Abstract
In the U.S., black patients are less likely than whites to receive biologic treatment for their psoriasis. We conducted a qualitative freelisting study to identify patient-generated factors that may explain this apparent racial disparity in psoriasis treatment by comparing the perceptions of biologics and other psoriasis therapies between white and black adults with psoriasis. Participants included 68 white and black adults with moderate to severe psoriasis who had and had not received biologic treatment. Each participant was asked to list words in response to verbal probes querying five psoriasis treatments: self-injectable biologics, infliximab, methotrexate, apremilast, and phototherapy. Salience scores indicating the relative importance of each word were calculated, and salient words were compared across each race-treatment group. Participants who had experience with biologics generally associated positive words with self-injectable biologics. Among biologic-naïve participants, “apprehension”, “side effects”, and “immune suppression” were most salient. “Unfamiliar” and “dislike needles” were salient only among black participants who were biologic-naïve. Participants were generally unfamiliar with the other psoriasis therapies except phototherapy. Unfamiliarity with biologics particularly among black, biologic-naïve patients may, in part, explain the existing racial disparity in biologic treatment for psoriasis and might stem from lack of exposure to or poor understanding of biologics.
INTRODUCTION
Psoriasis is a chronic systemic inflammatory disease primarily of the skin that affects approximately 7.5 million Americans (National Psoriasis Foundation). Moderate to severe psoriasis, in particular, has major negative effects on the physical and psychosocial well-being of those affected (Rapp et al., 1999) and is associated with cardiometabolic (Azfar et al., 2012; Gelfand et al., 2009; Gelfand et al., 2006; Langan et al., 2012; Mehta et al., 2010; Wan et al., 2018) and other comorbid diseases (Chiesa Fuxench et al., 2016; Grewal et al., 2017; Kurd et al., 2010; Ogdie et al., 2018; Takeshita et al., 2018; Wan et al., 2013; Yeung et al., 2013) that may be modulated by psoriasis treatment (Ahlehoff et al., 2015; Bissonnette et al., 2017a; Bissonnette et al., 2017b; Mehta et al., 2018; Wu et al., 2017; Wu et al., 2018a; Wu et al., 2018b; Yang et al., 2016). Psoriasis is also associated with a large economic burden of up to $135 billion in total costs in 2013 dollars, of which up to $35.4 billion is attributed to indirect costs that are largely due to lost work productivity (Brezinski et al., 2015). Therefore, it is important on both individual and societal levels that patients with psoriasis receive adequate and effective treatment.
Despite a growing number of increasingly efficacious therapeutic options (primarily biologics) for treating moderate to severe psoriasis, most patients remain undertreated with only topical medications or no treatment at all (Lebwohl et al., 2014). Considering that, in the U.S., psoriasis may be more severe (Gelfand et al., 2005) and have a greater negative impact on quality of life (Shah et al., 2011) among racial/ethnic minorities than whites, undertreatment may disproportionately affect minority and other marginalized or disadvantaged individuals with psoriasis. For example, among Medicare recipients, black beneficiaries with moderate to severe psoriasis are 70% less likely to receive biologic treatment for their psoriasis than whites, independent of other demographic and socioeconomic factors, comorbidities, and differences in Medicare plans (Takeshita et al., 2015). A clinic-based study of patients with psoriasis in the U.S. also found similar racial differences in psoriasis treatment with biologics (Kerr et al., 2015). As biologic therapies increasingly become the mainstay of treatment for patients with more severe psoriasis, it is important to identify why black patients are less likely than whites to receive highly efficacious biologics for their skin disease and rectify any existing treatment disparity. Thus, our study aimed to understand psoriasis patients’ perceptions of biologics and other common or new psoriasis treatments by leveraging qualitative research methods which are aimed at generating testable hypotheses for why a racial disparity in biologic treatment for psoriasis exists in the U.S.
RESULTS
Participants
Sociodemographic and clinical characteristics of the 68 participants with moderate to severe psoriasis according to race-treatment groups are summarized in Table 1. Median age ranged from 48 to 55 years and was similar across race-treatment groups. Participants were predominantly female (70.6% of all participants). Most participants resided in Northeastern U.S. and were affiliated with the University of Pennsylvania Health System. Significantly more black participants were of lower income and education levels than whites. Differences in primary medical insurance were also seen across race-treatment groups.
Table 1.
