Abstract
Purpose:
Emerging data indicate that the timing and rhythms of energetic behaviors may influence metabolism and obesity risk. Our aim was to derive diurnal rest-activity patterns from actigraphy in adolescents and analyze associations with adiposity measures and cardio-metabolic risk factors.
Methods:
Adolescents in the Project Viva cohort wore a wrist actigraph over seven days. We derived markers of daily rest-activity patterns from actigraphy using non-parametric models, generating measurements of relative amplitude (RA). RA reflects the normalized difference in activity measured during the most active 10-hour period and the least active 5-hour period, averaged over multiple 24-hour periods. Using multivariable-adjusted linear regression models, we estimated associations of RA and its components with markers of adiposity (body mass index, waist circumference, skinfolds, dual-energy X-ray absorptiometry fat mass) and cardio-metabolic health (cardio-metabolic risk score, derived as the mean of five sex-specific internal z-scores for waist circumference, systolic blood pressure, HDL-cholesterol scaled inversely, and log-transformed triglycerides and HOMA-IR).
Results:
778 adolescents provided at least five days of valid actigraphy data. The average age was 13.2 (±0.9) years, 52% were female and the average RA was 0.9 (±0.1). A higher RA reflecting higher activity during wakefulness and lower activity during the night was associated with more favorable indices of adiposity (e.g. −0.35 kg/m2 lower BMI per each 0.04 units increment of RA; 95% CI: −0.60, −0.09).
Conclusions:
In this large sample of adolescents, a higher RA emerged as a novel biomarker, associated with more favorable cardio-metabolic profiles.
Keywords: actigraphy, rest-activity patters, obesity, adolescents
Introduction
Obesity is prevalent in more than 20% of US adolescents ages 12 to 19 years. Obesity that begins during adolescence increases the risk of obesity and its complications later in life (1). Adolescence is also a particularly critical developmental period for the emergence of behaviors that increase risk for obesity; therefore, identification of new behavioral risk factors could provide novel intervention targets. Both sleep duration and physical activity have been identified as modifiable risk factors for obesity (2, 3). There is also an emerging literature demonstrating a relationship between the timing of sleep and weight regulation in children and adolescents (4). In addition, irregular sleep patterns and circadian misalignment are risk factors for obesity in children and adolescents (5–8) and may be inter-related to sleep-wake activity patterns.
While both activity and sleep play key roles in energy metabolism through physiological as well as behavioral mechanisms (9, 10), little is known regarding the combined effect of activity and sleep in adolescents across 24-hour-periods on risk factors associated with adiposity.
Actigraphy is an objective method for estimating patterns of activity and sleep in large cohorts and can be used to derive reliable estimates of 24-hour rest-activity rhythms (11). We used actigraphy data to estimate relative amplitude (RA), a composite measure of the strength of rest and activity rhythms over a 24-hour day that has previously been associated with body mass index (BMI) among adults (12). RA reflects the difference between the most active 10-hour period and the least active 5-hour period. It is a marker that captures four dimensions: the timing and average level of activity during sleep and also during the day, with a normalized value between 0 and 1. A higher RA is indicative for more restful sleep, higher activity during the day, but also stronger rhythm or rhythm fit (more robust rhythms have a higher RA). It provides a normalized value that allows comparison between individuals (13). Our overall objective was to test if RA and its components could provide informative markers reflective of energetic behaviors over the day and night (activity and rest), as well as indices of rest-activity timing and rhythm strength, that associate with obesity-related health outcomes. We hypothesized that an earlier timing of peak activity and rest periods and greater difference in the amplitude of activity in the active and rest periods (i.e., an overall stronger rest-activity patterns, or RA) would be associated with a lower BMI and more favorable markers of adiposity and cardio-metabolic function in community-dwelling adolescents. We analyzed data from Project Viva, a Boston-area cohort that included comprehensive actigraphy and cardio-metabolic measurements on 769 adolescents, ages 11 to 16 years.
Methods
Participants
Project Viva is a longitudinal pre-birth cohort study of 2,128 children and their mothers. Data collection started in 1999, when Project Viva recruited pregnant women at in-person visits in the first trimester of pregnancy from Atrius Harvard Vanguard Medical Associates, a multi-specialty group practice located in eastern Massachusetts. Mother-child in-person visits occurred in infancy, early childhood, mid-childhood, and early adolescence, and additional information was collected from medical records and annual questionnaires. Details of the study protocol and recruitment/retention procedures are available elsewhere (14). For this study, we included adolescents in attendance of the “Early Teen” visit (2012–2016), who provided valid actigraphy data and anthropometric measurements. Institutional review boards of Harvard Pilgrim Health Care and Brigham and Women’s Hospital approved the study protocols and all mothers provided written informed consent, and children provided verbal consent starting at the mid-childhood.
Actigraphy
The adolescents wore a triaxial GT3X+ actigraph (ActiGraph, Pensacola, FL) on their non-dominant wrist, for 24-hours per day (except when bathing or swimming) on seven to 10 consecutive days. Bedtimes, naps and any times when actigraphs were removed were reported in a paper diary. At the end of their wear period, the participants returned their actigraphs in the mail or in person and the data were downloaded using ActiLife software (version 6.7 [or later], ActiGraph, Pensacola, FL) and scored blinded to other information at the Brigham and Women’s Sleep Reading Center. Activity counts for the GT3X+ were collected in 1-minute epochs. The GT3X+ actigraphs were initialized at a sampling rate of 30 Hz. We recently validated the GT3X+ actigraph for detecting sleep and wakefulness versus polysomnography in adolescents, showing high sensitivity to detect sleep periods (15). We considered a day valid if >10 hours of activity counts were collected. Participants had to provide at least five days of recordings to be included in our analysis. We screened all data files visually and identified a main rest interval as the primary sleep period based on self-completed logs and observation of a sharp decrease/increase in activity. We considered the primary sleep period invalid if the device was removed for one hour or more within the in-bed interval. Sleep onset and offset were determined using the Cole-Kripke sleep algorithm (15), which determines if an epoch is sleep or awake based on a seven-minute window, which includes the four previous epochs and the two epochs after the current (16). We chose the Cole-Kripke algorithm because of its higher accuracy to identify sleep-wake periods in teenagers as compared to the Sadeh algorithm (15).
