Summary
The roundworm C. elegans reversibly arrests larval development during starvation [1], but extended early-life starvation reduces reproductive success [2, 3]. Maternal dietary restriction (DR) buffers progeny from starvation as young larvae, preserving reproductive success [4]. However, the developmental basis of reduced fertility following early-life starvation is unknown, and it is unclear how maternal diet modifies developmental physiology in progeny. We show here that extended starvation in first-stage (L1) larvae followed by unrestricted feeding results in a variety of developmental abnormalities in the reproductive system, including proliferative germ-cell tumors and uterine masses that express neuronal and epidermal cell-fate markers. We found that maternal DR and reduced maternal insulin/IGF signaling (IIS) increase oocyte provisioning of vitellogenin lipoprotein, reducing penetrance of starvation-induced abnormalities in progeny, including tumors. Furthermore, we show that maternal DR and reduced maternal IIS reduce IIS in progeny. daf-16/FoxO and skn-1/Nrf, transcriptional effectors of IIS, are required in progeny for maternal DR and increased vitellogenin provisioning to suppress starvation-induced abnormalities. daf-16/FoxO activity in somatic tissues is sufficient to suppress starvation-induced abnormalities, suggesting cell-nonautonomous regulation of reproductive system development. This work reveals that early-life starvation compromises reproductive development and that vitellogenin-mediated intergenerational insulin/IGF-to-insulin/IGF signaling mediates adaptation to nutrient availability.
Keywords: insulin, dietary restriction, starvation, vitellogenin, tumors, maternal provisioning
Graphical Abstract
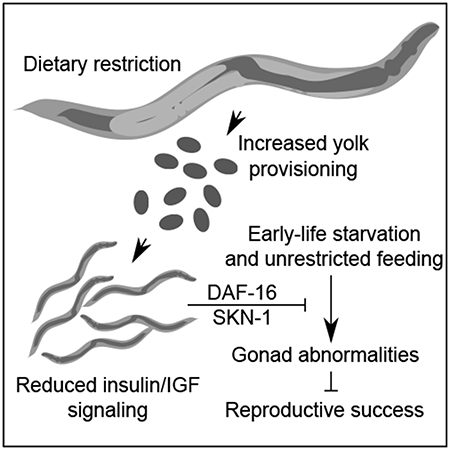
eTOC Blub:
Jordan et al. show that early-life starvation causes reproductive abnormalities in C. elegans, but that maternal dietary restriction increases progeny starvation resistance. Dietary restriction and reduced insulin/IGF signaling increase maternal vitellogenin provisioning which activates daf-16/FoxO in progeny to confer protection from starvation.
Results and Discussion
Early-life starvation causes reproductive abnormalities
To determine how extended larval starvation compromises reproductive success, we compared early adult worms starved for 8 days as L1-stage larvae to control adults that were starved briefly as L1 larvae (~12 hr for synchronization), hereafter referred to as “starved” and “control”, respectively. Approximately half of the starved worms displayed prominent abnormalities in their reproductive system despite being well fed after the starvation period into early adulthood (Figures 1A–1C). These abnormalities were highly variable and included endomitotic oocytes (Emo), abnormally shaped oocytes and embryos, gonad-migration and egg-laying defects, and internal hatching of progeny (Figures S1A–S1C). The most common abnormalities fell on a spectrum ranging from gonads enlarged with proliferative germ cell nuclei (identified with the germ-cell specific marker Pmex-5::H2B::mCherry [5] and referred to as “mex-5+”) to uterine masses consisting of largely mex-5- cells (Figures 1B, S1A and S1C). We collectively refer to these developmental abnormalities of the reproductive system as “gonad abnormalities”. The mex-5+ gonad enlargements resembled proximal germ-cell tumors caused by “latent niche” signaling [6, 7], and the mex-5- uterine masses were disorganized and appeared to contain differentiated cells reminiscent of teratoma-like tumors that form in aging adults [8]. The majority of uterine masses expressed elt-1 and unc-119 reporter genes, embryonic markers of epidermal and neuronal cell fates, respectively, confirming somatic differentiation (Figures 1D and E). We observed similar abnormalities in three wild isolates subjected to 8 d L1 starvation (Figure S1D). Individuals with abnormalities produced substantially smaller broods (Figure 1F), consistent with developmental abnormalities limiting reproductive success.
Figure 1. Early-life starvation followed by unrestricted feeding results in reproductive abnormalities.
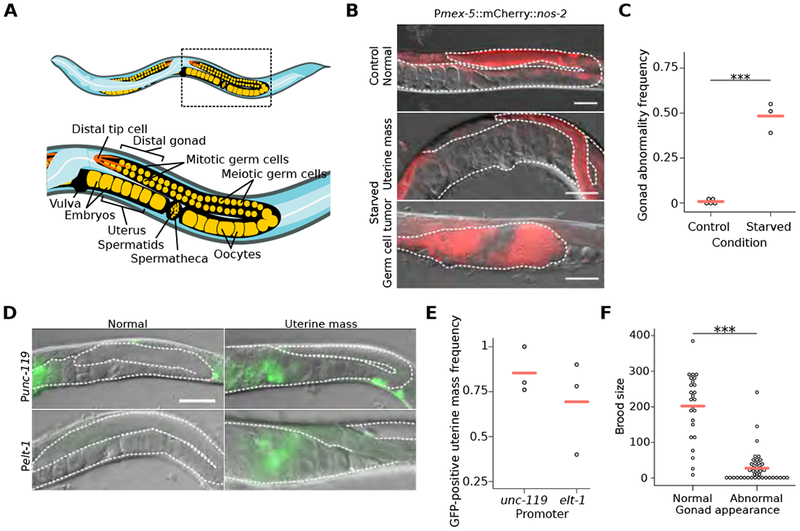
(A) Cartoon depicting organization of posterior gonad arm of an adult C. elegans hermaphrodite. Boxed area is enlarged to show region assessed for gonad abnormalities. (B) Representative images of control worms and previously starved adults with a germ-cell tumor or uterine mass. DIC and fluorescent images of the germ-cell reporter Pmex-5::H2B::mCherry (naSi2) are overlaid. (C) Frequency of all gonad abnormalities; at least 50 worms per condition per replicate. (D) Representative images of adults after L1 starvation with or without uterine masses. (E) Frequency of GFP-positive uterine masses; at least 20 masses were scored per condition per replicate. (F) Individual brood sizes of worms with either a normal or abnormal gonad; two biological replicates of at least 31 worms each. (B, D) Visible portions of the gonad and uterus are outlined with a white dashed line, animals are oriented as in (A), and scale bar is 50 microns. (C, F) ***p < 0.001; t-test on means of replicates. (C, E) Circles represent biological replicates. (C, E, F) Cross bars reflect the mean. See also Figure S1.
