Abstract
Objective:
To describe the association of implementing a HEART care pathway on use of hospital care and non-invasive stress testing as well as 30-day patient outcomes in community emergency departments (EDs).
Methods:
We performed a prospective interrupted time series study of adult encounters for patients evaluated for suspected acute coronary syndrome (ACS). The primary outcome was hospitalization/observation, non-invasive stress testing, or both within 30 days. The secondary outcome was 30-day all-cause mortality or acute myocardial infarction. A generalized estimating equation segmented logistic regression model was used to compare the odds of the primary outcome pre- and post-HEART implementation. All models were adjusted for patient and facility characteristics and fit using physicians as a clustering variable.
Results:
65,393 ED encounters (Pre: 30,522 and Post: 34,871) were included in the study. Overall 33.5% (Pre: 35.5% and Post: 31.8%) of ED chest pain encounters resulted in hospitalization/observation, noninvasive stress testing, or both. Primary adjusted results found a significant decrease in the primary outcome post-implementation (OR 0.984, 95% CI 0.974–0.995). This resulted in an absolute adjusted month-to-month decrease of 4.39% (95% CI: 3.72–5.07%) after 12 months follow-up with a continued trend downward. There was no difference in 30-day mortality or myocardial infarction (Pre: 0.6% vs. Post: 0.6%, OR 1.02, 95% CI 0.97–1.08)
Conclusion:
Implementation of a HEART pathway in the ED evaluation of patients with chest pain resulted in less inpatient care and non-invasive cardiac testing and was safe. Using HEART to risk stratify chest pain patients can improve the efficiency and quality of care.
INTRODUCTION
Background:
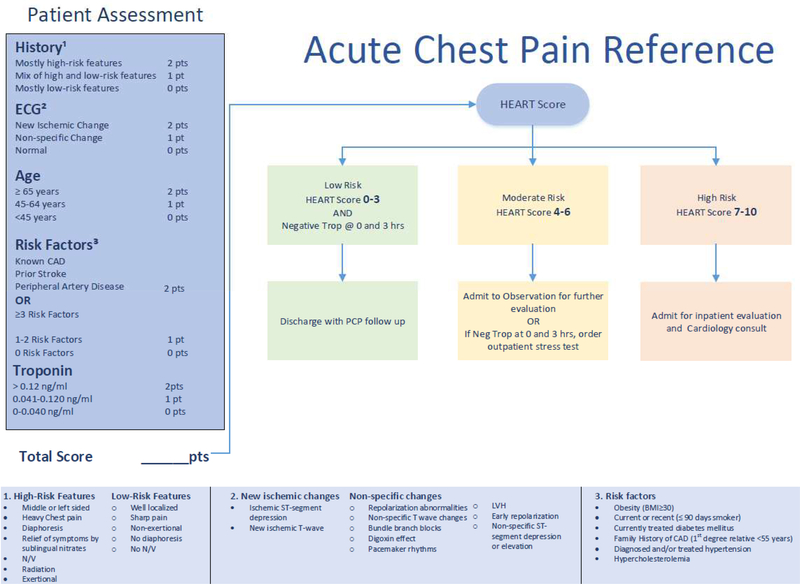
Heart disease is a leading cause of mortality1, and chest pain, a symptom that often triggers an evaluation for suspected acute coronary syndrome (ACS), is the second most frequent reason for all ED visits.2 This leads to over $3 billion in hospital costs each year to evaluate patients with possible ACS.3 However, there is no evidence that the current paradigm of acute care, which frequently includes hospital observation and cardiac stress testing, actually improves patient outcomes.4–6 Objective risk scores, such as HEART (H-history, E-EKG, A-age, R-risk factors, T-troponin), are designed to help clinicians identify which patients require further hospital based observation or testing, and who may be discharged safely.7 HEART is a validated 0–10 point ACS scoring system.7–9 Patients with a HEART score between 0–3 have been shown to have a low risk (<1%) of 30-day major adverse cardiac events (MACE).8,10–12
Importance:
Recent evidence suggests that risk stratification tools in the emergency department (ED) can identify patients with suspected acute coronary syndrome (ACS) who are at low risk and thus can defer further hospital observation or non-invasive cardiac testing. However, there is limited data on the impact of the adoption of such tools on health care utilization and patient outcomes. A single site randomized trial at an academic medical center suggests a HEART pathway to discharge low-risk patients may safely reduce admissions13, but a larger cluster randomized trial in the Netherlands resulted in no change in utilization.14 There are no reports to describe the safety and efficacy of similar care pathways in community hospitals.
