Abstract
This report compares self-report (SR) antiretroviral (ARV) adherence data to adherence data collected via Wisepill, a real-time electronic monitoring (EM) device, among young people living with HIV (YPLH) in the southern United States. Participants (n=66; ages 16 to 26) were followed for 14 weeks. Descriptive analyses were used to compare SR to EM data. Correlations and a linear regression were conducted to explore factors possibly associated with SR-EM discrepancies. We also examined associations between various levels of SR and EM adherence and viral suppression/non-suppression at 14 weeks. Rates of SR adherence were maintained between 87% and 92% while rates of EM adherence declined from 64% to 34%. YPLH who were ARV-experienced, had lower treatment motivation, and reported more frequent recent marijuana use, had a greater discrepancy between their SR and EM adherence levels compared to other YPLH. Higher rates of SR and EM adherence were independently associated with a decline in viral load. A sensitivity analysis also revealed that SR adherence was a better predictor of viral non-suppression, whereas EM adherence was a better predictor of viral suppression. These measurement approaches are discussed in the context of providing clinical care to YPLH.
Keywords: HIV, antiretroviral, adherence, measurement, youth
INTRODUCTION
Adolescents and young adults, ages 13-26 (herein referred to as young people), account for nearly a quarter of all new human immunodeficiency virus (HIV) infections in the United States each year, with the highest infection rates occurring in the South.(1) Young people living with HIV (YPLH) also represent a large proportion of individuals initiating antiretroviral (ARV) treatment.(2) Establishing and maintaining consistent ARV adherence is important in reducing disease morbidity and mortality; however, ARV adherence is particularly difficult for youth. For example, it has been estimated that rates of adherence among YPLH in North America range from 28.0% to 74.5%.(3, 4) Further, one study conducted in the United States with racial and ethnic minority youth found that nearly 40% of YPLH evidenced suboptimal adherence.(5) Poor ARV adherence among youth is troublesome as adherence plays an important role in achieving and maintaining viral suppression and inhibiting disease progression.(6, 7)
Elucidating patterns of youth ARV adherence is a critical first step to developing effective interventions which target adherence. Measurement issues, however, challenge our understanding of ARV use and non-use. There are many strategies to measure adherence. Commonly, researchers have relied on self-report (SR). Due to advancements in technology, electronic medication monitoring (EM) has also become a widely used approach. Associations between SR and EM measured adherence and biological outcomes (e.g. viral load, CD4+ T-cell count) have been demonstrated(8); however, no measure has been identified as the “gold standard,” as each method has its limitations. SR measures are subject to recall and social desirability biases and tend to overestimate actual adherence. EM devices can be costly, are subject to “pocket dosing” (i.e., removal of more than one dose when the device is opened), may fail due to device malfunctions (e.g., limited battery life), or can be lost by patients.(9) Such shortcomings result in an incomplete understanding of adherence patterns among youth. This includes how each measure relates to HIV-related health functioning.
Measuring adherence using both SR and EM methods simultaneously can capture different aspects of adherence and account for each one’s limitations. For example, SR measures have been found to accurately capture adherence among individuals that report missing doses(9) whereas some EM measures allow for real-time tracking of device usage patterns. Because of their different approaches, however, a discrepancy between each measure’s observation of adherence usually occurs. This has been confirmed in studies with adults. (10, 11) Rates of ARV adherence discrepancy range from 1% to 48.8% among persons living with HIV (PLWH).(11) More specifically, self-report adherence rates have been found to be consistently higher than those measured by EM, in some cases by as much as 10% to 20%.(11)
To help explain these discrepancies, one study has empirically examined factors associated with a SR-EM discrepancy score(10). Levine et al. (2006) found greater discrepancies in adults with decreased cognitive functioning and an external locus of control (10). Similar studies have yet to be conducted. Moreover, in a recent review of different approaches to measuring medication adherence, only one investigation examining ARV adherence, conducted by Farley et al. (2003), included children.(11, 12) While relevant to the current study, analyses in the Farley (2003) study were limited to assessing how EM rates compared to four other methods of adherence assessment: caregiver self-report, pharmacy refill records, appointment attendance and physician/nurse questionnaire.(12) Youth self-report was not assessed or compared to youth EM adherence.
