Abstract
Objectives:
To assess outcomes after secondary surgical resection in patients with recurrent uterine leiomyosarcoma (uLMS).
Methods:
We retrospectively identified all patients who had no evidence of disease after initial surgery for uLMS, who underwent surgery for a first recurrence at our institution between 1/1991-10/2013. We excluded patients who received any therapy for recurrence prior to secondary resection, and patients who underwent surgery soon after morcellation [of presumed benign fibroids] showed widespread disease. Overall survival (OS) was determined from time of first recurrence to death or last follow-up.
Results:
We identified 62 patients: 29 with abdominal/pelvic recurrence only, 30 with lung recurrence only, 3 with both. Median time to first recurrence was 18 months (95% CI: 13.3-23.3): 15.8 months (95% CI: 13.0-18.6) abdominal/pelvic recurrence; 24.1 months (95% CI: 14.5-33.7) lung-only recurrence (p=0.03). Median OS was 37.7 months (95% CI: 25.9-49.6) abdominal/pelvic recurrence; 78.1 months (95% CI: 44.8-11.4) lung recurrence (p = 0.02). Complete gross resection (CGR) was achieved in 58 cases (93%), with gross residual ≤1cm in 2 (3.5%) and >1cm in 2 (3.5%). Median OS based on residual disease was 54.1 months (95% CI: 24.9-83.3), 38.7 months (95% CI: NE), 1.7 months (95% CI: NE), respectively (P<0.001). In cases with CGR, neither adjuvant radiation (N=9), chemotherapy (N=8) nor hormonal therapy (N=10) was associated with improved OS.
Conclusions:
Secondary surgical resection of recurrent uLMS is reasonable in patients with a high probability of achieving CGR. Lung-only recurrences were associated with more favorable outcome. Following CGR, additional therapy may not offer benefit.
INTRODUCTION
Uterine leiomyosarcomas (uLMS) are rare smooth-muscle tumors that account for only 1% of all uterine malignancies, but approximately 70% of all uterine sarcomas [1]. The median age at diagnosis is 51 years, and most women will have disease confined to the uterus and/or cervix (Stage 1) [2]. These tumors are aggressive, and relatively resistant to both chemotherapy and radiation therapy [3–6]. Surgery remains the mainstay of treatment even for patients with distant metastases [7]. A total hysterectomy with intact uterine removal is recommended, with a goal of achieving complete gross resection (CGR) [8]. However, even after aggressive surgical resection, more than 90% of patients with advanced-stage disease will recur or progress [9, 10]. The majority of recurrences are in the abdomen and pelvis, but metastases to the lungs are also common [11].
The prognosis for patients with recurrent disease is poor, with 5-year overall survival (OS) of 40-60% [9, 12]. Management options in this setting are limited and poorly defined. Patients will often receive chemotherapy, typically gemcitabine and docetaxel (based on reportedly modest response rates) [13, 14]. Radiation therapy also appears to yield a limited response [15]. The benefits of secondary surgical resection have been demonstrated in other soft-tissue sarcomas [16–18]. Retrospective studies suggest that surgical resection for recurrent uLMS may offer a survival advantage in appropriately chosen cases [11, 19]. In a select group of patients from a slightly heterogenous cohort treated at our institution, Leitao et al. showed that optimal resection of recurrent uLMS may provide long-term survival [11]. Additionally, resection of isolated lung metastases in patients with recurrent uLMS has been associated with 5-year survival of approximately 40% [20, 21]. The objective of this study was to assess outcomes following secondary surgical resection in patients with recurrent uLMS.
METHODS
This study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center. We identified patients who underwent surgical resection for a first recurrence of uLMS between January 1991 and October 2013, using databases maintained by the Department of Surgery and Division of Gynecology. This dataset includes some cases reported in our earlier publication (which included cases from 1/1/91 to 3/30/01): 8 with abdominal/pelvic recurrence; 14 with lung-only recurrence [11]. Inclusion of these earlier cases permitted a more robust assessment of longer-term outcomes; however, only cases meeting our current, stricter inclusion criteria were considered in this analysis. The medical records of all patients meeting our current inclusion criteria were reviewed. Abstracted data included patient age, date of initial diagnosis, tumor histology and grade, residual tumor after all operations, any post-resection therapy, dates and sites of all recurrences, and disease status at last follow-up. The diagnosis of recurrent high-grade uLMS was made in patients with known history of primary uLMS. All patients underwent surgical resection for their first recurrence at our institution. We included only patients who had CGR at initial surgery for uLMS and were apparently disease-free following initial surgery. Only lung resections—not ablations—were included in the lung recurrence group. We excluded patients who received any non-surgical therapy for recurrence prior to their secondary resection, as well as patients who underwent surgery soon after morcellation showed widespread disease. The amount of residual disease was determined based on operative reports. OS was calculated from the date of first recurrence to the date of death or last follow-up. Survival was estimated using the Kaplan-Meier method, and estimates were compared using the log-rank test. All statistical tests were performed using SPSS 25.0 software.