Goals of this Investigation:
The primary aim of this study is to describe the impact of implementing a HEART care pathway designed to reduce hospitalization and non-invasive stress testing in 13 community EDs in Southern California. We also report rates of 30-day acute myocardial infarction (AMI) and all-cause death before and after the intervention. We hypothesized that the HEART pathway would decrease hospitalizations and noninvasive cardiac testing without adverse impact on 30-day AMI and death rates.
METHODS
Study design and setting:
We conducted a prospective interrupted time series study of all adult ED encounters for suspected ACS at 13 community hospitals between May 6, 2015 to June 3, 2017. Study sites were all part of Kaiser Permanente Southern California (KPSC). KPSC is an integrated health system providing health care for over four million members. KPSC hospitals provide care to over 1 million ED patients per year (study sites ranging from ≈25,000 to 95,000 ED visits per year). Of these ED visits, approximately 80% are health plan members. We excluded one new hospital that did not have pre-data for comparison and one hospital not staffed by KPSC physicians, therefore not trained or educated regarding the HEART recommendations. Our data set allows us to track detailed information for our members’ in-network encounters as well as capture claims data for out-of-network encounters.
Selection of Participants:
ED encounters were included for adult (≥ 18 years) patients health plan members who had both a troponin lab test and a chest pain diagnosis (Appendix A). All sites use the same troponin lab assay (Beckman Coulter Access AccuTnI+3). We only included health plan members because we do not have accurate follow up information for our outcomes for non-member patients. Our data set allows us to identify claims data for any hospital or ED encounter, as well as all in-network healthcare. We excluded patients with a do not resuscitate (DNR) status, who had an AMI identified in the ED, were transferred from another hospital, or who expired in the ED (Figure 1). Encounters that occurred May 6, 2015-May 5, 2016 were included in the pre-implementation period, May 6, 2016-June 2, 2016 was the washout period, and June 3, 2016-June 2, 2017 was the post-implementation period. KPSC adopted HEART to be used by ED physicians during the clinical evaluation and management of patients with possible ACS in January 2016. The implementation included clinical recommendations for patients with low (0–3), moderate (4–6) and high risk (7–10) HEART scores. An education module and plenary presentation at a local conference disseminated information to all emergency physicians summarizing current medical evidence related to the management of possible ACS as well as the expectation that physicians use HEART scores as part of routine clinical care. Decision support was embedded into the electronic health record (May 2016) and prompts alerted physicians to insert the history, EKG, and risk factors necessary to calculate a HEART score. The age and troponin values were automatically included to allow for an automated calculation of the HEART score.15 Each physician and ED maintained the autonomy to document HEART, follow the recommendations or adjust according to patient needs. There is no standard type or location of observation units, nor pathway to cardiology referrals for non-invasive testing among our study sites. A formal power calculation was not performed, but it was estimated that the approximately 30,000 ED chest pain encounters before and after implementation in our 13 EDs would provide sufficient data to detect a meaningful difference in outcomes, if such a difference existed.
Figure 1:
The HEART Pathway
Measurements:
Covariates included patient demographic information. Age, gender, and race data were obtained from administrative records, while education was proxied by the percentage of college-educated individuals at the census block level based on a patient’s home zip code. Clinical patient variables and physician data were similarly obtained by querying the structured electronic medical records. Cardiac risk factors such as hypertension and diabetes were defined using the Elixhauser index codes. The ICD-9 and ICD-10 codes used to define dyslipidemia, coronary artery disease (CAD), stroke, percutaneous coronary intervention (PCI), and coronary artery bypass graft (CABG) can be found in Appendix A. Body mass index (BMI) was measured from ED intake documentation or the most recently available visit, while smoking and family history of CAD were self-reported fields in electronic health records. Those with a history of PCI or CABG were considered to have had prior coronary vascularization. The KPSC medical center was recorded at the time of the ED encounter.