Rest-activity patterns:
Non-parametric analyses of actigraphy data stem from the assumption that the rest-activity rhythm may not be optimally modeled through a sinusoidal shape, but rather resembles an asymmetrical square shape (13). We estimated the following 5 characteristics of sleep patterns using non-parametric analysis:
L5: L5 is the average of the activity values for the 5 least active consecutive hours in the 24-hour cycle. Lower L5 counts indicate more consolidated sleep, whereas higher L5 counts indicate less restful sleep.
L5 midpoint: The L5 midpoint refers to the mean decimal clock time of the 5 consecutive hours with the lowest activity in the 24-hour cycle.
M10: M10 is the average of the activity values for the 10 most active consecutive hours in the 24-hour cycle. Lower M10 counts indicate lower activity levels, whereas higher M10 counts indicate higher activity levels.
M10 midpoint: The M10 midpoint indicates the mean decimal clock time of the 10 consecutive hours with the highest activity in the 24-hour cycle.
RA: The RA is computed as the difference between the activity level at M10 and that at L5, normalized by their sum. RA is calculated as follows: (M10-L5)/(M10+L5). Higher RA values indicate a more robust 24-hour rest-activity rhythm, reflecting both higher activity during wakefulness and lower activity (more restful sleep) during the night.
The code used for the extraction of the rest-activity patterns is publicly available as the Matlab/Octave toolbox ActiCircadian, at https://sleepdata.org/community/tools/acticircadian. For the rest-activity analysis, we used raw activity counts from axis 1. Before analysis, we excluded non-wear times using the Troiano algorithm (17). M10 and L5 were computed by filtering the average sleep profile over the recording days with a 10 and 5 hour long window, respectively, and selecting the highest and lowest value, respectively, and their relative time of occurrence.
Anthropometry
We assessed the participants’ height and weight using a calibrated stadiometer (Shorr Productions, Olney, MD) and scale (model TBF-300A, Tanita Corporation of America, Inc., Arlington Heights, IL) and derived BMI in kg/m2. Age- and sex-specific BMI z scores were derived from US national reference data (18). We further evaluated adiposity by using dual-energy X-ray absorptiometry (DXA) and calculated the trunk and total fat mass index (kg/m2). We measured waist circumference (cm) using a Lefkin woven tape. We measured subscapular and triceps skinfold thicknesses using Holtain calipers (Holtain LTD, Crosswell, United Kingdom) and calculated the sum of the skinfold thicknesses. Research assistants performed all measurements following standardized techniques and participated in in-service training to ensure measurement validity.
Cardio-metabolic biomarkers
We measured fasting insulin, glucose, triglycerides and high-density lipoprotein cholesterol (HDL-C) from fasting blood samples. Electrochemiluminescence immunoassay was performed for insulin, while glucose, triglycerides, and HDL-C were measured enzymatically (Roche Diagnostics, Indianapolis, IN). We calculated insulin resistance by using the homeostatic model assessment (HOMA-IR = fasting insulin [μU/mL] × fasting glucose [mg/dL]/405). We measured blood pressure using automated oscillometric monitors (Dinamap Pro100, Tampa, FL). Trained research assistants obtained five measurements at one-minute-intervals from which we calculated the mean systolic blood pressure. We derived a cardio-metabolic risk score, as reported previously, as the mean of five sex-specific internal z-scores for systolic blood pressure, waist circumference, log-transformed HOMA-IR, log-transformed triglycerides and HDL-C (scaled inversely) (19). A positive score indicates a higher cardio-metabolic risk than the Project Viva cohort average.
Other measures
We calculated physical activity by taking the sum of the reported activity hours of the following two questions: “In the past month, how many hours per week does your child spend engaged in light or moderate recreational activities or sports such as biking, skateboarding, dancing, gymnastics, baseball, playing outdoors, or other similar activities? (Do not include walking.)” and “In the past month, on average, how many hours per week does your child spend engaged in vigorous recreational activities or sports such as swimming, running, basketball, soccer, hockey, football, rollerblading, tennis, karate, or other similar activities?” Mothers reported the pubertal stage of their child using a validated rating scale for pubertal development (20). The score was defined as a mean score of the sex-specific puberty metrics separately for girls (breast development, menstruation, body hair, growth spurts, and acne) and boys (voice changes, facial hair, body hair growth spurts, and acne). We obtained demographic data using questionnaires or from caregiver interviews. We asked mothers to report the child’s average total sleep duration over 24-hours. We assessed TV (or screen) time by asking children the number of hours the child spent watching television on a TV, computer, or handheld device on an average weekday and weekend day in the past month. TV (screen) time was a weighted average (weighted 5:2 for weekday vs. weekend to account for the distribution of days in a week). We defined season when actigraphy was collected by a four-level categorical variable according to the meteorological definition of seasons determined by range of temperature: Spring: March 1st-May 31th; Summer: June 1st –August 31st, Autumn: September 1st – November 30th, Winter: December 1st – February 28th.