RESULTS
The clinical characteristics of all 62 patients, and sites of recurrence, are listed in Table 1. The median time to first recurrence after initial diagnosis of uLMS was 18.3 months (95% CI: 13.3-23.3 months). There were 29 patients with abdominal/pelvic recurrences, 30 with lung recurrences, and 3 with both abdominal/pelvic and lung recurrences. Following surgery for recurrence, 10 patients (16%) received postoperative chemotherapy, 10 (16%) received radiation therapy, and 10 (16%) received hormonal therapy, at the discretion of their treating physicians.
Table 1.
Clinical characteristics of the cohort of patients with recurrent uLMS (n=62)
| Variable | N (%) |
|---|---|
| Median age at diagnosis (yrs) | |
| Median (range) | 53.5 (32.0-74.0) |
| FIGO Stage at diagnosis | |
| 1 | 45 (71) |
| 2 | 7 (11) |
| 3 | 2 (3) |
| 4 | 4 (6) |
| N/A | 4 (6) |
| Adjuvant chemotherapy following primary surgery | 19 (31) |
| Doxorubicin | 1 (5) |
| Doxorubicin/Ifosfamide | 1 (5) |
| Gemcitabine/Docetaxel or Paclitaxel | 10 (53) |
| Gemcitabine/Docetaxel/Doxorubicin | 2 (10) |
| Cisplatin/Paclitaxel | 1 (5) |
| Cisplatin/Ifosfamide | 1 (5) |
| N/A | 2 (10) |
| Adjuvant radiation following primary surgery | 7 (11) |
| Whole Pelvic | 7 (100) |
| Hormonal therapy following primary surgery | 3 (4.8) |
| Aromatase Inhibitor | 3 (100) |
| Median time to recurrence (months) | |
| Median (95%CI) | 18.3 (13.3-23.3) |
| Median age at recurrence (years) (range) | 57.0 (35.2-76.9) |
| Residual at recurrence surgery | |
| None | 58 (94) |
| Gross | 4 (6.5) |
| <1cm | 2 (50) |
| ≥1cm | 2 (50) |
| Adjuvant chemotherapy following recurrence surgery | 10 (16) |
| Doxorubicin/Ifosfamide | 4 (40) |
| Gemcitabine | 2 (20) |
| Gemcitabine/Docetaxel or Paclitaxel | 4 (40) |
| Mesna, Doxorubicin, Ifosfamide, Dacarbazine | 1 (10) |
| Adjuvant radiation following recurrence surgery | 10 (16) |
| Whole Pelvic | 10 (100) |
| Hormonal therapy following recurrence surgery | 10 (16) |
| Aromatase Inhibitor | 6 (60) |
| Selective Estrogen Receptor Modulators | 1 (10) |
| Progesterone | 3 (30) |
| Overall Survival | |
| Median (95%CI) | 54.2 (25.8-82.4) |
| Dead of Disease | 45 (71) |
The clinical characteristics of patients with abdominal/pelvic recurrences (n=29) are listed in Table S1. The median time to first recurrence after initial diagnosis of uLMS was 15.8 months (95% CI: 13.0-18.6-months). Twenty-three of the 29 (79%) abdominal/pelvic recurrences were detected by routine radiographic surveillance. The remaining 6 patients with abdominal/pelvic recurrences presented with clinical symptoms or had an abnormal clinical examination which prompted imaging. Of the 32 patients with abdo/pelvic disease, 22 patients had solitary lesions on pre-operative imaging; 4 patients had 2 lesions; 6 patients had multiple (>2) implants. All except 1 patient underwent exploratory laparotomy for resection of recurrent disease; 1 patient underwent laparoscopic resection of liver lesions. Ten patients required some form of bowel surgery, and 5 required complex urologic procedures (partial cystectomy, ureteral re-implant, or other procedure). In 7 patients with a single site of recurrence, a multi-disciplinary team of surgeons was required for successful resection. Twenty-six patients had no residual disease following surgery for recurrence; 3 patients had gross disease (in 1 patient <1cm; in 2 patients ≥1cm). Seven patients received postoperative chemotherapy, 9 received radiation therapy, and 5 received hormonal therapy. Of these 7, 3 patients had a single site recurrence and the remaining 4 had multiple (>2) lesions at secondary surgery. Median OS was 37.7 months (95% CI: 25.9 – 49.6 months).
