Abstract
Stem cell differentiation towards a specific lineage is controlled by its microenvironment. Polymer scaffolds have long been investigated to provide microenvironment cues; however synthetic polymers lack the specific signaling motifs necessary to direct cellular responses on their own. In this study, we fabricated random and aligned poly(ε-caprolactone) nanofiber substrates, surface-functionalized with RGD via strain-promoted azide-alkyne cycloaddition, that were used to investigate the role of a covalently tethered bioactive peptide (RGD) and nanofiber orientation on neural differentiation of mouse embryonic stem cells. Gene and protein expression showed neural differentiation progression over 14 days, with similar expression on RGD random and aligned nanofibers for neurons and glia over time. The high levels of glial fibrillary acidic protein expression at early time points were indicative of neural progenitors, and occurred earlier than on controls or in previous reports. These results highlight the influence of RGD binding versus topography in differentiation.
Keywords: D3 mouse embryonic stem cells, neural differentiation, RGD, functionalized nanofibers, glial fibrillary acidic protein
Graphical Abstract

Introduction
Embryonic stem cells (ESCs) have unique properties of self-renewal and differentiation, making them suitable choices for in vitro models to study various pathologies. With the potential to differentiate into a wide variety of lineages, ESCs can be used for tissue culture models of diseases or for therapeutic approaches for neurodegenerative diseases1–2. Soluble bioactive agents combined with a compatible protein-coated surface are the typical strategy to support and direct differentiation. While use of adsorbed protein is convenient, this method suffers from batch-to-batch variation and the presentation of bioactive protein motifs is uncontrolled and highly variable. However, using rationally designed synthetic or biomimetic materials3, the topographical4–8 and bioactive cues5, 9–15 can be controlled tightly to regulate the differentiation of these ESC cultures. These physical and biochemical cues, inspired by the native extracellular matrix (ECM), can be used to study the differentiation process and design therapeutic devices which take advantage of the specific features.
As the ECM plays a crucial role in regulating cell behavior, synthetic polymer scaffolds have been fabricated to mimic ECM topography using nanofibers12, 16. Topographical factors, such as nanofiber orientation, have influenced proliferation17–18 and cell function for various tissues, e.g., nerve4, 9 and smooth muscle7. Random fiber orientations mimic the ECM structure more closely4, while an aligned fiber topography facilitates contact guidance13, cellular alignment, and directional migration, which are desirable responses for neuronal regeneration and neurite outgrowth13. Electrospinning of various synthetic polymers, including poly(ε-caprolactone) (PCL)10, 13, 19–20, poly(L-lactic acid)9, 20, poly(glycolic acid)20 and poly(DL-lactic-co-glycolic acid)20 has been used to develop nanofiber substrates for bone21, vascular tissue7, 22, and nerve9 tissue engineering. However, the aforementioned synthetic polymer substrates are hydrophobic and lack bioactive cell-recognition sites, resulting in low cell adhesion and proliferation. Generally, bioactivity is added via addition of whole proteins during10, 19, 22,23–25 or after26–27 electrospinning. Although whole proteins are useful, as they add multiple binding sites for cells, electrospinning or adsorbing proteins eliminates control over the presentation of the bioactive sites, increasing the batch-to-batch variation in the preformed substrates. By functionalizing synthetic nanofibers after electrospinning with a tethered synthetic peptide, the bioactivity and concentration of the peptide can be maintained28–30.
Covalently tethering bioactive peptides to the surface of the nanofiber is an attractive alternative to adsorbed proteins. Strain-promoted azide-alkyne cycloaddition (SPAAC) reactions has been used widely to functionalize the surfaces of nanofibers due to the high efficiency, mild reaction conditions and orthogonality of the reactants29–31. This rapid and convenient method of bioconjugation is scalable and highly reproducible in comparison to other surface-tethering techniques such as plasma treatment, wet chemical methods, surface graft polymerization31–32. The SPAAC method of surface modification of the nanofibers post electrospinning affords precise control over the amount of surface available functionality33. The use of 4-dibenzocyclooctynol (DIBO) as an initiator of the ε-caprolactone polymerization results in end-functionalized PCL, where the reactive handle survives the electrospinning process28. Strained cyclooctynes, such as DIBO, react quickly with azides due to ring strain,34 allowing fast surface functionalization in metal-free conditions. In addition, the aromatic rings afford characterization of the peptide surface concentration by UV-visible spectroscopy28.
We have shown previously9, 13 that aligned nanofibers functionalized with tethered GYIGSR peptide mimicked adsorbed laminin during mESC neural differentiation. Therefore, we were interested in investigating how other bioactive laminin peptides influenced the neural differentiation process. RGD is an ubiquitous peptide that has been previously shown to mimic fibronectin35, but is also found on the a chain of laminin36. The RGD peptide has been used in studies of mESC pluripotency25 and neural stem cell differentiation37 and has clear integrin interaction sites with cells38. Here, we investigated RGD-tethered nanofibers to compare random and aligned nanofiber orientations on mESC neural differentiation.
Materials and Methods
Materials used for Nanofiber Synthesis and Cell Study
Nanofibers
All materials were used as received unless otherwise stated. Tetrahydrofuran (anhydrous, ≥99.9%, inhibitor-free), chloroform (anhydrous, contains amylenes as stabilizer, ≥99%), and calcium hydride (reagent grade, 95%) were purchased from Sigma-Aldrich (St. Louis, MO). Phenylacetaldehyde (98%, stabilized), lithium di-isopropylamide mono(tetrahydrofuran) (1.5 M solution in cyclohexane, AcroSeal™), iodotrimethylsilane (95–97%), n-butyllithium (2.5 M solution in hexanes, AcroSeal™), hexanes and methylene chloride were purchased from Fisher Scientific (Houston, TX). Sodium thiosulfate pentahydrate (Proteomics grade, 99%) was purchased from Amresco, LLC (Solon, OH). 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) was purchased from Oakwood Products, Inc. (Estill, SC). Sodium sulfate anhydrous (ACS grade) and methanol (ACS grade), hydrochloric acid (36.5–38%, ACS Grade) were purchased from VWR International (Radnor, PA).
Dry toluene (HPLC Grade, 99.7%, Alfa Aesar) for polymerization was purified and dried on an Inert Pure Solv system (MD Solvent Purification system, model PS-MD-3) and degassed using three cycles of the freeze-vacuum-thaw. ε-Caprolactone (ε-CL, 99%, ACROS Organics™) was dried over calcium hydride under nitrogen overnight and distilled under reduced pressure. Magnesium 2,6-di-tert-butyl-4-methylphenoxide catalyst [Mg(BHT)2(THF)2]39, 4-dibenzocyclooctynol (DIBO)28 initiator and DIBO-end functionalized poly(ε-caprolactone) were synthesized using methods described previously13. Resins for peptide synthesis (Novabiochem®) were purchased from EMD Millipore (Billerica, MA). Fmoc-amino acids were purchased from Aapptec (Louisville, KY).
Square (22 × 22 mm) and round (8 mm) Fisherbrand™ borosilicate cover glasses (#1.5) were washed with methanol / toluene / methanol, dried with nitrogen, and cleaned with UV light (355 nm) for 3 min prior to use. After nanofibers were collected on the glass coverslips, the nanofiber mats were glued to the edges of a glass slide by a silicone sealant and dried under vacuum overnight.
Cell study
Materials used during this study included the following: D3 mESCs (ATCC); ES-qualified 0.1% gelatin (Embryomax, Millipore); D3 growth medium consisted of Dulbecco’s Modification of Eagle’s Medium (DMEM) (Corning) prepared with sodium bicarbonate (Sigma), supplemented with 10% ES-qualified fetal bovine serum (Millipore), 10−4 M β-mercaptoethanol (Millipore Embryomax), 4 mM L-glutamine (Life Technologies), 4.7 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Hyclone GE Healthcare), and 1000 units/mL human recombinant leukemia inducing factor (GlobalStem); trypsin-EDTA (Sigma Aldrich); flow cytometry antibodies: anti-SSEA1 (BioLegend 125606) and isotype control (BioLegend 401611); Neural differentiation medium consisted of 70% DMEM/F-12 (Corning), 20% neurobasal medium (Life Technologies), 1X N2 supplement, 20% PluriQ serum replacement (GlobalStem), 10−3 M sodium pyruvate (Sigma), 2 mM L-glutamine (Life Technologies), and 2 μM retinoic acid (Sigma). TRIzol reagent (Life Technologies); cDNA Synthesis Kit (Quantabio), SYBR Green (Quantabio). Paraformaldehyde (Fisher Scientific); Triton-X (Sigma); sodium borohydride (MP Biomedicals); Primary antibodies: NES (Abcam 134017; 1:10,000), SSEA1 (DSHB MC480; 1:8), OCT4 (Abcam 198857; 1:1000), SOX1 (Cell Signaling Tech 4194; 1:200), GFAP (BioLegend 82401; 1:1000), and TUBB3 (Abcam 78078; 1:500) for early time points (days 1 and 3); GFAP, TUBB3, MAP2 (Abcam 11267; 1:500), GAP43 (Abcam 16053; 1:500), and OLIG1 (Abcam 53041; 1:500). Nuclei stain: H33342 (Life Technologies H1399); Secondary antibodies were diluted at 1:400; goat anti-mouse IgM AF546 (Life Technologies A21045), donkey anti-rabbit IgG AF647 (Life Technologies A31573), goat anti-chicken IgY AF488 (Life Technologies A11039), goat anti-mouse IgG2a AF546 (Life Technologies A21133), and goat anti-mouse IgG1 AF546 (Life Technologies A21123).