Statistical analysis
Sample characteristics were described as means and standard deviations for the continuous variables, and frequencies and percentages for the categorical variables. We used multivariable linear regression models to examine the associations of rest-activity patterns with adiposity measures and the cardio-metabolic risk score. Our first model, model 1, included the following covariates that we hypothesized based on a priori knowledge suggesting that they associate with cardio-metabolic health or could operate as a design variable: maternal education and annual household income; child age, sex, race/ethnicity, and parent-reported puberty score; and season of data collection. We then adjusted for parent-reported physical activity of the child (model 2), parent-reported sleep duration of the child (model 3), and child-reported TV time (model 4). We decided to adjust for parent-reported physical activity rather than for actigraphy-recorded physical activity in order to avoid intercorrelation since all exposures were obtained from actigraphy. We carried out a sensitivity analysis adjusting for light and moderate-to-vigorous physical activity based on actigraphy. This analysis showed that the associations did not vary substantially with adjustment for actigraphy-derived physical activity as compared to parent-reported physical activity. In addition, we adjusted the cardio-metabolic risk score for BMI z-score in model 4 to examine associations independent of overall adiposity. We performed all analyses using SAS 9.4 (SAS Institute, Inc., Cary, NC).
Results
Characteristics of the study sample (N=778) are shown in Table I. The average age of the participants was 13.2 (±0.9) years and the sample included approximately equal proportions of males and females. Approximately one-third of adolescents were of minority ethnicity/race and 14% were obese defined by a BMI ≥ 95th percentile (21). Compared to the 778 participants in this analysis, non-participants (N=1,350) showed small differences in maternal educational attainment (60% versus 73% college-educated) and annual household income (59% versus 66% > $70,000).
Table I.
Descriptive characteristics of the study population (N=778).
| Early teen BMI percentile category | ||||
|---|---|---|---|---|
| Overall | Normal<85th %tile | Overweight 85th-<95th %tile | Obese ≥95th %tile | |
| N (%) | ||||
| Mother | ||||
| College graduate | 564 (72.6) | 440 (77.7) | 77 (70.0) | 46 (46.5) |
| Annual household income >$70,000 | 467 (65.5) | 355 (68.0) | 73 (70.9) | 38 (43.7) |
| Adolescent | ||||
| Female | 403 (51.8) | 291 (51.3) | 64 (58.2) | 47 (47.5) |
| Race/ethnicity. White | 497 (64.0) | 382 (67.5) | 65 (59.1) | 49 (49.5) |
| . Black | 123 (15.8) | 74 (13.1) | 19 (17.3) | 29 (29.3) |
| . Hispanic | 35 (4.5) | 21 (3.7) | 8 (7.3) | 6 (6.1) |
| . Asian | 21 (2.7) | 21 (3.7) | 0 (0.0) | 0 (0.0) |
| . Other | 101 (13.0) | 68 (12.0) | 18 (16.4) | 15 (15.2) |
| Mean (SD) | ||||
| Age, years | 13.2 (0.9) | 13.2 (0.9) | 13.2 (0.8) | 13.2 (0.9) |
| Pubertal development scale (units, range 1–4) | 2.5 (0.8) | 2.4 (0.8) | 2.8 (0.8) | 2.8 (0.8) |
| Adiposity and cardio-metabolic risk score | ||||
| BMI (kg/m2) | 20.9 (4.6) | 18.7 (2.0) | 23.8 (1.4) | 30.2 (4.3) |
| BMI z-score | 0.36 (1.08) | −0.12 (0.82) | 1.31 (0.18) | 2.06 (0.29) |
| Waist circumference (cm) | 72.9 (11.9) | 67.5 (6.3) | 79.9 (5.5) | 95.7 (9.9) |
| DXA total fat mass index (kg/m2) | 6.3 (3.1) | 4.8 (1.3) | 8.1 (1.6) | 12.2 (2.8) |
| DXA trunk fat mass index (kg/m2) | 2.4 (1.5) | 1.7 (0.5) | 3.2 (0.9) | 5.2 (1.5) |
| Subscapular + triceps skinfold (mm) | 28.3 (13.9) | 22.0 (6.9) | 36.7 (9.0) | 55.5 (10.4) |
| Cardio-metabolic risk score | −0.03 (0.60) | −0.25 (0.48) | 0.35 (0.45) | 0.74 (0.49) |
| Sleep, physical activity & TV time | ||||
| Sleep duration (hours/day), parent-report | 8.8 (0.9) | 8.9 (0.9) | 8.7 (0.8) | 8.4 (1.0) |