One hypothesis is that the same factors associated with medication adherence might also explain discrepancies in observed medication adherence. ARV adherence is affected by demographic factors such as age and gender (13, 14); treatment factors such as treatment experience, motivation to take ARV as prescribed and social support; and behavioral health risks such as mental health problems and substance use. Poor social support, low ARV self-efficacy, advanced HIV disease, psychological distress, depression, and substance use have all been found to negatively impact adherence among YPLH.(4, 5, 14–17) Understanding these discrepancies and the factors that drive them is important for identifying YPLH who may be at risk for over- or underreporting adherence, or YPLH who are less likely to consistently use electronic devices to monitor their adherence. To our knowledge, however, there have been no studies that have examined similar factors as possible predictors of the discrepancy that is observed between SR and EM-based measures of youth ARV adherence.
The aims of this study are four-fold. First, we examine the discrepancy between rates of past 7-day SR ARV adherence and past 7-day EM ARV adherence measured among YPLH over a period of 14 weeks. Second, we explore possible factors associated with the discrepancy between the two measures of adherence. Next, we assess the ability of each adherence measure to predict change in HIV-related biological outcomes (viral load and CD4+ T-cell count) over 14 weeks. The final aim is to explore via a sensitivity analysis the association between various levels of SR and EM adherence and viral suppression/non-suppression. We hypothesized that there would be discrepancies between SR and EM measurements of adherence over time, and based on previous studies with adult populations,(10) that rates of SR adherence would remain consistently high over the course of the study period while EM-measured adherence will steadily decline. It was also expected that adherence, as measured by both SR and EM, would be significantly associated with changes in our biological outcomes – negatively associated with viral load and positively associated with CD4+ T-cell count.
METHODS
Procedures
All project procedures and materials were approved by institutional review boards at Rhode Island Hospital and University of Mississippi Medical Center (UMMC). Participants, ages 14-26, were recruited for a RCT testing the effects of an Information-Motivation-Behavior (IMB) theory-based iPhone app on ARV medication adherence and HIV-related biological outcomes from HIV clinics in the greater Jackson, MS area. To be eligible, study participants needed to be: (1) 14-26 years of age, inclusive; (2) in medical care for HIV and receiving antiretroviral treatment; (3) aware of their HIV status as per clinician and clinical record; (4) have a detectable viral load at study entry as measured by blood testing; (5) understand written and spoken English; and (6) able to give consent/assent. Participants were excluded from the study if it was determined that the participant was impaired by cognitive or medical limitations as per clinical assessment.
Research staff obtained consent from participants over the age of 18 and obtained parental consent and adolescent assent for those under 18 years of age. Participants completed audio computer-assisted self-interview assessments at a pre-baseline assessment, baseline (T1; two weeks after pre-baseline) and four (T2), eight (T3), and 14 (T4) weeks post-baseline. Study data were collected and managed using REDCap electronic data capture tools hosted at Lifespan's Department of Information Services. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.(18)
At the completion of the pre-baseline questionnaire participants were randomized to receive either an IMB-based multilevel gaming intervention, which included a HIV-related game and adherence-based text messages or a time- and attention-matched enhanced standard of care control intervention, which included a non-HIV related game. All participants received a 7-day EM device (Wisepill Technologies, Cape Town, South Africa), at the pre-baseline assessment. Randomization occurred via REDCap using stratification based on gender and daily gaming status (daily gamer versus non-daily gamer). Research staff routinely performed analyses to ensure equality among the two intervention groups.
Measures
Biological Outcomes:
HIV-1 Viral Load and CD4+ T-cell count.
Viral load (HIV-1 RNA PCR) and CD4+ T-cell count were measured by the medical clinic laboratory at study entry and within one month of the final assessment. To facilitate analyses, at the final assessment, participants who were undetectable (<20 copies of the virus) were given a viral load of 10. Change in viral load and CD4+ T-cell count was computed by subtracting the follow-up value from the baseline value.
ARV Adherence:
Self-report ARV adherence.
Youth reported the number of doses they were prescribed per day in the past seven days and estimated the number of doses they had missed in the past seven days. Past 7-day adherence at each time point was calculated and is reported as a proportion.
Adherence measured by EM.
Participants were given a Wisepill adherence device to use for the duration of the study. When the EM device is opened, information is wirelessly relayed to a secure network. EM assumes that each recorded opening represents an actual ingested dose at the time the device was opened. It is also assumed that only one dose was taken at each recorded opening. Past 7-day EM adherence was calculated by dividing the number of bottle openings by the total number of prescribed doses in the seven days prior to an individual’s in person assessment (when SR adherence is measured) and is reported as a proportion.
