Abstract
Cognitive slowing is a known but comparatively under-investigated neuropsychological complication of the epilepsies in relation to other known cognitive comorbidities such as memory, executive function and language. Here we focus on a novel metric of processing speed, characterize its relative salience compared to other cognitive difficulties in epilepsy, and explore its underlying neurobiological correlates. Research participants included 55 patients with temporal lobe epilepsy (TLE) and 58 healthy controls from the Epilepsy Connectome Project (ECP) who were administered a battery of tests yielding 14 neuropsychological measures, including selected tests from the NIH Toolbox-Cognitive Battery, and underwent 3T MRI and resting state fMRI. TLE patients exhibited a pattern of generalized cognitive impairment with very few lateralized abnormalities. Using the neuropsychological measures, machine learning (Support Vector Machine binary classification model) classified the TLE and control groups with 74% accuracy with processing speed (NIH Toolbox Pattern Comparison Processing Speed Test) the best predictor. In TLE, slower processing speed was associated predominantly with decreased local gyrification in regions rostral and caudal middle frontal gyrus, inferior precentral cortex, insula, inferior parietal cortex (angular and supramarginal gyri), lateral occipital cortex, rostral anterior cingulate, and medial orbital frontal regions, as well as three small regions of the temporal lobe. Slower processing speed was also associated with decreased connectivity between the primary visual cortices in both hemispheres and the left supplementary motor area, as well as between the right parieto-occipital sulcus and right middle insular area. Overall, slowed processing speed is an important cognitive comorbidity of TLE associated with altered brain structure and connectivity.
Keywords: TLE, epilepsy, cognition, MRI, processing speed
Graphical Abstract
1. INTRODUCTION
The domains of memory, language and executive function are among the most studied cognitive complications of the epilepsies (Helmstaedter & Witt, 2012; B. Hermann, Loring, & Wilson, 2017; Saling, 2009) with an increasing number of imaging investigations focused on the disrupted regions and networks associated with these cognitive anomalies (Diehl et al., 2008; He et al., 2018; Lin, Mula, & Hermann, 2012; McDonald et al., 2014; Reyes et al., 2018; Vlooswijk et al., 2011). Psychomotor slowing is also a common but arguably less investigated cognitive abnormality of the epilepsies. While known to be exacerbated by many anti-epileptic drugs (AED) (Kwan & Brodie, 2001; Loring, Marino, & Meador, 2007; Witt, Elger, & Helmstaedter, 2015), cognitive and/or psychomotor slowing is evident in new onset adult and pediatric patients prior to administration of AEDs (Baker, Taylor, Aldenkamp, & group, 2011; Oostrom et al., 2003), and has been observed to persist following remission of epilepsy and cessation of medication treatment (Aldenkamp et al., 1993; Berg et al., 2008). Thus, cognitive and psychomotor slowing is an inherent neuropsychological morbidity of the epilepsies.
At its most basic level, processing speed can be defined as either the amount of time it takes to process a specific quantity of information, or the quantity of information that can be processed within a specific unit of time (Kalmar & Chiaravalloti, 2008). There has been little consistency in the metrics used to assess cognitive and psychomotor slowing in epilepsy, as speed-based performances have been assessed with a variety of measures including simple and complex reaction time, finger tapping, mental scanning, motor assembly tasks, and others (Grevers, Breuer, Ijff, & Aldenkamp, 2016). One common approach, across diverse disorders, has been the use of digit symbol substitution tests, with applications to examine speeded performance in schizophrenia (Dickinson, Ramsey, & Gold, 2007; Knowles, David, & Reichenberg, 2010; Morrens, Hulstijn, & Sabbe, 2007), multiple sclerosis (Benedict, Morrow, Weinstock Guttman, Cookfair, & Schretlen, 2010), normal aging (Salthouse et al., 2000; Tucker-Drob & Salthouse, 2008) as well as epilepsy (Garcia-Ramos et al., 2018). Further investigation of the task has shown that it is driven in part by speed-dependent processes (graphomotor speed, perceptual speed), with contributions of visual scanning efficiency, learning/memory and executive function (Ashendorf & Reynolds, 2013; Joy, Fein, & Kaplan, 2003). An alternative measure of central processing speed is the Pattern Comparison Processing Speed Test (PCPS) of the NIH Toolbox Battery-Cognition (NIHTB-CB) which is an efficient visually-based measure of choice reaction time adapted for computerized presentation. This test has applicability across the lifespan, sound test-retest reliability, appropriate age-related performance characteristics, and demonstrated construct validity (Carlozzi et al., 2014). Furthermore, there is less confounding of psychomotor issues with quantification of central information processing speed compared to digit symbol substitution tests. Here we examine the clinical relevance of this metric compared to other clinical and experimental cognitive tests.
At least two issues pertinent to slowing of speed-dependent processes in epilepsy remain to be clarified and are a focus of this investigation. First, the relative salience of slowed processing speed relative to other potential cognitive abnormalities in epilepsy remains uncertain. Abnormalities in memory, language and executive function are of clear importance, but the set of abnormalities that most reliably discriminates persons with epilepsy compared to healthy controls, and the role of slowing of processing speed in this discrimination, remains to be determined. To address this issue we utilize machine learning to characterize the relative power of various cognitive abilities, including processing speed, to classify or discriminate patients with epilepsy compared to controls. As machine learning builds multidimensional models using multiple variables, it offers the ability to analyze neuropsychological measures together as a group, instead of individually. For example, a combination of several, individually non-significant features may classify two groups better than the most significant feature itself. Currently, there is enormous interest in applying machine learning techniques in many fields of medicine (Deo, 2015). Its strength comes from the ability to detect patterns in highly complex datasets. In supervised machine learning, the models learn from previous examples or, simply, predictor-outcome pairs. Various algorithms have been developed to solve this optimization problem (Caruana & Niculescu-Mizil, 2006), the most noteworthy at present is artificial neural network or deep learning (Schmidhuber, 2015). Broadly speaking, there are two goals in machine learning studies, achieving high accuracy and understanding the underlying significant predictors. In some cases, the goal is to obtain high accuracy with less emphasis on identifying the underlying predictors. Deep learning is a good example of this case as it is known to outperform other algorithms given enough data, but the complexity of the learned model may be difficult to comprehend or interpret. In other cases, the goal is to sort through multiple potential predictors to determine which factors contribute the most to the learned model. In this case, simpler algorithms such as Support Vector Machine (SVM), with a linear kernel, may be better (Cortes & Vapnik, 1995). In this investigation, we apply SVM to neuropsychological data to classify groups (epilepsy and controls) and identify the salient predictors.
Second, the presence and nature of the underlying neurobiology of cognitive and psychomotor slowing in epilepsy has yet to be fully characterized. Only limited research has addressed this issue. Dow et al. (2004) examined mental scanning speed in adults with temporal lobe epilepsy and found slowed performance related to reduced volume of total cerebral white matter. Alexander et al. (2014) reported increased fractional anisotropy (FA) of the left fornix related to faster processing speed in TLE patients without hippocampal sclerosis. Van Veenendaal et al. (2017) examined the relationship between central information processing speed and resting-state functional network efficiency and found no relationship between speed and network analysis in 55 patients with localization-related epilepsy. To inquire into the potential underlying neurobiology of processing speed abnormalities we characterized its association with measures of cortical volume, thickness, area, and gyrification, followed by examination of resting-state functional MRI (rs-fMRI) to understand the broader network abnormalities associated with psychomotor slowing.