| Physical activity (hours/day), parent-report | 1.2 (1.0) | 1.3 (1.0) | 1.2 (0.9) | 0.9 (0.9) |
| TV time (hours/day), child-report | 2.0 (1.3) | 1.8 (1.2) | 2.1 (1.2) | 2.9 (1.6) |
| Rest-activity patterns | ||||
| Relative amplitude | 0.9 (0.1) | 0.9 (0.0) | 0.9 (0.1) | 0.9 (0.1) |
| L5 (counts) | 56.8 (43.4) | 53.5 (37.4) | 59.4 (45.7) | 73.3 (64.5) |
| L5 midpoint (decimal hours) | 3.2 (1.4) | 3.2 (1.5) | 3.2 (1.3) | 3.2 (1.3) |
| M10 (counts) | 1787 (514) | 1813 (516) | 1746 (485) | 1704 (495) |
| M10 midpoint (decimal hours) | 14.2 (4.3) | 14.2 (4.2) | 14.8 (3.1) | 13.6 (5.5) |
Abbreviations: BMI=body mass index; DXA=dual-energy x-ray absorptiometry; SD=standard deviation
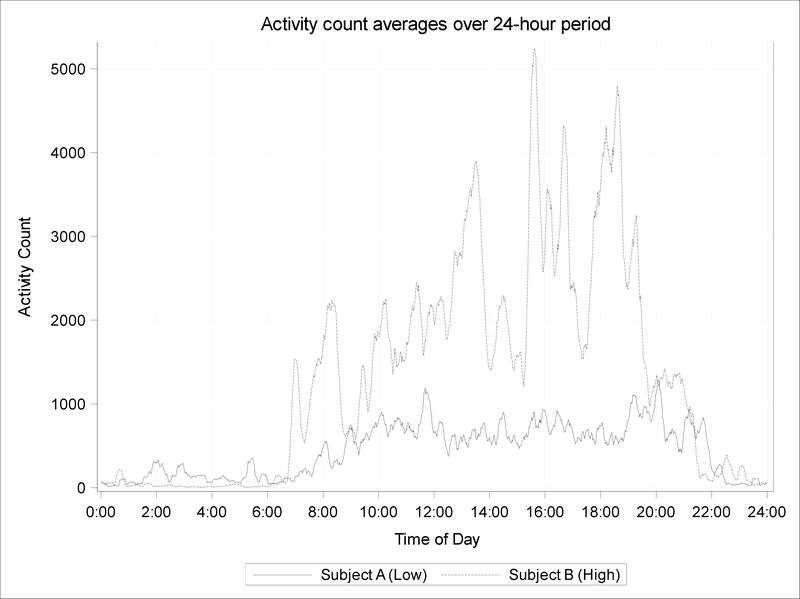
Associations of rest-activity patterns (per each inter-quartile range [IQR] increment) with adiposity measures and the cardio-metabolic risk score are shown in Table II. In multivariable analyses, adjusted for maternal education and annual household income; child age, sex, race/ethnicity, and parent-reported puberty score, season of data collection, and parent-reported physical activity, adolescents with higher log transformed L5 (nighttime activity) counts (IQR: 0.63 counts, log transformed) had higher BMI levels (model 2: 0.71; 95% CI: 0.33, 1.09), BMI-z-scores (model 2: 0.11; 95% CI: 0.02, 0.20) as well as had higher metrics of all other adiposity and cardio-metabolic health indices. Associations were slightly attenuated in subsequent models with further adjustment for parent-reported child sleep duration and child-reported TV time. Conversely, adolescents with higher M10 (daytime activity) counts (IQR: 630 counts) had smaller skinfold thicknesses (model 1: −1.45 mm; 95% CI: −2.73, −0.17). However, associations lost significance after additional adjustment for parent-reported physical activity (model 2 to 5). In all four models, adolescents with a higher RA (IQR: 0.04 units) had a lower BMI (model 4: −0.35 kg/m2; 95% CI: −0.60, 0.09) and lower adiposity (model 4: waist circumference [−1.09 cm; 95% CI: −1.75, −0.44], skinfold thicknesses [−0.85 mm; 95% CI: −1.65, −0.05], DXA total fat mass index [−0.36 kg/m2; 95% CI: −0.57, −0.15] and trunk fat mass index [−0.15 kg/m2; 95% CI: −0.25, −0.05]). A higher RA was also related to a lower cardio-metabolic risk score in models 1 and 2. Figure 1 shows two examples of adolescent with either low or high RA. Interestingly, we observed a negative association between L5 midpoint (IQR: 1.72 decimal hours) and skinfold thicknesses (model 4: −1.32 mm; 95% CI: −2.59, −0.05) in model 3 and 4, indicating that a later timing of lowest activity was associated with lower skinfolds. M10 midpoint did not associate with any markers of adiposity and cardio-metabolic risk.
Table II.
Associations of rest-activity patterns with obesity measures. Data from 778 participants, age 11–16 years, in Project Viva.
| Exposure (per IQR) | Outcome | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|
| Estimate (95% CI) | |||||
| Relative amplitude (per 0.04 units) | BMI (kg/m2) | −0.51 (−0.75, −0.26) | −0.48 (−0.73, −0.23) | −0.33 (−0.58, −0.08) | −0.35 (−0.60, −0.09) |
| BMI z-score | −0.08 (−0.14, −0.02) | −0.07 (−0.13, −0.01) | −0.05 (−0.11, 0.01) | −0.06 (−0.12, 0.01) | |
| Waist circumference (cm) | −1.46 (−2.11, −0.81) | −1.35 (−2.00, −0.70) | −1.06 (−1.71, −0.40) | −1.09 (−1.75, −0.44) | |
| Subscapular + triceps skinfold (mm) | −1.47 (−2.25, −0.70) | −1.25 (−2.04, −0.47) | −0.90 (−1.70, −0.10) | −0.85 (−1.65, −0.05) | |
| DXA total fat mass index (kg/m2) | −0.47 (−0.67, −0.27) | −0.46 (−0.67, −0.26) | −0.36 (−0.56, −0.15) | −0.36 (−0.57, −0.15) | |
| DXA trunk fat mass index (kg/m2) | −0.21 (−0.31, −0.12) | −0.21 (−0.31, −0.11) | −0.15 (−0.25, −0.05) | −0.15 (−0.25, −0.05) | |
| Cardio-metabolic risk score | −0.06 (−0.10, −0.01) | −0.06 (−0.10, −0.01) | −0.02 (−0.07, 0.02) | −0.01 (−0.05, 0.03) | |