Participant Characteristics
| White Biologic N = 19 |
White No Biologic N = 17 |
Black Biologic N = 16 |
Black No Biologic N = 16 |
P-Value | |
|---|---|---|---|---|---|
| Age (years), median (IQR) | 48 (35, 66) | 50 (36, 62) | 54 (46.5, 62) | 55 (46.5, 66.5) | 0.54 |
| Female | 12 (63.2) | 11 (64.7) | 14 (87.5) | 11 (68.8) | 0.39 |
| Annual household income | 0.005 | ||||
| <$50,000 | 6 (31.6) | 3 (17.6) | 10 (62.5) | 10 (62.5) | |
| ≥$50,000 | 12 (63.2) | 12 (70.6) | 3 (18.7) | 3 (18.7) | |
| Prefer not to answer | 1 (5.2) | 2 (11.8) | 3 (18.7) | 3 (18.7) | |
| Education | <0.001 | ||||
| Less than bachelor’s degree | 4 (21.0) | 3 (17.6) | 12 (75.0) | 11 (68.8) | |
| Bachelor’s degree or more | 15 (79.0) | 14 (82.4) | 4 (25.0) | 5 (31.2) | |
| Employment | 0.14 | ||||
| Unemployed/unable to work | 5 (26.3) | 0 (0) | 4 (25.0) | 2 (12.5) | |
| Part time | 2 (10.5) | 5 (29.4) | 1 (6.2) | 1 (6.2) | |
| Full time | 9 (47.4) | 9 (52.9) | 6 (37.5) | 7 (43.8) | |
| Retired | 1 (5.3) | 2 (11.8) | 5 (31.3) | 5 (31.3) | |
| Other | 2 (10.5) | 1 (5.9) | 0 (0) | 1 (6.2) | |
| Marital status | 0.08 | ||||
| Single | 6 (31.6) | 6 (35.3) | 5 (31.3) | 5 (31.3) | |
| Married/domestic partner | 11 (57.9) | 11 (64.7) | 6 (37.5) | 5 (31.3) | |
| Divorced/separated/widowed | 1 (5.3) | 0 (0) | 5 (31.3) | 5 (31.3) | |
| Other | 1 (5.3) | 0 (0) | 0 (0) | 0 (0) | |
| Prefer not to answer | 0 (0) | 0 (0) | 0 (0) | 1 (6.3) | |
| Residence in Northeast U.S. | 14 (73.7) | 10 (58.8) | 14 (87.5) | 15 (93.8) | 0.08 |
| Primary medical insurance | 0.004 | ||||
| Commercial | 8 (42.1) | 15 (88.2) | 9 (56.3) | 7 (43.8) | |
| Medicare/Medicaid | 9 (47.4) | 1 (5.9) | 7 (43.7) | 5 (31.3) | |
| Other | 0 (0) | 1 (5.9) | 0 (0) | 1 (6.2) | |
| None | 2 (10.5) | 0 (0) | 0 (0) | 0 (0) | |
| Prefer not to answer | 0 (0) | 0 (0) | 0 (0) | 3 (18.7) |
IQR, interquartile range
Psoriasis history and characteristics, with the exception of treatment history, were similar across all race-treatment groups (Table 2). Compared with participants who had experience with biologics, biologic-naïve participants were more likely to be currently receiving phototherapy or topical therapy only and were less likely to have been on oral systemic therapy in the past.
Table 2.
Psoriasis History
| White Biologic N = 19 |
White No Biologic N = 17 |
Black Biologic N = 16 |
Black No Biologic N = 16 |
P-Value | |
|---|---|---|---|---|---|
| Age (years) of psoriasis onset, median (IQR) | 0.46 | ||||
| <40 years old | 16 (84.2) | 11 (64.7) | 11 (68.8) | 10 (62.5) | |
| ≥40 years old | 3 (15.8) | 6 (35.3) | 5 (31.2) | 6 (37.5) | |
| Psoriasis duration (years), median (IQR) | 21 (14, 34) | 10 (7, 22) | 8 (5.5, 32.5) | 9 (2.25, 25) | 0.83 |
| Current psoriasis severity | 0.88 | ||||
| None/very little | 5 (26.3) | 3 (17.7) | 3 (18.8) | 3 (18.8) | |
| Mild | 5 (26.3) | 3 (17.7) | 2 (12.5) | 3 (18.8) | |
| Moderate | 6 (31.6) | 8 (47.1) | 7 (43.8) | 4 (25.0) | |
| Severe | 3 (15.8) | 3 (17.7) | 4 (25.0) | 6 (37.5) | |
| Psoriatic arthritis | 9 (47.4) | 4 (23.5) | 6 (37.5) | 3 (18.8) | 0.28 |
| Primary medical provider for psoriasis | 0.53 | ||||
| Dermatologist | 15 (79.0) | 16 (94.1) | 15 (93.8) | 15 (93.8) | |
| Rheumatologist | 3 (15.8) | 1 (5.9) | 1 (6.3) | 0 (0) | |
| Other | 1 (5.3) | 0 (0) | 0 (0) | 1 (6.2) | |
| Dermatology life quality index, median (IQR) | 7 (1, 11) | 5 (2, 9) | 9 (2.5, 15.5) | 5.5 (2, 13.5) | 0.62 |
| Current treatment | |||||
| Phototherapy | 0 (0) | 8 (47.1) | 2 (12.5) | 8 (50.0) | <0.001 |
| Oral systemic | 1 (5.3) | 4 (23.5) | 1 (6.3) | 2 (12.5) | 0.44 |
