Abstract
The present study explored how motor cortical activity was influenced by visual perception of complex environments that either afforded or obstructed arm and leg reactions in young, healthy adults. Most importantly, we focused on compensatory balance reactions where the arms were required to regain stability following unexpected postural perturbation. Our first question was if motor cortical activity from the hand area automatically corresponds to the visual environment. Affordance-based priming of the motor system was assessed using single-pulse Transcranial Magnetic Stimulation (TMS) to determine if visual access to a wall-mounted support handle influenced corticospinal excitability. We evaluated if hand actions were automatically facilitated and/or suppressed by viewing an available handle within graspable range. Our second question was if the requirement for rapid movement to recover balance played a role in modulating any affordance effect in the hands. The goal was to disentangle motor demands related to postural threat from the impact of observation alone. For balance trials, a custom-built, lean and release apparatus was used to impose temporally unpredictable postural perturbations. In all balance trials, perturbations were of sufficient magnitude to evoke a compensatory change-in-support response; therefore, any recovery action needed to carefully take into account the affordances and constraints of the perceived environment to prevent a fall. Consistent with our first hypothesis, activity in an intrinsic hand muscle was increased when participants passively viewed a wall-mounted safety handle, in both seated and standing contexts. Contrary to our second hypothesis, this visual priming was absent when perturbations were imposed and the handle was needed to regain balance. Our results reveal that motor set is influenced by simply viewing objects that afford a grasp. We suggest that such preparation may provide an advantage when generating balance recovery actions that require quickly grasping a supportive handle. This priming effect likely competes with other task-dependent influences that regulate cortical motor output. Future studies should expand from limitations inherent with single-pulse TMS alone, to determine if vision of our surrounding world influences motor set in other contexts (e.g. intensified postural threat) and investigate if this priming corresponds to overt behavior.
Keywords: Corticospinal excitability, Reactive balance, Anticipatory set, Transcranial magnetic stimulation, Affordance
1. Introduction
Considerable evidence from both animal and human research has shown that simply viewing objects can potentiate specific actions, suggesting that we put our surroundings into motor terms automatically (Buccino et al., 2009; Cardellicchio et al., 2011; Franca et al., 2012; Grafton et al., 1997; Grèzes et al., 2003; Makris et al., 2011; Tucker & Ellis, 1998, 2004). This concept, referred to as ‘affordances’ (Gibson, 1979), has been demonstrated in humans using various imaging techniques including functional Magnetic Resonance Imaging (Grafton et al., 1997; Grèzes et al., 2003) and Transcranial Magnetic Stimulation (TMS) (Buccino et al., 2009; Cardellicchio et al., 2011; Franca et al., 2012; Makris et al., 2011, 2013) as well as behavioural outcomes such as improved reaction time when afforded actions are subconsciously primed (Tucker & Ellis, 1998). The ability to automatically translate the visual world into potential action offers a big advantage to smoothly interact with our environment. The predictive nature of using visual information to prime specific actions is especially relevant given processing delays inherent with a large, complex nervous system. Thus, behaviours that must be quick yet goal-directed stand to become particularly more effective. Among the class of human behaviours that would benefit most profoundly from this arrangement is the control of balance.
Although balance was long thought to be controlled at a subcortical level (Magnus, 1926; Sherrington, 1910), a large body of evidence now attests to a contribution of the cerebral cortex when maintaining upright posture, and this includes compensatory reactions to unexpected postural challenge (Bolton et al., 2011; Bolton et al., 2012; Mochizuki et al., 2010; Wälchli et al., 2017). Perhaps most crucial are the balance reactions that require the limbs to establish a new support base and catch a falling centre of mass (Maki et al., 2008; Maki et al., 2003; Maki & McIlroy, 1997). Notably, these change-of-support reactions are the only line of defence when postural perturbations exceed a certain threshold. The fact that cortical networks can play a role in responding to unexpected external postural perturbations seems remarkable given how quickly these whole-body responses must take place to avoid a fall. Indeed, the rapid onset latencies of automatic postural reactions compared with slower voluntary reaction times have been an historical impediment to recognize that the brain could play a meaningful role in balance. This was likely influenced by the classical cognitive psychology framework where sensorimotor transformations were thought to rely entirely on serial processes, a perspective significantly revised in recent years (Cisek & Kalaska, 2010). Any serial process through cortical networks, particularly a process that only starts after perturbation, would be unavoidably slow. However, if suitable responses could be established prior to a fall, this would offer a viable solution for producing fast, yet sophisticated ‘context-appropriate’ reactions. Thus, motor affordances hold great promise as a mechanism that may bias specific recovery actions suited to our surroundings, even before the need for such action.
Research protocols currently in use make it difficult to effectively expose cortical roles in reactive balance. The status quo is to focus on relatively small perturbations in clutter-free environments, with an emphasis on fixed support (feet-in-place) reactions. However, when perturbations are large, change-of-support reactions are the only option to recover stability (Maki & McIlroy, 1997). Daily life often imposes obstacles and various movement options that can help us regain balance, which forces a selection process to effectively target a limb to a new support base if a loss of balance occurs. As the need for behavioral adaptation rises, so does the demand on higher brain resources, particularly when the arms or legs are used to establish a new base of support amid complex surroundings. To truly emphasize cortical roles in reactive balance, environmental complexity needs to be introduced while forcing a change-of-support strategy with the limbs. Another major problem in the traditional study of reactive balance is the almost exclusive reliance on external measures such as muscle onsets, ground reaction forces, and video motion capture to infer neural processes. Such external measures fail to allow direct insight into what the brain may actually be doing to help avoid a fall. In fact, this problem is compounded when you consider that much of what the brain may do to prevent a fall in complex settings likely happens before the fall. This includes predicting future instability (Slobounov et al., 2009), building visuospatial maps as we move through our environment (Maki & McIlroy, 2007), and possibly forming contingencies based upon the environment even without foreknowledge of a fall (Bolton, 2015). Exposing such preparation would be entirely inaccessible without use of direct neurophysiological probes. Study designs that emphasize direct neural measures and change-of-support reactions within cluttered environments pose significant methodological challenges. However, these study designs also have great potential to reveal cortical mechanisms for how falls are avoided in the complex settings encountered in daily life.
In the current study, existing limitations will be overcome by using a direct measure of brain activity (TMS), before postural perturbation and in situations where the limbs are required to establish a new base of support in a choice-demanding environment (i.e. step or reach to recover balance depending upon the available option). This combination of experimental features represents an important innovation in the field to expose how the brain contributes to fall resistance in complex, real-life settings. The proposed study will test if the concept of affordances applies to the preparation of postural recovery actions.
There is presently no direct evidence for an affordance effect in a postural context, nor is there evidence for affordance priming evoked by objects relevant for balance recovery (e.g. safety handle). The objective of this study is to determine if corticospinal excitability (CSE) increases in an intrinsic hand muscle, First Dorsal Interosseus (FDI), when viewing a wall-mounted safety handle commonly used to regain balance. We predict that viewing a safety handle will excite the hand projection from the primary motor cortex (M1) compared to conditions where the handle is not visible (i.e. handle covered). Corticospinal excitability will be measured immediately following visual access to a response environment with or without a safety handle within graspable range. Standing participants will be (a) thrown off balance or (b) remain unperturbed in separate test blocks to determine if an affordance effect occurs with observation alone, and if this effect is amplified by postural threat. The rationale for this study is that the successful completion of the proposed research will provide evidence of a fundamental link between viewing a supportive object and motor preparation relevant for balance. The expectation is that this mechanism will automatically prime compensatory arm reactions based upon our surroundings, even in a context of simple observation where the participants know there is no postural threat.