Other Factors:
Demographics.
Participants reported demographic information including age, gender, race, ethnicity, educational level, sexual orientation, current housing situation and stability of housing, time since starting ARVs (three months or less vs. more than 3 months), and health literacy (“how often do you need help understanding materials from a doctor or pharmacy?”).
Measures of related knowledge, attitudes and behaviors:
ARV Knowledge.
ARV treatment knowledge was assessed using three Likert-style items with five response options ranging from “Strongly Disagree” to “Strongly Agree”.(19, 20) Items included “I know what the possible side effects of each of my HIV medications are” and “I understand how each of my HIV medications works to fight HIV in my body.” Higher scores indicate greater knowledge.
ARV Treatment Motivation.
Personal and social motivations for ARV treatment were measured by four Likert-style items.(19, 20) Items included “I am worried that other people might realize that I am HIV infected if they see me taking my HIV medications” and “It upsets me that the HIV medications that I have been prescribed can cause side effects”. Response options ranged from “Strongly Disagree” to “Strongly Agree”. Higher scores indicate lower motivation for ARV treatment.
Self-Efficacy for ARV Use.
Five Likert-style items assessed perception of the ability to perform necessary ARV skills.(19, 20) Items included “How hard or easy is it for you to take your HIV medications when your usual routine changes?” and “How hard or easy is it for you to take your HIV medications when you do NOT feel good physically?”. Higher scores indicate greater perceived ability.
Adherence Social Support.
Six Likert-style items measured social support for taking medications, going to medical appointments and other tasks related to adherence using four-point scales. Higher scores indicate greater adherence social support.(21, 22)
Psychological Distress.
Psychological distress was assessed by the Global Symptom Index of the 18-item Brief Symptom Inventory (BSI-18).(23) Participants reported the severity of 18 symptoms in the past seven days. Higher total scores equaled greater psychological distress.
Sexual Activity.
The Adolescent Risk Behavior Assessment (ARBA) is designed to assess adolescent self-reported sex behaviors. (24) Participants reported on occurrence of any vaginal or anal sexual activity in the past three months (yes/no) and the occurrence of sex without a condom. Reliability for this assessment of condom use behavior and its comparability to more detailed measures have previously been demonstrated in adolescents.(25)
Substance Use.
The ASSIST is an eight-item questionnaire that screens for all levels of problem substance use. (26) The instrument covers tobacco, alcohol, cannabis, cocaine, amphetamine-type stimulants (including ecstasy), inhalants, sedatives, hallucinogens, opioids and “other drugs.” Participants were asked if they had ever used a substance. If endorsed, participants were asked to report the frequency of use of that substance in the past 3 months: never, once or twice, monthly, weekly, or daily or almost daily.
Plan of Analysis
First, rates of past 7-day ARV SR adherence were compared to rates of EM adherence including percentages of missing data and potential reasons for missing data. SR-EM discrepancy scores were then calculated for participants at each time point and averaged to create a mean SR-EM discrepancy score. Next, bivariate correlations were conducted to determine associations between the mean SR-EM discrepancy score and demographic variables, HIV-related treatment factors, and behavioral health risks. Significant correlates (p < .05) of discrepancy score were then entered simultaneously into a linear regression model to determine which factors had the strongest associations with discrepancy score above and beyond other factors. A second set of bivariate correlations were also conducted to determine associations between SR and EM adherence (averaged across time points) and changes in HIV-related health functioning (i.e., viral load and CD4 count) from the pre-baseline assessment to 14 weeks post-baseline (T4). Another linear regression was then conducted to examine the impact of mean adherence levels (SR and EM adherence, independently) on T4 viral load controlling for the effect of our intervention and pre-baseline viral load. To account for missing data not due to attrition (18%), we used multiple imputation with imputed data derived from the pooled results of 10 separate imputed datasets. We also compared our results from the imputed data set (N = 61) to results using the raw data set (N = 52). Finally, to explore the association between specific levels of adherence and a clinically meaningful indicator of viral load (i.e., viral suppression), a descriptive sensitivity analysis was performed. Analyses were conducted in SPSS 22.0.(27)
RESULTS
Descriptives
Demographics:
The majority of the sample identified as male (77%) with remaining participants identified as female. Their mean age was 22.36 years (SD = 2.48). Many had completed high school (38%) or received some college education (42%); fewer participants had not completed high school (12%) or had graduated from college (8%). Based on reports of sexual behavior and gender, 70% were classified as men who have sex with men (MSM). Most YPLH indicated that their housing situation was stable (85%). For our trial, 34 participants were randomly allocated to the multilevel IMB gaming intervention and 32 to the enhanced standard of care control condition.