| Biologic | 13 (68.4) | 0 (0) | 12 (75.0) | 0 (0) | <0.001 |
| Topicals only | 1 (5.3) | 7 (41.2) | 1 (6.3) | 5 (31.3) | 0.02 |
| Other | 1 (5.3) | 0 (0) | 1 (6.3) | 0 (0) | 0.86 |
| None | 3 (15.8) | 0 (0) | 1 (6.3) | 1 (6.3) | 0.36 |
| Past treatment | |||||
| Phototherapy | 12 (63.2) | 10 (58.8) | 11 (68.8) | 12 (75.0) | 0.82 |
| Oral systemic | 13 (68.4) | 4 (23.5) | 9 (56.3) | 1 (6.3) | <0.001 |
| Biologic | 16 (84.2) | 0 (0) | 15 (93.8) | 0 (0) | <0.001 |
| Topicals only | 0 (0) | 4 (23.5) | 0 (0) | 3 (18.8) | 0.02 |
| Other | 3 (15.8) | 1 (5.9) | 1 (6.3) | 0 (0) | 0.46 |
| None | 1 (5.3) | 2 (11.8) | 0 (0) | 1 (6.3) | 0.73 |
IQR, interquartile range
Freelisting Results
Self-Injectable Biologics
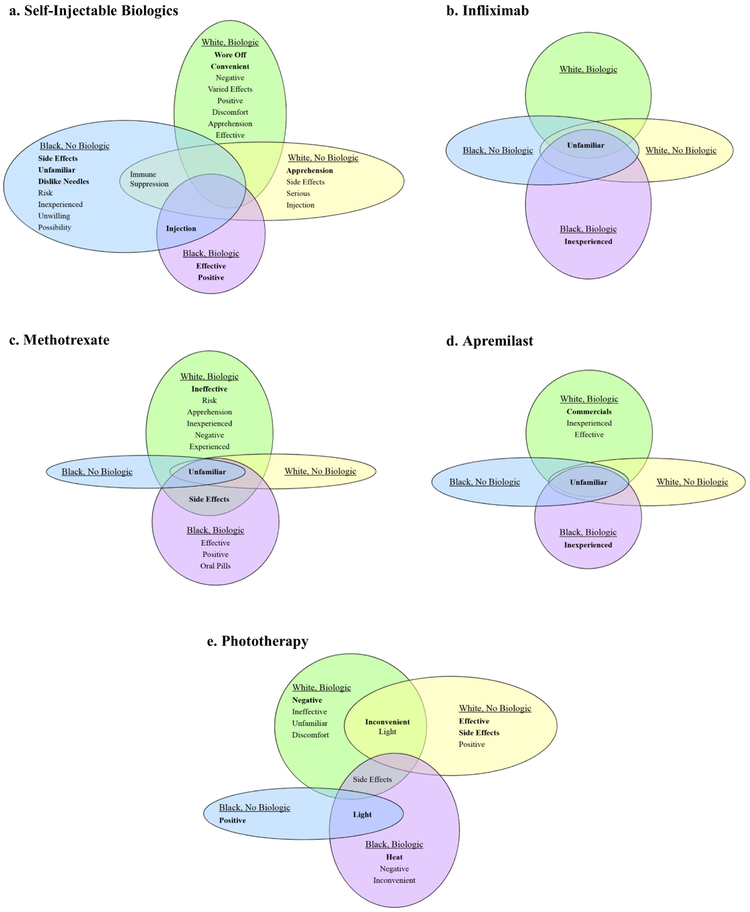
Salient words for self-injectable biologics varied across race-treatment groups (Figure 1a). Positive feelings (“positive”, “effective”, “convenient”) were salient only among participants who had experience with biologic treatment, but were mixed with some negative perceptions particularly among the white-biologic group. Among biologic-naïve participants, feelings of apprehension and concern over potential side effects and immune suppression were salient. Notably, lack of familiarity with self-injectable biologics and dislike of needles were each strongly salient for the black biologic-naive group only. Additionally, only black participants linked “injection”, which included the descriptive terms “shots” and “needles”, with self-injectable biologics.
Figure 1. Salient items from freelists for psoriasis therapies by race-treatment groups.
For each psoriasis therapy [(a) self-injectable biologics, (b) infliximab, (c) methotrexate, (d) apremilast, and (e) phototherapy] words are listed in order of salience from most to least salient. Bolded words are strongly salient; plain text words are moderately salient. Words are listed twice if strongly salient in one group and moderately salient in another.
Considering baseline differences in income and education among the race-treatment groups, we also compared freelist data for self-injectable biologics between higher and lower income and education groups (Table 3). “Unfamiliar” was not salient among any of the four groups. However, for both the lower income and education groups, “injection” was identified as the only salient item, whereas apprehension and concerns over side effects and immune suppression were salient for both the higher income and education groups.
Table 3.
Salient Items* From Freelists of Psoriasis Therapies by Income and Education Level
| Psoriasis Therapy |
Income^ | Education | ||
|---|---|---|---|---|
| Higher (N = 30) |
Lower (N = 29) |
Higher (N = 38) |
Lower (N = 30) |
|
| Self-Injectable Biologics | side effects | injection | apprehension | injection |
| apprehension | side effects | |||
| negative | immune suppression | |||
| immune suppression | ||||
| Infliximab | unfamiliar | unfamiliar | unfamiliar | unfamiliar |
| inexperienced | ||||
| Apremilast | unfamiliar | unfamiliar | unfamiliar | unfamiliar |
| commercials | ||||
| inexperienced | ||||
| Methotrexate | unfamiliar | unfamiliar | unfamiliar | unfamiliar |
| side effects | side effects | side effects | side effects | |
| risk | ||||
| ineffective | ||||
| Phototherapy | inconvenient | light | inconvenient | light |
| positive | negative | positive | ||
| light | effective | |||
| side effects | booth | |||
| unfamiliar | ||||
| side effects | ||||
| ineffective | ||||
Bolded words are strongly salient; plain text words are moderately salient.