Outcomes:
The primary outcome was admission to the hospital, which included patients admitted under observation status, and/or ordering of a non-invasive cardiac stress test. The stress testing was defined as either an ED referral to a cardiology department for a non-invasive stress test or a direct order for stress testing during the ED encounter. The secondary outcome was 30-day AMI or all-cause mortality (Appendix A). We considered any statistically significant increase in 30-day AMI or all-cause mortality would indicate failure and any improvement a success. We believed that a small increase (0.2%) in month to month hospitalizations/stress tests associated with improved AMI/Mortality, or a small decrease (0.2%) month to month in admissions/stress testing with no association with AMI/Mortality rates would have long-term benefits to our health system and members.
Analysis:
Continuous patient and encounter characteristics were summarized using means and standard deviations, while categorical characteristics were presented as frequencies and percentages. Forest plots were generated for each outcome to summarize variability by medical center in the pre- and post- period.
To assess changes in the odds of hospitalization and/or stress testing before and after HEART implementation, we fit a generalized estimating equation segmented logistic regression model as this strategy is a favored methodology to account for secular trends while assessing an intervention’s impact.16–20 The unit of analysis was ED encounter. The model included terms for the year-long pre-intervention baseline monthly trend of each outcome, as well as terms for the change in level during the 4-week washout period when the intervention was implemented (May 6, 2016-June 3, 2016), and the monthly trend in the year after the intervention was implemented. To account for known correlation among encounters for the same physician, we fit our model using physician as a clustering variable. All models were adjusted for the following characteristics: age, gender, race, medical center, college education, comorbidities expressed as Elixhauser index21, hypertension, diabetes, dyslipidemia, BMI, smoking, family history of CAD, CAD, prior coronary revascularization, and stroke. Results were summarized using Odds Ratios (ORs) and 95% Confidence Intervals.
We performed several sensitivity analyses to confirm our findings. We ran models using unique patient identifiers as an alternative clustering variable to provider and found nearly identical results. Since provider and medical center were not completely independent (some providers worked at more than one medical center, N=206), we present results from the model using physician as a clustering variable, using unique provider-medical center indicators. Potential associations by medical center were assessed to identify any heterogeneity in the change in outcomes after implementation.