HIV-related treatment factors:
Of the total sample, 36% were newly-initiated to ART. Most (94%) were taking ARVs once per day with 6% of YPLH having to take ARVs more than once per day. Twenty-four percent of the sample missed one or more doctor’s appointments in the past three months. One in six YPLH (17%) said they lived with another person who was HIV-positive. Most participants said they “never” (77%) or “rarely” (20%) needed someone to help them read healthcare instructions. Means levels of adherence-related knowledge, motivation, and behaviors ranging from one to five were 3.87 (SD = 1.08), 3.00 (SD = 1.02), and 3.69 (SD = 0.95), respectively; the mean level of adherence-related social support, with scores ranging from two to five, was 4.38 (SD = 0.73).
Behavioral health risks:
Participants had an average amount of psychological distress as measured by the GSI of 1.93 (SD = 0.94). Whereas a majority of YPLH reported having ever used alcohol (85%) or marijuana (64%), fewer YPLH said they had ever used other drugs (e.g., cocaine, opioids, sedatives; 18%). On a scale of “never” (1) to “daily” (5), average past 3-month alcohol use was 2.30 (SD = 1.12) and marijuana use was 2.38 (SD = 1.66). In the past three months, 37% reported having unprotected anal or vaginal sex.
ARV adherence and HIV-related related health functioning:
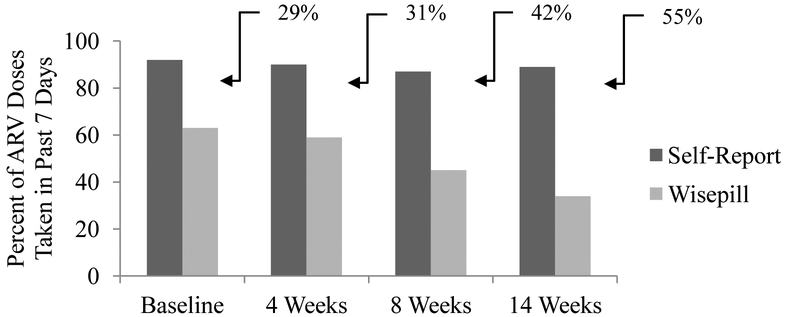
Figure 1 presents rates of past 7-day adherence. SR adherence remained consistent across time points (~90%) and was higher than EM adherence, which saw a decline from T1 to T4, leading to an increase across time points in the discrepancy between SR-EM adherence rates. Missing data also increased from T1 to T4 for EM observations, and could, in part, be attributed to confirmed EM device malfunctions (e.g., low battery) which were observed in 1.6% to 6.5% of observations across assessments. In addition, 19% of YPLH indicated that at some point in the study they stopped using the EM device for a period of time; however, length of non-use could not be ascertained.
Figure 1.
Rates of past 7-day ARV adherence as measured by self-report and Wisepill observations at baseline and four, eight, and 14 weeks post-baseline with discrepancies noted. Self-report n’s = 62, 62, 61, 60 and Wisepill n’s = 59, 57, 55, 50 for baseline, 4 weeks, 8 weeks, and 14 weeks post-baseline, respectively.
At pre-baseline, YPLH had a mean log10 viral load of 3.75 (SD = 1.23), and by 14 weeks post-baseline, this declined to 2.20 (SD = 1.69). At T4, 53% were virally suppressed. CD4 count increased from pre-baseline (M = 401.06, SD = 308.61) to 14 weeks post-baseline (M = 528.19, SD = 382.97).
Associations with SR-Wisepill discrepancy score
Bivariate correlations conducted between mean SR-EM discrepancy score (averaged across time points) and demographic variables, HIV-related treatment factors, and behavioral health risks revealed four significant correlates of the discrepancy score: male gender (vs. female gender; r = - 0.25, p = 0.049), ART experienced (vs. newly initiated; r = −0.43, p < 0.01), ARV treatment adherence motivation (r = 0.27, p = 0.04), and frequency of recent marijuana use (r = 0.31, p = 0.02). When entered simultaneously into a linear regression, all remained significant except for gender; specifically, YPLH newly-initiated to ART had a lower discrepancy score than ART-experienced YPLH (B = 0.22, 95% CI = −0.09 – 0.35, p = 0.001); greater HIV treatment motivation was associated with a lower discrepancy score (B = 0.07, 95% CI = 0.01 - 0.13, p = 0.03); and more frequent marijuana use was associated with a higher discrepancy score (B = 0.05, 95% CI = 0.01 – 0.09, p = 0.02) (see Table I).