Participants who did not provide income information (N=9) were excluded from analysis.
Infliximab
The most salient word associated with infliximab was “unfamiliar” across all race-treatment (Figure 1b), income, and education groups (Table 3). Lack of experience with infliximab treatment was also strongly salient for each of the black-biologic and lower education groups.
Methotrexate
“Unfamiliar” was strongly salient for methotrexate across all race-treatment (Figure 1c), income, and education groups (Table 3). Among white-biologic and black-biologic participants, who were also more likely to have been treated with methotrexate in the past, additional strongly salient items included “side effects” for both groups and “ineffective” for the white-biologic group. Moderately salient items among participants with history of biologic treatment were generally negative in nature among whites and positive among blacks.
Apremilast
Similar to infliximab and methotrexate, “unfamiliar” was also strongly salient for apremilast across all race-treatment (Figure 1d), income, and education groups (Table 3). Specifically, for the white-biologic group, reference to commercials for apremilast was strongly salient. “Commercials” remained strongly salient among whites, regardless of biologic treatment status, compared with all blacks (data not shown) and was also moderately salient for those in the higher education group.
Phototherapy
“Inconvenient” was the most salient item for phototherapy among white participants, regardless of biologic treatment history, whereas it was the least moderately salient item for the black-biologic group and was not at all salient for the black biologic-naïve group (Figure 1e). Comparing all white and black participants, “inconvenient” remained a strongly salient item for the former while “light” and “positive” were salient items for the latter (data not shown). Similarly, among participants who had experience with biologic treatment, negative feelings and “inconvenient” were strongly salient compared with biologic-naïve participants for whom positive feelings and “effective” were strongly salient (data not shown). A comparison of salient items between participants who were currently receiving versus not receiving phototherapy was performed as well and revealed a mix of both positive and negative words. Of note, the most salient words associated with those currently receiving phototherapy were positive or neutral whereas negative or neutral items were most salient for those not currently receiving phototherapy (data not shown). “Inconvenience” was also the most and only strongly salient item for the higher income and education groups (Table 3). The term “light” which included descriptors of phototherapy (e.g., “UV-ray”, “lights”, “sun lamp”) was strongly salient among all black participants and those in the lower income and education groups.
DISCUSSION
In our freelisting study of white and black patients with moderate to severe plaque psoriasis, we identified differences in perceptions and understanding of psoriasis therapies by race and treatment history that reveal potential reasons for the existing racial disparity in biologic treatment for psoriasis. Most strikingly, we found that for self-injectable biologics, lack of familiarity was a uniquely salient item among black participants who were biologic-naïve. Unfamiliarity with self-injectable biologics among this group was unlikely to be due to patients never having been treated with biologics as “unfamiliar” was not a salient item among white participants who were also biologic-naïve. Similarly, in analyses that compared participants by income and education levels, lack of familiarity with self-injectable biologics did not emerge as a salient item suggesting that neither of these measures of socioeconomic status were primary drivers of our findings among the black biologic-naïve group. Collectively, these results indicate that black patients with psoriasis, in particular, are less aware of biologics as therapeutic options despite biologics being highly efficacious treatments for psoriasis. We hypothesize that greater unfamiliarity with biologics among black versus white patients may be an important modifiable cause of the racial disparity in biologic treatment for psoriasis that we have previously observed (Takeshita et al., 2015).
Notably, “side effects” and “dislike needles” were also uniquely salient items for self-injectable biologics among the black, biologic-naïve group. This suggests that patients’ preference to avoid needles and greater concern about side effects compared with other race-treatment groups for whom these items were not salient may also drive different biologic treatment patterns. The idea that the risk-benefit balance for biologics may be different between whites and blacks is supported by a single study of rheumatoid arthritis patients that found blacks to be more risk-averse than whites when considering treatment with disease-modifying antirheumatic drugs (Constantinescu et al., 2009).
While treatment differences due to patient preferences, alone, do not constitute a disparity, it is important to remember that patient preferences are not always based on an accurate or adequate comprehension of health care. In fact, preferences that are founded on an inaccurate or inadequate understanding can be a source of disparity (Institute of Medicine, 2003). We cannot definitively determine from our study if the identified salient themes among race-treatment groups are based on similar levels of understanding of biologics. However, it is notable that among the salient items for self-injectable biologics, purely descriptive terms, as captured under the item “injection”, were highly salient among black but not white participants and also among participants of lower income and education levels. Among all salient items, a predominance of descriptive terms, some of which were provided in the freelisting prompts, over other words or phrases that express a feeling or represent deeper knowledge may support a hypothesis that the understanding of biologics is less comprehensive among the black, lower income, and lower education participants than white, higher income, and higher education participants, respectively. A similar pattern of descriptive salient terms (i.e., “light”) among black, lower income, and lower education participants was noted for phototherapy, even though a numerically higher percentage of black participants were currently receiving phototherapy.
