likely to invoke harmful inflammatory and immune response following transplantation. Menstrual-blood, bone marrow, adipose tissue, and peripheral blood-derived cells are considered valuable candidates for cell-based therapy. These cells are valued due to their autologous source being less likely to experience hindrance by ethical limitation. In addition to this, these cells provide stem cell markers and maintain multi-lineage differentiation potential, self-renewal, and the ability to ameliorate histological and behavioral impairments that are actuated by stroke (Borlongan et al., 2010; Kaneko et al., 2013; Rodrigues et al., 2011).
Despite cell transplantation possessing high potential for therapeutic use, many transplanted cells are killed within regions of ischemia due to hostile microenvironments generated as the result of stroke (Hicks et al., 2009). It is of critical importance that the success of cell transplant therapy is better ensured via successful induction of differentiation into cerebral cell types and increased survival of the grafted cells. Oligodendrocyte precursor cells are mobilized in response to ischemic injury, with the cells’ differentiation into a mature oligodendroglial lineage implicated as mitigating ischemic demyelination. Age detrimentally affects this white matter-related regenerative response, as well as the body’s overall capacity to produce new neurons after episodes of ischemia (Liang et al., 2016; Miyamoto et al., 2013). Thus, the production of important trophic factors such as osteogenic protein-1 or glial cell line-derived neurotrophic factors (GDNF) must be considered when transplanting cells (Chiang et al., 1999). The generation of trophic factors may serve as adjunctive therapy due to their dual ability to protect against ischemia-induced injury while also promoting grafted stem cell survival. Intrastriatal grafting of mouse bone marrow stem cells (BMSCs) within MCAO models has demonstrated a dose-dependent ability to repair BBB permeability and re-establish normal perfusion (Borlongan et al., 2004b). An additional therapeutic potential may reside in adult rat isolated CP grafts that undergo the process of alginate microencapsulation. As a result of this, motor deficits and infarct volume were markedly reduced suggesting a promising potential for hypoxic cells via the utilization of alginate microencapsulation methodology (Borlongan et al., 2004c). Amelioration of behavioral deficits, committal to neuronal lineage, and high GDNF secretion were observed with transplantation of a teratocarcinoma-derived Ntera2/D1 neuron-like (NT2N) cell line intracranial transplant into the rodent brain. NT2N transfection was observed with a different source for stroke cells being provided by nuclear receptor related-1 protein (Nurr1) transcription factor (Hara et al., 2008). MCAO models that have been intravenously transplanted with amniotic fluid-derived stem cells (AFSCs) have also been attributed to attenuation of histological and attenuated deficits, likely due to the upregulation of cell differentiation, proliferation, and endogenous repair mechanisms, all of which have been observed to occur within the dentate gyrus (DG) and SVZ (Tajiri et al., 2012). Within the striatum, different doses of intracerebral human neural stem cells (NSC) have been grafted and revealed a dose-dependent neurological and motor recovery in stroke induced rats (Tajiri et al., 2014c). Reduction of infarct volume was not detected within a study using NSC cell line transplantation, however, behavioral improvements were observed (Tajiri et al., 2014c). Although there may be some uncertainty about stem cell treatment’s mechanism of action, the transplantation of some NSC lines was showed to demonstrate improvement in functional recovery following stroke induction within rat models (Ishikawa et al., 2013). It has been suggested that vascular endothelial growth factor (VEGF) is responsible for the promotion of neurogenesis and vasculogenesis within stroke animals transplanted with endothelial cells. These findings were built upon the discovery that regenerative processes are prompted following the targeting of vascular repair within experimental stroke models (Ishikawa et al., 2013).
Faulty repair mechanisms post-stroke are associated with the aging of stem cells and acquired behavioral and motor deficits. Heterochronic parabiosis, a method that allows surgically linking the circulatory systems between two animals of different age, is used to assess the effect of aged stem cells in young mice and young stem cells in aged mice. In oculo transplantation is used to assess how host environment affects stem cell proliferation (Conboy et al., 2013). In oculo transplantation, a technique in which transplants are placed in the anterior chamber of the eye (allowing graft visualization), within the host environment demonstrates that circulating factors play a role in impairing neurogenesis and lowering efficiency of repair. Additionally, heterochronic parabiosis confirmed the presence of circulating factors that negatively impact stem cell proliferation, induce neural progenitor cell senescence, and alter lineage fate commitment (Katsimpardi et al, 2014). Young cells in aged mice exhibited increased proliferation, attributed to the activation of signaling pathways in NSCs (Conese et al., 2017). Young human mesenchymal stem cells (hMSCs) produce fewer microglia following transplantation in ischemic stroke, demonstrating that inflammation and immune response are more likely to produce efficient clean-up of tumor necrosis factors (TNFs) post-stroke. Histological analysis of aged hMSC transplantation revealed increased numbers of microglia and pericyte covered vessels, further supporting the lack of efficient control of inflammation (Yamaguchi et al., 2017). Aging has a mechanism of action that involves decreased Notch signaling which then causes downregulation of satellite cell regeneration. Parabiosis was used to further assess whether Notch signaling became reactivated when young stem cells were shared with aged mice. Restoration of Notch signaling, regenerative and proliferative capacity were observed, demonstrating that Notch activation plays a role in maintaining efficient stem cell activity in young mice (Conboy et al., 2005).
Grafting procedures need to be examined and optimized in addition to the development and exploration of therapeutic stem cell transplant’s mechanism of action following stroke. In specific cases, functional benefit in stroke models is served by the tailoring the effect of human bone marrow-derived CD133+ cells during transplantation (Borlongan et al., 2005). Additionally, functional benefits depend on the time of administration as well as graft survival route during cell transplantation procedures (Borlongan et al., 2005). Within this study, motor deficit reduction and graft survival demonstrated localization in models that used both delayed and immediate transplantation. However, immediate intravenous transplantation resulted in alleviation of behavioral deficits while delayed intravenous transplantation showed to be critical for the sustenance of graft survival (Borlongan et al., 2005). Thus, the results of this illuminate the importance of stem cell transplant regulation in order to provide the most efficient application of stem cell therapy for stroke patients (Borlongan et al., 2005).
Understanding of cell therapy mechanisms has advanced exponentially within the past four years since this topic was last reviewed (Savitz, 2015), and clinical trial design has been modified significantly in response to this expansion of knowledge. Initially, in 1998, FDA approval was granted due to the demonstration that safety and feasibility of NT2N cell transplantation therapy was provided in twelve stroke patients (Borlongan and Hess, 2006). At 27 months post-surgery, one of the twelve patients received a cell graft of NT2N neurons within proximity to a lacunar infarct. During the post-mortem assessment, there was no evidence of neoplasm within the transplanted area, demonstrating that there is no evidence of harmful effect following more than two years of NT2N neuron transplant (Nelson et al., 2002). On the contrary, a large clinical trial was initiated due to a major limiting factor of NT2N transplantation. It was found that NT2N neurons have the ability to cause neoplastic state reversion following transplantation. This neoplastic state reversion is attributed to their cancerous origin and property of high proliferation (Hara et al., 2008). Thus, it is imperative to explore approaches that enable the allocation of safer progenitor and stem cells as sources for graft alternatives (Borlongan and Hess, 2006). Fluorodeoxyglucose uptake (positron emission tomography) was found to be improved at the site of implantation of hNSC grafts of patients with basal ganglia stroke or motor deficits as the result of stroke (Kondziolka et al., 2000). Further demonstrating the safety and feasibility of this therapy (Bang, 2016), after hNSC transplantation, promotion of daily living was observed over six years post-transplantation (Kondziolka et al., 2005). Stereotactically implanted hNSCs have recently demonstrated the regaining of neurological function in patients affected by stroke (Kalladka et al., 2016). To further support the safety and feasibility of stem cell transplant in stroke patients, large amounts of evidence have shown that stem cell administration during different stroke phases is effective within a range of various administration routes (Nagpal et al., 2017). Various cell types, including allogeneic adipose cells, autologous bone marrow stromal cells, adipose cells, and endothelial progenitor cells have been investigated for use in stroke therapy (; ; ; ). Alleviation of acute stroke has shown to be most effective via intravenous transplantation. During the acute phase of stroke, upregulation of chemoattractant cues in the ischemic brain cause the homing of peripherally grafted cells to the site of injury. As mentioned earlier, inflammation and immune response increase in response to stroke. Intracranial administration of grafted cells to the site of injury is a more invasive route, likely responsible for agitation of inflammatory and immune response, thus, decreasing cell graft survival. By utilizing intravenous administration, the migration of cells via chemoattractant signals produce less damage at the site of injury and is an overall minimally invasive procedure.
During the stage of chronic stroke, a more invasive route of administration is best-- this being intracerebral transplantation. Intracerebral transplantation is considered to be an ideal approach due to ischemic repair requiring high viability and large populations of cells localized at the area of damage (Hara et al., 2007). Bone-marrow derived stem cells such as EPCs, MSCs, human telomerase-reverse transcriptase-immortalized mesenchymal stem cells, (hTERT-MSCs), HSCs, and very small embryonic-like stem cells (VSELs), have been investigated to study migration and tendency to senesce during intracerebral transplants following TBI and ischemia (Borlongan et al., 2011). Migration and viability must be maintained as long as possible, due to the gradual decrease of chemoattractant signaling. As time passes and chemoattractant signaling lessens, peripherally grafted cells migrate less efficiently to the site of injury as compared to how they would have during acute stroke. Because of this, intracerebral transplantation is the preferred method for delivering cells to the brain. This procedure allows for the chronic stage to undergo stabilization with less inflammatory and immune response as opposed to acute stage transplantation. An issue with intracerebral transplantation is that multiple transplants are not feasible due to the surgical trauma caused by the procedure (Hara et al., 2007). On the other hand, several studies have shown that successful migration of stem cells ensued following intravenous transplantation at a later time (Zhang et al., 2011). A study demonstrated that intravenously transplanted human bone marrow endothelial progenitor cells (hBMEPCs) endogenously enhance post-stroke vasculogenesis by migrating to the BBB for close involvement in vasculature repair. This finding emphasizes the importance of further considering intravenous transplantation as a means for therapeutic use in stroke (Garbuzova-Davis et al., 2017). The treatment of ischemia is currently in its initial stages of clinical trial testing using animal models. The majority of pre-clinical and early clinical stages of testing are focused around the premise that stem cell treatment of ischemia is feasible and safe. Additionally, a major obstacle for stem cell therapy in stroke is aging. A study found that intravenously administered human adipose-derived stem cells (hDASCs) were not nearly as efficient at migrating to the spleen in aged rats when compared to young. Stem cell migration to the spleen is important for neurotrophic secretion and efficient regulation of inflammatory response following stroke (Tajiri et al., 2014a). This finding further supports the need to examine differences between aged and young animal models, and to consider important factors such as inflammatory response, immune response, circulating factors, angiogenesis, and overall reparative response post-stroke. Moreover, stroke outcomes can be sex specific, and that secretion of hormones such as 17β-estradiol can exert protective or deleterious effects depending on age of the transplant recipient (Leon et al., 2012; Petrone et al., 2014). Careful consideration of sex and age differences should be pursued when developing stroke therapies to better suit a diverse patient population. By expanding scientific knowledge of these less obvious yet determinative differences between young and aged brains, novel strategies can be developed and better equipped for treating age-related damage and degeneration.
3.4. Traumatic Brain Injury
Worldwide, TBI is one of the major causes of disability and death, particularly within the United States. Studies indicate that in the United States alone, approximately 3.2 – 5.3 million individuals live with a TBI-related disability (Taylor et al., 2017). Such chronic disabilities affect one’s health and social environment. Additionally, severe fiscal consequences accrue as the result of medical bills and wages lost. Current TBI treatments are merely palliative in nature, as they do not lead to the regeneration of damaged neural architecture (Taylor et al., 2017). Drawing from this, novel treatments for acute and chronic TBI are needed. Historically, fluid percussion or controlled cortical impact injury (CCI) models have been employed within the field of TBI research (Hayashi et al., 2009). CCI is a preferential model because the resulting damage is closely comparable to cell death, edema, ischemia, excitotoxicity, and altered gene expression that occurs in human TBI (Hayashi et al., 2009).
Stem cells from different sources have recently been utilized as therapeutic modalities in the treatment of TBI in both pre-clinical and clinical investigations (Table 4). Stem cell transplants are most successful when performed quickly following the initial injury (Carroll and Borlongan, 2008). Following transplantation of bone marrow stromal cells (BMSCs) into the striatum, rats demonstrated functional improvements that correlated with cerebral reperfusion and increased BBB permeability (Borlongan et al., 2004b). Following peri-impact region injection, murine BMSCs survived and ameliorated neuronal behavior in rat TBI models (Shen et al., 2016). Grafts of BMSCs increased GAP-43-positive fibers as well as synaptophysin-positive varicosities. Rats subjected to TBI while treated with BM-MSCs showed decreased levels of BAX and BAD as well as heightened levels of GDNF protein (Allen et al., 2013). GDNF subsequently mitigated the induction of apoptosis and maintained neuron survival (Allen et al., 2013). It is, therefore, possible BMSC origin of GDNF is integral to neural remodeling as well as the attenuation of BAX/BAD apoptotic pathway signals.
Table 4: Traumatic Brain Injury.
In this table, all studies in PubMed from 2006 to 2018 with intracerebral transplant to treat TBI are referenced. In summary, these studies show that stem cell transplant following TBI potentiates neurogenesis and reduces inflammatory cytokines, reactive astrogliosis, and edema. These effects act together to improve motor function and recover some cognitive function.
