Abstract
Endometriosis is a disease in which tissue that normally grows inside the uterus grows outside the uterus and causes chronic pelvic pain and infertility. However, the exact mechanisms of the pathogenesis of endometriosis-associated infertility are unknown. Epigenetic dysregulation has recently been implicated in infertility. Here, we report a reduction of histone deacetylase 3 (HDAC3) protein amounts in eutopic endometrium of infertile women with endometriosis compared to a control group. To investigate the effect of HDAC3 loss in the uterus, we generated mice with conditional ablation of Hdac3 in progesterone receptor (PGR)-positive cells (Pgrcre/+Hdac3f/f; Hdac3d/d). Loss of Hdac3 in the uterus of mice results in infertility due to implantation failure and decidualization defect. Expression microarray and ChlP-seq analyses identified COL1A1 and COL1A2 as direct targets of HDAC3 in both mice and humans. Reduction of HDAC3 abrogated decidualization in a primary culture of human endometrial stromal cells (hESCs) similar to that observed in infertile patients with endometriosis. Whereas attenuation of HDAC3 resulted in p300 recruitment to Col1a1 and Col1a2 genes in the uterus of mice as well as hESCs, inhibition of p300 permitted hESCs to undergo decidualization. Collectively, we found attenuation of HDAC3 and overexpression of collagen type I in the eutopic endometrium of infertile patients with endometriosis. HDAC3 loss caused a defect of decidualization through the aberrant transcriptional activation of Col1a1 and Col1a2 genes in mice and COL1A1 and COL1A2 genes in humans. Our results suggest that HDAC3 is critical for endometrial receptivity and decidualization.
INTRODUCTION
Endometriosis is a benign gynecological disease in which endometrial cells are found outside the uterus. Endometriosis, which afflicts more than 10% of women of reproductive age, is a major cause of infertility, but its etiology is unclear. The current standard of care for endometriosis is surgical removal of lesions and hormonal suppression, although these therapies have various side effects and a high incidence of relapse. Therefore, identification of mechanisms involved in the pathogenesis of endometriosis and infertility is critical. Recently, many studies have focused on epigenetic aberrations and their contribution to endometrial function (1, 2).
Histone deacetylases (HDACs) play key roles in the epigenetic regulation of genes by modulating the acetylation of histone and nonhistone substrates (3). Class I HDACs (HDAC1, HDAC2, HDAC3, and HDAC8) are central in cell survival, proliferation, differentiation, and cancer development (4). The condensation of eukaryotic DNA into chromatin by HDACs plays a role in transcriptional repression by interfering with the accessibility of the DNA to the transcription factors (5). Various transcriptional coactivators, including lysine acetyltransferase 2A (KAT2A), p300, p300/CBP associated factor (PCAF), and steroid receptor coactivator 1 (SRC1), have intrinsic acetyltransferase activity that is critical for their function (6). p300 is crucial in the regulation of genes implicated in controlling cell proliferation, apoptosis, differentiation, cell cycle, and DNA repair (7). p300 is expressed in endometrium and involved in the regulation of the estrogen receptor function (8, 9). However, the detailed role of p300 in the formation of endometriosis is unknown.
Epigenetic regulation plays an important role in normal endometrial steroid hormone responses, which can affect endometrial function (10, 11). However, epigenetic modification of several genes has been reported in eutopic endometrium in women with endometriosis as well as in animal models (12–14). The expression of HDAC1 and HDAC2 proteins in endometriotic stroma cells and the regulation of their expression by steroid hormones have been reported (15, 16). HDAC3, similar to HDAC1 and HDAC2, is ubiquitously expressed. However, HDAC3 contains an unusual C terminus and, unlike the predominantly nuclear location of HDAC1 and HDAC2, localizes to the nucleus, cytoplasm, and plasma membrane, indicating that it is functionally distinct from other members of its class (17). By forming multiprotein complexes with the corepressors nuclear receptor corepressor (N-CoR) and silencing mediator for retinoid and thyroid hormone receptors (SMRT), HDAC3 acts as a corepressor for a diverse array of transcription factors, including nuclear receptors (3, 18). N-CoR and SMRT function as corepressors for diverse transcription factors, including estrogen receptors and progesterone receptor (PGR) (19, 20). HDAC3 not only forms a complex with N-CoR/SMRT but also requires interaction with the deacetylase-activating domain of N-CoR/SMRT for its enzyme activity (21). However, the function of HDAC3 in endometriosis and uterine biology remains unknown.
The present study revealed that the amounts of HDAC3 were decreased in endometrium from infertile women with endometriosis. Animal models allowed us to study the temporal sequence of events involved in disease establishment and progression. A baboon model has previously been developed to study the pathophysiology of endometriosis (22). The expression of HDAC3 was examined by sequential analysis of the eutopic endometrium in the same animals over time and with the progression of endometriosis in baboon model. We used human primary stromal cell and uterine-specific Hdac3 knockout mice to demonstrate that HDAC3 is necessary for implantation and decidualization. Our findings help to understand the etiology of female infertility and provide a molecular framework that should be useful for the design of new therapeutic strategies.
RESULTS
HDAC3 proteins are reduced in infertile women with endometriosis
To determine whether HDAC3 is related to endometriosis-related infertility, we first examined the expression of HDAC3 proteins in eutopic endometrium from infertile women with endometriosis using immunohistochemistry. HDAC3 protein was abundant throughout the menstrual cycle in human endometrial epithelial and stromal cells (Fig. 1A). However, HDAC3 expression was significantly lower (P < 0.001) in the endometrial epithelial and stromal cells of infertile women with endometriosis compared to controls in proliferative, early secretory, and mid-secretory phases (Fig. 1A). To assess the expression of HDAC3 during progression of endometriosis, we used a nonhuman primate model. We observed decreased expression of HDAC3 in the stromal cells of the eutopic endometrium from the same baboons over time and with progression of endometriosis (Fig. 1B). To determine whether HDAC3 expression is dysregulated after endometriosis development, endometriosis was surgically induced in 2-month-old female mice by using autologous uterine tissue transfer. First, we examined the expression of HDAC3 in mice during all phases of the estrous cycle as well as during early pregnancy. The expression of HDAC3 was consistently strong in each phase as well as in early pregnancy (fig. S1). However, the expression of HDAC3 proteins was significantly reduced (P < 0.01) in the stromal cells of the eutopic endometrium from the mice with endometriosis compared to the sham group (Fig. 1C). This result suggests that HDAC3 loss is associated with endometriosis development.
Fig. 1. Attenuation of HDAC3 in the eutopic endometrium from women with endometriosis as well as baboon and mouse models.
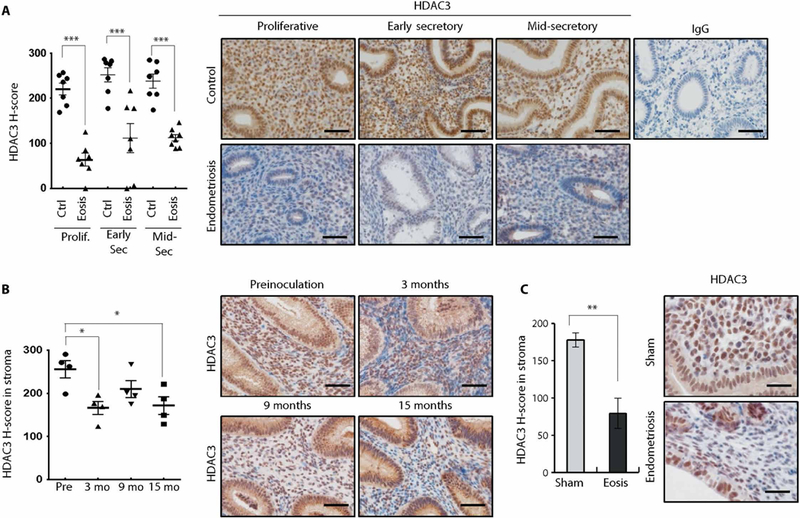
(A) Immunohistochemical H-score and representative photomicrographs of HDAC3 proteins in the proliferative, early secretory, and mid-secretory phase endometrium from women without and with endometriosis (n = 7 per phase for each group). (B) Immunohistochemical H-score in stroma and representative photomicrographs of HDAC3 in the endometriosis baboon model. Endometriosis was induced by intraperitoneal inoculation of menstrual endometrium. The expression of HDAC3 was examined in the baboon endometrium before inoculation and 3, 9, and 15 months after inoculation (n = 4). (C) Immunohistochemical H-score and representative photomicrographs of HDAC3 in the eutopic endometrium from the endometriosis mouse model. Endometriosis was surgically induced in mice, and then the expression of HDAC3 was examined by immunohistochemical analysis (n = 6). Nuclei were counterstained with hematoxylin (blue). Mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, Student’s t test for data with only two groups and ANOVA followed by Tukey or Bonferroni test for pairwise t test for data containing more than two groups. Scale bars, 50 μm. Ctrl, control; Eosis, endometriosis; Prolif, proliferative phase; Sec, secretory phase; IgG, immunoglobulin G.