Fabrication of Synthetic Nanofibers
Experimental Methods
Proton 1H nuclear magnetic resonance (NMR) (300 MHz and 500 MHz) spectra were recorded on Varian Mercury 300 and 500 spectrometers. The polymers were dissolved in CDCl3 solvent at 15 mg/mL, the relaxation time was 2 sec with 64 transients. Size exclusion chromatography (SEC) was used to determine molecular mass and molecular mass distributions (Đm). Chromatograms were collected on a Tosoh EcoSEC HLC-8320GPC using refractive index detector and N,N-dimethylformamide (DMF) containing 0.1 M lithium bromide as the eluent at a flow rate of 0.3 mL/min and 40 °C. The 2 columns were calibrated using narrow molecular mass poly(styrene) standards (20 standards from 0.5 kDa to 5,480 kDa). Nanofiber scaffolds were sterilized by ethylene oxide using an Anprolene benchtop sterilizer (Anderson Products, Inc., Haw River, NC) according to the manufacturer’s protocol for 12 h at room temperature and 35% humidity (concentration of ethylene oxide is about 0.5 g/L), purged for at least 48 h and stored in a vacuum desiccator until further use.
Electrospinning conditions and nanofiber collection
The electrospinning setup for aligned nanofiber scaffolds is shown in Figure 1 (B, D). For aligned fiber scaffolds, the DIBO-terminated PCL was dissolved in HFIP (17% (w/v)) to yield a clear, slightly viscous solution. The solution was placed in a 2 mL glass syringe with a 22 gauge needle for aligned and 23 gauge needle for random fibers (JG22–0.5X or GJ23–0.5X, Jensen Global Dispensing Solutions). A voltage of 15 kV was applied to the solution, and the tip-to-collector distance was set to 10 cm. Aluminum foil was used as the grounded collector for random fibers and metal plate with gaps (24 × 110 mm) for aligned fibers. Random nanofibers were collected on glass cover slides placed on aluminum foil. Aligned nanofibers were collected by placing cover glasses in between the gaps of the collector. The collected nanofiber mats were glued to the edges of a glass slide with a silicone sealant and dried under vacuum overnight.
Figure 1:
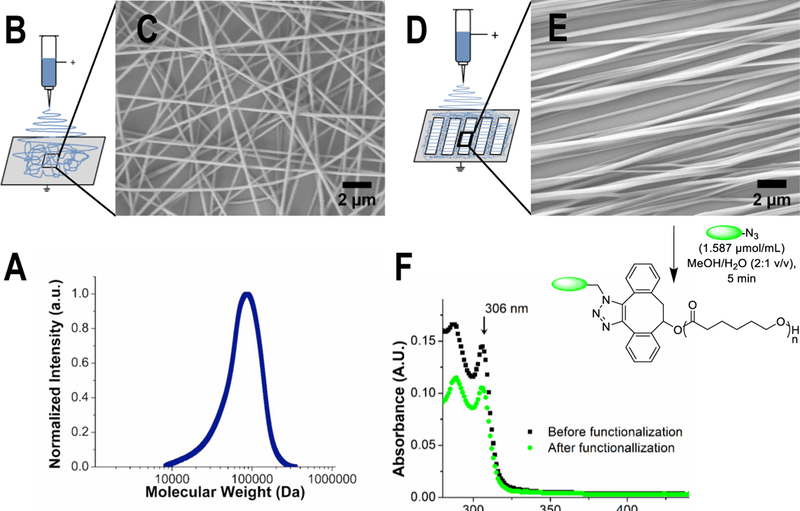
(A) Analysis by DMF size exclusion chromatography confirms successful synthesis of high molecular mass DIBO-terminated poly(ε-caprolactone) (Mn = 60,600 Da, Mw = 83,700 Da, Đm = 1.38). Molecular mass was determined against PS standards. (B, D) Nanofibers were fabricated by electrospinning from a solution of DIBO-terminated poly(e-caprolactone) in HFIP (17% w/v) and a voltage of 15 kV. Cover glasses were placed on aluminum foil or in the gaps of metal collector plate for collecting of random or highly aligned nanofibers. (C, E) Analysis of SEM images was performed to estimate topography of nanofibers. NIH ImageJ was used to estimate fiber diameter (ᴓ = 212 ± 63 nm for aligned and 219 ± 36 nm for random fibers) and alignment (Directionality™ plugin, average angle = 0 ± 6° for aligned and −2 ± 111° random nanofiber scaffolds). (F) Post-electrospinning modification with GRGDS or GRGES peptides via strain-promoted azidealkyne cycloaddition. The concentration of GRGDS or GRGES peptides was measured using UV–visible spectrophotometry by comparison of absorbance at 306 nm (peak corresponding to DIBO groups) before (black curve) and after (green curve) post-electrospinning modification.
Characterization of diameter and orientation
Nanofiber dimensions and alignment were imaged by scanning electron microscope (SEM) with an applied voltage of 5 kV (JSM-7401F, JEOL, Peabody, MA). Samples were sputter coated for 30 seconds with silver under nitrogen atmosphere prior to imaging. A UVO Cleaner, Model #42A UV light unit was used to clean the glass coverslips for nanofiber collection. High voltage power supply (ES30P-5W, Gamma High Voltage, Ormond Beach, FL) was used for electrospinning. The variation in nanofiber diameters was measured on at least 3 independent samples (5 images of each sample with >150 fibers per sample) using ImageJ and reported as an average ± standard deviation. The Directionality™ plugin of ImageJ was used to quantify the relative degree of alignment of the scaffolds by analyzing the angle distribution of fibers. The values are reported as an average ± standard deviation. Fityk 0.9.8 was used to fit a Gaussian function (red curve) and calculate average angle as the peak of the distribution fit. Angles for aligned fibers were normalized to 0. The highest peak was normalized to 1.
Solid phase peptide synthesis
N3-GRGDS and N3-GRGES peptides were synthesized using standard FMOC conditions on a CEM Discovery microwave peptide synthesizer. The N-terminus was derivatized with 6-azidohexanoic acid13, 40. The peptides were purified by precipitation from trifluoroacetic acid into cold diethyl ether and wash 3 times into cold diethyl ether, followed by dialysis against water for 3 days and lyophilization. The desired peptide product was confirmed by electrospray ionization mass spectrometry.
Nanofiber functionalization
Nanofiber covered glass slides were dipped into a solution of the respective azide-functionalized peptide (1.59 μmol/mL) in 1:2 water/methanol (v/v) solution for 5 min. The cover slips with functionalized nanofibers were rinsed with 1:2 water/methanol (v/v) solution, blown with nitrogen and dried overnight in a desiccator. Scaffolds were sterilized using an ethylene oxide exposure cycle for 12 h, degassed for 2 days and stored in a vacuum desiccator until further use.
The extent of functionalization with each peptide (reported as an average ± standard deviation) was confirmed using UV–visible spectrophotometry (Synergy™ MX plate reader from BioTek, with spectral resolution 1 nm), using chloroform as a solvent. The peak intensity at 306 nm (which corresponds to π-π* transition in alkyne bond in the DIBO-functionalized polymer) decreases after reaction with azide-functionalized peptide in comparison with fibers before functionalization. The surface concentrations of the GRGDS or GRGES peptide were calculated by dividing number of moles of reacted alkyne groups by surface area of the fibers. The surface area of the fibers was calculated by finding the volume of the fibers from measured mass of the fiber mats and measured by SEM fiber diameter. Density of the DIBO-PCL was assumed to be the same as of PCL (1.145 g/cm3).