Lack of familiarity with treatment was noted to be the most or second most salient item for all other psoriasis therapies (methotrexate, apremilast, infliximab), except for phototherapy. In contrast to self-injectable biologics, lack of familiarity with these other treatments was a similar theme across all race-treatment groups, and, at least in part, likely related to the fact that few participants were currently receiving methotrexate, infliximab, or apremilast in our study.
As with all studies, there are limitations to consider. The main limitation is that most study participants were from Northeastern U.S. and had been seen at a single, large, urban academic health center. As the intent of qualitative research is hypothesis generation rather than generalizability, our findings may not be representative of all patients with psoriasis throughout the U.S. who may be seen in different medical settings or who may have chosen not to participate in our study. However, many participants had a long history of psoriasis for which they had received care from multiple providers across different locations over their skin disease course. Second, there were differences in income and education level between the white and black participants that may explain our results. However, our additional analyses by income and education level suggest that our findings for the biologics are not entirely due to socioeconomic status differences. Third, our study was designed to identify differences in perceptions of psoriasis therapies by race-treatment groups primarily for biologics. Therefore, perceptions of other therapies and differences across other group categorizations require further study. Finally, as there are many potential causes of treatment disparities, understanding not only the patient’s experiences and perceptions but also the medical provider and health system factors that may contribute to disparities is important in future work.
In conclusion, we hypothesize that racial disparities in biologic treatment for psoriasis in the U.S. may be due to a greater level of unfamiliarity with biologics and preference to avoid needles among black patients compared with whites. Poor familiarity with biologics may stem from having less exposure to the treatments (e.g., not being offered a biologic by a medical provider, not seeing advertisements for biologics, among other reasons) or lack of recognition or understanding of biologics as treatments for psoriasis even when patients have been offered treatment with or have received information about them. Therefore, we propose that efforts to improve exposure to and understanding of biologic therapeutic options for psoriasis among black patients may be essential to minimizing existing disparities in treatment for this common and chronic inflammatory skin disease that is associated with major comorbid disease burden that may also benefit from biologic therapy. Future studies aimed at testing our hypothesis may involve developing and quantitatively testing, in a randomized controlled trial, treatment decision aids or other educational efforts to improve awareness of psoriasis therapies, especially biologics, among black patients with psoriasis. Such studies are critical to advancing efforts to reduce psoriasis treatment disparities and improve outcomes for all.
MATERIALS & METHODS
Study Overview and Design
We designed a qualitative study that used freelisting to understand and compare perceptions of biologics and other common or newer psoriasis therapies among white and black individuals with moderate to severe plaque psoriasis who have and have not received biologic treatment. Freelisting is a standard, systematic interviewing method that is well-established in anthropologic research and has been more recently applied to medical research (Ahmad et al., 2016; Andrew et al., 2003; Barg et al., 2009; Bennett et al., 2006; Fiks et al., 2011; Patterson et al., 2006; Schrauf and Sanchez, 2008). Freelisting allows one to identify how people with shared experiences (e.g., a disease such as psoriasis) perceive or define a topic (e.g., psoriasis treatments) (Dongre et al., 2009; Weller and Romney, 1988). This method involves asking respondents (e.g., individuals with psoriasis) to list all words that come to mind when they think of a specific word or phrase (e.g., biologics) (Dongre et al., 2009). Through freelisting, salient or important words and phrases are identified and considered to define a topic or domain (i.e., set of items or things that are of the same category) of interest based on the principles that the most relevant items will be mentioned more frequently and earlier than less important or relevant items (Bernard et al., 1986; Borgatti, 1999a; Handwerker and Borgatti, 1988; Weller and Romney, 1988). Salient words identified from freelists can also be compared among different groups of people. The freelisting study was embedded within a longer, in-depth, semi-structured interview study aimed at understanding the experience of psoriasis and its treatments from the patient perspective. This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Pennsylvania. Every participant provided informed consent; written consent was obtained for in person interviews and verbal consent was obtained for telephone interviews.
Study Sample
Study subjects were recruited both locally and nationally. Local recruitment was primarily from dermatologist referrals from outpatient clinics affiliated with and/or patient responses to study advertisements within the University of Pennsylvania Health System. National recruitment was primarily from individual responses to study advertisements sent to members of the National Psoriasis Foundation with select additional recruitment by dermatologists of psoriasis patients seen at the University of California San Francisco, Johns Hopkins University, Howard University, and Cooper University dermatology clinics. Inclusion criteria were as follows: (i) at least 18 years of age, (ii) self-report of current or prior diagnosis of moderate to severe plaque psoriasis from a dermatologist for at least six months, (iii) self-identified race as either white or black, (iv) resides in the United States, and (vi) is or has been under the care of a dermatologist or rheumatologist for psoriasis. Moderate to severe psoriasis was defined by at least one of the following: (i) study referral from a dermatologist who confirmed a history of moderate to severe psoriasis, or (ii) self-report of current or prior treatment with or recommendation by dermatologist to be treated with phototherapy, oral systemic, or biologic for psoriasis. Individuals were excluded if they did not speak English. Participants were interviewed either in person or via telephone; interview mode has not been shown to affect semi-structured interview responses (Gravlee et al., 2013; Vogl, 2013).