| Author | Model | Cell type | Cell Quantity | Outcomes |
| Shen et al., 2016 | CCI | mBM-MSCs | 1×107 cells/mL | Increased number of GAP-43-positive fibers and synaptophysin-positive varicosity; suppressed apoptosis; release of GDNF; improved neurological function. |
| Deng et al., 2017 | CCI | rBM-MSCs cultured with SDF-1 | 5 μl of cell suspension | Increase of BDNF, NGF, neuronal nuclear antigens; Increase of Brd-U-positive cells and hippocampal neurons; decrease of apoptosis and necrosis; reduced edema. |
| Harting et al., 2009 | CCI | rNSCs | 4×105 cells | Motor improvements but not cognitive recovery. |
| Gao et al., 2016 | CCI | Fetal hNSCs | 0.5×105 cells/μl | Decreased brain lesion volumes; reduced axonal injury; reduced microglial activation; increase in the brain M2/M1 ratio coupled with anti-inflammatory phenotype. |
| Haus et al., 2016 | CCI | hESC-NSCs | 2.5× 105 cells | Cognitive recovery without affecting either lesion volume or total cortical or hippocampal tissue volume; increase in host hippocampal neuron survival; differentiation of transplanted cells into mature neurons, astrocytes and oligodendrocytes. |
| Chen et al., 2017 | CCI | Embryonic rNSCs overexpressin g BDNF | 2×107 cells/mL | Increased expression of neurofilament 200, microtubule-associated protein 2, actin, calmodulin, and beta-catenin; neuronal survival; neurite growth; MAP2 expression in neuron-like cells differentiated from transplanted cells, but also in host cells after transplantation. |
| Ghazale et al., 2018 | CCI | Neonatal mNSCs with DHA pretreatment | 1×105 cells | Promoted neurogenesis; increase in glial reactivity and tyrosine hydroxylase positive neurons; attenuated calpain/caspase activation |
| Sullivan et al., 2017 | CCI | Adult mNSCs | 5×104 cells | Reduced reactive astrogliosis and mi croglial/macrophage activation in the corpus callosum |
| Skardelly et al., 2014 | CCI | Fetal hNPCs | 1×105 cells | Transient functional and antiinflammatory benefits. |
| Bonilla et al., 2014 | Weight drop | rBM-MSCs | 2×106 cells | MSCs survived in the host tissue, and some expressed neural markers; no long-term differences in neurological outcome, lesion size and neurotrophin production. |
| Mastro-Martinez et al., 2015 | Weight drop | rAD-MSCs | 2×105 cells | Improved recovery of motor function; increased neurogenesis and cell density in the hippocampus. |
| Lam et al., 2017 | CCI | rAD-MSCs | 1.5×106 cells | Improved functional outcome; triggered earlier astrocytosis and reactive microglia; TBI penumbra higher cellular proliferation and reduced neuronal damaged; higher cellular proliferation and suppressed apoptosis in hippocampus; Attenuated proteolytic neuronal and glial cells injury biomarkers; up-regulation of six genes related to axongenesis (Erbb2); growth factors (Artn, Ptn); cytokine (IL3); cell cycle (Hdac4); and notch signaling (Hes1); 7,943 genes were differentially expressed. |
| Cheng et al., 2015 | Weight drop | hUC-MSCs with WJ tissue | 1mm3 | Attenuated edema; reduced lesion volume; improved neurological function; promoted memory and cognitive recovery; increased expression of BDNF. |
| Tajiri et al., 2013 | CCI | Notch-Induced hBM-MSCs | 3×105 cells | Novel stem cell repair mechanism exerted by stem cells in the repair of the traumatically injured brain that involve their ability to harness a biobridge between neurogenic niche and injured brain site promoting long-distance migration of host cells and therefore promoting the endogenous repair mechanisms. |
| Shindo et al., 2006 | CCI | mESC-NPCs | 1×106 cells | Significant cholinergic differentiation; barely GFAP+ astrocytes within the grafts; presynaptic formations of graft-derived neurons; increase in neurotrophic factors. |
CCI – controlled cortical impact; BM-MSCs – bone marrow-derived mesenchymal stem cells; mBM-MSCs – mouse BM-MSCs; GDNF – glial cell-derived neurotrophic factor; SDF-1 – stromal cell-derived factor 1; BDNF – brain-derived neurotrophic factor; NGF – nerve growth factor; rBM-MSCs – rat BM-MSCs; NSCs – neural stem cells; rNSCs – rat NSCs; hNSCs – human NSCs; hESC-NSCs – human embryonic stem cell-derived NSCs; mNSCs – mouse NSCs; DHA – decosahexaenoic acid; hNPCs – human neuronal progenitor cells; rADMSCs – rat adipose tissue-derived MSCs; hUCBs – human umbilical cord blood cells; G-CSF – granulocyte colony stimulating factor; hUC-MSCs – human umbilical cord mesenchymal stem cells; WJ – Wharton’s jelly; hBM-MSCs – human BM-MSCs; mESC-NPCs – mouse embryonic stem cells-derived neuronal progenitor cells
The SDF-1/CXCR4 axis involved in a plurality of processes of which the regulation of tumor development and stem cell migration is noteworthy (Deng et al., 2018). Rat BM-MSCs with SDF-1-induced CXCR4-expression enhanced TBI repair and functional recovery. When transplanted, these cells led to significant increase in BDNF, NGF, neuronal nuclear antigens, and BrdU-positive cells. Furthermore, transplantation increased the number of hippocampal neurons while maintaining morphological architecture, attenuating apoptosis, decreasing necrosis, and reducing interstitial edema (Deng et al., 2018).
Neural stem cells (NSCs) derived from various origins have been suggested to serve as next-generation neurotherapeutic targets in TBI. NSCs are capable of producing mature and functional neural cells that compensate for defective neuroglia while conferring immunomodulatory benefit. While acute intracerebral NSC transplantation demonstrates enhanced functional recovery within moderate TBI rat models, delayed transplantation culminated more controversial results, including inconsistent motor and cognitive recovery (Harting et al., 2009).
Intracerebral transplantation of primed fetal human NSCs (hNSCs) steered host microglia/macrophages toward the anti-inflammatory M2 subtype which putatively contributed to neuroprotective effects after severe TBI (Gao et al., 2016). As compared to the control, treated animals demonstrated diminished axonal injury, mitigation of brain microglia/macrophage activation, and a modified M1:M2 ratio. hNSC grafts exhibited an overall anti-inflammatory effect by favoring microglia M2 differentiation, reducing levels of the TBI-induced pro-inflammatory cytokine receptor, IFN-γRβ, and increasing levels of anti-inflammatory cytokine receptor, IL-4Rα. (Gao et al., 2016). hNSCs are a uniquely attractive modality as compared to MSCs because of their commitment to neural lineage differentiation. In a recent study, hNSC grafts differentiated into neurons in injured mouse brains (Gao et al., 2016). Thus, the benefits of hNSC transplantation are multifaceted due to modulation of the post-injury microenvironment as well as replacement of lost neural cells.
Additonally, TBI animals that received human ESC-NSC (hESC-NSC) transplant showed significant improvement in cognitive function (Haus et al., 2016). Following transplantation of hNSCs, behavioral improvement was seen in the absence of reduced lesion volume or increased cortical/hippocampal tissue volume (Haus et al., 2016). In spite of these findings, a significant increase in host hippocampal neuron survival was noted in hNSC-transplanted animals. This is indicative of a correlation between cognitive performance and hippocampal neuron survival. Transplanted hNSCs survived for at least five months after transplantation and, in response to injury, differentiated into mature neurons, astrocytes, and oligodendrocytes. This may be significant in facilitating cognitive recovery after TBI (Haus et al., 2016). Even twenty weeks post-transplantation, a significant proportion of cells appeared undifferentiated and continued to express Nestin (Haus et al., 2016).
Levels of antioxidant enzymes such as catalase, glutathione, and superoxide dismutase are suppressed via post-transcriptional modification during normal aging in rat models (Itoh et al., 2013). Neural parenchyma has minute concentrations of antioxidant enzymes as compared to other tissue types, due to the resident NSC population dwindling with age (Itoh et al., 2013). Because TBI leads to the exacerbation of reactive oxygen species production, a more pronounced ebbing in the NSC population was seen following injury, indicating caspase-induced apoptosis (Itoh et al., 2013). Aging leads to the acquisition of senescent phenotypes and biomarkers such as p16, (cyclin-dependent kinase inhibitor 2a) in hematopoietic stem cells, (HSCs) and muscle stem cells (MuSCs) (Chang et al., 2016). As noted in a recent study, when HSC and MuSC populatios were treated with ABT263 (a specific inhibitor of the anti-apoptotic proteins BCL-2 and BCL-xL); apoptosis was disinhibited in senescent stem cells subsequently engendering a qualitative increase without quantitative decrease (Chang et al., 2016). Significant improvements were exhibited in single-cell clonogenicity, long-term repopulation ability, balancing of multilineage differentiation, as well as long-term engraftment ability (Chang et al., 2016). Such improvements were presumably observed due to the filling of vacated senescent stem cell niches by nonsenescent stem cells (Chang et al., 2016). Though the aforementioned study examined HSCs and MuSCs, these results point auspiciously toward potential neural corollary.
Brain-derived Neurotrophic Factor (BDNF), a member of the neurotrophin family, plays a vital role in furthering differentiation, maturation, and survival of neurons as well as in attenuating apoptosis in CNS cells (Allen et al., 2013). Primary NSCs overexpressing BDNF (BDNF/rNSCs) were administered into the injured cortex resulting in the heightened expression of neurofilament 200, microtubule-associated protein 2, actin, calmodulin, and β-catenin (Chen et al., 2017). These findings suggest that BDNF/rNSCs may possibly contribute to neuronal survival, growth, and differentiation at injury sites (Chen et al., 2017). Enhanced survival, growth, and differentiation of transplanted NSCs may be associated with the heightened expression of cytoskeletal proteins and activation of the Wnt/β-catenin signaling pathway.
Docosahexaenoic acid (DHA) is a common polyunsaturated structural lipid in the brain which furthers brain development via neuron differentiation, neurite enlargement, and formation of synapses (Tanaka et al., 2012). Pup mice-derived NSCs pre-treated with DHA mitigated TBI-induced motor deficits, enhanced neurogenesis, and elevated glial reactivity when transplanted at the site of injury in a murine TBI model (Ghazale et al., 2018). Treated animals also demonstrated decreases in both GFAP fragmentation and αII-spectrin proteolysis, both of which have recently been validated as potential biomarkers of necrotic calpain-mediated neural injury. This suggests a downregulation of calpain/caspase activation (Ghazale et al., 2018).
Though the applications of ESC and fetal-derived NSC treatments appear to be extremely promising, considerable limitations include ethical concerns and risk of tumorigenesis. For these reasons, adult NSCs are used as they afford fewer ethical objections and represent a lower risk of tumor generation (Amariglio et al., 2009; Cave et al., 2014; Ramos-Zuniga et al., 2012). An experimental TBI was utilized to review the effects of adult NSC intraventricular transplantation on the response of endogenous NSCs in the SVZ as well as the inflammatory response in the corpus callosum (Sullivan and Armstrong, 2017). This transplantation decreased astrogliosis and the activation of callosal microglia/macrophages after TBI, though Shh signaling was not involved (Sullivan and Armstrong, 2017). Additional investigations are needed to fully elucidate the mechanisms underpinning the immunomodulatory effects of NSCs as well as their therapeutic potential.
TBI animal models receiving local and systemic transplants of fetal human neural progenitor cells (fetal hNPCs) exhibited transient anti-inflammatory and functional improvements (Skardelly et al., 2014). Hopeful results were seen in animals receiving systemic transplants (Skardelly et al., 2014). Comparable to intraparenchymal injections in terms of efficacy, subarachnoid administration is proposed due to being minimally invasive and lower risk (Bonilla et al., 2014). Following subarachnoid transplantation, BM-MSCs survived, translocated to the injury cavity, and differentiated into mature neural cell classes for a minimum of six months post-transplantation, devoid of evidence suggesting long-term neurological change, lesion dimension, and neurotrophin output (Bonilla et al., 2014).
Additional investigations are needed in order to fully comprehend the long-term effects of stem cell therapies as well as the differences between their local and systemic administration after TBI. Additionally, human adipose-derived stem cells (hADSCs) demonstrated an age-dependent effect following intravenous administration in young (six months) and aged (twenty months) rat models of TBI (Tajiri et al., 2014a). In the young animals, such treatment resulted in the mitigation of cortical insult and hippocampal cell loss, which further correlated with significant amelioration of motor and cognitive functions (Tajiri et al., 2014a). It is noteworthy that hADSCs were found to have migrated to such peripheral organs (such as the spleen) in these animals (Tajiri et al., 2014a). As the spleen’s action has been implicated to facilitate MSC neuroprotective action, hADSCs represent a propitious treatment of TBI. Conversely, the diminished trafficking of these cells to the spleen may be the result of decreased efficacy of treatment in aged rats (Tajiri et al., 2014a).
Adipose derived mesenchymal stem cells (AD-MSCs) are harvested easily via minimally invasive techniques such as liposuction or abdominoplasty (Mastro-Martinez et al., 2015). In a rat TBI model, allogenic transplantation of AD-MSCs into the area surrounding the wound and below the surface of the cortex enhanced recovery of motor function, neurogenesis, and cell density of the hippocampus (Mastro-Martinez et al., 2015).
A new study demonstrated that AD-MSC transplantation in a rat TBI model enhanced functional outcomes, initiated astrocytosis and reactive microglia, attenuated apoptosis in the hippocampus, and minimized neuron damage in the peri-impact area (Lam et al., 2017). Further, levels of proteolytic biomarkers for neuronal and glial cell damage (αII-spectrin and GFAP-breakdown product) were diminished in cortical insult by rAD-MSCs (Lam et al., 2017). Greater than 7,900 genes of the topical MSC population were differentially expressed of which six genes were involved in axonogenesis. Growth factors Erbb2, Artn and Ptn, cytokine IL3, cell cycle Hdac4, and notch signaling Hes1 were regulated in the peri-impact area three days following MSC transplantation (Lam et al., 2017). Additionally, MSCs in the region surrounding the point of impact were found to express CXCR4, which is indicative of the likely involvement of the SDF-1/CXCR4 axis in the shuttling of MSCs (Lam et al., 2017).
In the realm of chronic TBI, an enticing therapeutic target to be considered involves the activation and mobilization of endogenous bone marrow stem/progenitor cells via administration of granulocyte colony stimulating factor (G-CSF) (Acosta et al., 2014). When administered as a joint therapy, human umbilical cord blood cells (hUCBCs), and G-CSF reduced neuroinflammation, heightened endogenous neurogenesis, and mitigated hippocampal cell loss in acute TBI (Acosta et al., 2014). This combined therapy led to robust and long-lasting motor function recovery, though such recovery was much more transient when hUCBCs or G-CSF were administered alone (Acosta et al., 2014). One possible mechanism underpinning the success of the joint therapy involves the generation of a microenvironment conducive to hUCBC integration with host tissue via the action of G-CSF and/or the mobilization of endogenous bone marrow stem cells to the locale of injury also via the action of G-CSF (Dela Pena et al., 2014). More promising results were affected by this synergistic treatment than by either stand-alone treatment (Dela Pena et al., 2014).
Of the plethora of stem cell types, human umbilical cord derived mesenchymal stem cells (hUC-MSCs) have pronounced advantages not limited to hearty auto-renewal and differentiation, ready availability, growth and proliferation in culture, elevated mitotic activity, anti-inflammatory qualities, and less ethical disquiet due to extra-embryonic tissues usually being discarded postpartum (Kim et al., 2008; Magatti et al., 2016). Human umbilical cord matrix Wharton’s jelly mesenchymal stem cells (WJ-MSCs), are acquired in a similar manner and could potentially secrete large amounts of factors involved in neuroprotection, neurogenesis, and angiogenesis (Hsieh et al., 2013).
Extracellular matrices (ECMs) are integral to tissue architectural support as well as cell anchoring, migration, proliferation, and differentiation (Xiao et al., 2016). Brain ECM has been shown to regulate neural plasticity and the regeneration of axons (Miyata and Kitagawa, 2017). Upon transplantation, WJ tissue, comprising stromal microenvironment as well as UC-MSCs, were shown to alleviate post-TBI brain edema, shrink lesion volume, increase neurologic function, elevate expression of BDNF, facilitate recovery of memory, and enhance cognitive recovery (Cheng et al., 2015). ECMs are pivotal to a new mechanism of stem cell repair referred to as “stem cell-paved biobridges.” Stem cells are able to streamline the long-distance migration of NSCs between the neurogenic SVZ niche and the site of brain insult by way of biobridges, facilitating endogenous repair systems (Tajiri et al., 2013). This recently found ability has expanded the current conception of repair mechanisms which were previously limited to the knowledge of either cell replacement or bystander effect being stem cells’ only means of repair (Tajiri et al., 2013). This newfound tenet manifested when cultures demonstrated that notch-induced human BM-MSCs (SB623) were found to mediate the migration of endogenous cells by way of a biobridge seven days post-TBI in an animal model (Tajiri et al., 2013). The biobridge was distinguished by observing elevated levels of extracellular metalloproteinases (MMPs) as well as by a stream of graft cells which was later supplanted by newly formed host cells (Tajiri et al., 2013). This novel mechanism of stem cell repair unveils the roles of MMPs and ECM in neural repair. Further, it implies that such biobridges may propel the migration of cells in other maladies, though this remains to be investigated (Tajiri et al., 2013). Additionally, other stem cells possess the ability to create biobridges similar to that created by SB623 as they demonstrate modified levels and functions of MMPs and ECM proteins (Cheng et al., 2015; Tajiri et al., 2013). Examples include cells derived from umbilical cord, umbilical cord blood, adult brain, and systemic blood (Cheng et al., 2015; Tajiri et al., 2013). Further investigation is required to more thoroughly elucidate the mechanisms underpinning the migration of newly formed host cells toward the site of injury, as well as the efficacy and safety of SB623 in non-acute TBI in order to fine-tune protocols for transplantation in forthcoming clinical trials (Tajiri et al., 2013).