Ablation of Hdac3 causes infertility due to defective embryo implantation and decidualization
To determine the function of HDAC3 in the uterus, we generated a mouse model with Hdac3 conditionally ablated in Pgr-positive cells (Pgrcre/+Hdac3f/f; Hdac3d/d). Ablation of HDAC3 in the uterus was confirmed by reverse transcription–quantitative polymerase chain reaction (RT-qPCR), Western blot, and immunohistochemical analyses (Fig. 2A). The expression of HDAC1 and HDAC2 was not changed in the uterus of Hdac3d/d mice, but HDAC3 was significantly decreased (P < 0.001) in the uterus of Hdac3d/d mice compared to control (Hdac3f/f) mice (Fig. 2A). In fertility tests of female Hdac3f/f and Hdac3d/d mice over 6 months, Hdac3f/f mice were fertile (average number of pups/litter; 7.44 ± 1.09), whereas Hdac3 d/d mice were sterile (table S1). Female Hdac3d/d mice showed normal ovulation, fertilization, and ovarian morphology (fig. S2 and table S2). These results suggest that the fertility defect is primarily due to a uterine defect.
Fig. 2. Defects of implantation and decidualization in Hdac3 d/d mice.
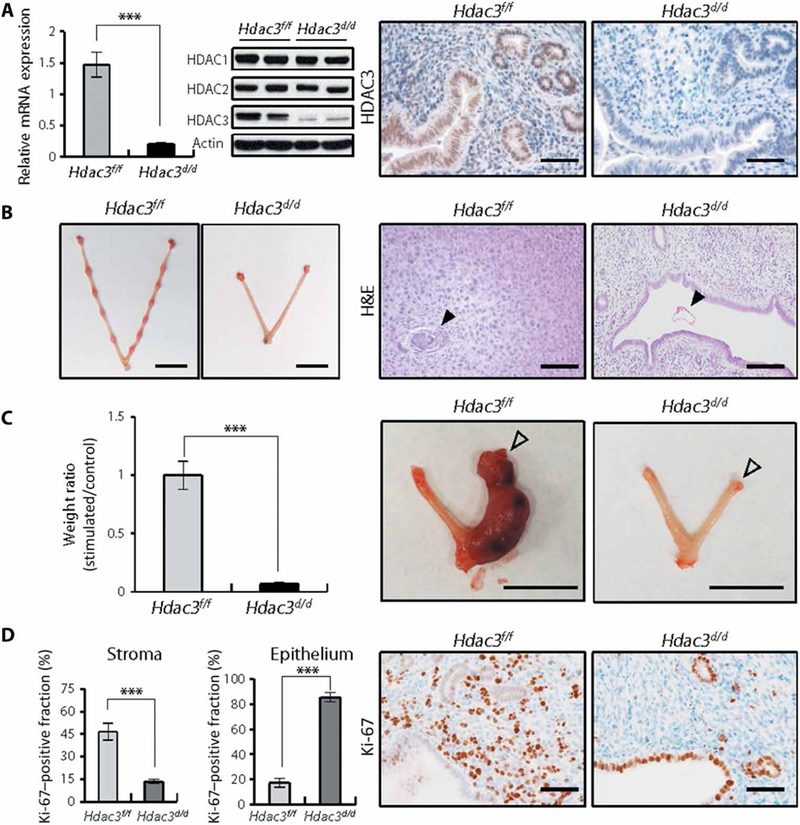
(A) Hdac3 ablation in the uteri of Hdac3d/d mice. RT-qPCR analysis of Hdac3 gene expression in the uterus of Hdac3f/f and Hdac3d/d mice at 2 months of age (n = 6 for each genotype). Western blot analysis of HDAC1, HDAC2, and HDAC3 proteins in the uteri of Hdac3f/f and Hdac3d/d mice at 2 months of age. Actin was used as sample loading control. Representative images of immunohistochemical staining for HDAC3 in the uteri of Hdac3f/f and Hdac3d/d mice at 2 months of age. Scale bars, 50 μm. (B) Implantation sites in uteri of Hdac3f/f and Hdac3d/d mice at GD 5.5. Scale bars, 1 cm. Representative images of hematoxylin and eosin (H&E) staining in uteri of Hdac3f/f and Hdac3d/d mice at GD 5.5. Arrowheads indicate embryos. Scale bars, 200 μm. (C) Ratio of uterine weight to body weight and representative photographs of uteri from Hdac3f/f (n = 6) and Hdac3d/d (n = 4) mice after artificially induced decidualization. Unfilled arrowheads indicate stimulated horns. Scale bars, 1 cm. (D) Percentages and representative photomicrographs of immunohistochemical staining for Ki-67 (brown), a proliferation marker, in endometrial epithelial and stromal cells from Hdac3f/f and Hdac3d/d mice at GD 3.5 (n = 6 for each genotype). Nuclei were counterstained with hematoxylin (blue). Scale bars, 50 μm. Mean ± SEM. ***P < 0.001, Student’s t test.
To determine the cause of infertility in Hdac3 d/d mice, we investigated whether ablation of Hdac3 alters implantation and decidualization. Implantation sites were not detected in the uterine horns of Hdac3 d/d mice (Fig. 2B). Histological analysis indicated that Hdac3f/f mice embryos were attached to the uterine horn and surrounded by decidualized cells, whereas in the uteri of Hdac3d/d mice, the embryos were free-floating within the uterine lumen and decidual cells were not evident (Fig. 2B).
We next examined the impact of ablation of Hdac3 on decidualization using an artificial decidualization model. Hdac3f/f mice displayed a decidual uterine horn that responded well to this artificial induction. However, Hdac3d/d mice exhibited a significant defect (P < 0.01) in the decidual response (Fig. 2C). This decidual defect was further confirmed by histological analysis and quantification of decidualization markers, bone morphogenetic protein 2 (Bmp2) and wingless-related mouse mammary tumor virus integration site 4 (Wnt4), in the Hdac3d/d mice (fig. S3).
In control Hdac3f/f mice, cell proliferation was reduced in epithelial cells before embryo attachment and increased in stromal cells undergoing decidualization in preparation for implantation at day 3.5 of gestation (GD 3.5). However, the proliferative responses in stromal and epithelial compartments of the uterus from Hdac3d/d mice were significantly altered (P < 0.001 and P < 0.001, respectively) at GD 3.5 compared to Hdac3f/f mice (Fig. 2D). These results suggest that Hdac3d/d mice were infertile due to defective embryo implantation and decidualization.