Cell Study
mESCs Culture
D3 mESCs were maintained pluripotent on feeder-free gelatin coated culture flasks in an incubator at 37 °C and 5% CO2. Growth medium for maintaining pluripotent mESCs was changed daily. The cells were passed once they reached 75–85% confluency; they were washed with 1x PBS and incubated in 1x trypsin EDTA for 2 min at 37 °C. The detached cells were neutralized with growth medium and centrifuged (160 g for 5 min at 4 °C) to collect in a pellet. Cells were seeded at 20,000 cells/cm2 for further culture; pluripotency was determined using flow cytometry by staining against SSEA1. Anti-SSEAI was incubated with 250,000 cells for 1 hour at 4 °C and then analyzed using flow cytometry against an isotype control. Cells which expressed an average of 98.8% SSEA1+ were used for neural differentiation of mESCs.
Neural Differentiation
Pluripotent mESCs were seeded in neural differentiation medium at a seeding density of 125,000 cells/cm2 on the substrates, and neural differentiation was induced using retinoic acid. The neural differentiation profile was analyzed at days 1, 3, 7, and 14 after seeding; pluripotent, neural progenitor, neuronal, and glial markers were analyzed using gene and protein expression.
Gene Expression
RNA isolation was performed using TRIzol Reagent and reverse transcribed using a cDNA synthesis kit, according to the manufacturer’s protocols. Quantitative PCR was performed using SYBR Green on pluripotent, neural progenitor, neuronal, and glial genes (Applied Biosciences 7500 qPCR system) using MIQE guidelines. Data analysis of ΔCt was calculated by subtracting the Ct of the gene of interest from housekeeping genes (β-actin and Gapdh) at the time point (days 1, 3, 7, or 14 of differentiation); ΔΔCt was calculated as the difference between the ΔCt(timepoint) - ΔCt(pluripotent). Data is represented as log2(fold change). Primer sequences can be found in Table 1. Note that we use standard gene and protein symbols, with italized symbols indicating genes while proteins are not italicized. Pou5f1 is seen in many reports as Oct4.
Table 1:
Summary of genes and their sequence utilized for gene analysis
| Gene | Sequence (Reverse) | Sequence(Forward) | |
|---|---|---|---|
| Pluripotent | Pou5f1 | GAA GCC GAC AAC AAT GAG AAC | GGC ACT TCA GAA ACA TGG TCT |
| Neural Progenitor | Nes | GGA AAG CCA AGA GAA GCC T | CAC CTC AAG ATG TCC CTT AGT C |
| Sox1 | GTA CAG TAT TTA TCG TCC GCA GA | GGC AGT CAT ACA AAA GTT GGC | |
| Neuronal | Pax6 | AAG GGC GGT GAG CAG ATG T | CAT GCT GGA GCT GGT TGG |
| Tubb3 | GTG GAC TTG GAA CCT GGA AC | CCT CCG TAT AGT GCC CTT TG | |
| Map2 | GAC CCA GAG TGT GTG AGT TTA T | CCA CTA ATG CCA GTT TCT CTC T | |
| Gap43 | TCA GGC ATG TTC TTG GTC AG | AGG AGG AGA AAG ACG CTG TA | |
| Glial | Gfap | GCG ATA GTC GTT AGC TTC GTG | CCA CCA GTA ACA TGC AAG AGA |
| Oligl | AGC AAC TAC ATC GCT CCT TG | TCC AGA CTT CTC TCC CAG AC | |
| Housekeeping | β-actin | CAC GGT TGG CCT TAG GGT TCA G | GCT GTA TTC CCC TCC ATC GTG |
| Gapdh | GTG GAG TCA TAC TGG AAC ATG TAG | AAT GGT GAA GGT CGG TGT G | |
Protein Expression
At the appropriate timepoint, samples were fixed with freshly prepared 4% buffered paraformaldehyde, washed, and stored in PBS at 4 °C. Cells were permeabilized in 0.5% and 0.1% Triton-X for 10 and 5 min respectively, and permeabilized with 1mg/mL of sodium borohydride twice for 4 min. Blocking was performed with BSA and 0.1% Triton-X for 1 hour. The cells were then stained with primary antibodies overnight at 4 °C, followed by staining with the appropriate secondary antibody overnight at 4 °C. Proteins of interest were NES, SSEA1, OCT4, SOX1, GFAP, and TUBB3 for early time points (days 1 and 3) and GFAP, TUBB3, MAP2, GAP43, and OLIG1 for later time points (days 7 and 14).
Analysis of Images
Images were captured on an inverted fluorescent microscope at exposure times set by controls that had only secondary antibodies and nuclei stain. At least five images were obtained from each sample. Image analysis was done on ImageJ (National Institutes of Health, v1.5h)41.
Cell Aggregate Size:
Using ImageJ, the sizes of mESCs aggregates were measured on at least 5 images for each sample for days 7 and 14 of neural differentiation. Aggregates were identified by nuclei stain and were outlined using either Oval or Polygon Selections tool. The outline was measured to determine the area of the aggregate. Cells that were close but did not touch each other were not considered aggregates.
Percent Positive Cells:
All images were captured at the same exposure as the respective control. A threshold for captured control images (no primary antibody) was set at <0.1% pixel intensity for each channel. The acquired brightness and contrast setting from the control was applied to the appropriate fluorescent channel on an image, so as to express only protein-positive cells. If necessary, the images were further adjusted until a distinct protein morphology was observed, and cells were then manually counted as protein positive. These positive cells were then normalized to the total number of cells labeled with any protein, to obtain a percent positive number. Results are expressed as + or – of the protein; for example, if a sample was labeled with SOX1, GFAP, and TUBB3 and showed only GFAP labeling, the result would be expressed as GFAP+ (SOX1- / TUBB3-).
Neurite Extension:
Neurite extension was quantified by tracing TUBB3+ neurites on aligned and random substrates. Using the NeuronJ plugin in ImageJ, these tracings were quantified and reported as total neurite length per mm2. In addition, the direction of neurite outgrowth was measured utilizing the Directionality™ plugin in ImageJ, similar to the nanofiber orientation analysis above. The direction of the aligned fibers was determined from the phase contrast image and set to 0°.
Statistical Analysis
Each experimental group consisted of at least three biological replicates, and quantitative data was represented as average ± standard deviation. Two-sample t-test with 95% confidence interval and a significance value of p > 0.05 was performed to prove that there was no statistical difference between diameters of random and aligned fibers as well as to show statistical difference between neurite alignment cultured on different substrates. Two-way ANOVA with Bonferroni post-hoc test was utilized to determine significance between gene expression using ΔΔCt, with p<0.05 considered significant. Kruskal-Wallis test with Bonferroni correction was utilized to determine significance in protein expression and neurite extension, with p<0.05 considered significant. Pearson’s correlation was performed to investigate correlations between protein expression (GFAP, TUBB3, and OLIG1), and number and size of aggregates.
Results
RGD and RGE Functionalized Synthetic Scaffolds
Synthesis of functionalized polymer
DIBO-end functionalized poly(ε-caprolactone) was synthesized by the ring-opening polymerization of ε-caprolactone using standard Schlenk techniques as shown in Scheme 1. DIBO was used as initiator to introduce functionality at the polymer chain end to facilitate efficient metal-free surface modification with peptides post-polymerization and post-electrospinning. The purity of the polymer and successful incorporation of DIBO into the polymer chain was shown by the 1H NMR spectra (the peaks at δ = 5.56, 3.10 and 2.93 ppm corresponding to protons from DIBO, Figure S1) and UV-visible spectroscopy (λmax = 306 nm corresponds to π-π* transition of the strained alkyne in DIBO, Figure S2). The use of Mg(BHT)2(THF)2 as a catalyst yielded a controlled polymerization with high (>90%) monomer conversion within short period of time (13 min) at 30 °C. The use of these catalytic conditions affords high molecular mass and narrow molecular mass distribution polymer (Mn = 60,600 Da, Mw = 83,700 Da, Đm = 1.38) as shown in Figure 1.
Scheme 1:
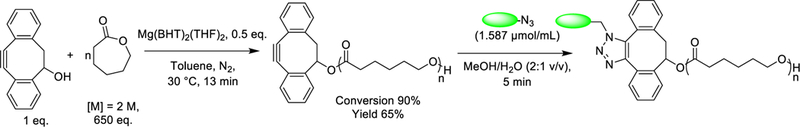
DIBO-end-functionalized poly(ε-caprolactone) was synthesized via ring-opening polymerization of ε-caprolactone using DIBO as an initiator and Mg(BHT)2(THF)2 as a catalyst. Surface of DIBO-PCL was modified post-electrospinning with GRGDS or GRGES peptides via strain-promoted azide-alkyne cycloaddition.