In summary, the core aims of this investigation are: 1) to determine the salience of cognitive and psychomotor slowing relative to other cognitive complications of epilepsy in discriminating patients with TLE from controls, and 2) to characterize the structural and functional connectivity patterns associated with slowed processing speed in TLE. These aims are addressed within the unique cohort of participants comprising the Epilepsy Connectome Project (ECP), which contains detailed neuroimaging, neuropsychological and clinical investigation of patients with chronic TLE and controls.
2. MATERIAL AND METHODS
The ECP is a two sites research project involving the Medical College of Wisconsin (MCW) and the University of Wisconsin-Madison. TLE patients enrolled in the ECP are between the ages of 18 and 60 (inclusive), demonstrate estimated full-scale intelligence quotient (IQ) at or above 70, speak English fluently, and have no medical contraindications to MRI. They have a diagnosis of TLE supported by 2 or more of the following: 1) described or observed clinical semiology consistent with seizures of temporal lobe origin, 2) EEG evidence of either Temporal Intermittent Rhythmic Delta Activity or temporal lobe epileptiform discharges, 3) temporal lobe onset of seizures captured on continuous EEG, or 4) MRI evidence of mesial temporal sclerosis or hippocampal atrophy. Patients with any of the following are excluded: 1) Presence of any lesions other than mesial temporal sclerosis causative for seizures and non-specific white matter abnormalities on 3 Tesla MRI, 2) an active infectious/autoimmune/inflammatory etiology of seizures, either suspected by treating clinician or documented through laboratory testing or response to immunosuppressive therapy. Supplemental Material 1 provides clinical and demographic information for the TLE participants.
Healthy controls underwent cognitive and behavioral assessment and a limited number underwent neuroimaging including MRI per research contract protocol. Participants were between the ages of 18 and 60 (inclusive). Exclusion criteria included: Edinburgh Laterality (handedness) Quotient (Oldfield, 1971) less than +50; primary language other than English; history of any learning disability; history of brain injury or illness, substance abuse, or major psychiatric illness (major depression, bipolar disorder, or schizophrenia); current use of psychoactive or vasoactive medications, as well as any medical contraindications to MRI. Presented here are data from a consecutive, prospectively enrolled series of 55 TLE patients and 58 healthy controls. This project was reviewed and approved by the Institutional Review Board (IRB) at MCW and all participants provided written informed consent.
2.1. Neuropsychological Assessment
All controls and TLE patients underwent neuropsychological assessment as part of the ECP protocol with testing aimed at known cognitive weaknesses of TLE patients (Table 2, Column 2). From this test battery a total of 14 cognitive indices were derived which included brief assessment of estimated intelligence (Wechsler Abbreviated Scale of Intelligence-2 vocabulary and block design subtests) (Wechsler, 2011), verbal learning and memory (Rey Auditory Verbal Learning Test, or RAVLT) including total words learned across trials (RAVLT-Total) and delayed recall (RAVLT-Delayed) (Rey, 1964), object naming (Boston Naming Test) (Kaplan, Goodglass, & Weintraub, 1983), letter fluency (Controlled Oral Word Association Test) (Heaton, Miller, Taylor, & Grant, 2004; Spreen & Benton, 1977), semantic (animal) fluency (Heaton et al., 2004; Strauss, Sherman, & Spreen, 2006), spatial orientation (Judgement of Line Orientation) (Benton, Hamsher, Varney, & Spreen, 1983), speeded fine motor dexterity (Grooved Pegboard, dominant and non-dominant hands) (Klove, 1963), and selected subtests from the NIHTB-CB including Pattern Comparison Processing Speed Test (PCPS), Dimensional Change Card Sort Test, List Sorting Working Memory Test, and Flanker Inhibitory Control and Attention Test. The measure of processing speed, PCPS, from the NIHTB-CB, requires the subject to identify whether two simultaneously presented visual patterns are the “same” or “not the same”. Patterns are either identical or vary in: 1) color, 2) adding/taking something away, or 3) one versus many. The score reflects the number of correct items (out of a possible 130) completed in 85 (Carlozzi, Beaumont, Tulsky, & Gershon, 2015; Carlozzi et al., 2014). For all 14 cognitive measures the age-corrected standard scores were used.
Table 2.
The 14 neuropsychological test scores are sorted by their average absolute weights (Column1 and 7) from the SVM analysis without feature selection. PCPS is the biggest contributor to the classification model, both collectively (SVM weight) and individually (effect size).
| No | Feature Name | TLE (Mean ± SD) | Control (Mean ± SD) | 2 sample t-test p-value | Effect Size d (Cohen’s) | Correlation with AED Count (ρ) | SVM Weight |
|---|---|---|---|---|---|---|---|
| 1 | Pattern Completion Processing Speed | 89.24±16.16 | 102.78±14.18 | < 0.001 | 1.27 | −0.122 | 0.66 |
| 2 | Grooved Peg board Dominant Hand | 87.35±16.69 | 100.72±14.27 | < 0.001 | 1.12 | −0.287* | 0.47 |
| 3 | Dimensional Change Card Sort | 86.87±15.30 | 97.88±15.68 | < 0.001 | 0.86 | −0.065 | 0.46 |
| 4 | Boston Naming Test |
96.47±13.28 | 108.00±16.19 | <0.001 | 0.71 | 0.059 | 0.41 |
| 5 | RAVLT Delayed Recall | 96.42±10.59 | 107.19±12.85 | <0.001 | 0.86 | −0.163 | 0.36 |
| 6 | Grooved Peg board Non-Dominant Hand | 90.22±17.49 | 97.95±17.13 | <0.001 | 1.07 | −0.271* | 0.36 |
| 7 | Flanker Inhibitory | 99.49±16.18 | 109.27±9.24 | 0.010 | 0.49 | −0.014 | 0.28 |
| 8 | WASI-II Vocabulary | 85.73±14.98 | 102.16±14.33 | <0.001 | 0.91 | −0.111 | 0.26 |
| 9 | RAVLT Total Words | 89.27±14.32 | 104.14±13.38 | <0.001 | 0.89 | −0.079 | 0.24 |
| 10 | Working Memory | 90.09±17.98 | 93.93±14.38 | 0.013 | 0.48 | −0.266* | 0.23 |
| 11 | WASI-II Block Design | 94.13±16.46 | 108.34±16.71 | <0.001 | 0.78 | −0.180 | 0.19 |
| 12 | Controlled Oral Word Association | 84.80±12.73 | 91.33±13.73 | 0.214 | 0.24 | −0.008 | 0.18 |
| 13 | Semantic Fluency | 94.91±16.54 | 102.36±14.66 | 0.019 | 0.45 | −0.104 | 0.16 |
| 14 | Judgement of Line Orientation | 89.11±21.53 | 115.14±19.24 | <0.001 | 0.74 | −0.188 | 0.16 |
2.2. Neuropsychological Discrimination of Epilepsy and Control Groups
The ability of the 14 neuropsychological tests to classify TLE and healthy control participants was tested using machine learning. Support Vector Machine (SVM) binary classification models (Cortes & Vapnik, 1995) were trained using the z-transformed standardized scores as the features. Supplemental Material 2 provides a diagram for the SVM training and testing procedures employed in this study. 10-fold cross validation was used, where 10% of the samples were kept as a testing set. Randomization seeds were used for repeatability. When feature selection was on, a cross validation (CV) loop was added and the feature with the lowest average absolute weight (or smallest contribution to the classification model) was removed per loop. The feature selection continued until the 10-fold classification loss reached the minimum. This “optimum set” of features was then used in the testing. The 10-fold accuracy was recorded. This entire procedure (in Supplemental Material 2) was repeated 10 times (10 iterations), both with and without the feature selection. The optimum sets of features were then analyzed based on their normalized weights, where the maximum absolute weight is one.