The expression of stromal estrogen receptor alpha (ESR1) and PGR was significantly decreased (P < 0.001 and P < 0.001, respectively) in Hdac3d/d mice compared to Hdac3f/f mice (Fig. 3A). The mRNA expression of progesterone (P4) target genes—Lrp2, Fst, Areg, and Il13ra2—was significantly lower (P < 0.05, P < 0.01, P < 0.05, and P < 0.01, respectively) in Hdac3d/d mice (Fig. 3B). Furthermore, the expression of stromal markers, vimentin and COUP-TFII, was significantly decreased (P < 0.001) in the stromal cells of Hdac3d/d mice (Fig. 3, C and D). These results suggest that ablation of Hdac3 in the uterus alters the characteristics of the uterine stromal cells.
Fig. 3. Decreased progesterone signaling in the uteri of Hdac3 d/d mice.
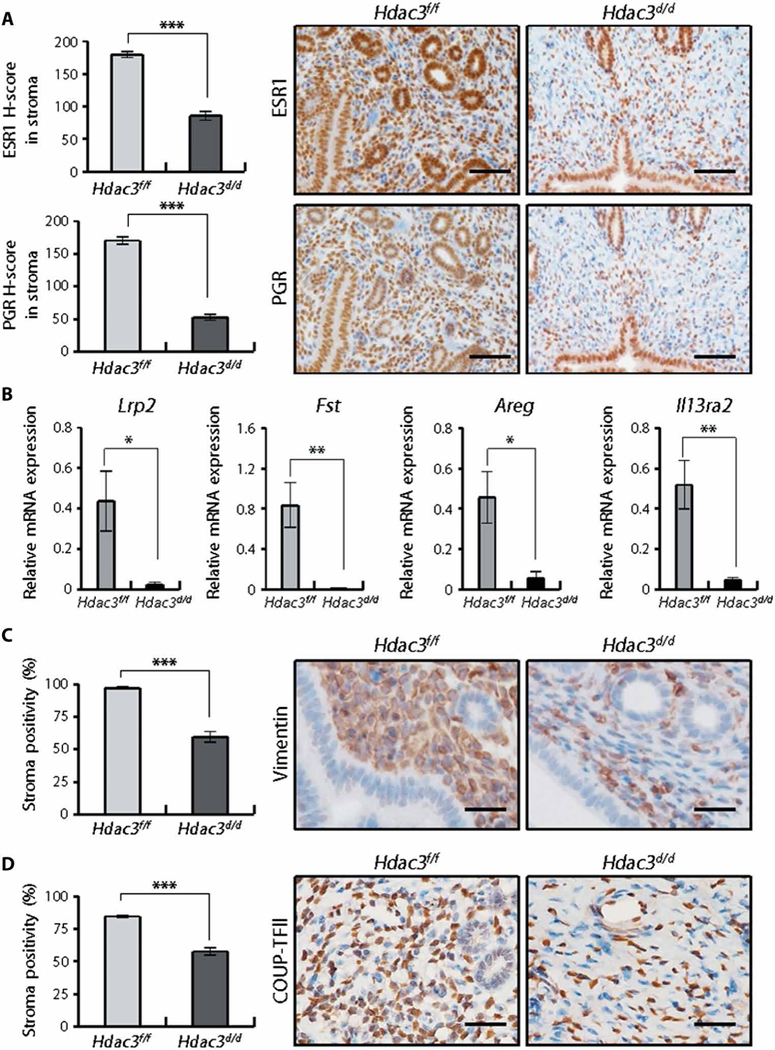
(A) Immunohistochemical H-score and representative photomicrographs of ESR1 and PGR in stromal cells in the endometrium from Hdac3f/f and Hdac3d/d mice at GD 3.5 (n = 6 for each genotype). Scale bars, 100 μm. (B) RT-qPCR analysis of expression of progesterone target genes Lrp2, Fst, Areg, and II13ra2 in the uteri of Hdac3f/f and Hdac3d/d mice at GD 3.5 (n = 6 for each genotype). (C) Percentages and representative photomicrographs of vimentin in stromal cells in the endometrium from Hdac3f/f and Hdac3d/d mice at GD 3.5 (n = 6 for each genotype). Scale bars, 25 μm. (D) Percentages and representative photo-micrographs of COUP-TFII in stromal cells in the endometrium from Hdac3f/f and Hdac3d/d mice at GD 3.5 (n = 6 for each genotype). Nuclei were counterstained with hematoxylin (blue). Scale bars, 25 μm. Mean ± SEM. * P < 0.05, **P < 0.01, and ***P < 0.001, Student’s t test.
Hdac3 ablation results in overexpression of collagen proteins in the uterus
To determine whether ablation of Hdac3 affected uterine development, we examined the uterine histology of Hdac3f/f and Hdac3d/d mice at 4, 6, and 8 weeks of age. Histological analysis and Masson’s trichrome staining revealed abundant collagen proteins in stromal cells of Hdac3d/d mice starting at 6 weeks of age (fig. S4). To identify direct target genes of Hdac3, we performed chromatin immuno-precipitation sequencing (ChIP-seq) and DNA microarray analysis of the uteri of Hdac3f/f and Hdac3d/d mice at 6 weeks of age (Gene Expression Omnibus accession code GSE83102). Motif analysis of HDAC3 interval sequences using the Cistrome motif database identified several transcription factor motifs, including E26 transformation-specific (ETS) domain, hormone nuclear receptor, BetaBetaAlpha-zinc finger (zf-C2H2), leucine zipper (bZIP), and helix-loop-helix (bHLH) family (fig. S5). Pathway analysis of the microarray results identified increases in the atherosclerosis and fibrosis pathways, which are related to collagen dysregulation (table S3). The microarray results were validated by RT-qPCR analysis (fig. S6A). By comparing the ChIP-seq and microarray results, we identified 113 genes that not only contain HDAC3-binding sites, but whose expression was altered in the uterus of Hdac3 d/d mice as compared to Hdac3f/f mice (fig. S6B). Collagen type I, alpha 1 and alpha 2 (Col1a1 and Col1a2) were up-regulated in Hdac3d/d mice and include conserved HDAC3-binding elements (HDAC3-BEs) in both the mouse and human (fig. S7A). The recruitment of HDAC3 onto Col1a1 and Col1a2 genes in the uterus of Hdac3f/f mice was confirmed by ChIP analysis (fig. S7B). To determine whether the recruitment of HDAC3 is dependent on the HDAC3-BE on COL1A1 and COL1A2 genes, substitutions and deletion mutations of the HDAC3-BE were made on pGL4.21-Basic plasmid including COL1A1 or COL1A2 gene (fig. S7, C and D), which was then transfected into human endometrial stromal cells (hESCs). The results of our ChIP assay showed that HDAC3-BE on COL1A1 and COL1A2 genes is critical for the recruitment of HDAC3 (fig. S7, E and F). Furthermore, the Hdac3d/d mice exhibited a significant increase in the intensity of Masson’s trichrome staining (P < 0.001) as well as COL1 proteins (P < 0.01) in stromal cells compared to Hdac3f/f mice (figs. S8 and S9). These results suggest that HDAC3 directly suppressed transcription of COL1A1 and COL1A2 genes in the endometrial stroma.
To determine whether collagen is dysregulated in our endometriosis mouse model, we performed Masson’s trichrome staining of the eutopic endometrium of mice with endometriosis. Masson’s trichrome staining detected collagen proteins in the mice with endometriosis but not the sham-operated mice (Fig. 4A). To determine whether type I collagen is dysregulated in human endometriosis, we performed Masson’s trichrome staining and COL1 immuno-histochemistry on endometrium from the proliferative, early secretory, and mid-secretory phases from women with and without endometriosis. The intensity of Masson’s trichrome staining was significantly increased in eutopic endometrium from women with endometriosis compared to controls in proliferative (P < 0.001), early secretory (P < 0.001), and mid-secretory phase (P < 0.001) (Fig. 4B). The amounts of COL1 proteins were consistent in endometrium from control women throughout the menstrual cycle (Fig. 4C). However, COL1 protein was significantly increased in eutopic endometrium from women with endometriosis compared to controls in proliferative (P < 0.001), early secretory (P < 0.05), and mid-secretory phases (P < 0.05) (Fig. 4C). We did not observe any difference in the intensity of Masson’s trichrome staining and COL1 protein content in the endometrium from women with or without endometriosis during the menstrual cycle (Fig. 4). These results suggest that Hdac3 plays a critical role in the pathogenesis of endometriosis through regulation of collagen homeostasis in the uterus.