Inappropriate proliferation of germ cells following extended L1 starvation
The Notch receptor GLP-1 and the RNA-binding protein GLD-1/QKI regulate germline differentiation [9, 10]. glp-1 activity maintains germline stem cells by preventing differentiation, and gain-of-function mutants develop germ-cell tumors [11, 12]. We found that the gonad abnormalities in starved wild-type worms resemble proximal tumors and other gonad abnormalities seen in glp-1(ar202) gain-of-function mutants raised at a semi-permissive temperature (Figure 2A) [11]. Starvation also increased penetrance of tumor formation in the mutant (Figure 2B). Animals heterozygous for the glp-1(e2072) loss-of-function mutation [13] did not display gonad abnormalities following L1 starvation (Figure 2B), suggesting development of abnormalities is sensitive to glp-1 dosage. We also tested gld-1, which promotes meiosis and differentiation; gld-1 null mutants develop germ-cell tumors [14]. In our control conditions, early-adult gld-1(q485) null mutants developed proximal germ-cell tumors but heterozygous mutants did not (Figures 2C and 2D), as expected. When subjected to 8 d L1 starvation, however, heterozygous mutants developed uterine masses, and the size of the tumors in homozygous mutants was substantially enlarged (Figures 2C and 2D). gld-1 mutants do not perform physiological apoptosis [15, 16]. Thus, the presence of large tumors in starved gld-1 homozygous mutants together with our glp-1 results suggests that extended L1 starvation followed by unrestricted feeding promotes inappropriate proliferation of germ cells.
Figure 2. Early-life starvation followed by unrestricted feeding results in the formation of germ-cell masses.
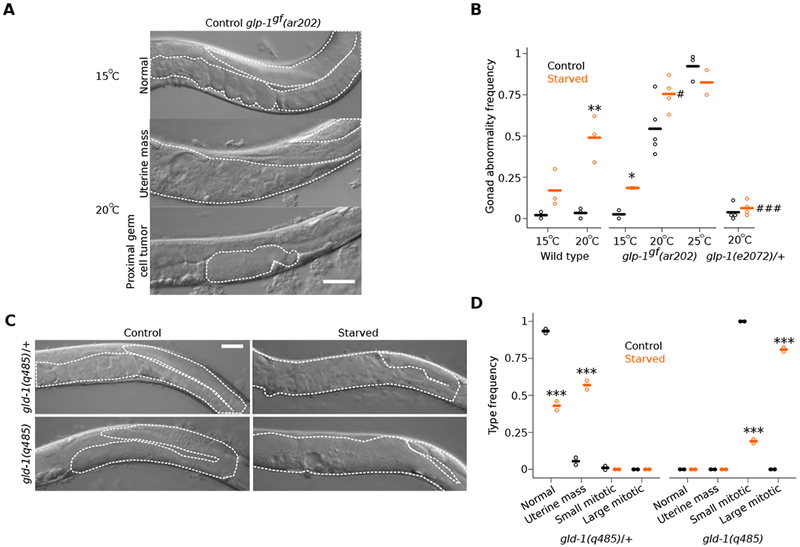
(A) Representative image of glp-1gf(ar202) from control conditions at permissive and semi-permissive temperatures. (B) Frequency of all gonad abnormalities; at least 40 worms per condition per replicate. *p < 0.05, ** < 0.01; t-test between control and starved. #p < 0.05, ### < 0.001; t-test between starved mutant and starved wild type. (C) Representative images of adult gld-1(q485) mutants from given conditions. (D) Frequency of indicated abnormalities; frequencies sum to one for each genotype; at least 30 worms per condition per replicate. ***p < 0.001; t-test between frequencies of control and starved abnormalities of given type. (A, C) Visible portions of the gonad and uterus are outlined with a white dashed line and scale bars are 50 microns. (B, D) Circles indicate biological replicates and cross bars reflect their means. See also Figure S2.
Developmental abnormalities involving somatic and germline tissues were evident (Figures S1A–C). Examination at 48 hr recovery suggested that somatic gonad development was often aberrant, likely contributing to gonad abnormalities observed later. In some animals sperm were observed throughout the somatic gonad and adjacent to the vulva, rather than the proximal oviduct prior to the first ovulation (Figure S2). We believe that extended starvation causes heterochronically discordant somatic and germline development, with abnormal somatic gonad-germline signaling possibly driving improper differentiation and tumorigenesis.
Maternal effects of dietary restriction and reduced IIS on starvation-induced abnormalities
Because maternal dietary restriction (DR) buffers progeny reproductive success from the costly effects of L1 starvation [4], we wondered whether maternal DR reduces the incidence of L1 starvation-induced gonad abnormalities. We obtained progeny from parents cultured in ad libitum (AL) or DR conditions, using bacterial dilution in a well-controlled liquid-culture system [4, 17], and starved progeny for 8 d as L1 larvae. Fewer progeny of maternal DR worms developed gonad abnormalities after starvation than progeny of maternal AL worms (Figure 3A), revealing an intergenerational effect of maternal diet on pathological consequences of early-life starvation.
Figure 3. Maternal DR and reduced IIS increase vitellogenin provisioning and reduce progeny IIS, protecting progeny from starvation-induced gonad abnormalities.
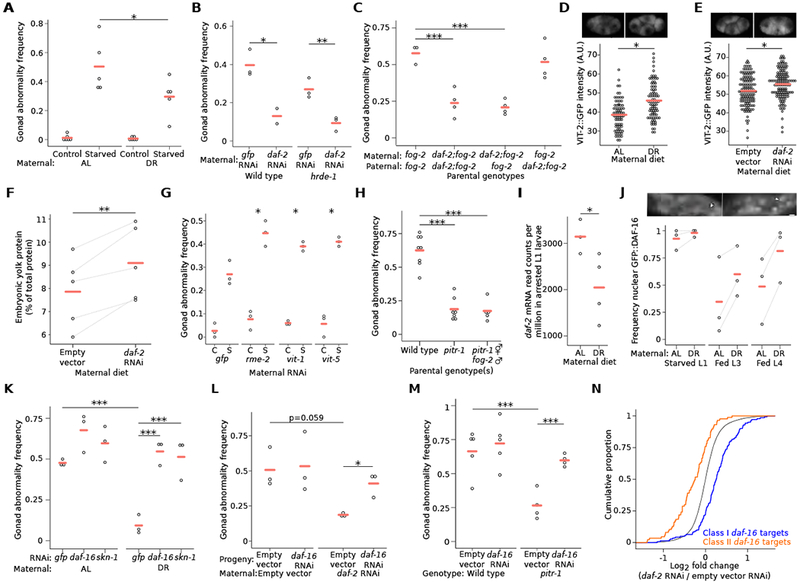
(A) Frequency of all gonad abnormalities; average of 49 progeny from ad libitum-fed (AL) or dietary restricted (DR) parents per replicate. *interaction p-value < 0.05; two-way analysis of variance (ANOVA). (B) Frequency of all gonad abnormalities in starved progeny from parents fed the indicated RNAi diet; at least 42 worms per replicate. (C) Frequency of all gonad abnormalities in starved cross progeny; at least 34 worms per replicate. (D, E) Circles indicate Pvit-2::VIT-2::GFP (pwIs23) fluorescence intensity of individual 1-4 cell embryos from parents raised in AL or DR conditions (D) and fed empty vector or daf-2 RNAi (E). Representative images of Pvit-2::VIT-2::GFP in 4-cell embryos are also provided for each condition. Three biological replicates of at least 24 embryos; cross bars reflect the mean of all embryos measured. A linear mixed-effect model was fit to all data with maternal diet as a fixed effect and biological replicate as a random effect; ***p < 0.001. Note that baseline discrepancy in VIT-2::GFP intensity is likely attributable to differences in culture methods (i.e., liquid versus solid media and E. coli HB101 versus HT115). (F) Summed density of wild-type yolk proteins 170, 115 and 88 relative to total protein on a gel. Lines connect results from five biological replicates. **p < 0.01; paired t-test. (G) Frequency of all gonad abnormalities in progeny from hrde-1 parents treated with the indicated RNAi; C = control, S = starved; at least 40 individuals were scored per condition. interaction p < 0.05, **p < 0.01; two-way ANOVA compared to gfp RNAi on means of replicates. (H) Frequency of all gonad abnormalities in starved self and cross progeny of the indicated genotype; at least 33 animals were scored per condition per replicate. (I) daf-2 mRNA read counts per million (CPM) in arrested L1 progeny of AL or DR parents. *q < 0.1. The full set of differentially expressed genes in progeny of parents fed AL and DR is listed in Table S1. Gene ontology (GO) terms for these genes are shown in Table S2. See Table S3 for daf-16 signature analysis of these genes. (J) Frequency of worms with intestinal nuclear GFP::DAF-16; 50 worms per condition per replicate; lines connect results from individual replicates. **p < 0.01; paired t-test of AL vs. DR across all replicates and stages. Insets are representative images of L1 larvae with cytoplasmic GFP::DAF-16 localization (left) or nuclear GFP::DAF-16 localization (right); white arrows indicate intestinal nuclei; scale bar is 5 microns. (K) Frequency of all gonad abnormalities in starved progeny of AL or DR parents raised on indicated RNAi food; at least 50 worms per replicate. (L) Frequency of all gonad abnormalities in starved hrde-1 mutants grown on the given RNAi food in each generation; at least 41 worms were scored per condition per replicate. (M) Frequency of all gonad abnormalities in starved progeny of the given genotype and raised on empty vector or daf-16 RNAi; at least 41 animals were scored per condition per replicate. (N) daf-16 target gene expression changes in progeny of hrde-1 parents fed empty vector or daf-2 RNAi. p = 3.664 × 10−15; Kolmogorov-Smirnov test. (A-C, F-M) Circles indicate biological replicates and cross bars reflect their mean. (A-C, F-H, K-M) * p < 0.05, ** p < 0.01, ***p < 0.001; t-test on means of replicates. See also Figure S3.