As is standard for qualitative studies, we used a purposive sampling strategy in selecting consecutive study subjects who indicated interest in participating in our study. Considering the study objective to understand racial disparities in biologic treatment for psoriasis, we aimed to achieve an approximately equal number of white and black participants who have and have not received biologic treatment for their psoriasis. Sampling in qualitative research is continued until data saturation is reached which indicates that no new information or important themes arise (Dongre et al., 2009). We evaluated for saturation by analyzing the freelist data when 80% and 100% of the data had been collected. No new salient words were identified between the two analyses, and the sample was considered to be sufficient. Each participant was provided $40 for his/her study participation.
Data Collection
The research team developed a freelist interview guide (Supplementary Appendix 1) based on their expertise and input from clinical experts in psoriasis. The guide included an initial description of the study, instructions for freelisting, and an example exercise to allow the study participant to practice with a question unrelated to the study. The guide included a written script for the interviewer in order to maintain consistency across interviews. Participants were asked to provide a verbal list of words in response to five questions about specific psoriasis therapies (self-injectable biologics, infliximab, methotrexate, apremilast, and phototherapy) by asking, “What words come to mind when you think of ‘X’ as a psoriasis treatment?” where X represents each of the psoriasis therapies of interest. Infliximab was queried separately from the other biologics due to being administered intravenously rather than being self-injectable. Brand names for biologics, infliximab, and apremilast were used. An optional prompt with a short description of each therapy was provided to those who expressed initial unfamiliarity. The questions were designed to identify perceptions and understanding of biologics and other commonly used or newer therapies for moderate to severe psoriasis among individuals with the disease.
Interviews were conducted from May 2017 to January 2018. All interviews were performed by one of two members of the research team (W.T.E. or V.T.R.) who were trained in conducting semi-structured interviews. The freelisting portion of the interview typically lasted up to 10 minutes. The interviewers met regularly with the principal investigator (J.T.) and anthropologist qualitative methods expert (F.K.B.) to address any questions regarding the interview process. Interviews also included questions about participants’ sociodemographic characteristics, psoriasis history, and skin disease related quality of life as measured by the Dermatology Life Quality Index (Bronsard et al., 2010; Finlay and Khan, 1994).
Data Management and Analysis
Each study participant’s word lists were transcribed and evaluated independently by three research team members (W.T.E., C.B., and L.H.) in order to group similar ideas into standardized single words or phrases while maintaining the order in which words were mentioned. For example, in response to biologics, the single, standard word “injection” was used to represent each mention of “needle”, “needles” and “shot”. Standardized and ordered word lists were reviewed and validated by the principal investigator, revised as necessary, and entered as text files into Anthropac 4.98 (Analytic Technologies, Lexington, Kentucky). For the primary analysis, a text file of word lists was created for each of the five questions about psoriasis treatments and each race-treatment group indicating the participant’s combination of race and treatment history with biologics (white-biologic, white-no biologic, black-biologic, and black-no biologic) for a total of 20 text files. In additional analyses, text files of word lists for each of the five psoriasis treatments and each of the following race, education, and income groups were also created: white and black race, higher (≥$50,000) and lower (<$50,000) annual household income, and higher (bachelor’s degree or higher) and lower (less than a bachelor’s degree) education level.
A salience index (Smith’s S) for each item (i.e., word or theme) in each list was calculated in Anthropac using the following formula: S=[∑((L−Rj+1)/L)]/N, where L is the length of each list, Rj is the rank of item j in the list, and N is the number of lists in the sample (Borgatti, 1999b; Smith and Borgatti, 1997). Smith’s S is a numeric measure of item importance and identifies salient items which represent those that are most important for defining a domain of interest (e.g., biologics) among members of a group (e.g., individuals with psoriasis). Salience values theoretically range from 0.0 (an item that is never mentioned in any list) to 1.0 (the first item on every list). Salience scores remained linked to their corresponding item and were sorted from high to low and plotted as scree plots with the y-axis representing Smith’s S. The plots were examined to identify the elbow that indicates the transition from a steep to flattened slope. All items linked to salience scores greater than or equal to the elbow value were considered salient (Borgatti, 1999b). Among salient items, those with the highest S values and largest spatial separation from words with lower values were considered strongly salient while the remaining words were considered moderately salient. The primary analysis compared salient items among subjects in the white-biologic, white-no biologic, black-biologic, and black-no biologic race-treatment groups. In additional analyses, we also compared salient words between the following participant groups: white and black race, higher and lower annual household income, and higher and lower education level.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Warren R. Heymann, MD; Wilson Liao, MD; and Ginette A. Okoye, MD; for their assistance with study recruitment and to our study participants for sharing their time and thoughts with us.
FUNDING/SUPPORT
This study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases K23-AR068433 (Takeshita).
ROLES OF SPONSORS
Funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
CONFLICT OF INTEREST
Dr. Takeshita receives research grants from Pfizer Inc. (to the Trustees of the University of Pennsylvania) and has received payment for continuing medical education work related to psoriasis that was supported indirectly by Eli Lilly and Novartis. Dr. Gelfand served as a consultant for BMS, Boehringer Ingelheim, GSK, Janssen Biologics, Novartis Corp, Regeneron, UCB (DSMB), Sanofi and Pfizer Inc., receiving honoraria; and receives research grants (to the Trustees of the University of Pennsylvania) from Abbvie, Janssen, Novartis Corp, Sanofi, Celgene, Ortho Dermatologics, and Pfizer Inc.; and received payment for continuing medical education work related to psoriasis that was supported indirectly by Eli Lilly and Ortho Dermatologics. Dr. Gelfand is a co-patent holder of resiquimod for treatment of cutaneous T cell lymphoma. All other authors have no conflicts to declare.