Characterization of diameter and orientation of nanofibers
DIBO-functionalized poly(ε-caprolactone) was used to fabricate highly aligned (with a narrow angular distribution 0 ± 6°, average ± standard deviation) and random (−2 ± 111°) nanofiber scaffolds (Figure S3) by using different electrospinning setups (Figure 1B, D). Using aluminum foil as a collector yielded random nanofibers. Use of a conductive metal frame with gaps afforded fabrication of aligned nanofibers. The quality of the Gaussian fit calculated by the Directionality™ plugin was high for aligned fibers (0.91 ± 0.06) and low for random fibers 0.41 ± 0.2, average ± standard deviation). Diameters of aligned and random fibers were not statistically different (ᴓ = 212 ± 63 nm and 219 ± 36 nm accordingly, p = 0.127). The distributions of nanofiber diameters are shown in Figure S4.
Solid phase peptide synthesis
End-functionalization of the respective peptides with 6-azidohexanoic acid serves as a spacer for the peptides to react with DIBO groups on the surface of the nanofibers. ESI mass spectrometry confirmed the mass and purity of each peptide; [M]+ of N3-GRGDS = 630.01 Da, yield = 84% and [M]+ of N3-GRGES = 644.27 Da (Figures S5 and S6 respectively).
Post-electrospinning surface modification and quantification
Post-electrospinning modification of nanofibers with either GRGDG or GRGES peptide via strain-promoted azide-alkyne cycloaddition was carried out by dipping nanofiber scaffolds in the water-methanol solution of azide-functionalized peptides at ambient temperature. The peak intensity at 306 nm (which corresponds to π-π* transition of DIBO) decreased after reaction with azide-functionalized peptide in comparison with fibers prior to functionalization (Figure 1F). Quantitative assessment of the amount of the peptides attached to the surface of nanofibers with different orientation gave comparable values. The surface concentration of GRGDS peptide was determined to be 10.8 ± 5.8 pmol/cm2 for random and 19.8 ± 2.1 pmol/cm2 for aligned nanofibers; and of GRGES peptide was measured to be 24.7± 8.4 pmol/cm2 for random and 8.4 ± 3.8 pmol/cm2 for aligned nanofibers. The degree of functionalization with GRGDS was calculated as the ratio of reacted DIBO groups to the total amount of polymer chains (0.10 ± 0.06 for random and 0.20 ± 0.02 for aligned nanofibers with GRGDS; and 0.24 ± 0.08 for random and 0.08 ± 0.04 for aligned nanofibers with GRGES).
mESC Response
Control nanofibers with RGE (null peptide), or too little RGD (1.4 ± 0.9 pmol/cm2 and 1.9 ± 1.5 pmol/cm2 on random and aligned nanofibers respectively), had insufficient cell adhesion for a complete analysis of gene expression (Figure S7) or any protein expression analysis.
Gene Expression
To investigate the neural differentiation of mESCs on the RGD nanofibers, we calculated fold change to describe the up or down regulation of genes via ΔΔCt using 2 housekeeping genes within each sample and pluripotent mESCs as the comparator. A log2(fold change) of 0 shows no difference between expression of pluripotent mESCs. First, as expected during the differenation process, the pluripotent gene, Pou5f1 expression (Figure S8A), decreased in all groups over time. By day 7, the gene was not expressed in random nanofibers in quantities that could be detected within 35 cycles.
Next, we investigated Sox1 expression (Figure 2A) to demonstrate differentiation toward neural progenitors. Sox1 is upregulated in all samples at days 1 and 3. At day 7, a statistical increase in Sox1 upregulation occurred in random nanofibers compared to days 1 and 3 that was sustained at day 14, while the upregulation at day 7 remained similar to days 1 and 3 in both aligned nanofibers and fibronectin-coated surfaces. For days 7 and 14, a fold change of 250 ± 180 and 31 ± 14 was found on random nanofibers, compared to fold change of 1 ± 0 and 2 ± 4 on aligned nanofibers, and 3 ± 10 and 9 ± 4 fibronectin-coated substrates.
Figure 2:
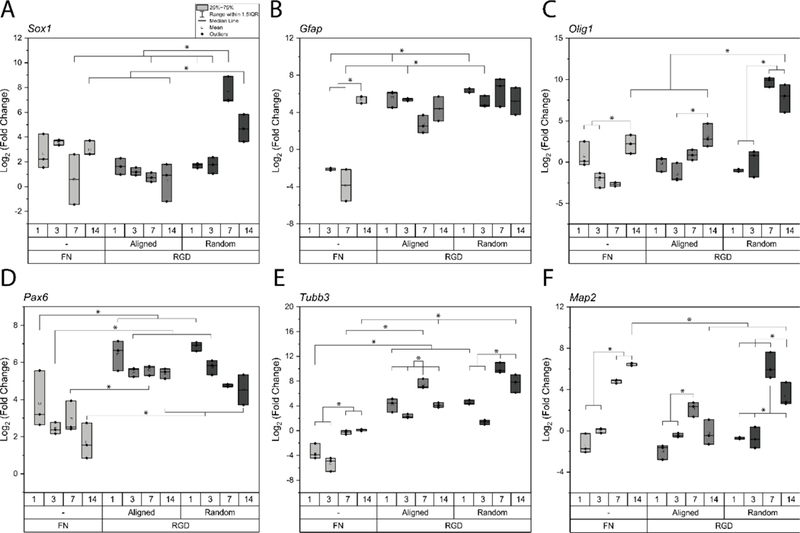
Summary of neural precursor (A), glial (B,C), and neuronal (D-F) gene expression over 14 days of neural differentiation of mouse embryonic stem cells, on fibronectin coated surfaces and RGD functionalized aligned and random nanofibers. Gene expression is represented as log2 (fold change). Statistically significances are highlighted between groups with p<0.05 considered significant.
Gfap expression (Figure 2B) was upregulated on both nanofiber topographies beginning at day 1. The upregulation was noted for days 1, 3, and 7 on both random and aligned nanofibers. A fold change of 102 ± 43 and 47 ± 33 was observed on random nanofibers at days 1 and 3 respectively, compared to 53 ± 21 and 38 ± 4 on aligned nanofibers. In contrast, Gfap on fibronectin-coated surfaces was downregulated until day 7. However, by day 14, cells on fibronectin-coated surfaces showed upregulated Gfap, that increased to the same level as the upregulation of cells on the nanofibers. At day 14, a fold change of 67 ± 52 was found on fibronectin-coated surfaces, compared to 30 ± 25 on aligned and 106 ± 112 on random nanofibers.
As Gfap was upregulated early, we were interested in the potential to derive oligodendrocytes. Oligodendrocyte expression was determined by Olig1 expression (Figure 2C); at days 3, 7, and 14, higher expression was found on both nanofiber topographies than fibronectin-coated surfaces. On fibronectin-coated substrates, cells had upregulated Olig1 by day 14, which was statistically higher than on fibronectin at days 3 and 7. Cells on aligned substrates had a similar response, with upregulation of Olig1 at day 14 that was statistically increased over day 3. However, the upregulation of Olig1 on random substrates was statistically increased earlier, and sustained these levels for day 14. For days 7 and 14, a fold change of 905 ± 222 and 284 ± 177 was found on random nanofibers, compared to a fold change of 1 ± 2 and 18 ± 20 on aligned nanofibers, and a fold change of −9 ± 5, and 5 ± 2 on fibronectin-coated substrates.
Expression of neuronal genes should advance from Pax6 to Tubb3 to Map2. Compared to mESCs, Pax6 was upregulated beginning at day 1 in all samples. Pax6 upregulation was consistently and statistically increased for both random and aligned nanofibers over fibronectin-coated substrates. Tubb3, however, was downregulated on days 1 and 3 on fibronectin-coated substrates. In contrast, cells on both aligned and random nanofibers samples showed statistical upregulation of Tubb3 beginning at day 1 (Figure 2E). At days 7 and 14, Tubb3 was slightly upregulated in cells on fibronectin-coated substrates, but was statistically higher on both aligned and random nanofibers for those time points. A fold change of 1226 ± 668 and 249 ± 147 was found on random nanofibers at days 7 and 14 respectively, compared to a fold change of 156 ± 75 and 24 ± 12 on aligned nanofibers, and −1 ± 2 and 0 ± 3 on fibronectin-coated substrates. Finally, for Map2, cells on all substrates had downregulated expression on days 1 and 3, and by day 7, the expression was upregulated (Figure 2F). The upregulation was higher on fibronectin-coated substrates and random nanofibers than on aligned nanofibers. A fold change of 29 ± 9 was found on fibronectin-coated substrate, compared to 105 ± 78 on random nanofibers, and 4 ± 1 on aligned nanofibers for day 7 of neural differentiation.
Results from the remaining genes from Table 1 can be found in Figure S8.