The clinical characteristics of patients with lung-only recurrences (n=30) are listed in Table S2. The median time to first recurrence after initial diagnosis of uLMS was 24.1 months (95% CI: 14.5-33.7 months). All 30 patients were free of gross residual disease after surgical resection. Three patients received chemotherapy and 1 received radiation therapy. Median OS in this group was 78.1 months (range, 44.8-111.4 months). Twenty-four patients had unilateral lung lesions and 6 had bilateral lesions. Patients with resected unilateral lesions had a median OS of 80.9 months (95% CI: 54.1-107.7); those with bilateral lesions had a median OS of 30.7 months (95% CI: 3.1-58.3). This difference in median OS was not statistically significant (Figure S1; p=0.13). The median OS for patients with lung-only recurrences was 78.1 months (95% CI: 44.8-111.4 months), compared to 37.7 months (95% CT25.9-49.6 months) for those with abdominal/pelvic recurrences (p=0.02) (Figure 1).
Figure 1.
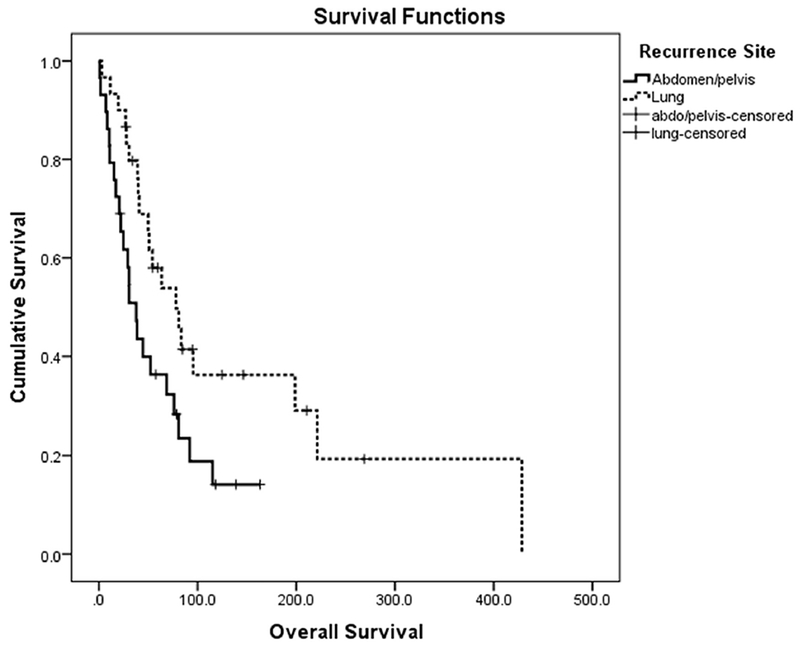
Overall survival based on site of recurrence (P=0.02)
The clinical characteristics of patients with both abdominal/pelvic and lung recurrences (n=3) are listed in Table S3. The median time to first recurrence after initial diagnosis was 65.7 months (95% CI: 0-145.6 months). Two patients (66%) had no residual disease after surgery; 1 patient (33%) had gross disease <1cm. Median OS for this group was not estimable. Because of their clinical similarities to patients with abdominal/pelvic recurrences, these 3 patients were included with the 29 patients in the abdominal/pelvic recurrence group for additional analyses.
The extent of surgical resection was a highly significant predictor of survival (Figure 2). Patients who had no gross residual disease (n=58) had better survival compared to patients whose disease was not amenable to complete resection (2 patients <1cm; 2 patients ≥1cm). The median OS based on residual disease (CGR, <1cm, >1cm) was 54.1 months (95% CI: 24.9-83.3), 38.7 months (95% CI: NE), and 1.7 months (95% CI: NE), respectively (P<0.001) (Table 2). Chemotherapy, radiation therapy, or hormonal therapy following surgery—irrespective of whether there was residual disease upon completion of surgery—demonstrated no association with improved survival (Table 3).