Since DR in our bacterial-dilution culture system reduces IIS, and reduction of maternal IIS buffers progeny reproductive success [4], we hypothesized that reduction of maternal IIS protects progeny from starvation-induced abnormalities. RNAi of the only known IIS receptor daf-2/InsR reduced penetrance of starvation-induced abnormalities in progeny (Figure 3B). However, since RNAi itself is heritable [18], it is unclear if this result reflects maternal or zygotic function of daf-2. We therefore performed RNAi in a hrde-1/AGO1 (heritable RNAi defective) mutant [19] to reduce or eliminate zygotic effects of RNAi in progeny. Maternal daf-2 RNAi in this background also reduced penetrance of abnormalities in progeny (Figure 3B), consistent with our hypothesis. However, this experiment does not distinguish between maternal and paternal effects of IIS. We therefore used a fog-2 (feminization of germline) mutant, which has a male-female mating system [20], to perform crosses with daf-2(e1370) to identify parent-of-origin effects. Maternal but not paternal mutation of daf-2 reduced starvation-induced abnormalities in progeny (Figure 3C), consistent with a maternal effect. In summary, reduction of maternal IIS protects progeny from pathological consequences of early-life starvation.
Vitellogenin provisioning mediates maternal effects
Maternal provisioning of vitellogenin lipoprotein supports L1 starvation resistance and is affected by maternal age [21, 22]. DR worms produce larger embryos [4], suggesting oocyte provisioning could be affected as a maternal-effect mechanism. Two different VIT-2 vitellogenin reporters were brighter in early (1-4 cell) embryos from DR parents than embryos from AL parents (Figures 3D and S3A), consistent with maternal DR increasing vitellogenin concentration. In addition, daf-2/InsR RNAi increased VIT-2 reporter intensity (i.e., protein concentration), consistent with reduced IIS increasing vitellogenin provisioning (Figure 3E). Further, embryo lysate contained more total vitellogenin protein compared to all protein (Figures 3F and S3B), and more of the vit-2 product YP170 (Figure S3C), when parents were subjected to daf-2/InsR RNAi.
To address physiological significance of altered vitellogenin provisioning, we used RNAi and mutants to alter provisioning. We used RNAi to target rme-2, which is required in developing oocytes for receptor-mediated endocytosis of vitellogenin [23], as well as two of six vitellogenin genes (vit-1 and vit-5). rme-2 RNAi was apparently inherited, as germline development was affected in progeny whether starved or not (Figure S3D), complicating interpretation. We therefore used a hrde-1/AGO mutant background to reduce or eliminate inheritance of silencing [19], allowing us to isolate maternal function of each gene. In this background, maternal RNAi of rme-2 increased penetrance of starvation-induced gonad abnormalities (Figure 3G). Maternal RNAi of vit-1 and vit-5 in the hrde-1 background had a similar but weaker effect (Figure 3G). We examined a pitr-1/SLC20A1 mutant, which has increased vitellogenin oocyte provisioning [24], and the effects of L1 starvation on reproductive development were suppressed (Figure 3H). Maternal disruption of pitr-1 function was sufficient for suppression (Figures 3H and S3E). Together these results suggest that nutrient availability and IIS affect maternal vitellogenin provisioning, and that increased vitellogenin levels protect progeny from pathological consequences of early-life starvation.
Maternal dietary restriction affects progeny IIS
Analysis of transcript abundance by RNAseq revealed intergenerational effects of maternal DR on progeny gene expression. 114 genes were differentially expressed on the first day of L1 starvation in DR progeny compared to AL progeny (q < 0.1; Table S1). Gene Ontology terms related to IIS, including “innate immune response” and “regulation of dauer entry”, were enriched (Table S2). Moreover, expression of daf-2/InsR was decreased in DR progeny (Figure 3I). DAF-2/InsR antagonizes function of the FoxO transcription factor DAF-16 in response to IIS [25], and regulatory targets of daf-16/FoxO define a transcriptional signature of reduced IIS [26]. Despite relatively few differentially expressed genes, genes up-regulated in DR progeny were significantly enriched for Class I daf-16 targets (activated by daf-16; p < 0.05), and down-regulated genes were significantly enriched for Class II targets (repressed by daf-16; p < 0.001) (Table S3), consistent with reduced IIS in DR progeny. DAF-2 regulates localization of DAF-16, with DAF-16 shifting from cytoplasmic to nuclear when IIS is reduced [25]. GFP::DAF-16 displayed strong nuclear localization during L1 starvation, as expected [27], but it was even more completely localized to nuclei in DR progeny (Figure 3J). GFP::DAF-16 was relatively more cytoplasmic in fed than starved larvae, as expected, but it was relatively more nuclear in fed DR progeny than in fed control progeny (Figure 3J). These results collectively support the conclusion that maternal DR decreases IIS in progeny.