Protein Expression
The profile of protein expression was tracked over 14 days using pluripotent, early and late neural markers (Figure S9). We saw a significant decrease of pluripotent markers (SSEA1 and POUF51) by day 3 of neural differentiation on both random and aligned nanofibers (Figure S10A and S10B respectively). By day 3, POUF51 was expressed on 60 ± 20% of the cells on aligned nanofibers, compared to 22 ± 16% of the cells on random nanofibers. In addition, a decrease in dual labeling of SSEA1+ / POUF51+ / NES- labeling was also observed by day 3 for random and aligned nanofibers. As pluripotent marker expression decreased, neural precursor marker expression increased. An increase in NES+ expression was found from day 1 to day 3, although not statistically significant (Figure S10C). NES expression increased from 0 ± 1% to 16 ± 27% of the cells on aligned nanofibers, compared to 9 ± 8% to 11 ± 16% of the cells on random nanofibers. Compared to day 1, a decrease in SOX1 was observed by day 3 on random nanofibers, while SOX1 expression remained similar on aligned nanofibers (Figure S10D); SOX1 expression decreased from 30 ± 22% to 7 ± 15% of the cells on random nanofibers at days 1 and 3 respectively, compared to 20 ± 15% and 31 ± 5% for aligned nanofibers.
Similar to the gene expression data, we found that 81 ± 15% and 79 ± 15% of the cells were GFAP labelled on random and aligned nanofibers, respectively, at day 1 of neural differentiation (Figure 3A). Co-labeling with SOX1 was also evident at day 1 (Figure S11A) In addition, GFAP labeling was consistently high over the course of 14 days, with only a decrease in GFAP+ (TUBB3- / OLIG1-) expression on random fibers at day 7 compared to day 3. GFAP expression decreased from 84 ± 20% to 35 ± 18% of the cells on random nanofibers at day 3 and 7, respectively, while expression increased slightly from 53 ± 36% to 70 ± 28% of the cells on aligned nanofibers. Total OLIG1+ expression of 14 ± 19% to 19 ± 22% of the cells on random nanofibers at day 7 and 14 respectively, compared to 6 ± 10% to 15 ± 20% of the cells on aligned nanofibers (Figure 3C).
Figure 3:

Protein quantification of glial (A, C) and neuronal (B, D) proteins. Cells were considered positive for the respective protein if they possessed the appropriate protein morphology. Prior to morphology assessment, the images were thresholded according to the brightness and contrast settings of control images, which were samples stained with secondary antibodies and nuclei stain only. Protein positive cells were normalized to the total number of cells expressing at least one protein label, and expressed as a percentage. Data is represented as average of single and double labeled protein ± std dev of total labeled proteins. Double labeling of GFAP is noted with SOX1 (days 1 and 3) and OLIG1 (days 7 and 14); TUBB3 with SOX1; OLIG1 with GFAP; and MAP2 with GAP43. * represents statistical difference between respective topography timepoints and groups, with a p<0.05 considered significant.
Next, we compared neuronal protein expression between nanofiber topographies. We found significantly higher TUBB3+ (SOX1- / GFAP- and GFAP- / OLIG1-) expression at day 7 (Figure 3B) compared to days 1, 3, and 14 on random nanofibers. TUBB3+ expression increased from 6 ± 7% to 49 ± 21% on random nanofibers, compared to 13 ± 6% to 32 ± 36% on aligned nanofibers from days 3 to 7 of neural differentiation. We also investigated the expression of mature neuronal markers MAP2+ (Figure 3D) and GAP43+ (Figure S10I) at days 7 and 14. We observed similar MAP2+ (GFAP- / GAP43-) expression on both aligned and random nanofibers. Cells were also double labeled (GFAP- / MAP2+ / GAP43+), on both nanofiber topographies (Figure 3D and Figure S11B), with similar total labeling on 48 ± 12% and 29 ± 9% on aligned nanofibers, and 35 ± 15% and 37 ± 29% on random nanofibers, at days 7 and 14 respectively. In addition, cells were distinctly different in their glial and neuronal morphologies (Figure 4) and very few cells were multi-labeled with glial and neuronal proteins (GFAP+ / MAP2+ / GAP43- or GFAP+ / MAP2- / GAP43+ or GFAP+ / MAP2+ / GAP43+).
Figure 4:
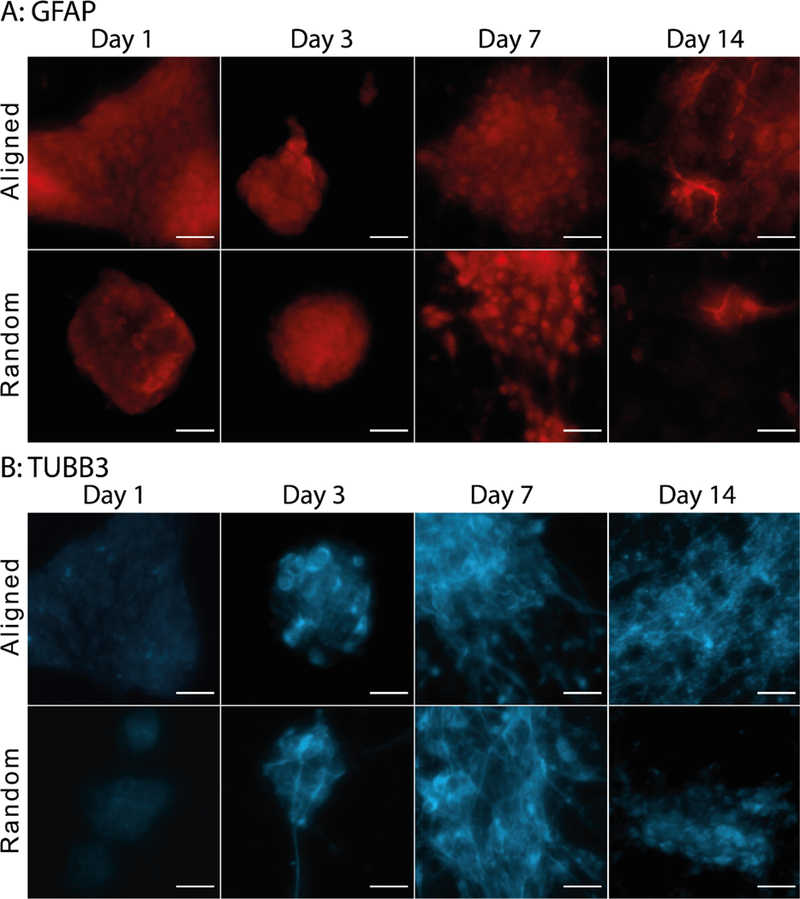
Glial (A) and neuronal (B) protein expression of mESCs for 14 days of neural differentiation. Images have been adjusted to control thresholds to highlight cells expressing positive markers, and have been enhanced for display. At early time points, cells expressed GFAP, however more distinct glial morphology was seen at later time points. Similarly, neuronal expression was also found at early time points, however more distinct neurites were found at later time points of neural differentiation. Scale bar of 20 μm.
Cell morphology, neurite extension and alignment
Both glia and neurons have distinct morphology that developed with time during culture. Neurite morphology showed by day 3 and glial morphology by day 14 (Figure 4). We compared neurite extension length by quantifying total neurite length per mm2 of traced TUBB3+ neurites on random and aligned topographies of RGD functionalized nanofibers. At day 7 of neural differentiation, total neurite length of 185.99 ± 213.35 mm per mm2 was found on aligned nanofibers, compared to 672.33 ± 497.38 mm per mm2 on random nanofibers (Figure 5). However, these differences were not significant. Neurites followed the nanofiber direction on aligned fibers, but extended in all directions on random topography (average orientation angle was 2.1 ± 16.4° and −11.9 ± 78.1° accordingly, Figure 5C, F). The full width of the Gaussian peak at the half of its maximum was much narrower on aligned (30.4°) than on random fibers (115.8°). The small peaks around 45° on both topographies correspond to the square or rectangular shape of analyzing images and are in accordance with previous analysis13.
Figure 5:

Neurite extension tracings. TUBB3 from day 7 on (A) aligned and (B) random nanofibers were used for (B, E) neurite tracings and (C, F) directionality measurements. (B) The aligned traces were rotated to orient the aligned fibers (from phase image) at 0°, and directionality of these neurite traces was measured. Gaussian fit (the red curve) was applied to measure neurite orientation. Scale bar of 50 μm.