Figure 2.
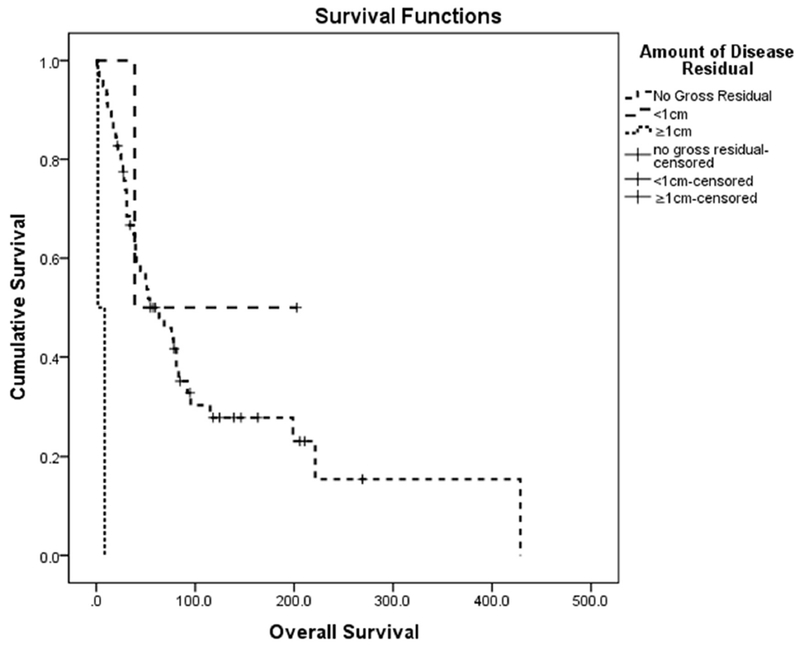
Overall survival based on residual disease following secondary surgery for recurrent uLMS (P<0.001)
Table 2.
Survival outcomes in patients with and without residual disease following secondary surgery for recurrent uLMS (n=58).
| Variable | N | Median OS (months) (95% CI) | P value |
|---|---|---|---|
| Residual disease | |||
| None | 58 | 54.1 (24.9-83.3) | |
| Any | 4 | 8.5 (0-44.7) | 0.5a |
| <1cm | 2 | 38.7 (NE) | |
| > 1cm | 2 | 1.7 (NE) | <0.001b |
- test of equality of survival distributions for none versus any residual disease
- test of equality of survival distributions for none versus ≥ 1cm residual disease
OS – Overall Survival
NE – Not Estimable
Table 3.
Survival outcomes based on treatment following secondary surgery in patients with recurrent uLMS (n=62) and in those with no gross residual following secondary surgery (n=58).
| Entire Cohort | No gross residual | |||||
|---|---|---|---|---|---|---|
| N | Median OS (months) (95% CI) | P value | N | Median OS (months) (95% CI) | P value | |
| Chemotherapy | ||||||
| No | 52 | 76.1 (46.7-105.5) | <0.001 | 50 | 76.1 (41.3-110.9) | 0.001 |
| Yes | 10 | 20.5 (3.8-37.2) | 8 | 20.5 (5.6-35.5) | ||
| Radiation | ||||||
| No | 52 | 63.5 (31.5-95.5) | 0.18 | 49 | 63.5 (28.1-98.9) | 0.2 |
| Yes | 10 | 38.7 (27.9-49.5) | 9 | 44.7 (24.3-65.1) | ||
| Hormonal Therapy | ||||||
| No | 52 | 52.3 (26.5-78.1) | 0.94 | 48 | 54.1 (29.7-78.6) | 0.96 |
| Yes | 80.9 (NE) | 10 | 80.9 (NE) | |||
NE – Not Estimable
DISCUSSION
Uterine LMS is a rare disease with a poor prognosis. In the setting of uterus-limited, completely resected disease, nearly half of women are cured by surgery alone. Others will develop recurrent disease and are generally not considered to be curable. Management options for recurrent disease include additional resection, ablation, chemotherapy, radiation therapy, hormonal therapy, or a combination of these. Treatment decisions are based on the number of metastases and sites of metastases, the patient’s disease-free interval, disease burden, organ function, prior therapies, and patient preferences [22]. The purpose of this study was to assess outcomes following secondary surgical resection in patients with recurrent uLMS, who were disease-free after initial surgery, and underwent a second surgery for their first recurrence, prior to receiving any additional therapy.