To determine whether intergenerational reduction of IIS caused by maternal DR has functional consequences, we examined the effects of reduced daf-16/FoxO and skn-1/Nrf, both effectors of IIS [25], in progeny. RNAi of each gene caused a modest, insignificant increase in the penetrance of abnormalities in AL progeny starved for 8 d as L1 larvae (Figure 3K). However, daf-16 RNAi and skn-1 RNAi both disrupted the protective effect of maternal DR on progeny (Figure 3K), indicating that their function is required in progeny to reduce starvation-induced abnormalities. Further, maternal daf-2/InsR RNAi (in a hrde-1/AGO1 background) required daf-16 function in progeny to suppress starvation-induced gonad abnormalities (Figure 3L). Together these results support the conclusion that maternal DR reduces progeny IIS, protecting progeny from pathological consequences of early-life starvation by promoting the activity of daf-16/FoxO and skn-1/Nrf. Moreover, daf-16 was required for the pitr-1/SLC20A1 mutant to suppress gonad abnormalities (Figure 3M). This result supports the conclusion that vitellogenin provisioning protects progeny from starvation at least in part by reducing IIS. Furthermore, RNAseq analysis showed that maternal daf-2 RNAi in a hrde-1 background increased daf-16 class I and decreased class II target gene expression in starved L1 progeny (Figure 3N), consistent with maternal IIS affecting progeny IIS. Maternal pitr-1 RNAi in a hrde-1 background had a qualitatively similar, though smaller effect on daf-16 target gene expression (Figure S3F), consistent with a relatively weak effect of maternal pitr-1 RNAi (compared to the mutant) on suppression of starvation-induced abnormalities (Figure S3E). These results suggest that dietary restriction and reduced IIS increase vitellogenin provisioning to oocytes, reducing progeny IIS and engaging daf-16 to protect progeny from L1 starvation-induced developmental abnormalities.
Reduced IIS suppresses development of starvation-induced gonad abnormalities
Recovering starved L1 larvae in conditions that reduce IIS suppresses development of gonad abnormalities. Dramatically fewer starved larvae recovering in DR or in conditions that induce dauer diapause [28, 29] (followed by recovery from dauer in AL conditions) displayed abnormalities (Figures 4A and S4A). These results are consistent with reduced IIS supporting developmental integrity after starvation, but other pathways affected by these conditions could also be involved. daf-2(e1370) mutants also developed fewer abnormalities (Figure 4B), as did skn-1/Nrf gain-of-function mutants in daf-16/FoxO-independent fashion (Figures 4C and S4B). Though consistent with reduced IIS suppressing starvation-induced abnormalities, it is unclear when during the life cycle these mutations exert their effect. We therefore used RNAi to perturb gene function during development after L1 starvation. RNAi of daf-2/InsR (or double RNAi of daf-2 and gfp) reduced the frequency of all types of abnormalities (Figures 4D, 4E and S4C), demonstrating that reducing IIS during recovery from starvation suppresses development of abnormalities. Suppression depended on daf-16 and skn-1 (Figure 4E), as seen with maternal DR (Figure 3K). These results demonstrate that reduced IIS preserves developmental integrity following early-life starvation.
Figure 4. Reducing somatic IIS signaling during recovery from early-life starvation suppresses gonad abnormalities.
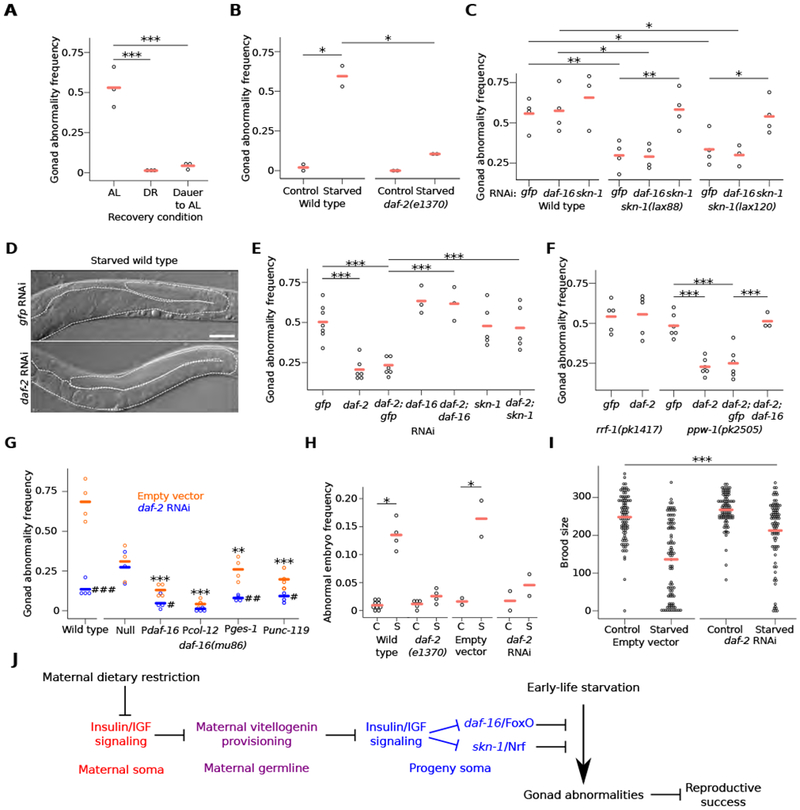
(A) Frequency of all gonad abnormalities in worms starved for 8 d and recovered in ad libitum (AL), dietary restriction (DR), or dauer-forming followed by AL conditions (Dauer to AL); at least 40 worms per condition per replicate. (B) Frequency of all gonad abnormalities in control or starved worms of the given genotype; at least 38 animals per condition per replicate. (C) Frequency of all gonad abnormalities in starved wild type and skn-1 gain-of-function mutants raised on the indicated RNAi; at least 50 worms per condition per replicate. (D) Representative images of starved worms raised on gfp or daf-2 RNAi. Visible portions of the gonad and uterus are outlined with a dashed white line. Scale bar is 50 microns. (E, F) Frequency of all gonad abnormalities in starved worms raised on the indicated single or double RNAi (E) or given genotype raised on the indicated RNAi (F); at least 50 worms per condition per replicate. (G) Transgenic rescue of daf-16; frequency of all gonad abnormalities in starved worms of the given genotype; at least 50 worms per condition per replicate. Pdaf-16 is an overexpression (gain-of-function) line. Pcol-12, Pges-1 and Punc-119 drive expression exclusively in the epidermis, intestine and nervous system, respectively. daf-16(mu86) null mutants without transgenic rescue do not survive 8 days of L1 starvation. Therefore, these animals were starved for three days; other genotypes were starved for eight days. Asterisks indicate comparisons between wild type and the other genotypes fed empty vector. Pound signs indicate comparisons within genotypes between empty vector and daf-2 RNAi; #p < 0.05, ## < 0.01, ### < 0.001; t-test. (H) Frequency of abnormally shaped embryos produced by starved worms of the given genotype or RNAi treatment; C = control, S = starved; at least 100 embryos per condition per replicate. (A-C, E-H) Circles indicate biological replicates and cross bars reflect their means. *p < 0.05, ** < 0.01, *** < 0.001; t-test on means of replicates. (I) Circles indicate individual brood sizes from five biological replicates of ~18 individuals per condition per replicate; cross bars reflect the mean of all individuals. A linear mixed-effect model was fit to all data with RNAi and length of starvation as fixed effects and biological replicate as a random effect. ***interaction p-value < 0.001. (J) A model of how IIS and vitellogenin provisioning mediate intergenerational adaptation to nutrient stress is presented. See also Figure S4.
We wondered where in the animal IIS functions to affect reproductive development, so we performed RNAi in mutants that largely restrict RNAi to the germline or soma [30, 31]. The effects of daf-2/InsR and daf-16/FoxO RNAi were abrogated in a mutant that restricts RNAi primarily to the germline (rrf-1) but were retained in a mutant that restricts RNAi to the soma (ppw-1; Figure 4F), suggesting that reduced IIS in the soma suppresses development of starvation-induced abnormalities. Transgenic rescue of a daf-16 null mutant showed that overexpression of daf-16 (using its own promoter) without daf-2 RNAi is sufficient to suppress abnormalities, and that daf-2 RNAi enhances suppression (Figure 4G). These results corroborate results using double RNAi (Figures 4E and 4F). Using heterologous promoters for tissue-specific expression revealed that daf-16 can function cell-nonautonomously in the epidermis, intestine or neurons to regulate reproductive development following L1 starvation (Figure 4G). These sites of action have been identified for daf-16 in regulating developmental arrest and aging [32–35]. In conclusion, reduction of somatic IIS during development protects worms from pathological consequences of early-life starvation.