Fig. 4. Overexpression of collagen in the endometrium of mice and women with endometriosis.
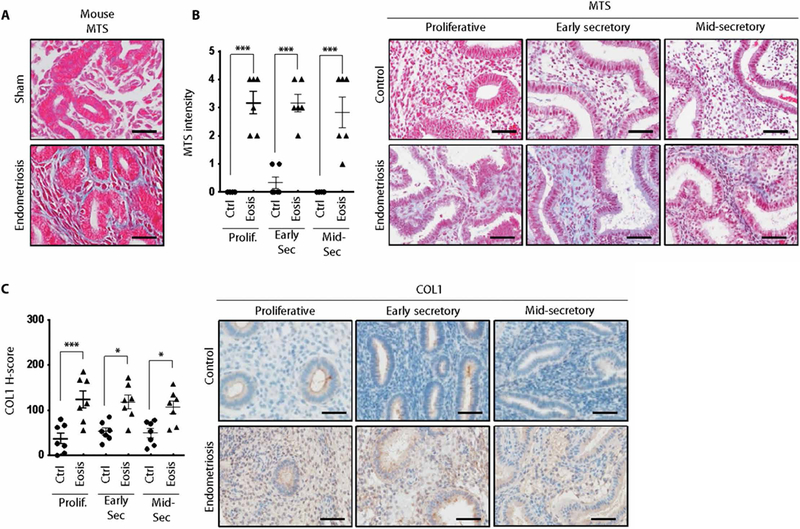
(A) Masson’s trichrome staining (MTS) in the endometriosis mouse model. (B) Intensity of Masson’s trichrome staining and representative photomicrographs of the endometrium from women with and without diagnosed endometriosis (n = 6 for each group per phase). Scale bars, 25 μm. (C) Immunohistochemical H-score and representative photomicrographs of COL1 in the eutopic endometrium from women with and without endometriosis (n = 7 for each group per phase) at proliferative, early secretory, and mid-secretory phases. Nuclei were counterstained with hematoxylin (blue). Scale bars, 50 μm. Mean ± SEM. *P < 0.05 and ***P < 0.001, Student’s t test.
HDAC3 attenuation results in recruitment of p300 to HDAC3-BE of type I collagen genes
Infertile women with endometriosis display markedly reduced decidualization and impaired uterine receptivity (23). Therefore, we examined the amounts of HDAC3 in hESCs from women with or without endometriosis. The expression of HDAC3 was lower in hESCs from women with endometriosis compared to controls (Fig. 5A). To determine the role of HDAC3 in decidualization, we induced in vitro decidualization in hESCs. The expression of decidualization marker genes, insulin-like growth factor-binding protein 1 (IGFBP1) and prolactin (PRL), was reduced in hESCs treated with HDAC3 small interfering RNA (siRNA) as compared to control (Fig. 5B). The expression of PGR was also decreased in hESCs treated with HDAC3 siRNA (Fig. 5C). To decipher the underlying mechanism of enhancement of uterine collagen biosynthesis by HDAC3 attenuation, we examined the roles of HDAC3 in the transcriptional regulation of collagen genes using primary human stromal cells. The mRNA expression of both COL1A1 and COL1A2 was reduced during the decidualization. However, inhibition of HDAC3 induced derepression of both COL1A1 and COL1A2 genes (Fig. 5D). These results suggest that HDAC3 plays an important role for decidualization in hESCs.
Fig. 5. The effect of HDAC3 knockdown on decidualization in hESCs.
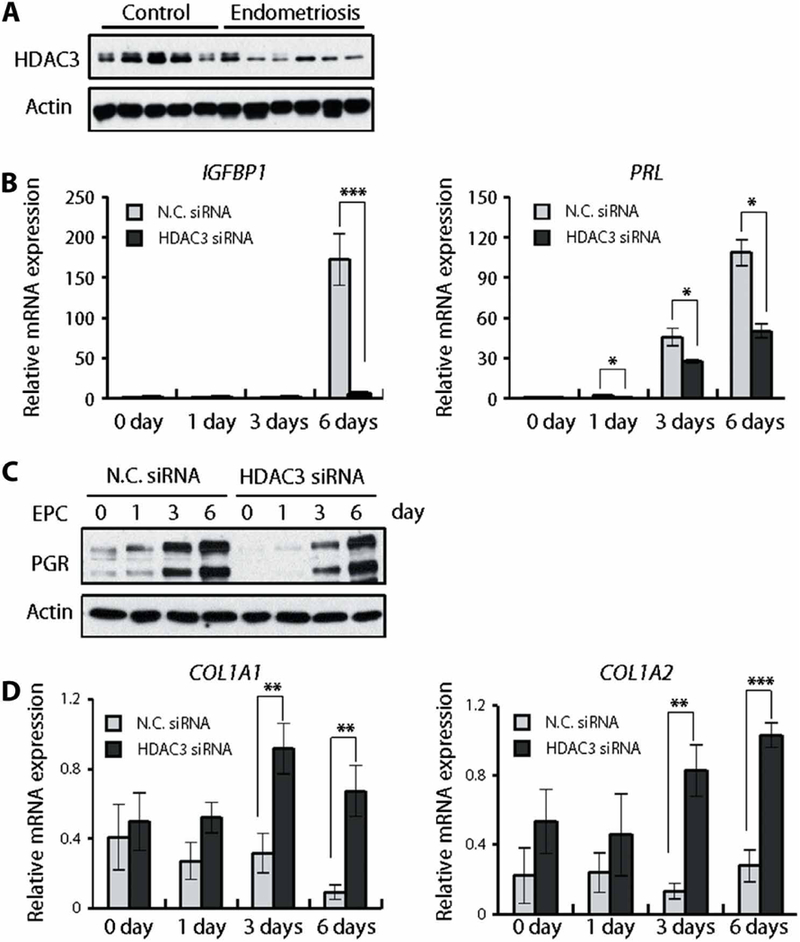
(A) Western blot analysis of HDAC3 in hESCs from infertile women with (n = 5) or without (n = 6) endometriosis. (B) RT-qPCR analysis of expression of decidualization marker genes, IGFBP1 and PRL, during in vitro decidualization of hESCs treated with nontargeting negative control siRNA (N.C. siRNA) or HDAC3 siRNA (n = 6). (C) Western blot analysis of PGR during in vitro decidualization of hESCs treated with negative control siRNA or HDAC3 siRNA in OPTI-MEM medium containing 10 nM estradiol, 1 mM medroxyprogesterone acetate, and 50 μM cAMP (EPC). (D) Knockdown of HDAC3 induces derepression of both COL1A1 and COL1A2 mRNA expression. Human stromal cells were transfected with negative control siRNA or HDAC3 siRNA and cultured for the indicated duration. The expression of indicated genes was analyzed by RT-qPCR (n = 6). Mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, ANOVA followed by Tukey or Bonferroni test for pairwise t test for data containing more than two groups.
The transcriptional regulation is reversibly regulated by both HDAC and histone acetyltransferase (HAT) protein complexes (24–26). Thus, we examined whether HAT proteins are involved in the transcriptional activation of collagen genes upon HDAC3 knockdown. HDAC3 knockdown significantly (P < 0.01) decreased HDAC3 binding to the HDAC3-BE of COL1A1 and COL1A2 genes (Fig. 6A). A marked increase of p300 recruitment to HDAC3-BE was observed in HDAC3 knockdown when compared with other HAT proteins (Fig. 6A). Similarly, the robust increase of p300 recruitment to HDAC3-BE was also induced in the uteri of Hdac3f/f mice (Fig. 6B).
Fig. 6. The effect of HDAC3 knockdown on the transcriptional regulation of collagen genes in hESCs.
