Abstract
Posttraumatic stress disorder (PTSD) symptoms are highly prevalent among individuals with substance use disorders (SUD), presenting a difficult-to-treat, complex comorbidity. Prognostic factors for treatment outcomes may characterize heterogeneity of the treated population and/or implicate mechanisms of action that are salient for improving treatments. High-frequency heart rate variability (HF-HRV) is a suggested biomarker for emotion regulation: the ability to generate appropriate emotional responses via the influence of the parasympathetic nervous system on the heart. This initial study investigated the utility of baseline resting HF-HRV for predicting PTSD symptoms and substance use outcomes following treatment of 37 SUD participants with comorbid PTSD symptoms. Participants completed either standard cognitive-behavioral therapy (CBT) for SUD or a novel Treatment of Integrated Posttraumatic Stress and Substance use (TIPSS) that combined CBT for SUD with cognitive processing therapy for PTSD. Analyses demonstrated that higher HF-HRV predicted greater reduction in PTSD symptoms following both types of treatment. This suggests prognostic value of HF-HRV as a predictor of PTSD treatment outcomes; those with poorer autonomic emotional regulation may not respond as well to psychotherapy in general. This hypothesis-generating analysis identifies a putative biomarker that might have utility in treatment prediction.
Keywords: heart rate variability, trauma, PTSD, substance use, treatment
1. Introduction
At least 90% of individuals with substance use disorders (SUDs; Brown, Stout, & Mueller, 1999) report trauma exposure. Sequelae of trauma may include the development of SUD and/or posttraumatic stress disorder (PTSD) symptoms characterized by intrusive thoughts, avoidance, negative alterations in cognitions and mood, and arousal (APA, 2013). Epidemiologically, 30-50% of individuals with a SUD meet full criteria for PTSD (Ford, Hawke, Alessi, Ledgerwood, & Petry, 2007), and rates of comorbidity are higher among treatment-seeking samples (Mccauley, Killeen, Gros, Brady, & Back, 2012). Individuals with diagnostic or subthreshold PTSD symptoms have worse SUD treatment outcomes than those with SUD alone (Najavits et al., 2015; Norman, Tate, Anderson, & Brown, 2007; Ouimette, Moos, & Finney, 2003); however, the mechanisms responsible for poorer treatment outcomes observed among individuals with this comorbidity are not clear. Prognostic variables predicting treatment outcomes could guide personal treatment tailoring, ultimately improving outcomes for this difficult to treat clinical presentation (Insel et al., 2010; Jacobsen, Southwick, & Kosten, 2001)
At least four basic etiological pathways have been established with regard to SUD/PTSD comorbidity (Stewart & Conrod, 2003). First, individuals with PTSD may develop SUD in the aftermath of trauma as an attempt to self-medicate the painful and intense emotionality associated with PTSD (‘self-medication model’; Khantzian, 1999; Reed, Anthony, & Breslau, 2007). Second, individuals with SUD, by virtue of lifestyles that inherently place them at increased risk of violence and harm, are at increased risk of experiencing trauma and subsequently developing PTSD (‘high-risk hypothesis’; Acierno, Resnick, Kilpatrick, Saunders, & Best, 1999; Bonin, Norton, Asmundson, Dicurzio, & Pidlubney, 2000; Chilcoat & Breslau, 1998; Kaysen, Neighbors, Martell, Fossos, & Larimer, 2006; North et al., 1999). Third, the disorders develop concurrently, in the aftermath of trauma, due to common underlying biopsychosocial processes (‘shared liability model’; Breslau, Davis, Andreski, Peterson, & Schultz, 1997; Cottler et al., 2011; Fassino et al., 2004; Krueger & Markon, 2006; Wolf et al., 2010). Fourth, individuals with SUD tend to experience increased anxiety and arousal secondary to chronic substance use (e.g., withdrawal symptoms), and this arousal coupled with poor coping, may increase risk for developing PTSD in the aftermath of trauma (‘susceptibility model’; Jacobsen et al., 2001; Sharkansky, Brief, Peirce, Meehan, & Mannix, 1999; Stewart, Conrod, Barton Samoluk, Pihl, & Dongier, 2000). The self-medication pathway is the most well-known, well-supported, and well-studied (e.g., Coffey et al., 2002; O’Hare & Sherrer, 2011; Saladin et al., 2003; Simpson, Stappenbeck, Varra, Moore, & Kaysen, 2012; Waldrop, Back, Verduin, & Brady, 2007). While use of substances might alleviate PTSD and related symptoms in the short term, substance use is an ineffective long-term coping strategy as it is associated with greater emotional avoidance, escalating substance use, and limited occasions to develop adaptive emotion regulation strategies (Littleton, Horsley, John, & Nelson, 2007; Ouimette et al., 2003; Ullman, Relyea, Peter-Hagene, & Vasquez, 2013; Victorson, Farmer, Burnett, Ouellette, & Barocas, 2005). Indeed, the extent to which an individual has difficulties with emotional regulation appears likely to influence treatment outcomes in both disorders (Bonn-Miller, Vujanovic, Boden, & Gross, 2011; McDermott, Tull, Gratz, Daughters, & Lejuez, 2009; Weiss, Tull, Viana, Anestis, & Gratz, 2012). Thus, emotion regulation may serve as a mechanism to predict or maintain course and treatment outcome for co-occurring SUD/PTSD (Gratz & Tull, 2010; Seligowski, Lee, Bardeen, & Orcutt, 2015; Tull, Barrett, McMillan, & Roemer, 2007).
A promising modality for informing treatment design and outcome, physiological measures are not subject to reporting bias, do not depend on individual insight, and provide a foundation for a biologically informed approach for treatment development. Heart rate variability, the variability in the time interval between heart beats, may serve as a useful physiological marker for investigating the influence of emotional regulation abilities on SUD/PTSD treatment outcomes (Appelhans & Luecken, 2006; Balzarotti, Biassoni, Colombo, & Ciceri, 2017). Resting high frequency heart rate variability (HF-HRV) reflects increases and decreases in heart rate that occur with respiration, putatively indicating the strength of parasympathetic influences on the heart through the vagus nerve (Thayer, Hansen, Saus-Rose, & Johnsen, 2009). The vagal nerve acting as a tonic “brake” on heart rate, can quickly regulate the frequency of cardiac cycles to match the demands of a given situation (Porges, 2001). High levels of resting HF-HRV theoretically indicate greater flexibility in adjusting emotional arousal to meet situational demands, while low levels may indicate autonomic inflexibility implying a vulnerability in adapting to autonomically challenging situations (Appelhans & Luecken, 2006). Consistent with this, resting HF-HRV is low in both PTSD and SUD populations (Chalmers, Quintana, Abbott, & Kemp, 2014; H. Cohen et al., 2000; Ingjaldsson, Laberg, & Thayer, 2003) and has been linked to hypervigilance and excessive worry in anxiety disorders (Lyonfields, Borkovec, & Thayer, 1995; Thayer, Friedman, & Borkovec, 1996). Evidence suggests that low resting HF-HRV may be a risk factor for PTSD: one study found that low HF-HRV predicts susceptibility to PTSD following combat-related trauma in veterans (Minassian et al., 2015). Individuals prone to develop PTSD symptoms may exhibit less functional parasympathetic adaptation, resulting in the diminished ability to self-regulate emotional arousal after a traumatic experience. SUD may develop as an attempt to regulate levels of arousal.