We used a gld-1/QKI mutant to investigate the effect of IIS on germ-cell proliferation. L1 starvation increased the size of proximal tumors in this differentiation-defective mutant (Figures S4D and S4E), as before (Figures 2C and 2D). daf-2/InsR RNAi suppressed the effect of starvation, decreasing tumor size, in daf-16/FoxO-dependent fashion (Figures S4D and S4E). These results suggest that reduction of IIS inhibits a hyperproliferative state of germ cells induced by extended L1 starvation.
In addition to reducing brood size, extended L1 starvation results in production of abnormally shaped, small embryos with reduced hatching efficiency [2]. Reduction of IIS suppressed production of abnormal embryos and increased brood size in starved worms (Figures 4H and 4I). These results further demonstrate that the starvation-induced gonad abnormalities we describe compromise reproductive success, presumably reducing organismal fitness, and they show that reducing IIS following early-life starvation increases reproductive success.
Conclusions
We show that early-life starvation in C. elegans leads to developmental abnormalities in the reproductive system, including germ-cell tumors as well somatic defects, and that these abnormalities reduce reproductive success (Figure 4J). Our results suggest that extended starvation followed by unrestricted feeding disrupts coordination of somatic and germline development, leading to development of gonad abnormalities. Remarkably, maternal DR, and specifically maternal reduction of IIS, mitigates these pathological consequences of starvation. Consistent with a maternal effect, we show that DR and reduced IIS increase vitellogenin oocyte provisioning and reduce IIS in progeny. We also show that somatic reduction of IIS following L1 starvation suppresses germ cell hyperproliferation and development of gonad abnormalities via activation of daf-16/FoxO and skn-1/Nrf, preserving developmental integrity and reproductive success. Together with our previous work showing that DR acts through maternal IIS to affect progeny size and starvation resistance [4], this work suggests that IIS in one generation can indirectly affect IIS in the next generation to mediate intergenerational adaptation to nutrient availability. Insulin signaling is frequently altered in mammalian models of maternal dietary effects on offspring [36], suggesting conservation of a central role of IIS in mediating physiological trade-offs between generations.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the corresponding author, L. Ryan Baugh (ryan.baugh@duke.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Worm maintenance
Worms were maintained under standard laboratory conditions at 20°C unless otherwise noted. Animals were faithfully passaged without dietary restriction (thinning of the E. coli lawn) or starvation for many generations (greater than five) prior to commencing experiments unless otherwise noted.
Strains used in this study
N2 (Bristol), GC1171 naSi2[pGC550(Pmex-5<mCherry::H2B::nos-2 3’UTR<GFP::H2B::nos-2 3’UTR - unc-119(+))] unc-119(ed3), DP132 edIs6 [Punc-119::GFP + rol-6(su1006)], OP354 unc-119(tm4063); wgIs354 [Pelt-1::TY1::EGFP::3xFLAG + unc-119(+)], LRB366 daf-2(e1370); fog-2(oz40), RT130 pwIs23[Pvit-2::VIT-2::GFP], BCN9070 vit-2(crg9070[vit-2::gp]), FX01200 hrde-1(tm1200), CB1370 daf-2(e1370), SPC167 dvIs19; skn-1(lax120), SPC168 dvIs19; skn-1(lax188), CF1038 daf-16(mu86), NK1228 qyIs288; daf-16(mu86); unc-119(ed4), NK1229 qyIs290; daf-16(mu86); unc-119(ed4), NK1231 qyIs291; daf-16(mu86); unc-119(ed4), NK1233 qyIs293; daf-16(mu86); unc-119(ed4), NL2550 ppw-1(pk2505), NL2098 rrf-1(pk1417), JK3025 gld-1(q485)/hT2 [bli-4(e937) let-?(q782) qIs48], GC833 glp-1(ar202), JK1505 unc-32(e189) glp-1(e2072)/eT1, DH1191 pitr-1(b1028), and wild-type isolates: CB4856, ED3077 and JU561.
METHOD DETAILS
Recovery from starvation assay
C. elegans embryos were prepared with sodium hypochlorite treatment, washed several times, and allowed to hatch in virgin S-basal (no ethanol or cholesterol) on a tissue culture roller drum at ~22°C so they hatch and enter L1 arrest. Animals were cultured for one or eight days (unless otherwise noted) after hypochlorite treatment prior to being plated on lawns of E. coli OP50 or HT115 (for RNAi experiments). Animals were incubated at 20°C unless otherwise noted and then assayed after three or four days of development, as indicated.
Germline Microscopy and scoring of gonad abnormalities
Adult worms were picked at random into 2.5 mM sodium azide on 4% noble agar pads. Worms were then examined using Nomarski microscopy on an AxioImager compound microscope (Zeiss) at 200-400× magnification. Wild-type worms starved for eight days as L1 larvae developed one or more of a variety of abnormalities at varying rates including defective distal tip-cell migration, vulval morphogenesis, and egg laying; as well as internal hatching, stacked and abnormally shaped oocytes, endomitotic oocytes and uterine masses, abnormally shaped embryos, and germ-cell masses (Figures S1A–C). Animals with any of these abnormalities in the gonad region were scored as abnormal. A majority of the animals scored as abnormal had masses that varied in the extent of differentiation, ranging from mex-5+ germ-cell tumors to highly differentiated, mex-5-uterine masses that are likely derived from endomitotic oocytes in the uterus (Figure 1B) [37]. When scoring glp-1gf(ar202), dumpy individuals were not scored. Development of mutants and animals subjected to different experimental conditions was scored to match stages (up to 5 d after initiating recovery from L1 arrest). Animals were censored if they were delayed to the extent that they were not adults at the time of scoring. For Figure 1E, individual uterine masses were assessed for the presence or absence of Pelt-1::GFP or Punc-119::GFP at 400-1000×. Homozygous gld-1 mutant tumors were classified based on their size and location for Figure 2D.
Gonad imaging
Worms were imaged at 400x magnification, using an AxioCam camera equipped with Zen software, on an AxioImager compound microscope (Zeiss). Images were merged using Fiji. Images were cropped and features were added using Inkscape.
Determination of brood size
Animals were prepared as described above and plated onto the indicated bacterial lawn. After 48 hr of recovery from L1 arrest, animals were singled onto plates seeded with the corresponding bacteria. Worms were transferred daily onto fresh plates until egg laying ceased and progeny were counted. Animals that could not be found were censored. For Figure 1F, worms were inspected for gonad abnormalities after 72 hr of recovery with a stereomicroscope and otherwise scored as described.
Abnormalities in progeny of AL and DR parents
Progeny from AL and DR parents were generated as previously described for use in this study [4]. Briefly, following synchronization by brief L1 arrest, worms were cultured in S-complete at a density of 10 per mL with 25 mg/ml (AL) or 3.13 mg/ml (DR) E. coli HB101 at 20°C and 180 rpm for 96 hr. Progeny embryos were prepared by hypochlorite treatment.