| L5 (per 0.63 counts, log transformed) | BMI (kg/m2) | 0.74 (0.36, 1.11) | 0.71 (0.33, 1.09) | 0.57 (0.20, 0.94) | 0.56 (0.19, 0.93) |
| BMI z-score | 0.12 (0.03, 0.21) | 0.11 (0.02, 0.20) | 0.10 (0.01, 0.19) | 0.10 (0.01, 0.19) | |
| Waist circumference (cm) | 2.13 (1.13, 3.12) | 2.10 (1.11, 3.08) | 1.83 (0.86, 2.81) | 1.78 (0.82, 2.75) | |
| Subscapular + triceps skinfold (mm) | 1.77 (0.57, 2.96) | 1.58 (0.38, 2.78) | 1.26 (0.07, 2.45) | 1.12 (−0.05, 2.30) | |
| DXA total fat mass index (kg/m2) | 0.59 (0.28, 0.90) | 0.59 (0.27, 0.90) | 0.49 (0.18, 0.79) | 0.45 (0.15, 0.76) | |
| DXA trunk fat mass index (kg/m2) | 0.28 (0.14, 0.43) | 0.29 (0.13, 0.44) | 0.23 (0.08, 0.38) | 0.21 (0.07, 0.36) | |
| Cardio-metabolic risk score | 0.07 (0.00, 0.14) | 0.07 (0.00, 0.14) | 0.04 (−0.03, 0.11) | 0.02 (−0.04, 0.08) | |
| M10 (per 630 counts) | BMI (kg/m2) | −0.21 (−0.61, 0.20) | −0.04 (−0.46, 0.39) | 0.07 (−0.34, 0.47) | 0.06 (−0.34, 0.47) |
| BMI z-score | −0.03 (−0.13, 0.06) | 0.01 (−0.09, 0.11) | 0.02 (−0.08, 0.11) | 0.01 (−0.08, 0.11) | |
| Waist circumference (cm) |
−0.89 (−1.97, 0.18) | −0.36 (−1.45, 0.74) | −0.15 (−1.22, 0.93) | −0.18 (−1.24, 0.88) | |
| Subscapular + triceps skinfold (mm) | −1.45 (−2.73, −0.17) | −0.65 (−1.97, 0.67) | −0.38 (−1.68, 0.93) | −0.31 (−1.60, 0.97) | |
| DXA total fat mass index (kg/m2) | −0.32 (−0.65, 0.00) | −0.19 (−0.53, 0.15) −0.12 (−0.44, 0.21) | −0.11 (−0.43, 0.21) | ||
| DXA trunk fat mass index (kg/m2) | −0.13 (−0.29, 0.03) | −0.08 (−0.24, 0.09) | −0.04 (−0.19, 0.12) | −0.03 (−0.19, 0.12) | |
| Cardio-metabolic risk score | −0.07 (−0.14, 0.00) | −0.06 (−0.14, 0.01) | −0.05 (−0.12, 0.02) | −0.04 (−0.10, 0.02) | |
| L5 midpoint (per 1.72 decimal hours) | BMI (kg/m2) | −0.15 (−0.55, 0.25) | −0.13 (−0.53, 0.27) | −0.24 (−0.64, 0.15) | −0.25 (−0.65, 0.15) |
| BMI z-score | −0.03 (−0.13, 0.06) | −0.03 (−0.13, 0.06) | −0.04 (−0.14, 0.05) | −0.05 (−0.14, 0.05) | |
| Waist circumference (cm) | −0.55 (−1.62, 0.51) | −0.47 (−1.53, 0.58) | −0.69 (−1.73, 0.34) | −0.70 (−1.76, 0.35) | |
| Subscapular + triceps skinfold (mm) | −1.01 (−2.28, 0.25) | −1.05 (−2.32, 0.21) | −1.37 (−2.62, −0.12) | −1.32 (−2.59, −0.05) | |
| DXA total fat mass index (kg/m2) | −0.15 (−0.49, 0.20) | −0.16 (−0.51, 0.19) | −0.25 (−0.59, 0.09) | −0.26 (−0.61, 0.09) | |
| DXA trunk fat mass index (kg/m2) | −0.07 (−0.23, 0.10) | −0.07 (−0.24, 0.10) | −0.12 (−0.28, 0.04) | −0.13 (−0.30, 0.04) | |
| Cardio-metabolic risk score | 0.01 (−0.06, 0.08) | 0.00 (−0.07, 0.08) | −0.03 (−0.10, 0.05) | −0.01 (−0.08, 0.05) | |
| M10 midpoint (per 2.48 decimal hours) | BMI (kg/m2) | −0.08 (−0.27, 0.11) | −0.07 (−0.26, 0.11) | −0.08 (−0.27, 0.11) | −0.06 (−0.25, 0.13) |
| BMI z-score | −0.01 (−0.05, 0.03) | −0.01 (−0.05, 0.04) | 0.00 (−0.05, 0.04) | 0.00 (−0.04, 0.05) | |
| Waist circumference (cm) | −0.29 (−0.79, 0.22) | −0.23 (−0.72, 0.26) | −0.29 (−0.78, 0.20) | −0.24 (−0.73, 0.25) | |
| Subscapular + triceps skinfold (mm) | −0.13 (−0.73, 0.48) | −0.13 (−0.72, 0.46) | −0.19 (−0.79, 0.41) | −0.12 (−0.71, 0.48) | |
| DXA total fat mass index (kg/m2) | −0.11 (−0.29, 0.07) | −0.10 (−0.28, 0.08) | −0.15 (−0.33, 0.03) | −0.16 (−0.34, 0.03) | |
| DXA trunk fat mass index (kg/m2) | −0.05 (−0.14, 0.03) | −0.05 (−0.13, 0.04) | −0.07 (−0.16, 0.02) | −0.07 (−0.16, 0.01) | |
| Cardio-metabolic risk score | −0.01 (−0.04, 0.03) | 0.00 (−0.04, 0.03) | −0.01 (−0.04, 0.03) | −0.01 (−0.03, 0.02) | |
Model 1 includes adjustment for maternal education and annual household income; child age, sex, race/ethnicity, and parent-reported puberty score; and season of data collection. Each subsequent model includes the adjustments in the preceding model, with the following further adjustments: model 2 includes parent-reported physical activity, model 3 includes parent-reported sleep duration, and model 4 includes child-reported TV time and BMI-z-score (only for the cardio-metabolic risk score). Abbreviations: BMI=body mass index; DXA=dual-energy x-ray absorptiometry.