2.3. MRI Acquisition and Preprocessing
MRI was performed on 3T GE 750 scanners at both institutions. T1-weighted images were acquired using a magnetization prepared gradient echo sequence (TR/TE=604ms/2.516ms, TI=1060.0ms, flip angle=8°, FOV=25.6cm, 0.8mm isotropic). Cube T2-weighted images were also acquired (TR/TE=2,500ms/94.641ms, flip angle=90°, FOV=25.6cm, 0.8mm isotropic).
Rs-fMRI images were acquired using whole-brain simultaneous multi-slice (SMS) imaging (8 bands, 72 slices, TR/TE=802ms/33.5ms, flip angle=50°, matrix=104 ×104, FOV=20.8cm, voxel size 2mm isotropic) (Moeller et al., 2010). The participants were asked to focus on a white cross at the center of black background. Time-series from four 5-minute runs acquired in a single session were concatenated.
All images were pre-processed using the Human Connectome Project (HCP) minimal preprocessing pipelines (Glasser et al., 2013). In brief, the function of this pipeline is to align the T1w and T2w images, perform a B1 (bias field) correction, register them to the (Montreal Neurological Institute (MNI)) space, segment the volume into predefined structures, reconstruct white and pial cortical surfaces, and perform FreeSurfer’s standard folding-based surface registration to their surface atlas (fsaverage). Once the pial surface is created, an outer surface that does not enter the sulcal folds is determined. For each vertex a 10 mm diameter circular region is formed on the outer smoothed surface along with a corresponding perimeter on the pial surface. Local gyrification index (LGI) is calculated at each vertex as the area ratio of the area of the cortex following the sulcal folds to the area of the cortex on the outer visible cortex. Each subject’s LGI values are then mapped to the fsaverage surface without additional smoothing. All structural data were inspected pre and post processing for quality assurance.
The functional portion of the HCP pipelines removes spatial distortions using spin echo field maps, realigns volumes to compensate for subject motion, registers the fMRI data to the structural, reduces the bias field, normalizes the 4D image to a global mean, masks the data with the final brain mask and maps the voxels within the cortical gray matter ribbon onto the native cortical surface spaces. More details on the HCP processing pipelines can be found in Glasser et al (2013).
2.4. fMRI Processing
Rs-fMRI images are heavily affected by subject motion in the scanner. We used three different motion metrics to determine if a run was successful: relative mean root-mean-squared (RMS), absolute mean RMS, and derivative of variance RMS (DVARS) (Power et al., 2014). These are common quality control measures for rs-fMRI scans, where the RMS’s measure the pure subject motion and DVARS measures combination of the motion and the scanner instabilities. These three measures were calculated per each run of 5 minutes. The measures were each transformed into the standard scores, and subjects who had z > 3 on any of these three measures in any of the four runs being concatenated were defined as motion outliers.
Then, additional pre-processing was performed on the rs-fMRI images using Analysis of Functional Neuro-Images (AFNI) (Cox, 1996), which included motion regression using 12 motion parameters, and regression-based removal of signal changes in the white matter, cerebrospinal fluid, and the global signal. For the brain atlas, a combination of 360 cortical regions defined by the HCP’s Glasser parcellation (Glasser et al., 2016) and 19 subcortical regions from the FreeSurfer subcortical segmentation (Fischl et al., 2002) was used. Blood-oxygen-level-dependent (BOLD) time series from these 379 regions were extracted per subject. The pairwise Pearson correlations were calculated and transformed to Fisher’s Z scores to generate connectivity matrices.
2.5. Structural MRI Analysis
Whole cortex vertex-wise analysis of cortical surface features (volume, thickness, area and LGI) was conducted using FreeSurfer’s statistical tool QDEC (Query, Design, Estimate, Contrast). This applies the General Linear Model with age as a covariate at each vertex to measure the correlation of speed with each cortex morphological feature. Prior to analysis, each measure was mapped to a standardized vertex space defined by the fsaverage atlas. The measures of thickness, area and volume were smoothed with a 15 mm full-width-at-half-maximum (FWHM) kernel. LGI values were fit to fsaverage without additional smoothing due to the inherent smoothing of the LGI calculation. Analyses were corrected for total intracranial volume (ICV), age and gender, and were very similar with or without ICV as covariate. A second level correction for multiple comparisons was performed by setting the threshold of detection of false discovery rate (FDR) corrections at p < 0.05, more conservative than cluster size corrections used in prior publication on cortical thickness correlations (Dabbs, 2013).
2.6. fMRI Analysis
All 71,631 resting connections were tested for their correlation with the age-corrected PCPS scores. For multiple comparisons, Benjamini-Hochberg false discovery rate (FDR) correction method was used (Benjamini & Hochberg, 1995).
3. RESULTS
3.1. Demographics
Research participants included 55 TLE patients and 58 healthy controls. The difference in the mean age (p<0.01) between the TLE (range 19–60 years) and control groups (range 18–56 years) was addressed by using age-corrected cognitive scores. The two groups did not significantly differ with regard to gender (p=0.85), with a modest trend in years of education (p=0.06). In the TLE group, 14 subjects had right TLE, 26 had left TLE, and 2 had bilateral onsets based on either interictal EEG, imaging (hippocampal sclerosis) or ictal monitoring. Thirteen subjects had uncertain lateralization. TLE participants were taking 0 to 4 AEDs with a mean of 2.05, with chronic epilepsy (mean= 20 years) characterized by onset in late adolescence (mean= 19 years). A subset of the sample underwent Wada Testing or fMRI language assessment and none showed reversed cerebral dominance.
3.2. Cognitive Performance
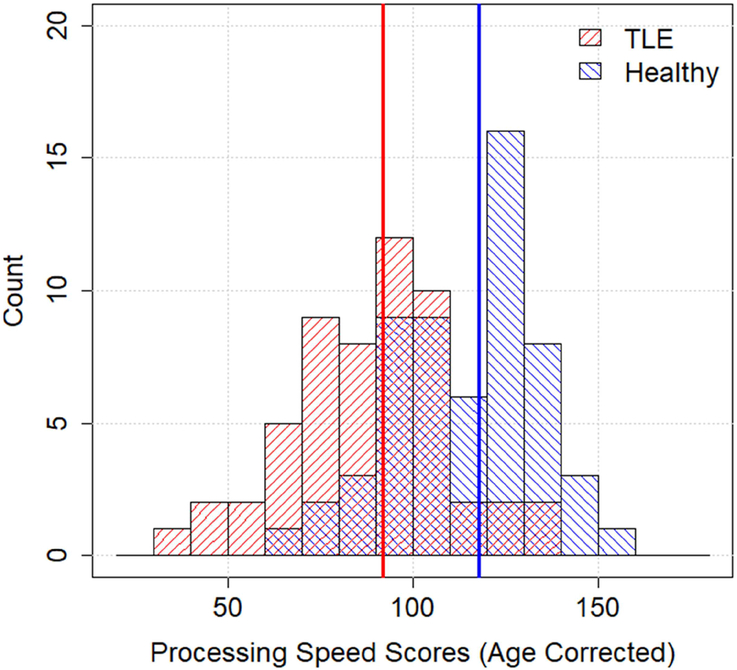
All test scores were normally distributed in both TLE and control groups (ps>0.15, Kolmogorov-Smirnov test), except for the Judgement of Line Orientation test (ps<0.05). Therefore, the Wilcoxon Rank-Sum Test was performed on this test and two-sample t-tests on the others. Patients with TLE as a group performed significantly worse on 13 of the 14 administered neuropsychological tests (Table 2, Column 5). PCPS had the largest effect size (1.27) followed by Grooved Pegboard (dominant hand 1.12, non-dominant hand 1.07), WASI-II Vocabulary (0.91), RAVLT (total words 0.89, delayed recall 0.86), and Dimensional Change Card Sort Test (0.86). Medium effect sizes were evident for WASI-II Block Design (0.78), Judgement of Line Orientation, and Boston Naming Test (0.71). Small effect sizes were observed for Flanker (0.49), Working Memory (0.48), Semantic Fluency (0.45) and COWA (0.24). The distribution of PCPS scores for the TLE and control groups is shown in Figure 1.