Currently, stem cell treatments lead to less functional and successful outcomes in chronic stage TBI as compared to acute stage TBI. Chronic TBI microenvironments are vastly less conducive to stem cell longevity than acute TBI environments (Harting et al., 2009) which demonstrate significantly higher levels of neurotrophic factors (Shindo et al., 2006). Intravenous delivery is likely the most appropriate method during acute stage TBI as it forgoes the trauma implicit in an intracerebral route of administration. Conversely, a more direct intracerebral route may effect more efficacious brain repair in chronic stage TBI at which point chemoattractant signals have diminished and the brain has stabilized.
Both acute and chronic TBI are being treated with the use of intrathecal and intravenous stem cell administration and is currently undergoing clinical trial testing. Cell types include: autologous bone marrow progenitor cells (BMPC) (; ; ; ) and, more recently, hUC-MSCs and BM-MSC-derived NSC-like cells (Miao et al., 2015; Wang et al., 2017). Erythropoietin (), G-CSF (Acosta et al., 2014), and other stem cell mobilizing agents lead to elevated levels of endothelial progenitor cells (EPCs).
In a Phase-I clinical trial involving patients with severe pediatric TBI, intravenously administered autologous bone marrow-derived mononuclear cells (BM-MNCs) led to a substantial drop in the Pediatric Intensity Level of Therapy (PILOT) score which is a metric of the overall therapeutic effort invested in the controlling of intracranial pressure (ICP) (Liao et al., 2015). A similar trend was detected in the Pediatric Logistic Organ Dysfunction (PELOD) score, which is recognized as being a valid metric of pediatric multiple organ dysfunction (Liao et al., 2015). It is reasonable to conclude that BM-MNC treatment could alleviate the caustic effects of inflammation in the acute post-TBI period. The safety and logistic viability of autologous intravenous BM-MNC administration were confirmed in a Phase-I/IIa study of severe adult TBI in which brain parenchyma preservation was correlated with functional outcomes (Cox et al., 2017). This modality likely mitigated the global inflammatory reaction, which was denoted by the downregulation of key inflammatory cytokines (Cox et al., 2017). Another study showed the betterment of both clinical and functional measures as well as the amelioration of PET scan results following intrathecal administration of autologous BM-MNCs in patients living with chronic TBI (Sharma et al., 2015).
A case study following the progress of a female fifteen-year-old TBI patient demonstrated the absence of any inimical consequence following autologous BM-MNC intrathecal administration and exhibited marked improvement in higher function, memory, speech, fine motor skills, and cognitive ability (Sharma et al., 2017). Six months following administration, brain PET scan results additionally corroborated these improvements (Sharma et al., 2017). Findings such as these emphasize that the quality of life of individuals living with chronic TBI could be bolstered by BM-MNC transplantation.
Additionally, human umbilical cord derived mesenchymal stem cells (hUC-MSCs) constitute a more auspicious cell source thanks to their greater ease of procurement, rapid proliferation, and lower antigenicity as compared to BM-MNCs. The abilities of hUC-MSCs have been demonstrated in both animal and pre-clinical trials (Hsieh et al., 2013; Kim et al., 2013; Magatti et al., 2016; Miao et al., 2015). The safety and efficacy of intrathecal hUC-MSC administration was demonstrated by a clinical study following one hundred patients with TBI (Miao et al., 2015). An additional Phase-I study demonstrated that both intravenous and intrathecal transplantation of autologous BM-MSC-derived NSC-like cells are safe and feasible for patients with severe TBI (Wang et al., 2017). Furthermore, following transplantation, heightened serum levels of neurotrophic factors NGF and BDNF were detected, and correlated with marked functional improvement within the majority of individuals (Wang et al., 2017).
As previously noted, studies and clinical trials probing the effects of the intracerebral administration of stem cells in PD, HD, and stroke will afford findings applicable to TBI treatment development. Additionally, aging and TBI both lead to the acquisition of senescent phenotypes and, therefore, a shrinking of the endogenous NSC population. However, the administration of radical scavengers in particular may be able to partially or completely reverse the senescent phenotypes seen in aging and TBI leading to a more robust, populous, and phenotypically-young NSC population. Further, the administration of naïve stem cells would naturally be free of such senescent markers and, thus, exhibit a more long-lasting effect as compared to naturally or traumatically aged stem cells. Administration of stem cells to the peri-impact area may engender the rescue of neural cells succumbing to the secondary death cycle of TBI.
3.5. Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease in which motor neurons degenerate and are unable to initiate voluntary muscle movement (Hefferan et al., 2011). There is no known cure, and current treatments are unable to halt the effects of the progressive disease (Glass et al., 2012). However, transgenic rodent models have provided an example to study the mechanisms of familial ALS, including the superoxide dismutase 1 (SOD1) variant (Hefferan et al., 2011). These findings have opened the door for new therapies such as stem cell treatment (Table 5). Bone marrow mononuclear cells (BMMNCs) injected in the spine were found to reduce degeneration rates. Increased doses were more effective in protecting motor neurons, as the stem cells mediated treatment through paracrine factors (; ).
Table 5: Amyotrophic Lateral Sclerosis.
In this table, promising pre-clinical studies were selected in PubMed from 2012–2017 which investigated stem cell therapy for ALS. Moreover, the clinical trials presented were selected due to 1) their recency and 2) their availability of published results. In summary, these milestone studies show that stem cell transplant in mouse models of ALS resulted in increased lifespan, improvement in motor neuron survival, and some motor function improvement. In humans with ALS, stem cells implants were shown to be safe, improved lifespan, and reduced disease progression.
| Author | Model | Cell type | Cell Quantity | Outcome |
|---|---|---|---|---|
| Nizzardo et al., 2016 | SOD1 G93A mice | iPSC-derived NSC | 1×106 cells | Significant increase in life span. |
| Zhong et al., 2017 | SOD1 G93A mice | NSC | 2.5×105 cells | Significant increase in life span, great motor neuron survival |
| Srivastava et al., 2014 | SOD1 G93A mice | NSC | Two injections of 1×105 cells | Transient improvement in motor function, total transplant cell death by 120 days. |
| ALS patients, n=63 | Autologous BM-MNC | Not Specified | Positive safety outcomes at 6 months | |
| Oh et al., 2015 | ALS patients, n=8 | Autologous BMSC | Two injections of 1×106/kg cells | Positive safety outcomes at 12 months |
| Mazzini et al., 2015 | ALS patients, n=6 | hNSC | 7.5×105 or 1.5 × 106 cells | Cessation of disease progression up to 18 months |
| Martinez et al., 2016 | ALS patients, n=39 | PBMNC | Not Specified | Increase in lifespan and improved Function Rating Scale scores. |
iPSC – induced pluripotent stem cell; NSC – neural stem cell; BM-MNC – bone marrow mononuclear cell; BMSC – bone marrow stem cell; hNSC – human neural stem cel; PBMNC – peripheral blood mononuclear cell
BMMNCs, while promising, are not the only form of stem cell therapy considered for ALS patients. Human spinal cord-derived neural stem cells (hSSCs), for example, produce more amino acid transporters to support reduced glutamate transporter expression and possess elevated glutamate concentrations outside of their cells due to the lack of transporters (), (O’Donovan et al., 2017). A subpopulation of iPSC-derived neural stem cells (LewisX+CXCR4+β1-integrin+) promoted survival and continued axonal sprouting in cultures of toxic ALS astrocytes and neurons from ALS patients (Nizzardo et al., 2016). Using the same culture of cells, mice with the SOD1 variant received this treatment and demonstrated improved strength of neuromuscular communication and axonal growth (Nizzardo et al., 2016). Mice with the same variation also displayed improved ALS symptoms when they received a treatment of neural stem cells and nerve growth factor (Zhong et al., 2017). Unfortunately, the transplanted grafts of stem cells have low survival rates, shown by in vivo magnetic resonance imaging. However, the effects of neuronal stem cell implants are still therapeutic (Srivastava et al., 2017). Stem cell therapy that utilizes hSSCs were paired with the delivery of immunosuppressants, and rat models with SOD1 variation exhibited higher graft survival rates (Hefferan et al., 2011). Thus, neural stem cells, whether they are spinal-derived or not, are able to protect and support motor movement through paracrine and immunomodulatory mechanisms.
Beyond murine models, human stem cell treatments have entered the first phases of clinical trials. Successful intraspinal delivery of hSSCs were administered to twelve ALS patients, and after thorough evaluation, termination of degeneration was observed (Glass et al., 2012). The treatment was deemed safe in phase I, with the absence of progression being favorable for phase II trials (Glass et al., 2012). Humans given autologous MSCs also reported positive outcomes in longevity as well as increased capability, quantified via the ALS functional rating scale (Martinez et al., 2016). For the treatment of ALS, a myriad of different stem cell types has been utilized. To produce neurons and supporting glial cells, human spinal cord-derived neural stem cells (; ), human neural stem cells (), and bone marrow-derived neural stem cells (Nafissi et al., 2016) are being used. Bone marrow derived stem cells, autologous BMSCs (; ; ; ), peripheral blood stem cells (Li et al., 2017; ), autologous hematopoietic stem cells (), autologous MSCs (; ; ; Staff et al., 2016; Sykova et al., 2017), and HLA-haplo matched allogenic bone marrow-derived stem cells (). Lastly, some miscellaneous stem cells include umbilical cord MSCs (), adipose-derived MSCs (Staff et al., 2016), induced pluripotent stem cells (iPSCs) (), and stem cells combined with G-CSF and nerve growth factor to encourage success in treatment (). There is a lot of data that have not yet been reported due to continuing studies or absence of publication on which further work is being conducted in a clinical setting (; ; ; ; ; ; ; ; ). ALS has yielded promising results for stem cell therapies, and the scientific community will continue to receive new information as more clinical trials are released.
Studying the effects of aging in ALS patients has proved to be difficult due to the rapid progression and fatality of the neurodegenerative disease. However, research is being done to compare early onset ALS to common late onset ALS, seen in adults aged 40–70 years (Japtok et al., 2015). Although ALS is more frequent in older generations, it is more severe within young onset models. A mutation in the FUS gene is responsible for 5% of familial ALS cases and leads to the accumulation of FUS protein within the cell. In the young onset model (patient aged 29 years), the pathological phenotype showed increased severity compared to the late onset (patient aged 58 years) (Japtok et al., 2015). However, it is acknowledged that FUS protein accumulates more in the cell with age, so it does play a role in the observed severity. iPSCs were used as the progenitor for cortical neurons that were used in the study, rather than for treatment (Japtok et al., 2015). Apart from aging effecting the severity of ALS observed, stem cells are being used to increase the lifespan of those diagnosed with the progressive disease.
ALS significantly shortens longevity in patients because the effects of aging are accelerated. Motor neurons and astrocytes exhibit reduced function like that of an much older individual, however, as of late, stem cells have become a promising therapy to bring in newer and younger cells that are able to support neurons. Human adipose-derived stem cells (ASCs) were transplanted into SOD1G93A mouse models of ALS intravenously (IV) and intracerebroventricularly (ICV). The onset of symptoms was delayed by 26 days in ICV mice, and their longevity was extended by 24 days (Kim et al., 2014). Additionally, motor neurons were derived from human neural stem cells (NSCs), then were intrathecally transplanted into SOD1G93 mouse models, and the lifespan was once again significantly prolonged (20 days) (Lee et al., 2014). Stem cells are able to resist the accelerated aging of ALS via the high secretion of neurotrophic factors.
Two trophic factors, glial-cell derived (GDNF) and vascular endothelial growth factor (VEGF), are particularly helpful in preventing the augmented aging of motor neurons and astrocytes experienced by patients with ALS. GDNF is released by genetically modified stem cells, both human neural progenitor cells (hNPCs) and hMSCs (Das et al., 2016; Das and Svendsen, 2015; Krakora et al., 2016). hNPCs released GDNF after injection into the spinal cord of aging rats. hNPCs most frequently differentiated into astrocytes to support and protect neurons from age-related deficits (Das et al., 2016). The genetically modified NPCs are used in murine models of ALS and have successfully protected motor neurons, which suggests that ALS and aging follow similar mechanisms. In familial models of ALS in rats, astrocytes develop a senescent phenotype at a much higher rate, leaving neurons vulnerable to damage and cell death (Das and Svendsen, 2015). Rat astrocytes were shown to be susceptible to accelerated aging due to the neurodegenerative progression; however, GDNF has been shown to partially reverse the damage (Das and Svendsen, 2015). Replacing old astrocytes with new stem cells that release GDNF is a promising therapeutic strategy. Lastly, one study was successful in using modified hMSCs to release GDNF to halt the progression of ALS. This continuing project experimented with the efficacy of VEGF versus GDNF, as well as a combination of VEGF and GDNF (Krakora et al., 2013). VEGF was found to be just as effective as GDNF in regards to protecting neurons against ALS and prolonged survival of murine models, thus fighting the aging process (Krakora et al., 2013). Together, VEGF and GDNF were more efficient than either individually, demonstrating a strong synergism (Krakora et al., 2013).
In conclusion, ALS accelerates the aging of neurons, but the newer stem cell therapies offer positive outcomes of neuroprotection and extended longevity through the release of trophic factors. Age of onset also contributes to the severity of the disease, which is something researchers need to consider when determining the dosage of stem cell injections. Stem cells can help reduce the detrimental effects of aging, especially in those experiencing rapid degeneration due to ALS.
3.6. Multiple Sclerosis
Stem cells possess the ability to remyelinate axons, allowing for increased traction within the research community with the absence of treatment options for multiple sclerosis (Karussis et al., 2006). Stem cell treatments can be injected intraventricularly and induce immunomodulatory effects that have been shown to mitigate aspects of MS pathology (Table 6) (Karussis et al., 2006). Two important features of oligodendrocyte maturation; 1) the upregulation of glial lineage protein markers and 2) migration to areas of white matter inflammation have both been shown to hinder the progression of MS (Karussis et al., 2006). The migration of BM-MSCs to sites of inflammation and subsequent differentiation into astrocytes, neuronal, or glial cells has been reliably shown in mice treated intravenously or intraventricularly (Karussis et al., 2006). Invasion of lymphocytes were minimized and white matter was shown to be preferentially rescued (Karussis et al., 2006). In addition to treatment with BM-MSCs, neurotrophic factor-secreting mesenchymal stem cell (NTF-SCs)/hADSCs transplantations may represent a possible therapeutic intervention (Razavi et al., 2018). Transplantation of MSCs into mice with MS demonstrated the ability to target inflammatory pathways by increasing interleukin-10 (IL-10), and suppressing myeloperoxidase and tumor necrosis factor-alpha, suggesting the presence of neuroprotective effects (Mahfouz et al., 2017). When compared to BM-MSCs, human embryonic stem cell-derived mesenchymal stromal cells (hE-MSCs) were shown to curb the progression of MS in mice whereas BM-MSCs alone were unsuccessful (Wang et al., 2014). hE-MSCs possess two potentially therapeutic characteristics: 1) their ability to cross the blood-brain barrier/blood-spinal cord barrier (BBB/BSCB) and migrate to CNS lesions and 2) low expression of IL-6, a major inflammatory cytokine (Wang et al., 2014). The administration of a combination of genetically modified adipose-derived MSCs and interferon-beta also demonstrated a reduction of inflammation as well as MS symptoms (Marin-Banasco et al., 2017). Laboratory research also revealed that hE-MSCs prevented demyelination while leaving axons undisturbed (Wang et al., 2014).