Abbreviations: BMI=body mass index; DXA=dual-energy x-ray absorptiometry.
Figure 1.
Actigraphic measurements in two adolescents displaying different RA patterns. The solid line represents the raw activity scores of one adolescent with a low RA of 0.73 (Subject A) in comparison with an adolescent with a high RA of 0.99 (Subject B, dashed line). Data are smoothed by applying a moving average of 15 minutes. Note that the adolescent with a lower RA compared to higher RA has modestly higher activity overnight and markedly lower activity during the day.
Discussion
This study of 24-hour actigraphic rest-activity patterns, adiposity and cardio-metabolic health in a community-based sample of adolescents identified several novel associations. RA, an indicator of a more robust 24-hour rest-activity rhythm, was consistently associated with a range of measures of adiposity and a cardio-metabolic risk score. Those associations persisted after controlling for parent-reported physical activity and sleep duration and child-reported TV time, suggesting that this marker is independently associated with cardio-metabolic health. Given that RA is determined by M10 (daytime activity) and L5 (nocturnal activity), we also observed significant associations of L5 with adiposity outcomes in the expected directions. For example, teens with obesity had about 23% higher L5 counts as compared to their peers with normal weight. The more consistent associations for L5 than M10 suggest that restful sleep rather than increased daytime activity may be a strong driver for the association of RA with adiposity.
Our data support a growing literature suggesting that adiposity and cardio-metabolic health are closely related to rest-activity rhythms (22–24). These findings are consistent with a previous study from our group that showed in an adult sample an inverse association between RA and BMI independent of multiple confounders as well as the duration of rest and/or activity (22). In adults, a more flattened RA has also been related to lower weight loss in overweight and obese women enrolled in a weight reduction program (25). Of note, rest-activity patterns vary across the lifespan and there is thus a need to also consider age-related changes in rhythm and their impact on obesogenic behavior. For example, we previously reported that older children compared to children aged 5–9-year-olds had a lower RA (26). However, the current literature on actigraphy-based rest-activity patterns, particularly using RA, in adolescents is sparse. Research on RA in adolescents had been mostly from psychiatric studies. For example, children and adolescents with major depressive disorder had a lower RA compared to controls (27). Interestingly, overweight and obese children and adolescents are more likely to experience psychological comorbidity than their normal-weight peers and genetic studies suggest a biological overlap of sleep traits with metabolism and psychiatric traits (37). However, it remains unclear as to whether psychiatric disorders and psychological problems are a cause or a consequence of childhood obesity or whether common factors promote both obesity and psychiatric disturbances in susceptible children and adolescents (28).
A number of biological theories may help explain why a high RA could be considered as a predictor of a healthier weight status, including its ability to index activity patterns in both day and nighttime and relate each to the other. First, individuals with longer periods of consolidated sleep (low L5, high RA) may have a shorter daily eating duration and thus may have lower caloric intake. Supporting this, a research study that employed actigraphy and a mobile app in healthy adults found that the total overnight fasting duration paralleled the time of inactivity at night (29). Second, higher RA may reflect higher physical activity level during the day, with attendant positive health effects (30). Third, the RA reflects overall strength of activity rhythms which are partially determined by circadian clocks. Disturbed rest-activity alterations may reflect disruptions in circadian rhythms, which are known to be closely linked to energy regulation and metabolic physiology (31).
Contrary to our working hypothesis, the timing of the most active period (M10 midpoint) was not associated with any adiposity outcome and a later timing of rest (L5 midpoint) was related to lower skinfolds. The midpoint of L5 and M10 provides reliable information about the timing of the rhythm, similar to that given by the acrophase of the cosinor method (32). Later M10 midpoint was related to obesity and metabolic risk in a recent study from Europe including 1044 adolescents (23). A limitation of that study was that the actigraphy recordings included only daytime measures and not 24-hour patterns (23). In a previous study of adults, we found that later acrophase (calculated using the cosinor approach), but not L5 and M10 midpoint, was associated with BMI (33). Differences in results highlight the possibility that measures of rest-activity and their relationship with obesity may differ between children and adults.
We observed an association between later L5 midpoint and skinfolds that was in the reverse direction than what we had hypothesized. There is however, controversy over the benefits of early vs late chronotype, and while some research suggests metabolic benefits of being a “lark” (34), recent research reported that genetic variants associated with later sleep timing were associated with lower BMI (35). Further research using 24-hour activity measurements along with chronotype information may clarify this association.