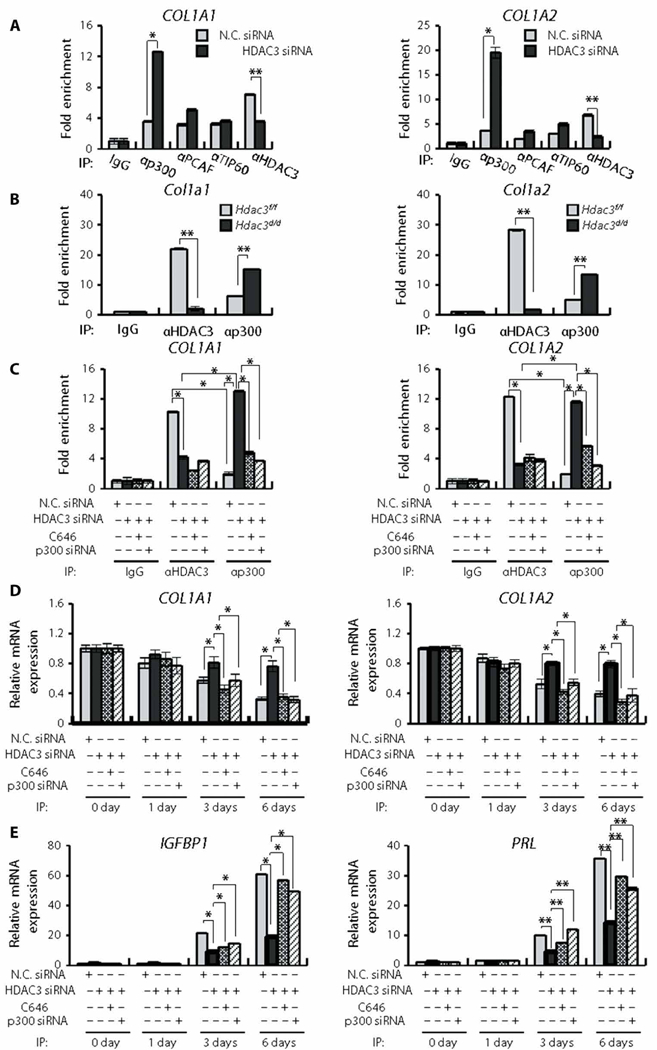
(A) HDAC3 knockdown enhances the selective recruitment of p300 to the HDAC3-BE of collagen genes. hESCs were transfected with nontargeting negative control siRNA (N.C. siRNA) or HDAC3 siRNA. ChIP assay was performed with the indicated antibodies. DNA samples were analyzed by qPCR (n = 3). (B) Recruitment of p300 to the HDAC3-BE is increased by Hdac3 ablation in mouse uteri. ChIP assay was performed using the indicated antibodies. DNA samples were analyzed by qPCR (n = 3). (C) p300 knockdown represses the transcriptional activation of collagen genes through knockdown of HDAC3. hESCs were transfected with negative control siRNA or HDAC3 siRNA and then treated with C646, a p300 inhibitor, or transfected with negative control siRNA or p300 siRNA. ChIP assays were performed with the indicated antibodies. DNA samples were analyzed by qPCR (n = 3). (D) The treatment of hESCs with C646 or p300 siRNA inhibits the transcriptional activation of both COL1A1 and COL1A2 genes during in vitro decidualization of hESCs with HDAC3 siRNA. hESCs were transfected with negative control siRNA or HDAC3 siRNA and then treated with C646 or transfected with negative control siRNA or p300 siRNA. The expression of the indicated genes was analyzed by RT-qPCR (n = 3). (E) Inhibition of p300 function reversed the decreased transcription of decidualization marker genes, IGFBP1 and PRL, upon HDAC3 knockdown during in vitro decidualization of hESCs. hESCs were transfected with negative control siRNA or HDAC3 siRNA and then treated with C646 or transfected with negative control siRNA or p300 siRNA. The expression of indicated genes was analyzed by RT-qPCR (n = 3). Mean ± SEM. *P < 0.05 and **P < 0.01, ANOVA followed by Tukey or Bonferroni test for pairwise t test for data containing more than two groups.
To determine whether transcriptional activation of COL1A1 and COL1A2 genes by p300 impairs human decidualization, we combined this system with a p300 inhibitor and an siRNA loss-of-function approach. When C646, a p300 inhibitor, was applied to hESCs treated with HDAC3 siRNA, p300 binding to HDAC3-BE was significantly reduced (P < 0.05; Fig. 6C). C646 treatment significantly decreased the amounts of COL1A1 and COL1A2 mRNAs (P < 0.05; Fig. 6D) and significantly increased IGFBP1 and PRL mRNAs compared to hESCs treated with HDAC3 siRNA (P < 0.05 and P < 0.01, respectively; Fig. 6E). Furthermore, transfection of the cells with HDAC3 siRNA and p300 siRNA also significantly decreased COL1A1 and COL1A2 mRNAs (P < 0.05; Fig. 6D) and significantly increased the abundance of IGFBP1 and PRL transcripts compared to hESCs treated with HDAC3 siRNA (P < 0.5 and P < 0.01; Fig. 6E).
DISCUSSION
This study reveals that the expression of HDAC3 is decreased in eutopic endometrium from women with endometriosis compared to controls. Epigenetic modifications are involved in the pathogenesis of endometriosis. The transcription factor specific protein 1 (SP1) is a downstream target of P4-dependent paracrine signals to induce the expression of 17βHSD2 gene in the endometrium. The function of HSD17B2 is critical to metabolize the biologically potent estrogen (E2) for normal endometrial growth and differentiation. However, endometriosis appears to lack P4-mediated secretion of factors that induce SP1 production and regulate HSD17B2 expression (27). Transcriptional repression of HDAC3 is also mediated by degradation of SP1 protein (28). We observed attenuation of HDAC3 in the eutopic endometrium after induction of endometriosis in mouse and baboon endometriosis models. Therefore, endometriosis development may result in the attenuation of HDAC3 through SP1. However, the molecular mechanism of HDAC3 attenuation in the etiology and pathophysiology of endometriosis will require further study.
We found that the attenuation of HDAC3 in endometriosis and HDAC3 loss causes infertility due to an implantation failure in the mouse uterus. However, we could not demonstrate a specific relationship of HDAC3 loss in endometriosis-associated infertility. The endometrial stroma is mainly replaced by large amounts of fibrous tissue in Hdac3d/d mice as well as in endometriosis patients. During the development and progression of endometriotic lesions, excess fibrosis may lead to scarring, chronic pain, and altered tissue function (29, 30). Despite this knowledge, the cellular and molecular mechanisms of fibrosis in endometriosis remain to be clarified. Therefore, identifying the mechanisms involved in the early pathogenesis of endometriosis-related infertility, especially during the onset of the disease, is required.
Hdac3d/d mice revealed aberrant activation of epithelial proliferation and dysregulation of PGR and ESR1 in the uterus. E2 and P4 meditate these changes by activating transcription of target genes through binding to their cognate receptors (31). Loss of epithelial E2 action is essential for implantation at the secretory phase in all eutherian mammal species that have been studied (32). Active epithelial proliferation at the preimplantation stage causes implantation failure in several knockout mouse studies (33, 34). Aberrant activation of epithelial proliferation is observed in the endometrium of infertile women with endometriosis (32, 35). P4 resistance implies aberrant gene expression in response to E2 and P4, and such an impaired P4 response is seen in the endometrium of women with endometriosis (36). P4 resistance is associated with early secretory phase deficiency, early pregnancy loss, or infertility due to endometriosis (32). Similarly, abnormal epithelial proliferation and attenuation of PGR and ESR1 are among the causes for early pregnancy loss in Hdac3d/d mice.