1.1. Research hypotheses
Viewing a support handle will result in facilitated CSE in the FDI muscle of the right hand when compared to trials where the handle is blocked in trials where there is no postural threat.
When postural threat is present, there will be greater CSE facilitation in FDI when the handle is present compared with observation alone.
2. Material and methods
2.1. Participants
A total of 63 young, healthy usable participants (65% Female, 35% Male) ranging between 18–27 years of age (mean = 21.6 +/− 2.2 years) were included in the final analysis. See Appendix for the power analysis. Once all participants were collected, prior planned analyses were used to determine if adequate power had been attained to address Hypothesis 2. Participants were recruited from the student population at Utah State University. Participants were right handed, as verified using the Edinburgh Handedness Inventory (Oldfield, 1971). All participants provided written informed consent to the procedures prior to testing. All procedures described herein received approval from the Institutional Review Board at Utah State University and were conducted in accordance with the Declaration of Helsinki. Participants with neurological illness were excluded from the study. Furthermore, participants were screened prior to testing to assess their suitability for TMS using guidelines developed by a consortium of experts (Rossi, Hallett, Rossini, Pascual-Leone, & Safety of TMS Consensus Group, 2009).
2.2. Data acquisition
Electromyography (EMG) was recorded using Delsys DE-2.1 differential surface electrodes, which contain preamplifiers potted in polycarbonate enclosures (Delsys Inc., Boston, MA, USA). The electrode configuration includes 2 silver bars each 10 mm long by 1 mm in width. EMG signals were amplified (gain = 1000) using a Delsys Bagnoli-4 amplifier (Delsys Inc., Boston, MA, USA). Data was acquired and bandpass filtered (10–1000 Hz) using Signal Software and a Cambridge Electronic device (Power 1401, Cambridge Electronic Design, Cambridge, UK).
The specific muscles for this study were selected based on their relevance to a rapid reach-to-grasp action or forward stepping action. EMG was collected from two intrinsic hand muscles on the right hand and ankle dorsiflexors on both legs. The FDI and Opponens Pollicus (OP) were measured given the important role of these muscles in gripping, and past TMS-based studies exploring hand affordance on intrinsic hand muscles (Buccino et al., 2009; Cardellicchio et al., 2011; Franca et al., 2012; Makris et al., 2011). OP was used to detect hand response onset, while FDI addressed the main research question of changes in CSE. To detect stepping responses, the Tibialis Anterior (TA) on both legs was monitored throughout testing. Electrogoniometers (Biometrics Ltd, Newport, UK) measured ankle dorsiflexion during the forward leaning start position for each trial.
2.3. Testing Apparatus
2.3.1. Lean and release system:
A custom-made lean-and-release cable system was used to impose temporally unpredictable forward perturbations. The lean-and-release device has been successfully used in healthy adult populations as well as in clinical populations to assess reactive balance (Lakhani, Mansfield, Inness, & McIlroy, 2011; A. Mansfield et al., 2011; A. Mansfield, Inness, Lakhani, & McIlroy, 2012). While some aspects of the postural perturbation were predictable, for example the direction and amplitude, the exact trials where perturbations occurred was unknown to participants, and the onset of the perturbation was also unpredictable.
All testing was conducted with participants standing in a forward lean position depicted in Figure 1. This forward lean position was maintained by means of a body harness attached to a cable, which was then secured to the wall behind the participant. The cable was fastened posteriorly at mid-thoracic level to the body harness. At the start of each trial, participants were placed with their feet approximately hip width apart. The experimenter had the ability to suddenly release the cable tension thereby perturbing the participant forward. In addition to a wall-mounted ‘release’ cable attached to the body harness, participants were secured via support cables to girders in the ceiling. This secondary support system ensured that participants were prevented from falling to the ground in the event that their own compensatory response was inadequate. Throughout testing participants were instructed to remain relaxed and react only if the cable released.
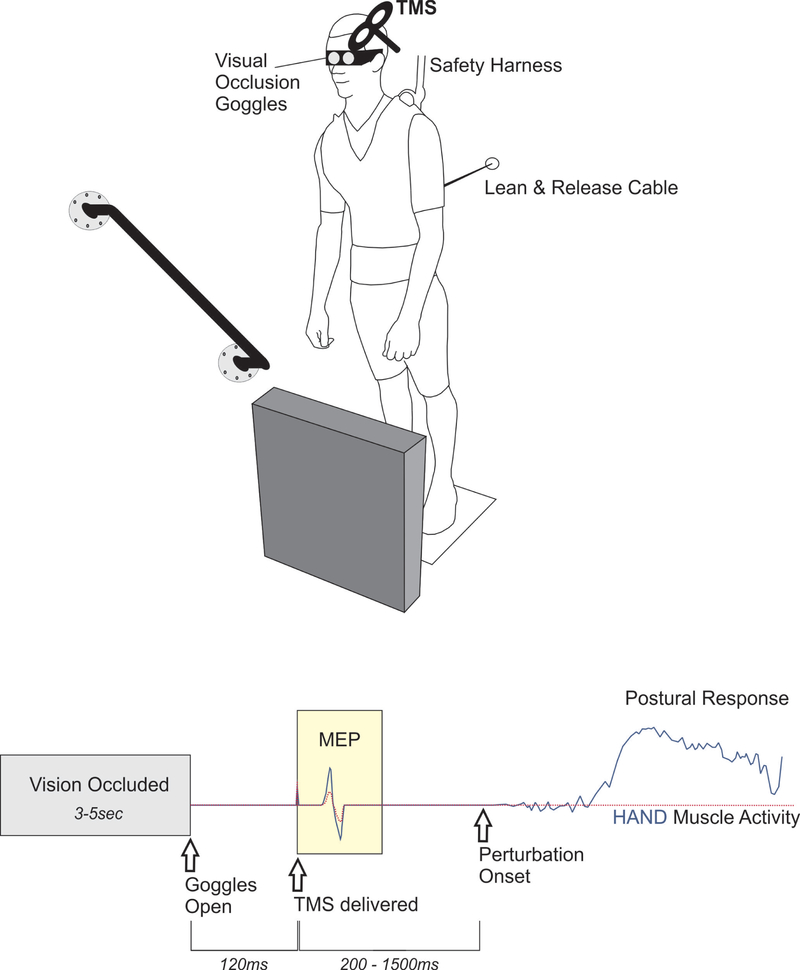
Figure 1: TMS-based method to investigate the impact of perceiving environmental affordances and/or constraints on motor preparation.
TOP. A ‘Lean and Release’ apparatus released participants in an unpredictable manner (perturbation test blocks only). The magnitude of perturbation required a rapid change of support reaction, using either the arm or leg to re-establish a stable base of support by either reaching to a secure handhold, or taking a forward step. In between trials, vision was occluded using liquid crystal occlusion spectacles and objects in the foreground was rearranged at random. BOTTOM. The timeline depicts when visual access to the environment became available and the timing of TMS probes relative to both visual access and the perturbation. The peak-to-peak amplitude of the muscle response to TMS (i.e. motor-evoked potential, MEP) provided an index of corticospinal excitability in the time period before perturbation. This figure presents theoretical response data to demonstrate the hypothesized impact of an affordance for hand action (solid, blue line) versus a trial where the handle is covered (dotted, red line). In this figure, both trials/conditions are overlaid to illustrate the hypothesized effect of preparing motor output to either facilitate or suppress potential action based on a particular environmental context.