Correlations
As we observed many cells growing in aggregates, and a high deviation in mature glial (GFAP+ and OLIG+) and neuronal (TUBB3+) protein expression, we investigated if there was any correlation between protein expression, aggregate size, and number of aggregates found on the substrates. At days 7 and 14 of neural differentiation, we found a significantly high inverse correlation between GFAP+ and TUBB3+ expression (−0.826 and −0.628, respectively) (Figures S12A and S12C respectively). Strong positive correlations were found between TUBB3+ expression and aggregate area (0.595) at day 7 and TUBB3+ expression and number of aggregates (0.682) at day 14. Furthermore, at day 14 of neural differentiation, we also found a strong correlation between GFAP+ and OLIG1+ (0.570) expression (Figure S12D).
Discussion
Control of stem cell differentiation into a specific neural lineage is important to push stem cell therapy to clinical practice in treatment neurological diseases, however it has been a great challenge. There are some reports investigating various factors that can influence stem cells neural differentiation. For example, nanofiber topography has been demonstrated to play an important role in ESC neural differentiation42. In particular, neural stem cell differentiation was faster on nanofibers than on microfibers43. The rate of neural differentiation on aligned fibers was increased in some reports29, 42, and was similar to random fibers in other reports43. It was also demonstrated that aligned orientation of fibers could guide the neurite outgrowth42–44, and have the potential to restore cellular architecture that is lost after nerve injury42. To investigate the effect of nanofiber orientation on neural differentiation of mESC, we fabricated random and aligned nanofibers. By using different electrospinning setups (Figure 1), we were able to fabricate nanofibers different in orientation, but similar in diameters.
While unfunctionalized PCL fibers have been found to support limited differentiation,42–43 coating of fibers with whole ECM proteins enhanced the response8, 45–46. However, the use of animal-or human-derived proteins limits their clinical application due to potential immune response. Short peptides can be used as a mimic of whole proteins in order to advance application of stem cell therapy to clinical practice. Chemical tethering of bioactive molecules is preferred over physical adsorption since it can prevent their loss over time, but typically requires multi-step processing and harsh conditions28. Reaction conditions including solvent system is important when choosing method of surface functionalization so nanofiber topography could be preserved. SPAAC used for surface modification of nanofibers, allowed scalable and efficient method that can be performed under simple metal-free conditions: ambient temperature, short times, and water/methanol-based solvent system28. This surface modification was achieved by reaction of DIBO incorporated into the polymer chain and azide-functionalized peptide, involving synthetic molecules without any animal or human components. Introducing azide functionality to the peptide could be easily performed by using azide-containing acid and allows to attach different amino acid sequences to the nanofiber surface in a controlled manner, allowing to induce different cellular responses and making the method versatile. Another regulatory limitation is the lack of direct methods for surface-bonded peptide quantification. Using DIBO as a functional group incorporated in the polymer allows easy quantitative assessment of the amount of peptide that attached to the surface via SPAAC by UV-visible spectroscopy29–30. Thus, to have an insight into which factors can help to control ESC differentiation to a specific neural lineage, we investigated the influence of topography (aligned or random orientation) and tethered peptide (RGD) on ESCs neural differentiation. This result was achieved through material design using SPAAC and different electrospinning setups that allow to fabricate nanofibers with similar diameters and amount of tethered peptide, but different orientation. While other groups attempted to control differentiation to specific lineage by topography or bioactive species, there are only few reports investigating influence of their combination and achieved control of both chemically tethered peptide and topography.
RGD was selected as the binding peptide for this study to further characterize potential binding sites on laminin to control neural differentiation. However, it is clear that laminin is not the only protein of interest when studying RGD, as fibronectin is generally the target protein when studying RGD35 and RGD peptide was found to be a primary interaction site for mESCs on collagen scaffolds25. Here, we found that RGD at low concentrations, 1.4 ± 0.9 pmol/cm2 and 1.9 ± 1.5 pmol/cm2 on random and aligned nanofibers respectively, did not support cellular attachment or proliferation. This result matched our controls with RGE peptide, where too few cells were found for analysis. While these results are in contrast to a previous report6 using unfunctionalized fibers, the previous group did not change the media during differentiation, and therefore, did not lose cells if they were loosely adherent. With regular media changes, the lack of specific binding sites (RGE) or low concentrations of binding sites (RGD) on the fibers reduced cell numbers over time, limiting our ability to characterize differentiation. In addition, neural stem cells (NSCs) have been found to require between 5–11 pmol/cm2 of RGD to be comparable to laminin-coated substrates47. Our RGD concentration of at least 6.4 pmol/cm2 is similar to this previous result for NSCs, and we were able to characterize 14 days of the neural differentiation progress of mESCs on the substrates with sufficient RGD.
In neural differentiation, mESCs begin to express neural lineage markers and lose their pluripotency markers by both gene and protein expression. Our findings suggest that mESCs begin to differentiate within 1 day of exposure to retinoic acid, as pluripotency genes were downregulated and neural precursor genes were upregulated on all substrates. Downregulation of Pou5f1 and upregulation of Sox1 and Pax6 was observed on day 1 for all substrates, which is similar to previous work13 with D3 mESCs. The progression continued and many of the markers investigated followed with both gene and protein expression, along with the typical morphology for glia and neurons, suggesting both the differentiation and maturation of the cells over 14 days, similar to other in vitro assessments of neural differentiation of mESCs4, 48. The correlations between TUBB3+ and GFAP+ cells were appropriate within the neural differentiation process, and moderate positive correlations between TUBB3+ cells and aggregate size agreed with other culture systems49 demonstrating mESCs neuronal differentiation was altered with cell contacts. Overall, the cells progressed through neuronal differentiation as would be expected.
In contrast to neuronal differentiation, we found significant upregulation of the glial marker, Gfap, on both orientations of RGD-functionalized nanofibers by day 1, without concomitant upregulation on fibronectin-coated glass substrates. On both nanofiber substrates, GFAP expression was evident early and consistently throughout the 14 days of neural differentiation. These results potentially indicated that peptide-tethered nanofibers, regardless of alignment, enhanced GFAP expression. However, our previous study13 on GYIGSR-tethered nanofibers had similar gene expression at day 1 to protein-coated substrates, with Gfap expression initially downregulated. Although gene expression is not always indicative of protein expression, we found similarly high GFAP protein expression at early timepoints on both nanofiber topographies for RGD-tethered scaffolds. This result, too, was in contrast to GYIGSR nanofibers13, indicating that nanofiber topographies alone are not responsible for GFAP upregulation. So, rather than the topography being the influencer, we looked to the influence of RGD peptide. While a previous study of neural stem cell differentiation on RGD lipid-bilayer substrates showed similar levels of GFAP and TUBB3 as adsorbed laminin after a 5 day differentiation50, RGD has been found to be a mechanosensitive interaction for a variety of stem cells and differentiation51, and topography, such as nanofibers, can further induce mechanotransduction38. For example, using RGD as an interaction point, neural stem cells have been shown to preferentially differentiate to glia at increased mechanical stiffnesses37, 52. In addition, the downregulation of P1 integrin, one binding site for RGD, has been previously shown to increase gliogenesis53 and retinoic acid exposure has been found to reduce β1 expression in some cells54. Taken together, these results indicated that the combination of retinoic acid with the specificity of the RGD-tethered nanofibers, regardless of alignment, are likely responsible for the upregulation of GFAP. Further work would be necessary to demonstrate if this early upregulation of GFAP is due to interactions between the integrins on mESCs and RGD or soluble factors in differentiation.
As GFAP expression was seen at day 1, we also wondered if this early expression was indicative of neural progenitor cells rather than glial differentiation. Previous studies have found early GFAP expression on both neuronal and glial cell lineages, demonstrating that GFAP can act as a neural precursor marker55–57. Here, at day 3, up to 14% of the cells on random and aligned nanofibers were dual labeled with SOX1 and GFAP (Figure 3A). Therefore, the protein expression evaluation indicated that GFAP at early stages was labeling neural progenitor cells. Previous studies showed GFAP labeling by day 3 on laminin-coated substrates and aligned YIGSR-nanofibers13, but did not show distinct glial morphology until day 14, and then only on laminin-coated substrates. At later time points during differentiation, GFAP is considered mature astrocyte marker55–57, and can be matched via morphology, as we saw in Figure 4A.
In addition to observed astrocyte morphology at later stages of differentiation, we observed oligodendrocyte expression on both fiber orientations via gene and protein expression at days 7 and 14. This OLIG1 expression was earlier than in previous studies58, including on YIGSR nanofibers, were we did not observe OLIG1 on aligned nanofibers at day 1413. An increased expression of oligodendrocytes may be related to fiber diameter, as NSCs on 283 nm fibers had increased oligodendrocytes after 5 days compared to tissue culture plastic or 749 nm fibers59, although our previously aligned YIGSR fibers were also in this smaller size range13. Additionally, fiber alignment was found to influence oligodendrocyte survival, with improved survival on random nanofibers over aligned nanofibers12, which agreed with our gene expression results (Figure 3C). In contrast, we found oligodendrocytes via protein expression largely within aggregates as has been previously reported4, 6, where the percentage of oligodendrocytes was similar on random and aligned substrates. The RGD-tethered nanofibers supported oligodendrocytes regardless of fiber orientation, but more work is needed to determine how influential the RGD peptide and integrin binding or nanofiber orientation is in the process.