Several studies have evaluated the feasibility of resecting metastases in patients with recurrent uLMS [11, 20, 21]. Guintoli et al. studied 128 patients with recurrent uLMS and demonstrated that secondary cytoreductive surgery was associated with significantly improved disease-specific survival (p<0.001) [19]. They also reported that optimal secondary surgical resection was associated with a significantly improved outcome. In the current study, we compared the outcomes of patients who underwent secondary surgery resulting in no gross residual disease, to the outcomes of patients who had gross residual disease after surgery. CGR was achieved in 58 cases (93%), with gross residual disease ≤1cm in 2 (3.5%) cases and >1cm in 2 (3.5%) cases. The median OS based on residual disease (CGR, gross residual <1cm, gross residual >1cm) was 54.1 months (95% CI: 24.9-83.3), 38.7 months (95% CI: NE), and 1.7 months (95% CI: NE), respectively (P<0.001). Consistent with Giuntoli et al., complete secondary surgical resection in patients with recurrent uLMS was associated with a significant improvement in OS [19]. Rigorous evaluation should determine the appropriateness of secondary surgery for each patient, as disease should be amenable to complete resection. In our study, 22 patients with abdominal-pelvic disease had solitary lesions on pre-operative imaging; 4 patients had 2 lesions and 6 patients had multiple (>2) implants. Even for patients who had solitary lesions, 5 patients required complex urologic procedures and 10 required intestinal resection. In 7 patients with a single site of recurrence, a multi-disciplinary team of surgeons was required for successful resection, emphasizing the surgical complexity of these cases.
We also compared patients with abdominal/pelvic recurrence to those with lung-only recurrence. The median time to first recurrence was 15.8 months (95% CI: 13.0-18.6) for those with abdominal/pelvic recurrence versus 24.1 months (95% CI: 14.5-33.7) for those with lung-only recurrence (p=0.03). Median OS was 37.7 months (95% CI: 25.9-49.6) for abdominal/pelvic recurrence compared to 78.1 months (95% CI: 44.8-11.4) for lung-only recurrence (p = 0.02). The more favorable outcomes in patients with lung-only recurrences is interesting and, perhaps speaks to the biology of the tumor rather than anatomic site of recurrence. Most patients (n=24; 80%) with lung metastases had unilateral lesions and showed a trend towards longer median OS, compared to those with bilateral lesions (80.9 months vs. 30.7 months; p=0.13). The lack of statistical significance is likely related to the fact that there were not enough cases in the current study to demonstrate significance. At our institution, patients are typically offered surgery for potentially resectable recurrences. In several retrospective reviews of recurrent uLMS and other soft-tissue sarcomas, surgical resection of pulmonary metastases has been shown to offer a survival benefit. Patients with lung metastases amenable to surgical resection should be identified and offered surgery, even in the setting of bilateral lesions [20, 23–26]. All our patients underwent surgical resection, but other studies have reported success with use of ablation techniques in select patients [27, 28]. The goal of this series was to highlight the benefit of thoracic surgery in this group of patients.
Chemotherapy in uLMS has been largely reserved for recurrent or metastatic disease that is not amenable to complete resection. Gemcitabine and docetaxel have demonstrated the highest objective response rates as first-line or second-line treatment for metastatic disease, with an OS of 14.7 months in second-line treatment [13]. More recently, the monoclonal antibody olaratumab, in combination with doxorubicin, was approved by the Food and Drug Administration (FDA) for the treatment of advanced sarcomas. Median OS was 26.5 months in patients randomized to doxorubicin-olaratumab versus 14.7 months in patients receiving doxorubicin alone (95% CI: 0.3-0.71, p=0.0003) [29]. Agents such as trabectedin, dacarbazine, ifosfamide, temozolomide and pazopanib have also shown activity in advanced uLMS [30, 31]. In our study, 10 patients (15.6%) received chemotherapy, 10 (15.6%) received radiation therapy, and 10 (15.6%) received hormonal therapy following surgery. Consistent with other reports, patients who received chemotherapy, radiation or hormonal treatment following complete surgical resection did not have improved outcomes compared to those treated with surgical resection alone (Table 3). Similarly, Giuntoli et al. reported that neither adjuvant chemotherapy nor radiation therapy was associated with improved survival after secondary surgical resection (HR 1.92) [32]. As such, the use of chemotherapy, radiation, and hormonal blockade should be considered only if complete surgical resection is not feasible, or in the setting of persistent residual disease after attempted surgical resection.