The change in hospitalization and stress testing associated with the intervention was graphically depicted by plotting the predicted values obtained from the model over the pre- and postimplementation periods. Furthermore, we plotted the predicted values had the intervention not occurred along with the values of the effect with HEART implementation.
The same analysis approach was used to assess changes in our secondary outcome, 30-day AMI or death, to analyze any potential changes pre- and post-implementation of HEART. Lastly, we analyzed a subset of the post-intervention sample with documented HEART scores to report primary and secondary outcomes stratified by low, moderate and high-risk groups.
All analyses were conducted with SAS (version 9.3; SAS Institute, Inc., Cary, NC). All tests of statistical significance were 2-sided with α=0.05. This study was approved by the Kaiser Permanente Southern California Institutional Review Board.
RESULTS
Characteristics:
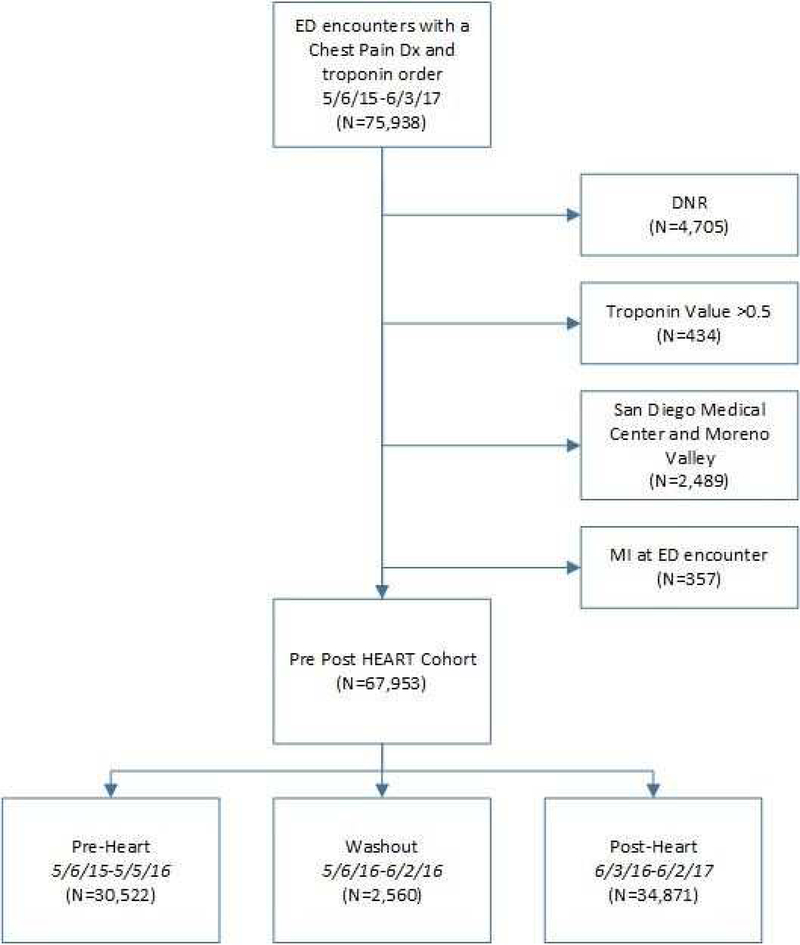
A total of 67,953 encounters were included in the analysis (Pre- 30,522 and Post- 34,871) (Figure 2). Table 1 shows patient characteristics from ED encounters in the pre- and post-implementation periods. The distribution of age, gender, and race was similar across periods. The mean age of the population was 58 years (Pre: 57.9, Post: 58.0), female (Pre: 57.6%, Post: 57.1%), and white (Pre: 52.3%, Post: 50.9%). BMI varied little before and after implementation, and most patients were either overweight (33.8%) or obese (42.5%). Similar prevalence of cardiac-specific comorbidities, including CAD, prior coronary revascularization, and stroke were observed in the pre- and post- periods (Table 1). Overall 33.5% of encounters resulted in the primary outcome (Pre: 35.5% and Post: 31.8%). Decreases in both hospitalization/observation (Pre: 14.7 vs Post: 13.2) and non-invasive stress testing (Pre: 27.8% vs Post: 24.3%) contributed to the overall reduction (Table 2).
Figure 2:
Flow diagram of the study cohort used for analysis.
Table 1-.
Characteristics of ED ACS encounters before HEART implementation (May 6, 2015-May 5, 2016) and after HEART implementation (June 3, 2016-June 2, 2017 (post-intervention)
| Patient Level Characteristics* | Encounter Level Characteristics | |||||
|---|---|---|---|---|---|---|
| Pre- HEART N=27,726 | Post- HEART N=31,481 | Total N=59,207 | Pre- HEART N=30,522 | Post- HEART N=34,871 | Total N=65,393 | |
| Mean Age (SD) | 57.6 (16.25) | 57.6 (16.29) | 57.6 (16.27) | 57.9 (16.29) | 58.0 (16.35) | 58.0 (16.32) |
| Gender (F) Race | 16031 (57.8%) | 18053 (57.3%) | 34084 (57.6%) | 17569 (57.6%) | 19905 (57.1%) | 37474 (57.3%) |
| Race | ||||||
| Alaska Native/Pacific Islander | 502 (1.8%) | 525 (1.7%) | 1027 (1.7%) | 549 (1.8%) | 585 (1.7%) | 1134 (1.7%) |