Gaze fixation was standardized across participants to maintain a consistent handle presence in the peripheral visual field. The handle was placed ~ 30° to the right of central vision (Note: This is a placement based upon research demonstrating the efficacy of peripheral vision to shape reach-to-grasp actions following postural perturbation (Akram, Miyasike-daSilva, Ooteghem, & McIlroy, 2013)). Moreover, body position was set to ensure that the handle was clearly within a graspable range. The experimenter instructed participants to lean as far forward as the cable allowed while keeping both feet in contact with the floor. This position required anterior rotation about the ankle, as the rest of the body remained aligned. The exact forward lean position for each participant was determined as the minimal lean angle where a change-of-support reaction (i.e. forward step) was necessary to recover balance upon cable release. Once we established this position, the ankle angle was measured using electrogoniometers. Participants were monitored throughout testing to ensure the same ankle angle was maintained across trials.
2.3.2. Affordances and constraints:
A support handle was positioned on the wall to the right and slightly forward of participants while they leaned into the cable. For half of the trials the handle was freely available and visible. In the event of perturbed balance, this handle acted as a stable support surface to target a compensatory reach-to-grasp. On the trials where the support handle was available, a block was also present directly in front of the participant’s legs to obstruct potential stepping reactions. Such placement of leg blocks has been suggested as a valuable method to force reliance on a reach-to-grasp action during postural perturbation tests (K. C. Cheng et al., 2009). Although the leg block was intended to impede movement, it is important to note that it was not rigidly fixed in place and could be displaced in the case of limb contact to avoid potential injury with stepping into the obstacle. For trials where the support handle was not available to grasp, a black tarp covered the handle to block it from view. The handle remained mounted at the same location; however, it was physically blocked to prevent direct visual access and to prevent any supportive grasp. For trials without a support handle, no leg block was present. In this situation, a freely available step path afforded a rapid change of leg support to regain balance in the event of perturbed balance.
2.3.3. Control of vision:
Visual access to a complex (i.e. choice-demanding) environment was limited to a time window immediately before postural perturbation. Access to vision was manipulated in this study by use of liquid crystal goggles (Translucent Technologies Inc. Toronto, ON, Canada). These goggles can be programed to open at precise time points, allowing a means for controlling the onset of visual stimuli in the environment. While closed, these goggles allowed an illuminated view without access to the visual scene therefore participants were unaware of the upcoming response setting. During this visual occlusion period, the configuration of obstacles and handholds were changed for each trial. Therefore, participants needed to quickly perceive and adapt their movements to a novel environment once the goggles opened for viewing. The handle cover and leg block were moved into position via computer-triggered, servo motors at the start of each trial regardless of condition. The consistent sound of the motors across trials, in addition to ear plugs and occluded vision, was intended to avoid any advanced cueing for the upcoming condition.
2.4. TMS protocol
In this study, single-pulse TMS was delivered over the hand motor cortical representation while participants stood in a leaning position. TMS pulses occurred in a manner time-locked to the opening of the liquid crystal goggles for all experimental conditions. The purpose was to investigate the influence on motor preparation immediately upon receiving visual access to the environment. It is critical to note that TMS was delivered soon after visual access, but prior to any movement (in trials where movement was required). Recall that the essential feature of this study was the preparatory state of the motor system related to perception of the environment, which means that TMS pulses were not delivered at any time after the body was set in motion.
Magnetic stimuli were delivered to the left primary motor cortex (M1) by a Magstim 200 (monophasic waveform) stimulator (Magstim Company Ltd., Whitland, UK). Stimulation was applied using a figure of eight D702 Coil (Double 70mm2 Coil - Magstim Company Ltd., Whitland, UK), located at the optimal position to obtain a motor evoked potential (MEP) in a representative grasp muscle of the contralateral hand. Specifically, TMS pulses were delivered over the optimal site, the hotspot, to elicit an MEP for the right FDI. The stimulating coil was oriented at approximately 45 degrees to the sagittal plane, thus inducing posterior to anterior current flow across the motor strip (Kammer, Beck, Thielscher, Laubis-Herrmann, & Topka, 2001; Kantak et al., 2013). To allow hotspot localisation and consistent coil placement, markings were made directly on the scalp. Once the hotspot was located, a test stimulus intensity was determined, which was a stimulus intensity where the average MEPs were approximately 1–1.5 millivolts peak-to-peak. The TMS coil remained fixed on the hotspot for all trials and the coil position was reset following any head motion associated with a corrective balance response. Note that test stimulus intensity was determined while subjects were in a standing, forward-lean position (but no cable release) to control for any postural state influence on CSE.
2.5. Experimental design
2.5.1. Main study:
Participants were briefly familiarized with reaching to the handle and stepping forward from a leaning position prior to testing. Once testing commenced, they were instructed to remain relaxed and still unless prompted to move by a sudden cable release. In the event of cable release, participants were required to regain stability by either reaching for the secure support handle or stepping forward. All trials were divided into distinct test blocks where participants were informed to either (a) remain still and observe (OBS) or (b) avoid falling by means of a compensatory balance response (BAL) with their arms or legs to establish a new support base. OBS blocks were tested before BAL blocks to maximize the sense of stability participants had in a supported lean position. For BAL trials, participants were instructed to only move if the cable was released thus requiring a compensatory reaction. For these BAL trials, the cable was randomly released on 27% of the trials (8 of the 30). It is important to recognize that participants were aware that a sudden cable release would occasionally occur during BAL blocks; however, they were unable to predict the onset of perturbation, nor were they aware of which specific trials required a response. The BAL condition was intended to create a general context of imminent postural threat without providing advance cues for perturbation onset.
Each trial began with participants instructed to look directly at a fixation point on the floor, about 3 metres in front of them, while holding their head in a comfortable position. For all conditions, goggles closed at the start of each trial so that different environmental configurations for handles and obstacles could be automatically positioned using the motorized handle cover and leg block system. These configurations were randomly controlled by the data collection program so that participants remained unaware of the forthcoming response environment. After a randomly assigned ‘closed’ period of 3 to 4 seconds, the goggles opened offering a full view. The participant response environment included one of two possible configurations: (a) no stepping obstacle/no support handle (STEP), or (b) stepping obstacle present/support handle present (REACH). For the REACH condition, a support handle was visible/available to the right, and slightly in front of the participant on the wall at a comfortable reach distance. In this condition, a stepping obstacle was also placed in front of both legs. This setting offered a mixture of affordance for arm action while specifically blocking any potential stepping response. The intention of this setting was to impose a context where the only option available was to quickly grasp the available support handle with their right arm. TMS pulses were delivered 120 ms after opening the goggles but prior to any perturbation that occurred (see 2.5.2. preliminary testing below for rationale for this specific time point). On trials where a perturbation did occur, the cable released between 200 to 1000 ms after the trial began. In addition to the two visual conditions listed above, ‘no-vision’ reference trials were randomly interspersed throughout collection blocks to deliver TMS without opening the goggles. The purpose of this condition was to provide a baseline reference to account for any task-related changes in motor activity (e.g. heightened arousal). These reference trials offered a baseline for normalizing MEP amplitudes in this study.