Importantly, 23 of 29 (79%) of the abdominal/pelvic recurrences were detected by routine radiographic surveillance. The remaining 6 patients presented with clinical symptoms or had an abnormal clinical examination which then prompted imaging. This underscores the importance of routine surveillance in the management of uLMS. The optimal type of imaging, and interval between scans, have not been studied in uLMS; however, at our institution, surveillance in asymptomatic patients typically consists of a CT scan of the chest, abdomen and pelvis every 3 months.
In conclusion, surgery remains an important intervention for uLMS, even in the recurrent setting. Secondary surgical resection of recurrent uLMS is a reasonable option if there is a high probability of achieving CGR. Lung-only recurrences appear to be associated with more favorable outcomes, even in the setting of bilateral lung metastases. Following complete resection, additional systemic or radiation therapy may not offer benefit, and patients can return to surveillance alone. Systemic cytotoxic therapies should be saved for the treatment of unresectable metastatic disease.
Supplementary Material
Highlights.
Data regarding optimal management of recurrent uterine leiomyosarcoma are scant
Secondary surgical resection should be considered if there is high probability of CGR
Compared to abdominal/pelvic recurrences, lung-only recurrences have better outcomes
Following complete resection, systemic therapy or radiation may not offer benefit
Acknowledgments
Funding: This study was funded in part through the NIH/NCI Support Grant P30 CA008748 (CORE GRANT - Craig Thompson).
Disclosures:
Dr. Long Roche reports other* from Intuitive Surgical Inc., outside the submitted work (*airfare to a survivorship conference, where she spoke).
Dr. Chi reports personal fees from Bovie Medical Co., personal fees from Verthermia Inc., personal fees from C Surgeries, from Intuitive Surgical Inc., outside the submitted work.
Dr. Abu-Rustum reports grants from Stryker/Novadaq, grants from Olympus, grants from GRAIL, outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
None of the authors declare conflicts of interest.
REFERENCES
- 1.Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: Epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol 2017;145:208–216. [DOI] [PubMed] [Google Scholar]
- 2.Leitao MM, Sonoda Y, Brennan MF, et al. Incidence of lymph node and ovarian metastases in leiomyosarcoma of the uterus. Gynecol Oncol 2003;91:209–212. [DOI] [PubMed] [Google Scholar]
- 3.Sutton G, Blessing JA, Malfetano JH. Ifosfamide and doxorubicin in the treatment of advanced leiomyosarcomas of the uterus: a Gynecologic Oncology Group study. Gynecol Oncol 1996;62:226–229. [DOI] [PubMed] [Google Scholar]
- 4.Omura GA, Major FJ, Blessing JA, et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 1983;52:626–632. [DOI] [PubMed] [Google Scholar]
- 5.Muss HB, Bundy B, DiSaia PJ, et al. Treatment of recurrent or advanced uterine sarcoma. A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the Gynecologic Oncology Group). Cancer 1985;55:1648–1653. [DOI] [PubMed] [Google Scholar]
- 6.Piver MS, Lele SB, Marchetti DL, et al. Effect of adjuvant chemotherapy on time to recurrence and survival of stage I uterine sarcomas. J Surg Oncol 1988;38:233–239. [DOI] [PubMed] [Google Scholar]
- 7.Burt BM, Ocejo S, Mery CM, et al. Repeated and aggressive pulmonary resections for leiomyosarcoma metastases extends survival. Ann Thorac Surg 2011;92:1202–1207. [DOI] [PubMed] [Google Scholar]
- 8.Dinh TA, Oliva EA, Fuller AF Jr, et al. The treatment of uterine leiomyosarcoma. Results from a 10-year experience (1990–1999) at the Massachusetts General Hospital. Gynecol Oncol 2004;92:648–652. [DOI] [PubMed] [Google Scholar]
- 9.Zivanovic O, Leitao MM, Iasonos, et al. Stage-specific outcomes of patients with uterine leiomyosarcoma: a comparison of the international Federation of gynecology and obstetrics and american joint committee on cancer staging systems. J Clin Oncol 2009;27:2066–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leitao MM Jr, Zivanovic O, Chi DS, et al. Surgical cytoreduction in patients with metastatic uterine leiomyosarcoma at the time of initial diagnosis. Gynecol Oncol 2012;125:409–413. [DOI] [PubMed] [Google Scholar]