Abnormalities in cross progeny of daf-2: fog-2 and fog-2
Homozygous males were allowed to mate with homozygous virgin females overnight, and cross progeny were obtained by standard hypochlorite treatment. Progeny were then starved and assessed for gonad abnormalities as described above.
Quantification of VIT-2::GFP in embryos from AL and DR parents
Embryos were prepared by standard hypochlorite treatment of worms raised in AL or DR liquid culture conditions for 96 hr and were mounted on 4% noble agar pads. Images were taken at 1000x magnification using an AxioImager compound microscope equipped with an AxioCam camera (Zeiss). VIT-2::GFP was quantified using the ‘Measure’ function in Image J. A threshold that included the embryos but not the surrounding agar was manually identified for each reporter strain and culture system (AL/DR liquid culture and RNAi on plates). That threshold was used to remove background for all embryos. Average pixel intensity for the area above background was reported.
Quantification of vitellogenin protein via gel electrophoresis
Embryos were plated on empty vector or daf-2 RNAi plates, allowed to develop for 72 hr, and their embryos were collected via bleaching. Protein was extracted using Laemmli buffer (70°C for 15 minutes with periodic vortexing). Total protein was quantified using a Pierce assay (Thermo Scientific) per manufacturer’s instructions. Nominally equivalent amounts of protein were loaded along with Benchmark protein ladder (Life Technologies) onto a 4-12% Bis-Tris gel (Invitrogen) and the gel was run per manufacturer’s instructions (200V for 50 min.) in MOPS buffer. Gel was stained using SYPRO Ruby (Invitrogen) per manufacturer’s instructions. Gel was imaged and relative density of bands of interest compared to total protein on the gel was determined using Quantity One software. Total yolk protein was determined by summing the densities of YP170, YP115, and YP88. Yolk protein bands of interest were identified by molecular weight and their expected depletion following rme-2 RNAi (data not shown). The actin loading control band was identified by molecular weight.
RNAseq in progeny from AL and DR parents
Progeny from AL and DR parents were cultured in S-basal for 24 hr so they hatched and entered L1 arrest, and then they were flash frozen in liquid nitrogen. RNA was prepared with Trizol (Invitrogen) according to the manufacturer’s instructions except that sand was included in homogenization. Libraries were prepared from 500 ng of total RNA and 12 PCR cycles using NEB Next Ultra RNA Library Preparation Kit (New England Biolabs). Sequencing was performed on an Illumina HiSeq 4000.
GFP::DAF-16 localization in the progeny of AL and DR parents
Progeny of AL and DR parents were prepared as described for an N-terminal DAF-16 translational fusion reporter gene strain (NK1228 [42]). Localization of intestinal GFP was scored at 400x or 1000x depending on stage using an Axiolmager compound microscope (Zeiss). Localization changes upon mounting worms on microscope slides, so scoring was done rapidly on relatively small numbers of animals at a time.
RNAseq in progeny from daf-2 and pitr-1 RNAi parents
Progeny from hrde-1 mutant worms raised on HT115 empty vector, daf-2, or pitr-1 RNAi plates for 72 hr were cultured in S-basal for 24 hr so they hatched and entered L1 arrest, and then they were flash frozen in liquid nitrogen. RNA was prepared with Trizol (Invitrogen) according to the manufacturer’s instructions except that sand was included in homogenization. Libraries were prepared from 15 ng of total RNA and 15 PCR cycles using NEB Next Ultra II RNA Library Preparation Kit (New England Biolabs). Sequencing was performed as described above for RNAseq of AL/DR progeny.
Starvation and DR recovery
Animals were starved as described above, except instead of recovery on plates, animals were recovered in DR liquid culture as described and gonad abnormalities were scored at 96 hr.
Starvation and Dauer to AL recovery
Wild-type animals were starved and then recovered in dauer-forming conditions [28, 44] (5 worms per μl and 1 mg/ml HB101 in S-complete at 20°C and 180 rpm) for 5 d then recovered on plates with an OP50 lawn (AL). After animals reached early adulthood, their gonads were assessed for the presence or absence of gonad abnormalities as described above.
RNAi
Single HT115 colonies carrying plasmids for RNAi of various genes grown on 100 μg/ml carbenicillin and 12.5 μg/ml tetracycline LB plates were inoculated into LB starter cultures with the same antibiotics. These were used to inoculate larger cultures grown in 50 μg/ml carbenicillin. Cells from large cultures were spun down at 4°C and resuspended at a concentration of 25 mg/ml in 15% glycerol S-complete medium. Aliquots were frozen and thawed only once and seeded onto NGM plates containing 25 μg/ml carbenicillin and 1 mM IPTG. For double RNAi experiments, equal volumes of RNAi suspension were mixed. Lawns were allowed to grow overnight at room temperature before adding worms. Empty vector (pAD12), daf-2 (pAD48), and daf-16 (pAD43) clones were gifts from Coleen Murphy. The skn-1 clone came from the Ahringer library. To perform maternal RNAi, hrde-1(tm1200), which are defective for heritable RNAi [19], were raised from hatching on RNAi food for 72 hr. Progeny were then collected and cultured as described previously.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistics and presentation
t-tests were performed using R or Microsoft Excel. Bartlett’s tests were performed to determine if variance could be pooled. Two-way ANOVA was performed using R. The R package Linear and Nonlinear Mixed Effects Models “nlme” was used to compute interaction p-values (Figures 3D, 3E, 4I and S3A). Plots were generated using the R package ggplot2.
Analysis of RNAseq data from progeny of AL/DR parents
Bowtie was used to map reads to the WS210 genome, including transcripts annotated in WS220 mapped back to WS210 [38, 39]. HTSeq was used to generate count tables for each library [40]. edgeR was used on count tables to determine differentially expressed genes. Detected genes were considered those with counts per million (CPM) > 1 across seven libraries. This resulted in 12,082 genes for differential expression analysis. The “calcNormFactors” function was used and tagwise dispersion estimate was used for differential expression. Genes with a false-discovery rate of less than 0.1 were considered differentially expressed.
daf-16 target gene enrichment analysis
A consensus background gene set was produced by intersecting all genes detected in our RNAseq data with those reported by Tepper et al [26] using the R package gplots. A total of 11,496 genes were detected in both data sets. Overlap was determined between differentially expressed genes (up- or down-regulated) in the progeny of DR parents compared to AL parents (FDR < 0.1) and high confidence daf-16 target genes as determined by Tepper et al (FDR < 0.0001) [26]. Hypergeometric p-values were determined using the Graeber lab calculator (http://systems.crump.ucla.edu/hypergeometric/index.php).
Gene Ontology term enrichment analysis
Genes differentially expressed in the progeny of DR parents (n = 114) were analyzed for enrichment of Gene Ontology terms using the 13,439 RNAseq-detected genes (criteria detailed above) as background using Gorilla [41].
Analysis of RNAseq data from progeny from daf-2 and pitr-1 RNAi parents
Count data was filtered to include 12,395 reliably-detected genes. Batch correction and differential expression analysis were performed in edgeR using the GLM method as described in section 4.2 of the edgeR manual [43]. Count tables were subsetted to include only the libraries of interest, including empty vector and daf-2 RNAi samples, or empty vector and pitr-1 RNAi samples. The “calcNormFactors” function was used to normalize libraries. The GLM model was set up using the command model.matrix(~condition + replicate), where condition denotes the RNAi condition, and replicate denotes biological replicate (or batch). The dispersion was estimated, then the model was fit using the “glmQLFit” function and differential expression was determined using “glmQLFTest”. Class I and Class II targets of daf-16 were defined from Tepper et al., 2013 using an FDR cutoff of 1 × 10−8. The distribution of log2 fold changes of the class I and class II targets that were considered expressed (included in the 12,395 genes from differential expression analysis) in the comparison of interest were assessed using the Kolmogorov-Smirnov test. The distributions were plotted with stat_ecdf function as part of the ggplot2 package in R.