In concert with the prognostic associations of HF-HRV in SUD and PTSD, evaluating this putative etiology requires convergent evidence regarding the association between baseline resting HF-HRV and treatment outcomes. Leading treatments for PTSD involve cognitive processing of the trauma-related beliefs or imaginal exposure to the trauma memories during therapy (Halligan, Michael, Clark, & Ehlers, 2003). Extended/suppressed emotional responses during processing of or exposure to trauma might worsen treatment outcomes (Jaycox, Foa, & Morral, 1998). For example, the ability to regulate negative mood during skills training prior to treatment predicts an improved response to subsequent exposure therapy for PTSD (Cloitre et al., 2002). Similarly in SUD treatment, emotion regulation deficits, and specifically poor ability to tolerate negative emotions at baseline, predict frequency of alcohol and cocaine use during treatment for these disorders, while post-treatment emotion regulation deficits also predict alcohol use at follow-up (Berking et al., 2011; Stotts et al., 2015).
Consistent with this, baseline resting HF-HRV might also be expected to predict post-treatment outcomes for both SUD and PTSD symptoms. Indeed, one study found that those with higher resting baseline HF-HRV displayed improved self-reported depression after writing about a traumatic experience than those with lower HF-HRV (Sloan & Epstein, 2005). Similarly, another study reported that individuals with higher baseline resting HF-HRV benefited (i.e., improved scores on self-reported depression) more from writing about bereavement than those with low HF-HRV (O’Connor, Allen, & Kaszniak, 2005). Notably, neither study was conducted in individuals diagnosed with PTSD or SUD. To date, no study has investigated the potential of baseline resting HF-HRV to predict outcomes in cognitive-behavioral treatment for individuals with co-occurring SUD/PTSD symptoms. A few studies have demonstrated that HRV reactivity (e.g., in response to trauma or substance cues) predicts substance use treatment outcomes and anxiety reduction (Garland, Franken, & Howard, 2012; Libby, Worhunsky, Pilver, & Brewer, 2012; Mathewson et al., 2013); however, HRV reactivity and baseline resting HF-HRV differ in their associations with psychological constructs (Muhtadie, Koslov, Akinola, & Mendes, 2015).
The current study examined baseline resting HF-HRV in an early-phase, clinical trial of a novel treatment for trauma-exposed individuals with SUD who also displayed clinically significant PTSD symptoms (at least 4 PTSD symptoms; Vujanovic, Smith, Green, Lane, & Schmitz, 2018; Vujanovic, Smith, Tipton, & Schmitz, 2018). Individuals were allocated to two conditions: standard Cognitive Behavioral Therapy for substance use (CBT) or the Treatment of Integrated Posttraumatic Stress and Substance Use (TIPSS). Of note, the TIPSS intervention contained psychoeducation regarding PTSD/SUD comorbidity and modules focusing on cognitive-emotional processing of the trauma, while standard CBT did not include trauma-related material. We hypothesized that individuals with higher baseline resting HF-HRV would show greater improvement in both SUD and PTSD symptoms at the end of treatment. Furthermore, we expected that this relationship would be stronger in the TIPSS condition, as this integrated treatment required explicit cognitive processing of the trauma, while standard CBT did not. Theoretically, higher baseline resting HF-HRV may be indicative of more flexible regulation of emotions during the processing of trauma, rather than emotional regulation reliant on suppression or avoidance. Therefore, individuals with higher resting HF-HRV might derive more benefit from the TIPSS condition in particular.
2. Method
2.1. Design
The current study is a secondary analysis of data collected from a pilot clinical trial (Vujanovic, Smith, Green, et al., 2018) investigating a novel treatment for comorbid PTSD and SUD (ClinicalTrials.gov Identifier: NCT02461732). Procedures relevant to the current investigation are reported here, with main design published elsewhere (Vujanovic, Green, Lane, & Schmitz, 2018). The relevant institutional review boards approved all procedures, which were conducted in accord with the Declaration of Helsinki.
2.2. Participants
Fifty-three participants1 with substance dependence per DSM-IV and at least four PTSD symptoms per DSM-5 (APA, 2000; APA, 2013) were enrolled in the study (this study was initiated shortly after the transition between DSM-IV and DSM-5, so DSM-IV was used for SUD diagnoses to remain consistent with other ongoing studies in the center, but PTSD diagnoses were based upon DSM-5 criteria). Of the 53 participants, 12 participants were non-randomized1 and 41 participants were randomized to either CBT (n = 32) or TIPSS (n = 21). Six participants from each condition dropped out of the study, while HRV data were missing for 4 participants2, leaving a total of 37 participants in the final analysis (23 in CBT and 14 in TIPSS). Participants were recruited from the greater Houston area. Participants were English-speaking, 18-65 years of age, reporting a history of trauma, and treatment-seeking for substance use and trauma-related issues. Individuals were excluded based on the following criteria: exclusive (only) nicotine dependence, alcohol or opioid dependence requiring supervised detoxification, current or past bipolar I or major psychotic disorder, past 6-month psychotic symptoms, major medical conditions, past 30-day suicidal or homicidal ideation with intent or plan, pregnancy, or inability to provide consent.
2.3. Measures
2.3.1. Baseline Resting High-Frequency Heart Rate Variability
Collection of electrocardiograms (ECG) occurred during a baseline experimental session prior to treatment. HF-HRV data were extracted from a 5-minute rest period and in response to a scripted cue-reactivity paradigm. As HF-HRV did not change as a function of cue type (Vujanovic, Wardle, Bakhshaie, et al., 2018), only the 5-minute baseline (pre-cue) resting period is described here. Electrocardiograms were collected using disposable Ag/AgCL electrodes in a modified Lead II placement on the inner forearms. Signals were amplified by a Biopac ECG100C amplifier (Biopac Systems, Inc., Goleta, CA) with a 35Hz low pass notch filter and 0.05Hz high pass filter, and sampled at 1.000kHz by a Biopac MP150. R-waves were detected in Biopac AcqKnowledge software, using auto threshold detection with a noise rejection interval of 5% of the peak-to-peak range, and windowing of 40 to 120 beats-per-minute. Trained research assistants edited the resulting inter-beat intervals for irregular beats using CardioEdit software (Brain-Body Center, University of Illinois at Chicago, Chicago IL). HF-HRV was quantified from these inter-beat interval sequences using CardioBatch software (Brain-Body Center, University of Illinois at Chicago, Chicago IL) and the moving polynomial method (Porges, 1985; Porges & Bohrer, 1990), with standard adult HF-HRV settings: 2Hz sample rate, frequency window of 0.12-0.40Hz, and 30s epoch length. The data are segmented into 30-second epochs and then averaged across those epochs in order to reduce distortion. The final values represent the average of the natural logarithm transformed variance of each epoch.
2.3.2. PTSD Symptoms
Clinician Administered PTSD Scale for DSM-5 (CAPS-5; Weathers et al., 2013).
The CAPS-5 is a structured interview containing 20 items to assess PTSD symptomology. Each PTSD symptom is rated on a 5-point Likert scale (0 = absent to 4 = extreme/incapacitating) with a total possible score of 80 points. A past-month time-frame was used to assess current PTSD symptom severity at baseline (α = .72) and at visit 12 (α = .82). CAPS-5 formed the basis of a PTSD diagnosis at both time-points. The principal investigator of the main treatment study conducted training to criterion with a group of 5-7 clinicians, who would administer the CAPS-5 for both groups and pre-and post- assessments. The clinician that was assigned as the study therapist was not permitted to administer the CAPS-5. Each participant’s CAPS-5 scores were reviewed for standardized administration and procedural adherence by the principal investigator, who was blind to the study condition.
2.3.3. Substance Use
Timeline Follow Back (TLFB; Sobell & Sobell, 1992).