The defective decidualization responses of eutopic and ectopic endometrial stromal cells in endometriosis patients (35, 37) are similar to those observed in Hdac3d/d mice as well as hESCs from women without disease with the knockdown of HDAC3. Furthermore, knockdown and inhibition of p300 changed decidualization from impaired to normal in hESCs with the knockdown of HDAC3. Our loss-of-function experiments in normal hESCs demonstrate a critical role for HDAC3 in decidualization for uterine receptivity. In addition, a gain-of-function experiment demonstrated reversal of the phenotype of abnormal decidualization to the normal phenotype in hESCs from women with disease.
The stromal cells of Hdac3d/d mice showed an increase of collagenous fibers. Histologically, endometriosis is characterized by dense fibrous tissue surrounding the endometrial glands and stroma (38). However, the molecular mechanisms of fibrosis in endometriosis remained unknown. We found an aberrant increase of collagen and fibrous stroma in eutopic endometrium from endometriosis patients. Furthermore, HDAC3 loss increased the expression of collagen in the mouse uterus as well as in hESCs. Therefore, our results suggest that HDAC3 loss may mediate the mechanisms of fibrogenesis in endometriosis. Knowledge of these mechanisms will be indispensable for the development of strategies to prevent and treat endometriosis, and further preclinical research will be required to investigate whether inhibition of collagen may be effective for this purpose.
Collagens are major structural protein components of the extracellular matrix. The collagen content is altered in the human uterus during pregnancy (39). Specific types of collagen have distinct spatio-temporal expression patterns during implantation. Collagen types I, III, and V are reduced at implantation sites compared to nonimplantation sites in the rat uterus (40), but they are not expressed in the decidual zones (41). Type VI is lost from rat stromal cells undergoing decidualization (42). Furthermore, collagen deposition is indispensable for fibrosis that is commonly found in patients with endometriosis and is associated with chronic pain and pelvic morbidity (43). Other studies have shown that collagen type I is increased in a mouse endometriosis model (44). In our results, both collagen type I genes (Col1a1 and Col1a2) were identified as direct target genes of HDAC3. Therefore, overexpression of collagen type I caused a decidualization defect in Hdac3d/d mice. It is also reported that abnormally increased deposition of collagen impairs uterine function (45). In our results, HDAC3 attenuation caused a defect of decidualization through overexpression of COL1A1 and COL1A2 in hESCs. Therefore, our results suggest that transcriptional repression of COL1A1 and COL1A2 genes is critical to induce decidualization of endometrial stromal cells.
The results of our HDAC3 siRNA experiment showed transcriptional activation of COL1A1 and COL1A2 in hESCs. Several members of the nuclear receptor family appear to exert critical physiologic roles by actively repressing gene transcription (46). HDACs play important roles in the epigenetic regulation of gene expression in cells and are emerging therapeutic targets for treating a wide range of diseases (47, 48). HDAC3 forms multiprotein complexes with N-CoR and SMRT to regulate transcription of genes as well as other nontranscriptional functions (3, 18). Several of these complexes contain additional transcriptional corepressor proteins with functional ties to chromatin structure (49). This result suggests a possible involvement of other transcriptional coregulators for COL1A1 and COL1A2 genes in hESCs.
In conclusion, we found the attenuation of HDAC3 in human eutopic endometrium from women with endometriosis. Using non-human primate and mouse models for endometriosis, we showed down-regulation of HDAC3 expression after endometriosis induction. Loss of Hdac3 in the murine uterus resulted in infertility due to implantation and decidualization defects. Our results suggest that loss of HDAC3 causes endometriosis-related infertility due to a non-receptive endometrium. Therefore, our studies offer a conceptual framework for understanding abnormal endometrial homeostasis, with implications for the diagnosis and treatment of nonreceptive endometrium in endometriosis-related infertility.
MATERIALS AND METHODS
Study design
The main objective of this study was to evaluate the role of HDAC3 in the endometrium during early pregnancy. First, the expression of HDAC3 was assessed in eutopic endometrium of infertile women with endometriosis compared to fertile women. To determine whether endometriosis affects HDAC3 expression, we examined HDAC3 expression in a nonhuman primate model of endometriosis. Subsequently, we investigated the role of HDAC3 in pregnancy by analyzing fertility, implantation, and decidualization in Hdac3d/d and littermate control mice. Last, transcriptional regulation of HDAC3 was characterized on COL1A1 and COL1A2 genes in hESCs. The control and treatment groups and the number of biological replicates (sample sizes) for each experiment are specified in the figure legends. Animal numbers for each study type were determined by the investigators on the basis of previous experience with the standard disease models that were used or from pilot studies. Animals were randomly allocated to the control and treatment groups and housed together to minimize environmental differences and experimental bias. Analysis of endpoint readouts was carried out in a blinded fashion.
Ethics statement
The study was approved by the institutional review board of Michigan State University, Greenville Health System, and University of North Carolina. All protocols relating to mice were overseen and approved by the Institutional Animal Care and Use Committee at Michigan State University. The endometriosis baboon animal model was reviewed and approved by the Institutional Animal Care and Use Committees (IACUCs) of both the University of Illinois at Chicago and Michigan State University.
Human endometrium samples
For experiments examining expression patterns of HDAC3 and COL1 in the endometrium, we used samples of human endometrium from endometriosis patients with infertility (n = 21) and fertile disease-free control women undergoing tubal ligation (n = 21). Endometrial biopsies were obtained at the time of surgery from women between the age of 18 and 45 with regular cycles. The presence or absence of endometriosis was confirmed during surgery. Women who were laparoscopically negative for this disease were placed into the control group, whereas women who were laparoscopically positive were placed in the endometriosis group. Use of an intrauterine device or hormonal therapies in the 3 months preceding surgery was exclusionary for this study. Histologic dating of endometrial samples was done on the basis of the criteria of Noyes et al. (50) and confirmed by subsequent histopathological examination by an experienced fertility specialist (B.A.L.).
hESC culture and in vitro decidualization
hESCs were obtained from the Michigan State University’s Center for Women’s Health Research Female Reproductive Tract Biorepository. The induction of in vitro decidualization has been described previously (51). For HDAC3 knockdown, siRNAs (L-003496–00-0005, Dharmacon, GE Healthcare) were transfected using Lipofectamine RNAiMAX (Invitrogen Corp.) before in vitro decidualization.
Animals and tissue collection
For the early pregnancy study, female Hdac3f/f and Hdac3d/d mice at 8 weeks of age were mated with C57BL/6 male mice, and uterine samples from pregnant mice were obtained at different days of pregnancy. The morning of vaginal plug observation was designated as day 0.5 of gestation (GD 0.5). For the fertility studies, adult female Hdac3f/f and Hdac3d/d mice were placed with C57BL/6 male mice for 6 months, and the number of litters and pups born during that period was recorded. The amounts of progesterone and estrogen in serum were measured by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Induction of decidualization
Induction of the artificial decidual response has been described previously (52). Ovariectomized Hdac3f/f and Hdac3d/d female mice were used for the decidualization experiment.
Induction of endometriosis
Two-month-old female mice had either a surgical procedure to induce endometriosis or a sham surgery. Under anesthesia, a midline abdominal incision was made to expose the uterus in female mice, and the uterine horns were removed. In a petri dish containing phosphate-buffered saline (PBS), the uterine horns were opened longitudinally with scissors, cut into small fragments of about 1 mm3, and then injected back into the peritoneum of the same mouse. The abdominal incision was closed with sutures and wound clips, respectively. We performed the same surgery in the sham group without endometrial inoculation. After a designated time, the mice were euthanized, and endometriosis-like lesions were counted and removed. For the baboon model, endometriosis was induced by intraperitoneal inoculation of menstrual endometrium on two consecutive menstrual cycles and harvested using laparotomy via endometriectomy from four female baboons, as previously described (22).