Table 6: Multiple Sclerosis.
All studies indexed by PubMed from 2013 to 2018 with relevant findings regarding the use of stem cells being injected into MS animal models or patients with are referenced here. Stem cell transplantation in animal models of MS showed consistent beneficial effects, but in humans, results were mixed, with one study showing an increase in T-lymphocytes and one study showing decreased progression and relapse of symptoms.
| Author | Model | Cell type | Cell Quantity | Outcome |
|---|---|---|---|---|
| Kassis et al., 2013 | C57BL/6 mice | BM-MSCs | Not Specified | Reduced lymphocytic infiltrations and preserved axons. |
| Wang et al., 2014 | C57BL/6 mice | hE-MSCs and BM-MSCs | 1×106 cells/mouse | The ability to cross the blood-brain barrier/blood-spinal cord barrier and emigrate into inflamed CNS tissue. |
| Donders et al., 2015 | Rats | WJ-MSCs | Not Specified | Alleviation of symptoms through conferring trophic support and the lessening of autoantigen-induced T cell proliferation. |
| Li et al., 2014 | MS patients, n=23 | hUC-MSCs | three times in a 6-week period | Symptoms improved with a lower relapse rate. |
| Riordan et al., 2018 | MS patients, n=20 | hUC-MSCs | 1 per day for 7 days | Intravenous infusions of UC-MSCs were safe. Symptom improvements were notable 1 month after treatment. |
| Razavi et al., 2018 | 50 adult rats | NTF-SCs and hADSCs | Not Specified | Motor functional tests showed significant improvement function in cell transplantation groups. |
| Mahfouz et al., 2017 | Swiss mice | MSCs | 40 mg/kg | Increased interleukin 10 and suppressed myeloperoxidase and tumor necrosis factor-alpha. |
| Marin-Banasco et al., 2017 | Mice | AdMSCs | Not Specified | Relieved MS symptoms and decreased indications of peripheral and central neuro-inflammation. |
| Karnell et al., 2017 | MS patients, n=23 | Tregs and Th1 cells | Not Specified | Increased CD4 and CD8 T cells. |
| Muraro et al., 2017 | MS patients, n=281 | AHSCT | Not Specified | Half of the patients remained free from neurological progression. |
BM-MSCs – bone marrow mesenchymal stromal cells; hE-MSCs – human embryonic stem cells; WJ-MSCs – Wharton’s jelly-derived stromal cells; hUC-MSCs – human umbilical cord-derived mesenchymal stem cells; NTF-SCs – neurotrophic factor secreting cells; hADSCs – human adipose derived stem cells; MSCs – mesenchymal stem cells; AdMSCs – adipose-derived mesenchymal cells; Tregs – CD45RA-regulatory T cells; Th1 cells – T helper type 1; AHSCT – autologous hematopoietic stem cell transplantation
WJ-MSC transplants reduced autoantigen-induced T-cell propagation while exhibiting minimal immunogenicity and expressing anti-inflammatory molecules/neurotrophic factors, alleviating MS symptoms (Donders et al., 2015). In humans, similar results were found. Human UC-MSCs were infused intravenously three times over six weeks in 23 MS patients (Li et al., 2014). In the UC-MSC-treated patients, a phenotypic shift from the inflammatory type 1 T-helper cell (Th1) to the regenerative type 2 T-helper cell (Th2) was seen (Li et al., 2014). The role that Th2 cells play in slowing MS progression has been clinically confirmed (Karnell et al., 2017). In a long-term analysis of 281 MS patients, autologous HSC transplantation improved outcomes compared to controls in younger patients, boasted lower baseline EDSS scores, and demonstrated less of a need for additional therapies. UC-MSCs () and autologous BMSCs () are among the few potential MS therapies being tested in clinic trials.
Aging presents a therapeutic challenge for those with long-term demyelinating diseases such as MS. The demyelination seen in MS combined with the diminished ability of the body to compensate efficiently seen in aging are potential reasons for why the transition of relapsing remitting MS into progressive MS seems to be an age-dependent process (Tutuncu et al., 2013). Remyelination has been shown to be less efficient within the aged CNS due to the variable expression of growth factors in older individuals (Hinks and Franklin, 2000). Slower myelination of axons has been associated with differential expression of platelet-derived growth factor, impacting the aggregation of oligodendrocyte progenitor cells, as well as low peak production of IGF-1 and TGF-beta, hindering the subsequent differentiation of recruited oligodendrocyte progenitors into mature oligodendrocytes (Hinks and Franklin, 2000). Recently, it has been shown that oligodendrocyte progenitor maturation is partially influenced by the availability of monocytes recruited to the demyelinated site from the blood (Ruckh et al., 2012). Treatment of aged mice with youthful monocytes via parabiosis established more robust remyelinating activity, suggesting that aged oligodendrocyte progenitor cells are capable of being responsive to therapies combating the aging process (Ruckh et al., 2012). In addition to environmental signaling changes, cell-intrinsic mechanisms have been elucidated as contributors to impaired remyelination due to aging. Reduced ability of cells to recruit histone deacetylase (HDAC) to the promoter regions of differentiation inhibitor genes such as Hes5 has been implicated in aging (Shen et al., 2008). Specifically, the HDAC1 and HDAC2 isotypes have been deemed critical for efficient oligodendrocyte progenitor differentiation (Shen et al., 2008). Thus, a chromosomal environment exists within aged oligodendrocyte progenitor cells that makes differentiation more unlikely compared to youthful cells. Alterations to intrinsic signaling pathways in aging may also modify the ability of cells to remyelinate damaged axons. Sonic hedgehog (Shh) signaling is critical for NSC differentiation into the oligodendrocyte and neuronal lineages (Traiffort et al., 2016; Wang et al., 2008). The regulation of this pathway seems to go awry with age, which may accelerate the progression of MS symptoms by impacting NSC differentiation signaling (Carlson et al., 2008). In summary, the effects of aging on stem cells and their successive precursor cells are multifaceted as they relate to the course of MS and present many potential therapeutic targets.
3.7. Multiple System Atrophy
Multiple system atrophy (MSA) is a sporadic neurodegenerative disorder that affects both the autonomic nervous system as well as locomotion via cerebellar ataxia, pyramidal signs, and Parkinsonism. The presence of α-synuclein in glial cells (particularly oligodendrocytes) is a histopathological hallmark (Lee et al., 2012; Masliah et al., 2000). Due to the diseases rapid progression and fatal conclusion, there is a dire need for treatments that mitigate MSA’s progression (Lee et al., 2012). In one study, intra-arterial administration of MSCs provided protective effects for striatal and nigral neurons in 6-OHDA/quinolinic acid animal model of MSA (Na Kim et al., 2017), correlating with observed motor outcomes (Na Kim et al., 2017). In a limited clinical study, autologous MSCs were used to treat patients with cerebellar-type MSA (Lee et al., 2012). The MSC-treated patient group showed a smaller increase in deficits and less extensive degeneration at 360 days compared to the placebo group (Lee et al., 2012). However, the study reported small ischemic lesions following intra-arterial infusion (Lee et al., 2012). While pre-clinical data hold promise for the use of stem cells in the treatment of MSA, additional studies of thorough design are needed (Table 7).
Table 7: Multi-System Atrophy.
In this table, all studies indexed by PubMed from 2012 to 2018 with intra-arterial/intravenous injections of mesenchymal stem cells to treat Multiple Systems Atrophy are referenced. In these two studies, stem cell transplant in mice and in humans with MSA both ameliorated motor deficits and improved behavioral outcomes.
| Author | Model | Cell type | Cell Quantity | Outcome |
|---|---|---|---|---|
| Na Kim et al., 2017 | adult male or female Sprague Dawley rats | Human MSCs | 4 × 107 cells/60 kg | Increased nigrostriatal neuronal survival and a coincidental increase in motor behavior. |
| Lee et al., 2012 | MSA patients, n=33 | Autologous MSCs | 4 × 107/injection | Possible delay in neurological deficits. |
MSA – multi-system atrophy; MSC – mesenchymal stem cells
As of yet, a reliable mechanism of pathology has not been deduced for MSA. However, thanks to the emergence of iPSC techniques, patient-specific models of MSA are currently being used to elucidate the specific pathogenesis of MSA so that targeted treatments thereof may be developed (Abati et al., 2018; Devine et al., 2011). While several studies have demonstrated the neuroprotective effects of MSCs in the treatment of MSA (Lee and Park, 2009; Sunwoo et al., 2014), additional studies should be conducted with regard to the effects of aging on endogenous stem cell reservoirs and their participation (or lack thereof) in MSA. Additionally, because of MSA’s similar characteristics to synucleinopathies, advances made in the treatment of PD may be found relevant for the treatment of MSA. For example, just as intracerebral transplantations of stem cells have been shown to restore lost DA neurons, stem cells may also be able to replace damaged oligodendrocytes which have also fallen prey to accumulations of alpha-synuclein (Lindvall et al., 2012).
4. The Youthful Brain
Aging leads to the accrual of senescent phenotypes in both brain parenchyma as well as stem cell neurogenic niches. As has been noted above, such phenotypes generally detract from the efficacy of stem cell therapy. When considering treatment options for CNS disorders, such as stroke and TBI, the age of the transplant recipient may influence the therapeutic outcomes, Compared to the largely aging patient population of stroke, TBIs are primarily sustained by those of a younger age group (Dewan et al., 2018a).
The young brain compared to the aging brain is more amenable to neuroplasticity and regenerative medicine. With respect to age-dependent responses seen in TBI, while adult TBI is characterized by a starkly pro-inflammatory response, the young brain after TBI engenders an anti-inflammatory depression of cytokines predominantly in the contralateral hemisphere and, to a lesser degree, in the ipsilateral region (Tajiri et al., 2014c). This was observed in the brains of seven-day-old Sprague-Dawley rats which were euthanized immediately following TBI. Inflammatory cytokine suppression was observed in the brain parenchyma only, and no such downregulation was noted in plasma cytokine levels. This is indicative a highly localized anti-inflammatory response. When administered in the TBI-exposed young brain, the anti-inflammatory effect of stem cell therapy is thus reasonably expected to produce greater neuronal rescue from inflammation-induced death than the same treatment would afford in adult TBI (Dewan et al., 2018b). Additionally, the greater degree of neuroplasticity noted in young subjects would further enhance neuronal survival.
Anti-inflammatory trends similar to those noted in immature animals subjected to TBI have been observed in the context of stroke. Further, in cerebral hypoxic-ischemic models, neonate mice have been shown to have briefer stromal cell-derived factor 1 (SDF-1)-mediated BMSC chemotaxis as compared to their adult counterparts (Miller et al., 2005). SDF-1 cell homing was highest three to five days post-injury (Miller et al., 2005). This narrowed therapeutic window may be a result of higher levels of neuroplasticity and tighter SDF-1 regulation as compared to the more stable, less regulated adult brain (Miller et al., 2005).
Pathological triggers and therapeutic targets may reside in inflammatory cues and stem cells, especially when key cellular differences are recognized between young and aged microglia (Safaiyan et al., 2016). As noted above, the young brain compared to the aging brain is more amenable to neuroplasticity and regenerative medicine. Myelin clearance by microglia becomes more burdensome during aging, propelling the microglia to undergo senescence eventually succumbing to a pathological immune response (Safaiyan et al., 2016). In the setting of stroke, the neonatal brain upregulates endogenous cytokines and chemokines that create a conducive microenvironment for stem cell proliferation and migration, altogether aiding the host regenerative process (Bartley et al., 2005; Millet et al., 2005). With the young brain nurturing an endogenous repair mechanism, it is not surprising that viable stem cells are easily harvested from young sources than aged tissues (Batsali et al., 2017; Kim et al., 2013). Altogether, these findings underscore the intricate role of inflammation on stem cells.
5. Synopsis
A large body of research has shown that stem cell transplantation, especially via an intracerebral route, has beneficial effects for various neurological disorders. However, proper consideration of immune response and the potential for tumor formation is crucial for the safety of patients. Similar to organ transplantation, stem cell therapies might require immunosuppressive drugs to prevent rejection since differentiated stem cells can be immunogenic (Weder et al., 2014). In a Parkinsonian primate study, cyclosporine was not enough to prevent the immune rejection of DA hESCs (Emborg et al., 2013). This was indicated by the proliferation of CD68 and CD 45 at the surrounding injection site (Emborg et al., 2013). As hES-derived neural progenitors mature, they express more MHC antigens, which can be recognized as foreign agents by host cells (Emborg et al., 2013). Another confounding factor is the different immuno-microenvironment between the source of stem cells and the targeted therapy region (Weder et al., 2014). Matching human leukocyte antigen (HLA) between donor and recipient has been the gold standard for minimizing graft rejection, however it might not be possible for certain type of stem cells. Interestingly, in one study, co-transplantation of MSC with porcine neuroblasts (pNb) increases the survival rate of pNb (Leveque et al., 2015). MSCs were able to modulate the immune response through various aspects such as preventing B-cells from differentiating to plasma cells, slowing the maturation of T-cells, and inhibiting the antigen presenting process (Leveque et al, 2015) any factors can contribute to the tumorigenicity of stem cells such as stem cell sources, extraction and modification methods, the microenvironment, and pre-existing tumors (Weder et al., 2014). For example, MSCs may promote cancer-prone cells to proliferate and vice versa. On the other hand, MSCs might also inhibit tumor growth by modulating the immune environment (Weder et al., 2014). These important factors should be carefully considered and addressed to lower the risk factors associated with the stem cell therapy (Weder et al., 2014).
Another important factor in stem cell therapy is the distribution and migration of the transplanted stem cells to various tissues such as lung, spleen, muscle, etc. (Weder et al., 2014). Currently, the distribution of stem cells is not uniform and its significance remains to be understood. The different distributions between transplantations might lead to an increase risk for patients (Weder et al., 2014). However, an uneven homing of stem cells might also explain the immunomodulatory ability of stem cells and be preferable (Acosta et al., 2015).
Finally, choosing the number of cells for transplantation is a delicate decision. Generally, the survival rate of transplanted cells is low. Therefore, if the quantity of transplanted cells is not sufficient, the therapeutic outcomes might diminish (Weder et al., 2014). On the other hand, a large dosage of cells may lead to large amount of cells in unintended tissues. This increases the risk of complications associated with transplantation (Sohni and Verfaillie, 2013). More importantly, since the cells have the tendency to clump together, high dosages might also increase the risk of embolism and vascular occlusion causing ischemic stroke (Sohni and Verfaillie, 2013; Weder et al., 2014).
6. Future Directions
The area of research that focuses on treating neurological disorders with stem cells has been rapidly expanded since the discovery of stem cells and their first treatment application in PD in 1980. Stem cells have been found and modified from various sources. With the advancement in technologies, transplantation methods have also improved and advanced toward safe and reliable techniques. However, there are many questions which remain to be answered. These challenges include fully understanding the mechanism of action, selecting proper cell type, optimizing dosage and delivery methods for each disease, and scaling-up for the general population while maintaining safety standards. Further understanding of the mechanism of action will assist with clinical designs that provide safe and therapeutic outcomes. In order to expand the availability of stem cell therapy, the quality of mass-produced cells must be carefully monitored to maintain safety and efficacy. In the next five years, many ongoing clinical trials will be concluded and reported. These results will help to shape public opinion and guide the field to improve our understanding of cell therapy. In addition, further efforts must focus on the significant role of aging and its effect on pathologies of neurological disorders and stem cells.