The TLFB is a self-report measure of timing and amount of substance use. At the first visit, participants fill out a calendar indicating the substance and the amount of that substance that they used for each day for the past 30 days. Participants completed the TLFB each time they entered the clinic and reported substance use since the last visit. As the current sample included participants with differing primary SUDs, we calculated the percent usage reported during the baseline week and at visit 12 for each participant’s primary drug of choice. TLFB scores were determined by dividing the number of reported days of use by the number of days in the reporting period for both baseline and visit 12.
2.4. Procedure
2.4.1. Intake and Baseline.
Interested individuals were screened via telephone and potential participants entered the clinic for an in-person intake that included a medical exam, diagnostic interviews with study therapists, urine and breath samples, and self-report questionnaires. Eligible participants then completed a baseline visit the week before beginning treatment. During this visit, participants provided urine and breath samples, reported substance use over the past 2 weeks with TLFB, and completed the CAPS-5 interview. Physiological data were also collected from eligible participants during an experimental laboratory paradigm (for details, see Vujanovic, Wardle, et al., 2018). During this session, ECG was collected during a 5-minute period before completion of the experimental tasks in order to derive resting HF-HRV.
2.4.2. Treatment.
Eligible participants completed either standard CBT for SUD or TIPSS. Standard CBT for SUD involved motivational interviewing and skills training techniques to improve coping in response to high-risk situations. Specific topics covered in CBT included problem thinking, changing problem thinking, lifestyle balance, increasing non-substance activities, enhancing social support, and relapse prevention. TIPSS (see Vujanovic, Smith, Green, et al., 2018; Vujanovic, Smith, Tipton, et al., 2018) incorporated motivational interviewing, key topics relevant to CBT for SUD, and psychoeducation on the link between PTSD and substance use. Cognitive-emotional processing techniques were used including identification of trauma-related maladaptive beliefs related to trust, intimacy, esteem, power/control, and safety, Socratic questioning techniques to challenge those beliefs, and a written account of the trauma with in-session review and discussion. Each session also included a review of any between-session substance use, cravings, and trauma cues. Both treatments consisted of a total of 12 visits over 6 weeks. In addition to the baseline session, a TLFB interview was completed each time the participants visited the clinic. The CAPS-5 was administered at baseline and immediately following the final treatment session (visit 12).
2.5. Statistical Analyses
We first assessed the sample characteristics and tested if the groups differed on any demographic or baseline variables using independent samples t-tests or chi-square analyses. We also tested if HF-HRV differed between participants who dropped and those who completed the study. For the main analyses, we performed a series of regressions to measure the independent and interactive effects of baseline resting HF-HRV and treatment condition on the two primary outcome variables (CAPS-5 and TLFB at week 12). As initial starting points can bias estimates of treatment effects in the traditional “change score” approach (Senn, 2006; Vickers, 2001), we chose to use a method that accounts for the baseline distribution of scores (van Breukelen, 2013). To do so, we entered the baseline score (CAPS-5 or TLFB at baseline) as a covariate in predicting post-treatment score (CAPS-5 or TLFB at week 12). HF-HRV, baseline CAPS-5 and baseline TLFB were mean centered by subtracting the mean from each participant’s value, and treatment condition was coded utilizing orthogonal polynomial codes, to ensure interpretability of the main effects in the presence of an interaction (J. Cohen, Cohen, West, & Aiken, 2003). For visual purposes and interpretability only, we chose to graph the values as change scores (baseline minus final). After data exploration, we determined that the data might not fully meet normality assumptions for the TLFB analysis, as raw scores were slightly skewed. While violations of normality may not be a large concern (Vickers, 2005), we confirmed similar results in a separate analysis on traditional change scores, which did meet the normality assumption. Finally, we tested these models among two subgroups: 1) only those participants who met full diagnostic criteria for PTSD at baseline (n = 29) and 2) only those participants who were randomized to treatment condition (n = 31).
We also evaluated the strength of associations among various demographic and baseline variables (e.g., age, sex, addiction severity, etc.) and the primary variables of interest (CAPS-5, TLFB, HF-HRV, and treatment condition) to identify potential confounds. No demographic variables were associated with both a predictor (HF-HRV, treatment condition) and an outcome variable (PTSD symptoms and substance use), and therefore, we did not include any covariates in the main regression analyses (per guidelines on inclusion of covariates in analyses of clinical trials; see Assmann et al., 2000; Pocock, Assmann, Enos, & Kasten, 2002). However, as HF-HRV is often correlated with age, sex, and race, we performed a separate analysis with these covariates to test whether HF-HRV predicts treatment outcomes above and beyond basic demographic characteristics.
3. Results
3.1. Sample Statistics
The sample was 51% male with an average age of 46.95 (SD = 9.68). Racial and ethnic categories were reported as follows: 73% African American, 24.3% White, and 2.7% Hispanic. On average, participants reported 13 years of education. All participants reported at least 4 symptoms of PTSD, and 78.4% of the sample met full diagnostic criteria for PTSD. The most common traumas included assaults (i.e., sexual, physical, and weapon-related), natural disasters, and transportation accidents. A smaller (< 20%) percentage of participants reported combat or captivity related trauma. All participants met diagnostic criteria for SUD for at least one substance class, with 54% of the sample meeting diagnostic criteria for more than one SUD. The most common primary substance diagnosis was cocaine dependence (64.9%). While conditions were comparable on demographic characteristics, baseline resting HF-HRV was higher in the standard CBT for SUD condition than the TIPSS condition in the main analysis, but HF-HRV did not differ between conditions in the randomized-only analysis, t(46) = 1.815, p = 0.08. Dropout rates did not differ between the conditions, X2 = .698, p = 0.4033. Additionally, there was no difference in baseline resting HF-HRV between those who completed the study and those who dropped, t(46) = −.520, p = .605. Please see Table 1 for detailed sample characteristics.
Table 1.
Sample Characteristics
| CBT Condition (n = 23) | TIPSS Condition (n = 14) | P Value | |
|---|---|---|---|
|
Demographics | |||
| Age (SD) | 45.52 (9.29) | 49.29 (10.19) | 0.257 |
| Gender | 52% Male | 50% Male | 0.898 |
| Race | 73.9% African American | 71.4% African American | 0.869 |
| Ethnicity | 91.3% Not Hispanic | 85.7% Not Hispanic | 0.074 |
| Years of Education (SD) | 13.5 (1.71) | 13.21 (3.33) | 0.770 |
| Employment | 39.1% Unemployed | 42.9% Unemployed | 0.823 |
| Marital Status | 8.7% Married | 28.6% Married | 0.112 |
|
Substance Use Disorder Diagnoses | |||
| Cocaine Abuse/Dependence | 65.2% | 85.7% | 0.173 |
| Alcohol Abuse/Dependence | 34.8% | 64.3% | 0.081 |
| Sedative Abuse/Dependence | 4.3% | 7.1% | 0.715 |
| Cannabis Abuse/Dependence | 43.5% | 14.35% | 0.066 |
|
Trauma History | |||
| Number of Traumas | 6.78(3.83) | 8.07(3.17) | 0.464 |
| Natural Disaster | 65.2% | 64.3% | 0.954 |
| Transportation Accident | 65.2% | 78.6% | 0.389 |
| Physical Assault | 69.6% | 71.4% | 0.904 |
| Assault With Weapon | 82.6% | 64.3% | 0.208 |
| Sexual Assault | 73.9% | 57.1% | |
|
Descriptives | |||
| Baseline Resting HF-HRV | 6.20 (1.80) | 4.53 (2.43) | 0.023 |
| Baseline CAPS-5 | 35.26 (11.48) | 40.43 (13.77) | 0.226 |
| Final CAPS-5 | 26.56 (13.81) | 27.29 (18.34) | 0.893 |
| Baseline TLFB | 53.32% (39.81) | 62.26% (38.09) | 0.505 |
| Final TLFB | 33.23% (45.66) | 38.64% (41.81) | 0.721 |
Note. HF-HRV = high-frequency heart rate variability, CAPS-5 = Clinician Administered PTSD Scale, TLFB = Timeline Follow Back, CBT = Cognitive Behavioral Therapy, TIPSS = Treatment of Integrated Posttraumatic Stress and Substance Use. P Value column indicates significance levels for either chi square or independent samples t-test between groups.