Figure 1.
TLE patients (red) overall scored significantly lower than the age and education-matched healthy controls (blue) on PCPS. The vertical lines indicate the median scores for each group, which were 92 and 118.
There were few lateralized cognitive findings. 26 left TLE and 14 right TLE patients (See Supplemental Material 1) did not differ in age (p>0.10), gender (p=0.50), education (p=0.40), AED count (p=0.96), or duration of epilepsy (p=0.81). The right TLE group performed significantly worse than the left TLE group on the Dimensional Change Card Sort Test (p=0.027, t=2.30). There were no other significant lateralized cognitive findings. The majority of cognitive tests were significantly lower than controls in both the left TLE (11 of 14 tests, all ps<0.02) and right TLE (13 of 14 tests, all ps< 0.03) groups. Thus, cognitive anomalies were generalized in nature in the context of lateralized epilepsy.
Spearman correlations examined the relationship between the number of AEDs and cognitive performance (Table 2, Column 6). AED effects were observed on measures of dominant and non-dominant hand speeded fine motor dexterity (ρs=−0.287 and −0.271, ps=0.034 and 0.046 respectively) and working memory (ρ=−0.266, p=.049). There were no other significant associations between AED number and cognition including PCPS (ρ =10.122, p=0.374).
3.3. Machine Learning Classification
The 14 neuropsychological test scores were able to train an SVM model that reliably classified TLE and control participants with 73.4±2.7% accuracy without feature selection. The PCPS feature received the highest average absolute weight (w=0.66) among all 14 scores in all 10 testing loops, followed by Grooved Pegboard-Dominant (w=0.47) (Table 2, Column 7). With feature selection, PCPS was most reliably and repeatedly present in every cross validation loop (9 out of 10 iterations) in the optimum set of features, followed by Grooved Pegboard-Dominant (5 out of 10) and Boston Naming Test (1 out of 10).
3.4. Structural MRI Results
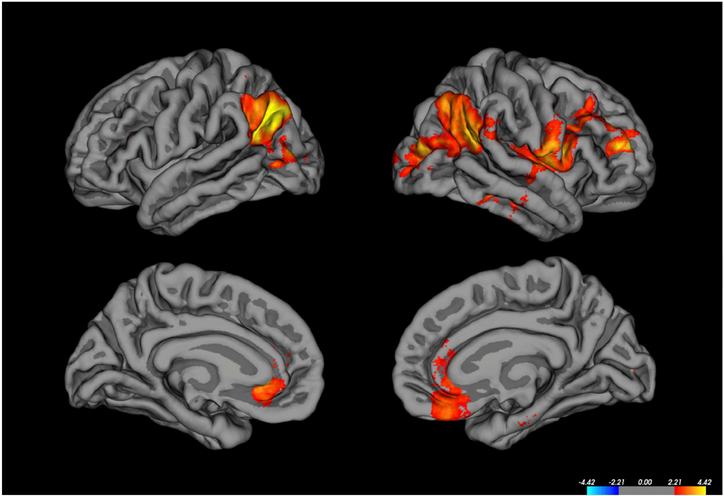
We examined associations of age-corrected PCPS scores in the TLE participants with metrics of cortical volume, thickness, white matter surface area, pial surface area and LGI, controlling for ICV, gender and age in the MRI structural analyses. After correction for multiple comparisons, no significant correlations were identified between PCPS and cortical thickness, area or volume. In contrast, LGI-PCPS correlations were significant in several cortical regions bilaterally, all of which were in the positive direction (Figure 2). In the left hemisphere these included the angular gyrus, lateral occipital cortex, and rostral anterior cingulate gyrus. In the right hemisphere, correlations with LGI were observed in rostral and caudal middle frontal gyrus, inferior precentral cortex, insula, inferior parietal cortex (angular and supramarginal gyri), lateral occipital cortex, rostral anterior cingulate, and medial orbital frontal regions, as well as three small regions of the temporal lobe (also see Supplemental Material 3).
Figure 2.
Only positive correlations between LGI and age-corrected processing speed scores were found in TLE participants (See text for details [Structural MRI Results])
3.5. fMRI Results
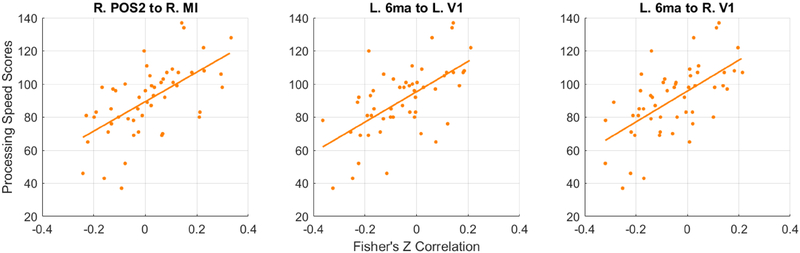
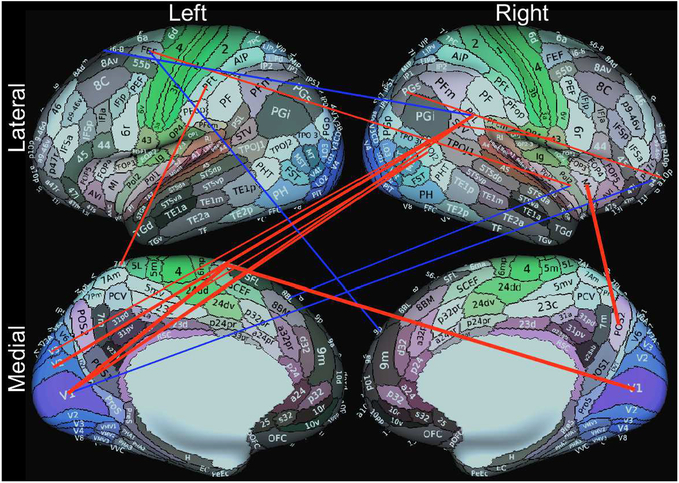
Only 3 subjects were defined as motion outliers as described in the methods and removed from the analyses. The correlation between PCPS and 3 of 71,631 resting state connections approached significance after multiple comparison corrections (r>0.6, pFDR=0.057) in 52 TLE patients. These included connections between the right parieto-occipital sulcus area 2 (POS2) and right middle insular area (MI), and between the primary visual cortices (V1) in both hemispheres and the left area 6m anterior (6ma, supplementary motor area). Scatter plots in Figure 3 show the relationships of PCPS scores vs. the Fisher’s Z correlation values from these 3 connections, with their fitted lines. Figure 4 shows 17 connections showing high correlations (r>0.5). Noticeably, from Figure 4, a group of connections from the right peri-sylvian language area (PSL) to the left visual cortices are positively correlated.
Figure 3.
Scatter plots of the age-corrected Processing Speed scores and the correlation strengths of the 3 resting state connections. All three connections positively correlated with the scores in TLE patients (r>0.6, pFDR=0.057).