| Asian | 2772 (10%) | 3090 (9.8%) | 5862 (9.9%) | 3023 (9.9%) | 3377 (9.7%) | 6400 (9.8%) |
| Black | 4437 (16%) | 4996 (15.9%) | 9433 (15.9%) | 5039 (16.5%) | 5753 (16.5%) | 10792 (16.5%) |
| Others | 5551 (20%) | 6847 (21.7%) | 12398 (20.9%) | 5963 (19.5%) | 7401 (21.2%) | 13364 (20.4%) |
| White | 14464 (52.2%) | 16023 (50.9%) | 30487 (51.5%) | 15948 (52.3%) | 17755 (50.9%) | 33703 (51.5%) |
| Hispanic Ethnicity | 10311 (37.2%) | 12060 (38.3%) | 22371 (37.8%) | 11282 (37.0%) | 13280 (38.1%) | 24562 (37.6%) |
| Some College Education** % (SD) | 57.1 (18.95) | 56.6 (19.21) | 56.8 (19.09) | 56.9 (18.96) | 56.4 (19.22) | 56.6 (19.10) |
| Elixhauser Comorbidity Index Mean (SD) | 3.8 (3.12) | 3.7 (3.04) | 3.7 (3.08) | 4.0 (3.26) | 4.0 (3.17) | 4.0 (3.21) |
| Hypertension | 15026 (54.2%) | 16827 (53.5%) | 31853 (53.8%) | 17127 (56.1%) | 19376 (55.6%) | 36503 (55.8%) |
| Diabetes | 7432 (26.8%) | 8725 (27.7%) | 16157 (27.3%) | 8490 (27.8%) | 10108 (29%) | 18598 (28.4%) |
| High Cholesterol | 16607 (59.9%) | 18839 (59.8%) | 35446 (59.9%) | 18694 (61.2%) | 21402 (61.4%) | 40096 (61.3%) |
| BMI Category | ||||||
| Underweight <18.5 | 295 (1.1%) | 327 (1%) | 622 (1.1%) | 347 (1.1%) | 402 (1.2%) | 749 (1.1%) |
| Normal weight 18.5–24.9 | 6148 (22.2%) | 6683 (21.2%) | 12831 (21.7%) | 6779 (22.2%) | 7447 (21.4%) | 14226 (21.8%) |
| Overweight 25–29.9 | 9409 (33.9%) | 10671 (33.9%) | 20080 (33.9%) | 10338 (33.9%) | 11795 (33.8%) | 22133 (33.8%) |
| Obese >30 | 11663 (42.1%) | 13551 (43%) | 25214 (42.6%) | 12845 (42.1%) | 14976 (42.9%) | 27821 (42.5%) |
| Unknown | 211 (0.8%) | 249 (0.8%) | 460 (0.8%) | 213 (0.7%) | 251 (0.7%) | 464 (0.7%) |
| Smoking Status | ||||||
| Never | 16778 (60.5%) | 19133 (60.8%) | 35911 (60.7%) | 18327 (60%) | 20991 (60.2%) | 39318 (60.1%) |
| Passive | 166 (0.6%) | 166 (0.5%) | 332 (0.6%) | 173 (0.6%) | 188 (0.5%) | 361 (0.6%) |
| Quit | 8266 (29.8%) | 9204 (29.2%) | 17470 (29.5%) | 9288 (30.4%) | 10453 (30%) | 19741 (30.2%) |
| Active | 1836 (6.6%) | 2071 (6.6%) | 3907 (6.6%) | 2036 (6.7%) | 2317 (6.6%) | 4353 (6.7%) |
| Missing | 680 (2.5%) | 907 (2.9%) | 1587 (2.7%) | 698 (2.3%) | 922 (2.6%) | 1620 (2.5%) |
| Family History of CAD | 9118 (32.9%) | 10702 (34%) | 19820 (33.5%) | 10187 (33.4%) | 12143 (34.8%) | 22330 (34.1%) |
| Coronary Artery Disease | 5435 (19.6%) | 6187 (19.7%) | 11622 (19.6%) | 6711 (22%) | 7770 (22.3%) | 14481 (22.1%) |
| Prior Coronary Revascularization | 381 (1.4%) | 415 (1.3%) | 796 (1.3%) | 524 (1.7%) | 571 (1.6%) | 1095 (1.7%) |
For patients with more than one encounter in the pre-implementation or post-implementation period, information from the first encounter was used
Some College Education Missing (Patient Level Pre: 49, Post: 39; Encounter Level Pre: 53, Post: 42)
Table 2-.
30-day outcomes of Emergency Department chest pain encounters pre- (May 6, 2015-May 5, 2016) and post-implementation (June 3, 2016-June 2, 2017) of a HEART care pathway.