3.2. Regression Analyses
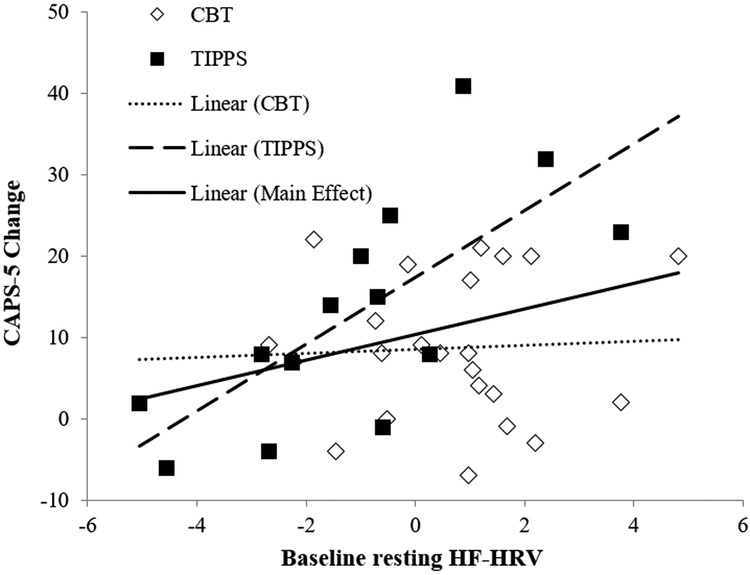
The overall regression model predicted CAPS-5 final scores, R2 = 0.816, F(4,32) = 15.89, p < 0.001. HF-HRV moderated the effect of treatment group (Table 2). In the TIPSS condition, higher HF-HRV predicted greater decrease in CAPS-5 final scores, r = −0.539, p = 0.047; the CBT condition failed to demonstrate a relationship between HF-HRV and CAPS-5 final scores r = 0.094, p = 0.669. The interaction and main effect of HF-HRV on CAPS-5 is presented in Figure 1. The overall model failed to predict TLFB final scores, R2 = 0.408, F(4,32) = 1.59, p = 0.200. HF-HRV did not moderate the effect of treatment group; therefore, simple effects were not investigated (Table 2). When analyses for the TLFB were repeated with a change score approach to meet normality assumptions, the model similarly failed to predict TLFB change scores, R2 = 0.084, F(3,33) = 1.01, p = 0.400.
Table 2.
Linear Regression Results
| PTSD Symptoms: CAPS-5 Final Scores | ||||
|---|---|---|---|---|
| B | SE B | β | p | |
| Variable | ||||
| Intercept | 23.28 | 1.72 | <0.001 | |
| HRV1 | −2.14 | 0.78 | −0.30 | 0.010 |
| Condition2 | −4.17 | 1.78 | −0.27 | 0.025 |
| Interaction3 | −1.97 | 0.78 | −0.26 | 0.017 |
| Baseline CAPS-54 | 0.91 | 0.13 | 0.73 | <0.001 |
| Substance Use: TLFB Final Scores | ||||
| B | SE B | β | p | |
| Variable | ||||
| Intercept | 34.97 | 7.76 | <0.001 | |
| HRV | 6.82 | 3.49 | 0.34 | 0.059 |
| Condition | 6.80 | 7.79 | 0.15 | 0.389 |
| Interaction | −2.06 | 3.54 | −0.10 | 0.565 |
| Baseline TLFB5 | 0.26 | 0.19 | 0.23 | 0.171 |
Note.
Baseline resting heart rate variability.
Treatment condition.
Heart rate variability multiplied by treatment condition.
Clinician Administered PTSD Scale
Timeline Follow Back.
Figure 1.
Improvement in PTSD Symptoms by baseline resting HF-HRV and treatment condition. Values are presented as change scores (baseline minus final, such that higher scores represent greater change) for interpretability and comparability. CAPS-5 = Clinician Administered PTSD Scale, HF-HRV = high-frequency heart rate variability, CBT = Standard Cognitive Behavioral Therapy for substance use disorders, TIPSS = Treatment of Integrated Posttraumatic Stress and Substance Use
Notably, repetition of these analyses with only those participants meeting full diagnostic criteria for PTSD (n = 29; 17 in CBT, 12 in TIPSS) demonstrated a similar pattern of results for both PTSD [R2 = 0.814, F(4,24) = 11.75, p < 0.001] and substance use outcomes [R2 = 0.491, F(4,24) = 1.91, p = 0.141]. In the sample among only those with full PTSD, higher HF-HRV was still related to greater decreases in CAPS-5 final scores in the TIPSS condition (r = −.663, p = .019), while analyses failed to document such an association in the CBT condition (r = −0.008, p = 0.975). Similarly, repetition of these analyses with only those participants who were randomized (n = 31) demonstrated a similar pattern of results for both PTSD [R2 = 0.816, F(4,26) = 12.95, p < 0.001] and substance use outcomes [R2 = 0.433, F(4,26) = 1.50, p = 0.230]. In the randomized sample, higher HF-HRV was still related to a greater decrease in CAPS-5 final scores in the TIPSS condition but not significantly (r = −0.467, p = 0.126), while analyses failed to support such a relationship in the CBT condition (r = 0.081, p = 0.741).
3.3. Analyses Including Demographic Covariates
Inclusion of covariates (age, sex, and race) in the first step of a two-step regression revealed similar results to the main regression analyses. Step 1 (only covariates) did not predict CAPS-5 final scores, R2 = 0.288, F(3,33) = .99, p = 0.407. Step 2 (HF-HRV, condition, the interaction, baseline CAPS-5) predicted CAPS-5 final scores, R2 = 0.832, F(7,29) = 9.31, p < 0.001. However, while HF-HRV and treatment group condition still both predicted CAPS-5 final scores, inclusion of the covariates rendered the interaction non-significant (Table 3). Step 1 (only covariates) did not predict TLFB final scores, R2 = 0.274, F(3,33) = .89, p = 0.454. Step 2 (HF-HRV, condition, the interaction, and baseline TLFB) did not predict TLFB final scores, R2 = 0.424, F(7,29) = .91, p = 0.514. Inclusion of the covariates did not change the null effect of HF-HRV and treatment group on TLFB final scores (Table 3).
Table 3.