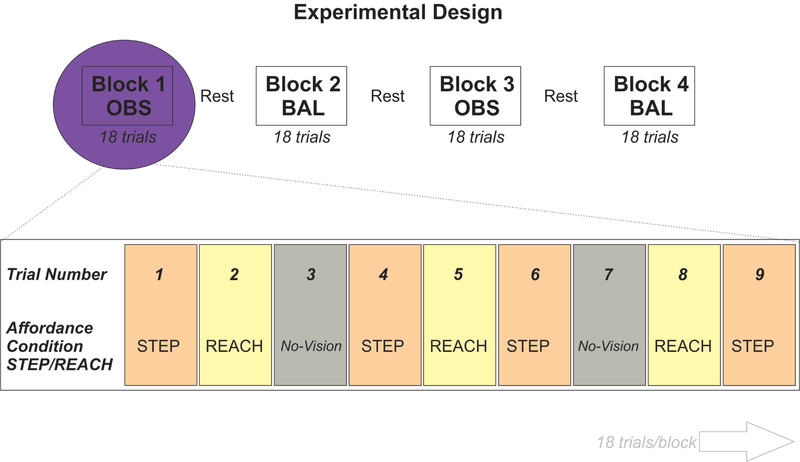
Test blocks consisted of 30 trials with two blocks per condition (OBS and BAL). Each test block consisted of 10 STEP trials, 10 REACH trials, and 10 No-Vision (NV) trials, randomly interspersed across the block, which resulted in a total of 120 trials for the main experiment. Each trial lasted 10 seconds, with a short pause between trials to allow participants a chance to reset as needed. For BAL blocks, the cable was randomly released on 8 of the 30 trials (4 STEP; 4 REACH). Participants were given a brief rest period in between each test block where they were allowed to sit down. The basic experimental design is depicted in Figure 2.
Figure 2: Experimental Design.
A visual representation of how trials were organized into blocks for either OBS (Observation only) or BAL (postural perturbations requiring compensatory balance response) with TMS over the hand area of the motor cortex. Brief rest periods were provided between each block. BAL blocks always took place first. Within each block, participants were exposed to different visual affordance conditions (STEP, REACH, No-vision) and single-pulse TMS was delivered 120ms following opening of the goggles. The particular affordance condition was randomized across trials.
2.5.2. Preliminary testing:
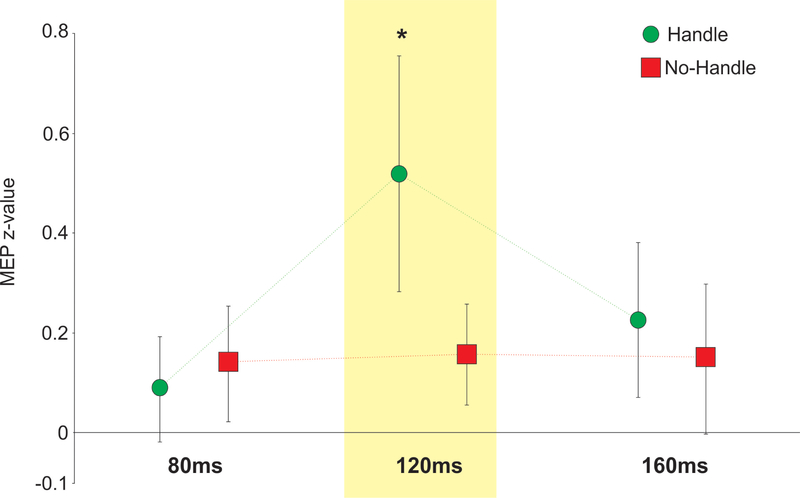
The proposed study extends from research limited to seated participants as they perform simple hand reactions to visual cues that are often displayed on a computer screen. Consequently, some initial testing was prudent to bridge the gap and determine if an affordance effect emerged in the presence of a support handle (prior to movement cues), as measured with TMS. To address this question, 25 young adults performed a seated reach-to-grasp task using a wall-mounted support handle placed directly in front of them and within a graspable range. TMS was delivered over the FDI hotspot using 120% resting motor threshold as a test stimulus (Note: Resting motor threshold was determined as the stimulator intensity where 5/10 MEPs exceeded 50 μV peak-to-peak). For this task, participants were required to reach for the handle only when hearing an auditory tone. In preliminary testing, single-pulse TMS was delivered at three distinct time points (80ms, 120ms and 160ms after the goggles opened) to address the following questions: (a) Does viewing a handle result in greater CSE relative to when the handle is not visible, (b) does this effect vary over time, and if so, (c) when was the effect most pronounced? Our results revealed greater MEP amplitudes when viewing the handle versus no-handle at 120ms (p < 0.05) following access to vision for the FDI and OP muscles (averaged) of the right hand (Figure 3). These preliminary results supported the proposed methods to measure affordance-based changes in CSE and indicated the timing where this effect could be readily exposed using TMS. Note: these results have recently been published in full (McDannald, Mansour, Rydalch, & Bolton, 2018).
Figure 3: Preliminary test results.
Data showing the difference in CSE for the handle versus no-handle trials in participants during a seated reach-to-grasp task for the FDI/OP muscles with standard error bars. *p < 0.05. Note: These results have been published (McDannald et al., 2018) following Stage 1 registered report acceptance.
2.5.3. Positive control:
At the end of each test session, a final test block was included to serve as an outcome-neutral, positive control. CSE of right-hand muscles was measured in seated participants while they directly fixated at the location of the safety handle (covered or uncovered). Past studies into motor affordances have investigated this effect in seated participants with direct vision of the viewed objects. Therefore, the purpose of this positive control was to replicate the existence of a ‘pure’ affordance effect based upon vision alone in a seated position. A single TMS pulse was delivered 120ms immediately following opening of the occlusion goggles. This timing is consistent with affordance priming in hand muscles when TMS was delivered at different time points ranging 120–180ms after visual presentation of objects (Franca et al., 2012). Importantly, this timing of 120ms is also consistent with our preliminary results outlined above (see section 2.5.2. Preliminary testing). The positive control block comprised 45 trials total: 15 ‘Handle’ trials, 15 ‘No-Handle’ trials, along with 15 ‘No-Vision’ trials to establish a baseline. The visual stimulus used the same wall-mounted handle as in the main study. However, in the positive control, participants were seated with the handle directly in front them within graspable range for the right hand. Once the occlusion goggles opened, participants could see either a handle or no handle (i.e. covered handle). Participants were instructed to remain relaxed at all times with both arms supported on armrests.
2.6. Data Analysis
2.6.1. Main study data processing:
An appropriate behavioral response was required to include a trial in the main analysis. An appropriate response was defined as (a) ‘reaching for the handle only following cable release and if a handle was available’ or (b) ‘stepping only following cable release and when the leg block was not present’. Consequently, any trials where the participant either (a) reached for the handle when it was covered, (b) stepped into the leg block, or (c) reached or stepped prior to the cable release were excluded from the main analysis. Responses prior to cable release were determined from the average amplitude of the full-wave rectified EMG signal. Specifically, a 100ms window prior to opening the goggles was compared with a 100ms window immediately after opening the goggles, but before TMS was delivered. This EMG measure was taken for the OP and TA muscles to detect early hand or leg activity. For each trial, a premature response was defined as trials where the average amplitude of the post-vision integrated EMG signal was greater than 2 standard deviations above the pre-vision average. Stepping or reaching errors after cable release were determined by force-sensitive resistors placed on the front of the leg block and the top surface of the safety handle, respectively (Note: In the event that the handle was covered, force applied to the top of the handle could be detected, while the cover still obstructed a secure grasp).
Background EMG was determined from the root mean square of EMG activity for the FDI muscle in a time window of 100ms immediately prior to TMS onset. If background EMG in this time window exceeded 10μV, the trial was discarded. Moreover, any trials producing a very small MEP amplitude (i.e. < 100μV peak-to-peak) were excluded. As a final step, outliers were identified as those values falling outside the threshold defined by 1.5 times the interquartile range, and these outliers were also excluded.