7. Conclusion
The growing of the aged population will likely be accompanied by higher incidence of neurological disorders. As we discussed in this review, there are currently no cures for many of these diseases. Aging plays many roles in enhancing disease progression such as the accumulation of harmful circulating factors, proteinaceous aggregation, and overall pathological phenotype. Aging in stem cells could best be described as a continuous positive feedback loop as aging progresses, error increases, thus, disease progresses. Ethical, practical and logistical approaches for slowing down the process of aging may be implemented to stave off erroneous driven disease states. With that being said, stem cell therapy may allow hopeful passage for alleviating the harmful effects of disease and aging. Over the past few decades, stem cell therapy has emerged as a potential therapeutic option for CNS disorders. However, to this day, there are no neurological disorders in which stem cell therapy is the first and best treatment option. Yet, enthusiasm must meet with expectation while translating stem cells from bench to bedside; especially for vulnerable patient populations with poor prognoses and limited treatment options. Of note, medical tourism is an ill-informed option because these advertised treatments are not regulated nor do they meet the standard of the US Food and Drug Administration (FDA) (Edelstein and Holmes, 2014). Instead, further rigorous research and well-designed clinical trials that stay in the framework of regulatory agencies are needed to ensure the safety of participants (California’s Stem Cell Organization, 2013). Unrealistic and faulty promises from some entities in the U.S. and abroad will stifle the progression of the field (Institute of Medicine and National Research Council, 2014). The field of stem cell therapy is advancing toward a critical point of providing meaningful outcomes for neurological disorders. However, stem cells are not silver-bullets for all diseases.
In this review, we highlighted the use of stem cell therapy in neurological disorders with an emphasis on the important role of aging. The current literature supports the notion of using stem cells as a treatment for many neurological disorders. Further pre-clinical and clinical studies are needed to ensure the safety and efficacy of these treatment options with consideration of the effects of aging on stem cells and the transplant recipients.
Abbreviations
- PD
Parkinson’s Disease
- HD
Huntington’s Disease
- TBI
traumatic brain injury
- ALS
amyotrophic lateral sclerosis
- MS
multiple sclerosis
- MSA
multiple system atrophy
- CNS
central nervous system
- IV
Intravenous
- IA
Intra-arterial
- DA
dopamine
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- iPSCs
induced pluripotent stem cells
- BBB
blood brain barrier
- CP
choroid plexus
- QA
quinolinic acid
- OECs
olfactory ensheathing cells
- GABA
γ-aminobutyric acid
- MSCs
mesenchymal stem cells
- MCAO
middle cerebral artery occlusion
- GDNF
glial cell line derived neurotrophic factor
- NT2N cells
Ntera2/D1 neuron-like cells
- SVZ
subventricular zone
- DG
dentate gyrus
- NSC
neural stem cell
- VEGF
vascular endothelial growth factor
- PET
positron emission tomography
- CCI
controlled cortical impact
- BMSCs
bone marrow stromal cells
- hESC
human embryonic stem cell
- hNSC
human neural stem cell
- BDNF
Brain-derived neurotrophic factor
- DHA
docosahexaenoic acid
- hADSCs
human adipose-derived stem cells
- hUCBCs
human umbilical cord blood cells
- G-CSF
granulocyte colony stimulating factor
- WJ-MSCs
Wharton’s jelly mesenchymal stem cells
- ECM
Extracellular matrix
- MMPs
extracellular matrix metalloproteinases
- BMPC
bone marrow progenitor cells
- hTERT-MSCs
human telomerase reverse transcriptase-immortalized mesenchymal stem cells
- VSELs
very small embryonic-like stem cells
- HSCs
hematopoietic stem cells
- hBMEPCs
human bone marrow endothelial progenitor cells
- hDASCs
human derived adipose stem cells
- IFN-γRβ
- EPCs
endothelial progenitor cells
- BM-MNCs
bone marrow-derived mononuclear cells
- ICP
intracranial pressure
- hSSCs
human spinal cord-derived neural stem cells
- NTF-SCs
neurotrophic factors secreting mesenchymal stem cell
- hE-MSCs
Human embryonic stem cell derived mesenchymal stromal cells
- hESCs
human embryonic stem cells
- GVHD
graft-versus-host disease
- pNb
porcine neuroblasts
- AD-MSCs
adipose-derived mesenchymal stem cells
- SB623
notch-induced human bone marrow derived mesenchymal stem cells
- BM-MSCs
bone marrow derived mesenchymal stem cells
- BMSCs
bone marrow stromal cells
- BM-MNCs
bone marrow-derived mononuclear cells
- PILOT
Pediatric Intensity Level of Therapy
- PELOD
Pediatric Logistic Organ Dysfunction
- hUC-MSCs
human umbilical cord derived mesenchymal stem cells
- BMMNCs
bone marrow mononuclear cells
- SOD1
superoxide dismutase 1
- ICV
intracerebroventricularly
- IV
intraveneously
- hNPCs
human neural progenitor cells
- IL-10
interleukin-10
- HDAC
histone deacetylase
- pNb
porcine neuroblasts
- FDA
US Food and Drug Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abati E, Di Fonzo A, Corti S, 2018. In vitro models of multiple system atrophy from primary cells to induced pluripotent stem cells. J. Cell. Mol. Med 22, 2536–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta SA, Tajiri N, Hoover J, Kaneko Y, Borlongan CV, 2015. Intravenous Bone Marrow Stem Cell Grafts Preferentially Migrate to Spleen and Abrogate Chronic Inflammation in Stroke. Stroke 46, 2616–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Sanberg PR, Sanchez-Ramos J, Song S, Kaneko Y, Borlongan CV, 2014. Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury. PLoS One 9, e90953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy C, Kaya FA, Kuskonmaz BB, Uckan D, Severcan F, 2014. Structural investigation of donor age effect on human bone marrow mesenchymal stem cells: FTIR spectroscopy and imaging. Age (Dordr) 36, 9691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK, 2013. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther 138, 155–75. [DOI] [PubMed] [Google Scholar]
- Altarche-Xifro W, di Vicino U, Munoz-Martin MI, Bortolozzi A, Bove J, Vila M, Cosma MP, 2016. Functional Rescue of Dopaminergic Neuron Loss in Parkinson’s Disease Mice After Transplantation of Hematopoietic Stem and Progenitor Cells. EBioMedicine 8, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, Toren A, Constantini S, Rechavi G, 2009. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 6, e1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambasudhan R, Dolatabadi N, Nutter A, Masliah E, McKercher SR, Lipton SA, 2014. Potential for cell therapy in Parkinson’s disease using genetically programmed human embryonic stem cell-derived neural progenitor cells. J. Comp. Neurol 522, 2845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachoud-Levi AC, Gaura V, Brugieres P, Lefaucheur JP, Boisse MF, Maison P, Baudic S, Ribeiro MJ, Bourdet C, Remy P, Cesaro P, Hantraye P, Peschanski M, 2006. Effect of fetal neural transplants in patients with Huntington’s disease 6 years after surgery: a long-term follow-up study. Lancet Neurol 5, 303–9. [DOI] [PubMed] [Google Scholar]
- Bachoud-Levi AC, Remy P, Nguyen JP, Brugieres P, Lefaucheur JP, Bourdet C, Baudic S, Gaura V, Maison P, Haddad B, Boisse MF, Grandmougin T, Jeny R, Bartolomeo P, Dalla Barba G, Degos JD, Lisovoski F, Ergis AM, Pailhous E, Cesaro P, Hantraye P, Peschanski M, 2000. Motor and cognitive improvements in patients with Huntington’s disease after neural transplantation. Lancet 356, 1975–9. [DOI] [PubMed] [Google Scholar]
- Baig AM, 2014. Designer’s microglia with novel delivery system in neurodegenerative diseases. Med. Hypotheses 83, 510–2. [DOI] [PubMed] [Google Scholar]
- Baker N, Boyette LB, Tuan RS, 2015. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone 70, 37–47. [DOI] [PubMed] [Google Scholar]
- Bang OY, 2016. Clinical Trials of Adult Stem Cell Therapy in Patients with Ischemic Stroke. J. Clin. Neurol 12, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsali AK, Pontikoglou C, Koutroulakis D, Pavlaki KI, Damianaki A, Mavroudi I, Alpantaki K, Kouvidi E, Kontakis G, Papadaki HA, 2017. Differential expression of cell cycle and WNT pathway-related genes accounts for differences in the growth and differentiation potential of Wharton’s jelly and bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther 8, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya N, Stubblefield P, 2013. Human Fetal Tissue Transplantation Springer; London. [Google Scholar]
- Bickford PC, Flowers A, Grimmig B, 2017. Aging leads to altered microglial function that reduces brain resiliency increasing vulnerability to neurodegenerative diseases. Exp. Gerontol 94, 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla C, Zurita M, Aguayo C, Rodriguez A, Vaquero J, 2014. Is the subarachnoid administration of mesenchymal stromal cells a useful strategy to treat chronic brain damage? Cytotherapy 16, 1501–1510. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Evans A, Yu G, Hess DC, 2005. Limitations of intravenous human bone marrow CD133+ cell grafts in stroke rats. Brain Res 1048, 116–22. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB, 2011. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog. Neurobiol 95, 213–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Hess DC, 2006. New hope for stroke patients: mobilization of endogenous stem cells. Cmaj 174, 954–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Kaneko Y, Maki M, Yu SJ, Ali M, Allickson JG, Sanberg CD, Kuzmin-Nichols N, Sanberg PR, 2010. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev 19, 439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, Carroll J, Hess DC, 2004a. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res 1010, 108–16. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, Carroll J, Hess DC, 2004b. Intracerebral xenografts of mouse bone marrow cells in adult rats facilitate restoration of cerebral blood flow and blood-brain barrier. Brain Res 1009, 26–33. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Skinner SJ, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF, 2004c. CNS grafts of rat choroid plexus protect against cerebral ischemia in adult rats. Neuroreport 15, 1543–7. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR, 1998. Cerebral ischemia and CNS transplantation: differential effects of grafted fetal rat striatal cells and human neurons derived from a clonal cell line. Neuroreport 9, 3703–9. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Thanos CG, Skinner SJM, Geaney M, Emerich DF, 2007. Transplants of Encapsulated Rat Choroid Plexus Cells Exert Neuroprotection in a Rodent Model of Huntington’s Disease. Cell Transplant 16, 987–992. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Howard BJ, LaFontaine MA, 2001. Brain oxidative stress in animal models of accelerated aging and the age-related neurodegenerative disorders, Alzheimer’s disease and Huntington’s disease. Curr. Med. Chem 8, 815–28. [DOI] [PubMed] [Google Scholar]
- California’s Stem Cell Organization, 2013. Patient Advisory for Stem Cell Therapy and Medical Tourism https://www.cirm.ca.gov/about-cirm/newsroom/press-releases/08192013/patient-advisory-stem-cell-therapy-and-medical-tourism (accessed June 2018).
- Carlson ME, Silva HS, Conboy IM, 2008. Aging of signal transduction pathways, and pathology. Exp. Cell. Res 314, 1951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Borlongan CV, 2008. Adult stem cell therapy for acute brain injury in children. CNS Neurol. Disord. Drug Targets 7, 361–9. [DOI] [PubMed] [Google Scholar]
- Casals JB, Pieri NC, Feitosa ML, Ercolin AC, Roballo KC, Barreto RS, Bressan FF, Martins DS, Miglino MA, Ambrosio CE, 2011. The use of animal models for stroke research: a review. Comp. Med 61, 305–13. [PMC free article] [PubMed] [Google Scholar]
- Cave JW, Wang M, Baker H, 2014. Adult subventricular zone neural stem cells as a potential source of dopaminergic replacement neurons. Front. Neurosci 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D, 2016. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med 22, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Yu Y, Tang LJ, Kong L, Zhang CH, Chu HY, Yin LW, Ma HY, 2017. Neural stem cells over-expressing brain-derived neurotrophic factor promote neuronal survival and cytoskeletal protein expression in traumatic brain injury sites. Neural Regen. Res 12, 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Yang B, Li D, Ma S, Tian Y, Qu R, Zhang W, Zhang Y, Hu K, Guan F, Wang J, 2015. Wharton’s Jelly Transplantation Improves Neurologic Function in a Rat Model of Traumatic Brain Injury. Cell. Mol. Neurobiol 35, 641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YH, Lin SZ, Borlongan CV, Hoffer BJ, Morales M, Wang Y, 1999. Transplantation of fetal kidney tissue reduces cerebral infarction induced by middle cerebral artery ligation. J. Cereb. Blood Flow Metab 19, 1329–35. [DOI] [PubMed] [Google Scholar]
- Choi KA, Choi Y, Hong S, 2018. Stem cell transplantation for Huntington’s diseases. Methods 133, 104–112. [DOI] [PubMed] [Google Scholar]
- Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT, 2014. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J. Transl. Med 12, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti F, Saporta S, Hauser RA, Parent M, Saint-Pierre M, Sanberg PR, Li XJ, Parker JR, Chu Y, Mufson EJ, Kordower JH, Freeman TB, 2009. Neural transplants in patients with Huntington’s disease undergo disease-like neuronal degeneration. Proc. Natl. Acad. Sci 106, 12483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisbani G, Maxan A, Kordower JH, Planel E, Freeman TB, Cicchetti F, 2017. Presence of tau pathology within foetal neural allografts in patients with Huntington’s and Parkinson’s disease. Brain 140, 2982–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators, G. D. a. I. I. a. P., 2017. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Rebo J, 2015. Systemic Problems: A perspective on stem cell aging and rejuvenation. Aging (Albany NY) 7, 754–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA, 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–4. [DOI] [PubMed] [Google Scholar]
- Conboy MJ, Conboy IM, Rando TA, 2013. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell 12, 525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conese M, Carbone A, Beccia E, Angiolillo A, 2017. The Fountain of Youth: A Tale of Parabiosis, Stem Cells, and Rejuvenation. Open Med (Wars) 12, 376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CS Jr., Hetz RA, Liao GP, Aertker BM, Ewing-Cobbs L, Juranek J, Savitz SI, Jackson ML, Romanowska-Pawliczek AM, Triolo F, Dash PK, Pedroza C, Lee DA, Worth L, Aisiku IP, Choi HA, Holcomb JB, Kitagawa RS, 2017. Treatment of Severe Adult Traumatic Brain Injury Using Bone Marrow Mononuclear Cells. Stem Cells 35, 1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currais A, 2015. Ageing and inflammation - A central role for mitochondria in brain health and disease. Ageing Res. Rev 21, 30–42. [DOI] [PubMed] [Google Scholar]
- Das MM, Avalos P, Suezaki P, Godoy M, Garcia L, Chang CD, Vit JP, Shelley B, Gowing G, Svendsen CN, 2016. Human neural progenitors differentiate into astrocytes and protect motor neurons in aging rats. Exp. Neurol 280, 41–9. [DOI] [PubMed] [Google Scholar]
- Das MM, Svendsen CN, 2015. Astrocytes show reduced support of motor neurons with aging that is accelerated in a rodent model of ALS. Neurobiol. Aging 36, 1130–9. [DOI] [PubMed] [Google Scholar]
- DeCarolis NA, Kirby ED, Wyss-Coray T, Palmer TD, 2015. The Role of the Microenvironmental Niche in Declining Stem-Cell Functions Associated with Biological Aging. Cold Spring Harb Perspect Med 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Pena I, Sanberg PR, Acosta S, Tajiri N, Lin SZ, Borlongan CV, 2014. Stem cells and G-CSF for treating neuroinflammation in traumatic brain injury: aging as a comorbidity factor. J. Neurosurg. Sci 58, 145–9. [PMC free article] [PubMed] [Google Scholar]
- Deleidi M, Jaggle M, Rubino G, 2015. Immune aging, dysmetabolism, and inflammation in neurological diseases. Front. Neurosci 9, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Porta MG, Alessandrino EP, Bacigalupo A, van Lint MT, Malcovati L, Pascutto C, Falda M, Bernardi M, Onida F, Guidi S, Iori AP, Cerretti R, Marenco P, Pioltelli P, Angelucci E, Oneto R, Ripamonti F, Bernasconi P, Bosi A, Cazzola M, Rambaldi A, Gruppo Italiano Trapianto di Midollo, O., 2014. Predictive factors for the outcome of allogeneic transplantation in patients with MDS stratified according to the revised IPSS-R. Blood 123, 2333–42. [DOI] [PubMed] [Google Scholar]
- Deng QJ, Xu XF, Ren J, 2018. Effects of SDF-1/CXCR4 on the Repair of Traumatic Brain Injury in Rats by Mediating Bone Marrow Derived Mesenchymal Stem Cells. Cell. Mol. Neurobiol 38, 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Devine MJ, Ryten M, Vodicka P, Thomson AJ, Burdon T, Houlden H, Cavaleri F, Nagano M, Drummond NJ, Taanman JW, Schapira AH, Gwinn K, Hardy J, Lewis PA, Kunath T, 2011. Parkinson’s disease induced pluripotent stem cells with triplication of the alpha-synuclein locus. Nat. Commun 2, 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, Rosenfeld JV, Park KB, 2018a. Estimating the global incidence of traumatic brain injury. J. Neurosurg 1–18. [DOI] [PubMed] [Google Scholar]
- Dewan S, Schimmel S, Borlongan CV, 2018b. Treating childhood traumatic brain injury with autologous stem cell therapy. Expert. Opin. Biol. Ther 18, 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders R, Vanheusden M, Bogie JF, Ravanidis S, Thewissen K, Stinissen P, Gyselaers W, Hendriks JJ, Hellings N, 2015. Human Wharton’s Jelly-Derived Stem Cells Display Immunomodulatory Properties and Transiently Improve Rat Experimental Autoimmune Encephalomyelitis. Cell Transplant 24, 2077–98. [DOI] [PubMed] [Google Scholar]
- Drago D, Cossetti C, Iraci N, Gaude E, Musco G, Bachi A, Pluchino S, 2013. The stem cell secretome and its role in brain repair. Biochimie 95, 2271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein SA, Holmes LP, 2014. Seeking Novel Treatments Abroad. Stem Cell Tourism: Risks, Legal Issues and Mitigation http://www.cellr4.org/article/700 (accessed June 2018).