Covariate Analysis
| PTSD Symptoms: CAPS-5 Scores | ||||
|---|---|---|---|---|
| B | SE B | β | p | |
| Model 1 | ||||
| Intercept | 28.01 | 2.90 | <0.001 | |
| Age | −0.25 | 0.30 | −0.16 | 0.413 |
| Sex | 2.74 | 2.74 | 0.18 | 0.325 |
| Race | −2.39 | 3.06 | −0.14 | 0.440 |
| Model 2 | ||||
| Intercept | 23.84 | 2.02 | <0.001 | |
| Age | −0.33 | 0.23 | −0.21 | 0.162 |
| Sex | 0.07 | 1.78 | 0.01 | 0.967 |
| Race | −0.37 | 1.92 | −0.02 | 0.847 |
| HRV1 | −2.88 | 0.94 | −0.41 | 0.005 |
| Condition2 | −4.01 | 1.80 | −0.26 | 0.034 |
| Interaction3 | −1.58 | 0.83 | −0.21 | 0.069 |
| Baseline CAPS-54 | 0.88 | 0.13 | 0.71 | <0.001 |
| Substance Use: TLFB Scores | ||||
| B | SE B | β | p | |
| Model 1 | ||||
| Intercept | 33.64 | 8.24 | <0.001 | |
| Age | −0.74 | 0.87 | −0.16 | 0.400 |
| Sex | 5.84 | 7.80 | 0.14 | 0.460 |
| Race | 3.91 | 8.71 | 0.08 | 0.657 |
| Model 2 | ||||
| Intercept | 34.45 | 9.18 | <0.001 | |
| Age | 0.16 | 1.07 | 0.04 | 0.882 |
| Sex | 5.56 | 8.37 | 0.13 | 0.512 |
| Race | 2.34 | 8.83 | 0.49 | 0.793 |
| HRV1 | 6.97 | 4.39 | 0.35 | 0.124 |
| Condition2 | 6.81 | 8.13 | 0.15 | 0.409 |
| Interaction3 | −1.62 | 3.87 | −0.80 | 0.678 |
| Baseline TLFB5 | 0.22 | 0.21 | 0.20 | 0.288 |
Note.
Baseline resting heart rate variability.
Treatment condition.
Heart rate variability multiplied by treatment condition.
Clinician Administered PTSD Scale.
Timeline Follow Back.
4. Discussion
This initial study investigated the prognostic utility of a physiological biomarker of emotional regulation, baseline resting HF-HRV, in predicting treatment outcomes in individuals with SUD and PTSD symptoms, a common and difficult-to-treat presentation. Results partially supported the original hypotheses. Higher baseline resting HF-HRV predicted greater improvement in PTSD symptoms from baseline to end of treatment for both CBT and TIPSS conditions. HF-HRV failed to predict improvement in self-reported substance use as a function of treatment. These results held when only including individuals who were randomized to therapy condition. The nearly identical results when including individuals with and without full diagnostic criteria for PTSD suggested that HF-HRV might be relevant to a broad, heterogeneous trauma-exposed population.
Overall, participants with higher resting baseline HF-HRV had greater improvement in PTSD symptoms compared to those with lower HF-HRV. We expected HF-HRV might more strongly relate to outcomes in the TIPSS condition, as individuals in the TIPSS condition were encouraged to process their trauma-related cognitions and emotions during therapy, a procedure that may be more effective when individuals are better able to appropriately regulate the autonomic response to the emotional experience. While the main regression analyses suggested this was true, inclusion of covariates that are commonly associated with HF-HRV (i.e., age, sex, and race) made the interaction fall short of statistical significance, leading us to interpret this result with caution. However, the main effect of HF-HRV remained significant, even when accounting for important demographic variables, indicating baseline resting HF-HRV predicts PTSD symptom change, above and beyond theoretically-relevant demographic variables. While we suspected that HF-HRV, a potential autonomic index of emotion regulation would predict outcomes more so for the TIPSS condition than the CBT condition, it is still possible that this autonomic index also plays a role in CBT outcomes as well. For example, emotion regulation predicts CBT treatment for alcohol use and depression (Berking et al., 2011; Slee, Spinhoven, Garnefski, & Arensman, 2008). Alternatively, the reduction in the significance of the interaction could be due to the lack of power to detect this effect, given the limited sample size with inclusion of a number of covariates. Although preliminary, this recommends HF-HRV for examination in larger treatment studies as a moderator of treatment outcomes in SUD/PTSD populations. Future studies should also examine whether individual differences in HF-HRV at baseline relate to differences in autonomic and subjective emotional regulation during actual therapy sessions to confirm the proposed mechanism by which baseline resting HF-HRV relates to treatment outcomes.
Future studies might examine the clinical utility of assisting individuals to bolster their ability to regulate negative emotion, as indexed by baseline resting HF-HRV, before participating in SUD/PTSD treatment. Indeed, preliminary studies have reported that HRV biofeedback alone increased HF-HRV and decreased PTSD symptoms (Tan, Dao, Farmer, Sutherland, & Gevirtz, 2011; Zucker, Samuelson, Muench, Greenberg, & Gevirtz, 2009). In combination with the current study, these findings suggest that utilizing biofeedback to improve HF-HRV prior to trauma-focused psychotherapies might improve outcomes.
The current findings for substance use outcomes were not as clear. No significant relationships between HF-HRV and substance use final scores were found, suggesting that HF-HRV was not related to substance use changes due to treatment condition in the current study. The main effect of HF-HRV was in the opposite direction as PTSD symptom change, but it is cautioned against interpreting such an effect (p>.05) in this limited sample. One potential explanation for the null results with regard to substance use outcomes includes the short duration of the study. As substances are often used to cope with PTSD symptoms, it is possible that a change in PTSD symptoms would occur before changes in substance use during treatment (Hien, Cohen, & Campbell, 2005). Therefore, it is plausible that the duration of the study did not allow adequate time to observe improvements in substance use. A longer treatment course or post-treatment follow-up period might be better suited for measuring associations between HF-HRV and treatment outcomes for SUD/PTSD populations. Previous studies have indicated that HRV biofeedback reduces craving for various substances (Eddie, Kim, Lehrer, Deneke, & Bates, 2015; Zucker et al., 2009); however, no study has reported actual change in substance use behaviors. Future studies might focus upon clarifying these conflicting findings.
While these findings are generally in line with previous research, there were several limitations to the current study. First, as this was a preliminary study, the sample size was limited. A larger sample might have increased the power to detect a relationship between HF-HRV and substance use outcomes. Second, participant reports of daily substance use assessed by TLFB each session were not biochemically verified. This was not feasible mainly because of the inclusion of participants using a broad array of substances with different detection periods (e.g., cannabis is detectable in urine for longer periods than cocaine, while alcohol is only detectable in breath for a matter of hours). However, according to a meta-analysis, the TLFB validly detects substance use patterns when compared to biological verification (Hjorthøj, Hjorthøj, & Nordentoft, 2012). Third, while the majority of the sample reported polysubstance use, cocaine was the most common primary substance followed by alcohol. Therefore, these results may not generalize to other substances such as opioids or cannabis. Additionally, the small frequencies of other substances did now allow investigation of group differences, a question future studies should address. Fourth, HF-HRV differed between the conditions at baseline, with the standard CBT for SUD condition manifesting higher HF-HRV than the TIPSS condition. Future replication studies might consider HF-HRV when randomizing individuals into treatment conditions. Fifth, due to technical issues affecting collection of respiration rate data, we were unable to control for potential influences of respiration rate on HF-HRV in this study (Berntson, Cacioppo, & Grossman, 2007). Therefore, we cannot rule out respiration rate as a potential generator of the individual differences observed and cannot draw firm conclusions about the neurophysiological processes (e.g., vagal tone) responsible for the differences in outcomes. Future studies should examine the separate contributions of HF-HRV and respiration rate to treatment outcomes. Finally, the individuals included in the study were restricted to treatment completers and therefore may not generalize to who dropped out, although HF-HRV did not differ between those who dropped out and those who completed the study.
In summary, these results suggest that HF-HRV might be useful in detecting which individuals with SUD and comorbid PTSD symptoms will respond better to treatment. Given that this presentation is both extremely common and very treatment resistant, these results may have important clinical implications. For example, those with high baseline resting HF-HRV might be better equipped physiologically to adjust their emotional arousal during therapy to achieve the proper amount of engagement needed for successful treatment, regardless of the type of treatment. Future studies should look to investigate this proposed mechanism by which HF-HRV predicts outcomes in larger samples that are powered to detect differences in treatment types. One potential path to investigate in the future includes the utility of HRV biofeedback prior to psychotherapy to better prepare the patient for regulating autonomic responses during treatment (Tan et al., 2011; Zucker et al., 2009).