MEP amplitude was determined as the rectified EMG area beginning at the positive EMG signal deflection for the FDI muscle and ending 50ms post TMS (range: ~15ms – 50ms). To help standardize data, average MEP amplitudes were converted into z-scores to reduce potential variability between test blocks within an individual and to reduce inter-subject variability (Hasbroucq et al., 1999; Klein-Flügge & Bestmann, 2012). The mean and standard deviations of the MEP amplitudes during ‘No-Vision’ trials for each test block were used as a reference, for each participant separately. The individual MEP amplitudes observed in the other two conditions (handle, no-handle) were converted into z-scores calculated from this reference. These normalized values were then grouped for statistical analysis. Note that all MEP analyses were limited to the FDI muscle whereas OP was used to monitor reaching behavior, and TA was used to detect stepping behavior.
2.6.2. Main Study statistical analysis:
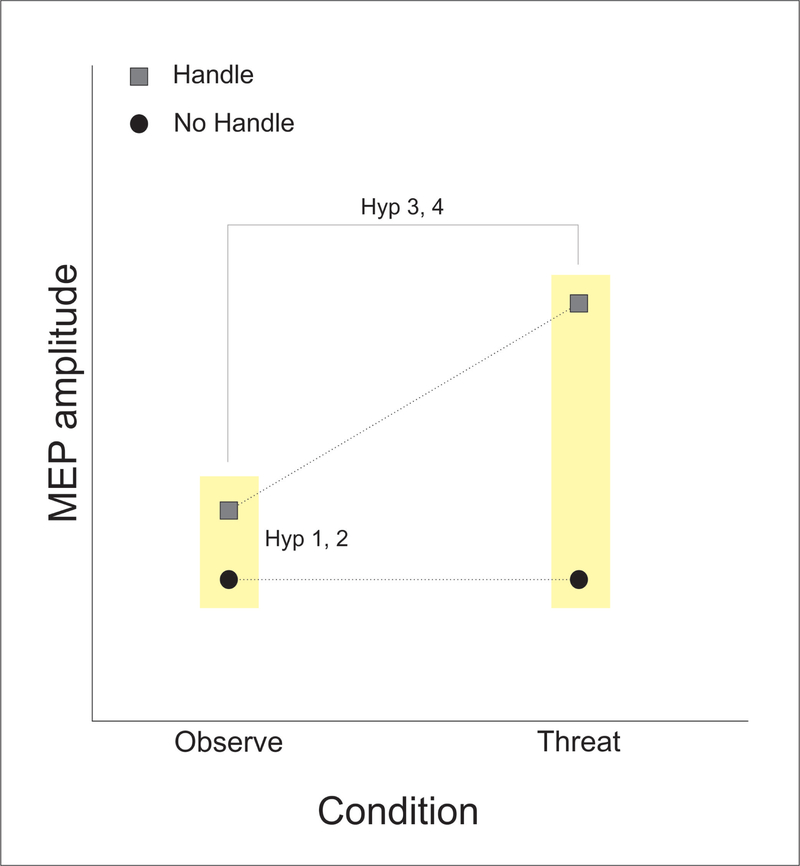
A 2 × 2 repeated measures ANOVA was used to test for interactions between factors ‘Condition’ (OBS, BAL) and ‘Affordance’ (STEP, REACH) for the MEP amplitude in the FDI muscle. First, a planned pairwise comparison was used to test Hypothesis 1, that viewing a handle without postural threat facilitates the FDI muscle relative to trials where the handle was covered. This analysis is essentially a paired t-test borrowing power from the other measures to more accurately estimate the pooled standard error when comparing affordance levels within the OBS condition. The interaction between condition and affordance addressed Hypotheses 2, that this affordance effect would be amplified when there was a postural threat. Both planned significance tests utilized one sided-alternative hypotheses (Fisher’s LSD, α < 0.05). Hypothesized effects for the FDI muscle are depicted in Figure 4.
Figure 4: Main study predictions (Hypotheses 1 and 2).
This diagram shows the predicted MEP changes in FDI when the handle was either (a) visible (blocks a step/affords a reach) or (b) covered (blocks a reach/affords a step). Hypotheses 1 tested the prediction that simply viewing a handle without postural threat would result in a significant increase in MEP amplitude relative to a covered handle). Hypotheses 2 tested the prediction that this affordance effect (i.e. handle MEP greater than no-handle MEP) would be significantly increased in the context of postural threat.
2.6.3. Positive control data processing and analysis:
The same steps for excluding trials and normalizing data described for the main experiment were used for the positive control data. A paired t-test was used to determine if the handle versus no-handle condition resulted in greater CSE. A one-tailed test (α < 0.05) was used for this comparison.
3. Results
The Stage 1 protocol received in-principle acceptance on 16th May 2018 and may be found at https://osf.io/qe4pm/. Raw data, the data acquisition/processing scripts, laboratory log, and guidance notes are also available on the Open Science Framework at the following link: https://osf.io/9z3nw/. A total of 65 participants completed testing, however one participant was removed for excessive EMG artifact and another failed to provide sufficient MEP data (due to screening criteria in the BAL condition). Notably, both of these participants were removed based on exclusion criteria specified in section 2.6.1. Main study data processing. This resulted in 63 participants for the final sample. Individual trials for each participant were screened (as outlined in the Methods section, 2.6.1. Main study data processing). From this screening process, 7.8 (+/−5.0) trials out of a possible 60 trials in the BAL condition were removed for each participant on average (of which 3.8 were response errors), 5.1 (+/−6.0) trials out of 60 in the OBS condition were removed, and 2.7 +/−2.2 trials out of 45 in the positive control condition were removed prior to data analysis. Average peak-to-peak MEP amplitudes for each condition were as follows: Positive control = 1.35 +/0.55 mV, OBS = 1.53 +/0.71 mV, BAL = 1.68 +/0.81 mV.
3.1. Main study results
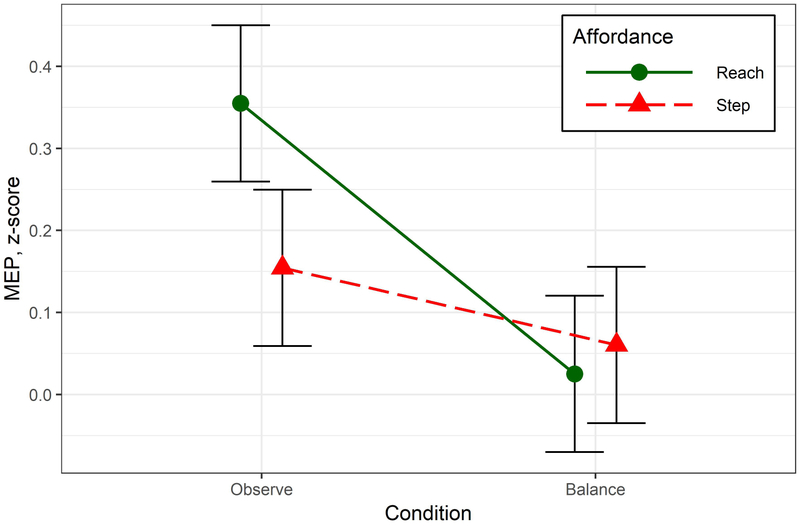
Two-way repeated measures ANOVA revealed an interaction between Condition and Affordance, F1, 62 = 5.69, p = .020, . To address our specific hypotheses, we used prior planned comparisons to determine if MEP amplitude in FDI was greater when the handle was present within each condition separately. For Hypothesis 1, planned comparisons were used to compare levels of Affordance (STEP, REACH) within the OBS condition and revealed a significant increase in amplitude when the handle was visible, t121 = 2.62, p = .010, Cohen’s d = 0.3. For Hypothesis 2, we had originally predicted an interaction, but in the opposite direction from what was found. Planned comparison of Affordance within the BAL condition showed no significant difference related to the presence of a handle, t121 = −0.46, p = .644. Instead of being augmented when postural perturbations were introduced, viewing the safety handle had no significant impact on MEP amplitude. Results are depicted graphically in Figure 5. A follow-up paired t-test comparison between BAL and OBS conditions showed a non-significant tendency for higher MEP amplitudes in the BAL condition t62 = 1.84, p = .07.