DATA AND SOFTWARE AVAILABILITY
RNAseq data sets can be found at GEO using accession numbers GSE114271 and GSE129088.
Supplementary Material
Table S1. Genes differentially expressed during L1 arrest in the progeny of DR parents compared to AL parents. Related to Figure 3.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| Escherichia coli OP50 | Caenorhabditis Genetics Center | WB ID: OP50 |
| Escherichia coli HB101 | Caenorhabditis Genetics Center | WB ID: HB101 |
| Escherichia coli HT115 pAD12 (vector) | Coleen Murphy Princeton University | N/A |
| Escherichia coli HT115 pAD43 (daf-16) | Coleen Murphy Princeton University | N/A |
| Escherichia coli HT115 pAD48 (daf-2) | Coleen Murphy Princeton University | N/A |
| Escherichia coli HT115 pPD126.25 (gfp) | Craig Hunter Harvard University |
Fire Lab Vector Kit pPD126.25 |
| Escherichia coli HT115 (skn-1) | Alejandro Aballay OHSU | Ahringer library T19E7.2/IV-2N18 |
| Escherichia coli HT115 (pitr-1) | Barth Grant Rutgers University |
Ahringer library C48A7.2/IV-3P05 |
| Critical Commercial Assays | ||
| NEB Next Ultra RNA Library Preparation Kit | New England Biolabs | Cat#E7530S |
| NEB Next Ultra II RNA Library Preparation Kit | New England Biolabs | Cat#E7770S |
| SYPRO Ruby Protein Stain | Thermo Fisher | Cat#S-12001 |
| NuPAGE 4-12% Bis-Tris Protein Gels | Thermo Fisher | Cat#NP0336BOX |
| Deposited Data | ||
| RNAseq data: arrested L1s from AL/DR mothers | This paper | GSE114271 |
| RNAseq data: arrested L1s from daf-2 and pitr-1 RNAi-treated mothers (in hrde-1 background) | This paper | GSE129088 |
| Experimental Models: Organisms/Strains | ||
| naSi2[pGC550(Pmex-5<mCherry::H2B::nos-2 3’UTR<GFP::H2B::nos-2 3’UTR - unc-119(+))] unc-119(ed3) III. | Jane Hubbard New York University |
WB strain: GC1171 [5] |
| edIs6 [Punc-119::GFP + rol-6(su1006)] | Caenorhabditis Genetics Center | WB Strain: DP132 |
| unc-119(tm4063) III; wgIs354 [Pelt-1::TY1::EGFP::3xFLAG + unc-119(+)] | Caenorhabditis Genetics Center | WB Strain: OP354 |
| daf-2(e1370) III; fog-2(oz40) V. | This paper | LRB366 |
| pwIs23[Pvit-2::VIT-2::GFP] | Caenorhabditis Genetics Center | WB Strain: RT130 |
| vit-2(crg9070[vit-2::gfp]) X. | Ben Lehner CRG, Barcelona |
BCN9070 |
| hrde-1(tm1200) III. | Shohei Mitani National Bioresource Project |
FX01200 |
| daf-2(e1370) III. | Caenorhabditis Genetics Center | WB Strain: CB1370 |
| dvIs19 III; skn-1(lax120) IV. | Caenorhabditis Genetics Center | WB Strain: SPC167 |
| dvIs19 III; skn-1(lax188) IV. | Caenorhabditis Genetics Center | WB Strain: SPC168 |
| daf-16(mu86) I. | Caenorhabditis Genetics Center | WB Strain: CF1038 |
| qyIs288; daf-16(mu86) I; unc-119(ed4) III. | David Sherwood Duke University |
NK1228 [42] |
| qyIs290; daf-16(mu86) I; unc-119(ed4) III. | David Sherwood Duke University |
NK1229 [42] |
| qyIs291; daf-16(mu86) I; unc-119(ed4) III. | David Sherwood Duke University |
NK1231 [42] |
| qyIs293; daf-16(mu86) I; unc-119(ed4) III. | David Sherwood Duke University |
NK1233 [42] |
| ppw-1(pk2505) I. | Caenorhabditis Genetics Center | WB Strain: NL2550 |
| rrf-1(pk1417) I. | Caenorhabditis Genetics Center | WB Strain: NL2098 |
| gld-1(q485) I/hT2 [bli-4(e937) let-?(q782) qIs48] (I;III). | Caenorhabditis Genetics Center | WB Strain: JK3025 |
| glp-1(ar202) III. | Caenorhabditis Genetics Center | WB Strain: GC833 |
| unc-32(e189) III; glp-1(e2072) III/eT1 | Caenorhabditis Genetics Center | WB Strain: JK1505 |
| pitr-1(b1028) IV. | Barth Grant Rutgers University |
DH1191 [24] |
| C. elegans wild-type isolate | Caenorhabditis Genetics Center | WB Strain: CB4856 |
| C. elegans wild-type isolate | Caenorhabditis Genetics Center | WB Strain: ED3077 |
| C. elegans wild-type isolate | Caenorhabditis Genetics Center | WB Strain: JU561 |
| Software and Algorithms | ||
| Bowtie | [38] | http://bowtie-bio.sourceforge.net/index.shtml |
| HTseq | [40] | https://htseq.readthedocs.io/en/release_0.11.1/ |
| edgeR | [43] | https://bioconductor.org/packages/release/bioc/html/edgeR.html |
| GOrilla | [41] | http://cbl-gorilla.cs.technion.ac.il |
Highlights:
Early-life starvation and unrestricted feeding result in reproductive abnormalities
Maternal dietary restriction protects progeny from starvation-induced abnormalities
Maternal diet and insulin/IGF signaling affect vitellogenin oocyte provisioning
Vitellogenin oocyte provisioning affects progeny insulin/IGF signaling
Acknowledgments
We thank the Duke University School of Medicine and the Center for Genomic and Computational Biology for use of the Sequencing and Genomic Technologies shared resource, which provided RNA sequencing service. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). The National Institutes of Health funded this work (LRB, R01GM117408; EJAH, R01GM061706). Figure 1A adapted from Caenorhabditis elegans hermaphrodite adult-en.svg from Wikimedia Commons by K. D. Schroeder (License: CC-BY-SA 3.0).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
Authors declare no competing interests.