The influence of the nanofiber topography was not obvious in neurogenesis or gliogenesis. By day 14, approximately 60% of the cells expressed GFAP+ while 40% expressed MAP2+ on random nanofibers, and 70% and 25% of the cells expressed GFAP+ and MAP2+, respectively, on aligned nanofibers. The progression of the differentiation over time was similar, and agreed with previous studies that compared neural differentiation process between random and aligned PCL6 and PLGA4 nanofibers. Both investigations on nanofibers found no significant differences between nanofiber orientations for pluripotent (POUF51), neural precursor (NES), neuronal (TUBB3), and glial (GFAP) markers. Consistently, differences noted in the literature between nanofiber orientation are with respect to contact guidance of neurite extension, where aligned nanofiber substrates have produced aligned neurites4, 6, 9–10. Total neurite extension, which was similar on both aligned and random fiber orientations, was comparable to the literature6, 13, 43. Ultimately, nanofibers may limit the total extension due to contact guidance limitations, however, topography can also act as a stimulus to direct and orient neurites as needed. Guidance of extending neurites or directing cell migration have been primary reasons to use aligned nanofibers, and our results further support this potential outcome.
Conclusion
In this study, we synthesized and characterized random and aligned PCL nanofibers with covalently attached RGD. Using DIBO as an initiator of ring-opening polymerization yielded an end-functionalized PCL that could be modified with RGD peptide post-electrospinning surface via strain-promoted azide-alkyne cycloaddition. This method of surface modification in combination with electrospinning resulted in fabrication of nanofibers with same diameter and amount of RGD functionalization, but with different orientation – random and aligned. Nanofibers with covalently tethered RGD increased GFAP expression at both gene and protein levels, likely due to a combination of the effect of retinoic acid and RGD peptide. At early time points, GFAP expression on RGD nanofibers likely serves as a neural progenitor marker rather than as a mature astrocyte marker. Oligodendrocytes were also formed by day 7 on RGD nanofibers, which is earlier than in previous reports. Neurite length on both fiber substrates was similar, but alignment of the neurites was significantly higher on aligned fibers due to contact guidance. The resulting differentiation of neurons progressed through mature neuronal markers, and was not influenced by the orientation of the nanofibers. Overall, the results indicated that the peptide-tethered nanofibers and soluble factors play an integrated role in the neural differentiation process, and that topography was less important in the outcomes.
Supplementary Material
Acknowledgments
This work is funded by the National Institutes of Health (R15-GM113155) and the National Science Foundation (CBET BME 1603832). R.K.W. acknowledges the generous support of the Margaret F. Donovan Endowed Chair for Women in Engineering. M.L.B is grateful for support from the W. Gerald Austen Endowed Chair in Polymer Science and Polymer Engineering from the John S. and James L. Knight Foundation.
Supporting Information
The Supporting Information is available on the ACS publication website and describes experimental details and characterization data including 1H MNR of the polymer, analysis of the fiber diameter and orientation and ESI spectra of the peptides. In addition, gene expression, protein expression, protein expression analysis, and graphs for correlations for all samples are included in SI.
References
- 1.Song B-W , In Vivo Assessment of Stem Cells for Treating Neurodegenerative Disease: Current Approaches and Future Prospects. Stem Cells Int. 2017, 2017, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lunn JS; Sakowski SA; Hur J; Feldman EL, Stem cell technology for neurodegenerative diseases. Annals of neurology 2011, 70 (3), 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen R; Hunt JA, Biomimetic materials processing for tissue-engineering processes. J. Mater. Chem. 2007, 17 (38). [Google Scholar]
- 4.Sperling LE; Reis KP; Pozzobon LG; Girardi CS; Pranke P, Influence of random and oriented electrospun fibrous poly(lactic-co-glycolic acid) scaffolds on neural differentiation of mouse embryonic stem cells. J. Biomed. Mater. Res. A 2017, 105 (5), 1333–1345. [DOI] [PubMed] [Google Scholar]
- 5.Sharifi F; Patel BB; Dzuilko AK; Montazami R; Sakaguchi DS; Hashemi N, Polycaprolactone Microfibrous Scaffolds to Navigate Neural Stem Cells. Biomacromolecules 2016, 17 (10), 3287–3297. [DOI] [PubMed] [Google Scholar]
- 6.Xie J; Willerth SM; Li X; Macewan MR; Rader A; Sakiyama-Elbert SE; Xia Y, The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials 2009, 30 (3), 354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu CYIR.; Kotaki M.; Ramakrishna S, Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials 2004, 25 (5), 877–886. [DOI] [PubMed] [Google Scholar]
- 8.Koh H; Yong T; Chan C; Ramakrishna S, Enhancement of neurite outgrowth using nanostructured scaffolds coupled with laminin. Biomaterials 2008, 29 (26), 3574–3582. [DOI] [PubMed] [Google Scholar]
- 9.Callahan LA; Xie S; Barker IA; Zheng J; Reneker DH; Dove AP; Becker ML, Directed differentiation and neurite extension of mouse embryonic stem cell on aligned poly(lactide) nanofibers functionalized with YIGSR peptide. Biomaterials 2013, 34 (36), 9089–95. [DOI] [PubMed] [Google Scholar]
- 10.Ghasemi-Mobarakeh L; Prabhakaran MP; Morshed M; Nasr-Esfahani MH; Ramakrishna S, Electrospun poly(epsilon-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29 (34), 4532–9. [DOI] [PubMed] [Google Scholar]
- 11.Koh HS; Yong T; Chan CK; Ramakrishna S, Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials 2008, 29 (26), 3574–82. [DOI] [PubMed] [Google Scholar]
- 12.Lim SH; Liu XY; Song H; Yarema KJ; Mao HQ, The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials 2010, 31 (34), 9031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silantyeva EA; Nasir W; Carpenter J; Manahan O; Becker ML; Willits RK, Accelerated neural differentiation of mouse embryonic stem cells on aligned GYIGSR-functionalized nanofibers. Acta Biomater. 2018, 75, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S; Hu F; Li J; Zhang S; Shen M; Huang M; Shi X, Design of electrospun nanofibrous mats for osteogenic differentiation of mesenchymal stem cells. Nanomedicine 2017. [DOI] [PubMed] [Google Scholar]
- 15.Wang X; Ding B; Li B, Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16 (6), 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levenberg S; Huang NF; Lavik E; Rogers AB; Itskovitz-Eldor J; Langer R, Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc. Natl. Acad. Sci. U.S.A. 2003, 100 (22), 12741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrams GA; Goodman SL; Nealey PF; Franco M; Murphy CJ, Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell and Tissue Res. 2000, 299 (1), 39–46. [DOI] [PubMed] [Google Scholar]
- 18.Zhao C; Tan A; Pastorin G; Ho HK, Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol. Adv. 2013, 31 (5), 654–68. [DOI] [PubMed] [Google Scholar]
- 19.Chong EJ; Phan TT; Lim IJ; Zhang YZ; Bay BH; Ramakrishna S; Lim CT, Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3 (3), 321–30. [DOI] [PubMed] [Google Scholar]
- 20.Li WJ; Cooper JA Jr.; Mauck RL; Tuan RS, Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006, 2 (4), 377–85. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimoto H; Shin YM; Terai H; Vacanti JP, A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24 (12), 2077–2082. [DOI] [PubMed] [Google Scholar]
- 22.Ma Z; He W; Yong T; Ramakrishna S, Grafting of Gelatin on Electrospun Poly(caprolactone) Nanofibers to Improve Endothelial Cell Spreading and Proliferation and to Control Cell Orientation. Tissue Eng. 2005, 11 (7–8), 1149–58. [DOI] [PubMed] [Google Scholar]