Figure 5: Main study results (Hypotheses 1 and 2).
Data showing the difference in CSE for the REACH (i.e. handle) versus STEP (i.e. no-handle) trials in the FDI muscle. This shows greater activity in the FDI muscle when the handle was present during the OBS condition but not the BAL condition. Error bars show the standard error of the mean.
3.2. Positive control results
A one-tailed, paired t-test was used to determine if MEP amplitude was greater when seated participants viewed the handle versus when the handle was covered. As predicted, MEP amplitude was significantly increased when the handle was visible, t62 = 2.58, p = .006, Cohen’s d = 0.33.
4. Discussion
Passively viewing a safety handle within graspable range resulted in increased muscle activity in an intrinsic hand muscle. This was found in seated participants as they directly viewed the handle, replicating our recent findings (McDannald et al., 2018), now also revealed in a standing context where the handle was visible in the periphery. Predictive biasing to grasp a supportive handle could in theory offer an advantage if this action needs to be summoned quickly (e.g. recovering from a stumble by grasping a nearby safety handle). Speculating along those lines, the second part of our study was designed to investigate if this motor affordance effect would be amplified in a situation where postural perturbations were imposed. Our original prediction was that visual priming—which presumably links the viewed handle with its associated motor action—would be increased in a context where the handle was needed. In contrast to our prediction, the affordance effect noted with passive observation was entirely absent during intensified postural threat. These results indicate that the subtle priming from viewing a graspable handle was inhibited or simply overshadowed by other factors that shape net CSE. It also suggests that factors affecting cortical motor set in a context of forthcoming compensatory balance reactions may be difficult to resolve using single-pulse TMS alone.
When interpreting present results, certain aspects of our task are important to consider. One key feature of our study design was to disentangle visual access to the environment from the imperative cue to move (i.e. cable release). This was necessary to evaluate visual priming in isolation from other processes that produce movement directly. In reaction-time studies such a paradigm is referred to as an instructed delay task, and when combined with TMS, this approach can be used to reveal changes in motor excitability from the moment of stimulus onset to the eventual motor response (Bestmann & Duque, 2016). An interesting result from such work is that CSE is temporarily suppressed during the delay period after the initial warning cue. The explanation for this seemingly paradoxical finding is that the selected action is held in check by the nervous system using a process of impulse control. Following up on this effect, Duque and colleagues (Duque, Labruna, Verset, Olivier, & Ivry, 2012) used paired-pulse TMS to demonstrate an important role for the premotor cortex in impulse control, and provided evidence that the inhibition appears to be exerted at a spinal, not cortical level. What this means is that cortical preparation for movement could potentially develop, while overt action is gated downstream until needed. Such a mechanism could allow for the benefits of advance cortical motor preparation while simultaneously withholding premature movement.
Another consideration when interpreting our results is the fact that our paradigm involves a choice-reaction. This added a ‘cognitive’ element to the task where rapid decision-making and response inhibition were required in combination to successfully avoid a forward fall. Our experiment was primarily intended to manipulate whether or not the handle was visible. However, one consequence of our study design was to potentially increase cognitive demands. Freeman and colleagues (Freeman, Itthipuripat, & Aron, 2016) found that the affordance effect, measured via electroencephalography, was abolished when participants were tasked with a higher working memory load. In a follow-up experiment, these same authors used paired-pulse TMS to show that higher working memory load was associated with greater intracortical suppression within the motor cortex. Increased intracortical suppression would make the motor cortex less responsive to any subtle affordance priming. While we did not challenge working memory per se, our modified lean and release task may have inadvertently burdened cognitive resources, resulting in tonic motor suppression.
Additionally, a leg block was presented in the lower visual field at the same time that the safety handle was displayed in the periphery. The purpose of the leg block was to strictly force a need to grasp the handle to regain balance (Note: Using a leg block is a common practice in studies that investigate compensatory arm responses to avoid a fall (K. C. Cheng et al., 2009; Kenneth C. Cheng, McKay, King, & Maki, 2012; King, McKay, Cheng, & Maki, 2010; Avril Mansfield & Maki, 2009)). However, a consequence of this arrangement is that the leg block would have required abrupt cessation of a stepping reaction. The imminent need to quickly prevent an automatic step may have exerted a strong, widespread suppressive influence across the entire motor system, a concept known as global suppression (Wessel & Aron, 2017). As an example, Majid and colleagues (Majid, Cai, George, Verbruggen, & Aron, 2012) revealed that when a highly automated hand response was suddenly prevented, a task-irrelevant leg muscle became simultaneously inhibited. It should be noted that studies investigating global suppression typically bias one specific, rapid response, making this action highly automatic and therefore difficult to inhibit. This contrasts our present approach where stepping and reaching responses were equally probably. Nonetheless, it is possible that global suppression to some degree may have dampened muscle activity in the hand.
Although TMS has been useful to understand neural processes that underlie the production of action (Bestmann & Duque, 2016), we acknowledge there are some clear limitations with our approach. In particular, the fact that we relied on single-pulse TMS limits what can be inferred from our data. As discussed above, this technique does not allow us to distinguish excitatory changes that develop within motor cortical networks from widespread changes throughout the entire corticospinal system. Furthermore, we delivered TMS at a single time point after visual access to the handle. This timing was based on preliminary research in our lab where we found that TMS delivered 120ms post-vision (but not at 80ms or 160ms) revealed an affordance effect in seated participants (McDannald et al., 2018). Although this provided us with an informed estimate for this registered report, our exclusive focus on one specific pulse timing invites the possibility that any affordance priming may go undetected at other time points. Our simplified TMS approach was deemed necessary to address specific research questions within practical time limits for a single test session. Future studies could employ paired-pulse TMS with expanded TMS timings to ascertain a more comprehensive picture of changes throughout the motor system when viewing a graspable object. Indeed, such an expanded approach using direct neurophysiological measures may ultimately be necessary to resolve predictive changes in motor set that emerge within the nervous system prior to postural perturbations (Dakin & Bolton, 2018).
As a final methodological consideration, we used a mode of perturbation that quickly released participants from a leaning start posture, which may raise the question of how much our model generalizes to real world falls. The lean-and-release technique has been previously used to gain valuable insight into reactive balance control (Thelen et al., 2000; Wojcik, Thelen, Schultz, Ashton-Miller, & Alexander, 1999), however, some peculiarities such as the leaning start position, and the fact that the direction and magnitude of the perturbation are known in advance, makes this scenario somewhat artificial. Moreover, the forward body displacement associated with the initial lean necessitates a pronounced step reaction when compared with steps initiated from an upright standing posture (Avril Mansfield & Maki, 2009). Despite these issues, this mode of perturbation was ideal for our purposes as it ensured consistent responses; most importantly, the forward reach when the handle was uncovered. The manner in which a perturbation was imposed was secondary and intended only to manipulate postural threat in a way that emphasized the relevance of the safety handle to recover balance. Of primary importance to our study, the safety handle was fixed in a constant spatial location to control vision of the handle to investigate visual priming.