Figure 4.
17 resting connections showed high correlations (r>0.5) with the age-corrected PCPS scores in 52 TLE patients. The picture contains 16 cortical connections, excluding one connection between right middle insula and left caudate (r=−0.51). Red lines indicate positive correlation (stronger connection in high performing patients) and vice versa for the blue lines. Line thickness corresponds to the relative strengths of correlation. The 3 most significant (r>0.6, pFDR=0.057) were connections between right parieto-occipital sulcus area 2 (POS2) and right middle insular area (MI), and between the primary visual cortices (V1) in both hemispheres and left area 6m anterior (6ma, SMA). The background brain images were generated with the Connectome Workbench and from Glasser et al. (Glasser et al., 2016).
4. DISCUSSION
The results of this investigation demonstrated that, in a non-surgical cohort of TLE participants, cognitive slowing is a powerful marker of TLE and is associated with functional anatomic anomalies in cortical surface features and functional connectivity.
4.1. Classifying TLE and Control Participants
4.1.1. The salience of processing speed
A comprehensive battery of 14 neuropsychological tests was administered to the ECP participants (Table 2, Column 2), with some tests representing traditional neuropsychological measures while others were incorporated from the NIH Toolbox-Cognitive Battery that have not been used in the epilepsy-neuropsychology literature to date. For the first time in this literature, a machine learning approach (SVM) was applied to the neuropsychological data in an attempt to classify or discriminate the TLE and control groups. Approximately 74% accuracy was reached with the cognitive tests and, of the administered measures, the Pattern Comparison Processing Speed Test, or PCPS, was the most powerful test in discriminating TLE patients from healthy controls. While it has been recognized that processing speed is among the cognitive morbidities of chronic epilepsy, its relative standing among the other cognitive morbidities of epilepsy has not been fully appreciated. In fact, it was the most salient measure in separating the TLE and control groups (Table 2, Column 7, SVM weight). Other measures of interest (Boston Naming Test, RAVLT) discriminated the TLE and control groups as expected, but not as powerfully as processing speed (Table 2, Columns 5 and 7). Even though SVM does not assume feature independence, it is still possible that if two features are highly correlated, the second will receive less attention or weight. Even with this in mind, we can conclude that PCPS is the best contributor to the classification model.
Slowed cognitive and psychomotor processing speed traditionally has been linked to anti-epilepsy drug factors (e.g., type, number) (Kwan & Brodie, 2001; Loring et al., 2007; Witt et al., 2015). Here we found the number of epilepsy medications/polytherapy to be associated with some measures of speeded performance (speeded fine motor dexterity) and working memory, but not central processing speed as assessed by the PCPS test. Increasingly aggressive medication treatment occurs in response to poor seizure control and or seizure severity, so the independent contribution of AED medication relative to problematic clinical seizure features cannot be isolated in a cross-sectional investigation of this type. As noted, cognitive and psychomotor slowing has been detected in drug-naïve new-onset pediatric and adult epilepsy (Baker et al., 2011; Oostrom et al., 2003) and has been found to persist following epilepsy remission and cessation of medication treatment (Aldenkamp et al., 1993; Berg et al., 2008). Thus, cognitive and psychomotor slowing is an inherent neuropsychological morbidity of the epilepsies including TLE. Further, slowing is among the measures that show the most decline (B. P. Hermann et al., 2006) or to be among the most impaired abilities (Taylor & Baker, 2010) in prospective neuropsychological investigations of chronic epilepsy patients on static therapy. An advantage of the processing speed test used from the NIH Toolbox Cognitive Battery is that it was minimally confounded by complex motor involvement, comes from a cognitive neuroscience background, and, again, was not impacted by polytherapy.
More broadly, it is worth mentioning that the participants with TLE exhibited a pattern of generally disrupted cognition, performing significantly worse on 13 of the 14 test measures compared to controls (Table 2, Columns 2 and 5), a pattern consistent with prior neuropsychological research (Oyegbile et al., 2004; Wang et al., 2011) and congruent with reports of widespread abnormalities in the integrity of cortical gray and white matter as well as subcortical structures and cerebellum (Deleo et al., 2018; Gross, 2011; Keller et al., 2015; Keller & Roberts, 2008; Lin et al., 2012). Lateralized cognitive abnormalities were few in number (problem solving in right TLE), with no lateralized effects across the remaining 13 of 14 test measures. Looking within the left and right TLE groups compared to controls, a similar generalized pattern of cognitive impairment was found. The majority of cognitive tests were significantly lower than controls in both the left TLE (11 of 14 tests, all differences <p 0.02) and right TLE (13 of 14 tests, all p’s < 0.03) groups. Thus, cognitive anomalies were generalized in nature in the context of lateralized epilepsy. It was in this context that processing speed was found to be a salient predictor of TLE. While few lateralized cognitive impairments were detected in this cohort, this is a finding that has been reported previously in prior examinations of general cognition in TLE (B. Hermann & Seidenberg, 1995; Ogden-Epker & Cullum, 2001) and specific cognitive domains in particular, including object naming (Cherlow & Serafetinides, 1976; Langfitt, 1995; Ogden-Epker & Cullum, 2001). Again, this may be related to the nonsurgical nature of this TLE sample.
4.1.2. Application of machine learning to the neuropsychology of epilepsy
In this work, machine learning was used to aid in finding cognitive patterns in the dataset. Machine learning remains a relatively new concept in many fields of study and certainly in the neuropsychology of epilepsy. Machine learning is a powerful set of tools that can detect patterns and synthesize data in a way that may prove useful to aid in human decision making, however, the complex interactions of variables invoked in machine learning impedes a traditional causative narrative familiar in science, making interpretation of results at times ambiguous (Obermeyer & Emanuel, 2016). As such, we were conservative in our approach, using it as a feature selection tool with 10-fold cross-validation to improve the generalizability of results. SVM analyzed the entire set of available features together, instead of treating features one at a time as in the traditional methods. In the future, with increased samples we will attempt to build machine learning regression models to predict neuropsychological performances with different types of structural and functional brain features. This may reveal valuable information that will reinforce or even correct our current understanding of brain-behavior relationships in TLE patients.
4.2. Neurobiological Correlates of Processing Speed
4.2.1. Cortical surface features
There is little published epilepsy literature addressing the neurobiological correlates of cognitive and psychomotor slowing in epilepsy. Here we examined this issue with measures of structure and resting connectivity. The structural findings showed that brain-behavior correlations were most clearly linked with the metric of gyrification, with increased gyrification associated with faster processing speed. The LGI-speed correlation was significant in several cortical regions (see Figure 2 and Supplemental Material 3) including left parietal, lateral occipital, rostral anterior cingulate; rostral, as well as the posterior aspect of the superior and middle temporal lobe. In the right hemisphere, LGI-speed correlations were significant in the rostral and caudal middle frontal regions, insula, three gyri of the temporal lobe, parietal, occipital, rostral anterior cingulate and medial orbital frontal regions. Other structural measures including cortical thickness, volume, and surface area were unassociated with processing speed.