| Pre- Implementation (N=30,522) | Post- Implementation (N=34,871) | Difference between Pre- and Post- Implementation (95%CI) | |||||
|---|---|---|---|---|---|---|---|
| HEART Score | All Post | ||||||
| Low | Intermediate | High | Total | ||||
| Admission and/or Stress | 13208 (43.3%) | 1807 (25.1%) | 2353 (51.2%) | 323 (69.2%) | 4483 (36.5%) | 13430 (38.5%) | −3.7% (−4.4%, −2.9%) |
| Admission to Hospital | 4480 (14.7%) | 172 (2.4%) | 983 (21.4%) | 270 (57.8%) | 1425 (11.6%) | 4592 (13.2%) | −1.5% (−2%, −1%) |
| Stress Test Within 30 Days | 8728 (28.6%) | 1734 (24.1%) | 1965 (42.8%) | 180 (38.5%) | 3879 (31.6%) | 8838 (25.3%) | −3.5% (−4.2%, −2.8%) |
| Expired or AMI Within 30 Days | 171 (0.6%) | 12 (0.2%) | 27 (0.6%) | 12 (2.6%) | 51 (0.4%) | 225 (0.6%) | 0.1% (−0.0%, 0.2%) |
Main Results:
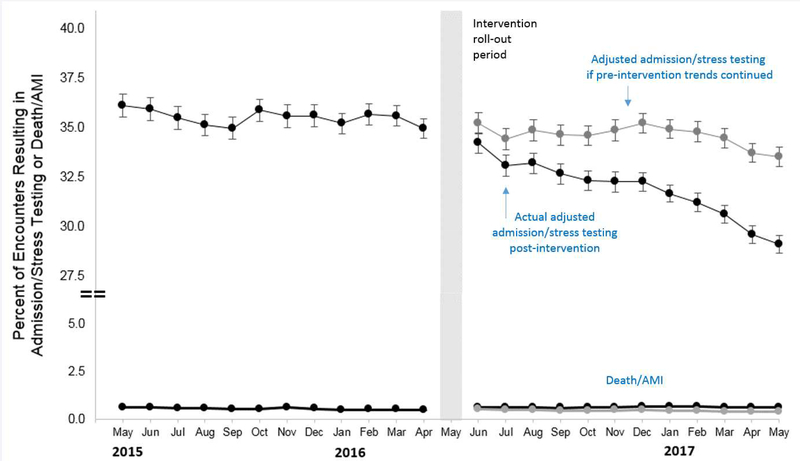
Primary analysis demonstrated a significant decrease in month-to-month trends post-implementation (OR 0.984, 95% CI 0.974–0.995) (Figure 3) (Appendix B). There was not a significant decreasing trend in the primary outcome month-to-month in the pre-period (OR 0.997, 95% CI 0.990–1.005), nor an immediate change between the end of the pre- period to the beginning of the post- period (OR 0.968, 95% CI 0.904–1.036). Overall, the HEART pathway resulted in a 4.39% adjusted decrease (95% CI: 3.72–5.07%) between expected results and the observed proportion of encounters resulting in the primary outcome after the intervention (Figure 3).
Figure 3:
Adjusted interrupted time series showing the changes in pre-and post-intervention month to month trends for ED chest pain encounters. The top black lines indicate encounters resulting in hospital admission and/or cardiac stress testing and the bottom black lines represent 30-day death and/or acute myocardial infarction (AMI) rates. The gray lines demonstrate the predicted results had the pre-intervention trends continued without the HEART care pathway.
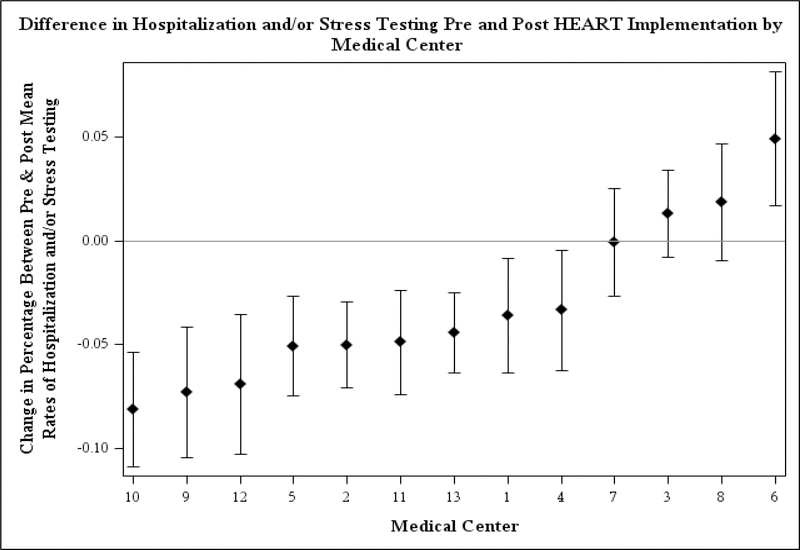
The difference in the proportion of encounters resulting in admission and/or stress testing between the pre- and post-implementation periods varied by medical center (Figure 4). Most medical centers decreased their percentage of encounters that resulted in admission and/or stress testing. There was no statistically significant association between the monthly time trend in either the pre- (p=0.93) or post- period (p=0.34) with medical center.