Western blot analysis
Membranes were blocked with casein (0.5%, v/v) before exposure to anti-HDAC3 (1:1000 dilution, sc11417, Santa Cruz Biotechnology), anti-PGR (1:1000 dilution, sc7208, Santa Cruz Biotechnology), anti-HDAC1 (1:1000 dilution, sc7872, Santa Cruz Biotechnology), anti-HDAC2 (1:1000 dilution, sc7899, Santa Cruz Biotechnology), or anti-β-actin (1:2000 dilution, sc1616, Santa Cruz Biotechnology) antibodies.
Immunohistochemistry analysis
Dewaxed hydrated paraffin-embedded tissue sections were immersed in 3% H2O2 and 100% methanol for 30 min at room temperature to quench endogenous peroxidase, and then the sections were blocked with 10% normal goat (for anti-HDAC3, Ki-67, ESR1, PGR, vimentin, and COUP-TFII antibodies) or rabbit (for anti-COL1 antibody) serum in PBS (pH 7.5) and incubated with anti-HDAC3 (1:1000 dilution, sc11417, Santa Cruz Biotechnology), anti–Ki-67 (1:1000 dilution, ab15580, Abcam), anti-ESR1 (1:500 dilution, M7047, DAKO), anti-PGR (1:500 dilution, A0098, DAKO), anti-vimentin (1:10,000 dilution, ab92547, Abcam), anti–COUP-TFII (1:500 dilution, PP-H7147–00, Perseus Proteomics), or anti-COL1 (1:1000 dilution, 1310–01, SouthernBiotech) antibodies overnight at 4°C. On the following day, the sections were incubated with secondary antibody conjugated to horseradish peroxidase (Vector Laboratories) for 1 hour at room temperature. Immunoreactivity was detected using diaminobenzidine (DAB; Vector Laboratories) and analyzed using microscopy software from NIS Elements Inc. (Nikon). The H-score was calculated using the following equation: H-score = Σ Pi (i), where i is the intensity of staining with a value of 1, 2, or 3 (weak, moderate, or strong, respectively) and Pi is the percentage of stained cells for each intensity, varying from 0 to 100%.
Masson’s trichrome staining
Dewaxed hydrated paraffin-embedded tissue sections were refixed in Bouin’s solution (HT10132; Sigma-Aldrich) and then stained with Weigert’s Iron Hematoxylin Set (HT10–79; Sigma-Aldrich) and Masson’s Trichrome Staining Kit (HT15; Sigma-Aldrich) for red muscle fibers and blue collagen, as described by the manufacturer.
RNA isolation and microarray analysis
Total RNA was extracted from the uterine tissues using the RNeasy Total RNA Isolation Kit (Qiagen). Microarray analysis was performed using GeneChip Mouse Genome 430 2.0 Arrays. Genes with an unadjusted P value of <0.01 and an absolute fold change of ≥1.5 were identified as differentially regulated. Ingenuity System Software (Ingenuity Systems Inc.) was used for pathway analysis.
Reverse transcription–quantitative PCR
The complementary DNAs (cDNAs) were synthesized with MMLV Reverse Transcriptase (Invitrogen Corp.) by using 1 μg of total RNA primed with random hexamer primers according to the manufacturer’s instructions. RT-qPCR was performed on cDNA to assess the expression of genes of interest with SYBR Green or TaqMan primers (table S4) with an Applied Biosystems StepOnePlus. Experimental gene expression data were normalized to 18S ribosomal RNA. Analysis of mRNA expression was first undertaken by the standard curve method, and results were corroborated by cycle threshold values assessing gene expression.
Chromatin immunoprecipitation followed by deep sequencing (ChIP-seq) and ChIP assay
Input and ChIP-DNA analyses were performed using the ChIP-IT High Sensitivity Kit (Active Motif) on uteri from 6-week-old mice. The model-based analysis of ChIP-Seq (MACS) peak-finding algorithm was used to normalize ChIP against the input control (53). ChIP assays were performed as described previously (33). For each ChIP reaction, 100 μg of chromatin was immunoprecipitated using 4 μg of antibodies against HDAC3 (sc11417, Santa Cruz Biotechnology), p300 (sc585, Santa Cruz Biotechnology), PCAF (sc13124, Santa Cruz Biotechnology), and TIP60 (sc25378, Santa Cruz Biotechnology). The sequences of the primers used for HDAC3 response element in the COL1A1 gene were 5’-GAGATGGCATCCCTGGAC-3’ and 5’-CCCATTGGACCTGAACCG-3’, and for HDAC3 response element in the COL1A2 gene, they were 5’-CTGGACTTCCTG-GCTTCAA-3’ and 5’-AGTTCACCCTTGGGACCAG-3’. Immuno-precipitation with normal rabbit IgG was performed as a negative control. The resulting signals were normalized to input DNA.
Statistical analysis
For data with only two groups, Student’s t test was used. For data containing more than two groups, an analysis of variance (ANOVA) test was used, followed by Tukey or Bonferroni test for pairwise t tests. All statistical analyses were performed using the Instat package from GraphPad. The original data are provided in table S5.
Supplementary Material
Acknowledgments:
We thank H. E. Teasley for editing the manuscript. The Pgrcre/+ mice were provided by F. J. DeMayo and J. P. Lydon, and Hdac3f/f mice were provided by E. N. Olson.
Funding: This work was supported by NICHD R01HD084478 to J.-W.J.; NICHD R01HD067721 to S.L.Y. and B.A.L.; National Research Foundation of Korea (NRF) grant funded by the Government of Korea (MSIT) (nos. NRF-2017R1E1A1A01072732 and 2018R1A5A2025079) to H.-G.Y.; NRF funded by the Ministry of Education, Science and Technology (MEST) (NRF-2016R1D1A1B03934346) to J.-Y.Y.; and NRF funded by the Korean government (MSIT) (NRF-2013R1A1A2059010 and NRF-2018R1A5A2020732) and Asan Institute for Life Sciences, Seoul, Korea (2015–575) to K.-C.C. University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core was supported by NICHD (NCTRI) grant P50-D28934.
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: The DNA microarray data of the uteri of Hdac3f/f and Hdac3d/d mice have been deposited to Gene Expression Omnibus (accession code GSE83102). Hdac3f/f mice are available from E. N. Olson under a material agreement with the University of Texas Southwestern Medical Center.