This gyrification index has been examined infrequently in relation to cognition in epilepsy, but it appears sensitive as we have found the same index to be uniquely associated with other measures of processing speed (digit symbol) in an independent cohort of children with new onset epilepsies (Bobholz et al., under reviewunder review). Studies of brain structure frequently focus on dimensional morphometric measures such as surface area, thickness, or volume over the cortical surface. Some studies have also included analyses of cortical folding, but this analysis is typically performed using a metric derived from local curvature and may be missing some of the more fine grain distinctions captured by an index of local gyrification (Shimony et al., 2016). Studies examining the cortical local gyrification index and its cognitive correlates have recently emerged, suggesting that cortical folding analysis has the capacity to reveal previously untapped relationships between brain structure and function (Chung, Hyatt, & Stevens, 2017; Forde et al., 2017; Green et al., 2018; Treble, Juranek, Stuebing, Dennis, & Fletcher, 2013). This metric appears particularly sensitive to speeded performance measures, and its wider utility in understanding cognitive anomalies in epilepsy appears promising.
4.2.2. Functional MRI
To our knowledge, there has only been one published study examining the relationships between resting state functional connections and metrics of processing speed in epilepsy, which used a different index of speed-based performance and reported no significant associations (van Veenendaal et al., 2017). Here we detected a modest number of associations that approached significance (Figure 4), perhaps the most salient involving connections with the primary visual cortex bilaterally with other regions, findings that appear congruent with the prominence of posterior (bilateral occipital-parietal) areas identified in the gyrification analyses. Connections from the right area PSL to the let visual cortices were found to be correlated with the processing speed performance in TLE population. While there is some suggestion that area PSL is involved in language processing, more strongly in the left hemisphere (Glasser et al., 2016), it is reasonable to suggest that more clinical/lesional, imaging and post-resection studies are required to confirm the role of the right area PSL in language function.
The regions implicated by the gryrification index and resting-state fMRI fit broadly within three networks 1) The bilateral primary and secondary visual processing streams (Goodale & Milner, 1992) 2) Dorsal and ventral visual attentional networks (Corbetta & Shulman, 2002) with involvment of parietal and frontal regions, often with greater right sided involvement (Fox, Corbetta, Snyder, Vincent, & Raichle, 2006). 3) Extended limbic system with orbital frontal, insular, and anterior cingulate areas. The first two networks correspond well with the demands of the PCPS task, given its heavy reliance on visual attention and visual processing. Thus prominence of visual processing streams reflected in the structural and functional imaging analyses is perhaps not surprising. The involvement of the extended limbic network is also expected in TLE (Reid & Staba, 2014). It follows that cognitive slowing in TLE would be associated with regions directly implicated in primary seizure network and also the broader secondary network effects specific to the PCPS task including association areas and visual processing/attention. These findings fit with the concept of focal epilepsy as a network disease with functional network disruptions beyond the primary seizure network with resultant cognitive deficits.
4.3. Limitations
One limitation of this investigation is that the TLE participants were not exclusively, or even primarily, surgical candidates, and therefore only a subset had prolonged EEG monitoring of spontaneous seizures to determine conclusively the laterality of seizure onset. On the other hand, surgical candidates represent a minority of TLE patients, and results based solely on presurgical samples may not be generalizable to the larger TLE population. The current sample was also not affected by the potential confound of identified, causative cerebral lesions present in many with surgically remediable TLE. Thus, this cohort is arguably more representative of the neurobiology associated with TLE. A second limitation is that the sample size is modest; similar investigations with a greater number of participants will help provide more robust assessments of innovative analytic procedures such as machine learning.
Finally, although the test battery was substantial and captured the main cognitive domains implicated in the epilepsy literature, different or additional cognitive indices may yield important additional findings (e.g., improved lateralized findings, enhanced machine learning outcomes).
5. CONCLUSIONS
For people with epilepsy the cognitive (and affective) comorbidities associated with the disorder create as much disability as the seizures themselves (Fisher et al., 2000; Perrine et al., 1995). In this study we demonstrate, in a non-surgical cohort of TLE patients, that cognitive slowing is a powerful marker of TLE and begin to elicudate the underlying functional anatomic correlates, including anomalies in cortical gyrification and functional connectivity. The implicated regions include parts of the extended limbic network as well as visual and motor association areas, in agreement with the network hypothesis of epilepsy that even focal epilepsy has widespread effects. These findings, combined with prior investigations, give credence to the relevance of cognitive slowing as an important marker and intrinsic aspect of TLE. Further studies aimed at replicating these findings and determining effective treatment paradigms should be considered.
Supplementary Material
Table 1.
Summary table of demographics
| Group | N | Mean Age (years) | Gender | Mean Education (years) | Mean Age of Onset (years) | Mean Epilepsy Duration (years) | Seizure Laterality |
|---|---|---|---|---|---|---|---|
| TLE | 55 | 40.1 ± 12.2 | F=30, M=25 | 14.2 ± 2.5 | 19.6 ± 12.3 | 20.5 ± 15.7 | Right=14, Left=26, Bilateral=2, Uncertain=13 |
| Control | 58 | 34.0 ± 10.6 | F=33, M=25 | 15.0 ± 2.1 | - | - | - |
6. ACKNOWLEDGMENTS
This work was supported by NIH U01 NS093650–0.
We thank Taylor M. McMillan, Courtney Forseth, Peter Kraegel, Megan Rozman, and Elizabeth Felton for their contributions to the project.
ABBREVIATIONS
- AED
Anti-Epileptic Drug
- DVARS
Derivative of Variance Root-mean-Squared
- ECP
Epilepsy Connectome Project
- EEG
Electroencephalogram
- FDR
False Discovery Rate
- FOV
Field-Of-View
- HCP
Human Connectome Project
- ICV
Intra-Cranial Volume
- LGI
Local Gyrification Index
- MRI
Magnetic Resonance Imaging
- NIH
National Institute of Health
- NIHTB-CB
NIH Toolbox Battery-Cognition
- PCPS
Pattern Comparison Processing Speed
- RAVLT
Rey Auditory Verbal Learning Test
- RMS
Root-Mean-Squared
- rs-fMRI
Resting State Functional MRI
- SVM
Support Vector Machine
- TE
Echo Time
- TI
Inversion Time
- TLE
Temporal Lobe Epilepsy
- TR
Repetition Time
- WASI
Wechsler Abbreviated Scale of Intelligence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aldenkamp AP, Alpherts WC, Blennow G, Elmqvist D, Heijbel J, Nilsson HL, … Wosse E (1993). Withdrawal of antiepileptic medication in children--effects on cognitive function: The Multicenter Holmfrid Study. Neurology, 43(1), 41–50. [DOI] [PubMed] [Google Scholar]