Figure 4:
Changes in hospitalization and/or stress testing Pre- and Post- a HEART care pathway was implemented at 13 emergency departments within an integrated health system.
Analysis of the secondary outcome (30-day AMI or all-cause mortality) showed no difference between pre- and post- periods (Pre: 0.6% vs Post: 0.6%). The adjusted analysis showed no baseline monthly trend pre-implementation (OR 0.987, 95% CI 0.946–1.029), no initial change pre- to post- (OR 1.183, 95% CI 0.782–1.789), and no overall change in monthly trends post-implementation (OR 1.024, 95% CI 0.967–1.084).
Subgroup analysis of post-intervention encounters demonstrated that 35.2% (12,267 of 34,871) of encounters had documented HEART scores. The majority were low-risk (58.7%) and the primary outcome increased with higher-risk HEART scores (low-risk 25.1%, moderate-risk 51.2% and high-risk 69.2%). Overall, patients with HEART scores had low rates of 30-day AMI or death (0.3%) and increasing HEART scores showed higher-risks of this secondary outcome (low-risk 0.2%, moderate-risk 0.6% and high-risk 2.6%).
LIMITATIONS
Variables with missing data and the strategies to account for each were as follows. Patients with missing zip codes to designate college education (N=95 or 0.1% of the pre/post encounters) were excluded from multivariate analyses. In addition, 13 encounters were missing a discharge status from the ED and were excluded from the analysis for admission to the hospital/observation (N=13) and the analysis for the combined primary outcome (N=7). Categorical outcomes with missing data in the pre- or post- periods (BMI N=464 and smoking N=1620) were included as an “Unknown” category. We acknowledge that our study population has a low rate of MACE and other patient populations with a higher MACE rate may find different results.
DISCUSSION
Our study found that implementation at community emergency departments of a care pathway using HEART to risk-stratify ED patients with suspected ACS safely reduced downstream hospital care and noninvasive cardiac testing. Our results should influence physicians, administrators, and policymakers to consider a standardized approach to the evaluation and management of patients with chest pain, or other presentations concerning for ACS.
These results confirm our hypothesis, that standard risk-stratification and clear care recommendations for low-risk patients can safely decrease hospital care and stress testing. Consistent with other reports, we found variability in the effect of the care pathway at each of the study sites.3,5 Our findings are consistent with similar strategies for other clinical conditions which have demonstrated benefits of validated decision instruments and care pathways.20 Furthermore, our study expands upon the findings of a small, single site RCT which used a similar HEART care pathway,13 but contradict the results of the cluster randomized trial from Dutch hospitals reporting no change in utilization.14
Despite the overall results and improvements in utilization, our sub-group analysis of low-risk HEART scores demonstrate there is still ample room for improvement. In fact, we even found one ED increased after implementation of our HEART pathway. This despite low-risk HEART scores with 30-day MACE risks of 0.2% which still resulted in hospitalization and/or stress testing for 25% of encounters. This represents opportunities for future research, and further implementation strategies to optimize patient care and resource utilization.
Observational quasi-experimental studies like this one cannot definitively attribute causality. However, we used an established and recommended interrupted time series design to account for this as much as possible.16,17 The study was also performed in an integrated health system that may offer better coordinated outpatient follow-up than other fee-for-service models in the United States. This may have some effect on the outcomes of ED patients after discharge and the generalizability of our findings. However, our baseline rates of hospital utilization are lower than national estimates (13.9% vs 16.2%)3 which indicates that similar care pathways might have even greater opportunities to reduce this care in different settings. In fact, as our findings suggest reduced utilization of testing is safe for patients with low-risk chest pain, it may be especially useful in resource constrained settings and safety-net hospitals.
In summary, implementation of HEART as a standard risk stratification tool in the ED evaluation of patients with chest pain resulted in less inpatient care and non-invasive cardiac testing without impacting patient safety. Using a tool to standardize the ED risk stratification of chest pain patients can improve the efficiency of care safely for patients.
Supplementary Material
Acknowledgments
Funding: Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL134647. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Ferencik was supported by the American Heart Association Fellow-to-Faculty Award (13FTF16450001).