4.1. Conclusions
Present results revealed an affordance effect in an intrinsic hand muscle when participants viewed a wall-mounted safety handle. When later faced with a context that occasionally required grasping the handle to avoid a fall, this effect was no longer evident. Visual priming appears to be one factor that influences hand muscle activity; however, additional task-dependent factors ultimately regulate net motor output. Impulse control, global suppression, and tonic suppression consequent to cognitive loading may in theory obscure an affordance effect, and even arousal associated with postural threat could potentially conceal any subtle priming when measured via net CSE. However, such speculation awaits experimental verification. We suggest that affordance priming for a grasp could in principle bias compensatory arm reactions before the need for such action arises. If true, this mechanism could yield balance reactions that are fast enough to avoid a fall, but also ecologically relevant to exploit opportunities for action (e.g. a new support base for the arm). Some caution is warranted however, as it remains a possibility that the affordance effect reported here and in past research may not actually have much of a direct functional impact. Further testing is needed to determine how motor set is dynamically shaped by our visual world and if indeed advance priming actually improves overt behavior as it relates to balance recovery.
Supplementary Material
Acknowledgements
The authors wish to thank Casey McPherson, Dayun Jeon, Haley Hayes, Elizabeth Edwards, James McJimsey, Ahmed Gaballah, Hunter Bell, and Paul Roberts for their assistance in collecting data for the pilot study. We also wish to thank Dr. Eadric Bressel and Dr. Chris Dakin for providing helpful insights into the study design. Research reported in this publication was supported by a grant to David Bolton by the National Institute On Aging of the National Institutes of Health under Award Number R21AG061688. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix – Protocol of Power Analysis
The sample size for this study was determined using G*Power (3.1.9.2): Statistical Power Analyses for Mac.
Positive Control and Hypothesis 1 (main study)
Power calculation for the positive control and Hypothesis 1 was based on results from a study by Makris et al. (2011). Experiment 2 of their study best approximates conditions where participants viewed graspable objects without a demand for movement. The ‘affordance’ effect size reported for MEP amplitude results was 0.3732. Based upon that effect size, power analysis indicated that for an alpha of .05, and a desired statistical power of 0.9, a total of 63 usable participants was required. The mean MEPs and standard deviations used for G*Power were reported by Makris et al. (2011) when participants viewed objects affording different grasp types (details shown below).
Specific parameters set into the program:
Test Family: t tests
Statistical test: Means: Difference between two dependent means (matched pairs)
Type of power analysis: A priori: compute required sample size – given α, power, and effect size
Tail(s): One
Effect Size dz = 0.3732
-Group 1: mean = 1.1, SD = 0.83
-Group 2: mean = 0.82, SD = 0.63
-Correlation between groups = 0.5
α = .05
Power = 0.9
Sample size = 63
Main study – Hypothesis 2
The present study attempted to bridge observations from past affordance research and determine if this concept applies when viewing a safety handle commonly used to avoid a fall. One challenge in estimating an appropriate sample size to test Hypothesis 2 (i.e. that postural threat will amplify the affordance effect) was the absence of a relevant effect size from which to base this calculation. The proposed solution was to collect the full sample (63) aimed at testing Hypothesis 1 and then use this sample to test the interaction between Condition (OBS, BAL) and Affordance (STEP, REACH). The observed effect size for the interaction would then be used to estimate the required final sample size needed to address Hypothesis 2. In the event that the study was underpowered at this stage to address Hypothesis 2, collection would continue with the stipulation of attaining a small effect size of 0.2 based on Cohen’s effect size classification (Cohen, 1988). Given that the interaction was found to be significant, testing was stopped at 63 participants.
J. Cohen Statistical power analysis for the behavioral sciences (2nd ed.), Lawrence Erlbaum Associates, Hillsdale, New Jersey (1988).
References for G*Power:
Faul, F., Erdfelder, E., Lang, A.-G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175-191.
Faul, F., Erdfelder, E., Buchner, A., & Lang, A.-G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41, 1149-1160.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Akram SB, Miyasike-daSilva V, Ooteghem KV, & McIlroy WE (2013). Role of peripheral vision in rapid perturbation-evoked reach-to-grasp reactions. Experimental Brain Research, 229(4), 609–619. 10.1007/s00221-013-3624-z [DOI] [PubMed] [Google Scholar]
- Bestmann S, & Duque J (2016). Transcranial Magnetic Stimulation: Decomposing the Processes Underlying Action Preparation. The Neuroscientist, 22(4), 392–405. 10.1177/1073858415592594 [DOI] [PubMed] [Google Scholar]
- Bolton DA (2015). The role of the cerebral cortex in postural responses to externally induced perturbations. Neuroscience and Biobehavioral Reviews, 57, 142–155. https://doi.org/S0149-7634(15)00232-8 [pii] [DOI] [PubMed] [Google Scholar]
- Bolton DA, Patel R, Staines WR, & McIlroy WE (2011). Transient inhibition of primary motor cortex suppresses hand muscle responses during a reactive reach to grasp. Neuroscience Letters, 504(2), 83–87. 10.1016/j.neulet.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Bolton DA, Williams L, Staines WR, & McIlroy WE (2012). Contribution of primary motor cortex to compensatory balance reactions. BMC Neuroscience, 13, 102-2202-13-102. 10.1186/1471-2202-13-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G, Sato M, Cattaneo L, Rodà F, & Riggio L (2009). Broken affordances, broken objects: A TMS study. Neuropsychologia, 47(14), 3074–3078. 10.1016/j.neuropsychologia.2009.07.003 [DOI] [PubMed] [Google Scholar]
- Cardellicchio P, Sinigaglia C, & Costantini M (2011). The space of affordances: A TMS study. Neuropsychologia, 49(5), 1369–1372. 10.1016/j.neuropsychologia.2011.01.021 [DOI] [PubMed] [Google Scholar]
- Cheng KC, McKay SM, King EC, Tung JY, Lee TA, Scovil CY, & Maki BE (2009). The moveable handhold: a new paradigm to study visual contributions to the control of balance-recovery reactions. Gait & Posture, 29(2), 339–342. 10.1016/j.gaitpost.2008.08.011 [DOI] [PubMed] [Google Scholar]
- Cheng Kenneth C., McKay SM, King EC, & Maki BE (2012). Reaching to recover balance in unpredictable circumstances: Is online visual control of the reach-to-grasp reaction necessary or sufficient? Experimental Brain Research, 218(4), 589–599. 10.1007/s00221-012-3051-6 [DOI] [PubMed] [Google Scholar]
- Cisek P, & Kalaska JF (2010). Neural mechanisms for interacting with a world full of action choices. Annual Review of Neuroscience, 33, 269–298. 10.1146/annurev.neuro.051508.135409; [DOI] [PubMed] [Google Scholar]