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/1¼74/eaaf7533/DC1
REFERENCES AND NOTES
- 1.Forte A, Cipollaro M, Galderisi U, Genetic, epigenetic and stem cell alterations in endometriosis: New insights and potential therapeutic perspectives. Clin. Sci. 126, 123–138 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Izawa M, Taniguchi F, Terakawa N, Harada T, Epigenetic aberration of gene expression in endometriosis. Front. Biosci. 5, 900–910 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Yoon HG, Choi Y, Cole PA, Wong J, Reading and function of a histone code involved in targeting corepressor complexes for repression. Mol. Cell. Biol. 25, 324–335 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reichert N, Choukrallah MA, Matthias P, Multiple roles of class I HDACs in proliferation, differentiation, and development. Cell. Mol. Life Sci. 69, 2173–2187 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schreiber SL, Bernstein BE, Signaling network model of chromatin. Cell 111, 771–778 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Struhl K, Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 12, 599–606 (1998). [DOI] [PubMed] [Google Scholar]
- 7.Goodman RH, Smolik S, CBP/p300 in cell growth, transformation, and development. Genes Dev. 14, 1553–1577 (2000). [PubMed] [Google Scholar]
- 8.Shiozawa T, Shih HC, Miyamoto T, Feng YZ, Uchikawa J, Itoh K, Konishi I, Cyclic changes in the expression of steroid receptor coactivators and corepressors in the normal human endometrium. J. Clin. Endocrinol. Metab. 88, 871–878 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Hanstein B, Eckner R, DiRenzo J, Halachmi S, Liu H, Searcy B, Kurokawa R, Brown M, p300 is a component of an estrogen receptor coactivator complex. Proc. Natl. Acad. Sci. U.S.A. 93, 11540–11545 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houshdaran S, Nezhat CR, Vo KC, Zelenko Z, Irwin JC, Giudice LC, Aberrant endometrial DNA methylome and associated gene expression in women with endometriosis. Biol. Reprod. 95, 93 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munro SK, Farquhar CM, Mitchell MD, Ponnampalam AP, Epigenetic regulation of endometrium during the menstrual cycle. Mol. Hum. Reprod. 16, 297–310 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Meyer JL, Zimbardi D, Podgaec S, Amorim RL, Abrão MS, Rainho CA, DNA methylation patterns of steroid receptor genes ESR1, ESR2 and PGR in deep endometriosis compromising the rectum. Int. J. Mol. Med. 33, 897–904 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE, Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5’ CpG island in endometriosis. J. Clin. Endocrinol. Metab. 92, 3261–3267 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW, Aberrant methylation at H0XA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am. J. Obstet. Gynecol. 193, 371–380 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Xiaomeng X, Ming Z, Jiezhi M, Xiaoling F, Aberrant histone acetylation and methylation levels in woman with endometriosis. Arch. Gynecol. Obstet. 287, 487–494 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Colón-Diaz M, Báez-Vega P, Garcia M, Ruiz A, Monteiro JB, Fourquet J, Bayona M, Alvarez-Garriga C, Achille A, Seto E, Flores I, HDAC1 and HDAC2 are differentially expressed in endometriosis. Reprod. Sci. 19, 483–492 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Ozawa Y, Lee H, Wen YD, Tan TH, Wadzinski BE, Seto E, Histone deacetylase 3 (HDAC3) activity is regulated by interaction with protein serine/threonine phosphatase 4. Genes Dev. 19, 827–839 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon HG, Chan DW, Huang ZQ, Li J, Fondell JD, Qin J, Wong J, Purification and functional characterization of the human N-CoR complex: The roles of HDAC3, TBL1 and TBLR1. EMBOJ. 22, 1336–1346 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M, Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103, 843–852 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Jeyakumar M, Petukhov S, Bagchi MK, A nuclear receptor corepressor modulates transcriptional activity of antagonist-occupied steroid hormone receptor. Mol. Endocrinol. 12, 513–524 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Guenther MG, Barak O, Lazar MA, The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol. Cell. Biol. 21, 6091–6101 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braundmeier AG, Fazleabas AT, The non-human primate model of endometriosis: Research and implications for fecundity. Mol. Hum. Reprod. 15, 577–586 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ, Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil. Steril. 85, 564–572 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adcock IM, Ford P, Ito K, Barnes PJ, Epigenetics and airways disease. Respir. Res. 7, 21 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Ruijter AJM,van Gennip AH, Caron HN, Kemp S, van Kuilenburg ABP, Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem. J. 370, 737–749 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK, Histone deacetylases and cancer: Causes and therapies. Nat. Rev. Cancer 1, 194–202 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Bulun SE, Cheng YH, Pavone ME, Yin P, Imir G, Utsunomiya H, Thung S, Xue Q, Marsh EE, Tokunaga H, Ishikawa H, Kurita T, Su EJ, 17Beta-hydroxysteroid dehydrogenase-2 deficiency and progesterone resistance in endometriosis. Semin. Reprod. Med. 28, 44–50 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kikuchi J, Wada T, Shimizu R, Izumi T, Akutsu M, Mitsunaga K, Noborio-Hatano K, Nobuyoshi M, Ozawa K, Kano Y, Furukawa Y, Histone deacetylases are critical targets of bortezomib-induced cytotoxicity in multiple myeloma. Blood 116, 406–417 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Matsuzaki S, Canis M, Darcha C, Dechelotte P, Pouly JL, Bruhat MA, Fibrogenesis in peritoneal endometriosis. A semi-quantitative analysis of type-I collagen. Gynecol. Obstet. Invest. 47, 197–199 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Nisolle M, Donnez J, Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 68, 585–596 (1997). [DOI] [PubMed] [Google Scholar]
- 31.Wetendorf M, DeMayo FJ, The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol. Cell. Endocrinol. 357, 108–118 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox C, Morin S, Jeong JW, Scott RT Jr., Lessey BA , Local and systemic factors and implantation: What is the evidence? Fertil. Steril. 105, 873–884 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim TH, Yoo JY, Wang Z, Lydon JP, Khatri S, Hawkins SM, Leach RE, Fazleabas AT, Young SL, Lessey BA, Ku BJ, Jeong JW, ARID1A is essential for endometrial function during early pregnancy. PLOS Genet. 11, e1005537 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY, COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLOS Genet. 3, e102 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lessey BA, Kim JJ, Endometrial receptivity in the eutopic endometrium of women with endometriosis: It is affected, and let me show you why. Fertil. Steril. 108, 19–27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN, Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obstet. Gynecol. Scand. 96, 623–632 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Yin X, Pavone ME, Lu Z, Wei J, Kim JJ, Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J. Clin. Endocrinol. Metab. 97, E35–E43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giudice LC, Kao LC, Endometriosis. Lancet 364, 1789–1799 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Morrione TG, Seifter S, Alteration in the collagen content of the human uterus during pregnancy and post partum involution. J. Exp.Med. 115, 357–365 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurst PR, Gibbs RD, Clark DE, Myers DB, Temporal changes to uterine collagen types I, III and V in relation to early pregnancy in the rat. Reprod. Fertil. Dev. 6, 669–677 (1994). [DOI] [PubMed] [Google Scholar]
- 41.Spiess K, Teodoro WR, Zorn TM, Distribution of collagen types I, III, and V in pregnant mouse endometrium. Connect. Tissue Res. 48, 99–108 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Mulholland J, Aplin JD, Ayad S, Hong L, Glasser SR, Loss of collagen type VI from rat endometrial stroma during decidualization. Biol. Reprod. 46, 1136–1143 (1992). [DOI] [PubMed] [Google Scholar]
- 43.Nunes FR, Ferreira JM, Bahamondes L, Prevalence of fibromyalgia and quality of life in women with and without endometriosis. Gynecol. Endocrinol. 30, 307–310 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Daftary GS, Zheng Y, Tabbaa ZM, Schoolmeester JK, Gada RP, Grzenda AL, Mathison AJ, Keeney GL, Lomberk GA, Urrutia R, A novel role of the Sp/KLF transcription factor KLF11 in arresting progression of endometriosis. PLOS ONE 8, e60165 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahima A, Soderwall AL, Uterine collagen content in young and senescent pregnant golden hamsters. J. Reprod. Fertil. 49, 161–162 (1977). [DOI] [PubMed] [Google Scholar]
- 46.Rosenfeld MG, Glass CK, Coregulator codes of transcriptional regulation by nuclear receptors. J. Biol. Chem. 276, 36865–36868 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Carter JW, Ready AE, Singhroy S, Duta E, Gerrard JM, The effect of exercise on bleeding time and local production of prostacyclin and thromboxane. Eur. J. Appl. Physiol. Occup. Physiol. 59, 355–359 (1989). [DOI] [PubMed] [Google Scholar]
- 48.Millard CJ, Watson PJ, Fairall L, Schwabe JWR, Targeting class I histone deacetylases in a “complex” environment. Trends Pharmacol. Sci. 38, 363–377 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Jones PL, Shi YB, N-CoR-HDAC corepressor complexes: Roles in transcriptional regulation by nuclear hormone receptors. Curr. Top. Microbiol. Immunol. 274, 237–268 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Noyes RW, Hertig AT, Rock J, Dating the endometrial biopsy. Am. J. Obstet. Gynecol. 122, 262–263 (1975). [DOI] [PubMed] [Google Scholar]
- 51.Lee JH, Kim TH, Oh SJ, Yoo JY, Akira S, Ku BJ, Lydon JP, Jeong JW, Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus. FASEB J. 27, 2553–2563 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finn CA, Martin L, Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol. Reprod. 7, 82–86 (1972). [DOI] [PubMed] [Google Scholar]
- 53.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M, Genome-wide analysis of estrogen receptor binding sites. Nat. Genet. 38, 1289–1297 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