What are the clinical implications of our study? Traditional public health approaches have targeted either day or nighttime activities. Our results highlight the importance of considering both day and nighttime patterns of activity, which together influence health outcomes, and the potential role of addressing 24 hour activity patterns as a component of anticipatory guidance for adolescents and their families. Engaging adolescents in discussions of rest-wake activity patterns, and employing interventions that target 24 hour rest-activity rhythms, such as timed light exposure and exercise interventions, and sleep interventions, may be particularly useful. The utility of such approaches is supported by several small studies. In patients with seasonal affective disorder, bright light therapy in the morning was found to normalize disturbed rest-activity patterns with an increase in RA (36). In adolescents, being more active in the immediate period after morning awakening was suggested as helping to entrain circadian rhythms (37). A bout of moderate-intensity aerobic exercise in the morning (7 AM) was shown to improve blood pressure and sleep quality compared to exercise in the afternoon (1 PM) or evening (7 PM) in prehypertensive subjects (38). In addition, moderate outdoor running in the morning for three consecutive weeks impacted positively on objective and subjective sleep and psychological functioning in healthy adolescents (39). This literature as well as our data showing that higher RA is associated with less adiposity, supports the importance of 24 hour rest-activity rhythms in health and well-being. There is a need for rigorous clinical trials to formally test the role of interventions informed by circadian biology in adolescents, a group who often experience circadian misalignment. The strong associations we observed between actigraphy-assessed RA and adiposity suggests that actigraphy RA may be used both to identify those adolescents at risk for cardiometabolic dysfunction and to monitor the effects of interventions that target rest-activity rhythms.
Strengths of our study are the relatively large sample size, the modeling of daily 24-hour rest-activity patterns derived from wrist-actigraphy, and the large set of objectively obtained measures of adiposity and cardio-metabolic health. We also acknowledge several limitations. First, due to the cross-sectional nature of the study design, causal inferences cannot be made. For example, we cannot determine if RA is a consequence of adiposity or whether RA contributed to the development of overweight and obesity and/or cardio-metabolic risk. Second, given that many exposures and outcomes were correlated with one another, we did not correct for multiple comparisons, thereby increasing the likelihood of spurious results. Third, we did not directly measure physiological markers of circadian phase, such as dim light melatonin onset. However, linear correlations between L5 and M10 midpoint obtained from actigraphy with dim light melatonin onset (the most accurate marker for assessing the circadian pace marker) are moderate to strong (40). Fourth, data collection was only performed over seven days and conducted at a single urban area (Eastern Massachusetts) and findings may differ in areas with different climate and geography.
In summary, our results highlight the potential utility of RA to identify adolescents at risk for metabolic disorders and suggest the need to consider activity and sleep patterns across the 24-hour period as future behavioral targets. Failure to only consider physical activity and sleep duration without considering the timing and quality of sleep may reduce the ability to optimally classify adolescents at high cardio-metabolic risk. Future research should determine how RA could be best manipulated for obesity prevention and intervention. For example, adiposity intervention programs may consider including morning light exposure and exercise in order to stabilize rest-activity patterns.
Implications and Contributions:
In a cross-sectional study of adolescents, relative amplitude, a composite measure of the strength of daily rest and activity patterns from actigraphy, was associated with more favorable cardio-metabolic profiles, independent of the duration of sleep or activity. Future research should determine how relative amplitude could be best manipulated for obesity prevention.
Acknowledgments:
This research was supported by the supported by the National Institute of Child Health and Human Development, the National Institute of Diabetes and Digestive and Kidney Diseases, and National Heart, Lung, and Blood Institute of the National Institutes of Health [grant numbers U54CA116847, R01HD034568, UG3OD023286, P30 DK092924, K24 DK10589, and R35 HL135818]. M.Q. was supported by a scholarship from the Tuebinger Program for the Advancement of Women in Science. S.M. was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health [grant number R24HL114473]. We thank the Project Viva participants and staff.
Abbreviations:
- BMI
Body mass index
- SD
Standard deviation
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- HDL-C
High-density lipoprotein cholesterol
- DXA
dual-energy X-ray absorptiometry
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Disclaimer: The author S.L.R. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
First draft: The funding agency had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; in the preparation, review, or approval of the manuscript; and in the decision to submit the manuscript for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth M. Cespedes Feliciano, Email: elizabeth.m.cespedes@kp.org.
Sheryl L. Rifas-Shiman, Email: sheryl_rifas@harvardpilgrim.org.
Sara Mariani, Email: SMARIANI@bwh.harvard.edu.
Emily R. Kaplan, Email: EKAPLAN1@partners.org.
Michael Rueschman, Email: MRUESCHMAN@bwh.harvard.edu.
Emily Oken, Email: emily_oken@harvardpilgrim.org.
Elsie M. Taveras, Email: elsie.taveras@mgh.harvard.edu.
Susan Redline, Email: SREDLINE@bwh.harvard.edu.