- Emborg ME, Zhang Z, Joers V, Brunner K, Bondarenko V, Ohshima S, Zhang SC, 2013. Intracerebral transplantation of differentiated human embryonic stem cells to hemiparkinsonian monkeys. Cell Transplant 22, 831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich DF, Thanos CG, Goddard M, Skinner SJ, Geany MS, Bell WJ, Bintz B, Schneider P, Chu Y, Babu RS, Borlongan CV, Boekelheide K, Hall S, Bryant B, Kordower JH, 2006. Extensive neuroprotection by choroid plexus transplants in excitotoxin lesioned monkeys. Neurobiol. Dis 23, 471–80. [DOI] [PubMed] [Google Scholar]
- Fangerau H, Fegert JM, Trapp T, 2014. Implanted Minds: The Neuroethics of Intracerebral Stem Cell Transplantation and Deep Brain Stimulation. Transcript Verlag [Google Scholar]
- Fink KD, Deng P, Torrest A, Stewart H, Pollock K, Gruenloh W, Annett G, Tempkin T, Wheelock V, Nolta JA, 2015. Developing stem cell therapies for juvenile and adult-onset Huntington’s disease. Regen. Med 10, 623–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores I, Blasco MA, 2010. The role of telomeres and telomerase in stem cell aging. FEBS Lett 584, 3826–30. [DOI] [PubMed] [Google Scholar]
- Freeman TB, Sanberg PR, Nauert GM, Boss BD, Spector D, Olanow CW, Kordower JH, 1995. The influence of donor age on the survival of solid and suspension intraparenchymal human embryonic nigral grafts. Cell Transplant 4, 141–54. [DOI] [PubMed] [Google Scholar]
- Gao J, Grill RJ, Dunn TJ, Bedi S, Labastida JA, Hetz RA, Xue H, Thonhoff JR, DeWitt DS, Prough DS, Cox CS Jr., Wu P, 2016. Human Neural Stem Cell Transplantation-Mediated Alteration of Microglial/Macrophage Phenotypes after Traumatic Brain Injury. Cell Transplant 25, 1863–1877. [DOI] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Haller E, Lin R, Borlongan CV, 2017. Intravenously Transplanted Human Bone Marrow Endothelial Progenitor Cells Engraft Within Brain Capillaries, Preserve Mitochondrial Morphology, and Display Pinocytotic Activity Toward Blood-Brain Barrier Repair in Ischemic Stroke Rats. Stem Cells 35, 1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazale H, Ramadan N, Mantash S, Zibara K, El-Sitt S, Darwish H, Chamaa F, Boustany RM, Mondello S, Abou-Kheir W, Soueid J, Kobeissy F, 2018. Docosahexaenoic acid (DHA) enhances the therapeutic potential of neonatal neural stem cell transplantation post-Traumatic brain injury. Behav. Brain. Res 340, 1–13. [DOI] [PubMed] [Google Scholar]
- Ghosh B, Zhang C, Smith GM, 2014. Bridging between transplantation therapy and neurotrophic factors in Parkinson’s disease. Front. Biosci. (Elite Ed) 6, 225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman EL, 2012. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells 30, 1144–51. [DOI] [PubMed] [Google Scholar]
- Glover LE, Tajiri N, Weinbren NL, Ishikawa H, Shinozuka K, Kaneko Y, Watterson DM, Borlongan CV, 2012. A Step-up Approach for Cell Therapy in Stroke: Translational Hurdles of Bone Marrow-Derived Stem Cells. Transl. Stroke Res 3, 90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogel S, Gubernator M, Minger SL, 2011. Progress and prospects: stem cells and neurological diseases. Gene Ther 18, 1–6. [DOI] [PubMed] [Google Scholar]
- Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Davalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millan M, Davis SM, Roy D, Thornton J, Roman LS, Ribo M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG, collaborators H, 2016. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387, 1723–31. [DOI] [PubMed] [Google Scholar]
- Guzik A, Bushnell C, 2017. Stroke Epidemiology and Risk Factor Management. Continuum (Minneap Minn) 23, 15–39. [DOI] [PubMed] [Google Scholar]
- Hara K, Matsukawa N, Yasuhara T, Xu L, Yu G, Maki M, Kawase T, Hess DC, Kim SU, Borlongan CV, 2007. Transplantation of post-mitotic human neuroteratocarcinoma-overexpressing Nurr1 cells provides therapeutic benefits in experimental stroke: in vitro evidence of expedited neuronal differentiation and GDNF secretion. J. Neurosci. Res 85, 1240–51. [DOI] [PubMed] [Google Scholar]
- Hara K, Yasuhara T, Maki M, Matsukawa N, Masuda T, Yu SJ, Ali M, Yu G, Xu L, Kim SU, Hess DC, Borlongan CV, 2008. Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Prog. Neurobiol 85, 318–34. [DOI] [PubMed] [Google Scholar]
- Harting MT, Sloan LE, Jimenez F, Baumgartner J, Cox CS Jr., 2009. Subacute neural stem cell therapy for traumatic brain injury. J. Surg. Res 153, 188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvard NeuroDiscovery Center, 2018. The Challenge of Neurodegenerative Diseases https://neurodiscovery.harvard.edu/challenge (accessed June 2018).
- Haus DL, Lopez-Velazquez L, Gold EM, Cunningham KM, Perez H, Anderson AJ, Cummings BJ, 2016. Transplantation of human neural stem cells restores cognition in an immunodeficient rodent model of traumatic brain injury. Exp. Neurol 281, 1–16. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kaneko Y, Yu S, Bae E, Stahl CE, Kawase T, van Loveren H, Sanberg PR, Borlongan CV, 2009. Quantitative analyses of matrix metalloproteinase activity after traumatic brain injury in adult rats. Brain Res 1280, 172–7. [DOI] [PubMed] [Google Scholar]
- Hefferan MP, Johe K, Hazel T, Feldman EL, Lunn JS, Marsala M, 2011. Optimization of immunosuppressive therapy for spinal grafting of human spinal stem cells in a rat model of ALS. Cell Transplant 20, 1153–61. [DOI] [PubMed] [Google Scholar]
- Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, Hovatta O, Jolkkonen J, 2009. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur. J. Neurosci 29, 562–74. [DOI] [PubMed] [Google Scholar]
- Hinks GL, Franklin RJ, 2000. Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats. Mol. Cell. Neurosci 16, 542–56. [DOI] [PubMed] [Google Scholar]
- Hirano N, 1990. Plaque assay and propagation in rat cell line LBC cells of rat coronavirus and 5 strains of sialodacryoadenitis virus. Zentralbl Veterinarmed B 37, 91–6. [DOI] [PubMed] [Google Scholar]
- Hsieh JY, Wang HW, Chang SJ, Liao KH, Lee IH, Lin WS, Wu CH, Lin WY, Cheng SM, 2013. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS One 8, e72604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Gebhart N, Richelson E, Brott TG, Meschia JF, Zubair AC, 2014. Mechanism of mesenchymal stem cell-induced neuron recovery and anti-inflammation. Cytotherapy 16, 1336–44. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, National Research Council, 2014. Stem Cell Therapies: Opportunities for Ensuring the Quality and Safety of Clinical Offerings: Summary of a Joint Workshop by the Institute of Medicine, the National Academy of Sciences, and the International Society for Stem Cell Research The National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Ishikawa H, Tajiri N, Shinozuka K, Vasconcellos J, Kaneko Y, Lee HJ, Mimura O, Dezawa M, Kim SU, Borlongan CV, 2013. Vasculogenesis in experimental stroke after human cerebral endothelial cell transplantation. Stroke 44, 3473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Imano M, Nishida S, Tsubaki M, Nakayama T, Mizuguchi N, Yamanaka S, Tabuchi M, Munakata H, Hashimoto S, Ito A, Satou T, 2013. Appearance of neural stem cells around the damaged area following traumatic brain injury in aged rats. J. Neural. Transm. (Vienna) 120, 361–74. [DOI] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT, 2006. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 443, 421–6. [DOI] [PubMed] [Google Scholar]
- Japtok J, Lojewski X, Naumann M, Klingenstein M, Reinhardt P, Sterneckert J, Putz S, Demestre M, Boeckers TM, Ludolph AC, Liebau S, Storch A, Hermann A, 2015. Stepwise acquirement of hallmark neuropathology in FUS-ALS iPSC models depends on mutation type and neuronal aging. Neurobiol. Dis 82, 420–429. [DOI] [PubMed] [Google Scholar]
- Jiang W, Buchele F, Papazoglou A, Dobrossy M, Nikkhah G, 2011. Multitract microtransplantation increases the yield of DARPP-32-positive embryonic striatal cells in a rodent model of Huntington’s disease. Cell Transplant 20, 1515–27. [DOI] [PubMed] [Google Scholar]
- Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW, 2016. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet 388, 787–96. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Dailey T, Weinbren NL, Rizzi J, Tamboli C, Allickson JG, Kuzmin-Nichols N, Sanberg PR, Eve DJ, Tajiri N, Borlongan CV, 2013. The battle of the sexes for stroke therapy: female-versus male-derived stem cells. CNS Neurol. Disord. Drug Targets 12, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnell FG, Lin D, Motley S, Duhen T, Lim N, Campbell DJ, Turka LA, Maecker HT, Harris KM, 2017. Reconstitution of immune cell populations in multiple sclerosis patients after autologous stem cell transplantation. Clin. Exp. Immunol 189, 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karussis D, Grigoriadis S, Polyzoidou E, Grigoriadis N, Slavin S, Abramsky O, 2006. Neuroprotection in multiple sclerosis. Clin. Neurol. Neurosurg 108, 250–4. [DOI] [PubMed] [Google Scholar]
- Kassis I, Petrou P, Halimi M, Karussis D, 2013. Mesenchymal stem cells (MSC) derived from mice with experimental autoimmune encephalomyelitis (EAE) suppress EAE and have similar biological properties with MSC from healthy donors. Immunol. Lett 154, 70–6. [DOI] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL, 2014. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CD, Sonnen JA, Swanson PD, Kopyov O, Leverenz JB, Bird TD, Montine TJ, 2007. Neural transplantation in Huntington disease: long-term grafts in two patients. Neurology 68, 2093–8. [DOI] [PubMed] [Google Scholar]
- Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV, 2013. Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int. J. Mol. Sci 14, 11692–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Lee HJ, An J, Kim YB, Ra JC, Lim I, Kim SU, 2014. Transplantation of human adipose tissue-derived stem cells delays clinical onset and prolongs life span in ALS mouse model. Cell Transplant 23, 1585–97. [DOI] [PubMed] [Google Scholar]
- Kim M, Lee ST, Chu K, Kim SU, 2008. Stem cell-based cell therapy for Huntington disease: a review. Neuropathology 28, 1–9. [DOI] [PubMed] [Google Scholar]
- Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M, 2012. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep 1, 703–14. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J, Decesare S, Jovin T, Zafonte R, Lebowitz J, Flickinger JC, Tong D, Marks MP, Jamieson C, Luu D, Bell-Stephens T, Teraoka J, 2005. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J. Neurosurg 103, 38–45. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, Jannetta P, DeCesare S, Elder EM, McGrogan M, Reitman MA, Bynum L, 2000. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 55, 565–9. [DOI] [PubMed] [Google Scholar]
- Krakora D, Mulcrone P, Meyer M, Lewis C, Bernau K, Gowing G, Zimprich C, Aebischer P, Svendsen CN, Suzuki M, 2013. Synergistic effects of GDNF and VEGF on lifespan and disease progression in a familial ALS rat model. Mol. Ther 21, 1602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L, 2011. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480, 547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Peruzzotti-Jametti L, Serapide MF, Testa N, Caniglia S, Balzarotti B, Pluchino S, Marchetti B, 2018. Neural Stem Cell Grafts Promote Astroglia-Driven Neurorestoration in the Aged Parkinsonian Brain via Wnt/beta-Catenin Signaling. Stem Cells [DOI] [PubMed] [Google Scholar]
- Lescaudron L, Rossignol J, Dunbar GL, 2014. Stem cells and neurodegenerative diseases CRC Press, Boca Raton. [Google Scholar]
- Lam PK, Wang KKW, Lo AWI, Tong CSW, Ching DWC, Wong K, Yang Z, Kong T, Lo KKY, Choy RKW, Lai PBS, Wong GKC, Poon WS, 2017. Interactome and reciprocal activation of pathways in topical mesenchymal stem cells and the recipient cerebral cortex following traumatic brain injury. Sci. Rep 7, 5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim KS, Ahn J, Bae HM, Lim I, Kim SU, 2014. Human motor neurons generated from neural stem cells delay clinical onset and prolong life in ALS mouse model. PLoS One 9, e97518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Xu K, Nguyen H, Guedes VA, Borlongan CV, Acosta SA, 2017. Stem Cell-Induced Biobridges as Possible Tools to Aid Neuroreconstruction after CNS Injury. Front. Cell Dev. Biol 5, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Lee JE, Kim HS, Song SK, Lee HS, Nam HS, Cheong JW, Jeong Y, Park HJ, Kim DJ, Nam CM, Lee JD, Kim HO, Sohn YH, 2012. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann. Neurol 72, 32–40. [DOI] [PubMed] [Google Scholar]