Acknowledgments
This study was funded by a National Institutes of Health KL2 Career Development Award (KL2TR000370-07: PI: Vujanovic). The work was also supported by the National Institute on Drug Abuse (P50 DA009262; PIs: Schmitz, Lane, Green) and a National Institutes of Health K08 Career Development Award (5K08DA040006-03, PI: Wardle). The authors would like to acknowledge Johann D’Souza and Sarwar Khan for their contribution to analyzing the heart rate variability data. The authors report no conflict of interest. Reprints can be sent to Anka Vujanovic at aavujano@central.uh.edu.
Footnotes
Twelve participants were collected during an extended pilot phase at the beginning of the study, and were assigned to treatment based on availability of a trained therapist, rather than being randomized. Of those 12, 10 received CBT and 2 of them received TIPSS. For the purposes of this analysis, we chose to include the non-randomized individuals for several reasons: 1) to maintain the largest sample size possible for the most accurate estimation of the effect of HF-HRV, 2) including the non-randomized individuals still resulted in balanced demographics across the groups, and 3) excluding them made no difference in the primary results.
Two participants did not complete the HRV session. Two more were excluded because at the time of HRV analysis, there were concerns about validity of one person’s outcome data and another person’s meeting inclusion criteria.
This was also true for the subgroup analysis including only randomized individuals, X2 = 1.916, p = 0.166.
References
- Acierno R, Resnick H, Kilpatrick DG, Saunders B, & Best CL (1999). Risk factors for rape, physical assault, and posttraumatic stress disorder in women: Examination of differential multivariate relationships. Journal of Anxiety Disorders, 13(6), 541–563. 10.1016/S0887-6185(99)00030-4 [DOI] [PubMed] [Google Scholar]
- Appelhans BM, & Luecken LJ (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10(3), 229–240. 10.1037/1089-2680.10.3.229 [DOI] [Google Scholar]
- Assmann SF, Pocock SJ, Enos LE, & Kasten LE (2000). Subgroup analysis and other (mis)uses of baseline data in clinical trials. The Lancet, 355(9209), 1064–1069. 10.1016/S0140-6736(00)02039-0 [DOI] [PubMed] [Google Scholar]
- Balzarotti S, Biassoni F, Colombo B, & Ciceri MR (2017). Cardiac vagal control as a marker of emotion regulation in healthy adults: A review. Biological Psychology, 130(April), 54–66. 10.1016/j.biopsycho.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Berking M, Margraf M, Ebert D, Wupperman P, Hofmann SG, & Junghanns K (2011). Deficits in emotion-regulation skills predict alcohol use during and after cognitive-behavioral therapy for alcohol dependence. Journal of Consulting and Clinical Psychology, 79(3), 307–318. 10.1037/a0023421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Grossman P (2007). Whither vagal tone. Biological Psychology, 74(2), 295–300. 10.1016/J.BIOPSYCHO.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Bonin MF, Norton GR, Asmundson GJ, Dicurzio S, & Pidlubney S (2000). Drinking away the hurt: The nature and prevalence of PTSD in substance abuse patients attending a community-based treatment program. Journal of Behavior Therapy and Experimental Psychiatry, 31(1), 55–66. 10.1016/S0005-7916(00)00008-2 [DOI] [PubMed] [Google Scholar]
- Bonn-Miller MO, Vujanovic AA, Boden MT, & Gross JJ (2011). Posttraumatic stress, difficulties in emotion regulation, and coping-oriented marijuana use. Cognitive Behaviour Therapy, 40(1), 34–44. 10.1080/16506073.2010.525253 [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL, & Schultz LR (1997). Sex differences in posttraumatic stress disorder. Archives of General Psychiatry, 54(11), 1044–1048. 10.1001/archpsyc.1997.01830230082012 [DOI] [PubMed] [Google Scholar]
- Brown PJ, Stout RL, & Mueller T (1999). Substance use disorder and posttraumatic stress disorder comorbidity: Addiction and psychiatric treatment rates. Psychology of Addictive Behaviors, 13(2), 115–122. 10.1037/0893-164X.13.2.115 [DOI] [Google Scholar]
- Chalmers JA, Quintana DS, Abbott MJA, & Kemp AH (2014). Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Frontiers in Psychiatry, 5(JUL), 80 10.3389/fpsyt.2014.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcoat HD, & Breslau N (1998). Posttraumatic stress disorder and drug disorders: Testing causal pathways. Archives of General Psychiatry, 55(10), 913–917. 10.1001/archpsyc.55.10.913 [DOI] [PubMed] [Google Scholar]
- Cloitre M, Koenen KC, Cohen LR, Luke ‘s S, Hospital R, & Han H (2002). Skills training in affective and interpersonal regulation followed by exposure: A phase-based treatment for PTSD related to childhood abuse. Journal of Consulting and Clinical Psychology\, 70(5), 1067–1074. 10.1037//0022-006X.70.5.1067 [DOI] [PubMed] [Google Scholar]
- Coffey SF, Saladin ME, Drobes DJ, Brady KT, Dansky BS, & Kilpatrick DG (2002). Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug and Alcohol Dependence, 65(2), 115–127. 10.1016/S0376-8716(01)00157-0 [DOI] [PubMed] [Google Scholar]
- Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, & Kotler M (2000). Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: Application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Research, 96(1), 1–13. 10.1016/S0165-1781(00)00195-5 [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, & Aiken LS (2003). Applied multiple regression/correlation analysis for the behavioral sciences (3rd ed.). Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Cottler LB, Ben Abdallah A, Cummings SM, Barr J, Banks R, & Forchheimer R (2011). Injury, pain, and prescription opioid use among former National Football League (NFL) players. Drug and Alcohol Dependence, 116(1–3), 188–194. 10.1016/j.drugalcdep.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddie D, Kim C, Lehrer P, Deneke E, & Bates ME (2015). A pilot study of brief heart rate variability biofeedback to reduce craving in young adult men receiving inpatient treatment for substance use disorders. Appl Psychophysiol Biofeedback, 39(3–4), 181–192. 10.1007/s10484-014-9251-z.A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassino S, Pierò A, Delsedime N, Busso F, Ferrero A, Mongelli E, … Daga GA (2004). Rehabilitative residential treatment in patients with severe mental disorders: personality features associated with short-term outcome. American Journal of Orthopsychiatry, 74(1), 33–42. 10.1037/0002-9432.74.1.33 [DOI] [PubMed] [Google Scholar]
- Ford JD, Hawke J, Alessi S, Ledgerwood D, & Petry N (2007). Psychological trauma and PTSD symptoms as predictors of substance dependence treatment outcomes. Behaviour Research and Therapy, 45(10), 2417–2431. 10.1016/J.BRAT.2007.04.001 [DOI] [PubMed] [Google Scholar]
- Garland EL, Franken IHA, & Howard MO (2012). Cue-elicited heart rate variability and attentional bias predict alcohol relapse following treatment. Psychopharmacology, 222(1), 17–26. 10.1007/s00213-011-2618-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz KL, & Tull MT (2010). Emotion regulation as a mechanism of change in acceptance- and mindfulness-based treatments (Baer RA, Ed.), Assessing mindfulness and acceptance processes in clients: Illuminating the theory and practice of change. Oakland, CA: New Harbinger Publications; 10.1037/a0022144 [DOI] [Google Scholar]