- Alexander RP, Concha L, Snyder TJ, Beaulieu C, & Gross DW (2014). Correlations between Limbic White Matter and Cognitive Function in Temporal-Lobe Epilepsy, Preliminary Findings. Frontiers in Aging Neuroscience, 6, 142. doi: 10.3389/fnagi.2014.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashendorf L, & Reynolds E (2013). Process analysis of the Digit Symbol task In Ashendorf L, Swenson R, & Libon D (Eds.), The Boston process approach to neuropsychological assessment: A practitioner’s guide (pp. 77–87). New York, NY: Oxford University Press. [Google Scholar]
- Baker GA, Taylor J, Aldenkamp AP, & group, S. (2011). Newly diagnosed epilepsy: cognitive outcome after 12 months. Epilepsia, 52(6), 1084–1091. doi: 10.1111/j.1528-1167.2011.03043.x [DOI] [PubMed] [Google Scholar]
- Benedict RH, Morrow SA, Weinstock Guttman B, Cookfair D, & Schretlen DJ (2010). Cognitive reserve moderates decline in information processing speed in multiple sclerosis patients. J Int Neuropsychol Soc, 16(5), 829–835. doi: 10.1017/S1355617710000688 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological, 57(1), 289–300. [Google Scholar]
- Benton AL, Hamsher KD, Varney NR, & Spreen O (1983). Contributions to Neuropsychological Assessment: A Clinical Manual. New York, NY: Oxford University Press. [Google Scholar]
- Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, Westerveld M, & Kulas J (2008). Residual cognitive effects of uncomplicated idiopathic and cryptogenic epilepsy. Epilepsy Behav, 13(4), 614–619. doi: 10.1016/j.yebeh.2008.07.007 [DOI] [PubMed] [Google Scholar]
- Bobholz S, Dabbs K, Almane D, Jones JE, Hsu D, Stafstrom C, … Hermann B (under review). Neurological substractes of processing speed in childhood epilepsy. Brain Imaging and Behavior. [DOI] [PubMed] [Google Scholar]
- Carlozzi NE, Beaumont JL, Tulsky DS, & Gershon RC (2015). The NIH Toolbox Pattern Comparison Processing Speed Test: Normative Data. Arch Clin Neuropsychol, 30(5), 359–368. doi: 10.1093/arclin/acv031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Tulsky DS, Chiaravalloti ND, Beaumont JL, Weintraub S, Conway K, & Gershon RC (2014). NIH Toolbox Cognitive Battery (NIHTB-CB): the NIHTB Pattern Comparison Processing Speed Test. J Int Neuropsychol Soc, 20(6), 630–641. doi: 10.1017/S1355617714000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruana R, & Niculescu-Mizil A (2006). An empirical comparison of supervised learning algorithms. Proceedings of the 23rd International Conference on Machine Learning, 161–168. [Google Scholar]
- Cherlow DG, & Serafetinides EA (1976). Speech and memory assessment in psychomotor epileptics. Cortex, 12(1), 21–26. [DOI] [PubMed] [Google Scholar]
- Chung YS, Hyatt CJ, & Stevens MC (2017). Adolescent maturation of the relationship between cortical gyrification and cognitive ability. Neuroimage, 158, 319–331. doi: 10.1016/j.neuroimage.2017.06.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci, 3(3), 201–215. doi: 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Cortes C, & Vapnik V (1995). Support-Vector Networks. Machine Learning, 20(3), 273–297. doi:Doi 10.1007/Bf00994018 [DOI] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Deleo F, Thom M, Concha L, Bernasconi A, Bernhardt BC, & Bernasconi N (2018). Histological and MRI markers of white matter damage in focal epilepsy. Epilepsy Res, 140, 29–38. doi: 10.1016/j.eplepsyres.2017.11.010 [DOI] [PubMed] [Google Scholar]
- Deo RC (2015). Machine Learning in Medicine. Circulation, 132(20), 1920–1930. doi: 10.1161/Circulationaha.115.001593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, & Gold JM (2007). Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry, 64(5), 532–542. doi: 10.1001/archpsyc.64.5.532 [DOI] [PubMed] [Google Scholar]
- Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, & Luders HO (2008). Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia, 49(8), 1409–1418. doi: 10.1111/j.1528-1167.2008.01596.x [DOI] [PubMed] [Google Scholar]
- Dow C, Seidenberg M, & Hermann B (2004). Relationship between information processing speed in temporal lobe epilepsy and white matter volume. Epilepsy Behav, 5(6), 919–925. doi: 10.1016/j.yebeh.2004.08.007 [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Vickrey BG, Gibson P, Hermann B, Penovich P, Scherer A, & Walker S (2000). The impact of epilepsy from the patient’s perspective I. Descriptions and subjective perceptions. Epilepsy Res, 41(1), 39–51. [DOI] [PubMed] [Google Scholar]
- Forde NJ, Ronan L, Zwiers MP, Alexander-Bloch AF, Faraone SV, Oosterlaan J, … Hoekstra PJ (2017). No Association between Cortical Gyrification or Intrinsic Curvature and Attention-deficit/Hyperactivity Disorder in Adolescents and Young Adults. Front Neurosci, 11, 218. doi: 10.3389/fnins.2017.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, & Raichle ME (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A, 103(26), 10046–10051. doi: 10.1073/pnas.0604187103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ramos C, Dabbs K, Meyerand ME, Prabhakaran V, Hsu D, Jones J, … Hermann B (2018). Psychomotor slowing is associated with anomalies in baseline and prospective large scale neural networks in youth with epilepsy. Neuroimage Clinical, 19, 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, … Van Essen DC (2016). A multi-modal parcellation of human cerebral cortex. Nature, 536(7615), 171–178. doi: 10.1038/nature18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, … Consortium WU-MH (2013). The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage, 80, 105–124. doi: 10.1016/j.neuroimage.2013.04.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, & Milner AD (1992). Separate visual pathways for perception and action. Trends Neurosci, 15(1), 20–25. [DOI] [PubMed] [Google Scholar]
- Green S, Blackmon K, Thesen T, DuBois J, Wang X, Halgren E, & Devinsky O (2018). Parieto-frontal gyrification and working memory in healthy adults. Brain Imaging Behav, 12(2), 303–308. doi: 10.1007/s11682-017-9696-9 [DOI] [PubMed] [Google Scholar]
- Grevers E, Breuer LE, Ijff DM, & Aldenkamp AP (2016). Mental slowing in relation to epilepsy and antiepileptic medication. Acta Neurol Scand, 134(2), 116–122. doi: 10.1111/ane.12517 [DOI] [PubMed] [Google Scholar]
- Gross DW (2011). Diffusion tensor imaging in temporal lobe epilepsy. Epilepsia, 52 Suppl 4, 32–34. doi: 10.1111/j.1528-1167.2011.03149.x [DOI] [PubMed] [Google Scholar]
- He X, Bassett DS, Chaitanya G, Sperling MR, Kozlowski L, & Tracy JI (2018). Disrupted dynamic network reconfiguration of the language system in temporal lobe epilepsy. Brain, 141(5), 1375–1389. doi: 10.1093/brain/awy042 [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, & Grant I (2004). Revised Comprehensive Norms for an Expanded Halstead Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz: Psychological Assessment Resources, Inc. [Google Scholar]
- Helmstaedter C, & Witt JA (2012). Clinical neuropsychology in epilepsy: theoretical and practical issues. Handb Clin Neurol, 107, 437–459. doi: 10.1016/B978-0-444-52898-8.00036-7 [DOI] [PubMed] [Google Scholar]
- Hermann B, Loring DW, & Wilson S (2017). Paradigm Shifts in the Neuropsychology of Epilepsy. J Int Neuropsychol Soc, 23(9–10), 791–805. doi: 10.1017/S1355617717000650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann B, & Seidenberg M (1995). Executive system dysfunction in temporal lobe epilepsy: effects of nociferous cortex versus hippocampal pathology. J Clin Exp Neuropsychol, 17(6), 809–819. doi: 10.1080/01688639508402430 [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, & Bell B (2006). Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol, 60(1), 80–87. doi: 10.1002/ana.20872 [DOI] [PubMed] [Google Scholar]
- Joy S, Fein D, & Kaplan E (2003). Decoding digit symbol: speed, memory, and visual scanning. Assessment, 10(1), 56–65. doi: 10.1177/0095399702250335 [DOI] [PubMed] [Google Scholar]