PREVIOUS OR PLANNED MEETING PRESENTATION
An abstract of the data contained in this manuscript was presented at the International Investigative Dermatology 2018 Meeting (May 2018) and the American Public Health Association 2018 Annual Meeting (November 2018).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahlehoff O, Skov L, Gislason G, Gniadecki R, Iversen L, Bryld LE, et al. (2015) Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol 29:1128–34. [DOI] [PubMed] [Google Scholar]
- Ahmad FS, Barg FK, Bowles KH, Alexander M, Goldberg LR, French B, et al. (2016) Comparing Perspectives of Patients, Caregivers, and Clinicians on Heart Failure Management. J Card Fail 22:210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew G, Patel V, Ramakrishna J (2003) Sex, studies or strife? What to integrate in adolescent health services. Reprod Health Matters 11:120–9. [DOI] [PubMed] [Google Scholar]
- Azfar RS, Seminara NM, Shin DB, Troxel AB, Margolis DJ, Gelfand JM. (2012) Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol 148:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg FK, Keddem S, Ginsburg KR, Winston FK. (2009) Teen perceptions of good drivers and safe drivers: implications for reaching adolescents. Inj Prev 15:24–9. [DOI] [PubMed] [Google Scholar]
- Bennett I, Switzer J, Aguirre A, Evans K, Barg K. (2006) 'Breaking it down': patient-clinician communication and prenatal care among African American women of low and higher literacy. Ann Fam Med 4:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard HR, Pertti JP, Werner O, Boster J, Romney AK, Johnson A, et al. (1986) The contruction of primary data in cultural anthropology. Curr Anthropol 27:382–96. [Google Scholar]
- Bissonnette R, Harel F, Krueger JG, Guertin MC, Chabot-Blanchet M, Gonzalez J, et al. (2017a) TNF-alpha Antagonist and Vascular Inflammation in Patients with Psoriasis Vulgaris: A Randomized Placebo-Controlled Study. J Invest Dermatol 137:1638–45. [DOI] [PubMed] [Google Scholar]
- Bissonnette R, Kerdel F, Naldi L, Papp K, Galindo C, Langholff W, et al. (2017b) Evaluation of Risk of Major Adverse Cardiovascular Events With Biologic Therapy in Patients With Psoriasis. J Drugs Dermatol 16:1002–13. [PubMed] [Google Scholar]
- Borgatti S (1999a) Cultural consensus theory In: The Ethnographic Toolkit (Schensul J, Weeks M, eds), Newbury Park, CA: Sage Publications, 115–51. [Google Scholar]
- Borgatti SP (1999b) Elicitation techniques for cultural domain analysis In: Enhanced ethnographic methods: Audiovisual techniques, focused group interviews, and elicitation techniques (Schensul JJ, LeCompte MD, Nastasi BK, Borgatti SP, eds), Walnut Creek, CA: Altamira Press, 115–51. [Google Scholar]
- Brezinski EA, Dhillon JS, Armstrong AW (2015) Economic Burden of Psoriasis in the United States: A Systematic Review. JAMA Dermatol 151:651–8. [DOI] [PubMed] [Google Scholar]
- Bronsard V, Paul C, Prey S, Puzenat E, Gourraud PA, Aractingi S, et al. (2010) What are the best outcome measures for assessing quality of life in plaque type psoriasis? A systematic review of the literature. J Eur Acad Dermatol Venereol 24 Suppl 2:17–22. [DOI] [PubMed] [Google Scholar]
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, Gelfand JM (2016) The Risk of Cancer in Patients With Psoriasis: A Population-Based Cohort Study in the Health Improvement Network. JAMA Dermatol 152:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu F, Goucher S, Weinstein A, Smith W, Fraenkel L. (2009) Understanding why rheumatoid arthritis patient treatment preferences differ by race. Arthritis Rheum 61:413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongre A, Deshmukh P, Kalaiselvan G, Upadhyaya S. (2009) Application of qualitative methods in health research: an overview. Online J Health Allied Sci 8:3. [Google Scholar]
- Fiks AG, Gafen A, Hughes CC, Hunter KF, Barg FK. (2011) Using freelisting to understand shared decision making in ADHD: parents' and pediatricians' perspectives. Patient Educ Couns 84:236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay AY, Khan GK (1994) Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol 19:210–6. [DOI] [PubMed] [Google Scholar]
- Gelfand JM, Dommasch ED, Shin DB, Azfar RS, Kurd SK, Wang X, et al. (2009) The risk of stroke in patients with psoriasis. J Invest Dermatol 129:2411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. (2006) Risk of myocardial infarction in patients with psoriasis. JAMA 296:1735–41. [DOI] [PubMed] [Google Scholar]
- Gelfand JM, Stern RS, Nijsten T, Feldman SR, Thomas J, Kist J, et al. (2005) The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol 52:23–6. [DOI] [PubMed] [Google Scholar]
- Gravlee CC, Bernard HR, Maxwell CR, Jacobsohn A. (2013) Mode effects in free-list elicitation: comparing oral, written, and web-based data collection. Soc Sci Comput Rev 31:119–32. [Google Scholar]
- Grewal SK, Wan J, Denburg MR, Shin DB, Takeshita J, Gelfand JM. (2017) The risk of IgA nephropathy and glomerular disease in patients with psoriasis: a population-based cohort study. Br J Dermatol 176:1366–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker WP, Borgatti SP (1988) Reasoning with numbers In: Handbook of Methods in Cultural Anthropology (Bernard HR, ed), Walnut Creek, CA: AltaMira Press, 549–93. [Google Scholar]