- Halligan SL, Michael T, Clark DM, & Ehlers A (2003). Posttraumatic stress disorder following assault: The role of cognitive processing, trauma memory, and appraisals. Journal of Consulting and Clinical Psychology, 71(3), 419–431. 10.1037/0022-006X.71.3.419 [DOI] [PubMed] [Google Scholar]
- Hien D, Cohen L, & Campbell A (2005). Is traumatic stress a vulnerability factor for women with substance use disorders? Clinical Psychology Review, 25(6), 813–823. 10.1016/J.CPR.2005.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorthøj CR, Hjorthøj AR, & Nordentoft M (2012). Validity of Timeline Follow-Back for self-reported use of cannabis and other illicit substances - Systematic review and meta-analysis. Addictive Behaviors, 37(3), 225–233. 10.1016/j.addbeh.2011.11.025 [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, & Thayer JF (2003). Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry, 54(12), 1427–1436. 10.1016/S0006-3223(02)01926-1 [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang P (2010). Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167(7), 748–751. 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM, & Kosten TR (2001). Substance use disorders in patients with posttraumatic stress disorder: A review of the literature. American Journal of Psychiatry, 158(8), 1184–1190. 10.1176/appi.ajp.158.8.1184 [DOI] [PubMed] [Google Scholar]
- Jaycox L, Foa EB, & Morral A (1998). Influence of emotional engagement and habituation on exposure therapy for PTSD. Journal of Consulting and Clinical Psychology, 66, 185–192. 10.1037//0022-006X.66.1.185 [DOI] [PubMed] [Google Scholar]
- Kaysen D, Neighbors C, Martell J, Fossos N, & Larimer ME (2006). Incapacitated rape and alcohol use: A prospective analysis. Addictive Behaviors, 31(10), 1820–1832. 10.1016/j.addbeh.2005.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ (1999). Treating addiction as a human process. (Aronson J, Ed.). Northvale, NJ. [Google Scholar]
- Krueger RF, & Markon KE (2006). Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology, 2(1), 111–133. 10.1146/annurev.clinpsy.2.022305.095213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby DJ, Worhunsky PD, Pilver CE, & Brewer JA (2012). Meditation-induced changes in high-frequency heart rate variability predict smoking outcomes. Frontiers in Human Neuroscience, 6, 54 10.3389/fnhum.2012.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton H, Horsley S, John S, & Nelson DV (2007). Trauma coping strategies and psychological distress: A meta-analysis. Journal of Traumatic Stress, 20(6), 977–988. 10.1002/jts.20276 [DOI] [PubMed] [Google Scholar]
- Lyonfields JD, Borkovec TD, & Thayer JF (1995). Vagal tone in generalized anxiety disorder and the effects of aversive imagery and worrisome thinking. Behavior Therapy, 26(3), 457–466. 10.1016/S0005-7894(05)80094-2 [DOI] [Google Scholar]
- Mathewson KJ, Schmidt LA, Miskovic V, Santesso DL, Duku E, McCabe RE, … Moscovitch DA (2013). Does respiratory sinus arrhythmia (RSA) predict anxiety reduction during cognitive behavioral therapy (CBT) for social anxiety disorder (SAD)? International Journal of Psychophysiology, 88(2), 171–181. 10.1016/J.IJPSYCHO.2013.03.016 [DOI] [PubMed] [Google Scholar]
- Mccauley JL, Killeen T, Gros DF, Brady KT, & Back SE (2012). Posttraumatic stress disorder and co-occurring substance use disorders: advances in assessment and treatment. Clinical Psychology: Science and Practice, 19(3), 283–304. 10.1111/cpsp.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MJ, Tull MT, Gratz KL, Daughters SB, & Lejuez CW (2009). The role of anxiety sensitivity and difficulties in emotion regulation in posttraumatic stress disorder among crack/cocaine dependent patients in residential substance abuse treatment. Journal of Anxiety Disorders, 23(5), 591–599. 10.1016/J.JANXDIS.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer MA, Risbrough VB, … Gorman P (2015). Association of predeployment heart rate variability with risk of postdeployment posttraumatic stress disorder in active-duty marines. JAMA Psychiatry, 72(10), 979–986. 10.1001/jamapsychiatry.2015.0922 [DOI] [PubMed] [Google Scholar]
- Muhtadie L, Koslov K, Akinola M, & Mendes WB (2015). Vagal flexibility: A physiological predictor of social sensitivity. Journal of Personality and Social Psychology, 109(1), 106–120. 10.1037/pspp0000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najavits LM, Thase ME, Harned MS, Barber JP, Butler SF, Crits-Christoph P, & Gallop RJ (2015). Six-month treatment outcomes of cocaine-dependent patients with and without PTSD in a multisite national trial. Journal of Studies on Alcohol and Drugs, 68(3), 353–361. 10.15288/jsad.2007.68.353 [DOI] [PubMed] [Google Scholar]
- Norman SB, Tate SR, Anderson KG, & Brown SA (2007). Do trauma history and PTSD symptoms influence addiction relapse context? Drug and Alcohol Dependence, 90(1), 89–96. 10.1016/J.DRUGALCDEP.2007.03.002 [DOI] [PubMed] [Google Scholar]
- North CS, Nixon SJ, Shariat S, Mallonee S, McMillen JC, Spitznagel EL, & Smith EM (1999). Psychiatric disorders among survivors of the Oklahoma City bombing. Journal of the American Medical Association, 282(8), 755–762. 10.1001/jama.282.8.755 [DOI] [PubMed] [Google Scholar]
- O’Connor M-F, Allen JJB, & Kaszniak AW (2005). Emotional disclosure for whom?: A study of vagal tone in bereavement. Biological Psychology, 68(2), 135–146. 10.1016/J.BIOPSYCHO.2004.04.003 [DOI] [PubMed] [Google Scholar]
- O’Hare T, & Sherrer M (2011). Drinking motives as mediators between PTSD symptom severity and alcohol consumption in persons with severe mental illnesses. Addictive Behaviors, 36(5), 465–469. 10.1016/j.addbeh.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Ouimette P, Moos RH, & Finney JW (2003). PTSD treatment and 5-year remission among patients with substance use and posttraumatic stress disorders. Journal of Consulting and Clinical Psychology, 71(2), 410–414. 10.1037/0022-006X.71.2.410 [DOI] [PubMed] [Google Scholar]
- Pocock SJ, Assmann SE, Enos LE, & Kasten LE (2002). Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. STATISTICS IN MEDICINE Statist. Med, 21, 2917–2930. 10.1002/sim.1296 [DOI] [PubMed] [Google Scholar]
- Porges SW (1985, December 30). Method and apparatus for evaluating rhythmic oscillations in aperiodic physiological response systems. U.S. Patents. Retrieved from https://patents.google.com/patent/US4510944A/en [Google Scholar]
- Porges SW (2001). The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology, 42(2), 123–146. 10.1016/S0167-8760(01)00162-3 [DOI] [PubMed] [Google Scholar]
- Porges SW, & Bohrer RE (1990). The analysis of periodic processes in psychophysiological research In Principles of psychophysiology: Physical, social, and inferential elements (pp. 708–753). Cambridge University Press; Retrieved from http://psycnet.apa.org/record/1990-98757-021 [Google Scholar]
- Reed PL, Anthony JC, & Breslau N (2007). Incidence of drug problems in young adults exposed to trauma and posttraumatic stress disorder. Archives of General Psychiatry, 64(12), 1435 10.1001/archpsyc.64.12.1435 [DOI] [PubMed] [Google Scholar]
- Saladin ME, Drobes DJ, Coffey SF, Dansky BS, Brady KT, & Kilpatrick DG (2003). PTSD symptom severity as a predictor of cue-elicited drug craving in victims of violent crime. Addictive Behaviors, 28(9), 1611–1629. 10.1016/j.addbeh.2003.08.037 [DOI] [PubMed] [Google Scholar]