- Dakin CJ, & Bolton DAE (2018). Forecast or Fall: Prediction’s Importance to Postural Control. Frontiers in Neurology, 9, 924 10.3389/fneur.2018.00924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Labruna L, Verset S, Olivier E, & Ivry RB (2012). Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. The Journal of Neuroscience, 32(3), 806–816. 10.1523/JNEUROSCI.4299-12.2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franca M, Turella L, Canto R, Brunelli N, Allione L, Andreasi NG, … Fadiga L (2012). Corticospinal facilitation during observation of graspable objects: a transcranial magnetic stimulation study. PloS One, 7(11), e49025 10.1371/journal.pone.0049025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Itthipuripat S, & Aron AR (2016). High Working Memory Load Increases Intracortical Inhibition in Primary Motor Cortex and Diminishes the Motor Affordance Effect. Journal of Neuroscience, 36(20), 5544–5555. 10.1523/JNEUROSCI.0284-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JJ (1979). The Ecological Approach To Visual Perception. Boston: Houghton Mifflin. [Google Scholar]
- Grafton ST, Fadiga L, Arbib MA, & Rizzolatti G (1997). Premotor Cortex Activation during Observation and Naming of Familiar Tools. NeuroImage, 6(4), 231–236. 10.1006/nimg.1997.0293 [DOI] [PubMed] [Google Scholar]
- Grèzes J, Tucker M, Armony J, Ellis R, & Passingham RE (2003). Objects automatically potentiate action: an fMRI study of implicit processing. European Journal of Neuroscience, 17(12), 2735–2740. 10.1046/j.1460-9568.2003.02695.x [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Osman A, Possamaï C-A, Burle B, Carron S, Dépy D, … Mouret I (1999). Cortico-spinal inhibition reflects time but not event preparation: neural mechanisms of preparation dissociated by transcranial magnetic stimulation. Acta Psychologica, 101(2), 243–266. 10.1016/S0001-6918(99)00007-4 [DOI] [PubMed] [Google Scholar]
- Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, & Topka H (2001). Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clinical Neurophysiology, 112(2), 250–258. https://doi.org/S1388-2457(00)00513-7 [pii] [DOI] [PubMed] [Google Scholar]
- Kantak SS, Wittenberg GF, Liao W-W, Magder LS, Rogers MW, & Waller SM (2013). Posture-related modulations in motor cortical excitability of the proximal and distal arm muscles. Neuroscience Letters, 533, 65–70. 10.1016/j.neulet.2012.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King EC, McKay SM, Cheng KC, & Maki BE (2010). The use of peripheral vision to guide perturbation-evoked reach-to-grasp balance-recovery reactions. Experimental Brain Research, 207(1–2), 105–118. 10.1007/s00221-010-2434-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Flügge MC, & Bestmann S (2012). Time-Dependent Changes in Human Corticospinal Excitability Reveal Value-Based Competition for Action during Decision Processing. Journal of Neuroscience, 32(24), 8373–8382. 10.1523/JNEUROSCI.0270-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani B, Mansfield A, Inness EL, & McIlroy WE (2011). Characterizing the determinants of limb preference for compensatory stepping in healthy young adults. Gait & Posture, 33(2), 200–204. 10.1016/j.gaitpost.2010.11.005 [DOI] [PubMed] [Google Scholar]
- Magnus R (1926). Cameron prize lectures on some results of studies in the physiology of posture. Lancet, 208(5377), 583–632. [Google Scholar]
- Majid DSA, Cai W, George JS, Verbruggen F, & Aron AR (2012). Transcranial Magnetic Stimulation Reveals Dissociable Mechanisms for Global Versus Selective Corticomotor Suppression Underlying the Stopping of Action. Cerebral Cortex, 22(2), 363–371. 10.1093/cercor/bhr112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki BE, Cheng KC, Mansfield A, Scovil CY, Perry SD, Peters AL, … McIlroy WE (2008). Preventing falls in older adults: new interventions to promote more effective change-insupport balance reactions. Journal of Electromyography and Kinesiology, 18(2), 243–254. https://doi.org/S1050-6411(07)00101-0 [pii] [DOI] [PubMed] [Google Scholar]
- Maki BE, & McIlroy WE (1997). The role of limb movements in maintaining upright stance: the “change-in-support” strategy. Physical Therapy, 77(5), 488–507. [DOI] [PubMed] [Google Scholar]
- Maki BE, & McIlroy WE (2007). Cognitive demands and cortical control of human balance-recovery reactions. Journal of Neural Transmission, 114(10), 1279–1296. 10.1007/s00702-007-0764-y [DOI] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE, & Fernie GR (2003). Change-in-support reactions for balance recovery. IEEE Engineering in Medicine and Biology Magazine, 22(2), 20–26. [DOI] [PubMed] [Google Scholar]
- Makris et al. (2013). Are object affordances fully automatic? A case of covert attention. Behavioral Neuroscience, 127(5), 797–802. [DOI] [PubMed] [Google Scholar]
- Makris S, Hadar AA, & Yarrow K (2011). Viewing objects and planning actions: on the potentiation of grasping behaviours by visual objects. Brain and Cognition, 77(2), 257–264. 10.1016/j.bandc.2011.08.002 [DOI] [PubMed] [Google Scholar]
- Mansfield A, Inness EL, Komar J, Biasin L, Brunton K, Lakhani B, & McIlroy WE (2011). Training rapid stepping responses in an individual with stroke. Physical Therapy, 91(6), 958–969. 10.2522/ptj.20100212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield A, Inness EL, Lakhani B, & McIlroy WE (2012). Determinants of limb preference for initiating compensatory stepping poststroke. Archives of Physical Medicine and Rehabilitation, 93(7), 1179–1184. 10.1016/j.apmr.2012.02.006 [DOI] [PubMed] [Google Scholar]
- Mansfield Avril, & Maki BE (2009). Are age-related impairments in change-in-support balance reactions dependent on the method of balance perturbation? Journal of Biomechanics, 42(8), 1023–1031. 10.1016/j.jbiomech.2009.02.007 [DOI] [PubMed] [Google Scholar]
- McDannald DW, Mansour M, Rydalch G, & Bolton DAE (2018). Motor affordance for grasping a safety handle. Neuroscience Letters, 683, 131–137. 10.1016/j.neulet.2018.05.040 [DOI] [PubMed] [Google Scholar]
- Mochizuki G, Boe S, Marlin A, & McIlRoy WE (2010). Perturbation-evoked cortical activity reflects both the context and consequence of postural instability. Neuroscience, 170(2), 599–609. 10.1016/j.neuroscience.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, & Safety of TMS Consensus Group. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–2039. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS (1910). Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. The Journal of Physiology, 40(1–2), 28–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov S, Cao C, Jaiswal N, & Newell KM (2009). Neural basis of postural instability identified by VTC and EEG. Experimental Brain Research, 199(1), 1–16. 10.1007/s00221-009-1956-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen DG, Muriuki M, James J, Schultz AB, Ashton-Miller JA, & Alexander NB (2000). Muscle activities used by young and old adults when stepping to regain balance during a forward fall. Journal of Electromyography and Kinesiology, 10(2), 93–101. 10.1016/S1050-6411(99)00028-0 [DOI] [PubMed] [Google Scholar]
- Tucker M, & Ellis R (1998). On the Relation between Seen Objects and Components of Potential Actions (Vol. 24). 10.1037/0096-1523.24.3.830 [DOI] [PubMed] [Google Scholar]
- Tucker M, & Ellis R (2004). Action priming by briefly presented objects. Acta Psychologica, 116(2), 185–203. 10.1016/j.actpsy.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Wälchli M, Tokuno CD, Ruffieux J, Keller M, & Taube W (2017). Preparatory cortical and spinal settings to counteract anticipated and non-anticipated perturbations. Neuroscience, 365, 12–22. 10.1016/j.neuroscience.2017.09.032 [DOI] [PubMed] [Google Scholar]
- Wessel JR, & Aron AR (2017). On the Globality of Motor Suppression: Unexpected Events and Their Influence on Behavior and Cognition. Neuron, 93(2), 259–280. 10.1016/j.neuron.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik LA, Thelen DG, Schultz AB, Ashton-Miller JA, & Alexander NB (1999). Age and Gender Differences in Single-Step Recovery From a Forward Fall. The Journals of Gerontology: Series A, 54(1), M44–M50. 10.1093/gerona/54.1.M44 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.