- Institute of Medicine (2003) Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, D.C [Google Scholar]
- Kerr GS, Qaiyumi S, Richards J, Vahabzadeh-Monshie H, Kindred C, Whelton S, et al. (2015) Psoriasis and psoriatic arthritis in African-American patients--the need to measure disease burden. Clin Rheumatol 34:1753–9. [DOI] [PubMed] [Google Scholar]
- Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM (2010) The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol 146:891–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN et al. (2012) Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol 132:556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebwohl MG, Bachelez H, Barker J, Girolomoni G, Kavanaugh A, Langley RG et al. (2014) Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol 70:871–81 e1-30. [DOI] [PubMed] [Google Scholar]
- Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. (2010) Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J 31:1000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. (2018) Effect of 2 Psoriasis Treatments on Vascular Inflammation and Novel Inflammatory Cardiovascular Biomarkers: A Randomized Placebo-Controlled Trial. Circ Cardiovasc Imaging 11 :e007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Psoriasis Foundation. https://www.psoriasis.org/content/statistics. Accessed May 30, 2018, 2018.
- Ogdie A, Grewal SK, Noe MH, Shin DB, Takeshita J, Chiesa Fuxench ZC et al. (2018) Risk of Incident Liver Disease in Patients with Psoriasis, Psoriatic Arthritis, and Rheumatoid Arthritis: A Population-Based Study. J Invest Dermatol 138:760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AE, Winch PJ, Gilroy KE, Doumbia S. (2006) Local terminology for medicines to treat fever in Bougouni District, Mali: implications for the introduction and evaluation of malaria treatment policies. Trop Med Int Health 11:1613–24. [DOI] [PubMed] [Google Scholar]
- Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr., Reboussin DM. (1999) Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 41:401–7. [DOI] [PubMed] [Google Scholar]
- Schrauf RW, Sanchez J (2008) Using freelisting to identify, assess, and characterize age differences in shared cultural domains. J Gerontol B Psychol Sci Soc Sci 63:S385–93. [DOI] [PubMed] [Google Scholar]
- Shah SK, Arthur A, Yang YC, Stevens S, Alexis AF. (2011) A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol 10:866–72. [PubMed] [Google Scholar]
- Smith JJ, Borgatti SP (1997) Salience counts and so does accuracy: correcting and updating a measure for free-list-item salience. J Linguist Anthropol 7:208–9. [Google Scholar]
- Takeshita J, Gelfand JM, Li P, Pinto L, Yu X, Rao P, et al. (2015) Psoriasis in the US Medicare Population: Prevalence, Treatment, and Factors Associated with Biologic Use. J Invest Dermatol 135:2955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita J, Shin DB, Ogdie A, Gelfand JM. (2018) Risk of Serious Infection, Opportunistic Infection, and Herpes Zoster among Patients with Psoriasis in the United Kingdom. J Invest Dermatol 138:1726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl S (2013) Telephone versus face-to-face interviews: mode effect on semistructured interviews with children. Sociol Methodol 43:133–77. [Google Scholar]
- Wan J, Wang S, Haynes K, Denburg MR, Shin DB, Gelfand JM. (2013) Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ 347:f5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan MT, Shin DB, Hubbard RA, Noe MH, Mehta NN, Gelfand JM (2018) Psoriasis and the risk of diabetes: A prospective population-based cohort study. J Am Acad Dermatol 78:315–22 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller SC, Romney AK (1988) Systematic Data Collection. Sage Publications: Newbury Park, CA. [Google Scholar]
- Wu JJ, Guerin A, Sundaram M, Dea K, Cloutier M, Mulani P. (2017) Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol 76:81–90. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Joshi AA, Reddy SP, Batech M, Egeberg A, Ahlehoff O, et al. (2018a) Anti-inflammatory therapy with tumour necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis. J Eur Acad Dermatol Venereol doi: 10.1111/jdv.14951. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Sundaram M, Cloutier M, Gauthier-Loiselle M, Guerin A, Singh R, et al. (2018b) The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: An observational cohort study. J Am Acad Dermatol 79:60–8. [DOI] [PubMed] [Google Scholar]
- Yang ZS, Lin NN, Li L, Li Y. (2016) The Effect of TNF Inhibitors on Cardiovascular Events in Psoriasis and Psoriatic Arthritis: an Updated Meta-Analysis. Clin Rev Allergy Immunol 51:240–7. [DOI] [PubMed] [Google Scholar]
- Yeung H, Takeshita J, Mehta NN, Kimmel SE, Ogdie A, Margoli DJ et al. (2013) Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol 149:1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.