Table I.
Multivariate Associations with the Self-Report/Wisepill ARV Adherence Discrepancy Score (N = 62)a
| B | SE | 95% CI | p | |
|---|---|---|---|---|
| Male | −0.13 | 0.08 | −0.28 - 0.02 | .09 |
| ARV-experienced | 0.22 | 0.07 | 0.09 - 0.35 | .001 |
| ARV adherence motivation (low) | 0.07 | 0.03 | 0.01 - 0.13 | .03 |
| Marijuana use (greater frequency) | 0.05 | 0.02 | 0.01 - 0.09 | .02 |
: Model R2 = 0.35
ARV adherence and HIV-related health functioning
Table II presents bivariate correlations between mean adherence levels (T1 through T4; SR and EM) and change in log10 viral load and CD4 count (pre-baseline subtracted from T4 health functioning). As shown, SR and EM adherence had significant negative correlations in similar magnitudes with change in log10 viral load (SR: r = −0.31, p = 0.03; EM: r = −0.29, p = 0.04); that is, higher adherence was associated with a lower change score (a decline in viral load). No significant associations were observed between adherence and change in CD4 count. SR and EM adherence had a small to moderate correlation with each other (r = 0.36; p < 0.01).
Table II.
Correlations between ARV Adherence and Change in Health Outcomesa
| Wisepill Adherence |
||||||
|---|---|---|---|---|---|---|
| Self-Reported Adherence |
Mean (T1-T4) |
p | Change in Viral Load |
p | Change in CD4 Count |
p |
| Mean (T1-T4) | 0.36 | <0.01 | −0.48 | <0.01 | 0.00 | 0.98 |
| Change in Viral Load | −0.31 | 0.03 | -- | -- | −0.29 | 0.04 |
| Change in CD4 Count | −0.01 | 0.95 | −0.29 | 0.04 | -- | -- |
: Ns = 51 to 62 due to missing data. Change in viral load and CD4 count refers to differences between pre-baseline and T4 observations.
To further explore the association between adherence and change in viral load, a linear regression predicting log10 viral load at T4 was conducted with an imputed data set (N = 61) (see Table III). Pre-baseline log10 viral load was included in the model to predict change in viral load. The model also controlled for the effect of the intervention. Results demonstrated that SR and EM adherence were independent predictors of change in viral load (SR: B = −4.10, 95% CI = −6.54 - −1.66, p = 0.001; EM: B = −1.42, 95% CI = −2.68 - −0.16, p = 0.03); a greater degree of adherence of either was associated with a decline in log10 viral load. We also compared our results from the imputed data set of 61 participants who provided follow-up data at the last assessment to results using the raw data set (N = 50). Effects remained similar; however, it is notable that the effect of EM adherence now trended towards significance (B = −1.30, 95% CI = −2.69 - 0.10, p = .07).
Table III.
ARV Adherence Predicting Log10 Viral Load at 14 Weeks Post-Baseline (N = 61)a
| B | SE | 95% CI | p | |
|---|---|---|---|---|
| Intervention | 0.05 | 0.37 | −0.68 - 0.79 | .89 |
| Log10 viral load (pre-baseline) | 0.32 | 0.14 | 0.04 - 0.60 | .03 |
| Self-Report (T1-T4) | −4.10 | 1.24 | −6.54 - −1.66 | .001 |
| Wisepill (T1-T4) | −1.42 | .64 | −2.68 - −0.16 | .03 |
: Model R2 = 0.38
Sensitivity analysis with ARV adherence and viral suppression
We examined associations between various levels of SR and EM adherence and viral suppression/non-suppression at T4 to establish adherence thresholds which could most accurately predict this clinically meaningful indicator of viral load. We discovered that a mean SR adherence (across time points) was a better predictor of viral non-suppression than viral suppression (data not shown). With a threshold of 90% SR adherence, we found that 80% of participants below threshold were virally non-suppressed, while only 65% above the threshold were virally suppressed. In contrast, EM was a better predicator of viral suppression compared to non-suppression. Participants with an EM adherence of greater than or equal to 80% had a rate of viral suppression of 100%; in comparison, among participants with an EM adherence below 80% or had instances of EM device non-use, only 56% were virally non-suppressed.