References
- [1].Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the united states, 1988–1994 through 2013–2014. JAMA 2016;315:2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guidolin M, Gradisar M. Is shortened sleep duration a risk factor for overweight and obesity during adolescence? A review of the empirical literature. Sleep Medicine 2012;13:779–786. [DOI] [PubMed] [Google Scholar]
- [3].Miguel-Berges ML, Reilly JJ, Moreno Aznar LA, et al. Associations Between Pedometer-Determined Physical Activity and Adiposity in Children and Adolescents: Systematic Review. Clin J Sport Med 2018;28:64–75. [DOI] [PubMed] [Google Scholar]
- [4].Miller AL, Lumeng JC, LeBourgeois MK. Sleep patterns and obesity in childhood. Current opinion in endocrinology, diabetes, and obesity 2015;22:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Broussard JL, Van Cauter E. Disturbances of sleep and circadian rhythms: novel risk factors for obesity. Current opinion in endocrinology, diabetes, and obesity 2016;23:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kjeldsen JS, Hjorth MF, Andersen R, et al. Short sleep duration and large variability in sleep duration are independently associated with dietary risk factors for obesity in Danish school children. Int J Obes (Lond) 2014;38:32–39. [DOI] [PubMed] [Google Scholar]
- [7].Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics 2011;127:e345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].He F, Bixler EO, Liao J, et al. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Med 2015;16:1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A 2013;110:5695–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med 2017;376:254–266. [DOI] [PubMed] [Google Scholar]
- [11].Calogiuri G, Weydahl A, Carandente F. Methodological issues for studying the rest-activity cycle and sleep disturbances: a chronobiological approach using actigraphy data. Biol Res Nurs 2013;15:5–12. [DOI] [PubMed] [Google Scholar]
- [12].Cespedes Feliciano EM, Quante M, Weng J, et al. Actigraphy-Derived Daily Rest-Activity Patterns and Body Mass Index in Community-Dwelling Adults. Sleep 2017;40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thomas KA, Burr RL, Spieker S. Maternal and infant activity: Analytic approaches for the study of circadian rhythm. Infant Behavior and Development 2015;41:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oken E, Baccarelli AA, Gold DR, et al. Cohort profile: project viva. Int J Epidemiol 2015;44:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Quante M, Kaplan ER, Cailler M, et al. Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms. Nature and science of sleep 2018;10:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cole RJ, Kripke DF, Gruen W, et al. Automatic sleep/wake identification from wrist activity. Sleep 1992;15:461–469. [DOI] [PubMed] [Google Scholar]
- [17].Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40:181–188. [DOI] [PubMed] [Google Scholar]
- [18].Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002;109:45–60. [DOI] [PubMed] [Google Scholar]
- [19].Cespedes EM, Rifas-Shiman SL, Redline S, et al. Longitudinal associations of sleep curtailment with metabolic risk in mid-childhood. Obesity (Silver Spring) 2014;22:2586–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. The Journal of adolescent health : official publication of the Society for Adolescent Medicine 1993;14:190–195. [DOI] [PubMed] [Google Scholar]
- [21].Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000. CDC Growth Charts for the United States: methods and development. Vital and health statistics Series 11, Data from the national health survey 2002:1–190. [PubMed] [Google Scholar]
- [22].Cespedes Feliciano EM, Quante M, Weng J, et al. Actigraphy-Derived Daily Rest-Activity Patterns and Body Mass Index in Community-Dwelling Adults. Sleep 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Garaulet M, Martinez-Nicolas A, Ruiz JR, et al. Fragmentation of daily rhythms associates with obesity and cardiorespiratory fitness in adolescents: The HELENA study. Clin Nutr 2016. [DOI] [PubMed] [Google Scholar]
- [24].Luik AI, Zuurbier LA, Hofman A, et al. Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int 2013;30:1223–1230. [DOI] [PubMed] [Google Scholar]
- [25].Bandin C, Martinez-Nicolas A, Ordovas JM, et al. Circadian rhythmicity as a predictor of weight-loss effectiveness. Int J Obes 2014;38:1083–1088. [DOI] [PubMed] [Google Scholar]
- [26].Mitchell JA, Quante M, Godbole S, et al. Variation in actigraphy-estimated rest-activity patterns by demographic factors. Chronobiol Int 2017;34:1042–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Armitage R, Hoffmann R, Emslie G, et al. Rest-activity cycles in childhood and adolescent depression. J Am Acad Child Adolesc Psychiatry 2004;43:761–769. [DOI] [PubMed] [Google Scholar]
- [28].Rankin J, Matthews L, Cobley S, et al. Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolescent health, medicine and therapeutics 2016;7:125–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gill S, Panda S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 2015;22:789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tremblay MS, Esliger DW, Tremblay A, et al. Incidental movement, lifestyle-embedded activity and sleep: new frontiers in physical activity assessment. Can J Public Health 2007;98 Suppl 2:S208–217. [PubMed] [Google Scholar]
- [31].Johnston JD, Ordovas JM, Scheer FA, et al. Circadian Rhythms, Metabolism, and Chrononutrition in Rodents and Humans. Adv Nutr 2016;7:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pérez JAM. An Introduction to Chronobiology In: Garaulet M, Ordovás JM, eds. Chronobiology and Obesity. New York, NY: Springer New York, 2013:11–28. [Google Scholar]
- [33].Cespedes Feliciano EM, Quante M, Weng J, et al. Actigraphy-Derived Daily Rest–Activity Patterns and Body Mass Index in Community-Dwelling Adults. Sleep 2017;40:zsx168–zsx168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Arora T, Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int J Obes 2015;39:39–44. [DOI] [PubMed] [Google Scholar]
- [35].Lane JM, Chang AM, Bjonnes AC, et al. Impact of Common Diabetes Risk Variant in MTNR1B on Sleep, Circadian, and Melatonin Physiology. Diabetes 2016;65:1741–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Winkler D, Pjrek E, Praschak-Rieder N, et al. Actigraphy in patients with seasonal affective disorder and healthy control subjects treated with light therapy. Biol Psychiatry 2005;58:331–336. [DOI] [PubMed] [Google Scholar]
- [37].Richardson CE, Gradisar M, Short MA, et al. Can exercise regulate the circadian system of adolescents? Novel implications for the treatment of delayed sleep-wake phase disorder. Sleep Med Rev 2017;34:122–129. [DOI] [PubMed] [Google Scholar]
- [38].Fairbrother K, Cartner B, Alley JR, et al. Effects of exercise timing on sleep architecture and nocturnal blood pressure in prehypertensives. Vasc Health Risk Manag 2014;10:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kalak N, Gerber M, Kirov R, et al. Daily morning running for 3 weeks improved sleep and psychological functioning in healthy adolescents compared with controls. J Adolesc Health 2012;51:615–622. [DOI] [PubMed] [Google Scholar]
- [40].Bonmati-Carrion MA, Middleton B, Revell VL, et al. Validation of an innovative method, based on tilt sensing, for the assessment of activity and body position. Chronobiol Int 2015;32:701–710. [DOI] [PubMed] [Google Scholar]