- Seligowski AV, Lee DJ, Bardeen JR, & Orcutt HK (2015). Emotion regulation and posttraumatic stress symptoms: A meta-analysis. Cognitive Behaviour Therapy, 44(2), 87–102. 10.1080/16506073.2014.980753 [DOI] [PubMed] [Google Scholar]
- Senn S (2006). Change from baseline and analysis of covariance revisited. Statistics in Medicine, 25(24), 4334–4344. 10.1002/sim.2682 [DOI] [PubMed] [Google Scholar]
- Sharkansky EJ, Brief DJ, Peirce JM, Meehan JC, & Mannix LM (1999). Substance abuse patients with posttraumatic stress disorder (PTSD): Identifying specific triggers of substance use and their associations with PTSD symptoms. Psychology of Addictive Behaviors, 13(2), 89–97. 10.1037/0893-164X.13.2.89 [DOI] [Google Scholar]
- Simpson TL, Stappenbeck CA, Varra AA, Moore SA, & Kaysen D (2012). Symptoms of posttraumatic stress predict craving among alcohol treatment seekers: Results of a daily monitoring study. Psychology of Addictive Behaviors, 26(4), 724–733. 10.1037/a0027169 [DOI] [PubMed] [Google Scholar]
- Slee N, Spinhoven P, Garnefski N, & Arensman E (2008). Emotion regulation as mediator of treatment outcome in therapy for deliberate self-harm. Clinical Psychology and Psychotherapy, 15(4), 205–216. 10.1002/cpp.577 [DOI] [PubMed] [Google Scholar]
- Sloan DM, & Epstein EM (2005). Respiratory sinus arrhythmia predicts written disclosure outcome. Psychophysiology, 42(5), 611–615. 10.1111/j.0048-5772.2005.347.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline Follow-Back In Measuring Alcohol Consumption (pp. 41–72). Totowa, NJ: Humana Press; 10.1007/978-1-4612-0357-5_3 [DOI] [Google Scholar]
- Stewart SH, & Conrod PJ (2003). Psychosocial models of functional associations between posttraumatic stress disorder and substance use disorder In Trauma and substance abuse: Causes, consequences, and treatment of comorbid disorders. BT - Trauma and substance abuse: Causes, consequences, and treatment of comorbid disorders. (pp. 29–55). Washington: American Psychological Association; 10.1037/10460-002 [DOI] [Google Scholar]
- Stewart SH, Conrod PJ, Barton Samoluk S, Pihl RO, & Dongier M (2000). Posttraumatic stress disorder symptoms and situation-specific drinking in women substance abusers. Alcoholism Treatment Quarterly, 18(3), 19–30. 10.1300/J020v18n03_04 [DOI] [Google Scholar]
- Stotts AL, Vujanovic A, Heads A, Suchting R, Green CE, & Schmitz JM (2015). The role of avoidance and inflexibility in characterizing response to contingency management for cocaine use disorders: A secondary profile analysis. Psychology of Addictive Behaviors, 29(2), 408–413. 10.1037/adb0000011 [DOI] [PubMed] [Google Scholar]
- Tan G, Dao TK, Farmer L, Sutherland RJ, & Gevirtz R (2011). Heart rate variability (HRV) and posttraumatic stress disorder (PTSD): A pilot study. Applied Psychophysiology Biofeedback, 36(1), 27–35. 10.1007/s10484-010-9141-y [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, & Borkovec TD (1996). Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry, 39(4), 255–266. 10.1016/0006-3223(95)00136-0 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, & Johnsen BH (2009, April 8). Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health Annals of Behavioral Medicine. Oxford University Press; 10.1007/s12160-009-9101-z [DOI] [PubMed] [Google Scholar]
- Tull MT, Barrett HM, McMillan ES, & Roemer L (2007). A preliminary investigation of the relationship between emotion regulation difficulties and posttraumatic stress symptoms. Behavior Therapy, 38(3), 303–313. 10.1016/j.beth.2006.10.001 [DOI] [PubMed] [Google Scholar]
- Ullman SE, Relyea M, Peter-Hagene L, & Vasquez AL (2013). Trauma histories, substance use coping, PTSD, and problem substance use among sexual assault victims. Addictive Behaviors, 38(6), 2219–2223. 10.1016/j.addbeh.2013.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breukelen GJP (2013). ANCOVA versus CHANGE From baseline in nonrandomized studies: The difference. Multivariate Behavioral Research, 48(6), 895–922. 10.1080/00273171.2013.831743 [DOI] [PubMed] [Google Scholar]
- Vickers AJ (2001). The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Medical Research Methodology, 1(1), 6 10.1186/1471-2288-1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers AJ (2005). Parametric versus non-parametric statistics in the analysis of randomized trials with non-normally distributed data. BMC Medical Research Methodology, 5, 35 10.1186/1471-2288-5-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victorson D, Farmer L, Burnett K, Ouellette A, & Barocas J (2005). Maladaptive coping strategies and injury-related distress following traumatic physical injury. Rehabilitation Psychology, 50(4), 408–415. 10.1037/0090-5550.50.4.408 [DOI] [Google Scholar]
- Vujanovic AA, Green CE, Lane SD, & Schmitz JM (2018). A novel, integrated cognitive-behavioral therapy for co-occurring posttraumatic stress and substance use disorders: A pilot randomized controlled trial. Manuscript Submitted for Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujanovic AA, Smith LJ, Green CE, Lane SD, & Schmitz JM (2018). Development of a novel, integrated cognitive-behavioral therapy for co-occurring posttraumatic stress and substance use disorders: A pilot randomized clinical trial. Contemporary Clinical Trials, 65, 123–129. 10.1016/j.cct.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujanovic AA, Smith LJ, Tipton KP, & Schmitz JM (2018, April 27). A novel, integrated cognitive-behavioral therapy for co-occurring posttraumatic stress and substance use disorders: A case study. Cognitive and Behavioral Practice. 10.1016/J.CBPRA.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujanovic AA, Wardle MC, Bakhshaie J, Smith LJ, Green CE, Lane SD, & Schmitz JM (2018). Distress tolerance: Associations with trauma and substance cue reactivity in low-income, inner-city adults with substance use disorders and posttraumatic stress. Psychology of Addictive Behaviors, 32(3), 264–276. 10.1037/adb0000362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop AE, Back SE, Verduin ML, & Brady KT (2007). Triggers for cocaine and alcohol use in the presence and absence of posttraumatic stress disorder. Addictive Behaviors, 32(3), 634–639. 10.1016/j.addbeh.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Blake D, Schnurr P, Kaloupek D, Marx B, & Keane T (2013). The clinician-administered PTSD scale for DSM-5 (CAPS-5). Interview Available from the National Center for PTSD at Www.Ptsd.va.Gov.
- Weiss NH, Tull MT, Viana AG, Anestis MD, & Gratz KL (2012). Impulsive behaviors as an emotion regulation strategy: Examining associations between PTSD, emotion dysregulation, and impulsive behaviors among substance dependent inpatients. Journal of Anxiety Disorders, 26(3), 453–458. 10.1016/J.JANXDIS.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf EJ, Miller MW, Krueger RF, Lyons MJ, Tsuang MT, & Koenen KC (2010). Posttraumatic stress disorder and the genetic structure of comorbidity. Journal of Abnormal Psychology, 119(2), 320–330. 10.1037/a0019035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker TL, Samuelson KW, Muench F, Greenberg MA, & Gevirtz RN (2009). The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: A pilot study. Applied Psychophysiology Biofeedback, 34(2), 135–143. 10.1007/s10484-009-9085-2 [DOI] [PubMed] [Google Scholar]