DISCUSSION
This report describes the discrepancy between two commonly used ARV adherence measurements, self-report (SR) and electronic monitoring (EM), among young people living with HIV (YPLH). Over the course of the 14-week study, SR rates of adherence remained high at all time points, while EM adherence rates declined creating a discrepancy between the two adherence measures. Rates of discrepancy between SR and EM measured adherence ranged from 29% to 55%. Our study adds to the understanding of ARV adherence in that adults and adolescents alike may have a similar degree of discrepancy in SR and EM adherence rates. Our findings also mirrored the consistent decline of EM adherence over time found by Levine and colleagues (2006) among adults. (10) It is possible that participants become less interested in using the EM device as the novelty of the device wears off. Also, some participants reported to staff that they may felt it was a burden to use because the device needed to be recharged, the container was not convenient for travel, or did not hold all of their medications. Although ARV adherence and its associated factors among YPLH have been well examined, our understanding of the intricacies of reported adherence and how adherence rates differ between various measures among YPLH remains understudied. This report is one of the first to describe the discrepancy that grew over a 14-week period between SR and EM measured ARV adherence among YPLH.
We further explored empirically which patient-level factors were associated with the SR-EM discrepancy. Greater adherence discrepancies were found among youth who were ARV experienced, had less motivation for ARV use, and used marijuana frequently. Our study adds to the only other published investigation of factors related to SR-EM discrepancies among persons living with HIV,(10) which was among adults and did not investigate substance use or motivation for ARV adherence. Our study does not provide an estimate of the “true” rates of adherence and substantial prior research indicates that all adherence assessments are prone to error. However, their data suggest that the difference in SR and EM adherence reports are likely to be most pronounced among those who use substances, have less motivation for ARV adherence, and have taken ARV previously. These factors can be taken into account by clinicians and researchers in their consideration of adherence reports.
Although there are no other studies that examine these psychosocial factors in relation to discrepancies of adherence assessment, our findings are consistent with the current literature regarding barriers to ARV adherence among YPLH. Substance use and less motivation have been found to be consistently associated with lower ARV adherence.(4, 5, 14, 19, 22) Frequent marijuana use may lower motivation and inhibit cognitive functioning and memory,(28) factors which could make it difficult for YPLH to accurately report on their ARV adherence and consistently use their EM device. In addition, those who are ARV-experienced may be fatigued by maintaining adherence behaviors, which is consistent with findings from Murphy et al. (2005).(14) On the other hand, ARV-naïve patients, who may feel compelled to follow medical advice out of serious concern for their potentially recent HIV diagnosis, are perhaps more likely to use the EM device consistently and precisely report on their ARV adherence. The magnitude of discrepancies observed in this report may also be attributed to the possibility that participants employed other methods, such as another pillbox, calendars, or alarms, to help them remember to take their medications rather than using the EM device.
Such discrepancies may make adherence tracking more difficult for HIV care providers. Because reports of adherence observations may dictate how clinicians form their treatment plans, variance between types of reports can create confusion on which measure to follow. Screening for these factors (e.g., ART experience, substance use, treatment motivation) may help clinicians or researchers using EM with youth populations understand who is at risk for adherence discrepancies and take steps to better capture their actual level of adherence.
This study also examined the ability of each adherence measure to predict change in HIV-related biological outcomes over time. Both adherence measures were found to be independently associated with a decrease in viral load over time, with the association stronger for SR compared to EM. The magnitude of these effects was consistent with findings from a recent meta-analysis of YPLH samples.(29) Interestingly, no significant associations were observed between adherence and change in CD4 count. This could possibly be attributed to our sample’s young age and relatively high CD4 counts at baseline.
Because both SR and EM were independently associated with viral load over time, further sensitivity analyses were conducted to explore the meaning and clinical utility of each measure of adherence when predicting viral load. Our findings suggest that SR may be a better predictor viral non-suppression, whereas EM may better predict viral suppression. Consistency of device use is likely a factor in this association. YPLH who are motivated to consistently use their device are also likely to be motivated to follow-up with other treatment recommendations (ARV use, clinic attendance) which results in better health outcomes. While measures of SR adherence are good predictors of change in viral load, they may be less reliable as predictors of viral suppression since YPLH may over-report their actual adherence. Overall, we found that each adherence measurement tool can provide valuable insight to understanding young people’s HIV-related health functioning.