Footnotes
Disclosures: There are no conflicts of interest to report for the following authors: ALS, AB, ES, RR, MSL, MF, SN, CZ, AK, MKG. Author, BCS, was a consultant for Medtronic.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Vedanthan R, Seligman B, Fuster V. Global perspective on acute coronary syndrome: a burden on the young and poor. Circ Res. 2014;114(12):1959–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevention CfDCa. National Hospital Ambulatory Medical Care Survey: 2010 Emergency Department Summary Tables. 2010.
- 3.Sabbatini AK, Nallamothu BK, Kocher KE. Reducing variation in hospital admissions from the emergency department for low-mortality conditions may produce savings. Health Aff (Millwood). 2014;33(9):1655–1663. [DOI] [PubMed] [Google Scholar]
- 4.Foy AJ, Liu G, Davidson WR Jr., Sciamanna C, Leslie DL. Comparative effectiveness of diagnostic testing strategies in emergency department patients with chest pain: an analysis of downstream testing, interventions, and outcomes. JAMA Intern Med. 2015;175(3):428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safavi KC, Li SX, Dharmarajan K, et al. Hospital variation in the use of noninvasive cardiac imaging and its association with downstream testing, interventions, and outcomes. JAMA Intern Med. 2014;174(4):546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinstock MB, Weingart S, Orth F, et al. Risk for Clinically Relevant Adverse Cardiac Events in Patients With Chest Pain at Hospital Admission. JAMA Intern Med. 2015;175(7):1207–1212. [DOI] [PubMed] [Google Scholar]
- 7.Fanaroff AC, Rymer JA, Goldstein SA, Simel DL, Newby LK. Does This Patient With Chest Pain Have Acute Coronary Syndrome?: The Rational Clinical Examination Systematic Review. JAMA. 2015;314(18):1955–1965. [DOI] [PubMed] [Google Scholar]
- 8.Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16(6):191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connor RE, Al Ali AS, Brady WJ, et al. Part 9: Acute Coronary Syndromes: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S483–500. [DOI] [PubMed] [Google Scholar]
- 10.Mark DG, Huang J, Chettipally U, et al. Performance of Coronary Risk Scores Among Patients With Chest Pain in the Emergency Department. J Am Coll Cardiol. 2018;71(6):606–616. [DOI] [PubMed] [Google Scholar]
- 11.Six AJ, Cullen L, Backus BE, et al. The HEART score for the assessment of patients with chest pain in the emergency department: a multinational validation study. Crit Pathw Cardiol. 2013;12(3):121–126. [DOI] [PubMed] [Google Scholar]
- 12.Sharp AL, Wu YL, Shen E, et al. The HEART Score for Suspected Acute Coronary Syndrome in U.S. Emergency Departments. J Am Coll Cardiol. 2018;72(15):1875–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8(2):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poldervaart JM, Reitsma JB, Backus BE, et al. Effect of Using the HEART Score in Patients With Chest Pain in the Emergency Department: A Stepped-Wedge, Cluster Randomized Trial. Ann Intern Med. 2017;166(10):689–697. [DOI] [PubMed] [Google Scholar]
- 15.Sharp AL, Broder BI, Sun BC. Improving emergency department care for low-risk chest pain. New England Journal of Medicine Catalyst 2018. [Google Scholar]
- 16.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. [DOI] [PubMed] [Google Scholar]
- 17.Fretheim A, Tomic O. Statistical process control and interrupted time series: a golden opportunity for impact evaluation in quality improvement. BMJ Qual Saf. 2015;24(12):748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fretheim A, Zhang F, Ross-Degnan D, et al. A reanalysis of cluster randomized trials showed interrupted time-series studies were valuable in health system evaluation. J Clin Epidemiol. 2015;68(3):324–333. [DOI] [PubMed] [Google Scholar]
- 19.Taljaard M, McKenzie JE, Ramsay CR, Grimshaw JM. The use of segmented regression in analysing interrupted time series studies: an example in pre-hospital ambulance care. Implement Sci. 2014;9:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharp AL, Huang BZ, Tang T, et al. Implementation of the Canadian CT Head Rule and Its Association With Use of Computed Tomography Among Patients With Head Injury. Ann Emerg Med. 2018;71(1):54–63 e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying Increased Risk of Readmission and In-hospital Mortality Using Hospital Administrative Data: The AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698–705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.