- 23.Ausubel LJ; Lopez PM; Couture LA, GMP scale-up and banking of pluripotent stem cells for cellular therapy applications. Methods Mol. Biol. 2011, 767, 147–59. [DOI] [PubMed] [Google Scholar]
- 24.Schnell E; Klinkhammer K; Balzer S; Brook G; Klee D; Dalton P; Mey J, Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials 2007, 28 (19), 3012–25. [DOI] [PubMed] [Google Scholar]
- 25.Hazenbiller O; Duncan NA; Krawetz RJ, Reduction of pluripotent gene expression in murine embryonic stem cells exposed to mechanical loading or Cyclo RGD peptide. BMC Cell Biol. 2017, 18 (1), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma W; Tavakoli T; Derby E; Serebryakova Y; Rao MS; Mattson MP, Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev. Biol. 2008, 8, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massumi M; Abasi M; Babaloo H; Terraf P; Safi M; Saeed M; Barzin J; Zandi M; Soleimani M, The effect of topography on differentiation fates of matrigel-coated mouse embryonic stem cells cultured on PLGA nanofibrous scaffolds. Tissue Eng. Part A 2012, 18 (5–6), 609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng J; Xie S; Lin F; Hua G; Yu T; Reneker DH; Becker ML, 4-Dibenzocyclooctynol (DIBO) as an initiator for poly(?-caprolactone): copper-free clickable polymer and nanofiber-based scaffolds. Polymer Chemistry 2013, 4 (7), 2215–2218. [Google Scholar]
- 29.Smith Callahan L. A.; Xie S; Barker IA; Zheng J; Reneker DH; Dove AP; Becker ML, Directed differentiation and neurite extension of mouse embryonic stem cell on aligned poly(lactide) nanofibers functionalized with YIGSR peptide. Biomaterials 2013, 34 (36), 9089–9095. [DOI] [PubMed] [Google Scholar]
- 30.Zheng J; Kontoveros D; Lin F; Hua G; Reneker DH; Becker ML; Willits RK, Enhanced Schwann Cell Attachment and Alignment Using One-Pot “Dual Click” GRGDS and YIGSR Derivatized Nanofibers. Biomacromolecules 2015, 16 (1), 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng J; Liu K; Reneker DH; Becker ML, Post-Assembly Derivatization of Electrospun Nanofibers via Strain-Promoted Azide Alkyne Cycloaddition. J. Am. Chem. Soc. 2012, 134 (41), 17274–17277. [DOI] [PubMed] [Google Scholar]
- 32.Yoo HS; Kim TG; Park TG, Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Advanced drug delivery reviews 2009, 61 (12), 1033–1042. [DOI] [PubMed] [Google Scholar]
- 33.Zheng J; Hua G; Yu J; Lin F; Wade MB; Reneker DH; Becker ML, Post-Electrospinning “Triclick” Functionalization of Degradable Polymer Nanofibers. ACS Macro Letters 2015, 4 (2), 207–213. [DOI] [PubMed] [Google Scholar]
- 34.Ning X; Guo J; Wolfert MA; Boons G-J, Visualizing Metabolically Labeled Glycoconjugates of Living Cells by Copper-Free and Fast Huisgen Cycloadditions. Angew. Chem. Int. Ed. 2008, 47 (12), 2253–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Main AL; Harvey TS; Baron M; Boyd J; Campbell ID, The three-dimensional structure of the tenth type III module of fibronectin: An insight into RGD-mediated interactions. Cell 1992, 71 (4), 671–678. [DOI] [PubMed] [Google Scholar]
- 36.Tashiro KI; Sephel GC; Greatorex D; Sasaki M; Shirashi N; Martin GR; Kleinman HK; Yamada Y, The RGD containing site of the mouse laminin A chain is active for cell attachment, spreading, migration and neurite outgrowth. J. Cell. Physiol. 1991, 146 (3), 451–459. [DOI] [PubMed] [Google Scholar]
- 37.Saha K; Keung AJ; Irwin EF; Li Y; Little L; Schaffer DV; Healy KE, Substrate modulus directs neural stem cell behavior. Biophys. J. 2008, 95 (9), 4426–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stukel JM; Willits RK, Mechanotransduction of Neural Cells Through Cell-Substrate Interactions. Tissue engineering. Part B, Reviews 2016, 22 (3), 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson JA; Hopkins SA; Wright PM; Dove AP, ‘Immortal’ ring-opening polymerization of [small omega]-pentadecalactone by Mg(BHT)2(THF)2. Polymer Chemistry 2014, 5 (8), 2691–2694. [Google Scholar]
- 40.Grandjean C; Boutonnier A; Guerreiro C; Fournier J-M; Mulard LA, On the Preparation of Carbohydrate-Protein Conjugates Using the Traceless Staudinger Ligation. J. Org. Chem. 2005. [DOI] [PubMed] [Google Scholar]
- 41.Abràmoff MDMPJ; Ram SJ , Image Processing with ImageJ. Biophotonics International 2004, 11 (7), 36–42. [Google Scholar]
- 42.Xie J; Willerth SM; Li X; Macewan MR; Rader A; Sakiyama-Elbert SE; Xia Y, The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials 2009, 30 (3), 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang F; Murugan R; Wang S; Ramakrishna S, Electrospinning of nano/micro scale poly(l- lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26 (15), 2603–2610. [DOI] [PubMed] [Google Scholar]
- 44.Silantyeva EA; Nasir W; Carpenter J; Manahan O; Becker ML; Willits RK, Accelerated Neural Differentiation of Mouse Embryonic Stem Cells on Aligned GYIGSR-functionalized Nanofibers. Acta biomaterialia 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel S; Kurpinski K; Quigley R; Gao H; Hsiao BS; Poo M-M; Li S, Bioactive Nanofibers: Synergistic Effects of Nanotopography and Chemical Signaling on Cell Guidance. Nano Lett. 2007, 7 (7), 2122–2128. [DOI] [PubMed] [Google Scholar]
- 46.Ghasemi-Mobarakeh L; Prabhakaran MP; Morshed M; Nasr-Esfahani M-H; Ramakrishna S, Electrospun poly (ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29 (34), 4532–4539. [DOI] [PubMed] [Google Scholar]
- 47.Saha K; Irwin EF; Kozhukh J; Schaffer DV; Healy KE, Biomimetic interfacial interpenetrating polymer networks control neural stem cell behavior. 2007, 81A (1), 240–249. [DOI] [PubMed] [Google Scholar]
- 48.Abranches E; Silva M; Pradier L; Schulz H; Hummel O; Henrique D; Bekman E, Neural differentiation of embryonic stem cells in vitro: a road map to neurogenesis in the embryo. PLoS One 2009, 4 (7), e6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joshi R; Thakuri PS; Buchanan JC; Li J; Tavana H, Microprinted Stem Cell Niches Reveal Compounding Effect of Colony Size on Stromal Cells-Mediated Neural Differentiation. Adv. Healthc. Mater. 2018, 7 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ananthanarayanan B; Little L; Schaffer DV; Healy KE; Tirrell M, Neural stem cell adhesion and proliferation on phospholipid bilayers functionalized with RGD peptides. Biomaterials 2010, 31 (33), 8706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higuchi A; Ling Q-D; Chang Y; Hsu S-T; Umezawa A, Physical Cues of Biomaterials Guide Stem Cell Differentiation Fate. Chem. Rev. 2013, 113 (5), 3297–3328. [DOI] [PubMed] [Google Scholar]
- 52.Seidlits SK; Khaing ZZ; Petersen RR; Nickels JD; Vanscoy JE; Shear JB; Schmidt CE, The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 2010, 31 (14), 3930–40. [DOI] [PubMed] [Google Scholar]
- 53.Pan L; North HA; Sahni V; Jeong SJ; McGuire TL; Berns EJ; Stupp SI; Kessler JA, β1-Integrin and Integrin Linked Kinase Regulate Astrocytic Differentiation of Neural Stem Cells. PLOS ONE 2014, 9 (8), e104335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chattopadhyay N; Ray S; Biswas N; Chatterjee A, Effect of All-trans-Retinoic Acid on Integrin Receptors of Human Cervical Cancer (SiHa) Cells. Gynecologic Oncology 1999, 75 (2), 215–221. [DOI] [PubMed] [Google Scholar]
- 55.Malatesta P; Gotz M, Radial glia - from boring cables to stem cell stars. Development 2013, 140 (3), 483–6. [DOI] [PubMed] [Google Scholar]
- 56.Kriegstein AR; Gotz M, Radial glia diversity: a matter of cell fate. Glia 2003, 43 (1), 37–43. [DOI] [PubMed] [Google Scholar]
- 57.Gotz M; Barde YA, Radial glial cells defined and major intermediates between embryonic stem cells and CNS neurons. Neuron 2005, 46 (3), 369–72. [DOI] [PubMed] [Google Scholar]
- 58.Visan A; Hayess K; Sittner D; Pohl EE; Riebeling C; Slawik B; Gulich K; Oelgeschläger M; Luch A; Seiler AEM, Neural differentiation of mouse embryonic stem cells as a tool to assess developmental neurotoxicity in vitro. NeuroToxicology 2012, 33 (5), 1135–1146. [DOI] [PubMed] [Google Scholar]
- 59.Christopherson GT; Song H; Mao H-Q, The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 2009, 30 (4), 556–564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