Our study has several limitations. Our small sample size limits the generalizability of our findings and may not be representative of all YPLH in the U.S. The measurement period for self-report and EM adherence was limited to the seven days leading up to the scheduled study appointment and may not be an accurate assessment of other weeks. Previous studies among people living with HIV have demonstrated a “white coat effect”, or improved adherence in anticipation of reporting to a clinician, in the days preceding a clinic visit. It is also important to note that the participants included in this study were connected to care. This sample may not be generalizable to all youth living with HIV. The participants in this study were recruited for a small pilot trial of a multilevel gaming intervention. All participants were given and encouraged to use an EM device. Participants may have been more motivated to use the EM device than YPLH in the community not enrolled in a research project.
Despite the limitations to this study, this report is the first to examine discrepancies between SR and EM ARV adherence and to assess factors associated with such discrepancies among YPLH. Our findings provide important insight to the current understanding of adherence and adherence measurement discrepancies, specifically between SR and EM. The use of multiple methods to capture ARV adherence among youth is recommended as each method, SR and EM, is independently associated with change in viral load over time and has a different pattern of association with viral suppression. Furthermore, our findings provide guidance to researchers and clinicians who provide care and seek to develop effective interventions to improve ARV adherence among YPLH. Identification of youth at risk for reporting discrepant adherence rates, particularly those with substance use problems, who have little motivation for ARV adherence or are ARV experienced, is crucial to understanding actual patterns of adherence among YPLH.
ACKNOWLEDGEMENTS
Research reported in this report was supported by the National Institute of Child Health and Human Development (R01HD074846), the National Institute of Mental Health of the National Institutes of Health (T32MH078788), and the Providence/Boston Center for AIDS Research (CFAR) (P30AI042853).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of Interest: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Center for Disease Control and Prevention (CDC). HIV Surveillance Report, 2015. 2015. November 2016. Report No. [Google Scholar]
- 2.Philbin MM, Tanner AE, DuVal A, Ellen J, Kapogiannis B, Fortenberry JD. Linking HIV-positive adolescents to care in 15 different clinics across the United States: Creating solutions to address structural barriers for linkage to care. AIDS care. 2014;26(1): 10.1080/09540121.2013.808730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S-H, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS (London, England). 2014;28(13):1945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reisner SL, Mimiaga MJ, Skeer M, Perkovich B, Johnson CV, Safren SA. A Review of HIV Antiretroviral Adherence and Intervention Studies Among HIV–Infected Youth. Topics in HIV medicine : a publication of the International AIDS Society, USA. 2009;17(1):14–25. [PMC free article] [PubMed] [Google Scholar]
- 5.MacDonell KK, Jacques-Tiura AJ, Naar S, Fernandez MI. Predictors of Self-Reported Adherence to Antiretroviral Medication in a Multisite Study of Ethnic and Racial Minority HIV-Positive Youth. Journal of pediatric psychology. 2016;41(4):419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahana S, Fernandez M, Wilson P, Bauermeister J, PhD MPH, Lee S, et al. Rates and Correlates of Antiretroviral Therapy Use and Virologic Suppression Among Perinatally and Behaviorally HIV-Infected Youth Linked to Care in the United States. Journal of acquired immune deficiency syndromes (1999). 2015;68(2):169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stricker SM, Fox KA, Baggaley R, Negussie E, de Pee S, Grede N, et al. Retention in Care and Adherence to ART are Critical Elements of HIV Care Interventions. AIDS and behavior. 2014;18(5):465–75. [DOI] [PubMed] [Google Scholar]
- 8.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-Report Measures of Antiretroviral Therapy Adherence: A Review with Recommendations for HIV Research and Clinical Management. AIDS and behavior. 2006;10(3):227–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paterson DL, Potoski B, Capitano B. Measurement of adherence to antiretroviral medications. Journal of acquired immune deficiency syndromes (1999). 2002;31 Suppl 3:S103–6. [DOI] [PubMed] [Google Scholar]