- Lee PH, Park HJ, 2009. Bone marrow-derived mesenchymal stem cell therapy as a candidate disease-modifying strategy in Parkinson’s disease and multiple system atrophy. J. Clin. Neurol 5, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, Kim M, 2006. Aging and neurodegeneration. Molecular mechanisms of neuronal loss in Huntington’s disease. Mech. Ageing Dev 127, 432–5. [DOI] [PubMed] [Google Scholar]
- Leon RL, Li X, Huber JD, Rosen CL, 2012. Worsened outcome from middle cerebral artery occlusion in aged rats receiving 17beta-estradiol. Endocrinology 153, 3386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveque X, Mathieux E, Nerriere-Daguin V, Thinard R, Kermarrec L, Durand T, Haudebourg T, Vanhove B, Lescaudron L, Neveu I, Naveilhan P, 2015. Local control of the host immune response performed with mesenchymal stem cells: perspectives for functional intracerebral xenotransplantation. J. Cell. Mol. Med 19, 124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HZ, Guo J, Gao J, Han LP, Jiang CM, Li HX, Bai SZ, Zhang WH, Li GW, Wang LN, Li H, Zhao YJ, Lin Y, Tian Y, Yang GD, Wang R, Wu LY, Yang BF, Xu CQ, 2011. Role of dopamine D2 receptors in ischemia/reperfusion induced apoptosis of cultured neonatal rat cardiomyocytes. J. Biomed. Sci 18, 18. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li JF, Zhang DJ, Geng T, Chen L, Huang H, Yin HL, Zhang YZ, Lou JY, Cao B, Wang YL, 2014. The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transplant 23 Suppl 1, S113–22. [DOI] [PubMed] [Google Scholar]
- Li XY, Liang ZH, Han C, Wei WJ, Song CL, Zhou LN, Liu Y, Li Y, Ji XF, Liu J, 2017. Transplantation of autologous peripheral blood mononuclear cells in the subarachnoid space for amyotrophic lateral sclerosis: a safety analysis of 14 patients. Neural Regen. Res 12, 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang AC, Mandeville ET, Maki T, Shindo A, Som AT, Egawa N, Itoh K, Chuang TT, McNeish JD, Holder JC, Lok J, Lo EH, Arai K, 2016. Effects of Aging on Neural Stem/Progenitor Cells and Oligodendrocyte Precursor Cells After Focal Cerebral Ischemia in Spontaneously Hypertensive Rats. Cell Transplant 25, 705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao GP, Harting MT, Hetz RA, Walker PA, Shah SK, Corkins CJ, Hughes TG, Jimenez F, Kosmach SC, Day MC, Tsao K, Lee DA, Worth LL, Baumgartner JE, Cox CS Jr., 2015. Autologous bone marrow mononuclear cells reduce therapeutic intensity for severe traumatic brain injury in children. Pediatr. Crit. Care Med 16, 245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Barker RA, Brustle O, Isacson O, Svendsen CN, 2012. Clinical translation of stem cells in neurodegenerative disorders. Cell Stem Cell 10, 151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Leon M, Outeiro TF, Goya RG, 2017. Cell reprogramming: Therapeutic potential and the promise of rejuvenation for the aging brain. Ageing Res Rev 40, 168–181. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magatti M, Abumaree M, Silini A, A. R, Saieva S, Russo E, T. ME, Rocca G,L, Parolini O, 2016. The Immunomodulatory Features of Mesenchymal Stromal Cells Derived from Wharton’s Jelly, Amniotic Membrane, and Chorionic Villi: In Vitro and In Vivo Data In: Parolini O, (Ed.), Placenta: The Tree of Life CRC Press, Boca Raton, pp. 91–128. [Google Scholar]
- Mahfouz MM, Abdelsalam RM, Masoud MA, Mansour HA, Ahmed-Farid OA, Kenawy SA, 2017. The neuroprotective effect of mesenchymal stem cells on an experimentally induced model for multiple sclerosis in mice. J. Biochem. Mol. Toxicol 31. [DOI] [PubMed] [Google Scholar]
- Marin-Banasco C, Benabdellah K, Melero-Jerez C, Oliver B, Pinto-Medel MJ, Hurtado-Guerrero I, de Castro F, Clemente D, Fernandez O, Martin F, Leyva L, Suardiaz M, 2017. Gene therapy with mesenchymal stem cells expressing IFN-ss ameliorates neuroinflammation in experimental models of multiple sclerosis. Br. J. Pharmacol 174, 238–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez HR, Gonzalez-Garza MT, Moreno-Cuevas J, Escamilla-Ocanas CE, Tenorio-Pedraza JM, Hernandez-Torre M, 2016. Long-term survival in amyotrophic lateral sclerosis after stem cell transplantation into the frontal motor cortex. Cytotherapy 18, 806–8. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L, 2000. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–9. [DOI] [PubMed] [Google Scholar]
- Mastro-Martinez I, Perez-Suarez E, Melen G, Gonzalez-Murillo A, Casco F, Lozano-Carbonero N, Gutierrez-Fernandez M, Diez-Tejedor E, Casado-Flores J, Ramirez-Orellana M, Serrano-Gonzalez A, 2015. Effects of local administration of allogenic adipose tissue-derived mesenchymal stem cells on functional recovery in experimental traumatic brain injury. Brain Inj 29, 1497–510. [DOI] [PubMed] [Google Scholar]
- Mazzini L, Gelati M, Profico DC, Sgaravizzi G, Projetti Pensi M, Muzi G, Ricciolini C, Rota Nodari L, Carletti S, Giorgi C, Spera C, Domenico F, Bersano E, Petruzzelli F, Cisari C, Maglione A, Sarnelli MF, Stecco A, Querin G, Masiero S, Cantello R, Ferrari D, Zalfa C, Binda E, Visioli A, Trombetta D, Novelli A, Torres B, Bernardini L, Carriero A, Prandi P, Servo S, Cerino A, Cima V, Gaiani A, Nasuelli N, Massara M, Glass J, Soraru G, Boulis NM, Vescovi AL, 2015. Human neural stem cell transplantation in ALS: initial results from a phase I trial. J. Transl. Med 13, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern Institute, 2018. Brain Disorders: By the Numbers http://mcgovern.mit.edu/brain-disorders/by-the-numbers (accessed June 2018).
- Miao X, Wu X, Shi W, 2015. Umbilical cord mesenchymal stem cells in neurological disorders: A clinical study. Indian J. Biochem. Biophys 52, 140–6. [PubMed] [Google Scholar]
- Miller JT, Bartley JH, Wimborne HJ, Walker AL, Hess DC, Hill WD, Carroll JE, 2005. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxicischemic injury. BMC Neurosci 6, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N, Pham LD, Hayakawa K, Matsuzaki T, Seo JH, Magnain C, Ayata C, Kim KW, Boas D, Lo EH, Arai K, 2013. Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke 44, 2573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Kitagawa H, 2017. Formation and remodeling of the brain extracellular matrix in neural plasticity: Roles of chondroitin sulfate and hyaluronan. Biochim. Biophys. Acta 1861, 2420–2434. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics, C., Stroke Statistics, S., 2015. Heart disease and stroke statistics−−2015 update: a report from the American Heart Association. Circulation 131, e29–322. [DOI] [PubMed] [Google Scholar]
- Mu S, Wang J, Zhou G, Peng W, He Z, Zhao Z, Mo C, Qu J, Zhang J, 2014. Transplantation of induced pluripotent stem cells improves functional recovery in Huntington’s disease rat model. PLoS One 9, e101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraro PA, Martin R, Mancardi GL, Nicholas R, Sormani MP, Saccardi R, 2017. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat. Rev. Neurol 13, 391–405. [DOI] [PubMed] [Google Scholar]
- Na Kim H, Yeol Kim D, Hee Oh S, Sook Kim H, Suk Kim K, Hyu Lee P, 2017. Feasibility and Efficacy of Intra-Arterial Administration of Mesenchymal Stem Cells in an Animal Model of Double Toxin-Induced Multiple System Atrophy. Stem Cells Transl. Med 6, 1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafissi S, Kazemi H, Tiraihi T, Beladi-Moghadam N, Faghihzadeh S, Faghihzadeh E, Yadegarynia D, Sadeghi M, Chamani-Tabriz L, Khanfakhraei A, Taheri T, 2016. Intraspinal delivery of bone marrow stromal cell-derived neural stem cells in patients with amyotrophic lateral sclerosis: A safety and feasibility study. J. Neurol. Sci 362, 174–81. [DOI] [PubMed] [Google Scholar]
- Nagpal A, Choy FC, Howell S, Hillier S, Chan F, Hamilton-Bruce MA, Koblar SA, 2017. Safety and effectiveness of stem cell therapies in early-phase clinical trials in stroke: a systematic review and meta-analysis. Stem Cell Res. Ther 8, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00254722, Safety of Autologous Stem Cell Treatment for Traumatic Brain Injury in Children https://ClinicalTrials.gov/show/NCT00254722 (accessed June 2018).
- NCT00801333, Derivation of Induced Pluripotent Stem Cells From an Existing Collection of Human Somatic Cells https://ClinicalTrials.gov/show/NCT00801333 (accessed June 2018).
- NCT01142856, Mesenchymal Stem Cells for Treatment of Amyotrophic Lateral Sclerosis (ALS) https://ClinicalTrials.gov/show/NCT01142856 (accessed June 2018).
- NCT01254539, Clinical Trial on The Use of Autologous Bone Marrow Stem Cells in Amyotrophic Lateral Sclerosis (Extension CMN/ELA) https://ClinicalTrials.gov/show/NCT01254539 (accessed June 2018).
- NCT01348451, Human Spinal Cord Derived Neural Stem Cell Transplantation for the Treatment of Amyotrophic Lateral Sclerosis https://ClinicalTrials.gov/show/NCT01348451 (accessed June 2018).
- NCT01363401, Safety and Efficacy Study of Autologous Bone Marrow Derived Stem Cell Treatment in Amyotrophic Lateral Sclerosis https://ClinicalTrials.gov/show/NCT01363401 (accessed June 2018).
- NCT01453829, Study to Assess the Safety and Effects of Autologous Adipose-Derived Stromal Cells in Patients After Stroke https://ClinicalTrials.gov/show/NCT01453829 (accessed June 2018).
- NCT01468064, Autologous Bone Marrow Stromal Cell and Endothelial Progenitor Cell Transplantation in Ischemic Stroke https://ClinicalTrials.gov/show/NCT01468064 (accessed June 2018).
- NCT01494480, The Clinical Trial on the Use of Umbilical Cord Mesenchymal Stem Cells in Amyotrophic Lateral Sclerosis https://ClinicalTrials.gov/show/NCT01494480 (accessed June 2018).
- NCT01575470, Treatment of Severe Adult Traumatic Brain Injury Using Bone Marrow Mononuclear Cells https://ClinicalTrials.gov/show/NCT01575470 (accessed June 2018). [DOI] [PMC free article] [PubMed]
- NCT01609283, A Dose-escalation Safety Trial for Intrathecal Autologous Mesenchymal Stem Cell Therapy in Amyotrophic Lateral Sclerosis https://ClinicalTrials.gov/show/NCT01609283 (accessed June 2018).
- NCT01640067, Human Neural Stem Cell Transplantation in Amyotrophic Lateral Sclerosis (ALS) https://ClinicalTrials.gov/show/NCT01640067 (accessed June 2018).
- NCT01678534, Reparative Therapy in Acute Ischemic Stroke With Allogenic Mesenchymal Stem Cells From Adipose Tissue, Safety Assessment, a Randomised, Double Blind Placebo Controlled Single Center Pilot Clinical Trial https://ClinicalTrials.gov/show/NCT01678534 (accessed June 2018). [DOI] [PubMed]
- NCT01714167, Autologous Bone Marrow Mesenchymal Stem Cell Transplantation for Chronic Stroke https://ClinicalTrials.gov/show/NCT01714167 (accessed June 2018).
- NCT01730716, Dose Escalation and Safety Study of Human Spinal Cord Derived Neural Stem Cell Transplantation for the Treatment of Amyotrophic Lateral Sclerosis https://ClinicalTrials.gov/show/NCT01730716 (accessed June 2018).
- NCT01758510, Safety Study of HLA-haplo Matched Allogenic Bone Marrow Derived Stem Cell Treatment in Amyotrophic Lateral Sclerosis https://ClinicalTrials.gov/show/NCT01758510 (accessed June 2018).
- NCT01825551, The Effect of GCSF in the Treatment of ALS Patients https://ClinicalTrials.gov/show/NCT01825551 (accessed June 2018).
- NCT01851083, Pediatric Autologous Bone Marrow Mononuclear Cells for Severe Traumatic Brain Injury https://ClinicalTrials.gov/show/NCT01851083 (accessed June 2018).
- NCT01898390, TRANSEURO Open Label Transplant Study in Parkinson’s Disease https://ClinicalTrials.gov/show/NCT01898390 (accessed June 2018).
- NCT01933321, Effect of Intrathecal Administration of Hematopoietic Stem Cells in Patients With Amyotrophic Lateral Sclerosis (ALS) https://ClinicalTrials.gov/show/NCT01933321 (accessed June 2018).
- NCT01937923, A Pre-Cellular Therapy Observational Study in Early Huntington’s Disease https://ClinicalTrials.gov/show/NCT01937923 (accessed June 2018).
- NCT01984814, Stem Cell Therapy for Amyotrophic Lateral Sclerosis https://ClinicalTrials.gov/show/NCT01984814 (accessed June 2018).
- NCT02028104, Stem Cell Therapy in Traumatic Brain Injury https://ClinicalTrials.gov/show/NCT02028104 (accessed June 2018).
- NCT02034188, Feasibility Study of Human Umbilical Cord Tissue-Derived Mesenchymal Stem Cells in Patients With Multiple Sclerosis https://ClinicalTrials.gov/show/NCT02034188 (accessed June 2018).
- NCT02116634, Mesenchymal Stem Cell Injection in Amyotrophic Lateral Sclerosis https://ClinicalTrials.gov/show/NCT02116634 (accessed June 2018).
- NCT02193893, Biological Treatment of Amyotrophic Lateral Sclerosis https://ClinicalTrials.gov/show/NCT02193893 (accessed June 2018).
- NCT02226848, Effect of Recombinant Erythropoietin on Numbers of Circulating Endothelial Progenitor Cells in People With Persistent Symptoms During the Subacute Period After Traumatic Brain Injury https://ClinicalTrials.gov/show/NCT02226848 (accessed June 2018).
- NCT03085706, Transplantation of Autologous Peripheral Blood Mononuclear Cells for Amyotrophic Lateral Sclerosis https://ClinicalTrials.gov/show/NCT03085706 (accessed June 2018).