- Kalmar JH, & Chiaravalloti ND (2008). Information processing speed in multiple sclerosis: A primary deficit? In John DeLuca PD, Jessica PD, & Kalmar JH (Eds.), Information processing speed in clinical populations. New York: Taylor and Francis. [Google Scholar]
- Kaplan EF, Goodglass H, & Weintraub S (1983). The Boston Naming Test (2nd ed.). Philadelphia, PA: Lea & Febiger. [Google Scholar]
- Keller SS, Richardson MP, O’Muircheartaigh J, Schoene-Bake JC, Elger C, & Weber B (2015). Morphometric MRI alterations and postoperative seizure control in refractory temporal lobe epilepsy. Hum Brain Mapp, 36(5), 1637–1647. doi: 10.1002/hbm.22722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, & Roberts N (2008). Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia, 49(5), 741–757. doi: 10.1111/j.1528-1167.2007.01485.x [DOI] [PubMed] [Google Scholar]
- Klove H (1963). Clinical Neuropsychology. Med Clin North Am, 47, 1647–1658. [PubMed] [Google Scholar]
- Knowles EE, David AS, & Reichenberg A (2010). Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry, 167(7), 828–835. doi: 10.1176/appi.ajp.2010.09070937 [DOI] [PubMed] [Google Scholar]
- Kwan P, & Brodie MJ (2001). Neuropsychological effects of epilepsy and antiepileptic drugs. Lancet, 357(9251), 216–222. doi: 10.1016/S0140-6736(00)03600-X [DOI] [PubMed] [Google Scholar]
- Langfitt JT (1995). Comparison of the psychometric characteristics of three quality of life measures in intractable epilepsy. Qual Life Res, 4(2), 101–114. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Mula M, & Hermann BP (2012). Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet, 380(9848), 1180–1192. doi: 10.1016/S0140-6736(12)61455-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring DW, Marino S, & Meador KJ (2007). Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychol Rev, 17(4), 413–425. doi: 10.1007/s11065-007-9043-9 [DOI] [PubMed] [Google Scholar]
- McDonald CR, Leyden KM, Hagler DJ, Kucukboyaci NE, Kemmotsu N, Tecoma ES, & Iragui VJ (2014). White matter microstructure complements morphometry for predicting verbal memory in epilepsy. Cortex, 58, 139–150. doi: 10.1016/j.cortex.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, & Ugurbil K (2010). Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med, 63(5), 1144–1153. doi: 10.1002/mrm.22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrens M, Hulstijn W, & Sabbe B (2007). Psychomotor slowing in schizophrenia. Schizophr Bull, 33(4), 1038–1053. doi: 10.1093/schbul/sbl051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeyer Z, & Emanuel EJ (2016). Predicting the Future - Big Data, Machine Learning, and Clinical Medicine. N Engl J Med, 375(13), 1216–1219. doi: 10.1056/NEJMp1606181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden-Epker M, & Cullum CM (2001). Quantitative and qualitative interpretation of neuropsychological data in the assessment of temporal lobectomy candidates. Clin Neuropsychol, 15(2), 183–195. doi: 10.1076/clin.15.2.183.1900 [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia, 9(1), 97–113. doi:Doi 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, Peters AC, Jennekens-Schinkel A, & Dutch Study Group of Epilepsy in, C. (2003). Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”--a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics, 112(6 Pt 1), 1338–1344. [DOI] [PubMed] [Google Scholar]
- Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R, … Hermann BP (2004). The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology, 62(10), 1736–1742. [DOI] [PubMed] [Google Scholar]
- Perrine K, Hermann BP, Meador KJ, Vickrey BG, Cramer JA, Hays RD, & Devinsky O (1995). The relationship of neuropsychological functioning to quality of life in epilepsy. Arch Neurol, 52(10), 997–1003. [DOI] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, & Petersen SE (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage, 84, 320–341. doi: 10.1016/j.neuroimage.2013.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AY, & Staba RJ (2014). Limbic networks: clinical perspective. Int Rev Neurobiol, 114, 89–120. doi: 10.1016/B978-0-12-418693-4.00005-4 [DOI] [PubMed] [Google Scholar]
- Rey A (1964). L’Examen clinique en psychologie, par André Rey,… 2e édition Paris: Presses universitaires de France (Vendôme Impr. des P.U.F.). [Google Scholar]
- Reyes A, Uttarwar VS, Chang YA, Balachandra AR, Pung CJ, Hagler DJ Jr., … McDonald CR (2018). Decreased neurite density within frontostriatal networks is associated with executive dysfunction in temporal lobe epilepsy. Epilepsy Behav, 78, 187–193. doi: 10.1016/j.yebeh.2017.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saling MM (2009). Verbal memory in mesial temporal lobe epilepsy: beyond material specificity. Brain, 132(Pt 3), 570–582. doi: 10.1093/brain/awp012 [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Toth J, Daniels K, Parks C, Pak R, Wolbrette M, & Hocking KJ (2000). Effects of aging on efficiency of task switching in a variant of the trail making test. Neuropsychology, 14(1), 102–111. [PubMed] [Google Scholar]
- Schmidhuber J (2015). Deep learning in neural networks: An overview. Neural Networks, 61, 85–117. doi: 10.1016/j.neunet.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Shimony JS, Smyser CD, Wideman G, Alexopoulos D, Hill J, Harwell J, … Neil JJ (2016). Comparison of cortical folding measures for evaluation of developing human brain. Neuroimage, 125, 780–790. doi: 10.1016/j.neuroimage.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, & Benton AL (1977). Neurosensory center comprehensive examination for aphasia: Manual of directions. revised edition Victoria, BC, Canada: Neuropsychology Laboratory, University of Victoria. [Google Scholar]
- Strauss E, Sherman E, & Spreen O (2006). A Compendium of Neuropsychological Tests (3rd Edition). New York: Oxford University Press. [Google Scholar]
- Taylor J, & Baker GA (2010). Newly diagnosed epilepsy: cognitive outcome at 5 years. Epilepsy Behav, 18(4), 397–403. doi: 10.1016/j.yebeh.2010.05.007 [DOI] [PubMed] [Google Scholar]
- Treble A, Juranek J, Stuebing KK, Dennis M, & Fletcher JM (2013). Functional significance of atypical cortical organization in spina bifida myelomeningocele: relations of cortical thickness and gyrification with IQ and fine motor dexterity. Cereb Cortex, 23(10), 2357–2369. doi: 10.1093/cercor/bhs226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, & Salthouse TA (2008). Adult age trends in the relations among cognitive abilities. Psychol Aging, 23(2), 453–460. doi: 10.1037/0882-7974.23.2.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veenendaal TM, DM IJ, Aldenkamp AP, Lazeron RHC, Hofman PAM, de Louw AJA, … Jansen JFA (2017). Chronic antiepileptic drug use and functional network efficiency: A functional magnetic resonance imaging study. World J Radiol, 9(6), 287–294. doi: 10.4329/wjr.v9.i6.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlooswijk MC, Vaessen MJ, Jansen JF, de Krom MC, Majoie HJ, Hofman PA, … Backes WH (2011). Loss of network efficiency associated with cognitive decline in chronic epilepsy. Neurology, 77(10), 938–944. doi: 10.1212/WNL.0b013e31822cfc2f [DOI] [PubMed] [Google Scholar]
- Wang WH, Liou HH, Chen CC, Chiu MJ, Chen TF, Cheng TW, & Hua MS (2011). Neuropsychological performance and seizure-related risk factors in patients with temporal lobe epilepsy: a retrospective cross-sectional study. Epilepsy Behav, 22(4), 728–734. doi: 10.1016/j.yebeh.2011.08.038 [DOI] [PubMed] [Google Scholar]
- Wechsler D (2011). Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II). San Antonio, TX: NCS Pearson. [Google Scholar]
- Witt JA, Elger CE, & Helmstaedter C (2015). Adverse cognitive effects of antiepileptic pharmacotherapy: Each additional drug matters. Eur Neuropsychopharmacol, 25(11), 1954–1959. doi: 10.1016/j.euroneuro.2015.07.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.