- 11.Leitao MM, Brennan MF, Hensley M, et al. Surgical resection of pulmonary and extrapulmonary recurrences of uterine leiomyosarcoma. Gynecol Oncol 2002;87:287–294. [DOI] [PubMed] [Google Scholar]
- 12.Zivanovic O, Jacks LM, Iasonos A, et al. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer 2012;118:660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hensley ML, Blessing JA, Mannel R, et al. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecol Oncol 2008;109:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hensley ML, Enserro D, Hatcher H, et al. Adjuvant Gemcitabine Plus Docetaxel Followed by Doxorubicin Versus Observation for High-Grade Uterine Leiomyosarcoma: A Phase III NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol 2018: JCO1800454 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed NS, Mangioni C, Malmstrom H, et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur J Cancer 2008;44:808–818. [DOI] [PubMed] [Google Scholar]
- 16.Daigeler A, Zmarsly I, Hirsch T, et al. Long-term outcome after local recurrence of soft tissue sarcoma: a retrospective analysis of factors predictive of survival in 135 patients with locally recurrent soft tissue sarcoma. Br J Cancer 2014;110:1456–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bautista N, Su W, O’Connell TX. Retroperitoneal soft-tissue sarcomas: prognosis and treatment of primary and recurrent disease. Am Surg 2000;66:832–836. [PubMed] [Google Scholar]
- 18.Stojadinovic A, Yeh A, Brennan MF. Completely resected recurrent soft tissue sarcoma: primary anatomic site governs outcomes. J Am Coll Surg 2002;194:436–447. [DOI] [PubMed] [Google Scholar]
- 19.Giuntoli RL 2nd, Garrett-Mayer E, Bristow RE, et al. Secondary cytoreduction in the management of recurrent uterine leiomyosarcoma. Gynecol Oncol 2007;106:82–88. [DOI] [PubMed] [Google Scholar]
- 20.Levenback C, Rubin SC, McCormack PM, et al. Resection of pulmonary metastases from uterine sarcomas. Gynecol Oncol 1992;45:202–205. [DOI] [PubMed] [Google Scholar]
- 21.Anraku M, Yokoi K, Nakagawa K, et al. Pulmonary metastases from uterine malignancies: results of surgical resection in 133 patients. J Thorac Cardiovasc Surg 2004;127:1107–1112. [DOI] [PubMed] [Google Scholar]
- 22.Reed NS. The management of uterine sarcomas. Clin Oncol (R Coll Radiol) 2008;20:470–478. [DOI] [PubMed] [Google Scholar]
- 23.McCormack PM, Martini N. The changing role of surgery for pulmonary metastases. Ann Thorac Surg 1979;28:139–145. [DOI] [PubMed] [Google Scholar]
- 24.Pfannschmidt J, Klode J, Muley T, et al. Pulmonary metastasectomy in patients with soft tissue sarcomas: experiences in 50 patients. Thorac Cardiovasc Surg 2006;54:489–492. [DOI] [PubMed] [Google Scholar]
- 25.Temple LK, Brennan MF. The role of pulmonary metastasectomy in soft tissue sarcoma. Semin Thorac Cardiovasc Surg 2002;14:35–44. [DOI] [PubMed] [Google Scholar]
- 26.Weiser MR, Downey RJ, Leing DH, et al. Repeat resection of pulmonary metastases in patients with soft-tissue sarcoma. J Am Coll Surg 2000;191:184–190. [DOI] [PubMed] [Google Scholar]
- 27.Jones RL, McCall J, Adam A, et al. Radiofrequency ablation is a feasible therapeutic option in the multi modality management of sarcoma. Eur J Surg Oncol 2010;36:477–482. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Matsumine A, Yamakado K, et al. Lung radiofrequency ablation in patients with pulmonary metastases from musculoskeletal sarcomas [corrected]. Cancer 2009;115:3774–3781. [DOI] [PubMed] [Google Scholar]
- 29.Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demetri GD, von Mehren M, Jones RL, et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J Clin Oncol 2016;34:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–1886. [DOI] [PubMed] [Google Scholar]
- 32.Giuntoli RL 2nd, Metzinger DS, DiMarco CS, et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy. Gynecol Oncol 2003;89:460–469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