- 10.Levine AJ. Adherence to antiretroviral medications in HIV: Differences in data collected via self-report and electronic monitoring. Health psychology. 2006;25(3):329–35. [DOI] [PubMed] [Google Scholar]
- 11.Shi L, Liu J, Koleva Y, Fonseca V, Kalsekar A, Pawaskar M. Concordance of Adherence Measurement Using Self-Reported Adherence Questionnaires and Medication Monitoring Devices. PharmacoEconomics. 2010;28(12):1097–107. [DOI] [PubMed] [Google Scholar]
- 12.Farley J Assessment of adherence to antiviral therapy in HIV-infected children using the Medication Event Monitoring System, pharmacy refill, provider assessment, caregiver self-report, and appointment keeping. Journal of acquired immune deficiency syndromes (1999).33(2). [DOI] [PubMed] [Google Scholar]
- 13.Murphy DA, Wilson CM, Durako SJ, Muenz LR, Belzer M. Antiretroviral medication adherence among the REACH HIV-infected adolescent cohort in the USA. AIDS care. 2001;13(1):27–40. [DOI] [PubMed] [Google Scholar]
- 14.Murphy DA, Belzer M, Durako SJ, Sarr M, Wilson CM, Muenz LR. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Archives of Pediatrics & Adolescent Medicine. 2005;159(8):764–70. [DOI] [PubMed] [Google Scholar]
- 15.Chenneville T, Machacek M, St. John Walsh A, Emmanuel P, Rodriguez C. Medication Adherence in 13- to 24-Year-Old Youth Living With HIV. Journal of the Association of Nurses in AIDS Care. 2017;28(3):383–94. [DOI] [PubMed] [Google Scholar]
- 16.Gross IM, Hosek S, Richards MH, Fernandez MI. Predictors and Profiles of Antiretroviral Therapy Adherence Among African American Adolescents and Young Adult Males Living with HIV. AIDS patient care and STDs. 2016;30(7):324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lall P, Lim SH, Khairuddin N, Kamarulzaman A. Review: An urgent need for research on factors impacting adherence to and retention in care among HIV-positive youth and adolescents from key populations. Journal of the International AIDS Society. 2015;18(2Suppl 1):19393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher JD. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health psychology. 2006;25(4):462–73. [DOI] [PubMed] [Google Scholar]
- 20.Team LP. The LifeWindows Information Motivation Behavioral Skills ART Adherence Questionnaire (LW-IMB-AAQ). . Storrs, CT: Center for Health, Intervention, and Prevention; 2006. [Google Scholar]
- 21.Martinez J, Harper G, Carleton RA, Hosek S, Bojan K, Glum G, et al. The Impact of Stigma on Medication Adherence Among HIV-Positive Adolescent and Young Adult Females and the Moderating Effects of Coping and Satisfaction with Health Care. AIDS patient care and STDs. 2012;26(2):108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naar-King S, Templin T, Wright K, Frey M, Parsons JT, Lam P. Psychosocial factors and medication adherence in HIV-positive youth. AIDS patient care and STDs. 2006;20(1):44–7. [DOI] [PubMed] [Google Scholar]
- 23.Derogatis LR. Brief Symptom Inventory: BSI; Administration, scoring, and procedures manual: Pearson; 1993. [Google Scholar]
- 24.Donenberg GR, Emerson E, Bryant FB, Wilson H, Weber-Shifrin E. Understanding AIDS-Risk Behavior Among Adolescents in Psychiatric Care: Links to Psychopathology and Peer Relationships. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(6):642–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houck C, Stewart A, Brown LK, Group PSS. Condom use measurement in adolescent HIV prevention research: Is briefer better? Int Public Health J. 2012;4(4):369–76. [Google Scholar]
- 26.Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, et al. Validation of the alcohol, smoking and substance involvement screening test (ASSIST). Addiction. 2008;103(6):1039–47. [DOI] [PubMed] [Google Scholar]
- 27.IBM Corp. IMB SPSS Statistics for Windows. Version 22.0 ed. Armonk NY: IBM Corp.; 2013. [Google Scholar]
- 28.Bonn-Miller MO, Oser ML, Bucossi MM, Trafton JA. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. Journal of Behavioral Medicine. 2014;37(1):1–10. [DOI] [PubMed] [Google Scholar]
- 29.Kahana SY, Rohan J, Allison S, Frazier TW, Drotar D. A Meta-Analysis of Adherence to Antiretroviral Therapy and Virologic Responses in HIV-Infected Children, Adolescents, and Young Adults. AIDS and behavior. 2013;17(1):41–60. [DOI] [PubMed] [Google Scholar]