References
- 1.Baugh LR (2013). To grow or not to grow: nutritional control of development during Caenorhabditis elegans L1 arrest. Genetics 194, 539–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jobson MA, Jordan JM, Sandrof MA, Hibshman JD, Lennox AL, and Baugh LR (2015). Transgenerational Effects of Early Life Starvation on Growth, Reproduction, and Stress Resistance in Caenorhabditis elegans. Genetics 201, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee I, A H., J Kim, J Yoshimoto, Y You (2012). Metabolic Rate Regulates L1 Longevity in C. elegans. PloS one 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hibshman JD, Hung A, and Baugh LR (2016). Maternal Diet and Insulin-Like Signaling Control Intergenerational Plasticity of Progeny Size and Starvation Resistance. PLoS Genet 12, e1006396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy D, Kahler DJ, Yun C, and Hubbard EJA (2018). Functional Interactions Between rsks-1/S6K, glp-1/Notch, and Regulators of Caenorhabditis elegans Fertility and Germline Stem Cell Maintenance. G3 (Bethesda). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Killian DJ, and Hubbard EJ (2005). Caenorhabditis elegans germline patterning requires coordinated development of the somatic gonadal sheath and the germ line. Dev Biol 279, 322–335. [DOI] [PubMed] [Google Scholar]
- 7.McGovern M, Voutev R, Maciejowski J, Corsi AK, and Hubbard EJ (2009). A “latent niche” mechanism for tumor initiation. PNAS 106, 11617–11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Zhao Y, Ezcurra M, Benedetto A, Gilliat AF, Hellberg J, Ren Z, Galimov ER, Athigapanich T, Girstmair J, et al. (2018). A parthenogenetic quasi-program causes teratoma-like tumors during aging in wild-type C. elegans. NPJ Aging Mech Dis 4, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimble J, and Crittenden SL (2005). Germline proliferaton and its control. WormBook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen D, and Schedl T (2013). Stem cell proliferation versus meiotic fate decision in Caenorhabditis elegans. Adv Exp Med Biol 757, 71–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepper A, Killian DJ, and Hubbard EJ (2003). Genetic Analysis of Caenorhabditis elegans glp-1 Mutants Suggests Receptor Interaction or Competition. Genetics 163, 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry LW, Westlund B, Schedl T (1997). Germ-line tumor formation caused by activation of glp-1, a Caenorhabditis elegans member of the Notch family of receptors. Development 124, 925–936. [DOI] [PubMed] [Google Scholar]
- 13.Kodoyianni V, Maine EM, and Kimble J (1992). Molecular Basis of Loss-of-Function Mutations in the glp-1 Gene of Caenorhabitis elegans. Molecular Biology of the Cell 3, 1199–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis R, Barton MK, Kimble J, and Schedl T (1995). gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics 139, 579–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinkston JM, Garigan D, Hansen M, and Kenyon C (2006). Mutations That Increase the Life Span of C. elegans Inhibit Tumor Growth. Science 313, 971–975. [DOI] [PubMed] [Google Scholar]
- 16.Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, and Hengartner MO (1999). Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 126, 1011–1022. [DOI] [PubMed] [Google Scholar]
- 17.Mair W, Panowski SH, Shaw RJ, and Dillin A (2009). Optimizing dietary restriction for genetic epistasis analysis and gene discovery in C. elegans. PLoS One 4, e4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, and Mello C (1998). Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811. [DOI] [PubMed] [Google Scholar]
- 19.Buckley BA, Burkhart KB, Gu SG, Spracklin G, Kershner A, Fritz H, Kimble J, Fire A, and Kennedy S (2012). A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature 489, 447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schedl T, and Kimble J (1988). fog-2, a Germ-Line-Specific Sex Determination Gene Required for Hermaphrodite Spermatogenesis in Caenorhabditis elegans. Genetics, 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez MF, Francesconi M, Hidalgo-Carcedo C, and Lehner B (2017). Maternal age generates phenotypic variation in Caenorhabditis elegans. Nature 552, 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chotard L, Mishra A, Sylvain M, Tuck S, Lambright D, and Rocheleau C (2010). TBC-2 Regulates RAB-5/RAB-7-mediated Endosomal Trafficking in Caenorhabditis elegans. Molecular Biology of the Cell 21, 2285–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant BH, D. (1999). Receptor-mediated Endocytosis in the Caenorhabditis elegans Oocyte. Molecular Biology of the Cell 10, 4311–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balklava Z, Rathnakumar ND, Vashist S, Schweinsberg PJ, and Grant BD (2016). Linking Gene Expression in the Intestine to Production of Gametes Through the Phosphate Transporter PITR-1 in Caenorhabditis elegans. Genetics 204, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy CT, and Hu PJ (2013). Insulin/insulin-like growth factor signaling in C. elegans. WormBook, 1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tepper RG, Ashraf J, Kaletsky R, Kleemann G, Murphy CT, Bussemaker HJ (2013). PQM-1 complements DAF-16 as a key transcriptional regulator of DAF-2-mediated development and longevity. Cell 154, 676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson ST, and Johnson TE (2001). daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Current Biology 11, 1975–1980. [DOI] [PubMed] [Google Scholar]
- 28.Baugh LR, Kurhanewicz N, and Sternberg PW (2011). Sensitive and precise quantification of insulin-like mRNA expression in Caenorhabditis elegans. PLoS One 6, e18086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu PJ (2007). Dauer. WormBook, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumsta C, and Hansen M (2012). C. elegans rrf-1 mutations maintain RNAi efficiency in the soma in addition to the germline. PLoS One 7, e35428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tijsterman M, O KL., Thijssen K., Plasterk RHA. (2002). PPW-1, a PAZ/PIWI Protein Required for Efficient Germline RNAi, Is Defective in a Natural Isolate of C. elegans. Current Biology 12, 1535–1540. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan RE, and Baugh LR (2016). L1 arrest, daf-16/FoxO and nonautonomous control of post-embryonic development. Worm 5, e1175196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan RE, Chen Y, Moore BT, Jordan JM, Maxwell CS, Schindler AJ, Baugh LR (2015). dbl-1/TGF-beta and daf-12/NHR Signaling Mediate Cell-Nonautonomous Effects of daf-16/FOXO on Starvation-Induced Developmental Arrest. PLoS Genet 11, e1005731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libina N, Berman JR, and Kenyon C (2003). Tissue-Specific Activities of C. elegans DAF-16 in the Regulation of Lifespan. Cell 115, 489–502. [DOI] [PubMed] [Google Scholar]
- 35.Zhang P, Judy M, Lee SJ, and Kenyon C (2013). Direct and indirect gene regulation by a life-extending FOXO protein in C. elegans: roles for GATA factors and lipid gene regulators. Cell Metab 17, 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rando OJ, and Simmons RA (2015). I’m eating for two: parental dietary effects on offspring metabolism. Cell 161, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGee MD, Day N, Graham J, and Melov S (2012). cep-1/p53-dependent dysplastic pathology of the aging C. elegans gonad. Aging 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langmead B, Trapnell C, Pop M, and Salzberg SL (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10, R25.21-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maxwell CS, Antoshechkin I, Kurhanewicz N, Belsky JA, and Baugh LR (2012). Nutritional control of mRNA isoform expression during developmental arrest and recovery in C. elegans. Genome Res 22, 1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anders S, Pyl PT, and Huber W (2015). HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eden E, Navon R, Steinfeld I, Lipson D, and Yakhini Z (2009). GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 10, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schindler AJ, Baugh LR, and Sherwood DR (2014). Identification of late larval stage developmental checkpoints in Caenorhabditis elegans regulated by insulin/IGF and steroid hormone signaling pathways. PLoS Genet 10, e1004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson MD, McCarthy DJ, and Smyth GK (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Webster AK, Jordan JM, Hibshman JD, Chitrakar R, and Baugh LR (2018). Transgenerational Effects of Extended Dauer Diapause on Starvation Survival and Gene Expression Plasticity in Caenorhabditis elegans. Genetics 210, 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Genes differentially expressed during L1 arrest in the progeny of DR parents compared to AL parents. Related to Figure 3.
Data Availability Statement
RNAseq data sets can be found at GEO using accession numbers GSE114271 and GSE129088.