- Neal EG, Acosta SA, Kaneko Y, Ji X, Borlongan CV, 2018. Regulatory T-cells within bone marrow-derived stem cells actively confer immunomodulatory and neuroprotective effects against stroke. J. Cereb. Blood Flow. Metab 271678X18766172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Kondziolka D, Wechsler L, Goldstein S, Gebel J, DeCesare S, Elder EM, Zhang PJ, Jacobs A, McGrogan M, Lee VM, Trojanowski JQ, 2002. Clonal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. Am. J. Pathol 160, 1201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niclis JC, Turner C, Durnall J, McDougal S, Kauhausen JA, Leaw B, Dottori M, Parish CL, Thompson LH, 2017. Long-Distance Axonal Growth and Protracted Functional Maturation of Neurons Derived from Human Induced Pluripotent Stem Cells After Intracerebral Transplantation. Stem Cells Transl. Med 6, 1547–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkhah G, Cunningham MG, Cenci MA, McKay RD, Bjorklund A, 1995. Dopaminergic microtransplants into the substantia nigra of neonatal rats with bilateral 6-OHDA lesions. I. Evidence for anatomical reconstruction of the nigrostriatal pathway. J. Neurosci 15, 3548–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova M, Small AL, Kimyon RS, Watters JJ, 2016. Age-dependent differences in microglial responses to systemic inflammation are evident as early as middle age. Physiol. Genomics 48, 336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizzardo M, Bucchia M, Ramirez A, Trombetta E, Bresolin N, Comi GP, Corti S, 2016. iPSC-derived LewisX+CXCR4+beta1-integrin+ neural stem cells improve the amyotrophic lateral sclerosis phenotype by preserving motor neurons and muscle innervation in human and rodent models. Hum. Mol. Genet 25, 3152–3163. [DOI] [PubMed] [Google Scholar]
- Norden DM, Muccigrosso MM, Godbout JP, 2015. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 96, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan SM, Sullivan CR, McCullumsmith RE, 2017. The role of glutamate transporters in the pathophysiology of neuropsychiatric disorders. NPJ Schizophr 3, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Lee YD, Wagers AJ, 2014. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat. Med 20, 870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KW, Moon C, Kim HY, Oh SI, Park J, Lee JH, Chang IY, Kim KS, Kim SH, 2015. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl. Med 4, 590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone AB, Simpkins JW, Barr TL, 2014. 17beta-estradiol and inflammation: implications for ischemic stroke. Aging Dis 5, 340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Zuniga R, Gonzalez-Perez O, Macias-Ornelas A, Capilla-Gonzalez V, Quinones-Hinojosa A, 2012. Ethical implications in the use of embryonic and adult neural stem cells. Stem Cells Int 2012, 470949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, 2016. How neuroinflammation contributes to neurodegeneration. Science 353, 777–83. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Schafer D, Vincent A, Blachere NE, Bar-Or A, 2015. Neuroinflammation: Ways in Which the Immune System Affects the Brain. Neurotherapeutics 12, 896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi S, Ghasemi N, Mardani M, Salehi H, 2018. Co-Transplantation of Human Neurotrophic Factor Secreting Cells and Adipose-Derived Stem Cells in Rat Model of Multiple Sclerosis. Cell J 20, 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve A, Simcox E, Turnbull D, 2014. Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res. Rev 14, 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan NH, Morales I, Fernandez G, Allen N, Fearnot NE, Leckrone ME, Markovich DJ, Mansfield D, Avila D, Patel AN, Kesari S, Paz Rodriguez J, 2018. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J. Transl. Med 16, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MC, Glover LE, Weinbren N, Rizzi JA, Ishikawa H, Shinozuka K, Tajiri N, Kaneko Y, Sanberg PR, Allickson JG, Kuzmin-Nichols N, Garbuzova-Davis S, Voltarelli JC, Cruz E, Borlongan CV, 2011. Toward personalized cell therapies: autologous menstrual blood cells for stroke. J. Biomed. Biotechnol 2011, 194720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolando C, Taylor V, 2014. Neural stem cell of the hippocampus: development, physiology regulation, and dysfunction in disease. Curr. Top. Dev. Biol 107, 183–206. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL, 2007. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447, 725–9. [DOI] [PubMed] [Google Scholar]
- Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ, 2012. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, Edinger AL, Jung S, Rossner MJ, Simons M, 2016. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci 19, 995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz SI, 2015. Developing Cellular Therapies for Stroke. Stroke 46, 2026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, Halbig TD, Hesekamp H, Navarro SM, Meier N, Falk D, Mehdorn M, Paschen S, Maarouf M, Barbe MT, Fink GR, Kupsch A, Gruber D, Schneider GH, Seigneuret E, Kistner A, Chaynes P, Ory-Magne F, Brefel Courbon C, Vesper J, Schnitzler A, Wojtecki L, Houeto JL, Bataille B, Maltete D, Damier P, Raoul S, Sixel-Doering F, Hellwig D, Gharabaghi A, Kruger R, Pinsker MO, Amtage F, Regis JM, Witjas T, Thobois S, Mertens P, Kloss M, Hartmann A, Oertel WH, Post B, Speelman H, Agid Y, Schade-Brittinger C, Deuschl G, Group ES, 2013. Neurostimulation for Parkinson’s disease with early motor complications. N. Engl. J. Med 368, 610–22. [DOI] [PubMed] [Google Scholar]
- Sharma A, Sane H, Kulkarni P, Gokulchandran N, Sawant D, Nivins S, Badhe P, 2017. Effect of cell transplantation in a chronic case of traumatic brain injury. Transplant Open 2, 1–4. [Google Scholar]
- Sharma A, Sane H, Kulkarni P, Yadav J, Gokulchandran N, Biju H, Badhe P, 2015. Cell therapy attempted as a novel approach for chronic traumatic brain injury - a pilot study. Springerplus 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Yin Y, Xia QJ, Lin N, Wang YC, Liu J, Wang HP, Lim A, Wang TH, 2016. Bone Marrow Stromal Cells Promote Neuronal Restoration in Rats with Traumatic Brain Injury: Involvement of GDNF Regulating BAD and BAX Signaling. Cell. Physiol. Biochem 38, 748–62. [DOI] [PubMed] [Google Scholar]
- Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJ, Casaccia-Bonnefil P, 2008. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci 11, 1024–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo T, Matsumoto Y, Wang Q, Kawai N, Tamiya T, Nagao S, 2006. Differences in the neuronal stem cells survival, neuronal differentiation and neurological improvement after transplantation of neural stem cells between mild and severe experimental traumatic brain injury. J. Med. Invest 53, 42–51. [DOI] [PubMed] [Google Scholar]
- Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ, 2014. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344, 649–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skardelly M, Gaber K, Burdack S, Scheidt F, Schuhmann MU, Hilbig H, Meixensberger J, Boltze J, 2014. Transient but not permanent benefit of neuronal progenitor cell therapy after traumatic brain injury: potential causes and translational consequences. Front. Cell. Neurosci 8, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohni A, Verfaillie CM, 2013. Mesenchymal stem cells migration homing and tracking. Stem Cells Int 2013, 130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squitieri F, Maglione V, Orobello S, Fornai F, 2011. Genotype-, aging-dependent abnormal caspase activity in Huntington disease blood cells. J. Neural Transm. (Vienna) 118, 1599–607. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Gross SK, Almad AA, Bulte CA, Maragakis NJ, Bulte JWM, 2017. Serial in vivo imaging of transplanted allogeneic neural stem cell survival in a mouse model of amyotrophic lateral sclerosis. Exp. Neurol 289, 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff NP, Madigan NN, Morris J, Jentoft M, Sorenson EJ, Butler G, Gastineau D, Dietz A, Windebank AJ, 2016. Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology 87, 2230–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Armstrong RC, 2017. Transplanted Adult Neural Stem Cells Express Sonic Hedgehog In Vivo and Suppress White Matter Neuroinflammation after Experimental Traumatic Brain Injury. Stem Cells Int 2017, 9342534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunwoo MK, Yun HJ, Song SK, Ham JH, Hong JY, Lee JE, Lee HS, Sohn YH, Lee JM, Lee PH, 2014. Mesenchymal stem cells can modulate longitudinal changes in cortical thickness and its related cognitive decline in patients with multiple system atrophy. Front. Aging Neurosci 6, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykova E, Rychmach P, Drahoradova I, Konradova S, Ruzickova K, Vorisek I, Forostyak S, Homola A, Bojar M, 2017. Transplantation of Mesenchymal Stromal Cells in Patients With Amyotrophic Lateral Sclerosis: Results of Phase I/IIa Clinical Trial. Cell Transplant 26, 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri N, Acosta S, Glover LE, Bickford PC, Jacotte Simancas A, Yasuhara T, Date I, Solomita MA, Antonucci I, Stuppia L, Kaneko Y, Borlongan CV, 2012. Intravenous grafts of amniotic fluid-derived stem cells induce endogenous cell proliferation and attenuate behavioral deficits in ischemic stroke rats. PLoS One 7, e43779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri N, Acosta SA, Shahaduzzaman M, Ishikawa H, Shinozuka K, Pabon M, Hernandez-Ontiveros D, Kim DW, Metcalf C, Staples M, Dailey T, Vasconcellos J, Franyuti G, Gould L, Patel N, Cooper D, Kaneko Y, Borlongan CV, Bickford PC, 2014a. Intravenous transplants of human adipose-derived stem cell protect the brain from traumatic brain injury-induced neurodegeneration and motor and cognitive impairments: cell graft biodistribution and soluble factors in young and aged rats. J. Neurosci 34, 313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri N, Duncan K, Antoine A, Pabon M, Acosta SA, de la Pena I, Hernadez-Ontiveros DG, Shinozuka K, Ishikawa H, Kaneko Y, Yankee E, McGrogan M, Case C, Borlongan CV, 2014b. Stem cell-paved biobridge facilitates neural repair in traumatic brain injury. Front. Syst. Neurosci 8, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri N, Hernandez D, Acosta S, Shinozuka K, Ishikawa H, Ehrhart J, Diamandis T, Gonzales-Portillo C, Borlongan MC, Tan J, Kaneko Y, Borlongan CV, 2014c. Suppressed cytokine expression immediatey following traumatic brain injury in neonatal rats indicates an expeditious endogenous anti-inflammatory response. Brain Res 1559, 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri N, Kaneko Y, Shinozuka K, Ishikawa H, Yankee E, McGrogan M, Case C, Borlongan CV, 2013. Stem cell recruitment of newly formed host cells via a successful seduction? Filling the gap between neurogenic niche and injured brain site. PLoS One 8, e74857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri N, Quach DM, Kaneko Y, Wu S, Lee D, Lam T, Hayama KL, Hazel TG, Johe K, Wu MC, Borlongan CV, 2014d. Behavioral and histopathological assessment of adult ischemic rat brains after intracerebral transplantation of NSI-566RSC cell lines. PLoS One 9, e91408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Farooqui AA, Siddiqi NJ, Alhomida AS, Ong WY, 2012. Effects of docosahexaenoic Acid on neurotransmission. Biomol. Ther. (Seoul) 20, 152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Sahli ZT, Wolny M, Mousa SA, 2014. Emerging therapies for Parkinson’s disease: from bench to bedside. Pharmacol. Ther 144, 123–33. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Bell JM, Breiding MJ, Xu L, 2017. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ 66, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traiffort E, Zakaria M, Laouarem Y, Ferent J, 2016. Hedgehog: A Key Signaling in the Development of the Oligodendrocyte Lineage. J. Dev. Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trokovic R, Weltner J, Noisa P, Raivio T, Otonkoski T, 2015. Combined negative effect of donor age and time in culture on the reprogramming efficiency into induced pluripotent stem cells. Stem Cell Res 15, 254–62. [DOI] [PubMed] [Google Scholar]
- Tutuncu M, Tang J, Zeid NA, Kale N, Crusan DJ, Atkinson EJ, Siva A, Pittock SJ, Pirko I, Keegan BM, Lucchinetti CF, Noseworthy JH, Rodriguez M, Weinshenker BG, Kantarci OH, 2013. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult. Scler 19, 188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermilyea SC, Guthrie S, Meyer M, Smuga-Otto K, Braun K, Howden S, Thomson JA, Zhang SC, Emborg ME, Golos TG, 2017. Induced Pluripotent Stem Cell-Derived Dopaminergic Neurons from Adult Common Marmoset Fibroblasts. Stem Cells Dev 26, 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kimbrel EA, Ijichi K, Paul D, Lazorchak AS, Chu J, Kouris NA, Yavanian GJ, Lu SJ, Pachter JS, Crocker SJ, Lanza R, Xu RH, 2014. Human ESC-derived MSCs outperform bone marrow MSCs in the treatment of an EAE model of multiple sclerosis. Stem Cell Reports 3, 115–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Imitola J, Rasmussen S, O’Connor KC, Khoury SJ, 2008. Paradoxical dysregulation of the neural stem cell pathway sonic hedgehog-Gli1 in autoimmune encephalomyelitis and multiple sclerosis. Ann. Neurol 64, 417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Luo Y, Chen L, Liang W, 2017. Safety of neural stem cell transplantation in patients with severe traumatic brain injury. Exp. Ther. Med 13, 3613–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weder N, Zhang H, Jensen K, Yang BZ, Simen A, Jackowski A, Lipschitz D, Douglas-Palumberi H, Ge M, Perepletchikova F, O’Loughlin K, Hudziak JJ, Gelernter J, Kaufman J, 2014. Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. J. Am. Acad. Child Adolesc. Psychiatry 53, 417–24 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LW, Wang YL, Christensen JM, Khalifian S, Schneeberger S, Raimondi G, Cooney DS, Lee WP, Brandacher G, 2014. Donor age negatively affects the immunoregulatory properties of both adipose and bone marrow derived mesenchymal stem cells. Transpl. Immunol 30, 122–7. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, 2016. Ageing, neurodegeneration and brain rejuvenation. Nature 539, 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Rao F, Guo ZY, Sun X, Wang YG, Liu SY, Wang AY, Guo QY, Meng HY, Zhao Q, Peng J, Wang Y, Lu SB, 2016. Extracellular matrix from human umbilical cord-derived mesenchymal stem cells as a scaffold for peripheral nerve regeneration. Neural Regen. Res 11, 1172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Horie N, Satoh K, Ishikawa T, Mori T, Maeda H, Fukuda Y, Ishizaka S, Hiu T, Morofuji Y, Izumo T, Nishida N, Matsuo T, 2017. Age of donor of human mesenchymal stem cells affects structural and functional recovery after cell therapy following ischaemic stroke. J. Cereb. Blood Flow. Metab 271678X17731964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara T, Matsukawa N, Hara K, Yu G, Xu L, Maki M, Kim SU, Borlongan CV, 2006a. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson’s disease. J. Neurosci 26, 12497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara T, Matsukawa N, Yu G, Xu L, Mays RW, Kovach J, Deans R, Hess DC, Carroll JE, Borlongan CV, 2006b. Transplantation of cryopreserved human bone marrow-derived multipotent adult progenitor cells for neonatal hypoxic-ischemic injury: targeting the hippocampus. Rev. Neurosci 17, 215–25. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Matsukawa N, Yu G, Xu L, Mays RW, Kovach J, Deans RJ, Hess DC, Carroll JE, Borlongan CV, 2006c. Behavioral and histological characterization of intrahippocampal grafts of human bone marrow-derived multipotent progenitor cells in neonatal rats with hypoxic-ischemic injury. Cell Transplant 15, 231–8. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li Y, Zhang C, Chopp M, Gosiewska A, Hong K, 2011. Delayed administration of human umbilical tissue-derived cells improved neurological functional recovery in a rodent model of focal ischemia. Stroke 42, 1437–44. [DOI] [PubMed] [Google Scholar]
- Zhong SJ, Gong YH, Lin YC, 2017. Combined intranasal nerve growth factor and ventricle neural stem cell grafts prolong survival and improve disease outcome in amyotrophic lateral sclerosis transgenic mice. Neurosci. Lett 656, 1–8. [DOI] [PubMed] [Google Scholar]